Abstract
Objective
Studies showed a decrease of the semen analysis parameters and an increase in the average age of first-time fathers over the past several decades. The aim of the present study was to assess the influence of paternal age on semen quality and fertilization outcomes in men with normal sperm DNA fragmentation and chromatin maturation index (DFI and CMI), reactive oxygen species (ROS), and total antioxidant capacity (TAC) levels.
Material and methods
The study was performed on 70 men with their wife’s age ≤38 years and normal sperm DFI, CMI, ROS, and TAC levels. None of the couples had a history of genital inflammation, chronic diseases, endocrine abnormality, chromosomal aberrations, Y chromosome microdeletion, azoospermia, and leukocytospermia. These men were separated into 2 groups according to their age (group A: age <45 years and group B: age ≥45 years). Semen analysis and fertilization outcome after using the intracytoplasmic sperm injection were assessed in both groups.
Result
Sperm concentration showed a significant reduction in group B (p=0.04). Although semen volume, sperm normal morphology, and progressive motility were decreased in group B, the reduction was not significant when compared with group A (p=0.09, p=0.47, and p=0.77, respectively). In addition, the differences of embryo quality with grades A, B, and C and 8-cell embryo formation were not statistically significant between the 2 groups.
Conclusion
These results demonstrated that in men with normal sperm DFI, CMI, ROS, and TAC levels, there were no significant changes in semen parameters and fertilization outcomes with an increasing age.
Keywords: Antioxidant, DNA fragmentation, fertilization, paternal age, reactive oxygen species
Introduction
The infertility problem cannot be clarified in approximately 15% of couples.[1] However, 30% of infertility is due to paternal problems that are identified on the basis of quantitative or qualitative sperm anomalies by semen analysis.[1]Some studies showed a worldwide decreasein the semen analysis parameters[2,3], and othersstated an increase of the average age of first-time fathers over the past several decades.[4]However, while maternal age and reduction ofthe ovarian reserve were recognized to be negative prognostic factors for assisted reproductiontechnologies, the paternal contribution was less well described in the literature.[5] Moreover, the effects of paternal age on sperm characteristics and fertilization outcome counting the quantification of free sperm DNA, reactive oxygen species (ROS), and total antioxidant capacity (TAC) levels have not been studied in humans and are still indistinct. Reports demonstrated that seminal volume, sperm motility, and morphology may be decreased with increasing paternal age.[6–11] In addition, the results of studies adjusting for duration of infertility and association between sperm concentration and increasing paternal age are conflicting.[12,13] Some studies showed the negative effect of paternal age older than 40[14], 50[15], and 60[12] years on pregnancy rates although others stated no effect of paternal age on pregnancy outcomes[13], especially when the wife was younger than 36 years.[16] In addition, a significant decrease in implantation rates was seen with paternal age in oligozoospermic patients that was not observed in normospermia.[17] As usually older men have a tendency to own older partners, it is difficult to control for the effect of maternal age on embryo quality. Therefore, unfortunately, the findings are still questionable. The main objective of the present study was to assess the influence of paternal age on sperm quality and fertilization outcomes by eliminating the effects of sperm DNA integrity/compaction, ROS, TAC, and maternal age.
