Abstract
Purpose:
To determine predictive factors and risk scores for conversion to overall advanced age-related macular degeneration (AMD), geographic atrophy (GA), neovascular disease (NV), and loss of vision, and to validate the model for AMD in an external cohort.
Methods:
Progression to advanced AMD was evaluated using stepwise survival analysis. Risk scores including genetic, demographic, behavioral, and ocular factors were derived for three AMD endpoints and were validated and calibrated in a large independent cohort. Vision loss of 15 or more letters was evaluated as a new endpoint in genetic analyses.
Results:
Eight common and rare variants in genes CFH, C3, ARMS2, COL8A1, and HSPH1/B3GALTL conferred a significantly higher risk of transition to advanced AMD. Three loci (C2, CFB, RAD51B) were associated with lower rate of progression. A protective effect was suggested for CTRB1 and PELI3. The age-adjusted area under the curve (AUC) for the composite model including 13 loci model was 0.900 over 12 years (0.896 in the validation cohort). Progressors had a higher risk category when genetic factors were considered and there was heterogeneity between models for GA and NV. The model was calibrated in the validation cohort. Determinants of visual loss included age, education, BMI, smoking, and several common and rare genetic variants.
Conclusion:
Eyes with the same macular grade had higher or lower risk of subsequent progression and visual loss based on the validated risk score. Identifying high risk individuals at an earlier stage using predictive modeling could lead to improved preventive and therapeutic strategies in the era of precision medicine.
INTRODUCTION
Age-related macular degeneration (AMD) is a progressive and degenerative disease affecting the central part of the retina, and is the leading cause of irreversible vision loss in the United States.1–3 The prevalence of this disease is rising with the growth of our aging population. Over 1.75 million individuals in the United States have the advanced forms of AMD, a number that is projected to rise dramatically.4 The world-wide prevalence for all AMD was estimated at 196 million people, and the global burden of disease will likely increase to 288 million by 2040.5 While the visual impact associated with early and intermediate stages of AMD can be minimal, some affected individuals progress to advanced disease. These advanced subtypes of geographic atrophy (GA) and neovascular disease (NV) are commonly associated with visual impairment and blindness, affecting quality of life and leading to loss of independence.6–8 Although anti-vascular endothelial growth factor (VEGF) injections are an effective treatment for many patients with NV,9 some patients do not respond, there is a large treatment burden, and visual loss continues over time. There is no treatment for GA. Thus, prevention of advanced disease and finding new and effective treatments remains a significant challenge. Identifying individuals with early and intermediate disease at high risk of progression to advanced stages would lead to earlier intervention and reduced burden of visual loss due to AMD.
The etiology of AMD is multifactorial, with both genetic and modifiable factors contributing to personal risk. The network of modifiable factors associated with reducing AMD risk is well established and highlights the importance of a healthy lifestyle.3 Despite the potential modification of disease risk through diet and healthy behaviors, genetic factors confer substantial risk in AMD onset and progression10 and cannot, at present, be modified. The identification of the impact of genetic risk factors on progression is therefore critical in the context of clinical care and disease management.
AMD is a common, polygenic disease wherein multiple common variants, defined as variants with a minor allele frequency ≥ 5%, contribute varying amounts to personal risk. Genome-wide association studies (GWAS) have been instrumental in the identification of these common variants, including complement factor H (CFH) Y402H, CFH (rs1410996), complement factor B (CFB), complement component 2 (C2), complement component 3 (C3), and complement factor I (CFI).11–16 These complement-related loci are now well established in their roles to confer AMD risk, and lend further support to the theory that inflammation and immune processes plays a critical role in the pathogenesis of AMD.17,18 Common loci in the angiogenesis, extracellular matrix, and immune pathways have also been identified as AMD risk factors.3,19–25
Rare and low frequency variation, defined as a minor allele frequency < 5%, is carried by a smaller proportion of the population, although these variants have larger effect sizes and functional impact. For instance, the first confirmed rare variant for AMD, CFH R1210C, confers the strongest genetic risk for AMD to date, with an odds ratio (OR) greater than 20.26 This mutation is also associated with an earlier age at advanced AMD diagnosis,26 extensive drusen accumulation throughout the macula, and extramacular drusen.27 Using methodology we proposed in 2010 to study densely affected families not explained by known loci,28 other rare variants in CFH were discovered.29–31 Rare variants in C3 (K155Q), CFI and complement component 9 (C9, P167S) also confer AMD risk,32,33 whereas the low frequency variant CFH1050Y and rare pellino E3 ubiquitin protein ligase family member 3 (PELI3) variant have shown a protective effect.19
These loci have been reported to confer varying levels of risk of AMD prevalence in case-control studies. However, many are also strong genetic predictors of progression of this disease over time, as determined by the analysis of large, prospective cohorts.34–40 Progression is typically defined as the transition from early and intermediate stages to advanced clinical phenotypes. CFH Y402H and ARMS2 were the first loci determined to be independently and significantly associated with progression,34 followed by CFH rs1410996, C2 E318D, CFB R32Q, C3 R102G, RAD51B, and COL8A1,35,36,39 as well as the highly penetrant, rare variants CFH R1210C and C3 K155Q.39,40 In a separate study evaluating specific transitions between AMD disease states, two additional variants in the lipid pathway were determined to affect progression from one stage to another.37 LIPC conferred a protective effect against transitioning from intermediate disease to NV, and ABCA1 was associated with a lower risk of progression from early to intermediate AMD.37
Many loci have been shown to be related to both GA and NV, namely those found in the complement pathway, and studies have assessed whether some variants may be more strongly associated with one advanced subtype compared to the other.34,37,41,42 ARMS2 was the first locus identified to confer a greater risk of developing NV compared to GA.41,42 A recent genome-wide association study confirmed this relationship for ARMS2, and suggested variants in MMP9, CETP, and SYN3-TIMP3 loci may also differ between the two advanced subtypes.43 No other variants associated with advanced disease have been determined to have a differential effect on the advanced AMD subtypes.
Gene therapy is not yet available for AMD prevention or management. However, since new genetic risk factors for AMD are being identified, particularly the rare variants with larger effect sizes, and gene therapies for AMD are currently being developed to target specific genotypes in the era of precision medicine, it is increasingly important to consider the utility of evaluating individual genetic susceptibility, especially for progression to advanced stages. Interest in predictive modeling is therefore growing and proper methodology is essential.44
Since 2006, we have developed a series of algorithms that predict risk for progression to advanced stages of AMD over time, and these models have achieved high predictability of up to 0.94, with perfect discrimination between groups indicated by a value of 1.0.14,34–40 Although numerous loci are associated with AMD risk, only a subset has been evaluated prospectively to determine associations with risk of progression to advanced disease. We hypothesize that a large subset of genetic factors will be predictive of progression to overall advanced AMD, together with other predictors, and will aid in accurately identifying high risk individuals who will develop vision-threatening AMD in the future. We also hypothesize that there will be differences in risk profiles for progression to the two distinct clinical manifestations of GA and NV.
It is not sufficient to identify the set of genetic factors that best determine which patients will progress to advanced stages of AMD and which patients will not. These risk factors must be validated in order to influence the management of this disease. Our study reported herein enhances the existing AMD literature by adding validation of the model using a large external cohort with similar baseline stages of AMD and the same co-variates in the model.
This study also expands the scope of predictive modeling to a functional endpoint, since despite the progressive visual impairment associated with advanced stages of AMD, the genetics underlying visual acuity (VA) loss have not been evaluated, and no composite model has been established for this endpoint. We, therefore, also test the hypothesis that several genetic factors are related to visual loss and calculate a prediction model for progression to this functional endpoint.
METHODS
OVERVIEW
Predictors of progression to advanced disease, GA and NV outcomes, and derivation of the risk prediction model were initially assessed in the AREDS cohort, the “derivation” cohort. The model was then validated using the independent Seddon Longitudinal Cohort, the “validation cohort”. Calibration of the derived model in the validation cohort was determined. The impact of genetic factors in addition to demographic, behavioral, and ocular predictors on risk of progression to advanced AMD was evaluated. A separate prediction model was derived for visual loss of 15 or more letters using the AREDS cohort. Sample cases with varying risk scores and subsequent outcomes are presented, which can be reviewed in the on-line risk calculator, www.seddonamdriskscore.org.45
We implemented rigorous methods in these prospective analyses, for both derivation of the prediction models and to ascertain the validity of our results. In previous studies we used eye specific analyses, including studies of risk factors for progression over time,27,46–50 which considers that individual eyes can progress to different stages of disease and at different time points. In contrast, person specific analyses results in classification of the individual as a progressor or non-progressor when the first eye progresses.
Validation of the derived composite model included the following: 1) application of a genetic risk model derived from AREDS to an external, independent cohort with the similar demographic, lifestyle, ocular and genetic data; 2) determination of the sensitivity and specificity of this risk model in the validation cohort to evaluate the accurate classification of the disease outcome; and 3) calibration of the model in the external cohort. We also report new analyses: calculation of the net reclassification improvement (NRI), a measure of model assessment and quantification of the contribution of genetic factors that has not been previously applied to AMD progression, and statistical assessment of the differences or heterogeneity of the models for GA and NV. Vali dation of risk factors enhances the likelihood that the prediction model will be generalizable and useful in detecting high risk individuals for inclusion in clinical trials and for potential discovery of new treatments.
DERIVATION COHORT: AGE-RELATED EYE DISEASE STUDY POPULATION
Data from the Age-Related Eye Disease Study (AREDS), a multicenter randomized clinical trial, were used in analyses to develop the model (referred to herein as the derivation cohort). All research adhered to the tenets of the Declaration of Helsinki and was performed under approved institutional review board protocol prior to the initiation of the study. The protocol was approved by a data and safety monitoring committee for 11 participating ophthalmic centers. Written informed consent was obtained from all participants prior to enrollment. This trial was registered at clinicaltrials.gov as NCT00594672.
Details of the AREDS have been previously reported.51 The AREDS evaluated the effect of antioxidant and mineral supplements on age-related macular degeneration (AMD) and cataract risk, and assessed progression to advanced stages of AMD. Participants were aged 55 to 80 years old at baseline and were required to have at least one eye with a VA no worse than 20/32. At least one eye was also required to be free from eye disease that might complicate the assessment of AMD, and the eye could not have had previous ocular surgery except for cataract extraction and unilateral photocoagulation for AMD. Participants were excluded from enrollment based on illness or other disorders that would complicate long-term longitudinal follow up or compliance with the study protocol. This study enrolled a total of 4,757 participants in the United States from 1992 to 1998. This analysis included 2,894 individuals or 5,600 eyes, of which 5,355 had complete genetic data. In this cohort, 1193 eyes progressed to advanced AMD: 599 to GA and 704 to NV (could also transition from GA). Among the 1149 progressing eyes with complete genetic data, 578 progressed to GA and 677 to NV. The mean follow-up time was 9.3 years and the interquartile range was 8 to 11 years.
VALIDATION COHORT: SEDDON LONGITUDINAL AMD REGISTRY AND BIOREPOSITORY COHORT
Risk prediction models derived from the AREDS cohort were validated using data from the Seddon Longitudinal Cohort, a large independent AMD cohort. All participants were enrolled in ongoing genetic and epidemiologic studies of AMD including a registry and biorepository of genetic and other biologic samples, as well as prospective assessment of progression and risk factors for disease, beginning in 1985 (J.M.S, Principal Investigator). Participants were derived from clinic populations and nationwide referrals and were prospectively followed. This research adhered to the tenets of the Declaration of Helsinki and was performed under approved institutional review board protocol. Written informed consent was obtained for all participants.
A total of 2,865 participants were recruited for the Seddon Longitudinal AMD Cohort. The selection criteria are outlined in Figure 1. Subjects were eligible for this study of disease progression if they had at least one eye with non-advanced AMD at baseline and at least one year of follow-up. Participants with advanced disease in both eyes at baseline could not progress to advanced AMD, and therefore were not eligible for inclusion in the analyses reported herein. In order to maintain consistency with AREDS (the derivation cohort), for these analyses only the subset of eligible participants in the validation cohort between 55 and 80 years at the baseline visit, were included. A total of 2,497 participants met the above inclusion criteria.
FIGURE 1.
Flow chart illustrating the selection of participants for inclusion in the validation cohort from the Seddon Longitudinal Age-Related Macular Degeneration Cohort.
In addition, exclusions were made for the following: 1) incomplete genetic data required for validation of the risk prediction algorithm derived from the AREDS cohort; and 2) incomplete lifestyle data, including assessments for level of education, BMI, and smoking. A total of 341 participants were excluded based on these criteria. The final validation cohort for analysis comprised 2156 participants and 3955 individual eyes. In the validation cohort with complete genetic data, 686 eyes progressed to advanced AMD: 357 to GA and 364 to NV. The mean follow up time was 9.8 years with an interquartile range of 7.9 to 12.0 years.
Ocular Examinations and Clinical Records
For the validation cohort, a baseline ocular examination was conducted upon enrollment into the study. The participant’s current ophthalmologist or another ophthalmologist who agreed to participate was recruited to perform the examination. Detailed protocols and standardized clinical data forms were designed by J.M.S. Refraction, best-corrected visual acuity, and cataract status were assessed, intraocular pressure was measured, and iris color was classified. A dilated examination, including a detailed evaluation of the macula was performed, and previous ocular records were obtained. Color fundus photographs were obtained in up to seven standard fields based on the modified Airlie House classification, adopted by the Early Treatment Diabetic Retinopathy Study (ETDRS) and previously described elsewhere.52 Briefly, images were obtained using 30° fundus cameras with the following standard field definitions: 1) centered on the optic disc; 2) centered on the macula; 3) temporal to the macula; and fields 4-7) tangential to horizontal lines passing through the upper and lower poles of the disc and to a vertical line passing through its center.
Study examination data and all available ocular images were evaluated by J.M.S., and subjects were assigned a baseline AMD grade in both eyes. Subsequent clinical records and imaging were obtained annually by the research team and these were assigned follow-up grades by J.M.S. to allow for prospective analyses of this cohort.
Risk Factor Data and Measurements
Demographic, behavioral, and medical data were collected at baseline for both cohorts using standardized risk factor questionnaires. Questionnaires included information related to demographic characteristics, cigarette smoking, and medical history. Height, weight, and blood pressure were measured at baseline. Body mass index (BMI, kg/m2) was calculated [weight (kg)/height (m)2].
PROGRESSION TO ADVANCED AMD
The Clinical Age-Related Maculopathy Staging (CARMS)53 system was applied to both the model-fitting and validation cohorts to determine AMD phenotypes in each eye at baseline and for all follow-up visits. In the AREDS cohort, phenotype data for all follow-up visits was based on the AREDS AMD Severity Scale.54 This classification system was used to categorize individual eyes using the CARMS system. CARMS grades were assigned as follows: grade 1 (no AMD, no drusen or only a few small drusen < 63 μm); grade 2 (early AMD, intermediate size drusen 63 to 124 μm); grade 3 (intermediate AMD, large drusen ≥ 125 μm). Grades 2 and 3 were further subdivided. For grade 2 eyes, three subtypes were delineated: grade 2A (several small drusen or <15 intermediate size drusen 63 to 124 μm); grade 2B (no drusen, abnormalities in the retinal pigment epithelium [RPE]); and grade 2C (drusen and RPE abnormalities). For grade 3 eyes, two subtypes were defined: grade 3A (several intermediate size drusen or any large drusen); and grade 3B (drusenoid RPE detachment). Advanced eyes were categorized into one of two advanced stages of disease: grade 4, the advanced dry subtype, GA, including both central and non-central forms, which was primary GA and not secondary or subsequent to treatment for NV; and grade 5, NV, or advanced exudative AMD, with choroidal neovascularization (CNV). In the validation cohort, non-advanced eyes were classified using the CARMS system based on all available phenotype data including the ocular examination, clinical records, and ocular imaging.
MACULAR PHENOTYPIC OUTCOMES
Three anatomic endpoints related to progression to advanced AMD and the advanced AMD subtypes (GA and NV) were prospectively evaluated during this study. Eyes that progressed were defined by a transition from no AMD, early AMD, or intermediate AMD to either GA or NV. The following criteria were used to classify progression: 1) no advanced disease was present at baseline, and an eye became advanced (GA or NV) during follow-up; 2) GA was present in a specific eye at baseline and developed NV during follow-up; and 3) no advanced disease was present at baseline, an eye developed GA during follow-up, and subsequently developed NV. Eyes that developed NV were censored and were considered to be an absorbing state, meaning that follow-up was terminated. Eyes could not retroactively develop GA in these analyses. Participants with advanced disease in both eyes at baseline were excluded from all analyses of progression to advanced disease.
VISUAL ACUITY
Visual acuity (VA) was evaluated as another outcome of interest in the derivation cohort. All AREDS participants had a best-corrected VA of 20/32 or better in at least one eye at baseline. VA was evaluated using the ETDRS LogMAR chart. VA was assessed every six months. Progression to visual loss over time was defined as decline in VA of 15 letters or more in an individual eye. Normal vision was defined as 95 to 100 letters. Visual loss was classified as mild (75 to 90 letters), moderate (55 to 70 letters), or severe (35 to 50 letters). Eyes with profound visual loss (15 to 30 letters) or near blindness (0 to 10 letters) at baseline were excluded from all analyses of visual acuity. Eyes were included in VA analyses regardless of baseline AMD grade.
DEMOGRAPHIC AND BEHAVIORAL COVARIATES
Baseline demographic, behavioral, and ocular characteristics were determined for each participant. The following covariates were evaluated as risk factors for progression and visual loss: age (55 to 64, 65 to 74, ≥ 75), sex, race (Caucasian, non-Caucasian [Black, Hispanic, Asian/Pacific Islander, other]) education (≤ high school, > high school), body mass index (BMI) (<25, 25 to 29, ≥ 30), and smoking status (never, past, current).
GENOTYPING AND GENETIC DATA
The DNA samples for the AREDS study population were purchased from the AREDS repository. The DNA samples for the validation cohort were obtained from enrolled study participants according to the standard study protocol. Genotypes were determined using array-based genotyping and gene sequencing platforms as previously described.14,19,25,26,32 All single nucleotide polymorphisms (SNPs) had a high genotype call rate (>98%), none deviated from Hardy-Weinberg equilibrium (P < 10-3), and none failed a differential missing test between groups being compared. PLINK was used to perform all quality control steps.55
Numerous SNPs were previously shown to be associated with AMD and provide the basis for evaluating the effects of different genes on each individual anatomic endpoint in a new predictive model. We classified genetic data into the following physiology-based categories: 1) complement pathway; 2) angiogenesis pathway; 3) lipid pathway; 4) immune/inflammatory pathway; 5) components of the extracellular matrix; and 6) DNA repair and protein binding. Although some of the SNPs are involved in more than one potential pathway, they were grouped as described below for these analyses.
Ten unique genetic loci were classified as complement pathway SNPs, including: complement factor H (CFH) Y402H (rs1061170); CFH rs1410996; CFH R1210C (rs121913059); CFH N1050Y (rs35274867); complement factor B (CFB) R32Q (rs641153); complement factor I (CFI) rs10033900; complement component 2 (C2) E318D (rs9332739); complement component 3 (C3) R102G (rs2230199); C3 K155Q (rs147859257); and complement component 9 (C9) P167S (rs34882957).
Two loci associated with the angiogenesis pathway were vascular endothelial growth factor A (VEGFA) rs94308025 and transforming growth factor beta receptor 1 (TGFBR1) rs334353.
Five SNPs were assessed as genetic risk factors in the lipid pathway: lipase C, hepatic type (LIPC) rs10468017; adenosine tri-phosphate binding cassette transporter 1 (ABCA1) rs1883025; cholesteryl ester transfer protein (CETP) rs3764261; apolipoprotein C1/apolipoprotein E (APOC1/APOE) rs4420638; and apolipoprotein H (APOH) rs1801689.
Six SNPs in our analyses have been reported to have some involvement in the immune/inflammatory pathway, including: age-related maculopathy susceptibility 2/high-temperature requirement A serine peptidase 1 (ARMS2) rs10490924; pellino E3 ubiquitin protein ligase family member 3 (PELI3) rs145732233; tumor necrosis factor receptor superfamily member 10A (TNFRSF10A) rs13278062; solute carrier family 16 member 8 >(SLC16A8) rs8135665; paired immunoglobin-like type 2 receptor beta/paired immunoglobin-like type 2 receptor alpha (PILRB/PILRA) rs11769700; and transmembrane protein 97/vitronectin (TMEM97/VTN) rs704.
Five loci have been associated with the extracellular matrix pathway: collagen type VIII alpha 1 chain (COL8AT) rs1309522625,37,39; collagen type IV alpha 3 chain (COL4A3) rs11884770; ADAM metallopeptidase with thrombospondin type 1 motif 9 (ADAMTS9) rs6795735; tissue inhibitor of metalloproteinase 3 (TIMP3) rs9621532; and chymotrypsinogen B1 (CTRBT) rs8056814.
Three genetic loci related to DNA repair and protein binding included: RAD51 paralog B (RAD51B) rs8017304; nuclear protein localization 4 homolog/tetraspanin 10 (NPLOC4/TSPAN10) rs9895741; and heat shock protein family H member 1/beta 3-glucosyltransferase (HSPH1/B3GALTL) rs9542236.
STATISTICAL ANALYSIS
All statistical analyses evaluated individual eyes, with both eyes contributing to the results, accounting for correlation of progression times in the two eyes.27,46–50 Rather than person-based analyses of the worst eye only, we account for eye-specific outcomes (progression to three distinct advanced AMD outcomes and visual acuity loss) and eye-specific covariates (such as baseline AMD grade). The application of eye-specific methodology also allows for the differentiation between participants who develop an outcome in a single eye compared to developing outcomes in both eyes. These methods result in a larger sample size with a resulting increase in statistical power.
