Abstract
Background
To review the obstetric outcomes of pregnancies with anencephalic fetuses when these pregnancies are not terminated and ongoing.
Methods
A retrospective chart review is made of the cases with a prenatal diagnosis of anencephaly and who were opted to continue the pregnancy in 1-year period. The evaluated data included gestational age at diagnosis, gestational age at birth, labor induction rates, cesarean delivery rates, stillbirth, shoulder dystocia rate, antepartum and postpartum hemorrhage.
Results
A total of 28 cases that were selected from 87 cases with the diagnosis of anencephaly are included in the study. The average gestational age at diagnosis was 18 weeks. The average gestational age at birth was 31 weeks (range 23 - 37 weeks). Stillbirths were reported in 32% (9/28). Polyhydramnios developed at six patients and two of them required amniodrainage due to severe polyhydramnios. Vaginal birth was achieved in 67% (19/28) of the patients. Labor induction was applied at total 17 patients and one of them had gone to cesarean section due to failed induction. There were two cases of shoulder dystocia. Nine patients had gone to cesarean delivery. Any other complication was not encountered.
Conclusions
Parents should be counseled in detail about continuation of an anencephalic pregnancy before making their decision. The information about “what an anencephalic pregnancy can lead”' should be given. The redundant cesarean deliveries, polyhydramnios and associated complications, obstetrical hemorrhage risk should be discussed with patients. It should also be emphasized that these maternal risks are for the sake of a non-life expectant baby.
Keywords: Anencephaly, Induction of labor, Prenatal counseling, Induced abortion
Introduction
Anencephaly is a lethal condition, which is part of the neural tube defect (NTD) spectrum. It is a serious developmental defect of the central nervous system. The brain and cranial bones are grossly malformed. Cerebral and cerebellar structures are reduced or grossly malformed, but the hindbrain is present. The etiology seems to be caused by genetic and environmental factors; however, many of which remains unknown. Its prevalence at birth ranges from 1 in 5,000 to 1 in 2,000 [1, 2]. The prevalence at birth has great geographic variation, with especially high rates in the British Isles, China, Mexico, and Turkey [3]. The widespread use of ultrasound has led to earlier bonding of parents to their unborn children because that’s very traumatic for parents to terminate babies that they have become attached.
If the anencephalic pregnancy is not terminated prenatally or diagnosis is not made prior to birth, live born infants typically survive less than 1 day [4]. It is an important aspect to address all the potential possibilities of maternal outcomes with parents, especially in case of pregnancy termination is not or no longer an option. The decision about pregnancy termination is associated with parents’ religious beliefs and restricted legal termination rules, in addition to other factors such as late detection of the anomaly and delayed fetal anatomy scans. Commonly, the major reason for continuation of pregnancy is the request of parents due to religious beliefs.
In our study, we aimed to review the obstetric outcomes after the continuation of pregnancies with anencephalic fetuses, to review the obstetric impact and natural history of anencephalic pregnancies, and to define the maternal risks in the continuation of pregnancies with non-life expectant babies.
Materials and Methods
This is a retrospective chart review of all prenatal diagnoses of anencephaly, made in Sanliurfa Education and Research Hospital, Sanliurfa, Turkey, in a 1-year period between May 2017 and April 2018. The unit is a busy tertiary center in the east of Turkey, which receives referral patients from the region, with about 35 - 40,000 deliveries per year. Also an important aspect of the hospital is that a great amount of health care is being provided to Syrian refugees due to the intensity of the Syrian refugees in the region. Approval and permission for the study regarding the provision of patient data were obtained from the institution (registration number: 96537014-00-3876). The study design is in accordance with the Helsinki Declaration (Association 2014), and conformed with the Committee on Publication Ethics Guidelines. The diagnosis of anencephaly was made through prenatal ultrasound by the same physician (Ekmekci E) and confirmed in postnatal neonatal examinations. The ultrasound device used was a Voluson E8 system (GE Healthcare, Milwaukee, WI) with an RAB 4 - 8-MHz transabdominal probe. Multiple gestations are not included in study.
