Abstract
Atractaspidines are poorly studied, fossorial snakes that are found throughout Africa and western Asia, including the Middle East. We employed concatenated gene-tree analyses and divergence dating approaches to investigate evolutionary relationships and biogeographic patterns of atractaspidines with a multi-locus data set consisting of three mitochondrial (16S, cyt b, and ND4) and two nuclear genes (c-mos and RAG1). We sampled 91 individuals from both atractaspidine genera (Atractaspis and Homoroselaps). Additionally, we used ancestral-state reconstructions to investigate fang and diet evolution within Atractaspidinae and its sister lineage (Aparallactinae). Our results indicated that current classification of atractaspidines underestimates diversity within the group. Diversification occurred predominantly between the Miocene and Pliocene. Ancestral-state reconstructions suggest that snake dentition in these taxa might be highly plastic within relatively short periods of time to facilitate adaptations to dynamic foraging and life-history strategies.
1. Introduction
Recently, several studies generated phylogenies of advanced African snakes, including colubrids, lamprophiids, elapids, and viperids [1–9]. In contrast, there has been only one morphology-based, phylogenetic study that focused on atractaspidines [10]. The Family Atractaspididae was originally erected by Günther [11] for species of Atractaspis, renowned for their unique and exceptionally long and mobile fangs [12]. Based on skull morphology, Bourgeois [13] created the subfamily Aparallactinae (within Colubridae) to accommodate Atractaspis, Aparallactus, and other closely related fossorial snakes. This grouping was supported by jaw musculature studies of Heymans [14–15], who transferred Atractaspis to the Subfamily Atractaspidinae (Atractaspininae, sensu Kelly et al. [16]). Several recent molecular [7–9] and morphological studies [17–18] recovered a monophyletic group containing both aparallactines and atractaspidines, and with few exceptions [19–21], current classification recognizes Aparallactinae and Atractaspidinae as sister taxa in the Family Lamprophiidae [2, 7–9, 22–25]. Phylogenetic relationships within atractaspidines are not well known, because many phylogenetic studies that included atractaspidines were limited by low sample sizes [2, 8–10, 21–23, 26–27].
Based on scale patterns and counts, Laurent [28] assigned the known species of Atractaspis into five groups (Sections A–E). Decades later, Underwood and Kochva [18] partitioned Atractaspis into two groups based on venom gland morphology and geographic distribution: the ‘bibronii’ group and the ‘microlepidota’ group. These authors defined the ‘bibronii’ group as having normal-sized venom glands and a sub-Saharan distribution, and it included the following species: A. aterrima, A. bibronii, A. boulengeri, A. congica, A. corpulenta, A. dahomeyensis, A. duerdeni, A. irregularis, and A. reticulata. The 2nd ‘microlepidota’ group has relatively elongated venom glands and is found in western, central and eastern Africa, including the distinctive horn of Africa, the Sinai Peninsula, and much of Arabia, Israel, and the Levant. This latter group consisted of the following species: A. engaddensis, A. engdahli, A. leucomelas, A. microlepidota, A. micropholis, and A. scorteccii. Moyer and Jackson [10] reconstructed phylogenetic relationships among 14 species of Atractaspis with morphological data, incorporating Macrelaps and Homoroselaps as outgroups, based on previous studies [18]. However, the two groups of Underwood and Kochva [18] were not supported [10]. More recent molecular phylogenetic studies suggest that Homoroselaps is sister to Atractaspis, whereas Macrelaps is closely related to Amblyodipsas and Xenocalamus [8–9, 27].
The diversification of burrowing asps is particularly interesting because of their unique front fangs, which are starkly different from other lamprophiids [21, 29–32]. It has been hypothesized that foraging for nestling mammalian prey was a major driver in the evolution of front fangs and “side-stabbing,” which are unique to Atractaspis [31, 33]. Both Atractaspis and Homoroselaps have front fangs, which differs from the rear-fang morphology that is common in their aparallactine sister group. Although Atractaspsis and Homoroselaps both contain front fangs, Atractaspis fang morphology is more similar to viperids (Atractaspis was previously and erroneously classified in the Viperidae), whereas Homoroselaps fang morphology is more similar to elapids [25, 31]. Underwood and Kochva [18] suggested a Macrelaps-like ancestor for aparallactines and atractaspidines, which may have foraged above ground and fed on a wide variety of prey items. Specialization on elongated prey items (e.g., squamates and invertebrates) may have taken different evolutionary routes within aparallactines and atractaspidines, which involved morphological changes that facilitated foraging, capture, and envenomation of prey items [31]. Burrowing asps and their sister group Aparallactinae are ideal groups to study fang evolution, because they possess many fang types (i.e., rear fang, fixed front fang, and moveable front fang) [25, 29–32]. Additionally, collared snakes (aparallactines) and burrowing asps make interesting models to study fang evolution because of their dietary specializations, especially prevalent within the Aparallactinae, which feed on prey ranging from earthworms to blind snakes [25, 31].
Herein, we employ phylogenetic hypotheses in conjunction with temporal biogeographic information to gain a more comprehensive understanding of the evolutionary history of Atractaspidinae. Specifically, we evaluate the following questions: Are currently recognized genera and species monophyletic? Are Atractaspis and Homoroselaps sister taxa? Are Atractaspis genetically partitioned into the ‘bibronii’ and ‘microlepidota’ groups as Underwood and Kochva [18] suggested? Additionally, we investigate patterns of diversification regarding character traits, including prey selection and fang morphology, within atractaspidines and aparallactines.
2. Materials and methods
2.1 Approvals and permissions
Permission for DFH, MB and EG to collect snakes in Uganda was obtained from the Uganda Wildlife Authority (UWA—permit no. 2888 issued on August 1, 2014, permit no. 29279 issued on August 11, 2015) and the Ministry of Tourism, Wildlife and Antiquities (permit no. GoU/008/2016). Permission for CK, WMM, MMA, and EG to collect snakes in Burundi was granted by the Institut National pour l’Environnement et la Conservation de la Nature (INECN—unnumbered permit from Directeur General de l’INECN dated December 27, 2011). Permission for CK, WMM, MMA, DFH, and EG to collect snakes in Democratic Republic of Congo (DRC) was granted by the Centre de Recherche en Sciences Naturelles (CRSN—LW1/28/BB/MM/BIR/050/07, unnumbered permit from 2008, LWI/27/BBa/MUH.M/BBY/141/09, LWI/27/BBa/MUH.M/BBY/023/10, LWI/27/BBa/MUH.M/BBY/001/011, LWI/27/BBa/CIEL/BBY/003/012, LW1/27/BB/KB/BBY/60/2014, LWI/27/BBa/BBY/146/014), Institut Congolais pour la Conservation de la Nature (ICCN—unnumbered permit by Provincial Director of ICCN, Equateur Province in Mbandaka in August 2013, 004/ICCN/PNKB/2013, 06/ICCN/PNKB/2014, 02/ICCN/PNKB/2015), and Institut Superieur d’Ecologie Pour la Conservation de la Nature (ISEC, Katana—ISEC/DG/SGAC/04/2015, ISEC/DG/SGAC/04/29/2016). The University of Texas at El Paso (UTEP) Institutional Animal Care and Use Committee (IACUC—A-200902-1) approved field and laboratory methods. Permits for WC to collect snakes in South Africa were granted by the Department of Economic Development, Environmental Affairs and Tourism (permit nos. CRO 84/11CR and CRO 85/11CR). Permits for MOR and JP to collect snakes in Mozambique were granted by the Gorongosa Restoration Project and the Mozambican Departamento dos Serviços Cientificos (PNG/DSCi/C12/2013; PNG/DSCi/C12/2014; PNG/DSCi/C28/2015). Additional specimens and samples were obtained from natural history museums and university collections (Table 1) that followed appropriate legal guidelines and regulations for collection and loans of specimens.
Table 1. Voucher numbers, localities, and GenBank accession numbers for genetic samples.
DRC = Democratic Republic of the Congo; RC = Republic of Congo; SA = South Africa; GNP = herpetological collection of the E. O. Wilson Biodiversity Center, Gorongosa National Park, Mozambique. Other collection acronyms are explained in Sabaj [108]. Note that Lawson et al. [109] erroneously listed the specimen of Atractaspis sp. as MVZ 228653.
| Species | Collection No. | Field No. | Locality | 16S | ND4 | cyt b | c-mos | RAG1 |
|---|---|---|---|---|---|---|---|---|
| Eutropis longicaudata | SAMA R38916 | — | Malaysia | — | AY169645 | DQ239139 | DQ238979 | — |
| Rena humilis | CAS 190589 | — | — | — | — | — | — | — |
| Boa constrictor | — | — | — | — | — | AF471036 | AF471115 | — |
| Acrochordus granulatus | — | — | — | — | U49296 | AF217841 | AF471124 | — |
| Agkistrodon piscivorus | — | — | — | — | AF156578 | AF471074 | AF471096 | — |
| Atheris nitschei | — | — | — | — | AY223618 | AF471070 | AF471125 | — |
| Crotalus viridis | — | — | — | — | AF194157 | AF471066 | AF471135 | — |
| Diadophis punctatus | — | — | — | — | AF258910 | AF471094 | AF471122 | — |
| Hypsiglena torquata | — | — | — | — | U49309 | AF471038 | AF471159 | — |
| Natrix natrix | — | — | — | — | AY873710 | AF471059 | AF471121 | — |
| Thamnophis sirtalis | — | — | — | — | AF420196 | AF402929 | DQ902094 | — |
| Boiga dendrophila | — | — | — | — | U49303 | AF471089 | AF471128 | — |
| Bamanophis dorri | — | — | — | — | AY487042 | AY188040 | AY188001 | — |
| Dolicophis jugularis | — | — | — | — | AY487046 | AY376740 | AY376798 | — |
| Dendroaspis polylepis | — | — | — | — | AY058974 | AF217832 | AY058928 | — |
| Naja kaouthia | — | — | — | — | AY058982 | AF217835 | AY058938 | — |
| Naja annulata | — | — | — | — | AY058970 | AF217829 | AY058925 | — |
| Bothrolycus ater | — | — | — | — | — | — | — | — |
| Gonionotophis brussauxi | IRSNB 16266 | — | Gabon: Ogooué-Lolo Province: Offoué-Onoy Department: Mount Iboundji | — | FJ404358 | AY612043 | AY611952 | — |
| Lycophidion capense | PEM R22890 | CMRK 275 | Botswana | — | DQ486320 | DQ486344 | DQ486168 | — |
| Bothrophthalmus lineatus | — | — | Uganda | — | — | AF471090 | AF471090 | — |
| Lycodonomorphus laevissimus | PEM R5630 | — | SA: Eastern Cape Province: Grahamstown District | — | DQ486314 | DQ486338 | DQ486162 | — |
| Lycodonomorphus rufulus | PEM R22892 | CMRK 236 | SA: Eastern Cape Province: Hole in the Wall | — | HQ207153 | HQ207111 | HQ207076 | — |
| Boaedon upembae | UTEP 21002 | ELI 205 | DRC: Haut-Lomami Province: Kyolo | — | KM519681 | KM519700 | KM519734 | KM519719 |
| Boaedon upembae | UTEP 21003 | ELI 208 | DRC: Haut-Lomami Province: Kyolo | — | KM519680 | KM519699 | KM519733 | KM519718 |
| Boaedon fuliginosus 1 | — | — | Burundi | — | FJ404364 | FJ404302 | AF544686 | — |
| Boaedon fuliginosus 2 | PEM R5639 | — | Rwanda: Butare District | — | HQ207147 | HQ207105 | HQ207071 | — |
| Boaedon fuliginosus 3 | PEM R5635 | — | Rwanda: Nyagatare District | — | HQ207148 | HQ207106 | HQ207072 | — |
| Psammophylax variabilis | — | IPMB J296 | Burundi | — | FJ404328 | AY612046 | AY611955 | — |
| Atractaspis andersonii | MVZ 236612 | — | Yemen: Lahi Governorate | — | — | MK621624 | — | — |
| Atractaspis andersonii | MVZ 236613 | — | Yemen: Lahi Governorate | MK621482 | MK621565 | MK621623 | — | — |
| Atractaspis andersonii | MVZ 236614 | — | Yemen: Lahi Governorate | — | — | MK621622 | — | — |
| Atractaspis cf. andersonii | — | TMHC 2013-10-336 | Oman: Dhofar Mts. | MK621475 | MK621552 | MK621609 | — | — |
| Atractaspis aterrima | IRD CI.208 | CI 208 | Ivory Coast: Drekro | MK621477 | MK621558 | MK621615 | MK621672 | MK621521 |
| Atractaspis aterrima | IRD CI.267 | CI 267 | Ivory Coast: Allakro | MK621478 | MK621557 | MK621614 | MK621671 | MG775793 |
| Atractaspis aterrima | IRD T.265 | TR 265 | Togo: Mt. Agou | — | — | MK621616 | MK621673 | — |
| Atractaspis aterrima | — | TR 649 | Mali | — | MK621559 | MK621617 | — | — |
| Atractaspis bibronii | MCZ-R 184426 | AMB 8268 | SA: Limpopo Province | MK621481 | MK621544 | MK621602 | — | — |
| Atractaspis bibronii | MCZ-R 184500 | AMB 8364 | SA: Limpopo Province | — | MK621545 | MK621603 | MK621667 | — |
| Atractaspis bibronii | MCZ-R 184505 | AMB 8369 | SA: Limpopo Province | — | MK621543 | MK621601 | — | MK621509 |
| Atractaspis bibronii | PEM R20775 | 624 | SA: Limpopo Province: Ngala | — | MK621534 | MK621593 | MK621663 | — |
| Atractaspis bibronii | PEM R9768 | 629 | Malawi: Mt. Mulanje | — | MK621535 | MK621594 | — | — |
| Atractaspis bibronii | PEM R20951 | MB 21278 | SA: Northern Cape Province: Kathu | — | MK621536 | MK621595 | — | MK621503 |
| Atractaspis bibronii | — | MB 21703 | SA: Mpumalanga Province: Madimola | MK621468 | — | MK621598 | MG775900 | MG775791 |
| Atractaspis bibronii | NMB R10815 | MBUR 00961 | SA: Limpopo Province: Tshipise region | MK621466 | MK621537 | MK621596 | MK621664 | MK621504 |
| Atractaspis bibronii | NMB R10866 | MBUR 20911 | SA: Northern Cape Province: Boegoeberg Dam | — | MK621538 | — | MK621665 | MK621505 |
| Atractaspis bibronii | — | MCZ-R 27182 | SA: Limpopo Province | — | MK621546 | MK621604 | MK621668 | — |
| Atractaspis bibronii | — | LV 004 | SA: North West Province: Lephalale | — | MK621541 | MK621599 | MK621659 | MK621510 |
| Atractaspis bibronii | — | RSP 489 | — | — | MK621540 | — | — | — |
| Atractaspis bibronii | — | TGE-T2-36 | SA: KwaZulu-Natal Province | MK621467 | MK621539 | MK621597 | MK621666 | MK621506 |
| Atractaspis bibronii rostrata | — | GPN 191 | Mozambique: Gorongosa National Park | MK621474 | MK621542 | MK621600 | MK621660 | MK621511 |
| Atractaspis bibronii rostrata | — | GPN 353 | Mozambique: Gorongosa National Park | MK621487 | — | — | — | — |
| Atractaspis bibronii rostrata | — | GPN 354 | Mozambique: Gorongosa National Park | MK621488 | — | — | — | — |
| Atractaspis bibronii rostrata | — | GPN 421 | Mozambique: Gorongosa National Park | MK621486 | — | — | — | — |
| Atractaspis bibronii rostrata | — | MTSN 8354 | Tanzania: Nguru Mts. | MK621490 | — | — | — | — |
| Atractaspis bibronii rostrata | — | MTSN 8473 | Tanzania: Usambara Mts. | MK621491 | — | — | — | — |
| Atractaspis bibronii rostrata | MUSE 13889 | — | Tanzania: Udzungwa Mts. | MK621489 | — | — | — | — |
