Abstract
Hepatocellular carcinoma (HCC) affects more than half a million people worldwide each year. Paraneoplastic syndromes associated with HCC include erythrocytosis, hypercalcemia, hypercholesterolemia, hypoglycemia and thrombocytosis. Thrombocytosis is a rare paraneoplastic syndrome in HCC mediated by thrombopoietin (TPO) production. We report a case of thrombocytosis as a paraneoplastic syndrome in a patient with HCC and hepatitis C cirrhosis. A 56-year-old patient was evaluated with abdominal distension and pain of 1-month duration. He had a history of hepatitis C infection with liver cirrhosis, CTP (Child-Turcotte-Pugh) class C, MELD (model for end-stage liver disease) score 22, methadone dependence, alcohol abuse and depression. His physical examination was remarkable for distended abdomen with shifting dullness, palpable hepatomegaly and scleral icterus. Routine laboratory tests showed a platelet count of 754 k/µL, white blood cell count 12.4 k/µL, serum sodium level 128 mEq/L, alanine aminotransferase 93 U/L, aspartate aminotransferase 871 U/L, total serum bilirubin 4.3 mg/dL, direct serum bilirubin 2.8mg/dL and albumin 2.6 g/dL. Computed tomography of the abdomen and pelvis revealed hepatomegaly with numerous hypodensities suspicious for HCC. Abdominal paracentesis was done, serum ascites albumin gradient (SAAG) was 2.4 g/dL consistent with portal hypertension, and spontaneous bacterial peritonitis was ruled out. Magnetic resonance imaging of the liver was consistent with infiltrating HCC, portal vein thrombosis and retroperitoneal lymphadenopathy. His alpha fetoprotein (AFP) level was 79,102 ng/mL and TPO level was 126 pg/mL. JAK2 mutation was negative and no other cause of reactive thrombocytosis could be identified. One year prior to this admission, the patient was noted to have a normal platelet count and AFP level. He was not considered a candidate for liver transplantation due to ongoing substance abuse, and expired 1 month later. Thrombocytosis is a rare paraneoplastic condition seen in HCC. It is presumed to be secondary to increased production of TPO by the tumor. We observed an elevated level of TPO in our patient. Thrombocytosis in HCC is associated with a high tumor burden, portal vein thrombosis (PVT), serum AFP levels and a poor prognosis. Thrombocytosis in a cirrhotic patient should alert the presence of HCC and is associated with poor outcomes.
Keywords: Hepatocellular carcinoma, Paraneoplastic syndrome, Thrombocytosis
Introduction
Hepatocellular carcinoma (HCC) is seen in more than half a million people worldwide each year with approximately 20,000 new cases in United States [1, 2]. It is the fifth most common malignancy in men and seventh most common malignancy in women. In the developing world, it is seen in association with hepatitis B virus infection. In the United States the incidence of HCC is on the rise and is seen increasingly in association with hepatitis C virus infection [3].
Thrombocytopenia has been reported as a risk factor and as a prognostic indicator for HCC [4], however, thrombocytosis is rare. There are a variety of paraneoplastic syndromes associated with HCC including erythrocytosis, hypercalcemia, hypercholesterolemia, hypoglycemia and Thrombocytosis [5]. Thrombocytosis is a rare paraneoplastic syndrome in HCC and is thought to be mediated by thrombopoietin (TPO) production.
Few cases have been reported in medical literature of thrombocytosis coexisting with HCC. We report a case of thrombocytosis as a paraneoplastic syndrome in a patient with HCC and hepatitis C cirrhosis.
Case Report
A 56-year-old man was evaluated in the emergency room of our hospital with abdominal pain, abdominal distension and bilateral leg swelling of 1-month duration. The patient reported that his symptoms started 1 month ago and had been progressively getting worse over the week prior to index presentation. He described the abdominal pain as constant, diffuse, non-radiating and moderate in intensity without any precipitating or relieving factors. He reported one episode of diarrhea which did not contain any blood. There was no nausea, vomiting, constipation, fever, and early satiety or appetite changes. He had a history of hepatitis C infection with cirrhosis of the liver, Child-Turcotte-Pugh (CTP) class C, model for end-stage liver disease (MELD) score 22, methadone dependence, alcohol abuse and depression. Patient was a former smoker, had a history of substance abuse and was enrolled in a methadone program. He did not have a family history of liver or colon cancer. His medications included albuterol, folic acid, multi-vitamins, thiamine, citalopram and methadone.
On examination, he was found to have temperature of 36.8 °C, blood pressure of 138/84 mm Hg, pulse rate of 93 beats per minute and the respiratory rate of 14 breaths per minute. The patient was noted to have scleral icterus along with yellowish discoloration of the skin, spider angiomata on the upper chest, head and upper extremities. The abdomen was distended, diffusely tender with a positive fluid thrill and shifting dullness. There was palpable hepatomegaly but no splenomegaly. The lower extremities had bilateral pitting edema, tenderness and erythema.