Material and methods
Sample collection and preparation
The oocytes and semen were obtained from couples who attended the Avicenna infertility clinic affiliated to Avicenna Research Institute, Tehran, Iran. Informed consent was obtained from all subjects. The use of human gametes was approved by the Medical Ethics Committee of Avicenna Research Institute. None of the couples had a history of genital inflammation, chronic diseases (e.g., diabetes and cancer), endocrine abnormality, chromosomal aberrations, Y chromosome microdeletion, azoospermia, and leukocytospermia. Paternal age was ≥24 and ≤60 years old, and maternal age was ≤38 years old. Semen specimen was collected the day of ovum pick up after 3–5 days of sexual abstinence by masturbation into a sterile container, in a room specially provided for this purpose and located adjacent to the laboratory. After liquefaction (30 min at 37°C), sperm DNA fragmentation index (DFI) and chromatin maturation index (CMI), semen TAC and ROS levels, and semen analysis were performed according to the World Health Organization guideline.[18] Samples with abnormal DFI, TAC, or ROS level were excluded from the study. Therefore, other variables that had a direct effect on ROS, such as smoking and medical or surgical history (varicocele),[19] were not included in this paper. Parameters were considered normal when sperm concentration was ≥15 million/mL, total sperm motility (progressive+nonprogressive) ≥40%, vitality ≥58%, and normal sperm morphology ≥4% by the World Health Organization criteria.[18] A total of 70 participants who fulfilled the inclusion criteria were classified according to male age into 2 groups: 35 patients with age <45 years and 35 patients with age ≥45 years. These two groups were determined based on the previous studies that separately showed the negative effect of paternal age on seminal parameters and pregnancy rates.[6–15]
Sperm DFI
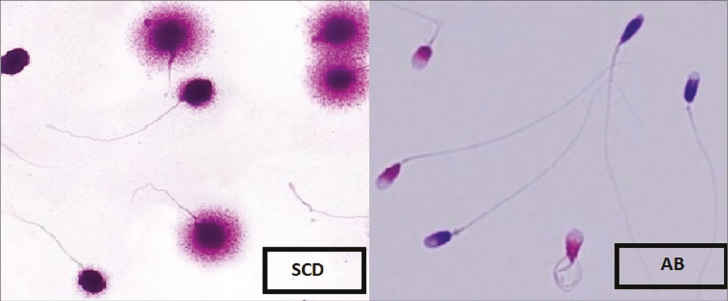
Sperm DNA fragmentation was assessed by the sperm chromatin dispersion test using a Sperm DNA Fragmentation Assay kit (Dain Bioassay Co., Iran).[20] The Halosperm assay was based on the sperm characteristics to produce a halo following acid denaturation and removal of nuclear proteins.[21] For the assay of DNA fragmentation, 50 μL washed sperm with a concentration of 1 × 106 sperm/mL was mixed with 50 μL agarose (6.5%). Then, 20 μL of the mixture was loaded onto a pretreated glass slide and placed on a cold surface (4°C) for 5 min. The slides were treated with denaturing solution for 7 min and lysing solution for 15 min. Then, the slides were washed with distilled water for 5 min, dehydration was performed using increasing concentrations of ethanol (70%, 90%, and 100%), and finally, the air-dried slide was stained. For assessment, a minimum of 200 sperms was assessed with a high-resolution 400× bright-field objective. Sperms with a large halo were classified as normal, and those with no or a small halo were classified as DNA-fragmented sperm. As shown in Figure 2, the DFI results were presented as the percentage of total sperm count.[21]
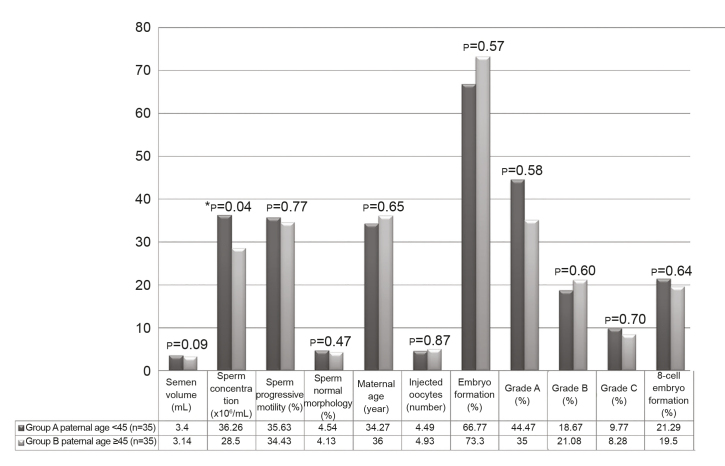
Figure 2.
Statistical comparisons of the semen parameters and ICSI outcome between the two groups. Data are expressed as mean±SD. p≤0.05, significant; p≤0.001, highly significant
SD: standard deviation; DFI: DNA fragmentation index; CMI: chromatin maturity index. The independent t-test was used to compare the parametric variables in both groups; ICSI: Intracytoplasmic sperm injection
Sperm CMI
Aniline blue test evaluates the degree of sperm chromatin compaction or maturation, and it is able to detect sperm chromatin defects related to their nucleoprotein content.[22] At first, 1 × 106 sperm/mL of each sample was centrifuged at 300 g for 5 min and processed with a fixed glutaraldehyde for 5 min at 4°C, and the thin smears were prepared. Each slide was stained with aniline blue at room temperature of 25°C. At least 200 sperms were evaluated in a different field of each slide using 1000 × magnification of a microscope. The pink and the blue sperms were classified as mature and immature, respectively. The percentage of total sperm count was reported as CMI (%) (Figure 1).
