Abstract
Context
The impact of long-term cross-sex hormone therapy (CSHT) in transgender men and women is still uncertain.
Objective
To perform a systematic review and meta-analysis and update the evidence regarding the effects of CSHT on bone mineral density (BMD) in transgender men and women.
Data Sources
Medline, Cochrane Central Register of Controlled Trials, and Embase were searched for studies published until August 2018.
Study Selection
Of 10,849 studies, 19 were selected for systematic review. All included patients were aged >16 years and received CSHT with BMD assessment by dual-energy X-ray absorptiometry (DXA).
Data Extraction
Data on BMD, CSHT, and clinical factors affecting bone mass were collected. A National Institutes of Health scale was used to assess the quality of studies.
Data Synthesis
Nineteen studies were meta-analyzed (487 trans men and 812 trans women). In trans men, mean BMD difference compared with natal women was not significant in any site in either cross-sectional or before-after studies. In trans women, mean BMD difference was not significant compared with natal men at the femoral neck, total femur, and lumbar spine in cross-sectional studies; before-after studies reported a slight but significant increase in lumbar spine BMD after 12 and ≥24 months of treatment.
Conclusions
Long-term CSHT had a neutral effect on BMD in transgender men. In transgender women, only lumbar spine BMD seemed to be affected after CSHT. This evidence is of low to moderate quality as a result of the observational design of studies, small sample sizes, and variations in hormone therapy protocols.
Keywords: gender incongruence, cross-sex hormone therapy, bone mineral density, DXA
Transgender people experience a deep and persistent sense of incongruence between their gender of identity and the sex attributed to them at birth, with distress lasting for ≥6 months [1–3]. Hormone therapy and gender-affirming surgery (GAS) are the main therapeutic strategies for gender transition. Cross-sex hormone therapy (CSHT) suppresses gonadal hormones and secondary sex characteristics of the biological sex while inducing body characteristics of the gender of identity [4]. Although gender transition has been associated with improvement in mental health and other areas of functioning [4–6], the full long-term effects of CSHT are still uncertain.
Sex steroids are major determinants of bone homeostasis. In boys, during puberty, testosterone stimulates periosteal apposition, leading to increased bone width and size compared with girls, despite the similar cortical thickness [7]. In turn, estrogen plays a main regulatory role in bone metabolism in both women and men, acting on bone remodeling and keeping it within physiological limits. Estradiol acts on the lifespan of osteoblasts, decreasing apoptosis and increasing the functional capacity of individual osteoblasts. In osteoclasts, estradiol induces apoptosis and decreases cellular differentiation [8]. Estrogen deficiency is associated with an imbalance between bone resorption and bone formation that is linked to osteoblast apoptosis, oxidative stress, and osteoblastic NF-κB (RANKL) activity.
Not much is known about the effects of CSHT on bone mass in transgender individuals [9]. Recent data from transgender men (female to male) and women (male to female) receiving hormone therapy have shown an increase in bone mineral density (BMD) after 12 months of treatment [10]. Another study on long-term testosterone therapy reported larger cortical bone size in trans men compared with natal females [11]. Conversely, trans women receiving estrogen therapy may lose lean mass in association with androgen deprivation, which over time can lead to smaller bones [12] and higher prevalence of low bone mass [13, 14].
To date, few studies evaluating the impact of CSHT on bone mass have been published, and a definitive conclusion has not been reached. A previous meta-analysis including 13 studies has assessed the relationship between hormone therapy and BMD. The results suggested that BMD was not significantly different in trans men and that lumbar spine BMD was increased in transwomen with CSHT [15]. Since then, however, new evidence has become available. Therefore, the aim of the present systematic review and meta-analysis was to update the available evidence regarding the effect of CSHT on BMD in transgender men and women.
1. Materials and Methods
This study was performed in accordance with Cochrane Collaboration guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis [16].
A. Eligibility Criteria, Search Strategy, and Study Selection
The research question was developed according to the PICOS strategy: the population (P) was defined as transgender individuals; the intervention (I) was defined as CSHT; the comparison group (C) corresponded to natal women and men with no gender incongruence; the outcome (O) was defined as BMD assessed by dual-energy X-ray absorptiometry (DXA); and the study design (S) was defined to include noninterventional case-control, cross-sectional, or cohort studies with ≥10 participants in each group.