Routine laboratory tests showed a platelet count of 754 k/µL, white blood cell count 12.4 k/µL, serum sodium level 128 mEq/L, alanine aminotransferase 93 U/L, aspartate aminotransferase 871 U/L, total serum bilirubin 4.3 mg/dL, direct serum bilirubin 2.8 mg/dL and albumin 2.6 g/dL. His alpha fetoprotein (AFP) level was 79,102 ng/mL, and thrombopoietin level was 126 pg/mL. JAK2 mutation was negative and no other cause of reactive thrombocytosis could be identified. One year prior to this admission, the patient was noted to have a normal platelet count and AFP level. Results of laboratory parameters are given in Table 1.
Table 1. Initial Laboratory Workup.
| Parameter | Initial Laboratory results | Reference range |
|---|---|---|
| Hemoglobin (g/dL) | 12.2 | 12 - 16 |
| Hematocrit (%) | 36.2 | 42 - 51 |
| Platelet count (k/µL) | 754 | 150 - 400 |
| White blood cell count (k/µL) | 12.4 | 4.8 - 10.8 |
| Sodium (mEq/L) | 128 | 135 - 145 |
| Potassium (mEq/L) | 4.9 | 3.5 - 5.0 |
| Bicarbonate (mEq/L) | 23 | 24 - 30 |
| Chloride (mEq/L) | 92 | 98 - 108 |
| Calcium (mEq/L) | 8.2 | 8.5 - 10.5 |
| Glucose (mg/dL) | 96 | 70 - 120 |
| Alpha fetoprotein (AFP) ng/mL | 79,102 | < 10 |
| Blood urea nitrogen (mg/dL) | 10 | 6 - 20 |
| Creatinine (mg/dL) | 0.5 | 0.5 - 1.5 |
| Total protein (g/dL) | 10.3 | 6 - 8.5 |
| Albumin (g/dL) | 2.6 | 3.2 - 4.8 |
| Alanine transaminase (U/L) | 93 | 5 - 40 |
| Aspartate transaminase (U/L) | 871 | 9 - 48 |
| Alkaline phosphatase (U/L) | 296 | 53 - 141 |
| Total bilirubin (mg/dL) | 4.3 | 0.2 - 1.2 |
| Direct bilirubin (mg/dL) | 2.8 | 0.2 - 1.2 |
| Lipase (U/L) | 76 | < 61 |
| Thrombopoietin | 126 | 7 - 99 |
Computed tomography of the abdomen and pelvis revealed hepatomegaly with numerous hypodensities suspicious for HCC (Fig. 1). Abdominal paracentesis was done, serum ascites albumin gradient (SAAG) was 2.4 g/dL consistent with portal hypertension, total protein was 1.2 g/dL, ascetic fluid white blood cell count was 440 cells/mL and the neutrophil count was 30 cells/mL. Spontaneous bacterial peritonitis (SBP) was ruled out. Magnetic resonance imaging of the liver was consistent with infiltrating HCC, portal vein thrombosis and retroperitoneal lymphadenopathy (Fig. 2.). He was not considered a candidate for liver transplantation due to ongoing substance abuse. He was started on ciprofloxacin for SBP prophylaxis, lactulose, completed antibiotic treatment for cellulitis and was referred to oncology. He expired 1 month later.
Figure 1.
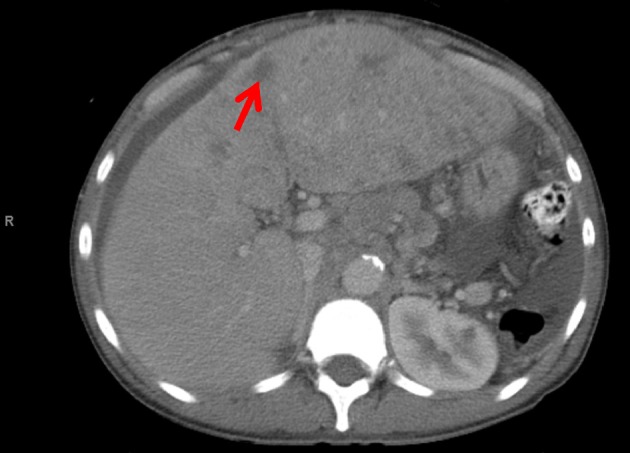
CT of the abdomen and pelvis reveals hepatomegaly with numerous hypodensities suspicious for HCC.
Figure 2.