The distributions of demographic, behavioral, ocular, and genetic risk factors were evaluated for progressors and non-progressors to advanced AMD (GA or NV), and separately for progression to GA and NV. Univariate associations between each risk factor and progression were evaluated using Generalized Estimating Equations (Table 1), allowing for the use of correlated data in these eye-specific analyses.
TABLE 1.
Univariate Associations Between Demographic, Behavioral, Ocular, and Genetic Factors and Progression to Advanced Age-Related Macular Degeneration, Geographic Atrophy, and Neovascular Disease in the Derivation Cohort.
| Overall advanced AMD | GA | NV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Progressors | Non- Progressors |
P valuea |
Progressors | Non- Progressors |
P valuea |
Progressors | Non- Progressors |
P valuea |
|
| Sample sizeb | n=1193 | n=4407 | n=599 | n=4407 | n=704 | n=4407 | |||
| Demographic | |||||||||
| Age (years) | |||||||||
| ≥ 75 | 224 (18.9) | 386 (8.8) | <.0001 | 112 (18.8) | 386 (8.9) | <.0001 | 139 (20.0) | 386 (8.9) | <.0001 |
| 65 to 74 | 773 (65.3) | 2808 (63.9) | 387 (64.9) | 2808 (63.9) | 457 (65.7) | 2808 (63.9) | |||
| 55 to 64 | 187 (15.8) | 1203 (27.4) | 97 (16.3) | 1203 (27.4) | 100 (14.4) | 1203 (27.4) | |||
| Sex | |||||||||
| Male | 508 (42.6) | 1915 (43.5) | 0.84 | 273 (45.6) | 1915 (43.5) | 0.45 | 280 (39.8) | 1915 (43.5) | 0.16 |
| Female | 685 (57.4) | 2492 (56.6) | 326 (54.4) | 2492 (56.6) | 424 (60.2) | 2492 (56.6) | |||
| Race | |||||||||
| Caucasian | 1184 (99.3) | 4197 (95.2) | <.0001 | 597 (99.7) | 4197 (95.2) | 0.0003 | 697 (99.0) | 4197 (95.2) | <.0001 |
| Non-caucasian | 9 (0.8) | 210 (4.8) | 2 (0.3) | 210 (4.8) | 7 (1.0) | 210 (4.8) | |||
| Behavioral | |||||||||
| Education | |||||||||
| ≤ High school | 458 (38.4) | 1355 (30.8) | <.0001 | 231 (38.6) | 1355 (30.8) | 0.001 | 280 (39.8) | 1355 (30.8) | <.0001 |
| > High school | 735 (61.6) | 3052 (69.3) | 368 (61.4) | 3052 (69.3) | 424 (60.2) | 3052 (69.3) | |||
| Body mass index | |||||||||
| < 25 | 362 (30.3) | 1519 (34.5) | 0.009 | 182 (30.4) | 1519 (34.5) | 0.05 | 213 (30.3) | 1519 (34.5) | 0.03 |
| 25 to 29.9 | 488 (40.9) | 1864 (42.3) | 240 (40.1) | 1864 (42.3) | 293 (41.6) | 1864 (42.3) | |||
| ≥ 30 | 343 (28.8) | 1023 (23.4) | 177 (29.6) | 1024 (23.2) | 198 (28.1) | 1024 (23.2) | |||
| Smoking | |||||||||
| Never | 468 (39.2) | 2223 (50.4) | <.0001 | 249 (41.6) | 2223 (50.4) | 0.0008 | 265 (37.6) | 2223 (50.4) | <.0001 |
| Past | 620 (52.0) | 1978 (44.9) | 304 (50.8) | 1978 (44.9) | 370 (52.6) | 1978 (44.9) | |||
| Current | 105 (8.8) | 206 (4.7) | 46 (7.7) | 206 (4.7) | 69 (9.8) | 206 (4.7) | |||
| Ocular | |||||||||
| Baseline AMD grade | |||||||||
| 1 | 29 (2.4) | 2156 (48.9) | <.0001 | 4 (0.7) | 2156 (48.9) | <.0001 | 25 (3.6) | 2156 (48.9) | <.0001 |
| 2 | 127 (10.7) | 1260 (28.6) | 49 (8.2) | 1260 (28.6) | 84 (11.9) | 1260 (28.6) | |||
| 3 | 1037 (86.9) | 991 (22.5) | 546 (91.2) | 991 (22.5) | 595 (84.5) | 991 (22.5) | |||
| Genetic loci | |||||||||
| Complement pathway | |||||||||
| CFH Y402H: rs1061170 | |||||||||
| TT | 187 (15.7) | 1584 (35.9) | <.0001 | 98 (16.4) | 1584 (35.9) | <.0001 | 103 (14.6) | 1584 (35.9) | <.0001 |
| CT | 540 (45.3) | 2027 (46.0) | 261 (43.6) | 2027 (46.0) | 325 (46.2) | 2027 (46.0) | |||
| CC | 466 (39.1) | 796 (18.1) | 240 (40.1) | 796 (18.1) | 276 (39.2) | 796 (18.1) | |||
| CFH: rs1410996 | |||||||||
| TT | 40 (3.4) | 719 (16.3) | <.0001 | 22 (3.7) | 719 (16.3) | <.0001 | 21 (3.0) | 719 (16.3) | <.0001 |
| CT | 361 (30.3) | 1988 (45.2) | 176 (29.5) | 1988 (45.2) | 211 (30.0) | 1988 (45.2) | |||
| CC | 790 (66.3) | 1694 (38.5) | 399 (66.8) | 1694 (38.5) | 471 (67.0) | 1694 (38.5) | |||
| CFH R1210C: rs121913059 | |||||||||
| CC | 1156 (98.8) | 4327 (99.7) | 0.009 | 582 (98.8) | 4327 (99.7) | 0.005 | 680 (98.7) | 4327 (99.7) | 0.009 |
| CT | 14 (1.2) | 12 (0.3) | 7 (1.2) | 12 (0.3) | 9 (1.3) | 12 (0.3) | |||
| C2 E318D: rs9332739 | |||||||||
| GG | 1151 (96.5) | 4056 (92.1) | <.0001 | 583 (97.3) | 4056 (92.1) | 0.0001 | 676 (96.0) | 4056 (92.1) | 0.001 |
| CG/CC | 42 (3.5) | 349 (7.9) | 16 (2.7) | 349 (7.9) | 28 (4.0) | 349 (7.9) | |||
| CFB R32Q: rs641153 | |||||||||
| CC | 1094 (92.5) | 3662 (83.8) | <.0001 | 552 (92.6) | 3662 (83.8) | <.0001 | 645 (92.7) | 3662 (83.8) | <.0001 |
| TC/TT | 89 (7.5) | 707 (16.2) | 44 (7.4) | 707 (16.2) | 51 (7.3) | 707 (16.2) | |||
| CFI: rs10033900 | |||||||||
| CC | 282 (23.6) | 1184 (26.9) | 0.004 | 141 (23.5) | 1184 (26.9) | 0.01 | 166 (23.6) | 1184 (26.9) | 0.02 |
| CT | 563 (47.2) | 2165 (49.2) | 272 (45.4) | 2165 (49.2) | 343 (48.7) | 2165 (49.2) | |||
| TT | 348 (29.2) | 1052 (23.9) | 186 (31.1) | 1052 (23.9) | 195 (27.7) | 1052 (23.9) | |||
| C3 R102G: rs2230199 | |||||||||
| CC | 569 (47.8) | 2714 (61.6) | <.0001 | 288 (48.2) | 2714 (61.6) | <.0001 | 335 (47.7) | 2714 (61.6) | <.0001 |
| CG | 510 (42.8) | 1497 (34.0) | 257 (43.1) | 1497 (34.0) | 299 (42.5) | 1497 (34.0) | |||
| GG | 112 (9.4) | 192 (4.4) | 52 (8.7) | 192 (4.4) | 69 (9.8) | 192 (4.4) | |||
| C3 K155Q: rs147859257 | |||||||||
| TT | 1117 (95.5) | 4287 (98.8) | <.0001 | 557 (94.6) | 4287 (98.8) | <.0001 | 664 (96.4) | 4287 (98.8) | <.0001 |
| GT | 53 (4.5) | 52 (1.2) | 32 (5.4) | 52 (1.2) | 25 (3.6) | 52 (1.2) | |||
| C9 P167S: rs34882957 | |||||||||
| GG | 1129 (96.5) | 4261 (98.2) | 0.004 | 566 (96.1) | 4261 (98.2) | 0.008 | 666 (96.7) | 4261 (98.2) | 0.02 |
| AG | 41 (3.5) | 78 (1.8) | 23 (3.9) | 78 (1.8) | 23 (3.3) | 78 (1.8) | |||
| CFH N1050Y: rs35274867 | |||||||||
| AA | 1148 (98.7) | 4146 (97.2) | 0.01 | 578 (98.8) | 4146 (97.2) | 0.03 | 677 (98.7) | 4146 (97.2) | 0.05 |
| TA | 14 (1.2) | 116 (2.7) | 6 (1.0) | 116 (2.7) | 9 1.3) | 116 (2.7) | |||
| TT | 1 (0.1) | 5 (0.1) | 1 (0.2) | 5 (0.1) | 0 (0.0) | 5 (0.1) | |||
| Angiogenesis pathway | |||||||||
| VEGFA: rs943080 | |||||||||
| CC | 231 (19.7) | 968 (22.3) | 0.12 | 117 (19.9) | 968 (22.3) | 0.48 | 137 (19.9) | 968 (22.3) | 0.11 |
| CT | 611 (52.2) | 2190 (50.5) | 312 (53.0) | 2190 (50.5) | 351 (50.9) | 2190 (50.5) | |||
| TT | 328 (28.0) | 1181 (27.2) | 160 (27.2) | 1181 (27.2) | 201 (29.2) | 1181 (27.2) | |||
| TGFBR1: rs334353 | |||||||||
| TT | 702 (60.0) | 2481 (57.2) | 0.17 | 335 (56.9) | 2481 (57.2) | 0.93 | 433 (62.8) | 2481 (57.2) | 0.02 |
| GT | 399 (34.1) | 1579 (36.4) | 216 (36.7) | 1579 (36.4) | 221 (32.1) | 1579 (36.4) | |||
| GG | 69 (5.9) | 279 (6.4) | 38 (6.5) | 279 (6.4) | 35 (5.1) | 279 (6.4) | |||
| Lipid pathway | |||||||||
| LIPC: rs10468017 | |||||||||
| CC | 654 (54.9) | 2267 (51.5) | 0.06 | 343 (57.3) | 2267 (51.5) | 0.01 | 363 (51.6) | 2267 (51.5) | 0.39 |
| TC | 467 (39.2) | 1784 (40.5) | 223 (37.2) | 1784 (40.5) | 296 (42.1) | 1784 (40.5) | |||
| TT | 71 (6.0) | 348 (7.9) | 33 (5.5) | 348 (7.9) | 44 (6.3) | 348 (7.9) | |||
| ABCA1: rs1883025 | |||||||||
| CC | 679 (56.9) | 2411 (54.8) | 0.19 | 345 (57.6) | 2411 (54.8) | 0.32 | 402 (57.1) | 2411 (54.8) | 0.16 |
| TC | 447 (37.5) | 1706 (38.8) | 219 (36.6) | 1706 (38.8) | 268 (38.1) | 1706 (38.8) | |||
| TT | 67 (5.6) | 284 (6.5) | 35 (5.8) | 284 (6.5) | 34 (4.8) | 284 (6.5) | |||
| CETP: rs3764261 | |||||||||
| CC | 471 (39.9) | 1934 (44.0) | 0.002 | 236 (39.6) | 1934 (44.0) | 0.03 | 273 (38.8) | 1934 (44.0) | 0.003 |
| AC | 549 (46.1) | 1986 (45.2) | 280 (47.0) | 1986 (45.2) | 323 (46.0) | 1986 (45.2) | |||
| AA | 170 (14.3) | 476 (10.8) | 80 (13.4) | 476 (10.8) | 107 (15.2) | 476 (10.8) | |||
| APOC1/APOE: rs4420638 | |||||||||
| AA | 855 (73.1) | 3085 (71.1) | 0.22 | 427 (72.5) | 3085 (71.1) | 0.46 | 506 (73.4) | 3085 (71.1) | 0.3 |
| GA | 315 (26.9) | 1254 (38.9) | 162 (27.5) | 1254 (38.9) | 183 (26.6) | 1254 (38.9) | |||
| APOH: rs1801689 | |||||||||
| AA | 1101 (94.7) | 3972 (93.1) | 0.07 | 557 (95.2) | 3972 (93.1) | 0.06 | 651 (94.9) | 3972 (93.1) | 0.1 |
| AC | 62 (5.3) | 292 (6.8) | 28 (4.8) | 292 (6.8) | 35 (5.1) | 292 (6.8) | |||
| CC | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | |||
| Immune/inflammatory pathway | |||||||||
| ARMS2/HTRA1: rs10490924 | |||||||||
| GG | 368 (31.0) | 2564 (58.2) | <.0001 | 184 (30.8) | 2564 (58.2) | <.0001 | 201 (28.7) | 2564 (58.2) | <.0001 |
| TG | 581 (48.9) | 1559 (35.4) | 297 (49.8) | 1559 (35.4) | 341 (48.6) | 1559 (35.4) | |||
| TT | 240 (20.2) | 284 (6.4) | 116 (19.4) | 284 (6.4) | 159 (22.7) | 284 (6.4) | |||
| PELI3: rs145732233 | |||||||||
| CC | 1159 (99.7) | 4221 (99.1) | 0.06 | 583 (99.8) | 4221 (99.1) | 0.06 | 684 (99.7) | 4221 (99.1) | 0.18 |
| TC | 3 (0.3) | 40 (0.9) | 1 (0.2) | 40 (0.9) | 2 (0.3) | 40 (0.9) | |||
| TNFRSF10A: rs13278062 | |||||||||
| TT | 335 (28.6) | 1179 (27.2) | 0.99 | 166 (28.2) | 1179 (27.2) | 0.32 | 207 (30.0) | 1179 (27.2) | 0.27 |
| GT | 556 (47.5) | 220 (50.7) | 266 (45.2) | 220 (50.7) | 338 (49.1) | 220 (50.7) | |||
| GG | 279 (23.9) | 960 (22.1) | 157 (26.7) | 960 (22.1) | 144 (20.9) | 960 (22.1) | |||
| SLC16A8: rs8135665 | |||||||||
| CC | 724 (61.9) | 2771 (63.9) | 0.15 | 360 (61.1) | 2771 (63.9) | 0.07 | 426 (61.8) | 2771 (63.9) | 0.11 |
| TC | 379 (32.4) | 1394 (32.1) | 192 (32.6) | 1394 (32.1) | 225 (32.7) | 1394 (32.1) | |||
| TT | 67 (5.7) | 174 (4.0) | 37 (6.3) | 174 (4.0) | 38 (5.5) | 174 (4.0) | |||
| PILRB/PILRA: rs11769700 | |||||||||
| TT | 739 (63.5) | 2701 (63.3) | 0.7 | 381 (65.1) | 2701 (63.3) | 0.57 | 424 (61.8) | 2701 (63.3) | 0.37 |
| CT | 376 (32.3) | 1397 (32.7) | 180 (30.8) | 1397 (32.7) | 234 (34.1) | 1397 (32.7) | |||
| CC | 48 (4.1) | 169 (4.0) | 24 (4.1) | 169 (4.0) | 28 (4.1) | 169 (4.0) | |||
| TMEM97/VTN: rs704 | |||||||||
| AA | 278 (23.9) | 1014 (23.7) | 0.65 | 139 (23.8) | 1014 (23.7) | 0.86 | 168 (24.5) | 1014 (23.7) | 0.64 |
| AG | 582 (50.0) | 2089 (49.0) | 294 (50.3) | 2089 (49.0) | 336 (49.0) | 2089 (49.0) | |||
| GG | 303 (26.1) | 1164 (27.3) | 152 (26.0) | 1164 (27.3) | 182 (26.5) | 1164 (27.3) | |||
| Extracellular matrix | |||||||||
| COL8A1: rs13095226 | |||||||||
| TT | 916 (76.8) | 3613 (82.0) | 0.0004 | 454 (75.8) | 3613 (82.0) | 0.002 | 538 (76.4) | 3613 (82.0) | 0.001 |
| CT | 255 (21.4) | 748 (17.0) | 131 (21.9) | 748 (17.0) | 155 (22.0) | 748 (17.0) | |||
| CC | 22 (1.8) | 44 (1.0) | 14 (2.3) | 44 (1.0) | 11 (1.6) | 44 (1.0) | |||
| COL4A3: rs11884770 | |||||||||
| CC | 638 (54.9) | 2243 (52.6) | 0.06 | 304 (52.0) | 2243 (52.6) | 0.69 | 395 (57.6) | 2243 (52.6) | 0.008 |
| TC | 452 (38.9) | 1675 (39.3) | 239 (40.9) | 1675 (39.3) | 253 (36.9) | 1675 (39.3) | |||
| TT | 73 (6.3) | 349 (8.2) | 42 (7.2) | 349 (8.2) | 38 (5.5) | 349 (8.2) | |||
| CTRB1: rs8056814 | |||||||||
| GG | 1017 (87.0) | 3504 (80.7) | <.0001 | 512 (86.9) | 3504 (80.7) | 0.0009 | 602 (87.5) | 3504 (80.7) | <.0001 |
| AG | 149 (12.8) | 786 (18.1) | 76 (12.9) | 786 (18.1) | 84 (12.2) | 786 (18.1) | |||
| AA | 3 (0.3) | 50 (1.2) | 1 (0.2) | 50 (1.2) | 2 (0.3) | 50 (1.2) | |||
| ADAMTS9: rs6795735 | |||||||||
| CC | 366 (31.3) | 1271 (29.3) | 0.17 | 190 (32.3) | 1271 (29.3) | 0.08 | 210 (30.5) | 1271 (29.3) | 0.49 |
| TC | 569 (48.6) | 2098 (48.4) | 289 (49.1) | 2098 (48.4) | 333 (48.3) | 2098 (48.4) | |||
| TT | 235 (20.1) | 970 (22.4) | 110 (18.7) | 970 (22.4) | 146 (21.2) | 970 (22.4) | |||
| TIMP3: rs9621532 | |||||||||
| AA | 1101 (92.3) | 3939 (89.5) | 0.01 | 553 (92.3) | 3939 (89.5) | 0.04 | 654 (92.9) | 3939 (89.5) | 0.01 |
| CA/CC | 92 (7.7) | 464 (10.5) | 46 (7.7) | 464 (10.5) | 50 (7.1) | 464 (10.5) | |||
| DNA repair/protein binding | |||||||||
| RAD51B: rs8017304 | |||||||||
| AA | 510 (43.6) | 1716 (39.6) | 0.0003 | 244 (41.4) | 1716 (39.6) | 0.05 | 310 (45.0) | 1716 (39.6) | 0.0002 |
| GA | 555 (47.4) | 2004 (46.2) | 290 (49.2) | 2004 (46.2) | 321 (46.6) | 2004 (46.2) | |||
| GG | 105 (9.0) | 619 (14.3) | 55 (9.3) | 619 (14.3) | 58 (8.4) | 619 (14.3) | |||
| NPLOC4/TSPAN10: | |||||||||
| rs9895741 | |||||||||
| GG | 456 (39.2) | 1822 (42.7) | 0.04 | 240 (41.0) | 1822 (42.7) | 0.37 | 263 (38.3) | 1822 (42.7) | 0.02 |
| AG | 526 (45.2) | 1880 (44.1) | 259 (44.3) | 1880 (44.1) | 310 (45.2) | 1880 (44.1) | |||
| AA | 181 (15.6) | 565 (13.2) | 86 (14.7) | 565 (13.2) | 113 (16.5) | 565 (13.2) | |||
| HSPH1/B3GALTL: | |||||||||
| rs9542236 | |||||||||
| TT | 347 (29.7) | 1453 (33.5) | 0.004 | 180 (30.6) | 1453 (33.5) | 0.02 | 199 (28.9) | 1453 (33.5) | 0.005 |
| CT | 555 (47.4) | 2108 (48.6) | 273 (46.4) | 2108 (48.6) | 336 (48.8) | 2108 (48.6) | |||
| CC | 268 (22.9) | 778 (17.9) | 136 (23.1) | 778 (17.9) | 154 (22.4) | 778 (17.9) | |||
AMD = age-related macular degeneration; GA = geographic atrophy; NV = neovascular disease.
P values calculated using Generalized Estimating Equations in order to account for inter-correlation in eye-specific analyses for 12 year progression.
Sample sizes for each genetic variable presented in Table 1 may not be equal to the overall sample size. Some participants do not have genetic information available for all genetic loci evaluated. Also note that the sum of the sample sizes for GA and NV do not equal the sample size for advanced AMD, as the sample for NV includes eyes that had GA at baseline.
Incidences of the various AMD outcomes were analyzed over the duration of available follow-up. Progression to each endpoint was evaluated using survival analysis methodology with the individual eye as the unit of analysis (using PROC PHREG with the aggregate option in SAS 9.4, allowing for the use of correlated data in eye-specific analyses).56 These associations were assessed using Cox proportional hazards models. Hazard ratios (HRs) were estimated and 95% CIs were calculated. Multivariate associations between progression to various endpoints and demographic, environmental, and ocular variables were evaluated (Table 2), and then associations between each individual SNP and overall AMD (Table 3) and GA and NV (Table 4) were assessed, adjusting for age, sex, education, BMI, baseline grade, and smoking.