| Atractaspis cf. bibronii rostrata | UTEP 21661 | ELI 038 | DRC: Haut-Katanga Province: Pweto | MK621459 | MK621532 | MK621591 | MK621661 | MK621507 |
| Atractaspis cf. bibronii rostrata | UTEP 21662 | ELI 144 | DRC: Haut-Katanga Province: Kabongo | MK621460 | MK621533 | MK621592 | MK621662 | MK621508 |
| Atractaspis boulengeri | — | IPMB J355 | Gabon: Ogooué-Maritime Province: Rabi | AY611833 | FJ404334 | AY612016 | AY611925 | — |
| Atractaspis boulengeri | — | 29392 | Gabon | MK621469 | MK621551 | MK621605 | MK621658 | MK621513 |
| Atractaspis boulengeri | RBINS 18606 | KG 063 | DRC: Tshopo Province: Longala | — | MK621550 | — | MK621657 | MK621512 |
| Atractaspis boulengeri | — | MSNS Rept 220 | Gabon: Ivindo National Park: Ipassa | MK621493 | — | — | — | — |
| Atractaspis boulengeri | IRSEN 00162 | MBUR 03483 | RC: Niari: Gnie-Gnie | MK621472 | — | — | — | — |
| Atractaspis congica | — | 633 | Angola: Soyo | MK621461 | MK621529 | MK621587 | MK621651 | MG775788 |
| Atractaspis congica | PEM R18087 | CT 375 | DRC | MK621462 | — | MK621588 | — | — |
| Atractaspis congica | PEM R22035 | PVPL5 WRB | Angola: Luanda | — | MK621574 | — | — | — |
| Atractaspis corpulenta | — | IPMB J369 | Gabon: Ogooué-Maritime Province: Rabi | AY611837 | FJ404335 | AY612020 | AY611929 | — |
| Atractaspis corpulenta | PEM R22707 | MBUR 03936 | RC: Niari: Tsinguidi | MK621465 | MK621548 | MK621606 | MK621654 | MG775790 |
| Atractaspis corpulenta kivuensis | RBINS 18607 | CRT 4264 | DRC: Tshopo Province: Lieki | — | MK621547 | — | MK621655 | — |
| Atractaspis corpulenta kivuensis | UTEP 21663 | ELI 2992 | DRC: Tshopo Province: Bombole | MK621471 | MK621549 | MK621607 | MK621656 | MK621514 |
| Atractaspis dahomeyensis | IRD 2193.N | 2193N Trape | Chad: Baibokoum | — | MK621561 | MK621619 | — | — |
| Atractaspis dahomeyensis | IRD 2197.N | 2197N Trape | Chad: Baibokoum | MK621479 | MK621560 | MK621618 | MK621674 | — |
| Atractaspis dahomeyensis | IRD 5011.G | 5011G Trape | Guinea: Kissidougou | MK621484 | MK621562 | — | — | — |
| Atractaspis duerdeni | — | MB 21346 | SA: Northern Cape Province: Kuruman region | MK621463 | MK621530 | MK621589 | MK621652 | MG775789 |
| Atractaspis duerdeni | — | MBUR 0229 | SA: Limpopo Province: Senwabarwana region | MK621464 | MK621531 | MK621590 | MK621653 | MK621502 |
| Atractaspis cf. duerdeni | — | — | Zimbabwe | — | U49314 | AY188008 | AY187969 | — |
| Atractaspis engaddensis | TAUM 16030 | — | Israel: Merav | — | MK621553 | MK621610 | — | — |
| Atractaspis engaddensis | TAUM 16542 | — | Israel: Hare Gilboa | — | MK621554 | MK621611 | MG775901 | MG775792 |
| Atractaspis engaddensis | TAUM 17072 | — | Israel: Yeroham | MK621476 | MK621555 | MK621612 | MK621669 | MK621519 |
| Atractaspis engaddensis | TAUM 17094 | — | Israel: Arad | — | MK621556 | MK621613 | MK621670 | MK621520 |
| Atractaspis engaddensis | — | 3258WW | Saudi Arabia: Algassim | MG746902 | — | — | — | — |
| Atractaspis irregularis | IRD 5010.G | 5010G | Guinea: Kissidougou | — | MK621573 | MK621625 | — | — |
| Atractaspis irregularis | ZMB 87809 | LI 10 104 | Liberia: Nimba County | — | MK621568 | MK621627 | MK621646 | MK621515 |
| Atractaspis irregularis | ZMB 87867 | LI 10 118 | Liberia: Nimba County | — | MK621569 | MK621628 | MK621647 | MK621516 |
| Atractaspis irregularis | ZMB 88015 | PLI 12 089 | Liberia: Nimba County | MK621473 | MK621570 | MK621629 | MK621648 | MK621517 |
| Atractaspis irregularis | IRD T.269 | T 269 | Togo: Mt. Agou | — | MK621566 | — | MK621649 | — |
| Atractaspis irregularis | IRD T.372 | T 372 | Togo: Diguengue | — | MK621567 | — | MK621650 | — |
| Atractaspis cf. irregularis | UTEP 21657 | AKL 392 | DRC: South Kivu Province: Lwiro | MK621492 | — | — | — | — |
| Atractaspis cf. irregularis | UTEP 21658 | EBG 1190 | DRC: South Kivu Province: Lwiro | — | MG776014 | MG746785 | MG775898 | — |
| Atractaspis cf. irregularis | UTEP 21659 | EBG 2671 | DRC: South Kivu Province: Lwiro | MK621457 | MK621572 | MK621631 | MK621645 | MK621518 |
| Atractaspis cf. irregularis | UTEP 21660 | EBG 2725 | DRC: South Kivu Province: Lwiro | MK621458 | — | — | — | — |
| Atractaspis cf. irregularis | UTEP 21654 | ELI 1208 | Burundi: Bubanza Province: Mpishi | MK621456 | MK621571 | MK621630 | MK621644 | MG775787 |
| Atractaspis cf. irregularis | UTEP 21655 | ELI 1635 | DRC: South Kivu Province: Lwiro | MG746901 | MG776015 | — | MG775899 | MG775786 |
| Atractaspis cf. irregularis | MUSE 10470 | — | DRC: South Kivu Province: Itombwe Plateau, Mulenge | MK621485 | — | MK621626 | — | — |
| Atractaspis microlepidota | No voucher | MBUR 08561 | Ethiopia: Benishangul-Gumuz Province: Kutaworke region | MK621496 | — | — | — | — |
| Atractaspis microlepidota | No voucher | MBUR 08365 | Ethiopia: Benishangul-Gumuz Province: Kutaworke region | MK621494 | — | — | — | — |
| Atractaspis microlepidota | No voucher | MBUR 08542 | Ethiopia: Benishangul-Gumuz Province: Kutaworke region | MK621495 | — | — | — | — |
| Atractaspis micropholis | IRD 1833.N | 1833N Trape | Chad: Arninga Malick | MK621483 | MK621575 | — | — | — |
| Atractaspis cf. micropholis | — | IPMB J283 | Togo | AY611823 | FJ404336 | AY612006 | AY611915 | — |
| Atractaspis reticulata heterochilus | UTEP 21664 | ELI 2882 | DRC: Tshopo Province: rd between Nia Nia and Kisangani | MK621470 | MK621528 | MK621586 | — | — |
| Atractaspis reticulata heterochilus | UTEP 21665 | ELI 3625 | DRC: Maniema Province: Katopa, near Lomami National Park | — | — | MK621608 | — | — |
| Atractaspis reticulata heterochilus | RBINS 18605 | KG 219 | DRC: Tshopo Province: Uma | — | MK621527 | MK621585 | MK621643 | — |
| Atractaspis reticulata heterochilus | — | KG 495 | DRC: Tshopo Province: Bagwase | — | MK621526 | MK621584 | MK621642 | MK621501 |
| Atractaspis watsoni | IRD 2523.N | 2523N Trape | Chad: Balani | MK621480 | MK621563 | MK621620 | MK621675 | MK621522 |
| Atractaspis watsoni | IRD 2565.N | 2565N Trape | Chad: Balani | — | MK621564 | MK621621 | MK621676 | MK621523 |
| Atractaspis sp. | MVZ 229653 | — | — | — | — | AF471046 | AF471127 | — |
| Homoroselaps dorsalis | PEM R:TBA | — | SA: Gauteng Province: Pretoria | MK621500 | — | — | — | — |
| Homoroselaps lacteus | — | 28676 | SA: Gauteng Province: Pretoria | MK621497 | — | MK621634 | — | — |
| Homoroselaps lacteus | LSUMZ 57229 | AMB 4483 | SA: Eastern Cape Province: Port Elizabeth | MK621498 | MK621581 | MK621638 | — | — |
| Homoroselaps lacteus | LSUMZ 55386 | — | — | — | AY058976 | — | AY058931 | — |
| Homoroselaps lacteus | — | MCZ-R 28142 | SA: Western Cape | — | MK621579 | MK621636 | — | — |
| Homoroselaps lacteus | — | MCZ-R 28271 | SA: Western Cape: Mauritzbaai | — | MK621580 | MK621637 | — | — |
| Homoroselaps lacteus | PEM R17097 | — | SA: Eastern Cape Province: Port Elizabeth | — | FJ404339 | MK621635 | FJ404241 | — |
| Homoroselaps lacteus | PEM R17128 | — | SA: Eastern Cape Province: Sundays River Mouth | — | MK621577 | MK621633 | — | MK621525 |
| Homoroselaps lacteus | PEM R17129 | — | SA: Eastern Cape Province: Sundays River Mouth | — | MK621576 | MK621632 | MK621677 | MK621524 |
| Homoroselaps lacteus | PEM R21097 | WC 2688 | SA: Eastern Cape Province: Thomas River | — | — | MK621640 | — | — |
| Homoroselaps lacteus | PEM R19176 | WC 10 092 | SA: Free State Province: Reitz | MK621499 | MK621583 | MK621641 | — | — |
| Homoroselaps lacteus | — | WC DNA 1261 | SA: Mpumalanga Province: Wakkerstroom | — | MK621582 | MK621639 | — | — |
| Amblyodipsas concolor | — | 634 | SA: KwaZulu-Natal Province | — | MG775916 | MG746801 | MG775806 | MG775720 |
| Amblyodipsas concolor | PEM R17369 | 618 | SA: KwaZulu-Natal Province: Cape Vidal | — | MG775917 | MG746802 | MG775807 | MG775721 |
| Amblyodipsas concolor | NMB R11375 | MBUR 01624 | SA: Limpopo Province: Wolkberg Wilderness Area | MG746916 | MG775920 | MG746804 | MG775810 | MG775724 |
| Amblyodipsas concolor | NMB R11376 | MBUR 01659 | SA: Limpopo Province: Wolkberg Wilderness Area | — | MG775918 | MG746803 | MG775808 | MG775722 |
| Amblyodipsas concolor | NMB R11377 | MBUR 01660 | SA: Limpopo Province: Wolkberg Wilderness Area | MG746915 | MG775919 | — | MG775809 | MG775723 |
| Amblyodipsas concolor | PEM R19437 | WC 373 | SA: Eastern Cape Province: Hluleka | — | MG775922 | MG746806 | MG775812 | MG775726 |
| Amblyodipsas concolor | PEM R19795 | WC 483 | SA: Eastern Cape Province: Dwesa Point | — | MG775923 | MG746807 | MG775813 | MG775727 |
| Amblyodipsas concolor | PEM R20284 | WC 975 | SA: Eastern Cape Province: Mazeppa Bay | — | MG775921 | MG746805 | MG775811 | MG775725 |
| Amblyodipsas dimidiata | — | CMRK 311 | Tanzania | — | DQ486322 | DQ486346 | DQ486170 | — |
| Amblyodipsas dimidiata | PEM R15626 | — | — | — | — | AY612027 | AY611936 | — |
| Amblyodipsas microphthalma | — | SP3 | SA: Limpopo Province: Soutpansberg | MG746914 | MG775927 | MG746808 | MG775818 | MG775729 |
| Amblyodipsas polylepis | — | AMB 6114 | SA: Limpopo Province: Farm Guernsey | — | MG775932 | — | MG775823 | MG775734 |
| Amblyodipsas polylepis | MCZ-R 190174 | AMB 7960 | Namibia: East Caprivi | — | MG775931 | MG746812 | MG775822 | MG775733 |
| Amblyodipsas polylepis | RBINS 18604 | UP 052 | DRC: Haut-Katanga Province: Kiubo | — | MG775929 | MG746810 | MG775820 | MG775731 |
| Amblyodipsas polylepis | PEM R22492 | MBUR 00353 | SA: Limpopo Province: Westphalia | MG746921 | MG775928 | MG746809 | MG775819 | MG775730 |
| Amblyodipsas polylepis | PEM R18986 | 632 | SA: Limpopo Province: Phalaborwa | — | MG775930 | MG746811 | MG775821 | MG775732 |
| Amblyodipsas polylepis | — | PVP9 WRB | Angola | MG746922 | MG775933 | MG746813 | — | — |
| Amblyodipsas polylepis | — | MTSN 7571 | Tanzania: Ruaha | MG746923 | — | MG746814 | — | — |
| Amblyodipsas polylepis | — | 3128WW | — | MG746924 | — | — | — | — |
| Amblyodipsas polylepis | PEM R23535 | WC 4651 | Angola: Moxico | MG746925 | — | — | — | — |
| Amblyodipsas unicolor | — | PB-11-502 | Guinea: Kankan | MG746917 | MG775924 | MG746815 | MG775814 | MG775728 |
| Amblyodipsas unicolor | ZMB 88018 | PGL-15-116 | Ivory Coast: Yamassoukro | — | — | MG746816 | MG775815 | — |
| Amblyodipsas unicolor | IRD 2209.N | 2209N Trape | Chad: Baibokoum | MG746918 | MG775925 | MG746817 | MG775816 | — |
| Amblyodipsas unicolor | IRD 2286.N | 2286N Trape | Chad: Baibokoum | — | MG775926 | MG746818 | MG775817 | — |
| Amblyodipsas ventrimaculata | PEM R23320 | WC 3920 | Angola: Moxico Province: Cuito River Source | MG746919 | — | MG746819 | — | — |
| Amblyodipsas ventrimaculata | — | R-SA | SA: Limpopo Province: Lephalale | MG746920 | — | — | — | — |
| Aparallactus capensis | MCZ-R 184403 | AMB 8180 | SA: Eastern Cape Province: Farm Newstead | MG746971 | MG776002 | MG746888 | MG775885 | — |
| Aparallactus capensis | MCZ-R 184404 | AMB 8181 | SA: Eastern Cape Province: Farm Newstead | — | MG776003 | MG746889 | MG775886 | — |
| Aparallactus capensis | MCZ-R 184501 | AMB 8365 | SA: Limpopo Province | — | MG776004 | MG746890 | MG775887 | — |
| Aparallactus capensis | — | GPN 134 | Mozambique: Gorongosa National Park | MG746988 | MG776000 | MG746886 | MG775883 | MG775781 |
| Aparallactus capensis | ZMB 83259 | GPN 310 | Mozambique: Gorongosa National Park | MG746983 | — | — | — | — |
| Aparallactus capensis | ZMB 83260 | GPN 333 | Mozambique: Gorongosa National Park | MG746979 | — | — | — | — |
| Aparallactus capensis | — | GPN 351 | Mozambique: Gorongosa National Park | MG746977 | — | — | — | — |
| Aparallactus capensis | — | GPN 352 | Mozambique: Gorongosa National Park | MG746978 | — | — | — | — |
| Aparallactus capensis | ZMB 83342 | GPN 359 | Mozambique: Gorongosa National Park | MG746976 | — | — | — | — |
| Aparallactus capensis | ZMB 83343 | GPN 394 | Mozambique: Gorongosa National Park | MG746981 | — | — | — | — |
| Aparallactus capensis | ZMB 83261 | GPN 429 | Mozambique: Gorongosa National Park | MG746975 | — | — | — | — |
| Aparallactus capensis | — | KB 2 | Rwanda: Akagera National Park | — | MG775996 | MG746882 | MG775879 | — |
| Aparallactus capensis | — | KB 5 | Rwanda: Akagera National Park | MG746987 | MG775995 | MG746881 | MG775878 | MG775777 |
| Aparallactus capensis | — | KB 8 | Tanzania: Kigoma | — | MG775998 | MG746884 | MG775881 | MG775779 |
| Aparallactus capensis | — | KB 23 | Rwanda: Akagera National Park | — | MG775997 | MG746883 | MG775880 | MG775778 |
| Aparallactus capensis | PEM R17909 | 648 | Malawi: Mt. Mulanje | — | MG775984 | MG746870 | MG775867 | MG775765 |
| Aparallactus capensis | — | 655 | SA: Eastern Cape Province: Middleton | — | MG775987 | — | MG775870 | MG775768 |
| Aparallactus capensis | PEM R17453 | 657 | DRC: Lualaba Province: Kalakundi | MG746970 | MG775986 | — | MG775869 | MG775767 |
| Aparallactus capensis | PEM R17332 | 659 | Tanzania: Klein’s Camp | — | MG775985 | MG746871 | MG775868 | MG775766 |
| Aparallactus capensis | HLMD J156 | — | SA | AY188045 | — | AY188006 | AY187967 | — |
| Aparallactus capensis | NMB R10885 | MBUR 01229 | SA: KwaZulu-Natal Province: Manyiseni | MG746985 | — | MG746878 | MG775876 | — |
| Aparallactus capensis | NMB R11380 | MBUR 01592 | SA: Limpopo Province: Haenetsburg region | — | MG775992 | MG746876 | MG775875 | MG775773 |
| Aparallactus capensis | NMB R11381 | MBUR 01593 | SA: Limpopo Province: Haenetsburg region | — | MG775991 | MG746875 | MG775874 | MG775772 |
| Aparallactus capensis | NMB R11382 | MBUR 01609 | SA: Limpopo Province: Haenetsburg region | — | — | MG746873 | MG775872 | MG775770 |
| Aparallactus capensis | NMB R11383 | MBUR 01642 | SA: Limpopo Province: Haenetsburg region | MG746984 | MG775993 | MG746877 | — | MG775774 |
| Aparallactus capensis | — | WC 1352 | Mozambique: Cabo Delgado Province: Pemba | — | MG775999 | MG746885 | MG775882 | MG775780 |
| Aparallactus capensis | PEM R20693 | WC 2612 | SA: Eastern Cape Province: Tsolwana | — | MG775994 | MG746880 | MG775877 | MG775776 |
| Aparallactus capensis | — | MCZ-R 27164 | SA: Limpopo Province | MG746973 | — | MG746892 | — | — |
| Aparallactus cf. capensis | PEM R18438 | 677 | SA: Limpopo Province | — | MG775988 | MG746872 | MG775871 | MG775769 |
| Aparallactus cf. capensis | NMB R10997 | MBUR 00871 | SA: Limpopo Province: Cleveland Nature Reserve | MG746986 | — | MG746879 | — | MG775775 |
| Aparallactus cf. capensis | NMB R11379 | MBUR 01554 | SA: Limpopo Province: near Sentrum | — | — | MG746874 | MG775873 | MG775771 |
| Aparallactus cf. capensis | — | MCZ-R 27805 | SA: Limpopo Province | MG746972 | MG776005 | MG746891 | — | — |
| Aparallactus cf. capensis | — | GPN 242 | Mozambique: Gorongosa National Park | MG746989 | MG776001 | MG746887 | MG775884 | MG775782 |
