Abstract
Objective
Histopathological changes in oral (buccal or lingual) mucosa after exposure to urine are still not completely understood. We evaluated these changes in free oral mucosal graft integrated in human urethra.
Material and methods
Total 19 patients with recurrent urethral stricture after oral mucosa urethroplasty (buccal 12 and lingual 7) were prospectively evaluated. Intraoperatively integrated buccal or lingual mucosal graft sample that was previously engrafted to urethra was completely excised along with healthy oral mucosa, and it was sample processed for histopathological evaluation by dedicated pathologist. Preoperative clinical data were properly collected from all the study participants.
Results
The mean age of the patients was 30 years, and the mean preoperative peak flow rate was 4.2 mL/s. Etiology of initial stricture was idiopathic in 13 (68.42%) patients and traumatic urethral catheterization in 6 (31.58%) patients. Mean interval from previous buccal mucosal urethroplasty to current urethroplasty was 21.9 months (range 12–46 months). On repeat urethroplasty, the mean stricture segment length was 59.2 (38–77) mm [60.08 (38–74.6) mm buccal, and 58.32 (39.6–77) mm lingual]. These integrated oral mucosal grafts maintained their histopathological characteristics in all patients except some kind of changes like submucosal fibrosis in seven (58.33%) cases of buccal and vacuolar degeneration in five (71.42%) cases of lingual mucosal urethroplasty.
Conclusion
Histopathological characteristics of integrated oral (buccal and lingual) mucosal grafts were maintained even on exposure to urine except some changes like submucosal fibrosis and vacuolar degeneration. Impact of these changes require further research.
Keywords: Buccal mucosa, lingual mucosa, urethral stricture
Introduction
Urethral stricture is one of the commonest urological diseases. To manage urethral strictures, many treatment modalities have been tried with variable degrees of success.[1] Substitution urethroplasty is a well-known and accepted procedure for treatment of long penobulbar urethral strictures (>2 cm).[2] Various tissues, like penile, prepucial as well as posterior auricular skin, small intestine submucosa and oral mucosa (buccal, lingual), have been used for substitution urethroplasty.[2–7] However, outcomes of all these materials vary; so there is no ideal material for urethral reconstruction.[7–9] In 1941, Humby first used buccal mucosa graft for urethral reconstruction. He used this in a case of penoscrotal fistula that had undergone multiple hypospadias repair.[10] However, after its initial description, it was not very popular until 1992 when Burger et al.[11] reintroduced this into clinical practice. Simonato et al.[12] first reported that lingual mucosa can be used as an alternative for substitution urethroplasty. Further studies have showed almost equal outcomes between these two kinds (buccal and lingual) of substitution urethroplasty.[8,12–14] Although nowadays these oral mucosal grafts are used in routine clinical practice, the exact mechanism that is responsible for incorporation of these grafts is still not completely understood. It is still unknown whether this mucosa acts as a scaffold for growth of urethral mucosa.[15–17] Reaction of oral mucosa after long-term exposure to urine is a matter of interest. This study aims to evaluate histopathological changes in free buccal or lingual mucosal graft integrated in human urethra and associated changes in these epitheliums.
Material and methods
After taking institutional review board approval (IRB approval number: 2432/MC/EC/2016), this descriptive observational study was conducted in our department of urology between November 2014 and December 2016. After taking informed and written consent, detailed history with physical examination was done in all the patients. We prospectively evaluated 19 patients with recurrent urethral stricture (long anterior urethral stricture) after buccal (12 patients) or lingual (7 patients) mucosal urethroplasty. Diagnosis of these strictures was made by uroflowmetry with combined retrograde and micturating cystourethrogram. Selected cases had also undergone urethrocystoscopy. Clinical data of all patients were collected preoperatively. All patients were operated in single stage by dorsolateral onlay graft urethroplasty. Intraoperatively, a sample of integrated buccal or lingual mucosal graft that was previously engrafted to urethra was completely excised, and it was taken for histopathological examination. A small piece of oral mucosa was also harvested from opposite side of oral cavity. In cases where past lingual tissue was taken for augmentation, lingual mucosa was harvested; whereas in other cases, it was taken from inner cheek. All patients were healthy and did not have any co-morbidity like diabetes and hypertension. Patients who had history of chewing betel nut or tobacco were excluded from this study. All samples were immediately transferred to 4% formalin solution, and slides were stained with hematoxylin and eosin stains.[18] After following standard protocol, these samples were evaluated histologically by same hospital-based pathologist under 10× and 40× magnifications. To overcome bias, these specimens were reviewed by another senior pathologist.