TABLE 2.
Multivariate Associations Between Demographic, Behavioral, and Ocular Factors and Progression to Overall Advanced Age-Related Macular Degeneration, Geographic Atrophy, and Neovascular Disease in the Derivation Cohort
| Overall advanced AMD | Progression to GA | Progression to NV | ||||
|---|---|---|---|---|---|---|
| HR (95% CI)a | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Demographic | ||||||
| Age (years) | ||||||
| ≥ 75 | Referent | Referent | Referent | |||
| 65 to 74.9 | 0.69 (0.59-0.81) | <.0001 | 0.72 (0.58-0.89) | 0.003 | 0.68 (0.57-0.83) | 0.0001 |
| 55 to 64.9 | 0.49 (0.40-0.62) | <.0001 | 0.55 (0.41-0.74) | 0.0001 | 0.46 (0.35-0.60) | <.0001 |
| Sex | ||||||
| Female | Referent | Referent | Referent | |||
| Male | 0.97 (0.34-1.12) | 0.64 | 1.20 (0.99-1.46) | 0.06 | 0.83 (0.69-0.99) | 0.04 |
| Race | ||||||
| Non-Caucasian | Referent | Referent | Referent | |||
| Caucasian | 4.11 (2.20-7.67) | <.0001 | 8.38 (2.11-33.33) | 0.003 | 2.72 (1.34-5.53) | 0.006 |
| Behavioral | ||||||
| Education | ||||||
| ≤ High school | Referent | Referent | Referent | |||
| > High school | 0.79 (0.69-0.91) | 0.0007 | 0.81 (0.67-0.97) | 0.03 | 0.79 (0.67-0.94) | 0.006 |
| Body mass index | ||||||
| < 25 | Referent | Referent | Referent | |||
| 25 to 29.9 | 1.16 (0.99-1.36) | 0.08 | 1.05 (0.84-1.32) | 0.66 | 1.18 (0.97-1.44) | 0.1 |
| ≥30 | 1.41 (1.19-1.68) | 0.0001 | 1.35 (1.06-1.71) | 0.01 | 1.35 (1.08-1.67) | 0.007 |
| Smoking | ||||||
| Never | Referent | Referent | Referent | |||
| Past | 1.26 (1.09-1.45) | 0.002 | 1.04 (0.85-1.27) | 0.7 | 1.40 (1.17-1.68) | 0.0002 |
| Current | 2.22 (1.71-2.88) | <.0001 | 1.46 (1.01-2.10) | 0.05 | 2.49 (1.83-3.40) | <.0001 |
| Ocular | ||||||
| Baseline AMD grade | ||||||
| 1 | Referent | Referent | Referent | |||
| 2 | 6.90 (4.46-10.67) | <.0001 | 18.92 (6.80-52.65) | <.0001 | 5.17 (3.18-8.39) | <.0001 |
| 3 | 46.8 (31.22-70.19) | <.0001 | 152.59 (57.05-408.17) | <.0001 | 25.97 (16.61-40.61) | <.0001 |
AMD = age-related macular degeneration; CI = confidence interval; GA = geographic atrophy; HR = hazard ratio; NV = neovascular disease.
HRs and 95% CIs were estimated using Cox proportional hazards models for 12 year progression using the individual eye as the unit of analysis.
HRs are adjusted for all variables listed in the Table.
TABLE 3.
Multivariate Associations Between Individual Genetic Loci and Progression to Advanced Age-Related Macular Degeneration in the Derivation Cohort.
| Multivariate Model Ia | Multivariate Model IIb | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Genetic loci | ||||
| Complement pathway | ||||
| CFH Y402H: rs1061170 | 1.41 (1.29-1.55) | <.0001 | 1.15 (1.02-1.30) | 0.02 |
| CFH: rs1410996 | 1.68 (1.50-1.89) | <.0001 | 1.47 (1.26-1.71) | <.0001 |
| CFH R1210C: rs121913059 | 2.34 (1.43-3.80) | 0.0007 | 4.37 (2.76-6.91) | <.0001 |
| C2 E318D: rs9332739 | 0.61 (0.43-0.87) | 0.007 | 0.61 (0.43-0.87) | 0.006 |
| CFB R32Q: rs641153 | 0.58 (0.44-0.75) | <.0001 | 0.72 (0.55-0.94) | <.0001 |
| CFI: rs10033900 | 1.08 (0.99-1.18) | 0.1 | 1.06 (0.97-1.16) | 0.22 |
| C3 R102G: rs2230199 | 1.28 (1.16-1.42) | <.0001 | 1.27 (1.14-1.41) | <.0001 |
| C3 K155Q: rs147859257 | 1.84 (1.35-2.50) | 0.0001 | 1.99 (1.48-2.70) | <.0001 |
| C9 P167S: rs34882957 | 1.12 (0.81-1.55) | 0.5 | 0.92 (0.68-1.25) | 0.59 |
| CFH N1050Y: rs35274867 | 0.61 (0.36-1.05) | 0.07 | 1.17 (0.71-1.93) | 0.54 |
| Angiogenesis pathway | ||||
| VEGFA: rs943080 | 0.98 (0.89-1.08) | 0.73 | 1.01 (0.92-1.11) | 0.87 |
| TGFBR1: rs334353 | 0.95 (0.85-1.06) | 0.35 | 0.92 (0.82-1.02) | 0.1 |
| Lipid pathway | ||||
| LIPC: rs10468017 | .94 (0.84-1.04) | 0.21 | 0.96 (0.86-1.07) | 0.43 |
| ABCA1: rs1883025 | .98 (0.88-1.09) | 0.72 | 0.98 (0.88-1.10) | 0.77 |
| CETP: rs3764261 | 1.10 (1.00-1.21) | 0.04 | 1.08 (0.98-1.19) | 0.14 |
| APOC1/APOE: rs4420638 | 1.00 (0.87-1.16) | 0.99 | 0.94 (0.81-1.09) | 0.41 |
| APOH: rs1801689 | 0.74 (0.55-1.00) | 0.05 | 0.87 (0.66-1.15) | 0.32 |
| Immune/inflammatory pathway | ||||
| ARMS2/HTRA1: rs10490924 | 1.55 (1.41-1.71) | <.0001 | 1.49 (1.35-1.64) | <.0001 |
| PELI3: rs145732233 | 0.32 (0.09-1.09) | 0.07 | 0.28 (0.07-1.12) | 0.07 |
| TNFRSF10A: rs13278062 | 1.08 (0.99-1.19) | 0.09 | 1.03 (0.93-1.13) | 0.61 |
| SLC16A8: rs8135665 | 1.05 (0.94-1.18) | 0.4 | 1.06 (0.95-1.19) | 0.28 |
| PILRB/PILRA: rs11769700 | 1.05 (0.94-1.17) | 0.42 | 1.04 (0.93-1.16) | 0.49 |
| TMEM97/VTN: rs704 | 0.98 (0.89-1.08) | 0.69 | 0.92 (0.84-1.02) | 0.1 |
| Extracellular matrix | ||||
| COL8A1: rs13095226 | 1.22 (1.06-1.41) | 0.005 | 1.18 (1.02-1.37) | 0.03 |
| COL4A3: rs11884770 | 0.95 (0.86-1.06) | 0.34 | 0.95 (0.86-1.06) | 0.36 |
| CTRB1: rs8056814 | 0.79 (0.65-0.96) | 0.02 | 0.85 (0.70-1.03) | 0.1 |
| ADAMTS9: rs6795735 | 0.98 (0.89-1.08) | 0.64 | 1.01 (0.92-1.11) | 0.86 |
| TIMP3: rs9621532 | 0.74 (0.58-0.94) | 0.02 | 0.81 (0.63-1.04) | 0.09 |
| DNA repair/protein binding | ||||
| RAD51B: rs8017304 | 0.86 (0.77-0.95) | 0.003 | 0.85 (0.77-0.95) | 0.003 |
| NPLOC4/TSPAN10: rs9895741 | 1.08 (0.98-1.19) | 0.1 | 1.06 (0.96-1.16) | 0.26 |
| HSPH1/B3GALTL: rs9542236 | 1.17 (1.07-1.29) | 0.0005 | 1.14 (1.04-1.25) | 0.005 |
AMD = age-related macular degeneration; CI = confidence interval; HR = hazard ratio.
Multivariate Model I: HRs for 12 year progression, risk per allele, adjusted for age, sex, race, education, and baseline AMD grade.
Multivariate Model II: HRs reflect risk per allele, adjusted for age, sex, race, education, baseline AMD grade, BMI, smoking status, and all other genetic loci in the table.
TABLE 4.
Multivariate Associations Between Individual Genetic Loci and Progression to Geographic Atrophy and Neovascular Disease Subtypes in the Derivation Cohort.
| GA | NV | |||||||
|---|---|---|---|---|---|---|---|---|
| Multivariate Model Ia | Multivariate Model IIb | Multivariate Model Ia | Multivariate Model IIb | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Genetic loci | ||||||||
| Complement pathway | ||||||||
| CFH Y402H: rs1061170 | 1.47 (1.29-1.67) | <.0001 | 1.05 (0.86-1.28) | 0.63 | 1.53 (1.36-1.72) | <.0001 | 1.17 (1.00-1.35) | 0.04 |
| CFH: rs1410996 | 1.76 (1.50-2.06) | <.0001 | 1.45 (1.14-1.85) | 0.003 | 1.87 (1.62-2.17) | <.0001 | 1.45 (1.19-1.76) | 0.0002 |
| CFH R1210C: rs121913059 | 2.84 (1.34-6.03) | <.0001 | 3.94 (1.90-8.15) | 0.001 | 2.66 (1.45-4.88) | 0.002 | 4.25 (2.05-8.79) | <.0001 |
| C2 E318D: rs9332739 | 0.45 (0.26-0.80) | 0.006 | 0.64 (0.34-1.21) | 0.17 | 0.66 (0.43-1.02) | 0.06 | 0.72 (0.48-1.10) | 0.13 |
| CFB R32Q: rs641153 | 0.53 (0.37-0.77) | 0.0007 | 0.74 (0.48-1.12) | 0.16 | 0.53 (0.37-0.75) | 0.0003 | 0.73 (0.52-1.03) | 0.07 |
| CFI: rs10033900 | 1.13 (0.99-1.29) | 0.08 | 1.14 (0.97-1.32) | 0.1 | 1.08 (0.96-1.21) | 0.2 | 1.00 (0.90-1.12) | 0.97 |
| C3 R102G: rs2230199 | 1.30 (1.12-1.50) | 0.0005 | 1.16 (0.98-1.39) | 0.09 | 1.34 (1.18-1.53) | <.0001 | 1.25 (1.09-1.42) | 0.001 |
| C3 K155Q: rs147859257 | 2.21 (1.49-3.28) | <.0001 | 2.59 (1.64-4.08) | <.0001 | 1.72 (1.08-2.73) | 0.02 | 1.24 (0.75-2.04) | 0.4 |
| C9 P167S: rs34882957 | 1.27 (0.80-2.01) | 0.32 | 0.87 (0.53-1.43) | 0.4 | 1.08 (0.71-1.63) | 0.73 | 0.83 (0.54-1.30) | 0.42 |
| CFH N1050Y: rs35274867 | 0.57 (0.26-1.25) | 0.16 | 0.94 (0.32-2.78) | 0.91 | 0.59 (0.28-1.23) | 0.16 | 1.26 (0.62-2.58) | 0.53 |
| Angiogenesis pathway | ||||||||
| VEGFA: rs943080 | 0.97 (0.85-1.11) | 0.64 | 0.98 (0.84-1.14) | 0.76 | 1.01 (0.90-1.14) | 0.85 | 1.07 (0.94-1.21) | 0.3 |
| TGFBR1: rs334353 | 1.03 (0.89-1.20) | 0.68 | 0.98 (0.82-1.17) | 0.79 | 0.89 (0.77-1.03) | 0.11 | 0.83 (0.72-0.96) | 0.01 |
| Lipid pathway | ||||||||
| LIPC: rs10468017 | 0.88 (0.76-1.02) | 0.09 | 0.90 (0.75-1.08) | 0.26 | 0.97 (0.85-1.10) | 0.63 | 1.02 (0.89-1.16) | 0.83 |
| ABCA1: rs1883025 | 0.97 (0.83-1.13) | 0.7 | 1.05 (0.88-1.26) | 0.58 | 0.95 (0.83-1.09) | 0.45 | 0.95 (0.83-1.10) | 0.51 |
| CETP: rs3764261 | 1.09 (0.96-1.24) | 0.2 | 1.02 (0.87-1.20) | 0.78 | 1.14 (1.01-1.29) | 0.03 | 1.08 (0.96-1.23) | 0.21 |
| APOC1/APOE: rs4420638 | 1.03 (0.84-1.27) | 0.77 | 0.98 (0.77-1.25) | 0.88 | 1.00 (0.83-1.20) | 1 | 0.93 (0.78-1.12) | 0.46 |
| APOH: rs1801689 | 0.62 (0.41-0.95) | 0.03 | 0.93 (0.60-1.44) | 0.74 | 0.72 (0.49-1.06) | 0.1 | 0.88 (0.58-1.34) | 0.56 |
| Genetic loci | ||||||||
| Immune/inflammatory pathway | ||||||||
| ARMS2/HTRA1: rs10490924 | 1.59 (1.39-1.81) | <.0001 | 1.42 (1.21-1.65) | <.0001 | 1.75 (1.55-1.97) | <.0001 | 1.57 (1.39-1.78) | <.0001 |
| PELI3: rs145732233 | 0.21 (0.04-1.22) | 0.08 | 0.26 (0.03-2.01) | 0.2 | 0.34 (0.06-1.96) | 0.23 | 0.39 (0.06-2.51) | 0.32 |
| TNFRSF10A: rs13278062 | 1.17 (1.02-1.33) | 0.03 | 1.12 (0.95-1.31) | 0.18 | 1.04 (0.92-1.17) | 0.55 | 0.92 (0.82-1.04) | 0.2 |
| SLC16A8: rs8135665 | 1.08 (0.92-1.26) | 0.35 | 1.11 (0.92-1.34) | 0.29 | 1.03 (0.89-1.19) | 0.69 | 1.01 (0.88-1.17) | 0.89 |
| PILRB/PILRA: rs11769700 | 1.03 (0.87-1.21) | 0.75 | 0.97 (0.79-1.19) | 0.78 | 1.10 (0.96-1.27) | 0.18 | 1.13 (0.98-1.31) | 0.09 |
| TMEM97/VTN: rs704 | 0.99 (0.86-1.13) | 0.82 | 1.06 (0.85-1.34) | 0.6 | 0.97 (0.86-1.09) | 0.59 | 0.94 (0.83-1.07) | 0.35 |
| Extracellular matrix | ||||||||
| COL8A1: rs13095226 | 1.34 (1.09-1.64) | 0.006 | 1.26 (0.98-1.61) | 0.07 | 1.24 (1.04-1.48) | 0.02 | 1.18 (0.99-1.41) | 0.07 |
| COL4A3: rs11884770 | 1.04 (0.89-1.20) | 0.65 | 1.13 (0.95-1.34) | 0.16 | .089 (0.78-1.01) | 0.07 | 0.86 (0.75-0.98) | 0.03 |
| CTRB1: rs8056814 | 0.79 (0.60-1.04) | 0.09 | 0.88 (0.65-1.19) | 0.4 | 0.74 (0.58-0.95) | 0.02 | 0.83 (0.65-1.06) | 0.14 |
| ADAMTS9: rs6795735 | 0.34 (0.82-1.07) | 0.34 | 0.90 (0.77-1.05.05) | 0.19 | 1.02 (0.90-1.15) | 0.8 | 1.08 (0.96-1.21) | 0.22 |
| TIMP3: rs9621532 | 0.71 (0.51-0.99) | 0.04 | 0.82 (0.56-1.19) | 0.3 | 0.66 (0.47-0.93) | 0.02 | 0.75 (0.53-1.06) | 0.1 |
| DNA repair/protein binding | ||||||||
| RAD51B: rs8017304 | 0.87 (0.76-1.01) | 0.06 | 0.97 (0.82-1.15) | 0.71 | 0.81 (0.72-0.92) | 0.002 | 0.81 (0.71-0.92) | 0.002 |
| NPLOC4/TSPAN10: rs9895741 | 1.06 (0.92-1.22) | 0.39 | 1.01 (0.86-1.19) | 0.89 | 1.13 (1.00-1.28) | 0.05 | 1.11 (0.99-1.25) | 0.08 |
| HSPH1/B3GALTL: rs9542236 | 1.19 (1.05-1.35) | 0.008 | 1.08 (0.93-1.26) | 0.29 | 1.23 (1.09-1.38) | 0.0007 | 1.12 (0.99-1.26) | 0.08 |
AMD = age-related macular degeneration; CI = confidence interval; GA = geographic atrophy; HR = hazard ratio; NV = neovascular disease.
Multivariate Model I: HRs for 12 year progression, risk per allele, adjusted for age, sex, race, education, and baseline AMD grade.
Multivariate Model II: HRs reflect risk per allele, and are adjusted for age, sex, race, education, baseline AMD grade, BMI, smoking status, and all other genetic loci in the Table.
Separate risk prediction models were determined for progression to advanced AMD, GA, and NV based on stepwise regression methods (Table 5). These stepwise regression models allow for the variables most predictive of a specific outcome to be determined based on an automatic procedure. The procedure involves each explanatory variable being separately considered for inclusion or exclusion from a predictive model based on a set of criteria that are specified a priori. The STEPWISE selection option of PROC PHREG was used, with P < .05 for a SNP to enter the model and P < .10 to remain in the model. Each model included age, sex, education, BMI, smoking history, and baseline grade. The non-genetic factors were included in all models given that they have been shown a priori to be predictive of progression to advanced AMD. All SNPs for which genotype data were obtained (n=31) were evaluated together and were subsequently included or not included in the final models. The stepwise procedure was used to select genetic loci that were most predictive of progression to each outcome after controlling for the non-genetic variables mentioned previously. Visual loss greater than 15 letters was assessed using the same methods as those described above for progression to advanced AMD.
TABLE 5.
Stepwise Selection of Genetic Factors Predictive of Progression to Overall Advanced Age-Related Macular Degeneration, Geographic Atrophy, Neovascular Disease, and Visual Acuity Loss in the Derivation Cohort.
| Overall advanced AMD | Progression to GA | Progression to NV | VA Loss ≥ 15 Letters | |||||
|---|---|---|---|---|---|---|---|---|
| n=1149/5355a | n=578/5355 | n=677/5355 | n=1423/4943 | |||||
| HR (95% CI)b | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Genetic loci | ||||||||
| Complement pathway | ||||||||
| CFH Y402H: rs1061170 | 1.14 (1.02-1.29) | 0.03 | 1.17 (1.01-1.36) | 0.04 | ||||
| CFH: rs1410996 | 1.46 (1.26-1.69) | <.0001 | 1.54 (1.28-1.86) | <.0001 | 1.40 (1.16-1.69) | 0.0004 | 1.30 (1.19-1.43) | <.0001 |
| CFH R1210C: rs121913059 | 4.18 (2.79-6.27) | <.0001 | 4.30 (2.10-8.83) | <.0001 | 4.02 (1.92-8.40) | 0.0002 | 3.01 (1.67-5.41) | 0.0002 |
| C2 E318D: rs9332739 | 0.60 (0.43-0.85) | 0.004 | ||||||
| CFB R32Q: rs641153 | 0.71 (0.54-0.93) | 0.01 | 0.69 (0.49-0.96) | 0.03 | ||||
| CFI: rs10033900 | 1.16 (1.00-1.34) | 0.06 | ||||||
| C3 R102G: rs2230199 | 1.27 (1.15-1.41) | <.0001 | 1.19 (1.00-1.41) | 0.05 | 1.24 (1.09-1.41) | 0.001 | 1.23 (1.12-1.35) | <.0001 |
| C3 K155Q: rs147859257 | 2.00 (1.50-2.66) | <.0001 | 2.66 (1.74-4.06) | <.0001 | 1.43 (1.05-1.94) | 0.02 | ||
| Angiogenesis pathway | ||||||||
| TGFBR1: rs334353 | 0.83 (0.72-0.96) | 0.01 | ||||||
| Immune/inflammatory pathway | ||||||||
| ARMS2/HTRA1: rs10490924 | 1.47 (1.34-1.62) | <.0001 | 1.44 (1.23-1.67) | <.0001 | 1.57 (1.39-1.77) | <.0001 | 1.33 (1.22-1.45) | <.0001 |
| PELI3: rs145732233 | 0.29 (0.07-1.18) | 0.08 | ||||||
| Extracellular matrix | ||||||||
| COL8A1: rs13095226 | 1.18 (1.03-1.37) | 0.02 | 1.29 (1.02-1.64) | 0.04 | 1.19 (1.00-1.42) | 0.05 | ||
| COL4A3: rs11884770 | 0.85 (0.74-0.98) | 2 | ||||||
| CTRB1: rs8056814 | 0.84 (0.69-1.02) | 0.07 | ||||||
| DNA repair/protein binding | ||||||||
| RAD51B: rs8017304 | 0.85 (0.77-0.94) | 0.001 | 0.81 (0.71-0.93) | 0.002 | 0.86 (0.79-0.94) | 0.001 | ||
| HSPH1/B3GALTL: rs9542236 | 1.14 (1.04-1.25) | 0.004 | ||||||
| AUC ± standard errorc | 0.90 ± 0.005 | -- | 0.87 ± 0.008 | -- | 0.86 ± 0.008 | -- | 0.72 ± 0.008 | -- |
AMD = age-related macular degeneration; AUC = area under the curve; CI = confidence interval; GA = geographic atrophy; HR = hazard ratio; NV = neovascular disease; VA = visual acuity.