| Aparallactus cf. capensis | — | GPN 357 | Mozambique: Gorongosa National Park | MG746982 | — | — | — | — |
| Aparallactus cf. capensis | ZMB 83344 | GPN 403 | Mozambique: Gorongosa National Park | MG746980 | — | — | — | — |
| Aparallactus cf. capensis | — | 2118 WW | SA: Limpopo Province: Bela Bela | MG746969 | — | — | — | — |
| Aparallactus cf. capensis | — | 2119 WW | SA: Limpopo Province: Bela Bela | MG746968 | — | — | — | — |
| Aparallactus cf. guentheri | — | MTSN 8341 | Tanzania: Nguru Mts | MG746974 | — | MG746899 | — | — |
| Aparallactus cf. guentheri | PEM R5678 | — | Tanzania: Usambara Mts | — | — | AY235730 | — | — |
| Aparallactus jacksonii | PEM R20739 | 649 | Tanzania: Mt. Kilimanjaro | MG746960 | MG775980 | MG746866 | — | — |
| Aparallactus jacksonii | PEM R17876 | 650 | Tanzania: Oldonyo Sambu | MG746962 | MG775983 | MG746869 | MG775866 | MG775764 |
| Aparallactus jacksonii | PEM R17874 | 651 | Tanzania: Oldonyo Sambu | MG746961 | MG775981 | MG746867 | MG775864 | MG775762 |
| Aparallactus jacksonii | PEM R17875 | 654 | Tanzania: Ndukusiki | — | MG775982 | MG746868 | MG775865 | MG775763 |
| Aparallactus jacksonii | — | MTSN 8301 | Tanzania: Nguru Mts | MG746963 | — | — | — | — |
| Aparallactus jacksonii | — | MTSN 8303 | Tanzania: Nguru Mts | MG746967 | — | — | — | — |
| Aparallactus jacksonii | — | MTSN 8323 | Tanzania: Nguru Mts | MG746964 | — | — | — | — |
| Aparallactus jacksonii | — | MTSN 8352 | Tanzania: Nguru Mts | MG746965 | — | — | — | — |
| Aparallactus jacksonii | — | MTSN 8353 | Tanzania: Nguru Mts | MG746966 | — | — | — | — |
| Aparallactus lunulatus | — | 653 | Tanzania: Nguru Mts | MG746991 | MG776006 | — | MG775891 | MG775784 |
| Aparallactus lunulatus | IRD 2158.N | 2158N | Chad: Baibokoum | — | MG776009 | MG746896 | MG775888 | — |
| Aparallactus lunulatus | IRD 2178.N | 2178N | Chad: Baibokoum | MG746993 | MG776010 | MG746897 | MG775889 | — |
| Aparallactus lunulatus | TMHC 2013-09-315 | — | Ethiopia: Borana | MG746992 | MG776008 | MG746895 | — | — |
| Aparallactus lunulatus | TMHC 2013-09-316 | — | Ethiopia: Simien Mts. | — | MG776007 | MG746894 | — | — |
| Aparallactus lunulatus | — | WBR 957 | NE of Lake Albert | MG746990 | — | MG746893 | MG775890 | MG775783 |
| Aparallactus modestus | — | IPMB J284 | Gabon: Ogooué-Maritime Province: Rabi | AY611824 | FJ404332 | AY612007 | AY611916 | — |
| Aparallactus modestus | MCZ-R 182624 | — | RC: Bomassa | — | — | MG746863 | MG775862 | — |
| Aparallactus modestus | MCZ-R 182625 | — | RC: Bomassa | — | MG775977 | MG746864 | MG775863 | — |
| Aparallactus modestus | MVZ 252411 | — | Ghana: Ajenjua Bepo | MG746957 | MG775978 | MG746865 | — | — |
| Aparallactus modestus | USNM 584365 | — | RC: Impongui | MG746949 | MG775958 | MG746844 | MG775844 | MG775747 |
| Aparallactus modestus | ZFMK 87627 | — | — | MG746959 | — | — | — | — |
| Aparallactus modestus | IRD 5009.G | 5009G Trape | Guinea: Kissidougou | MG746958 | MG775979 | — | — | — |
| Aparallactus modestus | RBINS 18608 | CRT 4045 | DRC: Tshopo Province: Bomane | — | MG775964 | MG746850 | MG775850 | — |
| Aparallactus modestus | — | CRT 4181 | DRC: Tshopo Province: Lieki | — | MG775966 | MG746852 | — | MG775752 |
| Aparallactus modestus | — | CRT 4256 | DRC: Tshopo Province: Lieki | — | MG775967 | — | — | MG775753 |
| Aparallactus modestus | UTEP 21609 | EBG 2609 | DRC: Ituri Province: Bazinga | MG746950 | MG775959 | MG746845 | MG775845 | — |
| Aparallactus modestus | UTEP 21605 | ELI 1379 | DRC: South Kivu Province: Kihungwe | MG746951 | MG775960 | MG746846 | MG775846 | MG775748 |
| Aparallactus modestus | UTEP 21606 | ELI 1419 | DRC: South Kivu Province: Kihungwe | MG746952 | MG775961 | MG746847 | MG775847 | MG775749 |
| Aparallactus modestus | No voucher | ELI 2138 | DRC: Equateur Province: Npenda Village | MG746948 | MG775957 | MG746843 | — | — |
| Aparallactus modestus | UTEP 21601 | ELI 2221 | DRC: Equateur Province: Npenda Village | MG746953 | MG775962 | MG746848 | MG775848 | — |
| Aparallactus modestus | UTEP 21602 | ELI 2222 | DRC: Equateur Province: Npenda Village | MG746954 | MG775963 | MG746849 | MG775849 | MG775750 |
| Aparallactus modestus | UTEP 21608 | ELI 2914 | DRC: Tshopo Province: Kisangani | MG746955 | MG775968 | MG746853 | MG775852 | — |
| Aparallactus modestus | — | KG 457 | DRC: Tshopo Province: Bagwase | — | MG775970 | MG746855 | MG775855 | MG775755 |
| Aparallactus modestus | — | KG 467 | DRC: Tshopo Province: Bagwase | — | MG775972 | MG746858 | MG775858 | MG775758 |
| Aparallactus modestus | — | KG 499 | DRC: Tshopo Province: Bagwase | — | MG775973 | — | MG775859 | MG775759 |
| Aparallactus modestus | — | KG 501 | DRC: Tshopo Province: Bagwase | — | MG775971 | MG746857 | MG775857 | MG775757 |
| Aparallactus modestus | — | KG 503 | DRC: Tshopo Province: Bagwase | — | MG775969 | MG746854 | MG775854 | MG775754 |
| Aparallactus modestus | — | KG 511 | DRC: Tshopo Province: Bagwase | — | MG775975 | MG746860 | MG775861 | MG775761 |
| Aparallactus modestus | — | KG 528 | DRC: Tshopo Province, Bagwase | — | — | MG746856 | MG775856 | MG775756 |
| Aparallactus modestus | — | KG 572 | DRC: Tshopo Province: Bagwase | — | MG775974 | MG746859 | MG775860 | MG775760 |
| Aparallactus modestus | — | MSNS REPT 34 | Gabon: Ogooué-Lolo Province: Mt. Iboundji | — | — | MG746862 | — | — |
| Aparallactus modestus | — | PB 11-733 | Guinea: Nzerekore | — | MG775976 | MG746861 | MG775853 | — |
| Aparallactus modestus | RBINS 18603 | UAC 038 | DRC: Tshopo Province: Yoko | — | MG775965 | MG746851 | MG775851 | MG775751 |
| Aparallactus modestus | PEM R22331 | MBUR 03449 | RC: Niari: Doumani | MG746956 | — | — | — | — |
| Aparallactus niger | IRD 8075.X | 8075X | Guinea: Nzerekore | MG746994 | MG776011 | MG746898 | MG775892 | — |
| Aparallactus werneri | FMNH 2504400 | — | Tanzania: Tanga | — | U49315 | AF471035 | — | — |
| Chilorhinophis gerardi | PEM R18882 | 635 | Zambia: Kalumbila | MG746995 | MG776012 | MG746900 | MG775893 | MG775785 |
| Macrelaps microlepidotus | PEM R20944 | — | SA: KwaZulu-Natal Province: Hillcrest | MG746927 | MG775938 | — | — | — |
| Macrelaps microlepidotus | — | 28666 | — | — | MG775935 | MG746821 | MG775824 | — |
| Macrelaps microlepidotus | PEM R19791 | WC DNA 511 | SA: Eastern Cape Province: Dwessa Nature Reserve | MG746926 | MG775934 | MG746820 | — | — |
| Macrelaps microlepidotus | PEM R20167 | WC DNA 928 | SA: Eastern Cape Province: Hogsback | — | MG775937 | MG746823 | — | — |
| Macrelaps microlepidotus | PEM R20295 | WC DNA 973 | SA: Eastern Cape Province: Mazeppa Bay | — | MG775936 | MG746822 | — | — |
| Micrelaps bicoloratus | — | CMRK 330 | — | — | — | DQ486349 | DQ486173 | — |
| Micrelaps muelleri | TAUM 15654 | — | Israel: Salti | — | — | MG746781 | — | — |
| Micrelaps muelleri | TAUM 16469 | — | Israel: Malkishua | — | — | MG746782 | MG775895 | — |
| Micrelaps muelleri | TAUM 16738 | — | Israel: Bet Nehemya | — | — | MG746783 | MG775896 | — |
| Micrelaps muelleri | TAUM 16944 | — | Israel: Ein Hod | — | MG776013 | MG746784 | MG775897 | — |
| Micrelaps cf. muelleri | TAUM 16426 | — | Israel: Afiq | — | — | MG746780 | MG775894 | — |
| Polemon acanthias | — | PEM R1479 | Ivory Coast: Haute Dodo | AY611848 | FJ404341 | AY612031 | AY611940 | — |
| Polemon acanthias | ZMB 88016 | PLI-12-053 | Liberia: Nimba County | — | MG775954 | MG746841 | MG775841 | MG775745 |
| Polemon acanthias | ZMB 88017 | PLI-12-208 | Liberia: Nimba County | MG746946 | MG775955 | MG746842 | MG775842 | MG775746 |
| Polemon acanthias | IRD T.266 | T266 Trape | Togo: Mt. Agou | MG746947 | MG775956 | — | MG775843 | — |
| Polemon ater | PEM R17452 | — | DRC: Lualaba Province: Kalakundi | MG746943 | MG775951 | MG746838 | MG775839 | MG775743 |
| Polemon ater | PEM R20734 | — | DRC: Lualaba Province: Fungurume | MG746944 | MG775952 | MG746839 | MG775840 | MG775744 |
| Polemon christyi | UTEP 21618 | DFH 535 | Uganda: Western Region: road to Budongo Central Forest Reserve | MG746945 | MG775953 | MG746840 | — | — |
| Polemon collaris | PEM R19893 | TB 28 | Angola: North-west region | MG746931 | MG775943 | MG746827 | MG775829 | — |
| Polemon collaris | UTEP 21612 | ELI 561 | DRC: South Kivu Province: vicinity of Byonga | MG746928 | MG775939 | MG746824 | MG775825 | MG775735 |
| Polemon collaris | UTEP 21613 | ELI 1317 | DRC: South Kivu Province: Fizi | MG746930 | MG775941 | MG746826 | MG775827 | MG775737 |
| Polemon collaris | UTEP 21614 | ELI 2464 | DRC: Tshuapa Province: Watsi Kengo, Salonga River | MG746929 | MG775940 | MG746825 | MG775826 | MG775736 |
| Polemon collaris | — | KG 523 | DRC: Tshopo Province: Bagwase | — | MG775944 | MG746828 | MG775830 | — |
| Polemon collaris | — | MSNS REPT 110 | Gabon: Ogooué-Lolo Province: Mt. Iboundji | MG746934 | — | MG746829 | — | — |
| Polemon collaris | RBINS 18544 | UAC 62 | DRC: Tshopo Province: Yoko | MG746933 | MG775942 | — | MG775828 | — |
| Polemon collaris | PEM R22747 | MBUR 03862 | RC: Niari: Tsinguidi region | MG746932 | — | — | — | — |
| Polemon fulvicollis | PEM R5388 | Gabon: Ogooué-Maritime Province: Rabi | AY611846 | FJ404342 | AY612029 | AY611938 | — | |
| Polemon fulvicollis laurenti | UTEP 21615 | ELI 3046 | DRC: Tshopo Province: Bombole Village | MG746942 | MG775949 | MG746837 | MG775837 | — |
| Polemon graueri | RBINS 18543 | CRT 4007 | DRC: Tshopo Province: Bomane | — | MG775947 | MG746833 | MG775834 | MG775740 |
| Polemon graueri | UTEP 21610 | EBG 1376 | DRC: South Kivu Province: Irangi | MG746940 | — | MG746835 | MG775836 | MG775742 |
| Polemon graueri | No voucher | EBG 2294 | DRC: Ituri Province: Komanda | MG746938 | — | MG746832 | MG775833 | — |
| Polemon graueri | UTEP 21611 | ELI 2842 | Uganda: Western Region: Rwenzori Mts National Park | MG746939 | MG775948 | MG746834 | MG775835 | MG775741 |
| Polemon graueri | — | MTSN 7378 | Rwanda: Nyungwe National Park | MG746941 | — | MG746836 | — | — |
| Polemon notatus | — | 29395 | Gabon | MG746935 | MG775950 | — | MG775838 | — |
| Polemon notatus | PEM R5404 | — | Gabon: Ogooué-Maritime Province: Rabi | AY611847 | FJ404343 | AY612030 | AY611939 | — |
| Polemon cf. robustus | UTEP 21617 | ELI 2594 | DRC: Equateur Province: Salonga River | MG746936 | MG775945 | MG746830 | MG775831 | MG775738 |
| Polemon robustus | UTEP 21616 | ELI 2069 | DRC: Mai-Ndombe Province: Isongo, Lake Mai-Ndombe | MG746937 | MG775946 | MG746831 | MG775832 | MG775739 |
| Xenocalamus bicolor | — | MCZ-R 27160 | SA: Limpopo Province | — | MG775911 | MG746794 | MG775800 | — |
| Xenocalamus bicolor | — | MCZ-R 27161 | SA: Limpopo Province | MG746905 | MG775912 | MG746795 | MG775801 | — |
| Xenocalamus bicolor | PEM R17377 | 615 | SA: Northern Cape Province: Kimberly | — | MG775903 | — | MG775795 | MG775710 |
| Xenocalamus bicolor | PEM R17438 | 616 | SA: KwaZulu-Natal Province | — | — | MG746787 | — | — |
| Xenocalamus bicolor | PEM R17438 | 647 | SA: Northern Cape Province: Kimberly, Rooipoort | — | MG775902 | MG746786 | MG775794 | MG775709 |
| Xenocalamus bicolor | NMB R10851 | MBUR 00925 | SA: Limpopo Province: Woudend | MG746904 | MG775910 | MG746793 | MG775799 | MG775716 |
| Xenocalamus bicolor | NMB R11418 | MBUR 01553 | SA: Limpopo Province: Sentrum | — | MG775907 | MG746790 | MG775797 | MG775714 |
| Xenocalamus bicolor | — | TGE T3 28 | SA: Northern Cape Province | — | MG775905 | MG746788 | MG775796 | MG775712 |
| Xenocalamus bicolor | — | TGE T3 29 | SA: Northern Cape Province | — | MG775908 | MG746791 | MG775798 | MG775715 |
| Xenocalamus bicolor | — | TGE T3 32 | SA: Northern Cape Province | — | MG775909 | MG746792 | — | — |
| Xenocalamus bicolor | — | TGE T4 14 | SA: Free State Province | — | MG775906 | MG746789 | — | MG775713 |
| Xenocalamus bicolor australis | PEM R22083 | — | SA: Northern Cape Province: Kimberly | MG746906 | MG775913 | MG746796 | MG775802 | — |
| Xenocalamus bicolor lineatus | — | 13321 | — | — | — | MG746797 | MG775803 | — |
| Xenocalamus bicolor machadoi | PEM R20771 | 666 | Angola: Moxico | MG746903 | MG775904 | — | — | MG775711 |
| Xenocalamus mechowii | PEM R23533 | WC 4654 | Angola: Moxico | MG746908 | — | — | — | — |
| Xenocalamus mechowii | PEM R23463 | WC 4695 | Angola: Cuando Cubango | MG746907 | — | — | — | — |
| Xenocalamus michelli | UTEP 21619 | ELI 209 | DRC: Haut-Lomami Province: Kyolo | MG746909 | MG775914 | MG746798 | MG775804 | MG775718 |
| Xenocalamus michelli | UTEP 21620 | ELI 355 | DRC: Tanganyika Province: near Manono airport | MG746910 | MG775915 | MG746799 | MG775805 | MG775719 |
| Xenocalamus transvaalensis | NMB R10888 | MBUR 01107 | SA: KwaZulu-Natal Province: Ndumo Game Reserve | MG746913 | — | MG746800 | — | MG775717 |
| Xenocalamus transvaalensis | — | FO57-51-51 | SA: KwaZulu-Natal Province: Maputaland | MG746911 | — | — | — | — |
| Xenocalamus transvaalensis | PEM R:TBA | — | SA: KwaZulu-Natal Province: Hluhluwe | MG746912 | — | — | — | — |
| Xenocalamus transvaalensis | PEM R12103 | — | SA: KwaZulu-Natal Province: Maputaland | AY611842 | FJ404344 | AY612025 | AY61193 | — |
2.2 Taxon sampling
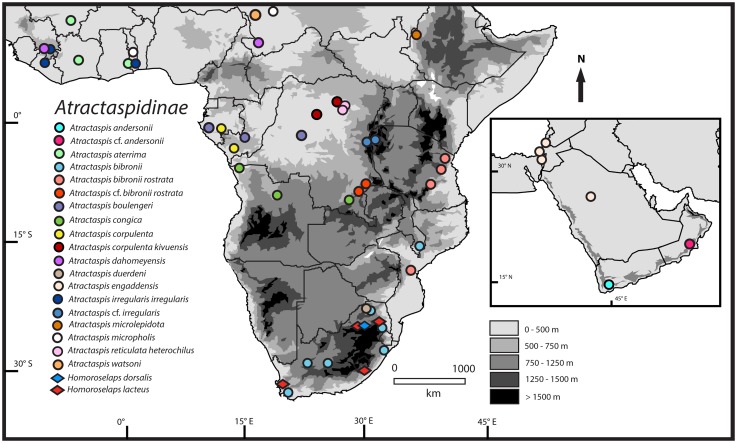
Specimens from the Subfamily Atractaspidinae were collected from multiple localities in sub-Saharan Africa (Fig 1). We generated sequences of three mitochondrial genes (16S, ND4, and cyt b) and two nuclear genes (c-mos and RAG1) for 91 atractaspidine individuals (Tables 1 and 2). This study included sequences from both atractaspidine genera (14/22 species of Atractaspis; 2/2 species of Homoroselaps) [24, 34]. Sequences from some of these individuals have been published previously [2, 7], and new sequences were deposited in GenBank (Table 1). Concatenated trees were rooted with Acrochordus granulatus (not shown on Fig 2). Three genera of Viperidae (Agkistrodon, Atheris, and Crotalus; not shown on Fig 2), two genera of Elapidae (Naja and Dendroaspis), six genera of Lamprophiinae (Boaedon, Bothrophthalmus, Bothrolycus, Gonionotophis, Lycodonomorphus, and Lycophidion), Psammophylax, and Micrelaps were used as outgroups for the concatenated analyses (Table 1, Fig 2). Additionally, we included sequences from six of the eight known aparallactine genera (6/9 species of Amblyodipsas; 7/11 species of Aparallactus; 1/2 species of Chilorhinophis; 1/1 species of Macrelaps; 7/14 species of Polemon; 4/5 species of Xenocalamus) [24, 35] for concatenated analyses and ancestral-state reconstructions. For divergence-dating analyses, additional samples from the squamate taxa Scincidae, Leptotyphlopidae, Viperidae, Colubrinae, and Dipsadinae were included (Table 1).