Statistical analysis
Statistical analysis was done using IBM Statistical Package for the Social Sciences (IBM SPSS Statistics Corp.; Armonk, NY, USA) version 21. Mean was used as a measure of central tendency, and range was used as a measure of dispersion.
Results
Table 1 shows the preoperative characteristics of all the patients. Mean age of the patients was 30 (16–45) years, whereas it was 32 (range 16–43) years in cases of buccal and 28 years (range 17–45) in cases of lingual mucosal urethroplasty. Mean preoperative peak flow rate was 4.2 mL/s (range 1.9–6.3). The etiology of initial stricture was idiopathic in 13 (68.42%) patients and traumatic urethral catheterization in 6 (31.58%) patients. Lichen sclerosus was not present in any patient. The mean interval from previous buccal mucosal urethroplasty to current urethroplasty was 21.9 months (range 12–46 months). And in cases of initial lingual mucosal urethroplasty, it was 20.8 months (range 16–39 months). Between two surgeries, direct visual internal urethrotomy (DVIU) was done in some patients (mean DVIU done was 1.8, range 0–4). Multiple areas of narrowing were also present in strictured segment. On redo urethroplasty, mean length of stricture segment was found to be 60.08 (38–74.6) mm and 58.32 (39.6–77) mm in cases of buccal and lingual mucosal urethroplasty, respectively, with mean stricture length of 59.2 (38–77) mm.
Table 1.
Preoperative clinical data of patients having recurrent urethral stricture following augmented oral mucosal urethroplasty
| Mean age in years (range) | 30 (16–45) |
|
| |
| Mean mL/s preop uroflow (ml/s)(range): | 4.2 (1.9–6.3) |
| Mean mL post-void residual urine (mL) (range) | 118.6 (0–350) |
|
| |
| Mean urethral stricture length (mm) (range) | 59.2 (38–77) |
| Buccal | 60.08 (38–74.6) |
| Lingual | 58.32 (39.6–77) |
|
| |
| No. of cases (%) | |
| Buccal | 12 |
| Lingual | 7 |
|
| |
| Mean no. of DVIUs (range) | 1.8 (0–4) |
|
| |
| Mean time duration to previous urethroplasty (months) | |
| Buccal | 21.9 (12–46) |
| Lingual | 20.8 (16–39) |
DVIU: direct visual internal urethrotomy
On gross (macroscopic) examination, integrated oral (buccal or lingual) mucosal graft was distinguished from urethra by its appearance of gray white color along with smooth surface of oral mucosal graft.
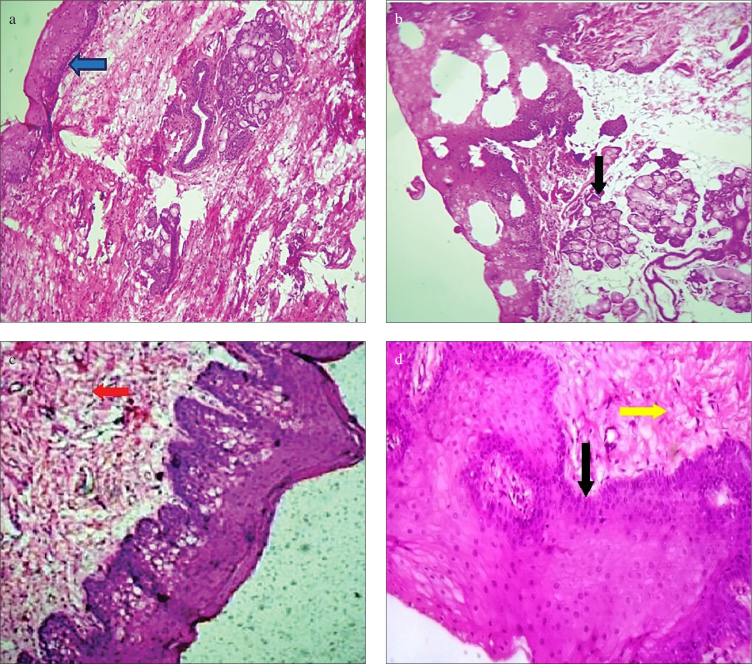
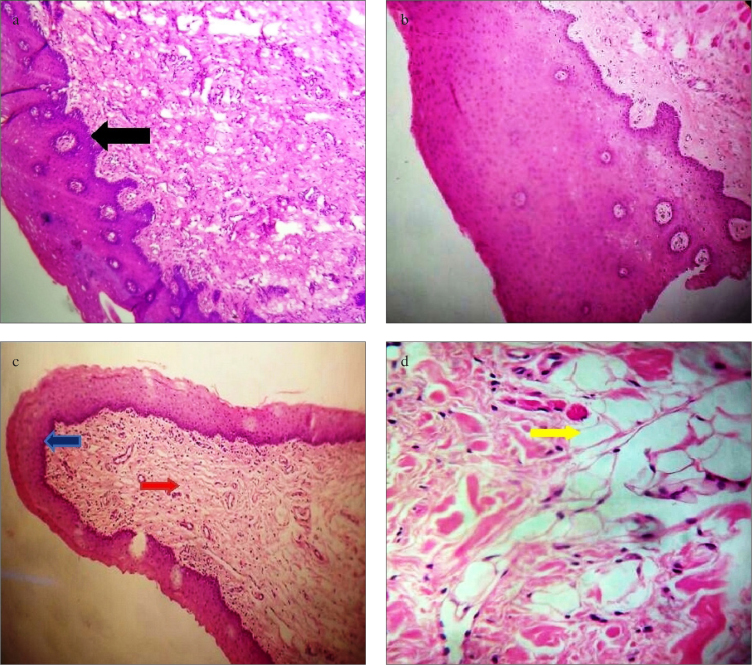
Newly harvested mucosa from inner surface of cheek and ventral surface of tongue showed characteristic stratified squamous epithelium (Figure 1 a, b and 2 a, b). We found that integrated buccal mucosal graft preserved the histopathological characteristics as normal oral mucosa in the form of nonkeratinized stratified squamous epithelium with normal basement membrane (Figure 1c). However, submucosal fibrosis was observed in many of these cases (58.33%) (Figure 1d). Similarly, in