Medline, Cochrane Central Register of Controlled Trials (accessed through Wiley Science), and Embase were searched for studies published until August 2018. We also searched http://ClinicalTrials.gov to retrieve randomized controlled trials with unpublished results. The following medical subject headings were used in the search: bone AND transsexualism OR “transgender person” OR “person, transgender” OR “persons, transgender” OR “transgender persons” OR “transgender” OR “transgenders” OR “transgendered persons” OR “person, transgendered” OR “persons, transgendered” OR “transgendered person” OR “transsexual persons” OR “person, transsexual” OR “persons, transsexual” OR “transsexual person.” There were no year or language restrictions. Studies with children and adolescents <16 years of age were not included. Previous GAS was not an exclusion criterion.
In case multiple reports of the same study were identified, the most complete report was chosen. If the abstracts did not provide enough information about inclusion and exclusion criteria, the full text was retrieved for evaluation.
Titles and abstracts of all articles retrieved were independently reviewed by two investigators to assess eligibility of the studies for inclusion in the systematic review and meta-analysis (T.M.F. and T.R.S.). The selected articles were read in full for confirmation of eligibility and data extraction. Disagreements were resolved by consensus or by consultation with a third reviewer (P.M.S.). If the required data were not located in the published article, authors were contacted to provide the missing information.
B. Data Extraction and Quality Control Assessment
The following data were extracted from each study: name of first author and study group, publication year, country, study design, number of participants, age, body mass index (BMI), smoking, alcohol consumption, physical activity, serum vitamin D levels, calcium intake, use of calcium and vitamin D supplements, duration of CSHT treatment, previous (GAS), duration of follow-up, bone mineral density, and T- score and Z-score for BMD at various sites and CSHT duration. Exclusion criteria for each study, where available, were also collected. DXA data of the forearm, total femur, femoral neck, and lumbar spine were extracted, as were the type of equipment and manufacturer.
A National Institutes of Health scale (retrieved in September 2018 from www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) was used to assess the quality of before-after (pre-post) and cross-sectional studies included in the meta-analysis. This scale includes items for evaluating potential flaws in the study methods or implementation, covering sources of bias, confounding, study power, the strength of causality in the association between interventions and outcomes, and other factors.
C. Statistical Analysis
Meta-analyses were performed separately for each outcome, with mean differences (MDs) used to evaluate CSHT effects. When SDs were missing, conservative imputations were made with the biggest SD observed in the other studies for the same outcome. Mean differences were pooled via random effects models with the DerSimonian and Laird variance estimator. The results were stratified by study design (cross-sectional or before-after) and follow-up time (12 and ≥24 months). I2 statistics and the Cochran Q test were used to assess heterogeneity among studies. All statistical tests were two-tailed, and significance was defined as P < 0.05. Statistical analyses were performed with R version 3.5.0 (R Foundation for Statistical Computing).
2. Results
A. Study Selection
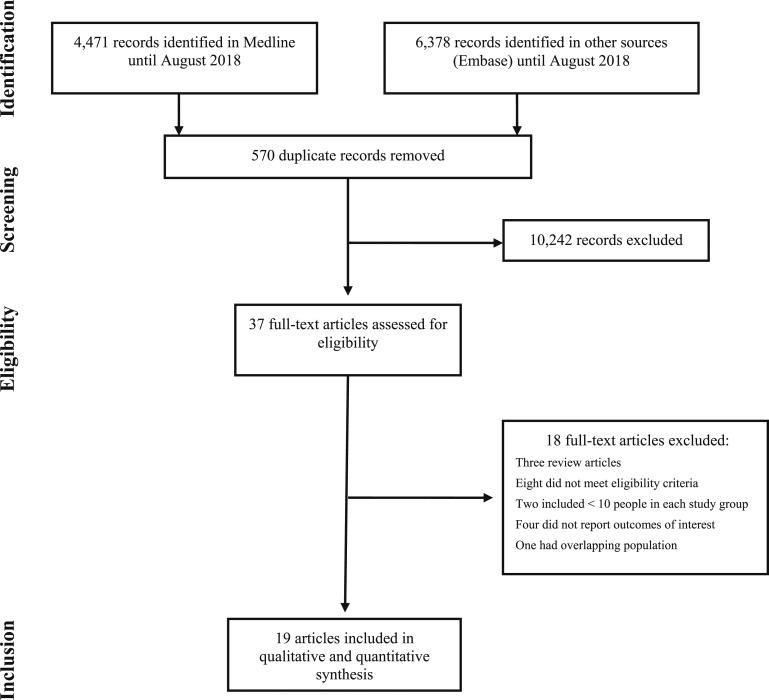
The primary search identified 10,849 articles. After title and abstract screening and exclusion of duplicates, 25 potentially eligible studies were retrieved for full-text analysis. Of these, 19 were included in the qualitative review (Fig. 1) and also in the meta-analyses [10–13, 17–31]. Three studies presented both cross-sectional and before-after data [13, 21, 29]; one of them was considered in the meta-analyses of both designs [13]. The other two were included only in before-after analyses, because participants were not using CSHT at the baseline evaluation [21, 29].