Fig 1. Map of sub-Saharan Africa and western Asia/Middle East, showing sampling localities for atractaspidines used in this study.
Table 2. Primers used for sequencing mitochondrial and nuclear genes.
| Gene Name | Primer Name | Primer Sequence ('5 to 3') | Primer Source |
|---|---|---|---|
| 16S | L2510 | CGCCTGTTTATCAAAAACAT | [110] |
| H3059 | CCGGTCTGAACTCAGATCACGT | ||
| L2510mod/16Sar | CCGACTGTTTAMCAAAAACA | [111] | |
| H3056mod/16Sbr | CTCCGGTCTGAACTCAGATCACGTRGG | ||
| ND4 | ND4 | CACCTATGACTACCAAAAGCTCATGTAGAAGC | [64, 112] |
| HIS1276 | TTCTATCACTTGGATTTGCACCA | ||
| cyt b | L14910 | GACCTGTGATMTGAAAAACCAYCGTTGT | [109, 113] |
| H16064 | CTTTGG TTTACAAGAACAATGCTTTA | ||
| c-mos | S77 | CATGGACTGGGATCAGTTATG | [114] |
| S78 | CCTTGGGTGTGATTTTCTCACCT | ||
| RAG1 | G396 (R13) | TCTGAATGGAAATTCAAGCTGTT | [115] |
| G397 (R18) | GATGCTGCCTCGGTCGGCCACCTTT |
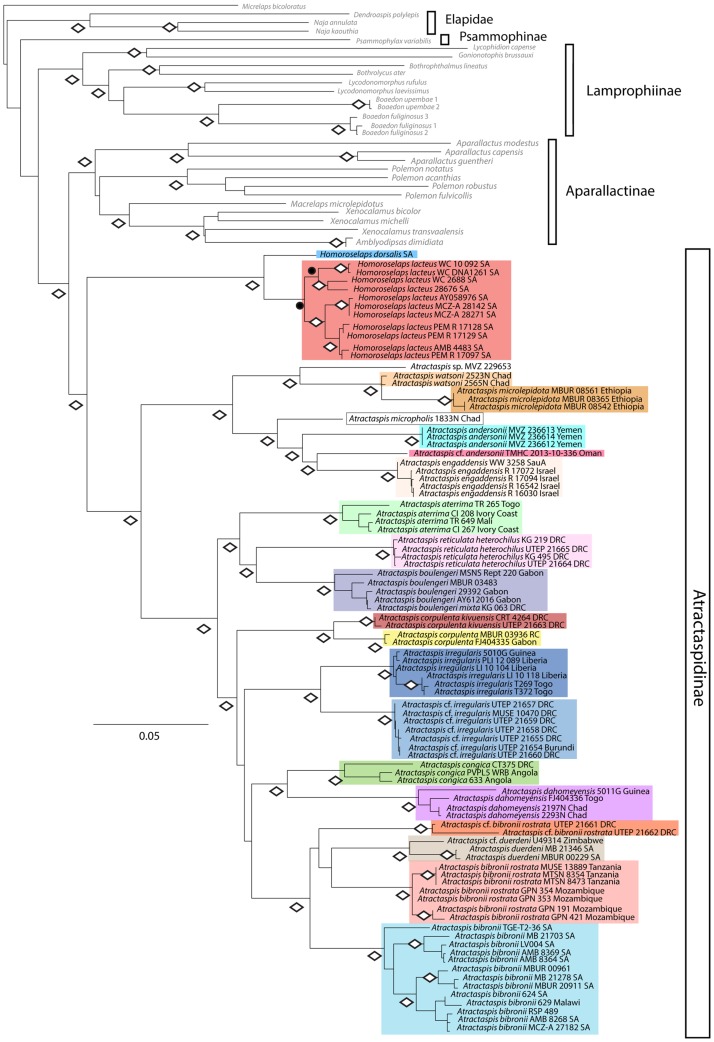
Fig 2. Maximum-likelihood phylogeny of Atractaspidinae with combined 16S, ND4, cyt b, c-mos, and RAG1 data sets.
Closed circles denote clades with Bayesian posterior probability values ≥ 0.95. Diamonds denote clades with strong support in both maximum likelihood analyses (values ≥ 70) and Bayesian analyses (posterior probability values ≥ 0.95).
2.3 Laboratory protocols
Genomic DNA was isolated from alcohol-preserved muscle or liver tissue samples with the Qiagen DNeasy tissue kit (Qiagen Inc., Valencia, CA, USA). Primers used herein are shown in Table 2. We used 25 μL PCR reactions with gene-specific primers with an initial denaturation step of 95°C for 2 min, followed by denaturation at 95°C for 35 seconds (s), annealing at 50°C for 35 s, and extension at 72°C for 95 s with 4 s added to the extension per cycle for 32 (mitochondrial genes) or 34 (nuclear gene) cycles. Amplification products were visualized on a 1.5% agarose gel stained with SYBR Safe DNA gel stain (Invitrogen Corporation, Carlsbad, CA, USA). Sequencing reactions were purified with CleanSeq magnetic bead solution (Agencourt Bioscience, La Jolla, CA) and sequenced with an ABI 3130xl automated sequencer at the University of Texas at El Paso (UTEP) Genomic Analysis Core Facility.
2.4 Sequence alignment and phylogenetic analyses
Phylogenetic analyses were conducted for our individual and five-gene concatenated data sets. Data were interpreted using the program SeqMan [36]. An initial alignment for each gene was produced in MUSCLE [37] in the program Mesquite v3.10 [38], and manual adjustments were made in MacClade v4.08 [39]. The Maximum Likelihood (ML) analyses of single gene and concatenated data sets were conducted using the GTRGAMMA model in RAxML v8.2.9 via the Cipres Science Gateway v3.3 [40]. All parameters were estimated, and a random starting tree was used. Support values for clades inferred by ML analyses were assessed with the rapid bootstrap algorithm with 1,000 replicates [40]. We also conducted Bayesian inference (BI) analyses with MrBayes v3.2.6 via the Cipres Science Gateway [40]. The model included 13 data partitions: independent partitions for each codon position of the protein-coding genes ND4, cyt b, c-mos, and RAG1, and a single partition for the mitochondrial gene 16S. Phylogenies were constructed based on concatenated data, which included 16S and the four protein-coding genes listed above. Concatenated data sets were partitioned identically for ML and BI analyses. The program PartitionFinder v1.1.1 [41–42] was used to find the model of evolution that was most consistent with our data for BI analyses. Bayesian analyses were conducted with random starting trees, run for 20,000,000 generations, and sampled every 1000 generations. Phylogenies were visualized using FigTree v1.3.1 [43].
2.5 Divergence dating
The program BEAST v1.8.3 via Cipres Science Gateway [40] was used to estimate divergence times across atractaspidine phylogenetic estimates. The five-gene data set was used to estimate divergence dates in BEAST. Substitution and clock models were unlinked for all partitions; trees were unlinked across the nuclear loci, but were linked for the two mitochondrial partitions because these evolve as a single unit. We implemented an uncorrelated log-normal relaxed clock model with a Yule tree prior. Two independent analyses were run for 100 million generations, sampling every 10,000 generations. Primary calibration points were obtained from Head et al. [44] and a secondary calibration point was obtained from Kelly et al. [7] including: the split between Scolecophidia and all other snakes (120–92 mya); split between Caenophidia and its nearest sister taxon, Booidea (72.1–66 mya); split between Colubroidea and its nearest sister taxon (Acrochordus + Xenodermatidae) (72.1–50.5 mya); the divergence of Colubridae + Elapoidea (30.9 ± 0.1 mya); and the split between Crotalinae and Viperinae (23.8–20.0 mya). All calibrations were constrained with a log-normal mean of 0.01, a normal standard deviation of 2.0 (first calibration point), and 1.0 (the last four calibration points). Parameter values of the samples from the posterior probabilities on the maximum clade credibility tree were summarized using the program TreeAnnotator v1.8.3 via Cipres Science Gateway [40].
2.6 Ancestral-state reconstructions
To understand the evolution of fang morphology and diet selection in atractaspidines, we reconstructed the pattern of character changes on the ML phylogeny herein. For ancestral-state reconstructions, we included all samples of aparallactines and atractaspidines available to us in order to better characterize fang and diet characters. All ancestral-state reconstructions were conducted by tracing characters over trees in Mesquite v3.10 [38]. We scored taxa using descriptions from the literature [25, 30–31, 45–55], and from our own data. We evaluated the following characters for fang morphology and diet selection: A. Fang morphology: (0) no fang, (1) rear fang, (2) fixed front fang, (3) moveable front fang, and (4) rear-front fang intermediate (anterior half of the maxilla, but not the anteriormost tooth); B. prey selection (0) rodents, (1) rodents, snakes, fossorial lizards, and amphibians, (2) snakes, (3) amphisbaenians, (4) snakes and fossorial lizards, (5) invertebrates, and (6) fish and amphibians. A ML approach was used for both analyses, because it accounts for and estimates probabilities of all possible character states at each node, thus providing an estimate of uncertainty [56]. A Markov K-state one-parameter model (Mk-1; [57]) that considers all changes as equally probable was implemented in our ancestral-state reconstructions. States were assigned to nodes if their probabilities exceeded a decision threshold; otherwise nodes were recovered as equivocal.
2.7 Morphology
Microcomputed tomography (CT) scans of specimens were produced using GE Phoenix V|Tome|X systems at the General Electric Sensing & Inspection Technologies in Scan Carlos, CA and University of Florida’s Nanoscale Research Facility. X-ray tube voltage and current, detector capture time, voxel resolution, and projection number were optimized for each specimen (S1 File). The radiographs were converted into tomograms with Phoenix Datos| R, and then rendered in three dimensions with volumetric rendering suite VGStudioMax 3.2 (http://www.volumegraphics.com). Tomogram stacks and 3D mesh files for all scans are available on Morphosource.org (S1 File).
3. Results
3.1 Concatenated gene tree analyses
Our data set consisted of 3933 base pairs (16S [546 bp], ND4 [679 bp], cyt b [1094 bp], c-mos [605 bp], and RAG1 [1009 bp]). Individuals with missing data were included in the concatenated sequence analyses, because placement of individuals that are missing a significant amount of sequence data can be inferred in a phylogeny, given an appropriate amount of informative characters [8, 58–60]. Furthermore, Jiang et al. [61] showed that excluding genes with missing data often decreases accuracy relative to including those same genes, and they found no evidence that missing data consistently bias branch length estimates.
The following models of nucleotide substitution were selected by PartitionFinder for BI analyses: 16S (GTR+G), ND4 1st codon position (GTR+G), ND4 2nd codon position (TVM+G), and ND4 3rd codon position (HKY+I+G); cyt b 1st codon position (TVM+G), cyt b 2nd codon position (HKY+I+G) and cyt b 3rd codon position (GTR+G); c-mos and RAG1 1st, 2nd and 3rd codon positions (HKY+I). Preferred topologies for the ML and BI analyses were identical, with similar, strong support values for most clades (Fig 2), and single-gene mtDNA analyses recovered similar topologies (not shown). The ML analysis likelihood score was –46340.867388. The relationships of Elapidae, Lamprophiinae, Micrelaps, and Psammophylax with respect to the ingroup Atractaspidinae, were not strongly supported in ML and BI analyses. However, Atractaspidinae was recovered in a strongly supported clade. Atractaspis and Homoroselaps were strongly supported as sister taxa (Fig 2). The genus Homoroselaps was recovered as a monophyletic group, and H. lacteus was partitioned into several well-supported clades. There were several strongly supported clades within Atractaspis: (1) Atractaspis andersonii, (2) Atractaspis aterrima, (3) A. bibronii, (4) A. bibronii rostrata, (5) A. cf. bibronii rostrata, (6) A. boulengeri, (7) A. congica, (8) A. corpulenta corpulenta, (9) A. corpulenta kivuensis, (10) A. dahomeyensis, (11) A. duerdeni, (12) A. engaddensis, (13) A. irregularis, (14) A. cf. irregularis, (15) A. reticulata heterochilus, and (16) A. microlepidota. There was strong support for a western Asia/Middle East and Africa clade containing A. andersonii, A. engaddensis, A. microlepidota, A. micropholis, A. watsoni, and A. sp. Atractaspis andersonii did not form a monophyletic group, because one of the samples from Oman (AF471127) was recovered as sister to a clade of A. engaddensis with strong support (Fig 2). The western African species A. aterrima was recovered with strong support as sister to a clade containing A. reticulata heterochilus and A. boulengeri. Atractaspis corpulenta kivuensis samples from eastern DRC were strongly supported as sister to A. corpulenta from northwestern Republic of Congo (near Gabon, the type locality). A well-supported clade of Atractaspis irregularis samples was partitioned by strongly supported central (A. cf. irregularis) and western African (A. irregularis) subclades. Atractaspis duerdeni was recovered within a well-supported A. bibronii complex. Atractaspis bibronii rostrata samples were partitioned into two highly divergent clades from southeastern DRC and Tanzania/Mozambique.