All parents were informed about the fetal status through detailed oral disclosure with photographs of sample anencephalic babies, and all were given the option of pregnancy termination, irrespective of gestational age. There is no restriction for termination of pregnancy in Turkey in cases of confirmed lethal or severe fetal anomalies with the approval of the hospital ethics board. Twenty-eight women who do not accept termination and opted to continue pregnancy were selected for the study. Pregnancies were followed up to 37 weeks of pregnancy; if labor did not start spontaneously, labor induction was started.
Outcome variables included gestational age at diagnosis, gestational age at birth, induction of labor, cesarean delivery, live births, stillbirths, shoulder dystocia, polyhydramnios, antepartum hemorrhage (APH), and postpartum hemorrhage (PPH). Polyhydramnios was defined as ultrasound estimation of amniotic fluid if the deepest vertical pocket is ≥ 8 cm and defined as severe polyhydramnios if ≥ 16 cm.
Results
Eighty-seven anencephaly cases from 27,754 deliveries were enrolled in the study during the 1-year period. A total of 3,885 deliveries were carried by Syrian refugees, 31 of which were anencephalic fetuses. The average maternal age was 27 years (range, 17 - 46 years). The median parity was 3 (range, 0 - 8). Thirteen percent (12/87) of the patients were primigravida. Three women had a history of previous anencephalic pregnancies. No other risk factors were identified. The mean gestational age at diagnosis was 18 weeks (range, 11 - 36 weeks). Forty-nine pregnancies were terminated after the diagnosis, according to family request.
Twenty-eight patients were rejected termination of pregnancy and opted for continuation of pregnancy, 11 (39%) of whom were Syrian refugees. The average gestational age at birth was 31 weeks (range, 23 - 37 weeks). Stillbirths were reported in 32% (9/28), 68% of the fetuses (19/28) were alive; however, all died within the first week of life. One baby was alive for 5 days in the neonatal intensive care unit, the remainder died in the first hour after delivery. The median gestational age of pregnancies with stillbirth was 27 weeks. The average birth weight was 1,526 ± 946 g (range, 450 - 3,170 g).
Vaginal birth was achieved in 68% (19/28) of the patients. Cesarean section (CS) was performed in nine patients (32%); seven patients had previous uterine surgery, one patient had a transverse presentation after membrane rupture at 32 weeks, and one patient had failed labor induction. Spontaneous labor was started in nine (32%) patients, two of them were underwent CS due to previous uterine surgery. Twelve (42%) patients reached 37 weeks with live fetuses and pregnancy was terminated during this week. Labor induction was performed in a total of 18 (64%) patients. Six (21%) patients developed polyhydramnios, and of these, two women were required serial amniodrainages due to severe polyhydramnios that was causing maternal dyspnea during follow-up. Amnioreduction was repeated twice for two patients.
Two (11%) vaginal deliveries were complicated by shoulder dystocia; both could be delivered vaginally by rotational maneuvers. One PPH was occurred due to uterine atony, which was resolved with medication and was not required surgery. There were no surgical complications associated with CS. There were no maternal postnatal complications. The complications associated with ongoing anencephalic pregnancies are listed in Table 1. The outcomes of the pregnancies are reported in a flow diagram in Figure 1.
Table 1. The Complications Associated With Ongoing Anencephalic Pregnancies.
| Number of cases | |
|---|---|
| Stillbirth | 9/28 |
| Polyhydramnios | 6/28 |
| Elective cesarean delivery | 7/28 |
| Cesarean delivery due to obstetrical indications | 2/28 |
| Labor induction | 18/28 |
| Shoulder dystocia | 2/19 |
| Antepartum/postpartum hemorrhage | 1/28 |
Figure 1.