Sample sizes reported as (numerator/denominator), where the numerator is equal to the number of eyes that progressed during follow up, and the denominator is equal to the number of eligible eyes at baseline, among participants with complete genetic data. Note that the number of eligible eyes does not equal two times the number of persons, as some people only contributed one eye to the analysis if the fellow eye had advanced AMD at baseline. Also note that the sum of the sample sizes for GA and NV disease do not equal the sample size for advanced AMD, as the sample for NV includes eyes that had GA and developed NV.
HRs for 12 year progression represent risk per allele, and are adjusted for age, sex, race, education, baseline AMD grade, BMI, smoking status, and all other SNPs in the table.
All area under the curve (AUC) statistics are age-adjusted in order to minimize confounding by age.
To assess heterogeneity, or whether there were differences in results between the two advanced outcomes, progression to GA or NV, we conducted analyses of the two subtypes and determined if any differences observed were statistically significant. Competing risks regression approaches were used based on the data duplication method of Lunn and McNeil,57 where a separate record was created to identify and compare risk factors consisting of genetic and non-genetic variables between progression to GA and NV. The set of genes considered in these analyses were all genes that were related to advanced AMD in the stepwise regression models.
Age-adjusted areas under the receiver operating curve (AUCs) were calculated for progression to overall advanced AMD, the two advanced subtypes, and VA loss using methodology previously described.39,58,59 The AUC is an index that evaluates how well a specific model can discriminate between progressors and non-progressors to each endpoint. The AUC for each endpoint was based on the risk prediction model determined by the STEPWISE methodology. The risk score for each endpoint was used to calculate the AUC that corresponds to the probability that a random progressing eye over a specific time period had a higher risk score than a random non-progressing eye that was followed for at least as long as that time period.
Risk scores for progression to each anatomic endpoint were calculated using regression coefficients of all demographic, behavioral, ocular, and genetic factors in the STEPWISE models. The hazard ratio for the ith subject is given from the Cox proportional hazards model by where βj is the regression coefficient for the jth variable and xij is the value of the jth variable for the ith subject. The corresponding estimate of the survival function for the ith subject is given by[S0 (t)]λ1 , where is S0(t) equal to the baseline survival function. It was estimated using the baseline option of PROC PHREG in SAS 9.4, where survival is defined as not having the outcome. The baseline survival function was estimated from a subject who was in the reference category for all covariates.
Probability of progression based on the risk score over specific time periods was defined as per our previous models: 1) very low (< 1%); 2) low (1% to < 10%); 3) medium (10% to < 30%); 4) high (30% to < 50%); 5) very high (≥ 50%). Probability of progression to advanced AMD, GA, and NV at 5 and 10 years from baseline was calculated, adjusted for competing mortality risks.
Risk score distributions including demographic, behavioral, ocular, and genetic variables were obtained for eyes which either progressed to advanced AMD within a 5 year period or did not progress and were followed for at least 5 years among eyes with intermediate AMD at baseline, and a boxplot was obtained comparing the risk score distributions for the two groups.
The composite risk scores derived from the AREDS (derivation) cohort were applied to the Seddon Longitudinal Cohort (validation cohort). The three models predicting progression to overall advanced AMD, GA, and NV were independently validated, and age-adjusted AUCs were calculated as described previously. The sensitivity and specificity for the prediction model for progression to overall advanced AMD were calculated for a variety of risk score cutoffs. Sensitivity was defined as the proportion of progressing eyes that had a risk score greater than or equal to a given threshold. Specificity was defined as the proportion of non-progressing eyes that had a risk score lower than a predetermined threshold and were followed for as long as the follow up interval (i.e., these eyes did not progress to advanced disease during the follow up interval). Our goal was to select a risk threshold where both sensitivity and specificity were at least 80%.
Calibration
We stratified the set of eyes with baseline intermediate AMD by risk decile according to the derivation sample risk score for progression to overall advanced AMD. For each decile, we fit a Kaplan-Meier curve and calculated the 5 year survival probability for overall AMD, and the corresponding 5 year incidence (1-survival estimate). We then multiplied these incidence rates by the number of people in each decile to obtain the observed count of progressors in each risk decile. Similarly, we calculated the baseline survival function from the derivation cohort = S0 (5) = survival probability at 5 years for eyes with zero values for all covariates. Then, for each eye in a risk decile in the validation sample, we calculated S (5) = S0 (5)exp(β * X), where X is a vector of risk factors, and β is a vector of regression coefficients corresponding to these risk factors, to obtain the estimated 5 year survival probability in an individual eye in a particular risk decile, and the corresponding 5 year incidence which equals 1-survival probability. We then added up the eye specific incidences of all eyes in a particular decile to obtain the expected count within a decile. We then used Poisson regression methods60 to compare the observed (O) to expected (E) decile specific counts, by regressing the observed count using an intercept only model with a log link, and the log [E] as an offset. The estimated E/O ratio is given by exp (−α) where α is the estimated intercept from the model, and the corresponding 95% confidence interval is given by exp [−α±1.96 x S.E (α)]. The p value is obtained from, 2 × [1 – Φ(∣z∣)] where z = α/SE(α) from the Poisson regression model, and Φ is the cumulative standard normal cumulative distribution function (c.d.f.)
Net Reclassification Improvement (NRI)
Net reclassification improvement (NRI) methods61,62 were used to compare the improvement in the performance of our composite risk prediction model based on the inclusion of genetic data. The NRI is calculated separately for progressors and non-progressors and quantifies the “correct” movement in risk of progression, specifically to a higher risk category for progressors and a lower risk category for non-progressors when genes are considered. For progressors, the NRI is calculated as the difference in the proportion of eyes with a higher risk category for the model with genes compared to the model without genes minus the proportion of eyes with a lower risk category for the model with genes compared to the model without genes. For non-progressors, the NRI is similarly defined as the difference in the proportion of eyes with a lower risk category for the model with genes compared to the model without genes minus the proportion of eyes with a higher risk category for the model with genes compared to the model without genes. An overall NRI was calculated by adding the individual NRIs for progressors and non-progressors. The NRI was calculated separately for 5 and 10 year incidence of progression, where eyes that progressed did so within the predetermined follow up interval and eyes that did not progress were followed for as long as the follow up interval. These analyses were done separately for the derivation and validation cohorts, based on the risk function of the derivation cohort as determined by the stepwise model risk functions shown in Table 5. In addition, we assessed the probability of progression for 24 representative eyes with intermediate AMD at baseline using models with or without genetic variables.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). P values less than .05 were considered statistically significant.
RESULTS
DERIVATION COHORT
The distributions of demographic, behavioral, ocular, and genetic characteristics within the derivation sample are presented in Table 1 for progression to overall advanced AMD, GA, and NV. The mean age of participants in the derivation cohort was 68.7 years. The sample was 43.6% male. For each outcome, progressors and non-progressors significantly differed in terms of their age, race, level of education, BMI, smoking status, and baseline AMD grade. Progressors to overall advanced AMD tended to be older (P < .0001) and Caucasian (P < .0001), and had a lower level of education (P < .0001), a higher BMI (P = .009), a history of past or current cigarette smoking (P < .0001), and more advanced stages of AMD at baseline (P < .0001). There was no difference observed between males and females (P = .84). Similar results were observed for progression to the GA and NV subtypes.
Genotypes for loci in the complement pathway differed between progressors and non-progressors. A higher number of risk alleles was associated with progression to overall advanced AMD, GA, and NV for common variants in CFH Y402H, CFH rs1410996, and C3 R102G (P < .0001 for all common complement SNPs), and the rare variants CFH R1210C (P = .009) and C3 K155Q (P < .0001). A significantly lower rate of progression was observed with protective alleles in C2 E318D and CFB R32Q. A protective relationship was observed between low frequency variant CFH N1050Y and overall advanced AMD and GA, and a suggestive effect was observed between this SNP and NV. Risk alleles in CFI and C9 P167S were also significantly associated with higher rates of progression to overall advanced AMD, GA, and NV. TGFBR1 was associated with a reduced rate of progression to NV, although no significant associations were observed for progression to overall advanced AMD and GA.
In the lipid pathway, protective alleles in LIPC were significantly associated with a lower rate of progression to GA (P = .01), with a suggestive result observed for overall advanced AMD (P = .06). APOH revealed a similar suggestive, protective effect for both endpoints (P = .07). Risk alleles in CETP were associated with a higher risk of progression to overall advanced AMD (P = .002), GA (P = .03), and NV (P = .003). No differences in progression were observed for ABCA1 or APOC1/APOE.
Evaluation of the genes present in the immune/inflammatory pathway revealed significant associations between risk alleles in ARMS2 and progression to overall advanced AMD, GA, and NV (all P < .0001). A beneficial effect of the protective allele in PELI3 was suggested for overall advanced AMD and GA (both P = .06), with a nonsignificant trend in the same direction observed for NV. Genotypes in TNFRSF10A, SLC16A8, PILRB/PILRA, and TMEM97/VTN did not differ between progressors and non-progressors for any of the three advanced AMD outcomes.
In the extracellular matrix pathway, a higher number of risk alleles in COL8A1 were associated with a higher rate of progression to overall advanced AMD (P = .0004), GA (P = .002), and NV (P = .001) endpoints. The CTRB1 variant had a protective effect for these outcomes (P < .0001 to .009). A similar protective relationship was observed for alleles in COL4A3 with regard to progression to NV (P = .008), with a suggestive effect for overall advanced AMD (P = .06). TIMP3 was also associated with a reduced rate of progression to all three outcomes (P = .01, .04, and .01 for overall advanced AMD, GA, and NV, respectively). No significant associations with progression to overall advanced AMD, GA, or NV was observed for ADAMTS9.
Three loci associated with DNA repair and protein binding were also evaluated. RAD51B was significantly associated with a protective effect against progression to overall advanced AMD (P = .0003) and NV (P = .0002). HSPH1/B3GALTL was associated with an increased rate of progression to each of these outcomes (P = .004 to .01). NPLOC4/TSPAN10 was associated with a higher rate of progression to overall advanced AMD (P = .04) and NV (P = .02) with an increasing number of risk alleles.
The multivariate associations between the demographic, behavioral, and ocular factors and progression are presented in Table 2 for each advanced AMD outcome. Participants who were older, Caucasian, obese (defined as a BMI ≥ 30), had a high school education or less, and a history of past or current cigarette smoking had a higher risk of progression to advanced AMD over time. A more advanced AMD grade at baseline was also significantly associated with progression to overall advanced AMD, GA, and NV.
Each genetic locus was evaluated for its independent association with progression over time. Associations between each genetic factor and progression to overall advanced AMD are displayed in Table 3, and are adjusted for age, sex, race, level of education, and baseline grade in the multivariate model I. A second multivariate model of progression adjusting for all 31 genetic features and all other covariates including smoking status and BMI, is also displayed (multivariate model II). This model which also adjusted for age, sex, race, education, baseline AMD status, education, BMI, and smoking status, served as the basis for each of our stepwise models of progression (later presented in Table 5). The adjusted associations with the various genetic factors for progression to the GA and NV subtypes separately are displayed in Table 4. Some differences were seen for loci in the complement pathway between the two adjusted models. The effects of CFH Y402H and CFH 1410996 were somewhat weaker in multivariate model II when all other loci were considered. On the other hand, the effect of the rare variant CFH R1210C was stronger in multivariate model II (HR: 2.34 and 4.37; P = .0007 and P < .0001, respectively). The variant CFH N1050Y exhibited a suggestive protective effect in multivariate model I (HR: .61; P = .07), but not in multivariate model II. Differences in effect were also noted for TIMP3, with a weaker association observed for this variant when all the other genes were considered.
Similar trends in the complement pathway to those reported above for the predictive variants CFH Y402H, CFH 1410996, CFH R1210C, and CFH N1050Y were observed for progression to GA and NV. In addition, the common C3 variant, R102G, exhibited a weaker effect in multivariate model II for both endpoints. Different relationships were observed for the rare C3 variant with regard to GA and NV. When adjusting for all other genes, the rare K155Q variant was a stronger predictor of progression to GA (HR: 2.59; P < .0001) and a weaker predictor for NV (HR: 1.24; P = .40). The loci identified as significantly associated with each endpoint were ultimately selected as the most predictive of progression in the multivariate STEPWISE risk prediction models.
STEPWISE RISK PREDICTION MODELS
Progression to Overall advanced AMD
A multivariate stepwise model identified 13 common and rare variants that were predictive of progression to overall advanced AMD. This model, in addition to the stepwise models for progression to GA, NV, and VA loss ≥ 15 letters, are presented in Table 5. Eight SNPs conferred a greater risk and five conferred a lower risk of progression. The following variants conferred a higher risk of progression: CFH Y402H (HR: 1.1; 95% CI: 1.0 to 1.3; P = .03), CFH rs1410996 (HR: 1.5; 95% CI: 1.3 to 1.7; P < .0001), CFH R1210C (HR: 4.2; 95% CI: 2.8 to 6.3; P < .0001), C3 R102G (HR: 1.3; 95% CI: 1.2 to 1.4; P < .0001), C3 K155Q (HR: 2.0; 95% CI: 1.5 to 2.7; P < .0001), ARMS2 (HR: 1.5; 95% CI: 1.3 to 1.6; P < .0001), COL8A1 (HR: 1.2; 95% CI: 1.0 to 1.4; P = .02), and HSPH1/B3GALTL (HR: 1.1; 95% CI: 1.0 to 1.3; P = .004). Variants in C2 E318D (HR: 0.6; 95% CI: 0.4 to 0.9; P = .004), CFB R32Q (HR: 0.7; 95% CI: 0.5 to 0.9; P = .01), and RAD51B (HR: 0.9; 95% CI: 0.8 to 0.9; P = .001) were significantly associated with a protective effect, with lower rates of progression to this endpoint. A protective effect was also suggested per effective allele for CTRB1 (HR: 0.8; 95% CI: 0.7 to 1.0; P = .07) and PELI3 A307V (HR: 0.3; 95% CI: 0.1 to 1.2; P = .08). The age-adjusted AUC for this composite risk model, including 13 genetic loci as well as demographic, behavioral, and ocular covariates, was .90 over 12 years. This high AUC indicates excellent discrimination between progressors to advanced AMD and non-progressors. Results of this multivariate stepwise model are also illustrated in Figure 2 (top).
FIGURE 2.
Genetic loci in the composite risk model associated with progression to advanced age-related macular degeneration over 12 years. The forest plot displays risk of progression per effective allele based on multivariate stepwise models. Hazard ratios (HRs.) and 95% confidence intervals (CIs) are presented for each locus on a log scale. Results are shown for the derivation cohort (top) and validation cohort (bottom).
Progression to GA
Seven SNPs were predictive of progression to the GA endpoint in the multivariate stepwise model, with an AUC of .87. A higher risk of progression to GA was observed per effective allele for CFH rs1410996 (HR: 1.5; 95% CI: 1.3 to 1.9; P < .0001), CFH R1210C (HR: 4.3; 95% CI: 2.1 to 8.8; P < .0001), C3 K155Q (HR: 2.7; 95% CI: 1.7 to 4.1; P < .0001), ARMS2 (HR: 1.4; 95% CI: 1.2 to 1.6; P < .0001), and COL8A1 (HR: 1.3; 95% CI: 1.0 to 1.6; P = .04). Higher risk was also suggested for CFI rs10033900 (HR: 1.2; 95% CI: 1.0 to 1.3; P = .06) and C3 R102G (HR: 1.2; 95% CI: 1.0 to 1.4; P = .05). The AUC for this model was .87.
Progression to NV
Ten variants were included in the multivariate model for progression to NV. Increased risk of progression was associated with a higher number of risk alleles for CFH Y402H (HR: 1.2; 95% CI: 1.0 to 1.4; P = .04), CFH rs1410996 (HR: 1.4; 95% CI: 1.2 to 1.7; P = .0004), CFH R1210C (HR: 4.0; 95% CI: 1.9 to 8.4; P < .0001); C3 R102G (HR: 1.2; 95% CI: 1.1 to 1.4; P = .001), ARMS2 (HR: 1.6; 95% CI: 1.4 to 1.8; P < .0001), and COL8A1 (HR: 1.2; 95% CI: 1.0 to 1.4; P = .05). Protective effects were observed for CFB R32Q (HR: 0.7; 95% CI: 0.7 to 1.0; P = .03) and RAD51B (HR: 0.8; 95% CI: 0.7 to 0.9; P = .002), as well as two newly identified variants that have not been previously associated with progression to advanced stages of AMD: TGFBR1 (HR: 0.8; 95% CI: 0.7 to 1.0; P = .01) and COL4A3 (HR: 0.8; 95% CI: 0.7 to 1.0); P = .02). The AUC for this multivariate model was .86. The stepwise model for VA loss of at least 15 letters is discussed below along with all results related to this outcome.
As described in the Methods, five categories were used to define rate of progression over specified time intervals: 1) very low (< 1%); 2) low (1% to < 10%); 3) medium (10% to < 30%); 4) high (30% to < 50%); 5) very high (≥ 50%). Figure 3 illustrates the cumulative incidence of progression to overall advanced AMD among eyes with intermediate AMD (CARMS grade 3) at baseline over a 5 and 10 year intervals, while Figure 4 illustrates the comparison between GA and NV. Rate of progression was calculated based on the composite risk score derived from the risk models for progression to each endpoint. Among eyes with the same grade of intermediate AMD at baseline, the 5 year cumulative incidence of progression to an advanced AMD outcome varied according to risk score profile. This profile reflects not only the underlying genetic disposition toward specific disease states, but also individual risk based on age, sex, race, education, BMI, and smoking. Approximately 59% of eyes were predicted to have a medium risk of progression to overall advanced AMD over 5 years based on the composite risk score. No eyes were predicted to have very low risk of progression, 13% had low risk, about 23% had high risk, and 5% had very high risk of progression. Similar results were observed for progression from intermediate AMD to the advanced subtypes GA and NV, with most eyes (57% and 54%, respectively), classified as having medium risk for progression to both advanced subtypes at 5 years.
FIGURE 3.
Probability of progression to advanced age-related macular degeneration endpoint (AMD) over 5 years and 10 years among eyes with intermediate disease at baseline based on demographic, behavioral, ocular, and genetic variables (the risk score) in the derivation cohort group. Probability of progression was defined as 1) very low (< 1%); 2) low (1% to < 10%); 3) medium (10% to < 30%); 4) high (30% to < 50%); 5) very high (≥ 50%).
FIGURE 4.
Probability of progression to geographic atrophy (GA) and neovascular disease (NV) endpoints over 5 years and 10 years among eyes with intermediate disease at baseline based on demographic, behavioral, ocular, and genetic variables (risk score) in the derivation cohort group. Probability of progression was defined as 1) very low (< 1%); 2) low (1% to < 10%); 3) medium (10% to < 30%); 4) high (30% to < 50%); 5) very high (≥ 50%).
A boxplot figure comparing the risk score distributions including demographic, behavioral, ocular, and genetic variables for progressors from intermediate AMD at baseline to advanced AMD within 5 years and non-progressors followed for at least 10 years among eyes is shown in Figure 5. Although there is some overlap between these distributions, the median value and risk score distributions of the progressing eyes are substantially higher than the median value and risk score distribution of non-progressing eyes. At 5 years, the separation between groups was slightly less. (Supplemental Figure 1)
FIGURE 5.
Boxplot depicting risk score percentile for progressors to advanced AMD over 10 years and non-progressors among eyes with intermediate disease at baseline. The percentiles were calculated from the sample of non-progressing eyes. The horizontal line represents the median and the + sign represents the arithmetic mean. The top and bottom of the box depicts the upper and lower 25th percentile.
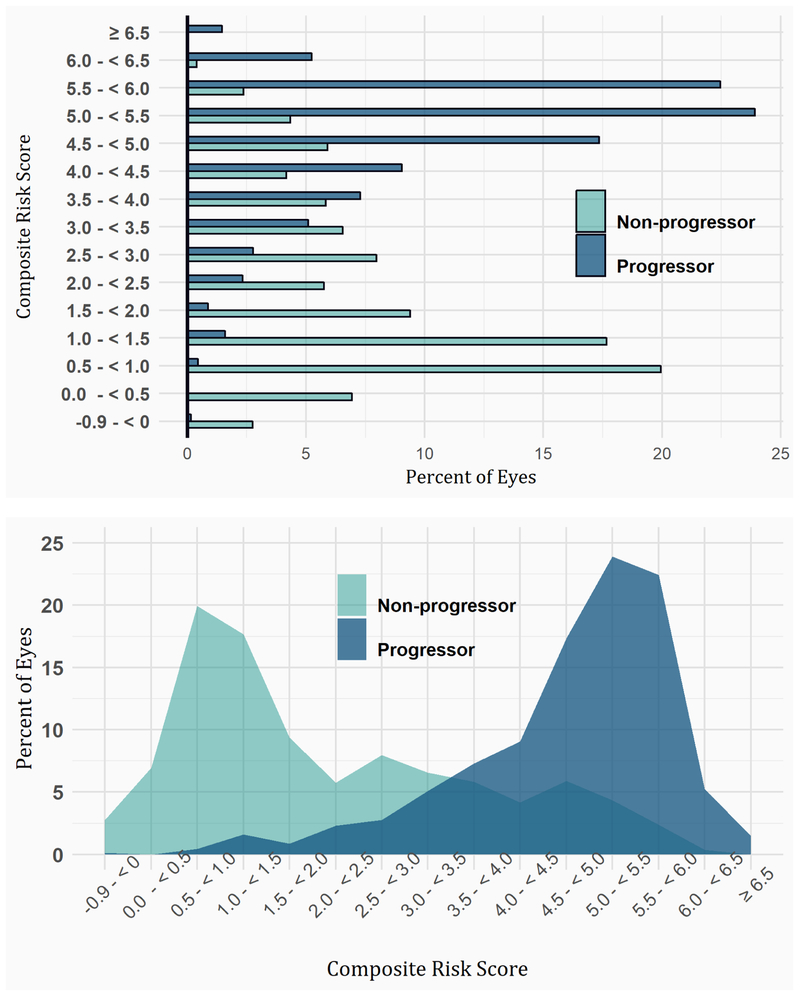
The percentages of eyes which progressed from intermediate AMD to advanced AMD over 5 or 10 years according to risk score deciles are displayed in Figure 6. For example in the 10 years period, there is approx. 20% progression in the lowest risk decile which increases to approximately 50% at the fifth risk decile and over 80% at the 10th risk decile. For the 5 years, there is approx. 10% progression in the lowest risk decile which increases to approximately 25% at the fifth risk decile and approx. 60% at the 10th risk decile.