For the analyses including all atractaspidine and aparallactine samples available to us (Fig 3), preferred topologies for the ML and BI analyses were identical, with similar, strong support values for most clades (Fig 3). The ML analysis likelihood score was –73090.650849. The concatenated ML and BI analyses recovered similar topologies to those from Portillo et al. [62] and Fig 2.
Fig 3. Maximum-likelihood phylogeny of Atractaspidinae and Aparallactinae with combined 16S, ND4, cyt b, c-mos, and RAG1 data sets.
Diamonds denote clades with maximum likelihood values ≥ 70 and Bayesian posterior probability values ≥ 0.95; closed circles denote clades with Bayesian posterior probability values ≥ 0.95.
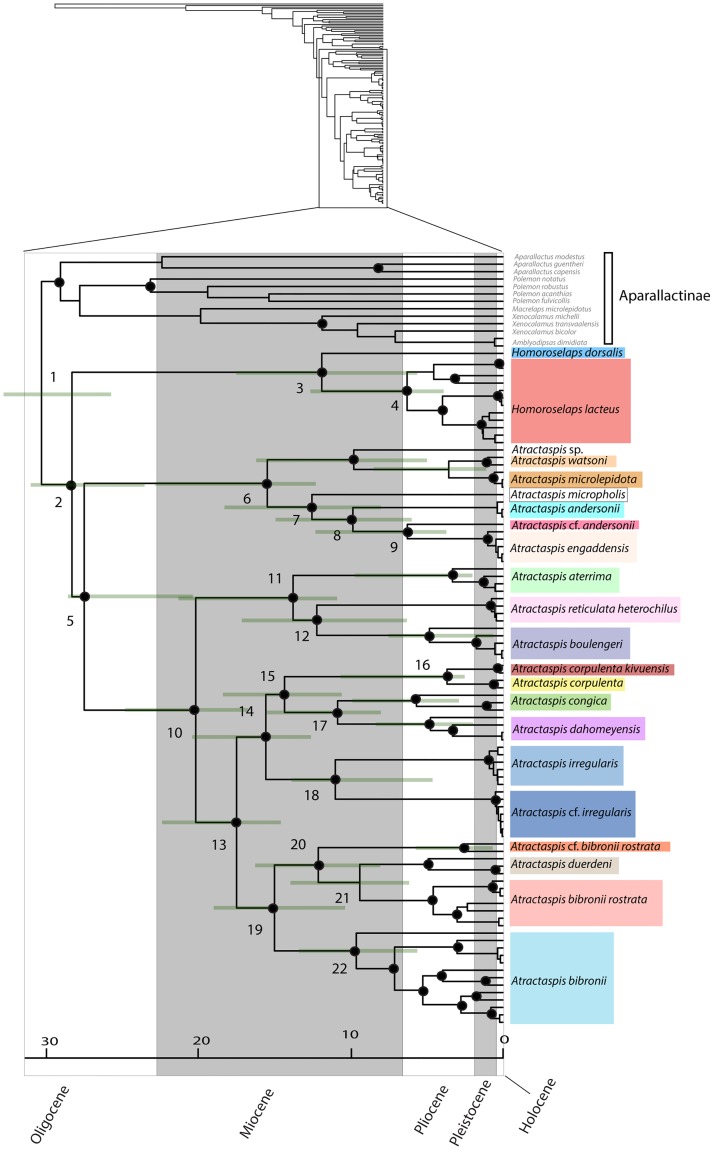
3.2 Divergence dating
Topologies from the BEAST (Fig 4) analyses were mostly consistent with the results from our concatenated tree analyses (Figs 2 and 3). BEAST results recovered A. corpulenta corpulenta/A. corpulenta kivuensis as sister to A. congica/A. dahomeyensis with strong support (Figs 2–4). Additionally, the relationship between Atractaspis irregularis and A. corpulenta/A. congica/A. dahomeyensis was strongly supported in BEAST analyses (Fig 4). Results from dating analyses suggested atractaspidines split from aparallactines during the early Oligocene around 29 mya (24.8–31.4 mya, 95% highest posterior densities [HPD]) (Table 3, Fig 4), which is similar to the results (34 mya) of Portillo et al. [62]. Subsequently, Atractaspis split from Homoroselaps in the mid-Oligocene, and most radiation events within each of the major clades associated with these genera occurred during the mid- to late Miocene and Pliocene (Fig 4). Specific dates with ranges are specified in Table 3.
Fig 4. Phylogeny resulting from BEAST, based on four calibration points.
Nodes with high support (posterior probability ≥ 0.95) are denoted by black circles. Median age estimates are provided along with error bars representing the 95% highest posterior densities (HPD) (Table 3).
Table 3. Estimated dates and 95% highest posterior densities (HPD) of main nodes.
Node labels correspond to those in Fig 4.
| Node | Event | Estimated age in mya (95% HPD) |
|---|---|---|
| 1 | Split between Aparallactinae and Atractaspidinae | 29.1 (24.8–31.4) |
| 2 | Split between Homoroselaps and Atractaspis | 27.2 (22.5–29.7) |
| 3 | Split between Homoroselaps dorsalis and H. lacteus | 11.4 (5.3–16.8) |
| 4 | Basal divergence of Homoroselaps lacteus | 6.0 (3.6–12.2) |
| 5 | Basal divergence of Atractaspis | 26.4 (19.6–27.4) |
| 6 | Split between A. watsoni/A. microlepidota/A. sp. and A. micropholis/A. andersonii/A. cf. andersonii/A. engaddensis | 14.8 (11.7–21.9) |
| 7 | Split between A. micropholis and A. cf. andersonii/A. engaddensis/A. andersonii | 12.1 (7.8–17.6) |
| 8 | Split between A. cf. andersonii/A. engaddensis and A. andersonii | 9.5 (5.7–14.4) |
| 9 | Split between A. cf. andersonii and A. engaddensis | 6.0 (3.6–11.7) |
| 10 | Split between A. aterrima/A. boulengeri/A. reticulata and the remainder of Atractaspis | 19.4 (16.1–23.7) |
| 11 | Split between A. aterrima and A. boulengeri/A. reticulata | 13.2 (10.5–20.4) |
| 12 | Split between A. boulengeri and A. reticulata | 11.7 (6.1–16.5) |
| 13 | Split between A. corpulenta/A. congica/A. dahomeyensis/A. irregularis and A. duerdeni/A. bibronii complex | 16.8 (14.1–21.5) |
| 14 | Split between A. corpulenta/A. congica/A. dahomeyensis and A. irregularis | 14.9 (12.1–19.6) |
| 15 | Split between A. corpulenta and A. dahomeyensis/A. congica | 13.8 (10.2–17.6) |
| 16 | Split between A. corpulenta corpulenta and A. corpulenta kivuensis | 3.6 (2.5–10.2) |
| 17 | Split between A. congica and A. dahomeyensis | 10.4 (7.6–14.8) |
| 18 | Split between A. irregularis irregularis and A. cf. irregularis | 10.5 (4.4–13.2) |
| 19 | Basal divergence of the A. bibronii complex | 14.4 (10.1–18.3) |
| 20 | Split between A. cf. bibronii rostrata and A. duerdeni/A. bibronii rostrata | 11.6 (7.6–15.7) |
| 21 | Split between A. bibronii rostrata and A. duerdeni | 9.0 (5.8–13.4) |
| 22 | Basal divergence of A. bibronii | 9.2 (5.6–12.9) |
3.3 Ancestral-state reconstructions
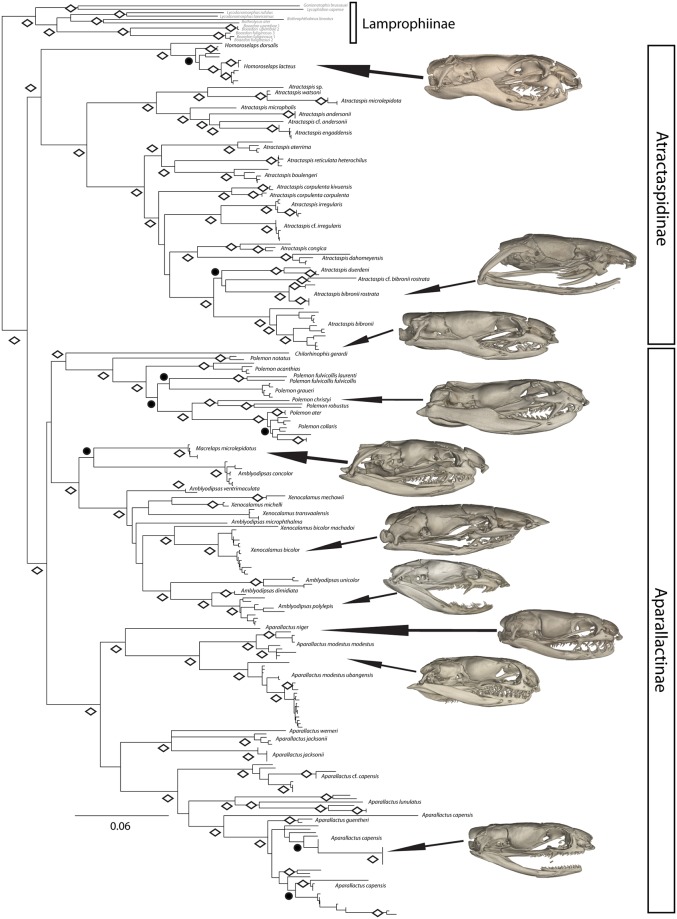
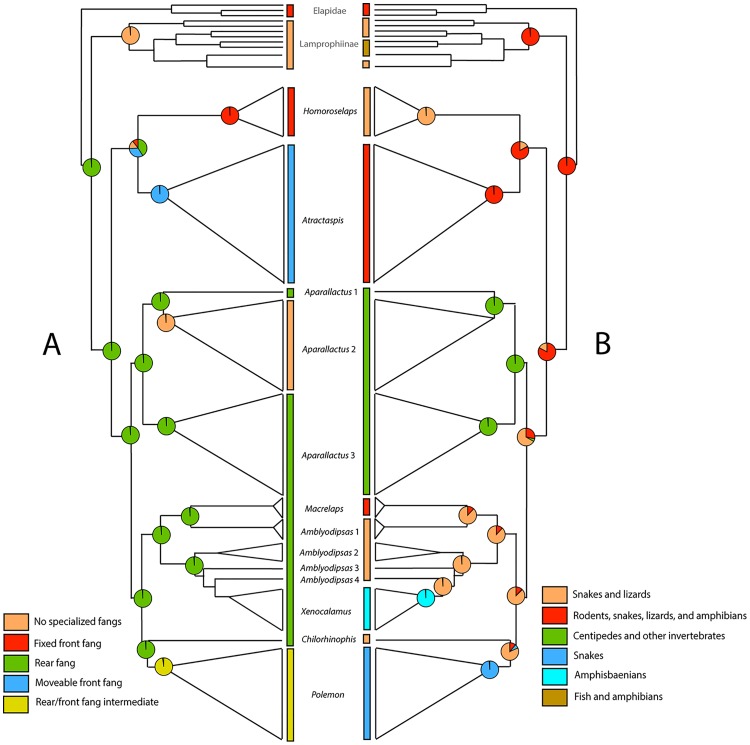
X-ray computer tomography of collared snakes and burrowing asps can be seen in Figs 3 and 5. Likelihood reconstructions of atractaspidine ancestral fang morphology inferred a rear fang condition for the ancestral condition of all lamprophiids (96.7%) (Fig 6[A]). Subsequently, the Subfamily Lamprophiinae lost a venom delivery fang condition. The common ancestor of aparallactines and atractaspidines was inferred to have a rear fang condition (97.8%). The analyses suggested a rear fang ancestor (72.5%) for the clade containing Homoroselaps and Atractaspis. The ancestor to Atractaspis was inferred to have a moveable front fang condition (97.4%). Results recovered a fixed front fang condition for the ancestor of all Homoroselaps (99.8%). The ancestor to all aparallactines was inferred to have a rear fang condition (99.6%), and this remained consistent throughout most aparallactine nodes with the exception of Polemon (rear/front fang intermediate, 97.8%) and Aparallactus modestus (no specialized fang, 99.7%).
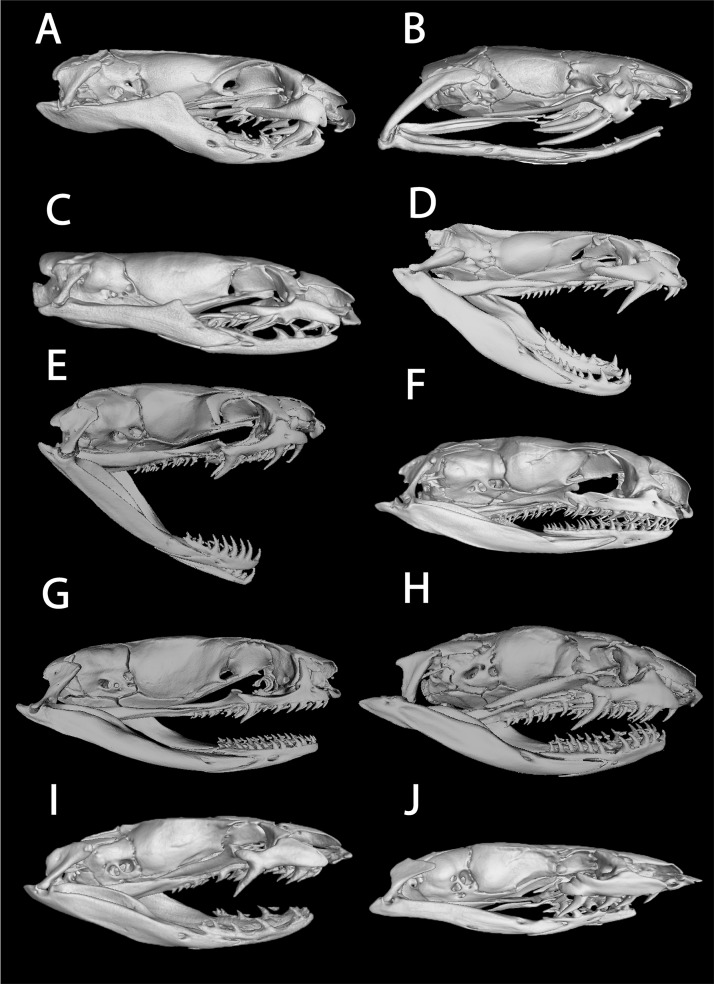
Fig 5. Computed tomography (CT) scans of aparallactine and atractaspidine genera.
Homoroselaps lacteus (CAS 173258) (A); Atractaspis bibronii (CAS 111670) (B); Chilorhinophis gerardi (CAS 159106) (C); Polemon christyi (CAS 147905) (D); Aparallactus niger (AMNH 142406) (E); Aparallactus modestus (CAS 111865) (F); Aparallactus capensis (G); Macrelaps microlepidotus (H); Amblyodipsas polylepis (CAS 173555) (I); Xenocalamus bicolor (CAS 248601) (J).
Fig 6. Ancestral-state reconstructions with ML optimization on the ML trees from the concatenated analyses shown in Fig 2.
(A) fang morphology, (B) dietary preference. Aparallactus 1 = A. niger; Aparallactus 2 = A. modestus; Aparallactus 3 = A. capensis, A. cf. capensis, A. guentheri, A. jacksonii, A. lunulatus, and A. werneri; Amblyodipsas 1 = A. concolor; Amblyodipsas 2 = A. dimidiata, A. polylepis, and A. unicolor; Amblyodipsas 3 = A. ventrimaculata; Amblyodipsas 4 = A. microphthalma.
For the analyses with diet data, likelihood reconstructions inferred a generalist diet of rodents, reptiles, and amphibians for the ancestral condition of all lamprophiids (99.7%) (Fig 6[B]). Several lamprophiines (Lycodonomorphus) subsequently adopted a more specialized diet of amphibians, reptiles, and fish. The common ancestor for aparallactines and atractaspidines was inferred to have a generalist diet of rodents, reptiles, and amphibians (92.4%). Results recovered a more specialized ancestral diet of snakes and lizards (64.5%) for aparallactines, which was favored over a generalist diet (27.7%). The condition of a snake and lizard diet (79.9%) was favored over a generalist diet (16.2%) for the ancestor of Polemon/Chilorhinophis and Amblyodipsas/Macrelaps/Xenocalamus. The latter dietary condition was retained for the ancestor of Polemon/Chilorhinophis (79.4%) and the ancestor of Amblyodipsas/Macrelaps/Xenocalamus (87.6%). Specialized dietary conditions were recovered for the genera Aparallactus (centipedes and other invertebrates, 99.7%), Polemon (snakes, 97.8%), and Xenocalamus (amphisbaenians, 98.8%). Results suggested a generalist diet for Atractaspidinae (92.3%). The ancestor of Homoroselaps was inferred to have a diet consisting of mostly lizards and snakes (99.9%), whereas the ancestor of Atractaspis was inferred to have a broader diet of rodents, reptiles, and amphibians (99.2%).