Figure 1.
Representative image of sperm chromatin dispersion and aniline blue staining test
Semen ROS level
Semen ROS levels were measured in a fresh liquefied semen using luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) oxidization at neutral pH, which was measured by chemiluminescence assay using Cytation™ 3 (BioTek, USA). For this assay, 1.2 μL of 5 mM luminol (dissolved in dimethyl sulfoxide, Sigma) was added to 40 μL of neat semen sample, and ROS levels were reported as RLU/s at 1-minute intervals after the addition of luminol, over a total period of 15 min in triplicate and then averaged for each sample. Phosphate-buffered saline solution as blank, phosphate-buffered saline solution + luminol as negative control, neat sample + luminol as test sample, and H2O2 + luminol as positive control were run in the same plate. The mean control value was subtracted from the mean semen value to eliminate any variation and give the true value of the test sample and reported as RLU/s/106 sperm. Accordingly, for seminal ROS levels, the subjects were selected by ROS ≤20 RLU/s/106 sperm.
Seminal plasma TAC level
The TAC test kit (Dain Bioassay Co.) was used to assess the TAC of seminal plasma (SP) based on the capability of aqueous and lipid antioxidants to inhibit the oxidation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) to ABTS+.[23] The antioxidant capacity of each sample to prevent ABTS oxidation was compared with standard Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid). Trolox standards, as well as samples, were assayed in duplicate.[23] According to kit protocol, 180 μL of freshly prepared reagent A containing ABTS and potassium persulfate was added to 10 μL of fresh Trolox standard or SP samples in the plate and read in OD0 at 660 nm wavelengths. Then, 20 μL of freshly prepared reagent B containing sodium acetate buffer was added to the wells and incubated in a dark place for 10 min. The plate read again in OD1 at 660 nm, and then the OD1-OD0 values were converted to μL/L and reported as TAC results.
Oocyte preparation, fertilization, and subsequent growth
The cumulus cell-oocyte complexes were retrieved transvaginal 36 h after treated with human chorionic gonadotropin (5000 or 10,000 IU) from clinically fertile women. The cumulus-corona cells of all oocytes had been removed chemically by a brief exposure (40 s) to medium (G-MOPS™ Plus, Vitrolife) containing 80 IU/mL hyaluronidase (Sigma, H1115000) with repetitious pipetting. Fertilization was done by using the intracytoplasmic sperm injection (ICSI) method. Sperm selection was performed by discontinuous PureSperm (Nidacon, Göteborg, Sweden) gradient. According to this, the sperm was injected into their partners (clinically fertile metaphase II oocytes). During the ICSI procedure, the oocytes were fixed using slight negative pressure in a holding pipette, and the injection pipette containing the sperm was deeply introduced into the ooplasm with the polar body at 6 or 12 o’clock position. Maturated oocytes were only injected after washing, and they were returned to medium and cultured in an incubator to observe a second polar body and two pronuclei and then moved to growth medium and followed up for cleavage success. All embryos were evaluated daily with an inverted phase-contrast microscope. They were graded according to a simple morphological classification including cell number in relation to the time of development, the percentage of cytoplasmic fragmentation, and cell symmetry on 48–72 h of post-ICSI technique.[24] The embryos were categorized as grade A (lacking fragmentation), grade B (fragmentation ≤20%), and grade C (fragmentation >20%) based on their quality.[25] The 8-cell embryo formation rate at day 3 was checked for all oocytes.
Statistical analysis
Statistical Package for the Social Sciences software (version 16; SPSS Inc.; Chicago, IL, USA) was applied for all analyzes. Data were assessed for normality test by Q-Q plots and expressed as mean ± standard deviation or percentages. Descriptive statistics were determined for all variables. The independent t-test and Pearson’s chi-square test were used to compare and correlate the parametric variables in both groups. In all statistical analyzes, a P-value <0.05 was considered statistically significant.