Figure 1.
PRISMA flow diagram of the study selection process.
Therefore, six cross-sectional studies analyzing the use of hormone therapy in transgender individuals vs controls [11–13, 17–19] and 14 studies evaluating BMD in transgender individuals before and after CSHT [10, 13, 20–31] were meta-analyzed.
B. Description of the Studies
Table 1 summarizes the characteristics of the six cross-sectional studies, and Table 2 describes the 14 before-after CSHT studies.
Table 1.
Characteristics of the Cross-Sectional Studies Included in the Meta-Analysis
| Study | Country | Comparison Group | Duration of Exposure (mo) | N | Age (y)a | BMI (kg/m2)a | Post-GAS (%) | Intervention |
|---|---|---|---|---|---|---|---|---|
| Transgender men | ||||||||
| Van Caenegem et al. 2012 [11] | Belgium | Natal women | 119 (9–264) | 50 | 37 ± 8 | 24.8 ± 3.8 | 100 | Testosterone esters every 2–3 wk or testosterone undecanoate 1000 mg every 12 wk or transdermal testosterone 50 mg/d |
| Broulik et al. 2018 [18] | Czech Republic | Natal women | 216 ± 36 | 35 | 47.22 ± 4 | 25.67 ± 3.73 | 100 | Testosterone isobutyrate 25 mg IM every wk, or testosterone propionate 250 mg every third wk IM, or testosterone undecanoate 4 × 40 mg daily |
| Transgender women | ||||||||
| Reutrakul et al. 1998 [19] | Thailand | Natal men | 13.9 | 11 | 21.2 ± 1.1 | NA | 0 | Estradiol valerate 10 mg IM 1–4 per mo, or mestranol 0.05 mg + norethisterone 1 mg/d, or ethinylestradiol and levonorgestrel, or cyproterone 1–4 tablets per d, or conjugated equine estrogen 1.25 mg 1 or 2 tablets per d |
| 59.8 | 17 | 24.1 ± 0.8 | NA | 0 | ||||
| Sosa et al. 2003 [17] | Spain | Natal men | 201 (36–420) | 27 | 43.0 ± 7.7 | 26.0 ± 4.7 | 0 | Ethinylestradiol + cyproterone acetate, or levonorgestrel, or oral conjugated equine estrogen, or depot estrogens (estradiol valerate or mestranol + norethisterone) |
| Lapauw et al. 2008 [12] | Belgium | Natal men | 96 (48–240) | 23 | 41 ± 7.0 | 24.4 ± 5.0 | 100 | Cyproterone acetate 50–100 mg/d + ethinylestradiol 25–50 μg/d |
| After surgery: ethinylestradiol 25–50 μg/d, estradiol valerate 2 mg/d, conjugated equine estrogens 1.25 mg/d, or transdermal estradiol | ||||||||
| Fighera et al. 2018 [13] | Brazil | Natal men | Undetermined; because 86.6% of participants were previously self-medicating with CSHT for variable periods of time, baseline assessment was performed 3 mo after the start of the standard treatment | 142 | 33.70 ± 10.29 | 25.37 ± 4.62 | 33 | Oral estradiol valerate 1–4 mg/d, or transdermal 17β estradiol 0.5–2.0 mg/d, or conjugated equine estrogen 0.625–2.500 mg/d with spironolactone 50–100 mg/d, or cyproterone acetate 50–100 mg/d |
| After GAS only estradiol was used |
Abbreviation: IM, intramuscular.
Mean ± SD.
Table 2.