4. Discussion
4.1 Biogeography
Atractaspidines are distributed throughout sub-Saharan Africa except for three species of Atractaspis that are found in western Asia/Middle East (Atractaspis andersonii, A. engaddensis, and A. microlepidota) [25, 29–31]. Based on our results, the most likely scenario for Atractaspis is an African origin with a vicariance or dispersal event into the western Asia/Middle East region in the late Miocene (Fig 4). Atractaspis from western Asia/Middle East and Africa last shared a common ancestor during the late Miocene around 12.1 mya (7.8–17.6). Other studies of African-western Asian/Middle Eastern complexes (e.g., Echis and Uromastyx) recovered similar dates during the late Miocene, with the Red Sea proving to be a strong biogeographic barrier [63–69]. However, lineages of Varanus from Africa and the Middle East split from each other 6.9 mya [70], and African and Middle Eastern Bitis arietans last shared a common ancestor around 4 mya [64]. These dating estimates suggest that there were multiple dispersal events, which were taxon specific. Many Middle Eastern amphibians and reptiles have common ancestors in the Horn of Africa [63–71]. Our study lacked multiple Atractaspis species from the Horn of Africa, and future studies should include samples of A. fallax, A. magrettii, A. leucomelas, and A. scorteccii to improve understanding of likely Africa–Asia biogeographic patterns in atractaspidines.
Atractaspis began to diversify around the mid-Oligocene simultaneously with many aparallactine genera [62]. Many of the modern species split from recent common ancestors during the mid- to late Miocene (Table 3, Fig 4). The late Miocene was characterized by considerable xeric conditions, which led to the expansion of savannas globally [72–73]. Other studies on Central and East African herpetofauna, including squamates (Adolfus, Atheris, Boaedon, Naja, Kinyongia, and Panaspis) and frogs (Amietia, Leptopelis, and Ptychadena), have shown similar trends of species diversification during the late Miocene [3–5, 62, 74–78].
The diversification of several western and central African Atractaspis was most likely a consequence of increasingly xeric conditions during the Miocene, when forest and other moist habitats were fragmented [72]. These Atractaspis were likely isolated in fragmented patches of forest during the mid- to late Miocene. Atractaspis irregularis is partitioned clearly by western African and central African lineages that diverged in the mid-Miocene, similar to Aparallactus modestus [62]. At this time, southern African and Middle Eastern Atractaspis also diversified. Atractaspis from the Near and Middle East (A. andersonii, A. engaddensis, and A. microlepidota) and southern Africa (A. bibronii and A. duerdeni) are not tropical forest species, and they inhabit deserts or semi-desert savannas and dry woodland [30, 79–80]. This adaptation to more xeric and open habitats would have allowed Near and Middle Eastern, and southern African Atractaspis, to disperse into these habitats during the dry conditions of the mid- to late Miocene. Studies on mammals and birds show most diversification events during the Pliocene [81–84], which is consistent with the timing of diversification for Atractaspis aterrima, A. congica, A. dahomeyensis, and populations of South African A. bibronii (Fig 4).
In contrast to Aparallactus jacksonii, Atractaspis bibronii rostrata showed no clear genetic partitioning between populations in the Nguru, Usambara, and Udzungwa Mountains [62]. Aparallactus jacksonii clearly exhibited deep divergence between an extreme northern Tanzanian population, and a population from the Nguru Mountains. These two populations diverged from each other during the late Miocene, suggesting that the habitats of this taxon were fragmented with increased aridity [62]. Other vertebrate taxa that have shown substantial divergences between populations found in extreme northern Tanzania (Usambara, Taita, and Pare Mountains) and those slightly south (Uluguru, Ukaguru, Nguru, and Malundwe Mountains), include the reed frog Hyperolius puncticulatus, the green barbet (Stactolaema olivacea), and the streaky canary (Serinus striolatus) [82, 85]. But like Atractaspis bibronii rostrata, the hyperoliid reed frog Hyperolius spinigularis and the aparallactine Aparallactus guentheri showed no clear biogeographic patterns between populations in different areas of the Eastern Arc Mountains. These results support the hypothesis that the evolutionary history of species from the Eastern Arc Mountains is lineage specific [85]. Atractaspis bibronii rostrata inhabit low-elevation woodlands and grasslands, and transitional habitats, rather than montane forest (i.e., Aparallactus jacksonii) [25]. This would allow taxa such as Atractaspis bibronii rostrata to continuously disperse between the different mountains of the Eastern Arcs, despite increased aridity. Additionally, ecological niche requirements may also explain the different biogeographic patterns seen in Aparallactus jacksonii and Atractaspis bibronii rostrata. Atractaspis bibronii has a generalist diet (mammals, squamates, and amphibians) and could have exploited more habitats than Aparallactus jacksonii, which is a centipede specialist [25].
4.2 Evolutionary relationships and taxonomy of Atractaspidinae
Our results indicate that both Atractaspis and Homoroselaps are strongly supported as monophyletic sister taxa. Results from Figueroa et al. [27] recovered a monophyletic group containing aparallactines and atractaspidines, but their results did not recover a monophyletic Atractaspis (A. irregularis was recovered as sister to aparallactines + atractaspidines). This sample was excluded from our analyses, because the only sequence available for this taxon was from BDNF, a gene not used herein. The results from Figueroa et al. [27] may be an artifact of sample size of atractaspidines, or incomplete lineage sorting of the BDNF nuclear gene. Results from our study indicate that A. irregularis is a monophyletic lineage within a strongly supported, monophyletic Atractaspis.
Underwood and Kochva [18] recognized two groups within Atractaspis: (1) the ‘bibronii’ group (represented in our study by A. aterrima, A. bibronii, A. boulengeri, A. congica, A. corpulenta, A. dahomeyensis, A. irregularis, and A. reticulata), characterized by a single posterior supralabial, three anterior infralabials, normal-sized venom glands, and a sub-Saharan distribution; and (2) the ‘microlepidota’ group (represented in our study by A. andersonii, A. engaddensis, A. microlepidota, and A. micropholis), characterized by two anterior temporals, highly elongated venom glands, and a North African/Near and Middle Eastern distribution. Whereas our study did not include genetic samples of all known species of Atractaspis, results herein (Fig 2) support partitioning of the genus into two groups sensu Underwood and Kochva [18]. Our results indicated a clear partition between a ‘Middle Eastern + African’ clade (including A. watsoni, a species that was not included by Underwood and Kochva [18]) and a ‘sub-Saharan African’ clade (Figs 2 and 4). These results strengthen the notion that venom gland size and length in Atractaspis are homologous. Our support for the ‘microlepidota’ group is consistent with the “Section A” (A. andersonii, A. fallax, A. leucomelas, A. microlepidota, and A. micropholis) of Laurent [28] and the A. micropholis/A. microlepidota/A. watsoni clade recovered by Moyer and Jackson [10]. However, our phylogeny (Fig 2) contrasts with the remaining “sections” of Laurent [28], most relationships depicted in the morphological phylogeny of Moyer and Jackson [10], and the molecular phylogenies of Pyron et al. [8–9] and Vidal et al. [22].
Based on relatively long branch lengths, several lineages of Atractaspis seem to be cryptic complexes of species. Because of the extensive geographic distribution of A. bibronii in central, eastern and southern Africa, it is unsurprising to find several highly divergent lineages that likely represent cryptic species. Given the proximity (ca. 167–333 km) of our Tanzanian localities of A. bibronii rostrata (Nguru, Usambara, and Udzungwa Mountains) to the insular type locality for this taxon (Zanzibar, Tanzania), the morphological similarity between our voucher specimens and the types [86], and the relatively long branch length and reciprocal monophyly of this clade compared to topotypic South African A. bibronii (Fig 2), it is likely that the former taxon is a valid species. However, additional comparisons to type specimens are needed to clarify the taxonomic status of populations in this clade, including samples from Haut-Katanga Province in southeastern DRC.
Our phylogenetic results indicated that several other species, including A. andersonii, A. boulengeri, A. congica, A. corpulenta, A. dahomeyensis, and A. irregularis likely represent more than a single species. For example, topotypic Angolan samples of A. congica are deeply divergent from our eastern DRC sample (Fig 2), which is likely attributable to A. congica orientalis [46]. Like Polemon fulvicollis fulvicollis (Gabon) and P. fulvicollis laurenti (DRC) [62], Gabonese Atractaspis corpulenta and eastern DRC populations of A. corpulenta kivuensis also showed marked genetic divergences between each other (Fig 2). The well-supported clade of A. irregularis from western Africa likely includes topotypic populations, because they straddle the type locality (Accra, Ghana) [87], whereas our Albertine Rift samples are likely attributable to one of the taxon’s many synonyms. One of these, Atractaspis bipostocularis from Mt. Kenya, was named for its two postocular scales, which distinguishes it from the single postocular of topotypic A. irregularis [88]. Because Mt. Kenya is located east of the Kenyan Rift, a major biogeographic barrier to several species of squamates [78], and moreover, all voucher specimens of A. cf. irregularis from the Albertine Rift have a single postocular (EG pers. obs.), A. bipostocularis is likely a distinct species that is endemic to the central Kenyan highlands. Other synonyms of A. irregularis that have one postocular and type localities in or near the Albertine Rift are likely attributable to our well-supported clade of A. cf. irregularis (Fig 2 in [87]), and include the following taxa: A. conradsi Sternfeld, 1908 (type locality: Ukerewe Island, Lake Victoria, Tanzania [89]), A. schoutedeni de Witte, 1930 (type locality: Goma, North Kivu, DRC [90]), A. babaulti Angel, 1934 (type locality: Kadjuju [1500 m elevation] on the western border of Lake Kivu, 15 km north of Katana, DRC [91]), and A. irregularis loveridgei Laurent, 1945 (type locality: Bunia, DRC [46]). Additional sampling and morphological analyses are in progress that will help clarify the correct taxonomy for these lineages. Because of the relative lack of fieldwork in Central Africa in recent decades [92–93] and the relatively rare encounters of these snakes above ground (EG, pers. obs.), it is likely that genetic samples from the above topotypic populations will remain elusive for many years.
4.3 Evolution of dietary preference and fang morphology
Burrowing asps and collared snakes have unique ecologies, particularly in terms of dietary preferences. Atractaspis in particular have very distinctive fangs (solenoglyphous fangs, similar to viperids) that have made their taxonomic history complicated (e.g., previously classified as viperids) [25, 31, 94]. The fangs of Homoroselaps resemble fangs of elapids more than vipers. In contrast, aparallactines tend to have rear fangs (Figs 3 and 6) [18, 25, 29–30]. Our ancestral-state reconstruction analysis of fang morphology suggested a rear fang ancestor for all collared snakes and burrowing asps (Aparallactinae and Atractaspidinae). Most lamprophiids are either rear fanged or lack fangs [25]. Our analyses also recovered dietary generalization as an ancestral-state for atractaspidines and aparallactines. Both of these conditions support the hypothesis proposed by Underwood and Kochva [18], which postulated that collared snakes and burrowing asps likely had a Macrelaps-like ancestor (large and rear fanged) that foraged above ground or in burrows of other organisms, and these taxa subsequently evolved into more specialized forms with specialized diets. Several aparallactines are dietary specialists [25, 31], that feed on the following: Aparallactus specialize on centipedes and possibly other invertebrates like earthworms; Chilorhinophis and Amblyodipsas consume snakes and other small, fossorial reptiles; Polemon are ophiophagous [25, 31, 95], but may occasionally consume other squamate prey items; Macrelaps consume reptiles, amphibians, and rarely mammals [25]; and Xenocalamus consume amphisbaenians [25, 31].
Unlike several aparallactines, Atractaspis are dietary generalists that consume a diverse variety of squamates, rodents (particularly nestling rodents), and occasionally amphibians [25, 31, 33, 52, 96–100]. The venom glands of Atractaspis are anatomically distinct from those of other front-fanged snakes such as viperids and elapids, because atractaspidines lack a distinct accessory gland and the presence of mucous-secreting cells at the end of each serous tubule [32, 101–103]. Similar to two other front-fanged snake groups (Elapidae and Viperidae), elongated venom glands have evolved within Atractaspis from western and northern African, and western Asia/Middle East species. These glands may be up to 12 cm long in A. engaddensis and 30 cm long in A. microlepidota [32]. Phylogenetically, Atractaspis is clearly partitioned according to venom gland length and geographic distribution (Figs 1 and 2). The purpose of these anatomical adaptations are unclear, although it is possible that they evolved to influence venom yield, as in Calliophis bivirgatus (Elapidae) [32]. The unique viper-like front fangs of Atractaspis may have evolved to facilitate the predation of rodent nestlings or squamates in tight burrows. Preying on animals in tight burrows limits mobility of the predator, because the body of the prey item can serve as a physical barrier, stopping the predator from further pursuit. Many lizards can detach their tails if a predator grabs the tails from behind. Shine et al. [31] postulated that it would be advantageous for a predator to push past the tail and envenomate or seize the prey by the body, a scenario ideal for Atractaspis. Deufel and Cundall [33] hypothesized that the evolution of the front fang in Atractaspis was likely the result of the following advantages: (1) greater envenomation efficiency resulting from the longer fangs; (2) closed mouth venom delivery system, allowing envenomation during head contact with any part of the prey; (3) capacity to quickly envenomate and release prey; and (4) potential for effective defense against adult rodents. Most prey consumed by Atractaspis (amphisbaenians, fossorial skinks, typhlopid snakes) [25] are also consumed by other atractaspidines and aparallactines, including Amblyodipsas, Chilorhinophis, Homoroselaps, Macrelaps, Polemon, and Xenocalamus [25, 31, 97]. These observations suggest that squamate prey are consumed across all atractaspidine and aparallactine genera, and therefore, they may not be the only selective force driving the evolution of the unique fang in Atractaspis. However, rodents and other mammals are not commonly preyed on by other burrowing asps and collared snakes [31, 104]. Deufel and Cundall [33] stated that it is unlikely that mammalian prey alone drove the evolution of a moveable front fang in Atractaspis, but the success and wide distribution of this genus may be partially attributed to mammalian prey. Unlike aparallactines, Atractaspis can quickly envenomate and dispatch all rodents in a nest [33]. A rear fang condition would require the snake to bite, hold and chew on every prey item, which is undoubtedly a more energetically costly form of envenomation compared with the predatory behavior of Atractaspis. Interestingly, in a feeding experiment, Atractaspis never attempted to ingest snake prey until the prey stopped reacting to fang pricks [33]. This observation suggests that Atractaspis will not risk injury until prey are completely immobilized. The unique fang and predatory behavior of Atractaspis has its functional trade-offs; Atractaspis lack large mandibular and maxillary teeth that allow snakes to quickly consume prey [33], and therefore, they take longer to ingest prey items. Because Atractaspis forage, kill, and consume prey in the soil and below the surface, there were likely no negative selective pressures acting against slow ingestion of prey. Because they are fossorial, Atractaspis may be relatively safe from predators while feeding, which is when non-fossorial snakes may be vulnerable to predation or attacks from other animals [25, 33].
Results from this study indicate that the rear-fang condition can cover a wide variety of dietary specializations. But this condition is not ubiquitous among aparallactines. Aparallactus modestus clearly lacks enlarged fangs (Figs 5 and 6), but previous studies have found venom glands in this taxon [105]. Additionally, the venom gland of A. modestus is reported to differ from the venom gland of A. capensis, but further details of the discrepancies were not discussed [32, 105, 106]. Interestingly, this species may prey on earthworms rather than centipedes (II pers. obs. [30]), explaining the loss of a rear-fang condition, which is present in all other Aparallactus species used for this study, including A. niger, the sister species to A. modestus (Figs 5 and 6).
Polemon fangs are not easily classified. The fangs of Polemon are located on the anterior half of the maxilla, rather than the more typical posterior end (Figs 5 and 6). These fangs are large and deeply grooved, and resemble a fixed front-fang condition, but yet they are positioned behind one or two smaller maxillary teeth. The ophiophagous diet of Polemon likely influenced the evolution of a front-fang condition in this genus. Polemon are known to prey on large and formidable snake prey, which can rival the predator in size [35, 48, 95, 107]. With large, deeply grooved fangs positioned on the anterior side of the maxilla, Polemon can quickly envenomate and kill relatively large and powerful prey (snakes) more effectively than they would with a rear-fang condition like Aparallactus. Snakes with rear fangs must typically chew in a forward orientation until the rear fang can penetrate the flesh of the prey item [25]. Several front-fanged, elapid genera prey heavily on snakes (e.g., Micrurus and Ophiophagus). The front-fang condition may be a favorable trait to feed on snakes, in order to immobilize and kill more quickly.