Results
A total of 70 participants who fulfilled the inclusion criteria were categorized into 2 groups according to their ages (35 patients with age <45 years and 35 patients with age ≥45 years). The ROS level of all samples was ≤20 in both groups. The mean percentages of sperm cells with fragmented DNA were 23.60±13.51% in group A and 23.53±15.74% in group B. In addition, the mean percentages of sperm with immature DNA were 13.85±7.10% in group A and 17.40±9.55% in group B. Furthermore, the TAC levels were 1460.04±70.45 and 1484.12±93.15 in groups A and B, respectively. Statistical description about the mean paternal age, semen volume, sperm concentration, normal morphology, progressive motility, and embryo quality of both groups were given in Figure 2. When conventional sperm parameters were compared between groups A and B (36.26±13.41 vs. 28.50±15.26), sperm concentration showed a significant reduction in group B (p=0.04). However, in comparison between groups A and B, respectively, semen volume (3.40±1.70 vs. 3.14±1.66), normal morphology (4.54±1.96 and 4.13±2.06), and progressive motility (35.63±14.40 and 34.43±15.71) were lower in group B, but the reduction was not statistically significant than in group A (p=0.09, p=0.47, and p=0.77, respectively). After ICSI, the embryo quality and 8-cell formation were assessed in the 2 groups. With the comparison of groups A and B, it was shown that differences of embryo quality with grade A (44.47%±37.05% vs. 35.00%±48.73%), grade B (18.67%±24.99% vs. 21.08%±5.89%), and grade C (9.77%±4.93% vs. 8.28%±2.63%) and 8-cell embryo formation (21.29%±36.05% vs. 19.50%±25.00%) were not statistically significant. The Pearson’s correlation coefficients of studied parameters and embryo quality with paternal age were investigated in these 70 semen samples and described in Table 1. The degree of correlation was assessed with the coefficient correlation (r) and P-value (P). In fact, there was no significant correlation between paternal age and studied parameters including semen volume, sperm progressive motility, normal morphology, embryo quality grade A, grade B, and grade C, and 8-cell embryo formations. Interestingly, it was noted that sperm concentration decreased significantly with an elevated paternal age (r=−0.15, p=0.041). In addition, routine semen parameters showed strong significant correlations with reproductive outcome. Correlations between sperm concentration and progressive motility (r=0.38, p=0.000), normal morphology (r=0.55, p=0.000), and embryo quality with grade A (r=0.24, p=0.002) revealed a strong positive relationship. Indeed, sperm progressive motility was positively associated with sperm normal morphology (r=0.48, p=0.000) and grade A embryo (r=0.24, p=0.001). The percentage of normal morphology augmented positive correlation with the percentage of grade A (r=0.30, p=0.000).
Table 1.
Correlation between paternal age, semen parameters, and ICSI outcomes
| S. volume (mL) | Con (×106/mL) | P. motility (%) | N. morphology (%) | Grade A (%) | Grade B (%) | Grade C (%) | 8-cell (%) | |
|---|---|---|---|---|---|---|---|---|
| Paternal age (year) | r=0.09 | r=−0.15* | r=−0.02 | r=−0.05 | r=−0.03 | r=−0.15 | r=−0.14 | r=−0.08 |
| p=0.319 | p=0.041 | p=0.766 | p=0.468 | p=0.738 | p=0.064 | p=0.071 | p=0.526 | |
|
| ||||||||
| S. volume (mL) | - | r=0.02 | r=0.08 | r=0.12 | r=0.11 | r=0.03 | r=0.02 | r=0.09 |
| p=0.808 | p=0.420 | p=0.190 | p=0.170 | p=0.870 | p=0.820 | p=0.420 | ||
|
| ||||||||
| Con (×106/mL) | - | r=0.38** | r=0.55** | r=0.24** | r=0.07 | r=−0.05 | r=0.18 | |
| p=0.000 | p=0.000 | p=0.002 | p=0.358 | p=0.630 | p=0.173 | |||
|
| ||||||||
| P. motility (%) | - | - | r=0.48** | r=0.24** | r=0.11 | r=−0.08 | r=0.06 | |
| p=0.000 | p=0.001 | p=0.160 | p=0.329 | p=0.680 | ||||
|
| ||||||||
| N. morphology (%) | - | - | - | r=0.30** | r=0.07 | r=−0.12 | r=0.06 | |
| p=0.000 | p=0.382 | p=0.130 | p=0.653 | |||||
|
| ||||||||
| Grade A (%) | - | - | - | - | r=0.26** | r=−0.05 | r=0.50** | |
| p=0.000 | p=0.510 | p=0.000 | ||||||
|
| ||||||||
| Grade B (%) | - | - | - | - | - | r=−0.02 | r=0.61** | |
| p=0.773 | p=0.000 | |||||||
|
| ||||||||
| Grade C (%) | - | - | - | - | - | - | r=−0.14 | |
| p=0.296 | ||||||||
The Pearson’s test was used to correlate the parametric variables in both groups. Data are expressed as mean±SD. P≤0.05, significant; p≤0.001, highly significant.