FIGURE 6.
Plot of percent of eyes which progressed over 5 or 10 years according to risk score deciles.
To identify eyes with higher risk of progression from intermediate to advanced AMD, we divided the composite risk scores into quintiles and estimated the hazard ratio of progression within 5 or 10 years. As shown in Table 6, the HR of progression compared with quintile 1 is 1.30 for quintile 2, (95% CI = 0.86 - 1.95, p=0.21), 2.71 for quintile 3 (95% CI = 1.89-3.90, p<0.001), 3.67 for quintile 4 (95% CI 2.59-5.20, p<0.001), 5.82 for quintile 5 (95% CI = 4.16-8.16, p<0.001). At 10 years, the HRs are somewhat higher and quintile 2 also becomes significantly associated with progression compared to quintile 1. Therefore, the composite risk score is strongly related to risk of progression and identifies a very high risk group with almost a 6-fold higher risk of progression in the top quintile compared to the lowest quintile. Thus, a combination of several common and a few rare variants plus the other variables in the model can identify a high risk population with a magnitude of risk comparable to the risk conferred by some single rare genetic variants.
TABLE 6.
Probability of Progression to Advanced Age-Related Macular Degeneration Over 5 or 10 Years Based on the Composite Risk Score in the Derivation Cohort
| Risk Score | HR | 95% CI | P value |
|---|---|---|---|
| 5 Years | |||
| Quintile 1 | Ref | ||
| Quintile 2 | 1.30 | 0.86 - 1.95 | 0.213 |
| Quintile 3 | 2.71 | 1.89 - 3.90 | <0.0001 |
| Quintile 4 | 3.67 | 2.59 - 5.20 | <0.0001 |
| Quintile 5 | 5.82 | 4.16 - 8.16 | <0.0001 |
| 10 Years | |||
| Quintile 1 | Ref | ||
| Quintile 2 | 1.72 | 1.30 - 2.27 | 0.0001 |
| Quintile 3 | 3.04 | 2.35 - 3.93 | <0.0001 |
| Quintile 4 | 4.11 | 3.19 - 5.29 | <0.0001 |
| Quintile 5 | 5.91 | 4.61 - 7.58 | <0.0001 |
HR = hazard ratio; CI = confidence interval.
Model for the risk score includes age, sex, race, education, BMI, smoking, baseline macular status, and 13 common and rare genetic variants.
The average percent of eyes that were projected to progress over 5 and 10 year follow-up intervals was also calculated. In this derivation cohort, the mean risk of progression to advanced AMD over 5 years was 23.7%, indicating medium risk. Over 10 years, the average rate of progression was high, and increased to 40.5%. Average risks for progression to GA and NV were 12% and 13%, respectively, at five years and 25.4% and 23.6%, respectively, at ten years.
VALIDATION COHORT
Among 2156 participants included in the validation cohort, the mean age was 68.8 years and sample was 54.3% female. These demographics are consistent with the population included in the derivation cohort analyses reported above. The distribution of demographic, behavioral, ocular, and genetic factors among progressors and non-progressors is shown in Table 7. Comparable to the derivation cohort, older age (P < .0001), Caucasian race (P = .01), a low level of education (P = .0004), and a history of past (P = .03) or current (P = .0002) cigarette smoking were significantly associated with a higher risk of progression to advanced AMD in the validation cohort. A more advanced stage of AMD at baseline was also a risk factor for progression (P < .0001). Interestingly, females had a lower risk of progression compared to males (P < .0001). Higher BMI was not significantly associated with progression to advanced AMD.
TABLE 7.
Multivariate Associations Between Demographic, Behavioral, and Ocular Factors and Progression to Overall Advanced Age-Related Macular Degeneration, Geographic Atrophy, and Neovascular Disease in the Validation Cohort.
| Progressors n=743 |
Non-Progressors n=3660 |
HR (95% CI)a | P value | |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | ||||
| ≥ 75 | 237 (31.9) | 622 (17.0) | Referent | |
| 65 to 74.9 | 384 (51.7) | 1964 (53.7) | 0.55 (0.45 – 0.67) | <.0001 |
| 55 to 64.9 | 122 (16.4) | 1074 (29.3) | 0.37 (0.28 – 0.50) | <.0001 |
| Sex | ||||
| Female | 413 (55.6) | 1856 (50.7) | Referent | |
| Male | 330 (44.4) | 1804 (49.3) | 1.32 (1.10 - 1.59) | 0.003 |
| Race | ||||
| Non-Caucasian | 4 (0.4) | 54 (1.5) | Referent | |
| Caucasian | 739 (99.5) | 3606 (98.5) | 0.27 (0.08 – 0.94) | 0.04 |
| Behavioral | ||||
| Education | ||||
| ≤ High school | 312 (42) | 1183 (32.3) | Referent | |
| > High school | 431 (58) | 2477 (67.7) | 0.76 (0.63 – 0.91) | 0.003 |
| Body mass index | ||||
| < 25 | 254 (34.2) | 1311 (35.8) | Referent | |
| 25 to 29.9 | 334 (45) | 1524 (41.6) | 0.97 (0.79 – 1.19) | 0.79 |
| ≥ 30 | 155 (20.9) | 825 (22.5) | 1.09 (0.86 – 1.39) | 0.48 |
| Smoking | ||||
| Never | 262 (35.3) | 1529 (41.8) | Referent | |
| Past | 420 (56.5) | 1908 (52.1) | 1.17 (0.97-1.41) | 0.10 |
| Current | 61 (8.2) | 223 (6.1) | 1.72 (1.19-2.48) | 0.004 |
| Ocular | ||||
| Baseline AMD grade | ||||
| 1 | 32 (4.3) | 2125 (58.1) | Referent | |
| 2 | 151 (20.3) | 842 (23) | 10.2 (6.65-15.5) | <.0001 |
| 3 | 560 (75.4) | 693 (18.9) | 36.4 (24.4-54.4) | <.0001 |
AMD = age-related macular degeneration; CI = confidence interval; GA = geographic atrophy; HR = hazard ratio; NV = neovascular disease.
HRs and 95% CIs were estimated using Cox proportional hazards models for 12 year progression using the individual eye as the unit of analysis. HRs are adjusted for all variables listed in the table.
Effect estimates for the 13 loci identified as most predictive of progression to overall advanced AMD in the derivation cohort are displayed in Table 8 for both the derivation and validation cohorts. CFH rs1410996, C2 E318D, C3 R102G, ARMS2, and COL8A1 variants were significantly associated with progression to overall advanced AMD in the validation cohort. All other variants trended in the expected direction based on the results from the derivation cohort. It is not surprising that rare and low frequency variants, which have a large impact on a smaller number of individuals, were not significantly associated with AMD risk in the validation analysis. Rare genotypes are often not validated in a relatively smaller sample size as they are carried by a small proportion of the population. Results of this multivariate stepwise model are illustrated in Figure 2 (bottom), and are in conjunction with the effect estimates observed in the derivation cohort. The direction of the effect is similar for most genes, and replicates the major genes with higher allele frequency.
TABLE 8.
Associations Between Specific Genetic Variants and Risk of Progression to Advanced Stages of Age-Related Macular Degeneration: Derivation and Validation Cohorts.
| Derivation Cohort | Validation Cohort | |||
|---|---|---|---|---|
| n=1149/5355a | n=686/3955 | |||
| HR (95% CI)b | P value | HR (95% CI) | P value | |
| Genetic loci | ||||
| Complement pathway | ||||
| CFH Y402H: rs1061170 | 1.14 (1.02-1.29) | 0.03 | 1.09 (0.92-1.29) | 0.32 |
| CFH: rs1410996 | 1.46 (1.26-1.69) | <.0001 | 1.38 (1.09-1.74) | 0.007 |
| CFH R1210C: rs121913059 | 4.18 (2.79-6.27) | <.0001 | 1.49 (0.77-2.77) | 0.23 |
| C2 E318D: rs9332739 | 0.60 (0.43-0.85) | 0.004 | 0.51 (0.32-0.83) | 0.007 |
| CFB R32Q: rs641153 | 0.71 (0.54-0.93) | 0.01 | 0.99 (0.72-1.39) | 0.99 |
| C3 R102G: rs2230199 | 1.27 (1.15-1.41) | <.0001 | 1.15(1.01-1.33) | 0.04 |
| C3 K155Q: rs147859257 | 2.00 (1.50-2.66) | <.0001 | 1.16 (0.49-2.73) | 0.74 |
| Immune/inflammatory pathway | ||||
| ARMS2/HTRA1: rs10490924 | 1.47 (1.34-1.62) | <.0001 | 1.49 (1.31-1.70) | <.0001 |
| PELI3: rs145732233 | 0.29 (0.07-1.18) | 0.08 | 0.48 (0.16-1.46) | 0.19 |
| Extracellular matrix | ||||
| COL8A1: rs13095226 | 1.18 (1.03-1.37) | 0.02 | 1.33 (1.09-1.64) | 0.006 |
| CTRB1: rs8056814 | 0.84 (0.69-1.02) | 0.07 | 0.87 (0.67-1.13) | 0.74 |
| DNA repair/protein binding | ||||
| RAD51B: rs8017304 | 0.85 (0.77-0.94) | 0.001 | 0.88 (0.77-1.02) | 0.09 |
| HSPH1/B3GALTL: rs9542236 | 1.14 (1.04-1.25) | 0.004 | 1.07 (0.94-1.23) | 0.31 |
Sample sizes reported as (numerator/denominator), where the numerator is equal to the number of eyes that progressed during follow up, and the denominator is equal to the number of eligible eyes at baseline, among participants with complete genetic data.
HRs for 12 year progression reflect risk per allele, and are adjusted for age, sex, race, education, baseline grade, BMI, smoking status, and all other SNPs in the table.
Risk models from the derivation cohort were applied to the independent validation cohort. The AUCs for progression to the overall advanced AMD, GA, and NV endpoints at 5, 10, and 12 years after baseline are shown in Table 9 for the validation and derivation samples. For the validation cohort, the AUCs (± SE) were .852 ± .011, .876 ± .008, and .896 ± .007 for progression to overall advanced AMD at 5, 10, and 12 years, respectively. These results are very comparable and only slightly lower than the results observed in the derivation cohort for the same outcomes: .873 ± .008, .900 ± .005, and .900 ± .005 at 5, 10, and 12 years. For progression to GA and NV subtypes separately, the AUCs were .859 ± .011 and .853 ± .011, respectively, at five years for the derivation sample, and were .870 ± .014 and .825 ± .017 at five years for the validation sample. The AUCs in the validation cohort were slightly higher for GA and slightly lower for NV.
TABLE 9.
Area Under the Curve Statistics for Progression to Overall Advanced Age-Related Macular Degeneration, Geographic Atrophy, and Neovascular Disease in the Derivation and Validation Cohorts at 5, 10, and 12 Years after Baseline.
| Derivation Cohort | Validation Cohort | |||||
|---|---|---|---|---|---|---|
| Progressors | Non-Progressors | AUC ± SE | Progressors | Non-Progressors | AUC ± SEa | |
| 5 years | ||||||
| Overall advanced AMD | 590 | 4758 | 0.873 ± 0.008 | 316 | 2995 | 0.852 ± 0.011 |
| GA | 280 | 5063 | 0.859 ± 0.011 | 162 | 2992 | 0.870 ± 0.014 |
| NV | 331 | 5012 | 0.853 ± 0.011 | 159 | 3119 | 0.825 ± 0.017 |
| 10 years | ||||||
| Overall advanced AMD | 1056 | 3559 | 0.900 ± 0.005 | 595 | 1752 | 0.876 ± 0.008 |
| GA | 579 | 3946 | 0.866 ± 0.008 | 315 | 1752 | 0.887 ± 0.010 |
| NV | 620 | 3908 | 0.861 ± 0.008 | 302 | 1910 | 0.828 ± 0.013 |
| 12 years | ||||||
| Overall advanced AMD | 1149 | 4206 | 0.900 ± 0.005 | 686 | 1268 | 0.896 ± 0.007 |
| GA | 578 | 4777 | 0.870 ± 0.008 | 357 | 1268 | 0. 899 ± 0.009 |
| NV | 677 | 4678 | 0.860 ± 0.008 | 364 | 1408 | 0.836 ± 0.012 |
AMD = age-related macular degeneration; AREDS = Age-Related Eye Disease Study; AUC = area under the curve; GA = geographic atrophy; NV = neovascular disease.
Calculated by applying the composite risk score derived from the derivation cohort to the independent validation cohort.
We calculated positive and negative predictive values for this composite risk model of progression, using a cutoff of approximately 80% for sensitivity and specificity. These values correspond to a risk score of 4. For the validation cohort, the sensitivity was 80.0%: out of a total of 686 eyes that progressed to advanced AMD, there were 549 eyes with a risk score ≥ 4. The specificity of the model was 81.9%; of 1268 eyes that did not progress, 1039 eyes had a risk score < 4. These sensitivity and specificity values were calculated over a follow up interval of 12 years for the validation cohort (Figure 7). Receiver operating characteristic curves for progression to overall advanced AMD at 5 and 10 years were similar. These models include age, sex, race education, baseline AMD grade, BMI, smoking status, and the 13 genetic factors determined to be most predictive of progression in the derivation sample: five common and rare loci in CFH and C3, seven common loci in C2, CFB, ARMS2, COL8A1, CTRB1, RAD51B, and HSPH1/B3GALTL, and a rare variant in PELI3. There was a moderate increase in the AUC between 5 and 10 years. AUCs for this model in the derivation and validation cohorts were similar.
FIGURE 7.
Receiver operating characteristic curve for 12 year progression to overall advanced age-related macular degeneration in the validation cohort according to the validated risk model that includes demographic, lifestyle, ocular, and genetic factors. AUC = 0.896.
A histogram and an area graph of the risk scores for progressors and non-progressors to overall advanced AMD over 12 years in the validation cohort are presented in Figure 8. The observed distribution indicates a good separation between the two groups based on the composite model including demographic, lifestyle, ocular, and genetic factors. Risk scores were substantially different between the two groups, with higher scores among eyes that progressed to advanced disease compared to eyes that did not, although there was some overlap between the two groups.
FIGURE 8.
Distribution of risk scores for progressors to incident advanced age-related macular degeneration over 12 years and for non-progressors separately in the validation cohort, according to the composite risk score based on demographic, behavioral, ocular, and genetic variables.
COMPARING RISK PREDICTION WITH AND WITHOUT GENES
Assessment of models with and without genetic factors are displayed in Tables 10-13 for both cohorts. Results of the NRI for the derivation cohort at 5 years are presented in Table 10. Among progressors, changes in risk score groups dependent on genetic predictors were primarily observed in the medium or high risk groups. Approximately 40% of progressing eyes changed from medium risk to a high or very high risk group in a model with genes compared to a model without genes, and 32% transitioned from high to very high risk (NRI: 0.25; P < .0001). There were no differences between models in the analyses of non-progressing eyes: some eyes moved to a higher or lower risk group in a model with genes, but these transitions occurred in approximately equal numbers (NRI: −0.001; P = .86). The overall NRI (calculated as the sum of the NRIs for progressors and non-progressors) was statistically significant (NRI: 0.25; P < .0001), indicating that when genetic factors were considered, progressors were in a higher risk group.
TABLE 10.
Net Reclassification Improvement Analysis Comparing Progression Probability Categories Among Progressors and Non-Progressors to Advanced Stages of Age-Related Macular Degeneration Over 5 Years Based on a Composite Risk Model with No Genes Versus a Composite Risk Model with Genes in the Derivation Cohort.
| Probability of Progression Based on the Composite risk model with genesc |
|||||||
|---|---|---|---|---|---|---|---|
| Probability of Progression |
Very low | Low | Medium | High | Very high | Total | |
|
Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 6 (85.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (1.2) |
| Low | 1 (1.6) | 52 (85.3) | 8 (13.1) | 0 (0.0) | 0 (0.0) | 61 (10.3) | |
| Medium | 0 (0.0) | 15 (4.0) | 216 (56.8) | 128 (33.7) | 21 (5.5) | 380 (64.4) | |
| High | 0 (0.0) | 0 (0.0) | 34 (24.6) | 59 (42.8) | 45 (32.6) | 138 (23.4) | |
| Very high | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 4 (0.7) | |
| Total | 7 (1.2) | 68 (11.5) | 258 (43.7) | 191 (32.4) | 66 (11.2) | 590 (100.0) | |
|
Non-Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 1909 (94.1) | 120 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2029 (42.6) |
| Low | 89 (6.7) | 1208 (90.6) | 36 (2.7) | 0 (0.0) | 0 (0.0) | 1333 (28.0) | |
| Medium | 0 (0.0) | 202 (16.9) | 767 (64.2) | 208 (17.4) | 18 (1.5) | 1195 (25.1) | |
| High | 0 (0.0) | 4 (2.0) | 98 (49.8) | 77 (39.1) | 18 (9.1) | 197 (4.1) | |
| Very high | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 2 (50.0) | 4 (0.1) | |
| Total | 1998 (42.0) | 1534 (32.2) | 903 (19.0) | 285 (6.0) | 38 (1.0) | 4758 (100.0) | |
Age-related macular degeneration = AMD; NRI = Net reclassification improvement.
NRI for progressors: .25; P < .0001
NRI for non-progressors: −.001; P = 0.86
Overall NRI: .25; P < .0001
Probability of progression was defined as 1) very low (< 1% risk); 2) low (1% to < 10% risk); 3) medium (10% to < 30% risk); 4) high (30% to < 50% risk); 5) very high (≥ 50% risk).
Composite risk model including age, sex, race, education, BMI, smoking status, and baseline AMD grade.
Composite risk model including includes age, sex, race, education, BMI, smoking status, baseline AMD grade, and 13 loci determined to be associated with progression to advanced stages of AMD.
TABLE 13.
Net Reclassification Improvement Analysis Comparing Progression Probability Categories Among Progressors and Non-Progressors to Advanced Stages of Age-Related Macular Degeneration Over 10 Years for a Composite Risk Model with No Genes Versus a Composite Risk Model with Genes in the Validation Cohort.
| Probability of Progression Based on the Composite risk model with genesc |
|||||||
|---|---|---|---|---|---|---|---|
| Probability of Progression |
Very low | Low | Medium | High | Very high | Total | |
|
Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 4 (30.8) | 9 (69.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (2.2) |
| Low | 2 (1.7) | 78 (66.1) | 38 (32.2) | 0 (0.0) | 0 (0.0) | 118 (19.8) | |
| Medium | 0 (0.0) | 1 (0.8) | 54 (40.3) | 58 (43.3) | 21 (15.7) | 134 (22.5) | |
| High | 0 (0.0) | 2 (0.7) | 62 (20.5) | 139 (45.9) | 100 (33.0) | 303 (50.9) | |
| Very high | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (18.5) | 22 (81.5) | 27 (4.5) | |
| Total | 6 (1.0) | 90 (15.1) | 154 (25.9) | 202 (34.0) | 143 (24.0) | 595 (100.0) | |
|
Non-Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 545 (73.0) | 202 (27.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 747 (42.6) |
| Low | 98 (15.6) | 468 (74.3) | 64 (10.2) | 0 (0.0) | 0 (0.0) | 630 (36.0) | |
| Medium | 0 (0.0) | 19 (12.3) | 75 (48.7) | 56 (36.4) | 4 (2.6) | 154 (8.8) | |
| High | 0 (0.0) | 0 (0.0) | 79 (39.3) | 88 (43.8) | 34 (16.9) | 201 (11.5) | |
| Very high | 0 (0.0) | 0 (0.0) | 12 (60.0) | 4 (20.0) | 4 (20.0) | 20 (1.1) | |
| Total | 643 (36.7) | 689 (39.3) | 230 (13.1) | 148 (8.5) | 42 (2.4) | 1752 (100.0) | |
Age-related macular degeneration, AMD; NRI, Net reclassification improvement.
NRI for progressors: .26; P < .0001
NRI for non-progressors: −.08; P < .0001
Overall NRI: .17; P < .0001
Probability of progression was defined as 1) very low (< 1% risk); 2) low (1% to < 10% risk); 3) medium (10% to < 30% risk); 4) high (30% to < 50% risk); 5) very high (≥ 50% risk).
Composite risk model including age, sex, race, education, BMI, smoking status, and baseline AMD grade.