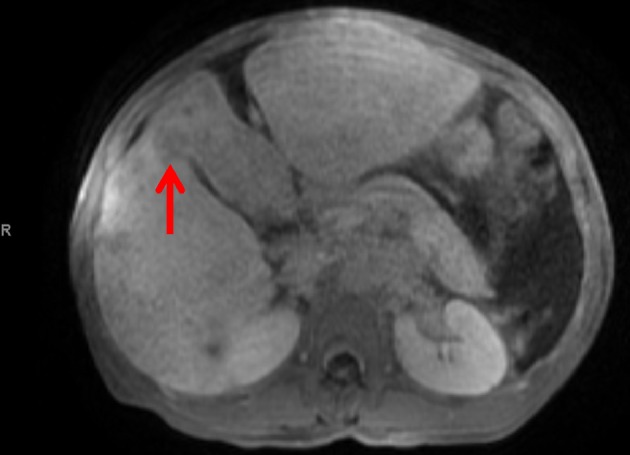
MRI of the abdomen and pelvis reveals abnormal T1 low appearance of the anterior segment of the right hepatic lobe and left hepatic lobe, and heterogeneous appearance of the right hepatic lobe with several hypointense foci on T1-weighted series.
Discussion
HCC is a highly malignant tumor that occurs in the background of chronic inflammation and liver injury due to hepatitis B, C, chronic alcohol use, hemochromatosis or non-alcoholic fatty liver disease (NAFLD) [4]. It is mostly associated with cirrhosis, but in case of hepatitis B infection, HCC can develop without developing cirrhosis. Long standing infection with hepatitis B increases the risk of HCC considerably as compared to the uninfected individuals, while in the presence of macronodular cirrhosis the risk of HCC is increased by 10-fold [6, 7]. Hepatitis B belongs to the Hepadnaviridae family and has direct oncogenic effects [8], and can cause HCC in the absence of micro or macro nodular cirrhosis. On the contrary hepatitis C causes HCC in the setting of cirrhosis only in most cases, and effective treatment and eradication of hepatitis C virus before cirrhosis development results in decreased risk of subsequent HCC [9]. The risk of HCC in HCV-infected individuals is 20 times higher as compared to non-infected individuals [10]. There are many paraneoplastic syndromes that manifest in the presence of HCC and the prevalence of paraneoplastic syndrome in HCC is 27% according to one study [11], and as high as 43% according to another [12]. These include erythrocytosis, hypercholesterolemia, hypercalcemia, hypoglycemia, demyelinating disease, pemphigus, polyarthritis, encephalomyelitis, and thrombocytosis [5, 11, 13-20]. Hypercholesterolemia, hypercalcemia, and erythrocytosis occur more commonly than others [21], and according to one study their prevalence is 24.5%, 5.3%, and 3.9%, respectively [11]. Paraneoplastic syndromes in HCC are seen in advanced disease, advanced TNM staging at diagnosis and higher AFP levels [11].
Thrombocytosis is a rare paraneoplastic condition seen in HCC [4, 5, 22, 23]. Human thrombopoietin (TPO) or the megakaryocyte growth factor is secreted by both hepatocytes and bone marrow cells [24, 25]. It is hypothesized that the thrombocytosis seen in association with HCC is secondary to increased production of TPO by the tumor cells [5]. Thrombocytosis in HCC is associated with a high tumor burden, portal vein thrombosis (PVT), serum AFP levels and a poor prognosis [5].
Our case is unique as we observed all features including an elevated level of TPO, high AFP levels, and PVT associated with thrombocytosis and a poor prognosis in HCC. Thrombocytosis in a cirrhotic patient should alert the presence of HCC, and when present is an indicator of poor outcomes.
Disclosure
None.
Abbreviations
- HCC
hepatocellular carcinoma
- TPO
thrombopoietin
- CTP
Child-Turcotte-Pugh
- MELD
model for end-stage liver disease
- AFP
alpha fetoprotein
- SBP
spontaneous bacterial peritonitis
- NAFLD
non-alcoholic fatty liver disease
- PVT
portal vein thrombosis
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: incidence - SEER 9 Regs research data, Nov 2009 Sub (1973-2007). Bethesda, MD: National Cancer Institute, April 2010.