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
Con: concentration; S. volume: semen volume; P. motility: progressive motility; N. morphology: normal morphology; SD: standard deviation; ICSI: intracytoplasmic sperm injection
Discussion
The present investigation was performed in 70 men with their wife’s age ≤38 years and normal sperm DFI, CMI, ROS, and TAC levels separated into 2 groups according to their age (group A: age <45 years and group B: age ≥45 years), aiming to examine the effect of paternal age on conventional semen parameters and ICSI outcomes after sperm injection to their wife oocyte. It was demonstrated that sperm concentration reduced by paternal age, but semen volume, sperm motility, and normal morphology had no significant changes by additional age. In addition, the percentage of embryo quality with grades A, B, and C and 8-cell embryo formation was not affected by paternal age.
In contrast to reports, our results demonstrated that seminal volume, sperm motility, and morphology were decreased with paternal age[9,10] and confirmed studies that showed decreasing sperm concentration with increasing age.[12,26] In addition, previously, it was demonstrated that paternal age ≥40 years had a negative effect on pregnancy rates.[14,27] However, a study stated no effect of paternal age on pregnancy outcomes when the wife was younger than 36 years.[16] The pregnancy rate reduction associated with increasing paternal age has been already described by other studies.[28,29] In this result, it was found that embryo quality and 8-cell embryo formation had no significant effect with increasing paternal age when their wife was younger than 38 years. Finally, the present study correlated paternal age with sperm concentration corresponding to reduced sperm maturation and growth. In addition, sperm DNA integrity had a negative correlation with embryo quality. Hence, DNA integrity might aid the assessment and management of male infertility. Many studies have highlighted the association between the level of sperm DNA fragmentation, embryo quality, and successful pregnancy after ICSI.[1,30] They found that when sperm DFI was increased, embryo quality and successful pregnancy decreased.[31,32] However, many studies have tried to determine the effect of DNA fragmentation on fertilization rate. Previously, it was reported that on conventional semen parameters, sperm concentration and rapid progressive motility before preparation affected the ICSI outcomes but not the rate of early embryo development.[33] In addition, the correlation of rapid progressive motility with embryo development was reported by investigators.[34] In our study, it was demonstrated that in men with normal sperm DFI, CMI, ROS, and TAC levels, there were no significant changes in semen parameters and fertilization outcome with increasing age (≥45 years). According to our previous review study, variables, such as body mass index (BMI), medical or surgical history (varicocele), and smoking, had a direct effect on ROS level.[19] In addition, chromatin compaction and integrity could be changed in different levels of ROS that might had a relationship with pregnancy outcome.[35,36] Therefore, in the present study, samples with abnormal DFI, TAC, or ROS level were excluded, and data on factors, such as food consumption trends and environmental exposure, and that had a direct effect on ROS were not recorded.