cases of integrated lingual mucosal grafts, epithelial characteristics were maintained with maintenance of normal basement membrane (Figure 2c). Vacuolar degenerative changes were observed in five cases (71.42% cases) with integrated lingual grafts (Figure 2d). Epithelial characteristics were maintained, and they were not associated with location of stricture segment and length of graft used. Thus, histopathological characteristics of the integrated oral (both buccal and lingual) mucosa transplants were completely preserved in all patients, and there were no histopathological feature that could suggest that urothelium had been overgrown these grafts. Mild chronic inflammatory reactions in the form of lymphocytic infiltration were observed in all specimens without any evidence of acute inflammatory changes (granulocytic infiltration) or neovascularity.
Figure 1.
a–d. Histopathological characteristics of buccal mucosa. (a): Graft harvested from inner check showing stratified squamous epithelium (blue arrow) (10× magnification). (b): Black arrow showing minor salivary glands in cases of inner check graft under 40× magnification (black arrow). (c): Integrated buccal mucosal graft showing lymphocytic infiltration (red arrow) (10× magnification). (d): Under 40× magnification, these integrated graft showing maintained epithelial characteristics (black arrow) with submucosal fibrosis (yellow arrow)
Figure 2.
a–d. Histopathological characteristics of lingual mucosa. (a, b): Graft harvested from ventral surface of tongue showing stratified squamous epithelium (black arrow) (10× and 40× magnification). (c): Integrated lingual mucosal graft showing lymphocytic infiltration (red arrow) with maintained epithelial characteristics (blue arrow) (10× magnification). (d): Under 40 magnification, these integrated graft showing vacuolar degenerations (yellow arrow)
Discussion
Since many years, urethral reconstruction has been a unique challenge to urologists as there are many options but the type of ideal material remains yet to be resolved.[19] Although lingual mucosa for urethral reconstruction is being used in recent years, buccal mucosal reconstruction is in practice for many years with good results.[11,12] Outcome of these grafts for urethral reconstruction is almost equivalent with excellent long-term results and limited donor side morbidity. These grafts are easily available in most patients, and harvesting procedure is simple.[13,14,19] There is little knowledge about the natural history of these integrated grafts into human urethra, and it is still not clear whether after exposure to urine these grafts are replaced by urothelium and are associated with any changes like fibrosis and degeneration. Risk factors like patient age, length, site, or etiology of stricture can contribute to failure of substitution urethroplasty.