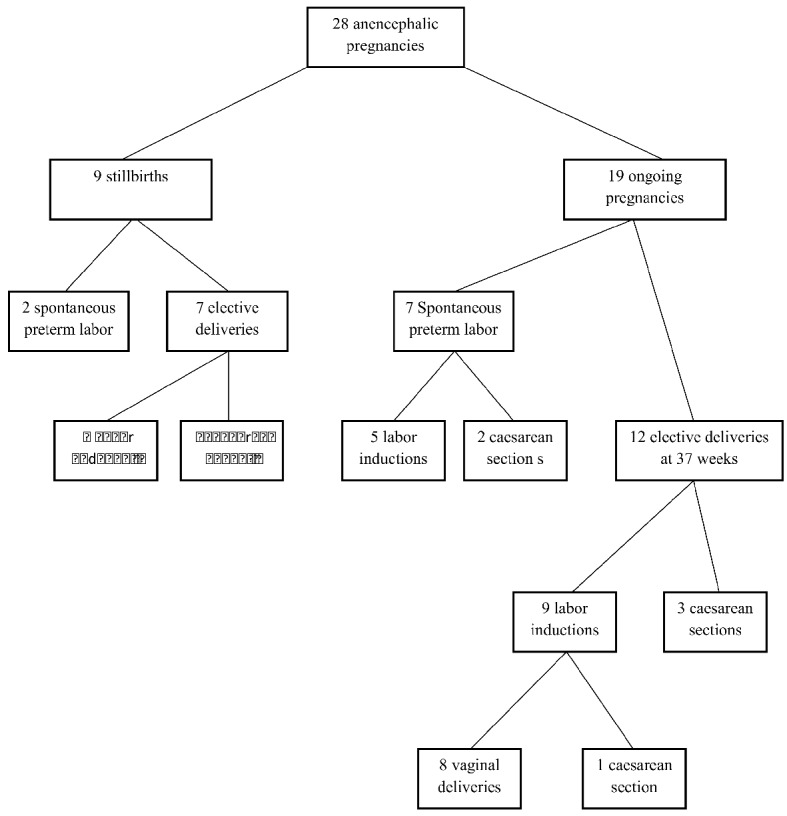
Outcome of anencephalic pregnancies in the study.
Discussion
In this study, we evaluated the outcome of anencephalic pregnancies and aimed to describe the redundant maternal risks for a non-viable baby. The major risk for the mother was redundant cesarean deliveries.
There is great geographic variation in the prevalence of fetal anencephaly and neural tube defects. Although this study was not a prevalence study, approximate prevalence can be calculated by using the annual birth numbers of the hospital. In a 1-year period, the total number of births was 27,754 in our hospital. If this is used for calculation, the prevalence is 3.1 per 1,000 live births. This prevalence is significantly higher compared with the reported prevalence in the literature. Obeid et al reported the prevalence as 3.52 per 10.000 live births on the basis of the surveillance data from all births covered by the full member countries of the European Surveillance of Congenital Anomalies (EUROCAT) [5]. There is a great variation in the prevalence compared to worldwide in our area. Also, in Northern Iran which is a close region to our area, the prevalence was reported as 12 per 10,000 live births, that’s very high again compared to worldwide, in a cross-sectional hospital-based study published in 2010 [6]. This significantly high prevalence may be misleading due to the non-homogenous study population, but the prevalence seems higher than the general population. The hospital is a tertiary center and accepting referral patients from the region. Also, there is an intensity of Syrian refugees in the region (about 500,000 Syrian refugees live in Sanliurfa province). Another aspect is that, in a recent study from the north west of Turkey, the prevalence of anencephaly is reported as 2.1 per 10,000 live births, which is similar to general worldwide data [7]. This great geographical variation at the prevalence of condition in the same country and same health policies cannot be only associated with environmental factors. Also, racial and genetic factors should be taken into account. We feel that further large population based studies are required to investigate this high prevalence in the region.
Another interesting aspect was about the prevalence of anencephaly in Syrian refugees. In this period, the total number of births of Syrian refugees was 3,885. Thirty-one anencephalic pregnancies were diagnosed among the Syrian refugees. The prevalence was 8 in 1,000 births (31/3885). Three pregnancies had a history of previous anencephalic pregnancies. Rates are very high when compared to world average and rates from the hospital. This may be evidence of destructive effects of environmental factors on the developing fetus, like war and malnutrition.