Conclusion
Our study suggested that in men with normal DFI, CMI, ROS, and TAC levels, increasing the paternal age had an effect on sperm concentration, but not on fertilization outcome. As usually older men have a tendency to own older partners, it was difficult for us to find men >45 years whose wife’s age was <38 years. If the number of samples was higher, we would certainly have acquired brighter results. The present study also did not record data on risk factors, such as food consumption trends, environmental exposure, BMI, and effect of ejaculatory abstinence duration. It has been suggested that nutritional antioxidant therapies with dietary intake of synthetic and natural food antioxidants might have a beneficial impact on semen quality in aged men. Future research should also focus on assessing other levels of ROS and their effects on semen quality in aged men. In addition, the effect of different antioxidants and their required doses to improve semen quality in aged male and female, lifestyle, food consumption trends, people habits, and even environmental exposures should be assessed.
Footnotes
Ethics Committee Approval: This study was approved by the Medical Ethics Committee of Avicenna Research Institute. IR.ACECR.Avicenna.REC.94.9.
Peer-review: Externally peer-reviewed.
Author Contributions: Design - S.D., M.D., H.R.K.K., M.R.S., M.M.A.; Data Interpretation - S.D., M.D., H.R.K.K., M.R.S., M.H., G.C., M.M.A.; Execution - S.D., M.D., M.H., G.C.; Data Analysis - S.D., M.D.; Manuscript Drafting and Revision - S.D., M.D., H.R.K.K., M.R.S., M.H., G.C., M.M.A.; Critical Discussion - H.R.K.K., M.R.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This project was financially supported by Avicenna Research Institute (Tehran, Iran).
References
- 1.Chapuis A, Gala A, Ferrières-Hoa A, Mullet T, Bringer-Deutsch S, Vintejoux E, et al. Sperm quality and paternal age: effect on blastocyst formation and pregnancy rates. Basic Clin Androl. 2017;27:2. doi: 10.1186/s12610-016-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26 609 men close to general population between 1989 and 2005 in France. Hum Reprodn. 2012;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikka SC, Ayaz A. Standardized Semen Analysis and Quality Control Management for Multicenter Male Reproductive Toxicology Clinical Trials. Elsevier; 2018. [Google Scholar]
- 4.Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A, Reproduction E, Force ST. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. 2011;18:29–43. doi: 10.1093/humupd/dmr040. [DOI] [PubMed] [Google Scholar]
- 5.Van Loendersloot L, Van Wely M, Limpens J, Bossuyt P, Repping S, Van Der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–89. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 6.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–48. doi: 10.1016/S0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 7.Eskenazi B, Wyrobek AJ, Sloter E, Kidd S, Moore L, Young S, et al. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–54. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom WJ, Overstreet JW, Sikka SC, Denne J, Ahuja S, Hoover AM, et al. Semen and sperm reference ranges for men 45 years of age and older. J Androl. 2006;27:421–8. doi: 10.2164/jandrol.05156. [DOI] [PubMed] [Google Scholar]
- 9.Stone BA, Alex A, Werlin LB, Marrs RP. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100:952–8. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95:1–8. doi: 10.1016/j.fertnstert.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Estofan G, Veron G, Tissera A, Estofan D, Molina R, Vazquez-Levin MI. The impact of age upon routine semen analysis and sperm kinematic parameters. Fertil Steril. 2017;108:e125–6. doi: 10.1016/j.fertnstert.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Luna M, Finkler E, Barritt J, Bar-Chama N, Sandler B, Copperman AB, et al. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril. 2009;92:1772–5. doi: 10.1016/j.fertnstert.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Whitcomb BW, Turzanski-Fortner R, Richter KS, Kipersztok S, Stillman RJ, Levy MJ, et al. Contribution of male age to outcomes in assisted reproductive technologies. Fertil Steril. 2011;95:147–51. doi: 10.1016/j.fertnstert.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De La Rochebrochard E, De Mouzon J, Thépot F, Thonneau P. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril. 2006;85:1420–4. doi: 10.1016/j.fertnstert.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90:97–103. doi: 10.1016/j.fertnstert.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Spandorfer SD, Avrech OM, Colombero LT, Palermo GD, Rosenwaks Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod. 1998;13:334–8. doi: 10.1093/humrep/13.2.334. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira RC, Braga DPDaF, De Souza Bonetti TC, Pasqualotto FF, Iaconelli A, Borges E. Negative influence of paternal age on clinical intracytoplasmic sperm injection cycle outcomes in oligozoospermic patients. Fertil Steril. 2010;93:1870–4. doi: 10.1016/j.fertnstert.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Who. WHO laboratory manual for the examination and processing of human semen, 2010. Switzerland: World Health Organization; [Google Scholar]