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Carr BI, Guerra V. Thrombocytosis and hepatocellular carcinoma. Dig Dis Sci. 2013;58(6):1790–1796. doi: 10.1007/s10620-012-2527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang SJ, Luo JC, Li CP, Chu CW, Wu JC, Lai CR, Chiang JH. et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10(17):2472–2477. doi: 10.3748/wjg.v10.i17.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson WS. The role of hepatitis B virus in the development of primary hepatocellular carcinoma: Part I. J Gastroenterol Hepatol. 1992;7(6):622–638. doi: 10.1111/j.1440-1746.1992.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 7.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2(8256):1129–1133. doi: 10.1016/S0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 8.Ringelhan M, O'Connor T, Protzer U, Heikenwalder M. The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets. J Pathol. 2015;235(2):355–367. doi: 10.1002/path.4434. [DOI] [PubMed] [Google Scholar]
- 9.Lim EJ, Torresi J. Prevention of hepatitis C virus infection and liver cancer. Recent Results Cancer Res. 2014;193:113–133. doi: 10.1007/978-3-642-38965-8_7. [DOI] [PubMed] [Google Scholar]
- 10.Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, Wu MH. et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157(8):674–682. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 11.Chang PE, Ong WC, Lui HF, Tan CK. Epidemiology and prognosis of paraneoplastic syndromes in hepatocellular carcinoma. ISRN Oncol. 2013;2013:684026. doi: 10.1155/2013/684026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh UY, Kim JH, Kim BH, Nam KD, Jang JY, Kim NH, Lee SK. et al. [The incidence and clinical significance of paraneoplastic syndromes in patients with hepatocellular carcinoma] Korean J Hepatol. 2005;11(3):275–283. [PubMed] [Google Scholar]
- 13.Goldberg RB, Bersohn I, Kew MC. Hypercholesterolaemia in primary cancer of the liver. S Afr Med J. 1975;49(36):1464–1466. [PubMed] [Google Scholar]
- 14.Sorlini M, Benini F, Cravarezza P, Romanelli G. Hypoglycemia, an atypical early sign of hepatocellular carcinoma. J Gastrointest Cancer. 2010;41(3):209–211. doi: 10.1007/s12029-010-9137-0. [DOI] [PubMed] [Google Scholar]
- 15.Oldenburg WA, van Heerden JA, Sizemore GW, Abboud CF, Sheedy PF 2nd. Hypercalcemia and primary hepatic tumors. Arch Surg. 1982;117(10):1363–1366. doi: 10.1001/archsurg.1982.01380340077018. [DOI] [PubMed] [Google Scholar]
- 16.Chang PE, Tan CK. Paraneoplastic erythrocytosis as a primary presentation of hepatocellular carcinoma. Indian J Med Sci. 2009;63(5):202–203. doi: 10.4103/0019-5359.53167. [DOI] [PubMed] [Google Scholar]
- 17.Walcher J, Witter T, Rupprecht HD. Hepatocellular carcinoma presenting with paraneoplastic demyelinating polyneuropathy and PR3-antineutrophil cytoplasmic antibody. J Clin Gastroenterol. 2002;35(4):364–365. doi: 10.1097/00004836-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Arguedas MR, McGuire BM. Hepatocellular carcinoma presenting with chronic inflammatory demyelinating polyradiculoneuropathy. Dig Dis Sci. 2000;45(12):2369–2373. doi: 10.1023/A:1005690908999. [DOI] [PubMed] [Google Scholar]
- 19.Hinterhuber G, Drach J, Riedl E, Bohler K, Ferenci P, Wolff K, Foedinger D. Paraneoplastic pemphigus in association with hepatocellular carcinoma. J Am Acad Dermatol. 2003;49(3):538–540. doi: 10.1067/S0190-9622(03)01581-0. [DOI] [PubMed] [Google Scholar]
- 20.Coeytaux A, Kressig R, Zulian GB. Hepatocarcinoma with concomitant paraneoplastic encephalomyelitis. J Palliat Care. 2001;17(1):59–60. [PubMed] [Google Scholar]
- 21.Luo JC, Hwang SJ, Wu JC, Li CP, Hsiao LT, Lai CR, Chiang JH. et al. Paraneoplastic syndromes in patients with hepatocellular carcinoma in Taiwan. Cancer. 1999;86(5):799–804. doi: 10.1002/(SICI)1097-0142(19990901)86:5<799::AID-CNCR15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Ryu T, Nishimura S, Miura H, Yamada H, Morita H, Miyazaki H, Kitamura S. et al. Thrombopoietin-producing hepatocellular carcinoma. Intern Med. 2003;42(8):730–734. doi: 10.2169/internalmedicine.42.730. [DOI] [PubMed] [Google Scholar]
- 23.Chen CC, Chang JY, Liu KJ, Chan C, Ho CH, Lee SC, Chen LT. Hepatocellular carcinoma associated with acquired von Willebrand disease and extreme thrombocytosis. Ann Oncol. 2005;16(6):988–989. doi: 10.1093/annonc/mdi171. [DOI] [PubMed] [Google Scholar]
- 24.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, Darbonne WC. et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 25.Lok S, Kaushansky K, Holly RD, Kuijper JL, Lofton-Day CE, Oort PJ, Grant FJ. et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369(6481):565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]