Although anencephalic fetuses are non-viable and termination of pregnancy is the most logical approach; due to religious beliefs, pregnancies are not being terminated commonly, compared to the usual. In Turkey, there is no restriction for pregnancy termination with an anencephalic fetus, but 32% (28/87) of patients were not terminated their pregnancies, primarily due to religious beliefs. When these pregnancies are ongoing, patients are subjected to various pregnancy complications specific to anencephalic pregnancies, in addition to general pregnancy complications.
The prenatal detection of anencephaly with ultrasound is high and reliable in the 10 - 14 weeks scan [8, 9]. In our study, the mean gestational age for the diagnosis of anencephaly was 18 weeks and the earliest gestational age was 11 weeks. Blaas et al reported that diagnosis of anencephaly could be made as early as the ninth week of pregnancy [10]. Almost all anencephalic fetuses can be diagnosed during the first trimester screening of 11 - 14 weeks. The sensitivity is 100% in the second trimester screening sonography.
In our study population, six (21%) patients required amnioreduction due to severe polyhydramnios. Polyhydramnios was also a significant prenatal complication in our study, as other authors have also found [1]. Obeidi et al reported that one-quarter of anencephalic pregnancies were complicated by polyhydramnios in their study [2]. The rate of stillbirth in our anencephaly fetuses was 32%. Al-Obaidly and Obeidi et al reported 40% and 42% stillbirth rates, respectively, in their studies during follow-up of anencephalic fetuses [2, 11]. Induction of labor might carry an increased risk for CS [12]. However, in our study, there were no differences in achieving vaginal birth in women who were induced compared with women who had spontaneous labor.
Shoulder dystocia in anencephalic pregnancies is an expected complication that can be attributed to diminished head size, which cannot dilate the cervix enough to deliver the fetal trunk and shoulders [13]. The shoulder dystocia rate was 2/19 (11%) in our study and all were relieved with rotational maneuvers. None of the patients in our study were diabetic, that supports that anencephaly could be solely itself a risk factor for shoulder dystocia.
The majority of maternal risks after an ongoing anencephalic pregnancy are associated with compulsory cesarean operations for non-viable babies. The risk of repeated CS should be addressed in women with a previous uterine scar, especially in anencephalic pregnancies because the risk about the prolongation of pregnancy to upper gestational age is increased due to fetal hypothalamic-pituitary axis dysfunction [14]. In our study, seven (25%) patients required CS due to previous uterine surgery, and two were underwent CS due to malpresentation and failed labor induction. In our clinic our management protocol is to follow anencephalic pregnancies up to 37 weeks and then to start the delivery. Therefore, we had no post-term pregnancies.
Although our study represents a shorter period of time than similar reports on this issue, our study population had a large cohort for this area. We could establish the outcomes of all patients because our hospital is the referral center for the region and all high-risk pregnancies are followed in our center. A major strength of our results is that, the study population contains patients who were all given the option to terminate pregnancies but opted to continue their pregnancies. There was no governmental restriction for pregnancy termination.
Study limitations
The limitation of our study is its retrospective nature; however, all medical data were directly achieved from case notes and other hospital records. Another limitation of our study is that we did not follow pregnancies to term due to the fact they were all terminated at 37 weeks. The pregnancies were not left to their natural course. On the other hand, there is no consensus in the literature regarding the time for termination of ongoing pregnancies with anencephalic fetuses.
Conclusions
In this study, our intention was not to discuss the ethical and moral issues in the management of anencephalic pregnancies, but rather to report the outcomes of a large number of cases complicated with anencephalic fetuses. We would like to draw attention to this problem, which is very common in our region for health professionals caring for patients with anencephalic pregnancies. The leading morbidity associated with ongoing anencephalic pregnancies seems to be associated with redundant cesarean deliveries. Also, polyhydramnios and postpartum hemorrhage are less frequent complications that should be kept in mind. These findings should assist in counseling parents more appropriately about the possible complications of ongoing anencephalic pregnancies and outcomes of these pregnancies.
Acknowledgments
None to declare.