Composite risk model including includes age, sex, race, education, BMI, smoking status, baseline AMD grade, and 13 loci determined to be associated with progression to advanced stages of AMD.
Even more striking results were observed at 10 years of follow up (Table 11). Most differentiation occurred in the medium or high risk groups, and there was movement to an even higher risk group based on the model with genes. Over 60% of progressing eyes within the medium risk category, according to the model with no genes, transitioned to a high or very high risk group in the model with genes, and over 40% in the high risk group transitioned from high to very high risk (NRI: 0.33; P < .0001). The net number of subjects moving higher vs moving lower was 344 of 1056 eyes (33%). Among non-progressing eyes, when comparing models with and without genes, the net number of subjects moving higher vs. lower was 247 out of 3559 subjects (7%), with NRI = 0.26; P < .0001. If addition of genetic loci was unrelated to progression, then these two values (33% and 7%) would be the same. However, the overall NRI=33% minus 7% =26% was significantly higher than 0, indicating that a risk model including genetic loci was a better discriminator between progressing and non-progressing eyes than a model without genetic loci.
TABLE 11.
Net Reclassification Improvement Analysis Comparing Progression Probability Categories Among Progressors and Non-Progressors to Advanced Stages of Age-Related Macular Degeneration Over 10 Years for a Composite Risk Model with No Genes Versus a Composite Risk Model with Genes in the Derivation Cohort.
| Probability of Progression Based on the Composite risk model with genesc |
|||||||
|---|---|---|---|---|---|---|---|
| Probability of Progression |
Very low | Low | Medium | High | Very high | Total | |
|
Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 3 (37.5) | 5 (62.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (0.8) |
| Low | 1 (0.9) | 60 (56.1) | 45 (42.1) | 1 (0.9) | 0 (0.0) | 107 (10.1) | |
| Medium | 0 (0.0) | 2 (1.2) | 59 (36.0) | 81 (49.4) | 22 (13.4) | 164 (15.5) | |
| High | 0 (0.0) | 2 (0.3) | 82 (11.9) | 310 (45.1) | 293 (42.7) | 687 (65.1) | |
| Very high | 0 (0.0) | 0 (0.0) | 2 (2.2) | 14 (15.6) | 74 (82.2) | 90 (8.5) | |
| Total | 4 (0.4) | 69 (6.5) | 188 (17.8) | 406 (38.5) | 389 (36.8) | 1056 (100.0) | |
|
Non-Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 816 (69.2) | 364 (30.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1180 (33.2) |
| Low | 198 (13.3) | 1139 (76.4) | 152 (10.2) | 1 (0.1) | 0 (0.0) | 1490 (41.9) | |
| Medium | 0 (0.0) | 36 (12.8) | 156 (55.3) | 74 (26.2) | 16 (5.7) | 282 (7.9) | |
| High | 0 (0.0) | 9 (1.6) | 209 (36.8) | 241 (42.4) | 109 (19.2) | 568 (16.0) | |
| Very high | 0 (0.0) | 0 (0.0) | 5 (12.8) | 17 (43.6) | 17 (43.6) | 39 (1.1) | |
| Total | 1014 (28.5) | 1548 (43.5) | 522 (14.7) | 333 (9.4) | 142 (4.0) | 3559 (100.0) | |
Age-related macular degeneration = AMD; NRI = Net reclassification improvement.
NRI for progressors: .33; P < .0001
NRI for non-progressors: −.068; P < .0001
Overall NRI: .26; P < .0001
Probability of progression was defined as 1) very low (< 1% risk); 2) low (1% to < 10% risk); 3) medium (10% to < 30% risk); 4) high (30% to < 50% risk); 5) very high (≥ 50% risk).
Composite risk model including age, sex, race, education, BMI, smoking status, and baseline AMD grade.
Composite risk model including includes age, sex, race, education, BMI, smoking status, baseline AMD grade, and 13 loci determined to be associated with progression to advanced stages of AMD.
In the validation cohort analyses, similar results were observed for progressors and non-progressors over five and ten years of follow up. At five years (Table 12), the NRIs for progressors and non-progressors were 0.18 and 0.009 (P < .0001 and P = .17, respectively), with an overall NRI of 0.19 (P < .0001). Table 13 displays the NRIs for progressors (0.26; P < .0001), non-progressors (−0.08; P < .0001), and overall NRI (0.17; P < .0001) in the validation cohort at 10 years which were similar to those observed in the derivation cohort.
TABLE 12.
Net Reclassification Improvement Analysis Comparing Progression Probability Categories Among Progressors and Non-Progressors to Advanced Stages of Age-Related Macular Degeneration Over 5 Years for a Composite Risk Model with No Genes Versus a Composite Risk Model with Genes in the Validation Cohort.
| Probability of Progression Based on the Composite risk model with genesc |
|||||||
|---|---|---|---|---|---|---|---|
| Probability of Progression |
Very low | Low | Medium | High | Very high | Total | |
|
Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 6 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (1.9) |
| Low | 0 (0.0) | 54 (91.5) | 5 (8.5) | 0 (0.0) | 0 (0.0) | 59 (18.7) | |
| Medium | 0 (0.0) | 19 (8.1) | 149 (63.4) | 56 (23.8) | 11 (4.7) | 235 (74.4) | |
| High | 0 (0.0) | 0 (0.0) | 2 (12.5) | 8 (50.0) | 6 (37.5) | 16 (5.1) | |
| Very high | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total | 6 (1.9) | 73 (23.1) | 156 (49.4) | 64 (20.3) | 17 (5.4) | 316 (100.0) | |
|
Non-Progressors |
|||||||
| Probability of Progression Based on the Composite risk model with no genesb | Very low | 1495 (96.0) | 62 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1557 (52.0) |
| Low | 55 (8.0) | 628 (91.3) | 5 (0.7) | 0 (0.0) | 0 (0.0) | 688 (23.0) | |
| Medium | 0 (0.0) | 111 (15.9) | 491 (70.3) | 91 (13.0) | 5 (0.7) | 698 (23.3) | |
| High | 0 (0.0) | 4 (9.3) | 12 (27.9) | 25 (58.1) | 2 (4.7) | 43 (1.4) | |
| Very high | 0 (0.0) | 2 (22.2) | 6 (66.7) | 1 (11.1) | 0 (0.0) | 9 (0.3) | |
| Total | 1550 (51.8) | 807 (26.9) | 514 (17.2) | 117 (3.9) | 7 (0.2) | 2995 (100.0) | |
Age-related macular degeneration, AMD; NRI, Net reclassification improvement.
NRI for progressors: .18; P < .0001
NRI for non-progressors: .009; P = 0.17
Overall NRI: .19; P < .0001
Probability of progression was defined as 1) very low (< 1% risk); 2) low (1% to < 10% risk); 3) medium (10% to < 30% risk); 4) high (30% to < 50% risk); 5) very high (≥ 50% risk).
Composite risk model including age, sex, race, education, BMI, smoking status, and baseline AMD grade.
Composite risk model including includes age, sex, race, education, BMI, smoking status, baseline AMD grade, and 13 loci determined to be associated with progression to advanced stages of AMD.
HETEROGENEITY OF RISK PROFILES BETWEEN GA AND NV
We evaluated whether risk models differed between the two types of advanced disease-GA and NV (Table 14). Genetic analyses were based on number of risk alleles in variants related to GA or NV in Table 4. There was higher HR for the ARMS2 variant for NV compared with GA, and both were significant, but the heterogeneity between HRs for NV and GA was not significant in these analyses. Three genes showed a significantly different HR for GA and NV. These included the RAD51B gene which showed a significant inverse association with NV, HR=0.76 (95% CI 0.66-0.87) but not GA (HR= 0.92, 95% CI 0.79-1.07). The two HR’s were significantly different, with P (het) = 0.034. In addition there was significant heterogeneity for COL4A3, which showed a borderline association with NV, HR=0.87 (95% CI 0.75-1.00) and no association with GA (HR=1.09, 95% CI 0.94-1.27), with P (het) =0.021. There was borderline significant heterogeneity for the gene TGFBR1, which showed a significant inverse association with NV, HR= 0.83 (95% CI 0.72-0.95) and no association with GA (HR=1.00, 95% CI 0.86-1.17), and the P (het) was 0.057.
TABLE 14.
Heterogeneity of Risk Profile Between Geographic Atrophy and Neovascular Disease in the Derivation Cohort.
| GA | NV | ||||
|---|---|---|---|---|---|
| HR (95% CI)a | P value | HR (95% CI)a | P value | P het b | |
| Genetic loci | |||||
| CFH Y402H: rs1061170 | 1.08 (0.91 - 1.27) | 0.397 | 1.17 (1.00 - 1.36) | 0.052 | 0.440 |
| CFH: rs1410996 | 1.49 (1.20 - 1.86) | 0.0003 | 1.47 (1.20 - 1.79) | 0.0002 | 0.894 |
| CFH R1210C: rs121913059 | 4.12 (2.09 - 8.10) | <0.0001 | 4.78 (2.59 - 8.83) | <0.0001 | 0.751 |
| C2 E318D: rs9332739 | 0.40 (0.21 - 0.75) | 0.004 | 0.68 (0.44 - 1.04) | 0.072 | 0.161 |
| CFB R32Q: rs641153 | 0.73 (0.50 - 1.07) | 0.106 | 0.62 (0.44 - 0.89) | 0.008 | 0.493 |
| CFI: rs10033900 | 1.08 (0.95 - 1.24) | 0.236 | 1.02 (0.91 - 1.15) | 0.694 | 0.483 |
| C3 R102G: rs2230199 | 1.24 (1.06 - 1.44) | 0.006 | 1.20 (1.05 - 1.38) | 0.008 | 0.772 |
| C3 K155Q: rs147859257 | 2.15 (1.43 - 3.23) | 0.0002 | 1.67 (1.07 - 2.62) | 0.024 | 0.402 |
| TGFBR1: rs334353 | 1.00 (0.86 - 1.17) | 0.999 | 0.83 (0.72 - 0.95) | 0.009 | 0.057 |
| ARMS2/HTRA1: rs10490924 | 1.38 (1.20 - 1.57) | <0.0001 | 1.58 (1.39 - 1.79) | <0.0001 | 0.105 |
| PELI3: rs145732233 | 0.23 (0.03 - 1.74) | 0.152 | 0.37 (0.05 - 2.61) | 0.318 | 0.727 |
| COL8A1: rs13095226 | 1.24 (0.99 - 1.55) | 0.063 | 1.17 (0.98 - 1.41) | 0.088 | 0.698 |
| COL4A3: rs11884770 | 1.09 (0.94 - 1.27) | 0.270 | 0.87 (0.75 - 1.00) | 0.055 | 0.021 |
| CTRB1: rs8056814 | 0.81 (0.61 - 1.08) | 0.153 | 0.76 (0.58 - 0.99) | 0.040 | 0.704 |
| RAD51B: rs8017304 | 0.92 (0.79 - 1.07) | 0.264 | 0.76 (0.66 - 0.87) | <0.0001 | 0.034 |
| HSPH1/B3GALTL: rs9542236 | 1.14 (0.99 - 1.30) | 0.061 | 1.16 (1.02 - 1.31) | 0.023 | 0.853 |
| Non- genetic variables | |||||
| Age | |||||
| 75+ | 1.00 (referent) | 1.00 (referent) | |||
| 55-64 | 0.48 (0.34 - 0.67) | <0.0001 | 0.40 (0.30 - 0.54) | <0.0001 | 0.385 |
| 65-74 | 0.65 (0.52 - 0.82) | 0.0002 | 0.70 (0.57 - 0.86) | 0.001 | 0.626 |
| Gender | |||||
| Female | 1.00 (referent) | 1.00 (referent) | |||
| Male | 1.17 (0.95 - 1.44) | 0.135 | 0.89 (0.74 - 1.08) | 0.240 | 0.035 |
| BMI | |||||
| <25 | 1.00 (referent) | 1.00 (referent) | |||
| 25-29 | 1.20 (0.94 - 1.52) | 0.146 | 1.21 (0.97 - 1.50) | 0.086 | 0.949 |
| 30+ | 1.50 (1.15 - 1.95) | 0.003 | 1.38 (1.08 - 1.76) | 0.009 | 0.614 |
| Smoking | |||||
| Never | 1.00 (referent) | 1.00 (referent) | |||
| Current | 1.72 (1.14 - 2.61) | 0.010 | 2.47 (1.75 - 3.48) | <0.0001 | 0.142 |
| Past | 1.09 (0.89 - 1.35) | 0.403 | 1.41 (1.16 - 1.71) | 0.001 | 0.058 |
| Race | |||||
| Non-Caucasian | 1.00 (referent) | 1.00 (referent) | |||
| Caucasian | 4.89 (1.22 - 19.67) | 0.025 | 1.51 (0.73 - 3.12) | 0.263 | 0.138 |
| Education | |||||
| ≤ High school | 1.00 (referent) | 1.00 (referent) | |||
| > High school | 0.81 (0.66 - 0.99) | 0.040 | 0.73 (0.61 - 0.87) | 0.001 | 0.397 |
| Baseline AMD grade | |||||
| 1 | 1.00 (referent) | 1.00 (referent) | |||
| 2 | 16.78 (6.02 - 46.81) | <0.0001 | 5.15 (3.05 - 8.69) | <0.0001 | 0.043 |
| 3 | 113.73 (42.28 - 305.94) | <0.0001 | 22.46 (13.75 - 36.69) | <0.0001 | 0.004 |
GA = geographic atrophy; NV = neovascular disease.
Based on a multivariate model for 12 year progression with all risk factors included. Hazard ratios for genetic loci calculated per minor allele.
P het = P value for heterogeneity based on a competing risk proportional hazards model, using the data duplication method of Lunn and McNeil, Biometrics, 1995.
In addition, there were two non-genetic variables that showed significant heterogeneity between GA and NV: gender and initial AMD grade. Compared to grade 1, there were significant associations between baseline grade 2 and 3 and progression for both GA and NV, with higher risk for progression with baseline grade 3 compared with baseline grade 2. However, the association between baseline grade 3 and progression was significantly stronger for progression to grade 4 compared with progression to grade 5, with P (het) = 0.004. Regarding gender, males had a slightly lower risk than females for NV and a slightly higher risk for GA. When assessing heterogeneity, the difference in gender associations between NV and GA was significant, with P (het) = 0.035.
CALIBRATION RESULTS
The prediction model was calibrated in the validation cohort using the coefficients from the derivation cohort model. As shown in Table 15 and Figure 9, we found an expected/observed (E/O) ratio of 0.99 (95% CI 0.87-1.11) and P=0.82, for testing the null hypothesis of an intercept = 0. Results indicate that the calibration of the model for intermediate AMD eyes for progression to advanced AMD is adequate in the validation sample.
TABLE 15.
Calibration of Risk Model for Progression to Advanced Age-Related Macular Degeneration. a
| Risk (decile) |
n | Observed # events |
Expected # events |
|---|---|---|---|
| 1 | 113 | 14.83 | 7.72 |
| 2 | 115 | 11.61 | 12.58 |
| 3 | 114 | 17.09 | 16.23 |
| 4 | 115 | 27.12 | 18.95 |
| 5 | 115 | 14.5 | 22.04 |
| 6 | 114 | 30.49 | 25.24 |
| 7 | 114 | 20.12 | 29.52 |
| 8 | 116 | 40.55 | 35.03 |
| 9 | 114 | 44.67 | 40.54 |
| 10 | 114 | 45.98 | 55.36 |
| total | 1144 | 266.98 | 263.21 |
Ratio (expected/observed) = 0.986 (95% CI 0.87, 1.11); Chi-square = 0.054; P value= 0.816 (indicating adequate calibration of the model).
Data was based on progression over 5 years comparing observed and expected number of eyes progressing in the validation dataset according to deciles of the risk score from the derivation sample among eyes with baseline intermediate AMD.
FIGURE 9.
Calibration of the risk model for progression to advanced age-related macular degeneration over 5 years comparing observed and expected number of eyes progressing in the validation cohort.
APPLICATION OF THE ONLINE RISK CALCULATOR
To facilitate clinical use of the algorithm in Table 5, we developed an online calculator which enhances our previous calculator.38 A physician can enter genotypes, together with baseline AMD grade and demographic factors to obtain a two, five and ten year risk of progression in individual eyes. The calculator allows for the evaluation of individual risk and can be used to assess varying risk profiles based on a specific subset of demographic, behavioral, ocular, and genetic factors. Our composite risk model for prediction progression to advanced AMD is available online at www.seddonamdriskscore.org.45
Representative results applying the online calculator are shown in Table 16, indicating wide variation in progression rates among individuals with the same AMD grade at baseline, which underscores the potential value of the risk score models in helping to counsel patients and assist with disease management and prognosis. The variation observed among individual eyes with the same baseline status is reinforced by the results of the NRI. In addition, for the 24 eyes in Table 16 with intermediate AMD, risk for 5 year progression was compared between models with and without genes. Results suggest that for 7 of the 24 eyes, the 5 year risk increased by 1 risk score group (as defined above) and for 1 eye, the 5 year risk decreased by 1 risk score group in the model with genes compared to the model without genes. This indicates that the clinical discussion with individual patients may vary depending on whether genetic variants are considered or not.
TABLE 16.
Cumulative Incidence of Progression to Advanced Age-Related Macular Degeneration at 2, 5, 10, and 12 Years Based on the Composite Risk Score, Adjusting for Mortality Risks for a Representative Sample of 24 Right and Left Eyes with Intermediate Stage Disease at Baseline.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eye | OD | OD | OD | OD | OD | OD | OD | OD | OD | OD | OD | OD |
| Age | 65-74 | ≥ 75 | ≥ 75 | ≥ 75 | < 65 | ≥ 75 | ≥ 75 | ≥ 75 | 65-74 | 65-74 | 65-74 | 65-74 |
| Sex | F | M | M | M | F | F | F | F | M | F | F | F |
| Education | > HS | ≤ HS | ≤ HS | > HS | ≤ HS | ≤ HS | ≤ HS | ≤ HS | ≤ HS | ≤ HS | ≤ HS | > HS |
| Body mass index | 25-29 | ≥ 30 | 25-29 | < 25 | ≥ 30 | 25-29 | < 25 | 25-29 | ≥ 30 | ≥ 30 | ≥ 30 | < 25 |
| Smoking | Never | Never | Past | Past | Never | Never | Never | Never | Past | Past | Never | Current |
| Complement pathway | ||||||||||||
| CFH Y402H: rs1061170 | TT | CT | CC | CC | TT | CT | CT | CT | TT | CC | CC | TT |
| CFH: rs1410996 | TT | CT | CC | CC | CT | CT | CT | CC | CT | CC | CC | CT |
| CFH R1210C: rs121913059 | CC | CC | CC | CC | CT | CC | CC | CC | CC | CC | CC | CC |
| C2 E318D: rs9332739 | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG |
| CFB R32Q: rs641153 | CC | CC | CC | CC | CC | CC | CC | CC | CT | CC | CC | CC |
| C3 R102G: rs2230199 | CG | CG | CC | CC | CC | CG | GG | CC | CC | CC | CC | CG |
| C3 K155Q: rs147859257 | TT | GT | TT | TT | TT | TT | TT | TT | TT | TT | TT | TT |
| Immune/inflammatory pathway | ||||||||||||
| ARMS2/HTRA1: rs10490924 | GT | GT | GG | TT | GT | GG | GT | GT | GT | GT | GT | GT |
| PELI3: rs145732233 | CC | CC | CC | CC | CC | CC | CT | CC | CC | CC | CC | CC |
| Extracellular matrix | ||||||||||||
| COL8A1: rs13095226 | TT | CT | TT | CT | TT | TT | TT | TT | CT | TT | TT | CT |
| CTRB1: rs8056814 | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG |
| DNA repair/protein binding | ||||||||||||
| RAD51B: rs8017304 | GA | GA | AA | AA | AA | AA | AA | GG | GA | AA | GG | AA |
| HSPH1/B3GALTL: rs9542236 | CT | CT | CT | CT | TT | TT | CT | CT | CT | CT | TT | CT |
| 2 year cumulative incidence, % | 4.2 | 27.1 | 14.1 | 20.6 | 21.6 | 8.1 | 5.1 | 11.7 | 6.6 | 19.4 | 10.2 | 15 |
| 5 year cumulative incidence, % | 10.6 | 52.1 | 30.4 | 40.8 | 47.8 | 19 | 12.3 | 26.1 | 16.3 | 42.4 | 24.4 | 34.6 |
| 10 year cumulative incidence, % | 21.1 | 67.6 | 45.4 | 54.4 | 76.7 | 32.6 | 22.1 | 41.1 | 31.5 | 65.6 | 44.4 | 58.6 |
| 12 year cumulative incidence, % | 25.5 | 69.4 | 48.5 | 56.2 | 84.3 | 36.7 | 25.4 | 44.5 | 37.6 | 71 | 51.3 | 65.7 |
| 5 year cumulative incidence no genes, % | 19.6 | 31 | 31.3 | 21.7 | 23.5 | 29.3 | 26.2 | 28.3 | 32.7 | 34.3 | 28.9 | 32.4 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| Eye | OS | OS | OS | OS | OS | OS | OS | OS | OS | OS | OS | OS |
| Age | 65-74 | < 65 | 65-74 | 65-74 | 65-74 | ≥ 75 | 65-74 | 65-74 | ≥ 75 | 65-74 | 65-74 | < 65 |
| Sex | F | F | F | F | F | M | F | M | F | M | F | M |
| Education | > HS | > HS | ≤ HS | > HS | ≤ HS | > HS | ≤ HS | ≤ HS | > HS | ≤ HS | > HS | > HS |
| Body mass index | ≥ 30 | ≥ 30 | < 25 | 25-29 | 25-29 | < 25 | < 25 | 25-29 | 25-29 | 25-29 | 25-29 | 25-29 |
| Smoking | Past | Past | Never | Past | Past | Past | Never | Never | Never | PAST | Past | Current |
| Complement pathway | ||||||||||||
| CFH Y402H: rs1061170 | CC | CT | TT | CC | CT | CC | CC | CC | CC | CT | CC | CT |
| CFH: rs1410996 | CC | CT | CT | CC | CT | CC | CC | CC | CC | CC | CC | CC |
| CFH R1210C: rs121913059 | CC | CC | CC | CC | CT | CC | CC | CC | CC | CC | CC | CC |
| C2 E318D: rs9332739 | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG | GG |
| CFB R32Q: rs641153 | CC | CC | CC | CC | CC | CT | CC | CC | CC | CC | CC | CC |
| C3 R102G: rs2230199 | CG | CG | CG | CG | CG | CC | GG | CC | CG | CC | CC | CC |
| C3 K155Q: rs147859257 | TT | GT | TT | TT | TT | TT | TT | TT | TT | TT | TT | TT |
| Immune/inflammatory pathway | ||||||||||||
| ARMS2/HTRA1: rs10490924 | GG | TT | GG | TT | GG | GG | GG | GT | GG | GT | GG | GG |
| PELI3: rs145732233 | CC | CC | CC | CC | CC | CC | CT | CC | CC | CC | CC | CC |
| Extracellular matrix | ||||||||||||
| COL8A1: rs13095226 | TT | TT | TT | TT | TT | CT | TT | TT | CT | TT | TT | TT |
| CTRB1: rs8056814 | GG | GG | GG | GG | GG | AG | GG | GG | AG | GG | GG | GG |
| DNA repair/protein binding | ||||||||||||
| RAD51B: rs8017304 | GA | AA | GG | GG | GA | GA | GG | AA | AA | GA | GG | GG |
| HSPH1/B3GALTL: rs9542236 | CC | TT | TT | CC | CT | TT | CC | CT | CT | CC | TT | CT |
| 2 year cumulative incidence, % | 13.7 | 20 | 3.6 | 18.7 | 28.7 | 4.4 | 4.5 | 12.6 | 11.8 | 11.5 | 6.1 | 7.3 |
| 5 year cumulative incidence, % | 32 | 45 | 9.3 | 40.9 | 58.7 | 10.5 | 11.3 | 29.1 | 26.2 | 27.2 | 15.2 | 18.2 |
| 10 year cumulative incidence, % | 55.3 | 73.9 | 19.5 | 63.4 | 83.6 | 18.8 | 22.4 | 49.6 | 41.1 | 48 | 30.5 | 36.7 |
| 12 year cumulative incidence, % | 62.6 | 82 | 24.3 | 68.6 | 88 | 21.5 | 27 | 55.8 | 44.6 | 54.9 | 37.1 | 44.8 |
| 5 year cumulative incidence no genes, % | 28.6 | 23.1 | 22.3 | 23.5 | 30.6 | 23.6 | 24.4 | 22.4 | 22.9 | 22.4 | 24.6 | 27.6 |
Results shown for derivation cohort. F = Female; M =Male; HS = high school.