In Xenocalamus, similar selective pressures (e.g., tight burrow foraging) that led to the evolution of fang and predatory behaviors in Atractaspis, may have led to the evolution of its unique quill-shaped snout [31]. Unlike Amblyodipsas polylepis, Xenocalamus possess relatively large maxillary teeth that gradually increase in size from the anterior to posterior side of the maxilla (Figs 3 and 5). This trait seems advantageous to improve their grasp of amphisbaenian prey.
It is not surprising that the rear fang and dietary generalist conditions were recovered as the ancestral-state condition for both atractaspidines and aparallactines, considering many lamprophiids are dietary generalists [25, 30]. Collared snakes and burrowing asps seem to have experienced the opposite of niche conservatism as results herein indicated that foraging behaviors and diet have heavily and rapidly influenced the evolution of fang morphology, dietary specializations, and snout shape. In collared snakes (aparallactines), dietary specializations seem to have shaped variation (and loss) of fangs and snout shape, particularly for Aparallactus, Polemon, and Xenocalamus. These genera tend to have more specialized diets than Macrelaps, Chilorhinophis and Amblyodipsas, all of which possess more typical rear fangs (Figs 3 and 5) [25, 30–31]. A fundamental controversy in snake evolution is whether front and rear fangs share the same evolutionary and developmental origin. Burrowing asps and collared snakes possess all known types of snake dentition (no fang, rear fang, fixed front fang, and moveable front fang). Our results lend credence to the hypothesis that rear fangs and front fangs share a common origin [94]. Our results also indicated that snake dentition, specifically alethinophidian groups such as atractaspidines and aparallactines, may be highly plastic within relatively short periods of time to facilitate foraging and life history strategies.
Supporting information
(XLSX)
Acknowledgments
Fieldwork by the last author in DRC was funded by the Percy Sladen Memorial Fund, an IUCN/SSC Amphibian Specialist Group Seed Grant, K. Reed, M.D., research funds from the Department of Biology at Villanova University, a National Geographic Research and Exploration Grant (no. 8556–08), UTEP, and the US National Science Foundation (DEB-1145459); EG, CK, WMM, and MMA thank their field companions M. Zigabe, A. M. Marcel, M. Luhumyo, J. and F. Akuku, F. I. Alonda, and the late A. M’Mema. We are grateful to F. B. Murutsi, former Chief Warden of the Itombwe Natural Reserve, for logistical support and permission for fieldwork in 2011; the Centre de Recherche en Sciences Naturelles and Institut Congolais pour la Conservation de la Nature provided project support and permits. We thank the Uganda Wildlife Authority of Kampala for necessary permits to work in Uganda, and Léonidas Nzigiyimpa of the Institut National pour l’Environnement et la Conservation de la Nature (INECN) of Burundi for logistical support and permit negotiations. Permits for samples from Gabon were granted by the Direction de la Faune et de la Chasse and CENAREST, Libreville. WC thanks National Geographic Okavango Wilderness Project (National Geographic Society grant number EC0715–15) for funding field work to Angola; Jan Venter, ex Eastern Cape Parks and Tourism Agency for fieldwork in the Wild Coast of South Africa, and Department of Economic Development, Environmental Affairs and Tourism (permit nos. CRO 84/11CR and CRO 85/11CR). MOR and JP thank all the respective West African institutions for collection and export permits; MOR is likewise grateful to the Gorongosa Restoration Project and the Mozambican Departamento dos Serviços Cientificos (PNG/DSCi/C12/2013; PNG/DSCi/C12/2014; PNG/DSCi/C28/2015) for support and permits. The fieldwork of ZTN in DRC was supported by the Belgian National Focal Point to the Global Taxonomy Initiative. Fieldwork in the Republic of Congo was part of a rapid biodiversity initiative, commissioned by Flora Fauna & Man, Ecological Services Ltd (FFMES). Jerome Gaugris of FFMES conducted the study organization and design. Permits were issued by the Groupe d’Etude et de Recherche sur la Diversité Biologique. We thank S. Meiri, E. Maza, J. Smid, H. Farooq, W. Wüster, J. R. Nicolau, R. Deans, L. Kemp, L. Verbugt, South African National Biodiversity Institute (SANBI), Steinhardt Museum, Museum of Vertebrate Zoology, University of California, Berkeley, and Museum of Comparative Zoology, Harvard University, for tissues. We acknowledge A. Betancourt of the UTEP Border Biomedical Research Center Genomic Analysis Core Facility for services and facilities provided. This core facility is supported by grant 5G12MD007592 to the Border Biomedical Research Center (BBRC) from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of NIMHD or NIH. Dr. William R. Branch passed away before the submission of the final version of this manuscript. Dr. Eli Greenbaum accepts responsibility for the integrity and validity of the data collected and analyzed.
Data Availability
The data included in this paper can be found on GenBank and Morphosource websites (access information is contained within the paper).
Funding Statement
This work was supported by the Percy Sladen Memorial Fund, an IUCN/SSC Amphibian Specialist Group Seed Grant, K. Reed, M.D., research funds from the Department of Biology at Villanova University, and UTEP (all to EG), National Science Foundation grant DEB-1145459 (to EG and KJ), National Geographic Research and Exploration Grant no. 8556-08 (to EG), National Geographic Okavango Wilderness Project no. EC0715-15 (to WC), Belgian National Focal Point to the Global Taxonomy Initiative (to ZTN). MOR is supported by the Gorongosa Restoration Project and the Mozambican Departamento dos Serviços Cientificos (PNG/DSCi/C12/2013; PNG/DSCi/C12/2014; PNG/DSCi/C28/2015). The University of Texas at El Paso (UTEP) Border Biomedical Research Center (BBRC) Genomic Analysis Core Facility is acknowledged for services and facilities provided. This core facility is supported by grant 5G12MD007592 to the (BBRC) from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH). MFB received payment as a herpetologist consultant with Flora Fauna & Man, Ecological Services Ltd. (FFMES). The funder provided support in the form of salaries for one author [MFB], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wüster W, Crookes S, Ineich I, Mané Y, Pook CE, Trape J-F, et al. The phylogeny of cobras inferred from mitochondrial DNA sequences: Evolution of venom spitting and the phylogeography of the African spitting cobras (Serpentes: Elapidae: Naja nigricollis complex). Mol. Phylogenet. Evol., 2007; 45: 437–453. 10.1016/j.ympev.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 2.Kelly CMR, Branch WR, Broadley DG, Barker NP, Villet MH. Molecular systematics of the African snake family Lamprophiidae Fitzinger, 1843 (Serpentes: Elapoidea), with particular focus on the genera Lamprophis Fitzinger 1843 and Mehelya Csiki 1903. Mol. Phylogenet. Evol., 2011; 58: 415–426. 10.1016/j.ympev.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 3.Menegon M, Loader SP, Marsden SJ, Branch WR, Davenport TRB, Ursenbacher S. The genus Atheris (Serpentes: Viperidae) in East Africa: Phylogeny and the role of rifting and climate in shaping the current pattern of species diversity. Mol. Phylogenet. Evol., 2014; 79: 12–22. 10.1016/j.ympev.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 4.Greenbaum E, Portillo F, Jackson K, Kusamba C. A phylogeny of Central African Boaedon (Serpentes: Lamprophiidae), with the description of a new cryptic species from the Albertine Rift. Afr. J. Herpetol., 2015; 64: 18–38. 10.1080/21564574.2014.996189 [DOI] [Google Scholar]
- 5.Wüster W, Chirio L, Trape J-F, Ineich I, Jackson K, Greenbaum E, et al. Integration of nuclear and mitochondrial gene sequences and morphology reveal unexpected diversity in the forest cobra (Naja melanoleuca) species complex in Central and West Africa (Serpentes: Elapidae). Zootaxa, 2018; 4455: 68–98. 10.11646/zootaxa.4455.1.3 [DOI] [PubMed] [Google Scholar]
- 6.Engelbrecht HM, Branch WR, Greenbaum E, Alexander GJ, Jackson K, Burger M, et al. Diversifying into the branches: Species boundaries in African green and bush snakes, Philothamnus (Serpentes: Colubridae). Mol. Phylogenet. Evol., 130: 357–365. 10.1016/j.ympev.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 7.Kelly CMR, Barker NP, Villet MH, Broadley DG. Phylogeny, biogeography and classification of the snake superfamily Elapoidea: A rapid radiation in the late Eocene. Cladistics, 2009; 25: 38–63. 10.1111/j.1096-0031.2008.00237.x [DOI] [PubMed] [Google Scholar]
- 8.Pyron RA, Burbrink FT, Colli GR, De Oca ANM, Vitt LJ, Kuczynski CA, et al. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol. Phylogenet. Evol., 2011; 58: 329–342. 10.1016/j.ympev.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Pyron RA, Burbrink FT, Wiens JJ. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol., 2013; 13: 93 10.1186/1471-2148-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moyer K, Jackson K. Phylogenetic relationships among the Stiletto Snakes (genus Atractaspis) based on external morphology. Afr. J. Herpetol., 2011; 60: 30–46. 10.1080/21564574.2010.520034 [DOI] [Google Scholar]
- 11.Günther ACLG. Catalogue of the Colubrine Snakes in the Collection of the British Museum. Trustees of the British Museum, London; 1858. [Google Scholar]
- 12.Vitt L. Caldwell JP. Herpetology: An Introductory Biology of Amphibians and Reptiles. 3rd Edition Academic Press, USA; 2009. [Google Scholar]
- 13.Bourgeois M. Contribution à la morphologie comparée du crâne des Ophidiens de l’Afrique Centrale. Publ. Univ. Off. Congo, 1986; XVIII: 1–293. [Google Scholar]
- 14.Heymans JC. La musculature mandibulaire et le groupe parotidien des Aparallactinae et Atractaspinae (Serpentes Colubridae) à majorité fouisseurs. Rev. Zool. Afr., 1975; 89: 889–905. [Google Scholar]
- 15.Heymans JC. Contribution a la phylogenèse des ophidiens de l’Afrique centrale. Ann. Soc. Royale Zool. Belgique, 1982; 112: 79–87. [Google Scholar]
- 16.Kelly CMR, Barker NP, Villet MR. Phylogenetics of advanced snakes (Caenophidia) based on four mitochondrial genes. Syst. Biol., 2003; 52: 439–459. 10.1080/10635150309313 [DOI] [PubMed] [Google Scholar]
- 17.McDowell SB. 1968. Affinities of the snakes usually called Elaps lacteus and E. dorsalis. J. Linn. Soc., Zoology, 1968; 47: 561–578. 10.1111/j.1096-3642.1968.tb00550h.x [DOI] [Google Scholar]
- 18.Underwood G, Kochva E. On the affinities of the burrowing asps Atractaspis (Serpentes: Atractaspididae). Zool. J. Linn. Soc., 1993; 107: 3–64. 10.1006/zjls.1993.1002 [DOI] [Google Scholar]
- 19.Vidal N, Hedges SB. Higher-level relationships of caenophidian snakes inferred from four nuclear and mitochondrial genes. C. R. Biol., 2002; 325: 987–995. 10.1016/S1631-0691(02)01509-3 [DOI] [PubMed] [Google Scholar]
- 20.Branch WR. Snakes of Angola: An annotated checklist. Amphib. Reptile Conserv., 2018; 12: 41–82. [Google Scholar]
- 21.Vidal N, Delmas AS, David P, Cruaud C, Couloux A, Hedges SB. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. C. R. Biol., 2007; 330: 182–187. 10.1016/j.crvi.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 22.Vidal N, Branch WR, Pauwels OSG, Hedges SB, Broadley DG, Wink M, et al. Dissecting the major African snake radiation: A molecular phylogeny of the Lamprophiidae Fitzinger (Serpentes, Caenophidia). Zootaxa, 2008; 1945: 51–66. 10.1016/j.crvi.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 23.Vidal N, Hedges SB. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. C. R. Biol., 2009; 332: 129–139. 10.1016/j.crvi.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 24.Uetz, P, Freed P, Hošek J (eds.). The Reptile Database, http://www.reptile-database.org, [accessed March 2019]; 2019.
- 25.Greene HW. Snakes: The Evolution of Mystery in Nature. University of California Press, Berkeley; 1997. [Google Scholar]
- 26.Nagy ZT, Vidal N, Vences M, Branch WR, Pauwels OSG, Wink M, et al. Molecular systematics of African Colubroidea (Squamata: Serpentes) In Huber B.A., Sinclair B.J., and Lampe K.-H. (Eds.), African Biodiversity: Molecules, Organisms, Ecosystems. Proceedings of the 5th International Symposium in Tropical Biology; Museum Koenig, Bonn; 2005. Pp. 221–228. [Google Scholar]
- 27.Figueroa A, McKelvy AD, Grismer LL, Bell CD, Lailvaux SP. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLoS ONE, 2016; 11: e0161070 10.1371/journal.pone.0161070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent RF. Révision du genre Atractaspis A. Smith. Mém. Mus. R. Hist. Nat. Belgique, 1950; 38: 1–49. [Google Scholar]
- 29.Branch WR. A Field Guide to the Snakes and Other Reptiles of Southern Africa, revised edition Struik Publishing, South Africa; 1998. [Google Scholar]
- 30.Marais J. A Complete Guide to the Snakes of Southern Africa. Struik Publishers, Cape Town; 2004. [Google Scholar]
- 31.Shine R, Branch WR, Harlow PS, Webb JK, Shine T. Biology of burrowing asps (Atractaspididae) from Southern Africa. Copeia, 2006; 2006: 103–115. 10.1643/0045-8511(2006)006[0103:BOBAAF]2.0.CO;2 [DOI] [Google Scholar]
- 32.Jackson TN, Young B, Underwood G, McCarthy CJ, Kochva E, Vidal N, et al. Endless forms most beautiful: The evolution of ophidian oral glands, including the venom system, and the use of appropriate terminology for homologous structures. Zoomorphology, 2017; 136: 1–24. 10.1007/s00435-016-0332-9 [DOI] [Google Scholar]
- 33.Deufel A, Cundall D. Feeding in Atractaspis (Serpentes: Atractaspididae): A study in conflicting functional constraints. Zoology, 2003; 106: 43–61. 10.1078/0944-2006-00088 [DOI] [PubMed] [Google Scholar]
- 34.Rödel M-O, Kucharzewski C, Mahlow K, Chirio L, Pauwels OSG, Carlino P, et al. A new stiletto snake (Lamprophiidae, Atractaspidinae, Atractaspis) from Liberia and Guinea, West Africa. Zoosyst. Evol., 2019; 95: 107–123. [Google Scholar]
- 35.Portillo F, Branch WR, Tilbury CR, Nagy ZT, Hughes DF, Kusamba C, et al. A cryptic new species of Polemon (Squamata: Lamprophiidae, Aparallactinae) from the miombo woodlands of Central and East Africa. Copeia, 2019; 107: 22–35. 10.1643/CH-18-098 [DOI] [Google Scholar]
- 36.Swindell SR, Plasterer TN. SEQMAN: Contig assembly. Method. Mol. Biol., 1997; 70: 75–89. 10.1385/0-89603-358-9:75 [DOI] [PubMed] [Google Scholar]
- 37.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res., 2004; 32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddison, WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. Version 3.2 http://mesquiteproject.org; 2017.
- 39.Maddison DR, Maddison WP. MacClade: Analysis of Phylogeny and Character Evolution. Sinauer Associates Inc, USA; 2005. [DOI] [PubMed] [Google Scholar]
- 40.Miller, MA, Pfeiffer W, Schwartz T. The CIPRES Science Gateway, Version 3.3 https://www.phylo.org; 2017.