Financial Disclosure
There has been no significant financial support for this work that could have influenced its outcome.
Conflict of Interest
We confirm that there are no known conflicts of interest.
Informed Consent
Obtained.
Author Contributions
Study conception and design: Ekmekci E and Gencdal S. Acquisition of data: Ekmekci E and Gencdal S. Analysis and interpretation of data: Ekmekci E and Gencdal S. Drafting of manuscript: Ekmekci E. Critical revision: Ekmekci E and Gencdal S.
References
- 1.Stone DH. The declining prevalence of anencephalus and spina bifida: its nature, causes and implications. Dev Med Child Neurol. 1987;29(4):541–546. doi: 10.1111/j.1469-8749.1987.tb02516.x. [DOI] [PubMed] [Google Scholar]
- 2.Obeidi N, Russell N, Higgins JR, O'Donoghue K. The natural history of anencephaly. Prenat Diagn. 2010;30(4):357–360. doi: 10.1002/pd.2490. [DOI] [PubMed] [Google Scholar]
- 3.Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16(1):6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaquier M, Klein A, Boltshauser E. Spontaneous pregnancy outcome after prenatal diagnosis of anencephaly. BJOG. 2006;113(8):951–953. doi: 10.1111/j.1471-0528.2006.01014.x. [DOI] [PubMed] [Google Scholar]
- 5.Obeid R, Pietrzik K, Oakley GP Jr, Kancherla V, Holzgreve W, Wieser S. Preventable spina bifida and anencephaly in Europe. Birth Defects Res A Clin Mol Teratol. 2015;103(9):763–771. doi: 10.1002/bdra.23400. [DOI] [PubMed] [Google Scholar]
- 6.Golalipour MJ, Najafi L, Keshtkar AA. Prevalence of anencephaly in Gorgan, northern Iran. Arch Iran Med. 2010;13(1):34–37. [PubMed] [Google Scholar]
- 7.Wyburn-Mason R. The nature of tic douloureux; treatment by alcohol block or section of the great auricular nerve. Br Med J. 1953;2(4828):119–122. doi: 10.1136/bmj.2.4828.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SP, Sebire NJ, Snijders RJ, Tunkel S, Nicolaides KH. Ultrasound screening for anencephaly at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1997;9(1):14–16. doi: 10.1046/j.1469-0705.1997.09010014.x. [DOI] [PubMed] [Google Scholar]
- 9.Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenat Diagn. 2009;29(4):402–411. doi: 10.1002/pd.2250. [DOI] [PubMed] [Google Scholar]
- 10.Blaas HG. Detection of structural abnormalities in the first trimester using ultrasound. Best Pract Res Clin Obstet Gynaecol. 2014;28(3):341–353. doi: 10.1016/j.bpobgyn.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Al-Obaidly S, Thomas J, Abu Jubara M, Al Ibrahim A, Al-Belushi M, Saleh N, Al-Mansouri Z. et al. Anencephaly and obstetric outcome beyond the age of viability. J Perinat Med. 2018;46(8):885–888. doi: 10.1515/jpm-2017-0363. [DOI] [PubMed] [Google Scholar]
- 12.Cammu H, Martens G, Ruyssinck G, Amy JJ. Outcome after elective labor induction in nulliparous women: a matched cohort study. Am J Obstet Gynecol. 2002;186(2):240–244. doi: 10.1067/mob.2002.119643. [DOI] [PubMed] [Google Scholar]
- 13.Bansal S, Guleria K, Agarwal N. Evaluation of sacral rhomboid dimensions to predict contracted pelvis: a pilot study of Indian primigravidae. J Obstet Gynaecol India. 2011;61(5):523–527. doi: 10.1007/s13224-011-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novy MJ, Walsh SW, Kittinger GW. Experimental fetal anencephaly in the rhesus monkey: effect on gestational length and fetal and maternal plasma steroids. J Clin Endocrinol Metab. 1977;45(5):1031–1038. doi: 10.1210/jcem-45-5-1031. [DOI] [PubMed] [Google Scholar]