- 19.Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, et al. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16:87. doi: 10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, et al. Halosperm® is an easy, available, and cost-effective alternative for determining sperm DNA fragmentation. Fertil Steril. 2005;84:860. doi: 10.1016/j.fertnstert.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Esteves SC, Sharma RK, Gosálvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–52. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 22.Barnard L, Aston KI. Spermatogenesis: Methods and Protocols. New York: Humana Press; 2012. [Google Scholar]
- 23.Mahfouz R, Sharma R, Sharma D, Sabanegh E, Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril. 2009;91:805–11. doi: 10.1016/j.fertnstert.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Darbandi S, Darbandi M, Khorshid HRK, Shirazi A, Sadeghi MR, Agarwal A, et al. Reconstruction of mammalian oocytes by germinal vesicle transfer: A systematic review. Int J Reprod Biomed. 2017;15:601–12. doi: 10.29252/ijrm.15.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JY, Kim JH, Jee BC, Lee JR, Suh CS, Kim SH. Can intracytoplasmic sperm injection prevent total fertilization failure and enhance embryo quality in patients with non-male factor infertility? Eur J Obstet Gynecol Reprod Biol. 2014;178:188–91. doi: 10.1016/j.ejogrb.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Aboulghar M, Mansour R, Al-Inany H, Abou-Setta AM, Aboulghar M, Mourad L, et al. Paternal age and outcome of intracytoplasmic sperm injection. Reprod Biomed Online. 2007;14:588–92. doi: 10.1016/S1472-6483(10)61050-4. [DOI] [PubMed] [Google Scholar]
- 27.Klonoff-Cohen HS, Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol. 2004;191:507–14. doi: 10.1016/j.ajog.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Hassan MA, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003;79:1520–7. doi: 10.1016/S0015-0282(03)00366-2. [DOI] [PubMed] [Google Scholar]
- 29.De La Rochebrochard E, Thonneau P. Paternal age≥ 40 years: an important risk factor for infertility. Am J Obstet Gynecol. 2003;189:901–5. doi: 10.1067/S0002-9378(03)00753-1. [DOI] [PubMed] [Google Scholar]
- 30.Sadeghi M, Lakpour N, Heidari-Vala H, Hodjat M, Amirjannati N, Hossaini Jadda H, et al. Relationship between sperm chromatin status and ICSI outcome in men with obstructive azoospermia and unexplained infertile normozoospermia. Rom J Morphol Embryol. 2011;52:645–51. [PubMed] [Google Scholar]
- 31.Simon L, Castillo J, Oliva R, Lewis SE. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod Biomed Online. 2011;23:724–34. doi: 10.1016/j.rbmo.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Muratori M, Tarozzi N, Cambi M, Boni L, Iorio AL, Passaro C, et al. Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: a possible new male predictive parameter of pregnancy? Medicine (Baltimore) 2016;95:e3624. doi: 10.1097/MD.0000000000003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges E, Setti A, Braga D, Figueira R, Iaconelli A. Total motile sperm count has a superior predictive value over the Who 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016;4:880–6. doi: 10.1111/andr.12199. [DOI] [PubMed] [Google Scholar]
- 34.Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod. 2001;16:918–24. doi: 10.1093/humrep/16.5.918. [DOI] [PubMed] [Google Scholar]
- 35.Darbandi M, Darbandi S, Agarwal A, Baskaran S, Sengupta P, Dutta S, et al. Oxidative stress-induced alterations in seminal plasma antioxidants: Is there any association with keap1 gene methylation in human spermatozoa? Andrologia. 2018;51:e13159. doi: 10.1111/and.13159. [DOI] [PubMed] [Google Scholar]
- 36.Darbandi M, Darbandi S, Agarwal A, Baskaran S, Dutta S, Sengupta P, et al. Reactive oxygen species-induced alterations in H19-Igf2 methylation patterns, seminal plasma metabolites, and semen quality. J Assist Reprod Genet. 2018 doi: 10.1007/s10815-018-1350-y. doi: 10.1007/s10815-018-1350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]