Except single human study, no other human study is available in this regard; however, some work has been done in animal models.[15–17] As seen in our study, these studies also showed complete integration of oral mucosal graft with maintained histopathological characteristics of oral mucosa in the form of stratified squamous epithelium with stratum spinosum layer.[15,17,20,21] As observed by Soave et al.[17], occasional lymphocytic infiltrations in lamina propria were also observed in all of our patients. This was in contrast to previous animal studies that showed acute granulocytic infiltration; however, they took biopsy after a short interval (6-month period), which could be one of the reason for acute inflammatory changes.[21] In contrast to Souza et al.[15], we did not find any neovascularity in these grafts. Soave et al.[17] did not found any fibrotic changes in these grafts except at the edges. However, in majority of our cases, we observed submucosal fibrosis in buccal grafts and vacuolar degeneration in lingual grafts. Histopathological changes of oral mucosa were not related with length of previous mucosal graft used, and it did not have any relation with location of stricture segment.
Even after exposure to urine, these grafts retained their histological characteristics and were not replaced by urothelium either partially or completely. However, some changes like submucosal fibrosis and vacuolar degeneration occurred in these grafts. This may be one of the reasons of failure of the surgery, but this hypothesis still requires more research for acceptance.
To the best of our knowledge, this is the first prospective human study to also evaluate the histopathological characteristics of lingual mucosa in addition to buccal mucosal characteristics. Although there were some limitations of this study, like small sample size, we did not obtain specimen from patients with successful urethroplasty cases that may show some histopathological difference with failed urethroplasty. We did not take oral mucosal biopsy at initial urethroplasty to rule out submucosal fibrosis and vacuolar degeneration that may be present at initial stage. We did not record the length of initial/primary buccal/lingual graft. Urethral stricture is a slow-going process, and the interval between previous and current urethroplasty was only 21 months, so a study with longer follow-up may be needed to confirm these results. In this study, we hypothesized that epithelial characteristic of integrated oral mucosa does not change with exposure to urine except that of some minor changes like submucosal fibrosis and vacuolar degeneration.
In conclusion, histopathological characteristics of integrated oral (buccal and lingual) mucosal grafts remained the same and did not have significant changes on exposure to urine, although some kind of changes like submucosal fibrosis and vacuolar degeneration occurred in these epitheliums. Outcome of these changes are still unproven and require further research.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of SMS Medical College (IRB approval number: 2432/MC/EC/2016).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – R.B., S.S.Y.; Design – R.B., S.S.Y.; Supervision – S.S.Y., V.T.; Resources – R.B., S.S.Y., V.T.; Materials – R.B., S.S.Y.; Data Collection and/or Processing – R.B., S.S.Y., V.T.;Analysis and/or Interpretation – R.B., S.S.Y.; Literature Search – R.B.;Writing Manuscript – R.B., S.S.Y.; Critical Review – S.S.Y., V.T.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Mundy AR. Management of urethral strictures. Postgrad Med J. 2006;82:489–93. doi: 10.1136/pgmj.2005.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YJ, Kim SW. Current Management of Urethral Stricture. Korean J Urol. 2013;54:561–9. doi: 10.4111/kju.2013.54.9.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palminteri E, Berdondini E, Fusco F, De Nunzio C, Salonia A. Long term results of small intestinal submucosa graft in bulbarurethral reconstruction. Urology. 2012;79:695–701. doi: 10.1016/j.urology.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 4.Manoj B, Sanjeev N, Pandurang PN, Jaideep M, Ravi M. Postauricular skin as an alternative to oral mucosa for anterior onlaygraft urethroplasty: a preliminary experience in patients with oralmucosa changes. J Urol. 2009;74:345–8. doi: 10.1016/j.urology.