The calculations described above assume complete data, including demographic, behavioral, ocular characteristics, and genetic data for each individual participant. In a clinical setting, it is possible that some patients may have some missing data for a variable number of risk factors. To address the issue of missing data, we created three distinct modules (A through C) that accounted for the availability of the data. These modules were defined as follows: A: all macular phenotypes and genetic variants known; B: all genetic variants known, all macular phenotypes unknown; C: all macular phenotypes known, genetic variables unknown. The NHANES 2009 data was used to impute values for missing demographic and behavioral factors. The proportion of participants with specific levels of education, smoking, and BMI as a function of age-sex groups was estimated. Missing genetic risk factors were imputed from the population prevalence of specific alleles.
The models described herein were evaluated with an additional adjustment for baseline drusen size in each eye. The results of these models are presented in Supplementary Table 1. Results for overall advanced AMD are similar. There were some minor differences noted for the specific GA and NV endpoints.
PROGRESSION TO LOSS OF VISUAL ACUITY
In addition to our analyses of advanced AMD endpoints, we evaluated the occurrence of VA loss of at least 15 letters over 12 years. The distributions of demographic, behavioral, ocular, and genetic characteristics among those who developed this level of visual loss and those who did not are presented in Table 17. Those experiencing VA loss ≥ 15 letters were older (P < .0001), Caucasian (P < .0001), had a lower level of education (P < .0001), a higher BMI (P = .0001), and a history of cigarette smoking (P < .0001). These participants also had more advanced stages of AMD at baseline (P < .0001). Visual loss was similar among males and females (P = .54).
TABLE 17.
Univariate Associations Between Demographic, Behavioral, Ocular, and Genetic Factors and Visual Acuity Loss in the Derivation Cohort.
| VA Loss ≤ 15 lettersa n=1650 |
VA Loss < 15 letters n=3620 |
P valueb | |
|---|---|---|---|
| Demographic | |||
| Age (years) | |||
| ≤ 75 | 893 (54.1) | 1275 (35.2) | <.0001 |
| 65 to 74 | 724 (43.9) | 2261 (62.5) | |
| 55 to 64 | 33 (2.0) | 84 (2.3) | |
| Sex | |||
| Male | 715 (43.3) | 1612 (44.5) | 0.54 |
| Female | 935 (56.7) | 2008 (55.5) | |
| Race | |||
| Caucasian | 1621 (98.2) | 3467 (95.8) | <.0001 |
| Non-caucasian | 29 (1.8) | 153 (4.2) | |
| Behavioral | |||
| Education | |||
| ≤ High school | 628 (38.1) | 1132 (31.3) | <.0001 |
| > High school | 1022 (61.9) | 2488 (68.7) | |
| Body mass index | |||
| < 25 | 481 (29.2) | 1244 (34.4) | 0.0001 |
| 25 to 29.9 | 716 (43.4) | 1537 (42.5) | |
| ≥ 30 | 453 (27.5) | 839 (23.2) | |
| Smoking | |||
| Never | 688 (41.7) | 1782 (49.2) | <.0001 |
| Past | 825 (50.0) | 1655 (45.7) | |
| Current | 137 (8.3) | 183 (5.1) | |
| Ocular | |||
| Baseline AMD grade | |||
| 1 | 258 (15.6) | 1624 (44.9) | <.0001 |
| 2 | 256 (15.5) | 958 (26.5) | |
| 3 | 903 (54.7) | 928 (25.6) | |
| 4 | 79 (4.8) | 16 (0.4) | |
| 5 | 134 (8.1) | 69 (1.9) | |
| Genetic loci | |||
| Complement pathway | |||
| CFH Y402H: rs1061170 | |||
| TT | 375 (22.7) | 1237 (34.2) | <.0001 |
| CT | 755 (45.8 ) | 1630 (45.0) | |
| CC | 520 (31.5) | 753 (20.8) | |
| CFH: rs1410996 | |||
| TT | 120 (7.3) | 570 (15.7) | <.0001 |
| CT | 577 (35.0) | 1574 (43.5) | |
| CC | 953 (57.8) | 1476 (40.8) | |
| CFH R1210C: rs121913059 | |||
| CC | 1634 (99.0) | 3609 (99.7) | 0.02 |
| CT | 16 (1.0) | 11 (0.3) | |
| C2 E318D: rs9332739 | |||
| GG | 1563 (94.7) | 3342 (92.3) | 0.007 |
| CG/CC | 87 (5.3) | 278 (7.7) | |
| CFB R32Q: rs641153 | |||
| CC | 1460 (88.5) | 3065 (84.7) | 0.001 |
| TC/TT | 190 (11.5) | 55 (15.3) | |
| CFI: rs10033900 | |||
| CC | 398 (24.1) | 926 (25.6) | 0.25 |
| CT | 810 (49.1) | 1179 (49.1) | |
| TT | 442 (26.8) | 915 (25.3) | |
| C3 R102G: rs2230199 | |||
| CC | 846 (51.3) | 2228 (61.5) | <.0001 |
| CG | 670 (40.6) | 1224 (33.8) | |
| GG | 134 (8.1) | 168 (4.6) | |
| C3 K155Q: rs147859257 | |||
| TT | 1596 (96.7) | 3566 (98.5) | 0.0001 |
| GT | 54 (3.3) | 54 (1.5) | |
| C9 P167S: rs34882957 | |||
| GG | 1605 (97.3) | 3545 (97.9) | 0.16 |
| AG | 45 (2.7) | 75 (2.1) | |
| CFH N1050Y: rs35274867 | |||
| AA | 1627 (98.6) | 3518 (97.2) | 0.007 |
| TA | 23 (1.4) | 98 (2.7) | |
| TT | 0 (0.0) | 4 (0.1) | |
| Angiogenesis pathway | |||
| VEGFA: rs943080 | |||
| CC | 330 (20.0) | 851 (23.5) | 0.02 |
| CT | 846 (51.3) | 1813 (50.1) | |
| TT | 474 (28.7) | 956 (26.4) | |
| TGFBR1: rs334353 | |||
| TT | 993 (60.2) | 2062 (57.0) | 0.08 |
| GT | 559 (33.9) | 1332 (36.8) | |
| GG | 98 (5.9) | 226 (6.2) | |
| Lipid pathway | |||
| LIPC: rs10468017 | |||
| CC | 878 (53.2) | 1889 (52.2) | 0.37 |
| TC | 666 (40.4) | 1464 (40.4) | |
| TT | 106 (6.4) | 267 (7.4) | |
| ABCA1: rs1883025 | |||
| CC | 919 (55.7) | 2015 (55.7) | 0.76 |
| TC | 636 (38.5) | 1377 (38.0) | |
| TT | 95 (5.8) | 228 (6.3) | |
| CETP: rs3764261 | |||
| CC | 685 (41.5) | 1615 (44.6) | 0.07 |
| AC | 770 (46.7) | 1606 (44.4) | |
| AA | 195 (11.8) | 399 (11.0) | |
| APOC1/APOE: rs4420638 | |||
| AA | 1196 (72.5) | 2853 (78.8) | 0.43 |
| GA | 454 (27.5) | 1037 (28.6) | |
| APOH: rs1801689 | |||
| AA | 1546 (93.7) | 3385 (93.5) | 0.72 |
| AC | 104 (6.3) | 233 (6.4) | |
| CC | 0 (0.0) | 2 (0.1) | |
| Immune/inflammatory pathway | |||
| ARMS2/HTRA1: rs10490924 | |||
| GG | 612 (37.1) | 2068 (57.1) | <.0001 |
| TG | 753 (45.6) | 1283 (35.4) | |
| TT | 285 (17.3) | 269 (7.4) | |
| PELI3: rs145732233 | |||
| CC | 1640 (99.4) | 3591 (99.2) | 0.43 |
| TC | 10 (0.6) | 29 (0.8) | |
| TNFRSF10A: rs13278062 | |||
| TT | 477 (28.9) | 979 (27.0) | 0.5 |
| GT | 808 (49.0) | 1847 (51.0) | |
| GG | 365 (22.1) | 794 (21.9) | |
| SLC16A8: rs8135665 | |||
| CC | 1022 (61.9) | 2310 (63.8) | 0.11 |
| TC | 544 (33.0) | 1167 (32.2) | |
| TT | 84 (5.1) | 143 (4.0) | |
| PILRB/PILRA: rs11769700 | |||
| TT | 1038 (62.9) | 2279 (63.0) | 0.89 |
| CT | 554 (33.6) | 1201 (33.2) | |
| CC | 58 (3.5) | 140 (3.9) | |
| TMEM97/VTN: rs704 | |||
| AA | 381 (23.1) | 873 (24.1) | 0.74 |
| AG | 823 (49.9) | 1760 (48.6) | |
| GG | 446 (27.0) | 987 (27.3) | |
| Extracellular matrix | |||
| COL8A1: rs13095226 | |||
| TT | 1305 (79.1) | 2940 (81.2) | 0.1 |
| CT | 324 (19.6) | 639 (17.7) | |
| CC | 21 (1.3) | 41 (1.1) | |
| COL4A3: rs11884770 | |||
| CC | 917 (55.6) | 1914 (52.9) | 0.05 |
| TC | 630 (382) | 1414 (39.1) | |
| TT | 103 (6.2) | 292 (8.1) | |
| CTRB1: rs8056814 | |||
| GG | 1420 (86.1) | 2952 (81.5) | 0.0004 |
| AG | 221 (13.4) | 625 (17.3) | |
| AA | 9 (0.5) | 43 (1.2) | |
| ADAMTS9: rs6795735 | |||
| CC | 510 (30.9) | 1062 (29.3) | 0.85 |
| TC | 770 (46.7) | 1783 (49.3) | |
| TT | 370 (22.4) | 775 (21.4) | |
| TIMP3: rs9621532 | |||
| AA | 1512 (91.6) | 3250 (89.8) | 0.04 |
| CA/CC | 138 (8.4) | 370 (10.2) | |
| DNA binding/protein repair | |||
| RAD51B: rs8017304 | |||
| AA | 727 (44.1) | 1453 (40.1) | 0.001 |
| GA | 756 (45.8) | 1642 (45.4) | |
| GG | 167 (10.1) | 525 (14.5) | |
| NPLOC4/TSPAN10: rs9895741 | |||
| GG | 683 (41.4) | 1506 (41.6) | 0.95 |
| AG | 741 (44.9) | 1611 (44.5) | |
| AA | 226 (13.7) | 503 (13.9) | |
| HSPH1/B3GALTL: rs9542236 | |||
| TT | 480 (29.1) | 1226 (33.9) | 0.005 |
| CT | 834 (50.5) | 1732 (47.8) |
AMD = age-related macular degeneration; VA = visual acuity.
Sample sizes for each genetic variable may not be equal to the overall sample size. Some participants do not have genetic information available for all genetic loci evaluated.
P values calculated using Generalized Estimating Equations in order to account for inter-correlation in eye-specific analyses.
The genotypes for several loci in the complement pathway differed with regard to VA loss. A higher number of risk alleles was associated with VA loss ≥ 15 letters for common variants in CFH Y402H (P < .0001), CFH rs1410996 (P < .0001), and C3 R102G (P < .0001), and the rare variants CFH R1210C (P = .02) and C3 K155Q (P = .0001). A significantly lower rate of visual loss was observed with protective alleles in C2 E318D (P = .007) and CFB R32Q (P = .001). A similar protective effect was observed with CFH N1050Y (P = .007). In the angiogenesis pathway, VEGFA was significantly associated with VA loss (P = .02), and a suggestive effect was observed for TGFBR1 (P = .08).
In the lipid pathway, a suggestive effect was observed for CETP (P = .07). In the immune pathway, significant associations were observed with risk alleles in ARMS2 (P < .0001). CTRB1 was associated with a protective effect against visual acuity loss (P = .0004) as were alleles in COL4A3 (P = .05) and TIMP3 (P =.04): these loci are associated with the extracellular matrix pathway. The DNA repair and protein binding genes RAD51B (P = .001), HSPH1/B3GALTL (P =.005) were also associated with this outcome.
The multivariate analyses for the endpoint of VA loss, adjusting for all of the variables (demographic, behavioral, and ocular factors) are presented in Table 18. Similar to the results of the univariate analyses, participants who had a higher risk of VA loss over time were older, Caucasian, had a higher BMI, a lower level of education, and a history of cigarette smoking. Multivariate associations between genetic factors and VA loss are reported in Table 19, including a multivariate model with all 31 genetic variants. These models were adjusted for age, sex, race, level of education, and baseline grade (model I), and the fully adjusted model also included BMI, smoking and all of the genetic loci (model II). The multivariate stepwise analysis for VA loss ≥ 15 letters led to the identification of six genetic variants associated with VA loss (shown in Table 5), which are the same genetic variants that were significantly related to VA loss in multivariate model II in Table 19. Four of the six were in the complement pathway, and were associated with a higher risk of visual loss: CFH rs1410996 (HR: 1.30; 95% CI:1.19 to 1.43; P < .0001), CFH R1210C (HR: 3.01; 95% CI: 1.67 to 5.41; P = .0002), C3 R102G (HR: 1.23; 95% CI: 1.12 to 1.35; P < .0001), and C3 K155Q (HR: 1.43; 95% CI: 1.05 to 1.94; P = .02). The ARMS2 variant increased risk of visual loss (HR: 1.33; 95% CI 1.22 to 1.45; P < .0001), and RAD51B was significantly associated with a protective effect against VA loss (HR: 0.86; 95% CI: 0.80 to 0.94; P = .001). All of the genes associated with VA loss were also significantly associated with progression to overall advanced AMD, although not all genes associated with AMD progression retained significance in the stepwise model for VA. The AUC for this composite predictive model for VA loss was .72.
TABLE 18.
Multivariate Associations Between Demographic, Behavioral, and Ocular Factors and Visual Acuity Loss in the Derivation Cohort
| HR (95% CI)a | P value | |
|---|---|---|
| Demographic | ||
| Age (years) | ||
| ≥ 75 | Referent | |
| 65 to 74.9 | 0.72 (0.63-0.82) | <.0001 |
| 55 to 64.9 | 0.41 (0.34-0.50) | <.0001 |
| Sex | ||
| Female | Referent | |
| Male | 0.90 (0.81-1.01) | 0.08 |
| Race | ||
| Non-Caucasian | Referent | |
| Caucasian | 1.57 (1.11-2.21) | 0.01 |
| Behavioral | ||
| Education | ||
| ≤ High school | Referent | |
| > High school | 0.86 (0.77-0.96) | 0.009 |
| Body mass index | ||
| < 25 | Referent | |
| 25 to 29.9 | 1.15 (1.01-1.31) | 0.04 |
| ≤ 30 | 1.35 (1.17-1.56) | <.0001 |
| Smoking | ||
| Never | Referent | |
| Past | 1.20 (1.06-1.34) | 0.003 |
| Current | 1.82 (1.48-1.56) | <.0001 |
| Ocular | ||
| Baseline AMD grade | ||
| 1 | Referent | |
| 2 | 1.52 (1.28-1.81) | <.0001 |
| 3 | 4.11 (3.56-4.74) | <.0001 |
| 4 | 10.25 (8.04-13.07) | <.0001 |
| 5 | 7.26 (5.70-9.25) | <.0001 |
AMD = age-related macular degeneration; CI = confidence interval; HR = hazard ratio; VA = visual acuity.
HRs and 95% CIs for 12 year progression were estimated using Cox proportional hazards models using the individual eye as the unit of analysis, adjusted for all variables listed in the table.
TABLE 19.
Associations Between Individual Genetic Loci and Visual Acuity Loss in the Derivation Cohort.
| Multivariate Model Ia | Multivariate Model IIb | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Genetic loci | ||||
| Complement pathway | ||||
| CFH Y402H: rs1061170 | 1.16 (1.01-1.25) | <.0001 | 0.97 (0.87-1.08) | 0.56 |
| CFH: rs1410996 | 1.29 (1.18-1.40) | <.0001 | 1.32 (1.17-1.49) | <.0001 |
| CFH R1210C: rs121913059 | 1.90 (1.12-3.21) | 0.02 | 2.93 (1.63-5.28) | 0.0003 |
| C2 E318D: rs9332739 | 0.84 (0.65-1.08) | 0.16 | 0.86 (0.66-1.11) | 0.25 |
| CFB R32Q: rs641153 | 0.87 (0.73-1.03) | 0.09 | 0.88 (0.73-1.06) | 0.18 |
| CFI: rs10033900 | 1.00 (0.93-1.08) | 0.99 | 1.00 (0.93-1.08) | 0.98 |
| C3 R102G: rs2230199 | 1.22 (1.12-1.33) | <.0001 | 1.22 (1.11-1.34) | <.0001 |
| C3 K155Q: rs147859257 | 1.28 (0.93-1.74) | 0.13 | 1.43 (1.04-1.98) | 0.03 |
| C9 P167S: rs34882957 | 0.92 (0.67-1.25) | 0.59 | 0.78 (0.55-1.11) | 0.17 |
| CFH N1050Y: rs35274867 | 0.64 (0.41-1.00) | 0.05 | 0.93 (0.60-1.44) | 0.74 |
| Angiogenesis pathway | ||||
| VEGFA: rs943080 | 1.07 (0.99-1.15) | 0.1 | 1.07 (0.98-1.16) | 0.13 |
| TGFBR1: rs334353 | 0.97 (0.88-1.06) | 0.47 | 0.94 (0.85-1.03) | 0.17 |
| Lipid pathway | ||||
| LIPC: rs10468017 | 0.98 (0.89-1.07) | 0.6 | 0.99 (0.91-1.09) | 0.9 |
| ABCA1: rs1883025 | 1.03 (0.94-1.23) | 0.52 | 1.01 (0.92-1.11) | 0.85 |
| CETP: rs3764261 | 1.05 (0.97-1.13) | 0.24 | 1.02 (0.94-1.11) | 0.66 |
| APOC1/APOE: rs4420638 | 1.01 (0.90-1.14) | 0.88 | 1.02 (0.90-1.15) | 0.81 |
| APOH: rs1801689 | 1.03 (0.83-1.28) | 0.77 | 1.15 (0.91-1.45) | 0.24 |
| Immune/inflammatory pathway | ||||
| ARMS2/HTRA1: rs10490924 | 1.37 (1.27-1.48) | <.0001 | 1.33 (1.23-1.45) | <.0001 |
| PELI3: rs145732233 | 0.97 (0.51-1.86) | 0.94 | 0.80 (0.38-1.70) | 0.56 |
| TNFRSF10A: rs13278062 | 1.03 (0.96-1.12) | 0.41 | 0.98 (0.90-1.06) | 0.57 |
| SLC16A8: rs8135665 | 1.01 (0.92-1.11) | 0.84 | 1.03 (0.93-1.13) | 0.6 |
| PILRB/PILRA: rs11769700 | 0.99 (0.90-1.09) | 0.79 | 1.00 (0.91-1.11) | 0.95 |
| TMEM97/VTN: rs704 | 1.02 (0.95-1.11) | 0.54 | 1.01 (0.93-1.10) | 0.78 |
| Extracellular matrix | ||||
| COL8A1: rs13095226 | 1.03 (0.91-1.16) | 0.66 | 1.04 (0.91-1.18) | 0.61 |
| COL4A3: rs11884770 | 0.96 (0.88-1.05) | 0.33 | 0.95 (0.87-1.05) | 0.3 |
| CTRB1: rs8056814 | 0.93 (0.81-1.08) | 0.36 | 0.92 (0.79-1.07) | 0.28 |
| ADAMTS9: rs6795735 | 1.01 (0.94-1.09) | 0.78 | 1.02 (0.94-1.11) | 0.63 |
| TIMP3: rs9621532 | 0.83 (0.68-1.00) | 0.05 | 0.89 (0.72-1.10) | 0.26 |
| DNA repair/protein binding | ||||
| RAD51B: rs8017304 | 0.89 (0.82-0.97) | 0.006 | 0.86 (0.79-0.94) | 0.0009 |
| NPLOC4/TSPAN10: rs9895741 | 0.99 (0.92-1.07) | 0.82 | 0.99 (0.91-1.07) | 0.74 |
| HSPH1/B3GALTL: rs9542236 | 1.07 (1.00-1.16) | 0.06 | 1.06 (0.98-1.15) | 0.13 |
AMD = age-related macular degeneration; CI = confidence interval; HR = hazard ratio.