- 41.Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol., 2012; 29: 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- 42.Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A. Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol. Biol., 2014; 14: 82 10.1186/1471-2148-14-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rambaut A, Drummond A. FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, UK; 2010. [Google Scholar]
- 44.Head JJ, Mahlow K, Müller J. Fossil calibration dates for molecular phylogenetic analysis of snakes 2: Caenophidia, Colubroidea, Elapoidea, Colubridae. Palaeontol. Electronica, 2016; 19: 1–21. [Google Scholar]
- 45.Loveridge R. Further revisions of African snake genera. Bull. Mus. Comp. Zool. (Harvard), 1944; 95: 121–247. [Google Scholar]
- 46.Laurent RF. Contribution à la connaissance du genre Atractaspis Smith. Rev. Zool. Bot. Afr., 1945; 38: 312–343. [Google Scholar]
- 47.de Witte GF, Laurent RF. Révision d'un groupe de Colubridae africains: Genres Calamelaps, Miodon, Aparallactus, et formes affines. Mém. Mus. R. Hist. Nat. Belgique (sér. 2), 1947; 29: 1–134. [Google Scholar]
- 48.Wilson VJ. The snakes of the eastern province of Zambia. The Puku, 1965; 3: 149–170. [Google Scholar]
- 49.Broadley DG. A revision of the African snake genera Amblyodipsas and Xenocalamus. Occas. Pap. Nat. Hist. Mus. Rhodesia, 1971; 4: 629–697. [Google Scholar]
- 50.Pitman CRS. A Guide to the Snakes of Uganda. Wheldon and Wesley; Codicote, UK; 1974. [Google Scholar]
- 51.Hughes B. A rare snake not so rare: Polemon neuwiedi in Ghana. Nigerian Field, 1978; 43: 86–88. [Google Scholar]
- 52.Douglas RM. A new size record and notes on feeding in captivity of Amblyodipsas concolor (A. Smith). J. Herpetol. Assoc. Africa, 1982; 28: 14–16. 10.1080/04416651.1982.9650108 [DOI] [Google Scholar]
- 53.Spawls S, Howell K, Drewes R, Ashe J. A Field Guide to the Reptiles of East Africa: Kenya, Tanzania, Uganda, Rwanda and Burundi. Natural World, London, UK; 2002. [Google Scholar]
- 54.Spawls S, Howell K, Hinkel H, Menegon M. Field Guide to East African Reptiles. Bloomsbury Natural History, London, UK; 2018. [Google Scholar]
- 55.Gower DJ, Rasmussen JB, Loader SP, Wilkinson M. The caecilian amphibian Scolecomorphus kirkii Boulenger as prey of the burrowing asp Atractaspis aterrima Günther: Trophic relationships of fossorial vertebrates. Afr. J. Ecol., 2004; 42: 83–87. 10.1111/j.1365-2028.2004.00495.x [DOI] [Google Scholar]
- 56.Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol., 1999; 48: 612–622. 10.1080/106351599260184 [DOI] [Google Scholar]
- 57.Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol., 2001; 50: 913–925. 10.1080/106351501753462876 [DOI] [PubMed] [Google Scholar]
- 58.Wiens JJ. Missing data, incomplete taxa, and phylogenetic accuracy. Syst. Biol., 2003; 52: 528–538. 10.1080/10635150390218330 [DOI] [PubMed] [Google Scholar]
- 59.Wiens JJ, Morrill MC. Missing data in phylogenetic analysis: Reconciling results from simulations and empirical data. Syst. Biol., 2011; 60: 719–731. 10.1093/sysbio/syr025 [DOI] [PubMed] [Google Scholar]
- 60.Anderson CG, Greenbaum E. Phylogeography of northern populations of the black-tailed rattlesnake (Crotalus molossus Baird and Girard, 1853), with the revalidation of C. ornatus Hallowell, 1854. Herpetol. Monogr., 2012; 26: 19–57. [Google Scholar]
- 61.Jiang W, Chen SY, Wang H, Li DZ, Wiens JJ. Should genes with missing data be excluded from phylogenetic analyses? Mol. Phylogenet. Evol., 2014; 80: 308–318. 10.1016/j.ympev.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 62.Portillo F, Branch WR, Conradie W, Rödel MO, Penner J, Barej M, et al. Phylogeny and biogeography of the African burrowing snake subfamily Aparallactinae (Squamata: Lamprophiidae). Mol. Phylogenet. Evol., 2018; 127: 288–303. 10.1016/j.ympev.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 63.Amer SA, Kumazawa Y. Mitochondrial DNA sequences of the Afro-Arabian spiny-tailed lizards (genus Uromastyx; family Agamidae): Phylogenetic analyses and evolution of gene arrangements. Biol. J. Linn. Soc., 2005; 85: 247–260. 10.1111/j.1095-8312.2005.00485.x [DOI] [Google Scholar]
- 64.Pook CE, Joger U, Stümpel N, Wüster W. When continents collide: Phylogeny, historical biogeography and systematics of the medically important viper genus Echis (Squamata: Serpentes: Viperidae). Mol. Phylogenet. Evol., 2009; 53: 792–807. 10.1016/j.ympev.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 65.Metallinou M, Arnold NE, Crochet PA, Geniez P, Brito J, Lymberakis C, et al. Conquering the Sahara and Arabian deserts: Systematics and biogeography of Stenodactylus geckos (Reptilia: Gekkonidae). BMC Evol. Biol., 2012; 12: 258 10.1186/1471-2148-12-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamar K, Scholz S, Crochet PA, Geniez P, Meiri S, Schmitz A, et al. Evolution around the Red Sea: Systematics and biogeography of the agamid genus Pseudotrapelus (Squamata: Agamidae) from North Africa and Arabia. Mol. Phylogenet. Evol., 2016; 97: 55–68. 10.1016/j.ympev.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 67.Kapli P, Lymberakis P, Crochet PA, Geniez P, Brito JC, Almutairi M, et al. Historical biogeography of the lacertid lizard Mesalina in North Africa and the Middle East. J. Biogeogr., 2014; 42: 267–279. 10.1111/jbi.12420 [DOI] [Google Scholar]
- 68.Šmíd J, Carranza S, Kratochvíl L, Gvoždík V, Nasher A, Moravec J. Out of Arabia: A complex biogeographic history of multiple vicariance and dispersal events in the gecko genus Hemidactylus (Reptilia: Gekkonidae). PLoS ONE, 2013; 8: e64018 10.1371/journal.pone.0064018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnold EN, Robinson MD, Carranza S. A preliminary analysis of phylogenetic relationships and biogeography of the dangerously venomous Carpet Vipers, Echis (Squamata, Serpentes, Viperidae) based on mitochondrial DNA sequences. Amphibia-Reptilia, 2009; 30: 273–282. [Google Scholar]
- 70.Portik DM, Papenfuss TJ. Monitors cross the Red Sea: The biogeographic history of Varanus yemenensis. Mol. Phylogenet. Evol., 2012; 62: 561–565. 10.1016/j.ympev.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 71.Portik DM, Papenfuss TJ. Historical biogeography resolves the origins of endemic Arabian toad lineages (Anura: Bufonidae): Evidence for ancient vicariance and dispersal events with the Horn of Africa and South Asia. BMC Evol. Biol., 2015; 15: 152 10.1186/s12862-015-0417-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouchenak-Khelladi Y., Maurin O, Hurter J, Van der Bank M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African acacias. Mol. Phylogenet. Evol., 2010; 57: 495–508. 10.1016/j.ympev.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 73.Schnitzler J, Barraclough TG, Boatwright JS, Goldblatt P, Manning JC, Powell M, et al. Causes of plant diversification in the Cape biodiversity hotspot of South Africa. Syst. Biol., 2011; 60: 343–357. 10.1093/sysbio/syr006 [DOI] [PubMed] [Google Scholar]
- 74.Portillo F, Greenbaum E, Menegon M, Kusamba C, Dehling JM. Phylogeography and species boundaries of Leptopelis (Anura: Arthroleptidae) from the Albertine Rift. Mol. Phylogenet. Evol., 2015; 82: 75–86. 10.1016/j.ympev.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 75.Larson T, Castro D, Behangana M, Greenbaum E. Evolutionary history of the river frog genus Amietia (Anura: Pyxicephalidae) reveals extensive diversification in Central African highlands. Mol. Phylogenet. Evol., 2016; 99: 168–181. 10.1016/j.ympev.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Medina MF, Bauer AM, Branch WR, Schmitz A, Conradie W, Nagy ZT, et al. Molecular phylogeny of Panaspis and Afroablepharus skinks (Squamata: Scincidae) in the savannas of sub-Saharan Africa. Mol. Phylogenet. Evol., 2016; 100: 409–423. 10.1016/j.ympev.2016.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes DF, Kusamba C, Behangana M, Greenbaum E. Integrative taxonomy of the Central African forest chameleon, Kinyongia adolfifriderici (Sauria: Chamaeleonidae), reveals underestimated species diversity in the Albertine Rift. Zool. J. Linn. Soc., 2017; 181: 400–438. 10.1093/zoolinnean/zlx005 [DOI] [Google Scholar]
- 78.Greenbaum E, Dowell Beer SA, Wagner P, Anderson CG, Villanueva CO, Malonza P, et al. Phylogeography of Jackson’s Forest Lizard Adolfus jacksoni (Sauria: Lacertidae) reveals cryptic diversity in the highlands of East Africa. Herpetol. Monogr., 2018; 32: 51–68. 10.1655/HERPMONOGRAPHS-D-18-00005.1 [DOI] [Google Scholar]
- 79.Al-Quran S. The Herpetofauna of the Southern Jordan. American-Eurasian J. Agric. & Environ. Sci., 2009; 6: 385–391. [Google Scholar]
- 80.Ismail M, Memish Z. Venomous snakes of Saudi Arabia and the Middle East: A keynote for travellers. Int. J. Antimicrob. Agents, 2003; 21: 164–169. [DOI] [PubMed] [Google Scholar]
- 81.Huhndorf MH, Kerbis Peterhans JC, Loew SS. Comparative phylogeography of three endemic rodents from the Albertine Rift, east central Africa. Mol. Ecol., 2007; 16: 663–674. 10.1111/j.1365-294X.2007.03153.x [DOI] [PubMed] [Google Scholar]
- 82.Fjeldså J, Bowie RCK. New perspectives on the origin and diversification of Africa’s forest avifauna. Afr. J. Ecol., 2008; 46: 235–247. 10.1111/j.1365-2028.2008.00992.x [DOI] [Google Scholar]
- 83.Voelker G, Outlaw RK, Bowie RCK. Pliocene forest dynamics as a primary driver of African bird speciation. Global Ecol. Biogeogr., 2009; 19: 111–121. 10.1111/j.1466-8238.2009.00500.x [DOI] [Google Scholar]
- 84.Demos TC, Kerbis Peterhans JC, Agwanda B, Hickerson MJ. Uncovering cryptic diversity and refugial persistence among small mammal lineages across the Eastern Afromontane biodiversity hotspot. Mol. Phylogenet. Evol., 2014; 71: 41–54. 10.1016/j.ympev.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 85.Lawson LP. The discordance of diversification: Evolution in the tropical-montane frogs of the Eastern Arc Mountains of Tanzania. Mol. Ecol., 2010; 19: 4046–4060. 10.1111/j.1365-294X.2010.04788.x [DOI] [PubMed] [Google Scholar]
- 86.Günther ACLG. Sixth account of new species of snakes in the collection of the British Museum. Ann. Mag. Nat. Hist., London, 1868; 1: 413–429 + pl. 17–19. [Google Scholar]
- 87.Wallach V, Williams KL, Boundy J. Snakes of the World: A Catalogue of Living and Extinct Species. CRC Press, Boca Raton, London and New York; 2014. [Google Scholar]
- 88.Boulenger GA. Description of a new snake of the genus Atractaspis from Mount Kenya, British East Africa. Ann. Mag. Nat. Hist., London, 1905; 15: 190. [Google Scholar]
- 89.Sternfeld R. Neue und ungenügend bekannte afrikanische Schlangen. Sitzungsber. Ges. Naturf. Freunde Berlin, 1908; 1908: 92–95. [Google Scholar]
- 90.de Witte GF. Description d’un vipéride nouveau du Kivu (Atractaspis Schoutedeni sp. n.). Rev. Zool. Bot. Afr., 1930; 19: 224–225 + figs. [Google Scholar]
- 91.Angel F. Description d’un vipéride nouveau du Congo belge, et de deux batraciens de Madagascar. Bull. Soc. Zool. Fr., Paris, 1934; 59: 169–172. [Google Scholar]
- 92.Tolley KA, Alexander GJ, Branch WR, Bowles P, Maritz B. Conservation status and threats for African reptiles. Biol. Cons., 2016; 204: 63–71. 10.1016/j.biocon.2016.04.006 [DOI] [Google Scholar]
- 93.Greenbaum E. Emerald Labyrinth: A Scientist’s Adventures in the Jungles of the Congo. ForeEdge, Lebanon, NH, USA; 2017. [Google Scholar]
- 94.Vonk FJ, Admiraal JF, Jackson K, Reshef R, de Bakker MAG, Vandershoot K, et al. Evolutionary origin and development of snake fangs. Nature (London), 2008; 454: 630–633. 10.1038/nature07178 [DOI] [PubMed] [Google Scholar]
- 95.Kusamba C, Resetar A, Wallach V, Lulengo K, Nagy ZT. Mouthful of snake: An African snake-eater’s (Polemon fulvicollis graueri) large typhlopid prey. Herpetol. Notes, 2013; 6: 235–237. [Google Scholar]
- 96.Deufel A, Cundall D. Feeding in stiletto snakes. Am. Zool., 2000; 40: 996–997. [Google Scholar]
- 97.Marais J. Natural History Notes—Atractaspididae—Atractaspis bibronii Smith, 1894 —Diet. African Herp News, 2010; 9. [Google Scholar]
- 98.Rasmussen JB. A review of the slender stiletto-snake, Atractaspis aterrima Günther 1863 (Serpentes Atractaspididae). Trop. Zool., 2005; 18: 137–148. 10.1080/03946975.2005.10531216 [DOI] [Google Scholar]
- 99.Broadley DG. A review of the southern African stiletto snakes of the genus Atractaspis A. Smith (Serpentes: Atractaspididae). Arnoldia, Zimbabwe, 1991; 9: 495–517. 10.1080/03946975.2005.10531216 [DOI] [Google Scholar]
- 100.Akani GC, Luiselli LM, Angelici FM, Corti C, Zuffi MAL. The case of rainforest stiletto snakes (genus Atractaspis) in southern Nigeria. Evidence of diverging foraging strategies in grossly sympatric snakes with homogeneous body architecture? Ethol. Ecol. Evol., 2001; 13: 89–94. 10.1080/08927014.2001.9522790 [DOI] [Google Scholar]
- 101.Kochva E, Shayer-Wollberg M, Sobol R. The special pattern of the venom gland in Atractaspis and its bearing on the taxonomic status of the genus. Copeia, 1967; 1967: 763–772. 10.2307/1441887 [DOI] [Google Scholar]
- 102.Wollberg M, Kochva E, Underwood G. On the rictal glands of some atractaspid snakes. Herpetol. J., 1998; 8: 137–143. 10.1655/0018-0831(2002)058[0001:OTRSOS]2.0.CO;2 [DOI] [Google Scholar]
- 103.Kochva E. The origin of snakes and evolution of the venom apparatus. Toxicon, 1987; 27: 65–106. [DOI] [PubMed] [Google Scholar]
- 104.Broadley DG. FitzSimons’ Snakes of Southern Africa. Delta Books, Johannesburg, South Africa; 1983. [Google Scholar]
- 105.Kochva E, Gans C. Salivary glands of snakes. Clin. Toxicol., 1970; 3: 363–387. 10.3109/15563657008990115 [DOI] [PubMed] [Google Scholar]
- 106.Kochva E, Wollberg M. The salivary glands of Aparallactinae (Colubridae) and the venom glands of Elaps (Elapidae) in relation to the taxonomic status of the genus. Zool. J. Linn. Soc., 1970; 49: 217–224. 10.1111/j.1096-3642.1970.tb00737.x [DOI] [Google Scholar]
- 107.Broadley DG, Craig DT, Wigge J. Snakes of Zambia. Chimaira, Frankfurt, Germany; 2003. [Google Scholar]
- 108.Sabaj, MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an Online Reference. Version 6.5 (16 August 2016). 2016; electronically accessible at http://www.asih.org/, American Society of Ichthyologists and Herpetologists, Washington, DC.
- 109.Lawson R, Slowinski JB, Crother BI, Burbrink FT. Phylogeny of the Colubroidea (Serpentes): New evidence from mitochondrial and nuclear genes. Mol. Phylogenet. Evol., 2005; 37: 581–601. 10.1016/j.ympev.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 110.Palumbi SR. Nucleic acids II: The polymerase chain reaction In: Hillis D.M., Moritz C., Mable B.K. (Eds.), Molecular Systematics. Sinauer Associates, MA, USA; 1996. Pp. 205–207. [Google Scholar]
- 111.Zaher H, Grazziotin FG, Cadle JE, Murphy RW, Moura-Leite JCD, Bonatto SL. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American xenodontines: A revised classification and descriptions of new taxa. Pap. Avulsos Zool., São Paulo, 2009; 49: 115–153. 10.1590/S0031-10492009001100001 [DOI] [Google Scholar]
- 112.Arévalo E, Davis SK, Sites JW Jr. Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in Central Mexico. Syst. Biol., 1994; 43: 387–418. 10.1093/sysbio/43.3.387 [DOI] [Google Scholar]
- 113.Burbrink FT, Lawson R, Slowinski JP. 2000. Mitochondrial DNA phylogeography of the polytypic North American rat snake (Elaphe obsoleta): A critique of the subspecies concept. Evolution, 2000; 54: 2107–2118. 10.1554/0014-3820(2000)054[2107:MDPOTP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 114.Slowinski JB, Lawson R. Snake phylogeny: Evidence from nuclear and mitochondrial genes. Mol. Phylogenet. Evol., 2002; 24: 194–202. 10.1016/S1055-7903(02)00239-7 [DOI] [PubMed] [Google Scholar]
- 115.Groth JG, Barrowclough GF. Basal divergences in birds and the phylogenetic utility of the nuclear RAG-1 gene. Mol. Phylogenet. Evol., 1999; 12: 115–123. 10.1006/mpev.1998.0603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
The data included in this paper can be found on GenBank and Morphosource websites (access information is contained within the paper).