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 5.Wisenbaugh ES, Gelman J. The Use of Flaps and Grafts in theTreatment of Urethral Stricture Disease. Adv Urol. 2015 doi: 10.1155/2015/979868. 979868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eppley BL, Keating M, Rink R. A buccal mucosal harvesting technique for urethral reconstruction. J Urol. 1997;157:1268–70. doi: 10.1097/00005392-199704000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman WB, Santucci RA. Buccal mucosa urethroplasty foradult urethral strictures. Indian J Urol. 2011;27:364–70. doi: 10.4103/0970-1591.85441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan S, Yadav SS, Tomar V. Outcome of buccal mucosa andlingual mucosa graft urethroplasty in the management of urethralstrictures: A comparative study. Urol Ann. 2016;8:36–41. doi: 10.4103/0974-7796.165715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villoldo GM, Loresi M, Giudice C, Damio O, Moldes JM, De Badiola F, et al. Histologic changes after urethroplasty using smallintestinal submucosa unseeded with cells in rabbits with injuredurethra. Urology. 2013;81:1380.e1. doi: 10.1016/j.urology.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Humby G. A one-stage operation for hypospadias repair. Br J Surg. 1941;29:84–92. doi: 10.1002/bjs.18002911312. [DOI] [Google Scholar]
- 11.Bürger RA, Müller SC, elDamanhoury H, Tschakaloff A, Riedmiller H, Hohenfellner R. The buccal mucosal graft for urethralreconstruction: a preliminary report. J Urol. 1992;147:662–4. doi: 10.1016/s0022-5347(17)37340-8. [DOI] [PubMed] [Google Scholar]
- 12.Simonato A, Gregori A, Lissiani A, Galli S, Ottaviani F, Rossi R, et al. The tongue as an alternative donor site for graft urethroplasty: A pilot study. J Urol. 2006;175:589–92. doi: 10.1016/S0022-5347(05)00166-7. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A, Dutta A, Jain DK. Initial experience with lingualmucosal graft urethroplasty for anterior urethral strictures. Med JArmed Forces India. 2013;69:16–20. doi: 10.1016/j.mjafi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumen N, Vierstraete-Verlinde S, Oosterlinck W, Hoebeke P, Palminteri E, Goes C, et al. Buccal Versus Lingual Mucosa Graft inAnterior Urethroplasty: A Prospective Comparison of Surgical Outcome and Donor Site Morbidity. J Urol. 2016;195:112–7. doi: 10.1016/j.juro.2015.07.098. [DOI] [PubMed] [Google Scholar]
- 15.Souza G, Calado AA, Delcelo R, Ortiz V, Macedo A., Jr Histopathological evaluation of urethroplasty with dorsal buccal mucosa: an experimental study in rabbits. Int Braz J Urol. 2008;34:345–54. doi: 10.1590/S1677-55382008000300012. [DOI] [PubMed] [Google Scholar]
- 16.Filipas D, Fisch M, Fichtner J, Fitzpatrick J, Berg K, Storkel S, et al. The histology and immunohistochemistry of free buccal mucosa and full-skin grafts after exposure to urine. BJU Int. 1999;84:108–11. doi: 10.1046/j.1464-410x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 17.Soave A, Steurer S, Dahlem R, Rink M, Reiss P, Fisch M, et al. Histopathologialcal Characteristics of Buccal Mucosa Transplantsin Humans after Engraftment to the Urethra: A Prospective Study. J Urol. 2014;192:1725–9. doi: 10.1016/j.juro.2014.06.089. [DOI] [PubMed] [Google Scholar]
- 18.Chan JK. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol. 2014;22:12–32. doi: 10.1177/1066896913517939. [DOI] [PubMed] [Google Scholar]
- 19.Mungadi IA, Ugboko VI. Oral mucosa grafts for urethral reconstruction. Ann Afr Med. 2009;8:203–9. doi: 10.4103/1596-3519.59572. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Xu Y, Song L, Zhang H. Combined buccal and lingual mucosa grafts for urethroplasty: an experimental study in dogs. J SurgRes. 2011;169:162–7. doi: 10.1016/j.jss.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Oliva P, Delcelo R, Bacelar H, Rondon A, Barroso U, Jr, Ortiz V, et al. The buccal mucosa fenestrated graft for Bracka first stageurethroplasty: experimental study in rabbits. Int Braz J Urol. 2012;38:825–32. doi: 10.1590/1677-553820133806825. [DOI] [PubMed] [Google Scholar]