Multivariate Model I: HRs for 12 year progression reflect risk per allele, and are adjusted for age, sex, race, education, and baseline AMD grade.
Multivariate Model II: HRs reflect risk per allele, and are adjusted for age, sex, race, education, baseline AMD grade, BMI, smoking status, and all other genetic loci in the Table.
DISCUSSION
We have developed several models, including the earliest model in 2006 with the three known genetics variants at the time (CFH, ARMS2, and C2), followed by inclusion of additional newly reported genes as well as demographic, behavioral, and ocular factors.14,34–40 This current work differs from and expands upon previous work in several ways: by adding recently discovered genetic variants, many of which had not been previously evaluated for their association with AMD progression, a completely independent validation cohort with similar baseline grades which has the same variables in the model as the derivation cohort allowing for valid comparisons of the risk models, new methods for assessing the impact of the polygenic model, and evaluation of VA loss as a functional outcome in risk prediction analyses.
The methodological approach of eye-specific analyses using both eyes, as we applied in analyses of separate topics previously, enhances the person-based analyses of the worst eye. The analysis of individual eyes accounts for eye-specific covariates, namely, level of macular disease severity, and additionally differentiates between participants who progress in a single eye compared with those who progress in both eyes.
We derived composite risk prediction models for progression to advanced AMD, as well as GA and NV separately, in the derivation cohort and achieved a high level of discrimination in a large, external validation cohort. We also demonstrated the added value of genes when comparing models with and without genes and this result was observed in both cohorts. Results add to the growing literature and evidence that individuals vary considerably in their risk of progression and visual loss over time depending on a combination of demographic, behavioral, ocular and genetic variables. These models can be used for future clinical care and management, selection of higher risk individuals for screening, identification of subjects at high risk of advanced disease and visual loss at an earlier stage for inclusion in randomized clinical trials testing new treatments, and for understanding the pathophysiology of the disease.
These prospective analyses also add new information regarding risk and protective genetic variants associated with progression of AMD. We identified 13 common or low frequency genetic variants independently associated with risk of progression to overall advanced AMD: 7 in the complement pathway, 2 in the immune/inflammatory pathway, 2 in the extracellular matrix pathway, and 2 in the DNA repair/protein binding pathway. A set of four loci in three genes were consistently predictive of each AMD outcome: the common, noncoding CFH variant (rs1410996), the rare variant CFH R1210C, and common variants in C3 R102G, and ARMS2. When assessing progression to GA and NV separately, there were some differences in the models with significant heterogeneity for genetic and non-genetic factors. Variants in TGFBR1 and COL4A3 were significantly associated with a higher risk of progression to the NV subtype, whereas the CFI variant was associated with higher risk of progression to GA. We previously reported that the genetic variant in ARMS2 on chromosome 10 conferred a larger risk for development of NV relative to GA, although it increases risk for both subtypes.
In addition to the obvious phenotypic differences between GA and NV, and a few possible genetic differences, there have been other differences noted between GA and NV. Response to supplementation with antioxidants differs: participants with NV have a more beneficial response to antioxidant supplementation than do those with GA. There are differences regarding various dietary modifications and gene-diet associations. 46–49,63–65 Distinct clinical features preceding each subtype have also been identified with OCT imaging50 and histopathologic observations in postmortem ocular tissue.66
Novel analyses were conducted to evaluate the impact of the risk model on predicting functional loss of vision. Many, but not all, of the genes related to progression to advanced disease were also related to this outcome. Common and rare variants in CFH and C3 were associated with a higher risk of VA loss, as was ARMS2, while the RAD51B variant conferred a protective effect. These six loci were consistently observed to be associated with AMD risk as well as visual loss, including progression to overall advanced AMD, GA, and NV. Differences existed for only two loci: the rare C3 K155Q was not associated with NV, and the protective RAD51B was not associated with GA. Demographic features, lifestyle factors including smoking and BMI, and baseline ocular status were also significantly associated with visual loss. The composite model achieved relatively high discrimination between those who lost vision over time and those who did not.
We demonstrated that a combination of several common and a few rare variants plus the other variables in the model can identify a very high risk population with a magnitude of risk comparable to the risk conferred by some single rare genetic variants. Seven of the 13 loci were in the complement pathway-5 common variants and 2 rare variants. The highest quintile of the risk score conferred almost a 6-fold higher risk of progression from intermediate to advanced disease. Although such a composite or polygenic model can identify high risk populations, it may not identify functional, actionable and “druggable” targets like the individual rare genetic variants. On the other hand, such a score can be useful to reduce the size and duration, and therefore cost, of clinical trials,36 and for incorporation into management or decision-making regarding timing of follow-up visits and preventive measures. These same methods outlined here and in our earlier models 39 could also be modified to include only genetic loci in a particular pathway, i.e. complement, lipid pathway, etc.
AUCs achieved in these analyses indicate a high discriminatory ability of the composite risk model to differentiate progressors from non-progressors or between those who lose vision and those who do not. The AUCs for our prediction models began at 0.70, and have since achieved excellent separation of groups as a result of incorporating additional common and rare variants, indicated by an AUC approaching 0.90.39,40 Our highest observed AUC was 0.94,67 and was calculated based on a model that incorporated plasma complement biomarkers as a predictive measure of AMD progression, in addition to demographic, behavioral, ocular, and genetic variables. To further enhance discrimination between groups, additional biomarkers could be added, such as optical coherence tomography parameters,50 or other functional data like low luminance vision.68 It is clear that AUCs for AMD are quite high relative to risk models for other common disorders. For example, AUCs for the Framingham Heart Study, a large longitudinal cohort evaluating risk factors for cardiovascular disease, reach only 0.65.69 In breast cancer, AUCs reach approximately 0.60, and are often lower.60,70
The relative contribution of genes and genetic susceptibility to AMD risk has been a subject of discussion. Macular phenotypes have been considered the primary predictors of future disease. However, drusen in the early and intermediate stages of AMD, as well as the presence of advanced disease in the fellow eye, are in the causal pathway for progression, and these features are also associated with the AMD genes. The inclusion of ocular covariates in our past and present prediction models attenuates the association between genetic factors and AMD risk, and underestimates the true effect of the genetic component. A model which included fellow eye was evaluated and most of the genes in the model were similar (Supplementary Table 2). It is clear that genes contribute to the risk burden associated with AMD advanced sub-types and progression, an observation that is further confirmed in validation analyses.
We previously reported a validation analysis for a risk prediction model containing seven loci,38 and conducted a split-sample validation in our 10 gene model.40 In both models, the AUCs for the total and validation samples were similar. The study reported herein differs in that the validation cohort is a large, independent and well-characterized prospective cohort with data on the same demographic, lifestyle, ocular, and genetic risk factors as the derivation cohort. AUC’s for the derivation and validation cohorts were similar. This report also includes evaluation of one of the largest subsets of genetic loci at the time of this submission, for their independent associations with progression to both advanced AMD and VA loss.
Determination of the sensitivity and specificity of our risk model in the validation cohort revealed that the model could highly discriminate between progressors and non-progressors. In addition to the new analyses, strengths of our study include the extensive follow-up time in both cohorts and a large number of eyes that progressed to each outcome during the follow-up interval in both the derivation and the validation cohorts.
CONCLUSION
New genetic variants associated with AMD risk in case-control studies were evaluated prospectively for their independent effects on progression from non-advanced to advanced AMD as well as subsequent visual loss. A subset of genes along with demographic, behavioral and ocular factors were determined to be most predictive of conversion to advanced AMD. A model was assessed for the functional outcome of visual loss of 15 or more letters, and predictors were some of the same covariates, including modifiable and genetic factors. When assessing GA and NV separately, there were some differences in the models with significant heterogeneity for genetic and non-genetic factors. The most important pathways associated with AMD progression and transition to advanced stages of GA and NV with visual loss involve the complement, inflammatory and immune systems, lipid and collagen matrix pathways, and DNA repair mechanisms.
Our composite risk models were highly predictive of changes over time to visually disabling forms of the disease, as evidenced by high AUC statistics and significant NRI statistics. The model also calibrated well in the external cohort. These assessments of NRI and calibration were conducted in a completely independent cohort with similar baseline grades and data on the same covariates that were used in deriving the model. Inclusion of genetic data enhanced the risk classification of individual eyes. This comprehensive external validation of the AMD risk model strengthens and underscores the generalizability and use of such a model in primarily Caucasian populations to identify patients at high risk of progression. Such individuals can have more intensive monitoring which will lead to earlier detection of the transition to late stages of AMD. New imaging modalities may contribute to this composite model to detect high risk subgroups.50 Anticipating that interventions will become available for high risk individuals with intermediate AMD who have not yet progressed, it is prudent to identify who they are so clinical trials can focus on this population. The precision medicine approach could facilitate the discovery and delivery of new treatments prior to visual loss.
Supplementary Material
Boxplot depicting risk score percentile for progressors to advanced AMD over 5 years and non-progressors among eyes with intermediate disease at baseline. The percentiles were calculated from the sample of non-progressing eyes. The horizontal line represents the median and the + sign represents the arithmetic mean. The top and bottom of the box depicts the upper and lower 25th percentile.
ACKNOWLEDGMENTS
Funding/Support: The research reported herein was supported by the following: National Eye Institute/National Institutes of Health R01-EY011309 (JMS) and R01-EY022445 (BR), Massachusetts Lions Research Fund, American Macular Degeneration Foundation.
Footnotes
Financial Disclosures: JMS: Gemini Therapeutics, Inc. employee; Laboratoire THEA, scientific advisory board; BR: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. The Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 2.Sobrin L, Seddon JM. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seddon JM. Macular Degeneration Epidemiology: Nature-Nurture, Lifestyle Factors, Genetic Risk, and Gene-Environment Interactions – The Weisenfeld Award Lecture. Invest Ophthalmol Vis Sci. 2017;58(14):6513–6528. doi: 10.1167/iovs.17-23544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol Chic Ill 1960. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564 [DOI] [PubMed] [Google Scholar]
- 5.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 6.Alexander MF, Maguire MG, Lietman TM, Snyder JR, Elman MJ, Fine SL. Assessment of Visual Function in Patients With Age-Related Macular Degeneration and Low Visual Acuity. Arch Ophthalmol. 1988;106(11):1543–1547. doi: 10.1001/archopht.1988.01060140711040 [DOI] [PubMed] [Google Scholar]
- 7.Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999;128(1):45–53. doi: 10.1016/S0002-9394(99)00169-5 [DOI] [PubMed] [Google Scholar]
- 8.Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The Psychosocial Impact of Macular Degeneration. Arch Ophthalmol. 1998;116(4):514–520. doi: 10.1001/archopht.116.4.514 [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 10.Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol Chic Ill 1960. 2005;123(3):321–327. doi: 10.1001/archopht.123.3.321 [DOI] [PubMed] [Google Scholar]
- 11.Klein RJ, Zeiss C, Chew EY, et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009;17(1):100–104. doi: 10.1038/ejhg.2008.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38(9):1055–1059. doi: 10.1038/ng1873 [DOI] [PubMed] [Google Scholar]
- 15.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39(10):1200–1201. doi: 10.1038/ng2131 [DOI] [PubMed] [Google Scholar]
- 16.Yates JRW, Sepp T, Matharu BK, et al. Complement C3 Variant and the Risk of Age-Related Macular Degeneration. N Engl J Med. 2007;357(6):553–561. doi: 10.1056/NEJMoa072618 [DOI] [PubMed] [Google Scholar]
- 17.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon J, Atkinson JP. Genetic Variants in the Complement System Predisposing to Age-Related Macular Degeneration: A Review. Mol Immunol. 2014;61(2):118–125. doi: 10.1016/j.molimm.2014.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291(6):704–710. doi: 10.1001/jama.291.6.704 [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Wagner EK, Souied EH, et al. Protective coding variants in CFH and PELI3 and a variant near CTRB1 are associated with age-related macular degeneration†. Hum Mol Genet. 2016;25(23):5276–5285. doi: 10.1093/hmg/ddw336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakawa S, Takahashi A, Ashikawa K, et al. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nat Genet. 2011;43(10):1001–1004. doi: 10.1038/ng.938 [DOI] [PubMed] [Google Scholar]
- 21.Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A. 2010;107(16):7395–7400. doi: 10.1073/pnas.0912019107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–439, 439e1-2. doi: 10.1038/ng.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77(3):389–407. doi: 10.1086/444437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107(16):7401–7406. doi: 10.1073/pnas.0912702107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Bhangale TR, Fagerness J, et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet. 2011;20(18):3699–3709. doi: 10.1093/hmg/ddr270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raychaudhuri S, Iartchouk O, Chin K, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011;43(12):1232–1236. doi: 10.1038/ng.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara D, Seddon JM. Phenotypic Characterization of Complement Factor H R1210C Rare Genetic Variant in Age-Related Macular Degeneration. JAMA Ophthalmol. 2015;133(7):785–791. doi: 10.1001/jamaophthalmol.2015.0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobrin L, Maller JB, Neale BM, et al. Genetic profile for five common variants associated with age-related macular degeneration in densely affected families: a novel analytic approach. Eur J Hum Genet EJHG. 2010;18(4):496–501. doi: 10.1038/ejhg.2009.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Triebwasser MP, Wong EKS, et al. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014;23(19):5283–5293. doi: 10.1093/hmg/ddu226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triebwasser MP, Roberson EDO, Yu Y, et al. Rare Variants in the Functional Domains of Complement Factor H Are Associated With Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2015;56(11):6873–6878. doi: 10.1167/iovs.15-17432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner EK, Raychaudhuri S, Villalonga MB, et al. Mapping rare, deleterious mutations in Factor H: Association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci Rep. 2016;6:31531. doi: 10.1038/srep31531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seddon JM, Yu Y, Miller EC, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45(11):1366–1370. doi: 10.1038/ng.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavanagh D, Yu Y, Schramm EC, et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015;24(13):3861–3870. doi: 10.1093/hmg/ddv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297(16):1793–1800. doi: 10.1001/jama.297.16.1793 [DOI] [PubMed] [Google Scholar]
- 35.Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction Model for Prevalence and Incidence of Advanced Age-Related Macular Degeneration Based on Genetic, Demographic, and Environmental Variables. Invest Ophthalmol Vis Sci. 2009;50(5):2044–2053. doi: 10.1167/iovs.08-3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon JM, Reynolds R, Yu Y, Daly MJ, Rosner B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011;118(11):2203–2211. doi: 10.1016/j.ophtha.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012;53(3):1548–1556. doi: 10.1167/iovs.11-8657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddon JM, Reynolds R, Yu Y, Rosner B. Validation of a prediction algorithm for progression to advanced macular degeneration subtypes. JAMA Ophthalmol. 2013;131(4):448–455. doi: 10.1001/jamaophthalmol.2013.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seddon JM, Reynolds R, Yu Y, Rosner B. Three new genetic loci (R1210C in CFH, variants in COL8A1 and RAD51B) are independently related to progression to advanced macular degeneration. PloS One. 2014;9(1):e87047. doi: 10.1371/journal.pone.0087047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seddon JM, Silver RE, Kwong M, Rosner B. Risk Prediction for Progression of Macular Degeneration: 10 Common and Rare Genetic Variants, Demographic, Environmental, and Macular Covariates. Invest Ophthalmol Vis Sci. 2015;56(4):2192–2202. doi: 10.1167/iovs.14-15841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobrin L, Reynolds R, Yu Y, et al. ARMS2/HTRA1 locus can confer differential susceptibility to the advanced subtypes of age-related macular degeneration. Am J Ophthalmol. 2011;151(2):345–352.e3. doi: 10.1016/j.ajo.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobrin L, Ripke S, Yu Y, et al. Heritability and genome-wide association study to assess genetic differences between advanced age-related macular degeneration subtypes. Ophthalmology. 2012;119(9):1874–1885. doi: 10.1016/j.ophtha.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritsche LG, Igl W, Bailey JNC, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134–143. doi: 10.1038/ng.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steyerberg EW, Uno H, Ioannidis JPA, van Calster B, Collaborators. Poor performance of clinical prediction models: the harm of commonly applied methods. J Clin Epidemiol. 2018;98:133–143. doi: 10.1016/j.jclinepi.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 45.AMD-Macular Degeneration Calculator. http://www.seddonamdriskscore.org/. Accessed July 6, 2018.
- 46.Merle BMJ, Silver RE, Rosner B, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015;102(5):1196–1206. doi: 10.3945/ajcn.115.111047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seddon JM, Silver RE, Rosner B. Response to AREDS supplements according to genetic factors: survival analysis approach using the eye as the unit of analysis. Br J Ophthalmol. 2016;100(12):1731–1737. doi: 10.1136/bjophthalmol-2016-308624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merle BMJ, Silver RE, Rosner B, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr. 2016;103(4):1135–1144. doi: 10.3945/ajcn.115.117606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merle BMJ, Silver RE, Rosner B, Seddon JM. Associations Between Vitamin D Intake and Progression to Incident Advanced Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2017;58(11):4569–4578. doi: 10.1167/iovs.17-21673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrara D, Silver RE, Louzada RN, Novais EA, Collins GK, Seddon JM. Optical Coherence Tomography Features Preceding the Onset of Advanced Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2017;58(9):3519–3529. doi: 10.1167/iovs.17-21696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol Chic Ill 1960. 2001;119(10):1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology. 1991;98(5):786–806. doi: 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- 53.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113(2):260–266. doi: 10.1016/j.ophtha.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 54.Davis MD, Gangnon RE, Lee L-Y, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol Chic Ill 1960. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic Epidemiol. 2012;19(3):159–165. doi: 10.3109/09286586.2012.674614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 58.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 59.Rosner B, Glynn RJ. Power and sample size estimation for the Wilcoxon rank sum test with application to comparisons of C statistics from alternative prediction models. Biometrics. 2009;65(1):188–197. doi: 10.1111/j.1541-0420.2008.01062.x [DOI] [PubMed] [Google Scholar]
- 60.Rosner BA, Colditz GA, Hankinson SE, Sullivan-Halley J, Lacey JV, Bernstein L. Validation of Rosner-Colditz breast cancer incidence model using an independent data set, the California Teachers Study. Breast Cancer Res Treat. 2013;142(1):187–202. doi: 10.1007/s10549-013-2719-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172; discussion 207-212. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 62.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awh CC, Lane A-M, Hawken S, Zanke B, Kim IK. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317–2323. doi: 10.1016/j.ophtha.2013.07.039 [DOI] [PubMed] [Google Scholar]
- 64.Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013;120(5):1020–1028. doi: 10.1016/j.ophtha.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awh CC, Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology. 2015;122(1):162–169. doi: 10.1016/j.ophtha.2014.07.049 [DOI] [PubMed] [Google Scholar]
- 66.Seddon JM, McLeod DS, Bhutto IA, et al. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016;134(11):1272–1280. doi: 10.1001/jamaophthalmol.2016.3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50(12):5818–5827. doi: 10.1167/iovs.09-3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Z, Guymer RH, Finger RP. Low luminance deficit and night vision symptoms in intermediate age-related macular degeneration. Br J Ophthalmol. 2016;100(3):395–398. doi: 10.1136/bjophthalmol-2015-306621 [DOI] [PubMed] [Google Scholar]
- 69.D’Agostino RB, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. [DOI] [PubMed] [Google Scholar]
- 70.Pfeiffer RM, Park Y, Kreimer AR, et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med. 2013;10(7):e1001492. doi: 10.1371/journal.pmed.1001492 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Boxplot depicting risk score percentile for progressors to advanced AMD over 5 years and non-progressors among eyes with intermediate disease at baseline. The percentiles were calculated from the sample of non-progressing eyes. The horizontal line represents the median and the + sign represents the arithmetic mean. The top and bottom of the box depicts the upper and lower 25th percentile.