Characteristics of the Before-After CSHT Studies Included in the Meta-Analysis
| Study | Country | Duration of Exposure | N | Agea (y) | BMI (kg/m2)a | Post-GAS (%) | Intervention |
|---|---|---|---|---|---|---|---|
| Transgender men | |||||||
| Van Kesteren et al. 1996 [25] | Netherlands | 12 mo | 35 | 25 (16–40) | 23 (17–32) | 0 | Testosterone esters every 2 wk IM (Sustanon 250 mg or Testoviron 180 mg) or testosterone undecanoate 160 mg/d (oral) |
| Van Kesteren et al. 1998 [24] | Netherlands | 38.2 mo [28–53] | 19 | 25 (16–39) | 22.1 ± 2.7 | 100 | Testosterone esters 250 mg every 2 wk |
| After GAS testosterone IM every 2–3 wk | |||||||
| Turner et al. 2004 [28] | USA | 24 mo | 15 | 37.0 ± 3.0 | NA | 33.3 | Testosterone esters IM (70.7 ± 4.5 mg weekly) |
| Haraldsen et al. 2007 [29] | Norway | 12 mo | 21 | 25.1 ± 4.8 | NA | 0 | Testosterone enanthate IM 250 mg every 3 wk |
| Mueller et al. 2010 [27] | Germany | 24 mo | 45 | 30.4 ± 9.1 | 24.1 ± 4.5 | 0 | Testosterone undecanoate 1000 mg IM every 12 wk |
| Pelusi et al. 2014 [31] | Italy | 12 mo | 15 | 30.9 (27.9–33.9) | 22.3 (19.9–24.6) | 0 | Testosterone enanthate IM 100 mg every 10 d |
| 15 | 29.4 (26.6–32.1) | 23.9 (21.1–26.6) | 0 | Testosterone gel 50 mg/d | |||
| 15 | 28.2 (25.6–30.9) | 22.1 (19.5–24.6) | 0 | Testosterone undecanoate 1000 mg every 12 wk | |||
| Van Caenegem et al. 2015 [20] | Belgium | 12 mo | 23 | 27 ± 9 | 24.5 ± 5.3 | 0 | Testosterone undecanoate 1000 mg IM at week 0, 6, 18 and every 12 wk after |
| Wiepjes et al. 2017 [10] | Belgium, Norway, Italy, Netherlands | 12 mo | 199 | 24 (21–31) | 23.9 (21.3–28.8) | 0 | Testosterone gel 50 mg/d or testosterone esters 250 mg IM every 2 wk, or testosterone undecanoate 1000 mg IM every 12 wk |
| Transgender women | |||||||
| Van Kesteren et al. 1996 [25] | Netherlands | 12 mo | 56 | 33 (16–69) | 22 (17-28) | 0 | Ethinylestradiol 100 µg/d or transdermal estradiol twice a wk, or estradiol valerate injection or oral conjugated estrogens with cyproterone acetate 100 mg/d or spironolactone |
| Van Kesteren et al. 1998 [24] | Netherlands | 45.5 mo [32–63] | 20 | 25.4 (16–38) | 22.1 ± 2.4 | 100 | Ethinylestradiol 100 μg/d with cyproterone acetate 100 mg/d until GAS, after which only estradiol was used |
| Dittrich et al. 2005 [22] | Germany | 24 mo | 60 | 38.37 ± 11.36 | 24.19 ± 4.34 | 0 | Oral estradiol-17β valerate 6 mg/d with 3.8 mg goserelin acetate every 4 wk |
| Mueller et al. 2005 [23] | Germany | 24 mo | 40 | 38.39 ± 11.09 | 24.02 ± 4.00 | 0 | Oral estradiol-17β valerate 6 mg/d with 3.8 mg goserelin acetate every 4 wk |
| Haraldsen et al. 2007 [29] | Norway | 12 mo | 12 | 29.3 ± 7.8 | NA | 0 | Ethinylestradiol 50 μg/d in the first 3 mo and 100 μg/d thereafter |
| Mueller et al. 2011 [30] | Germany | 24 mo | 84 | 36.3 ± 11.3 | 22.3 (21.7–23.0) | 0 | Estradiol 17β valerate 10 mg IM every 10 d with 3.8 mg goserelin acetate every 4 wk |
| Van Caenegem et al. 2015 [21] | Belgium | 24 mo | 49 | 33 ± 12 | NA | 0 | Oral estradiol valerate, 4 mg/d or transdermal 17β estradiol 100 µg/d (age >45 y) combined with oral cyproterone acetate 50 mg/d |
| Gava et al. 2016 [26] | Italy | 12 mo | 20 | 32.9 ± 9.4 | 22.0 | 0 | Transdermal estradiol 1–2 mg/d with cyproterone acetate 50 mg/d |
| 20 | 29.4 ± 10.2 | 21.9 | 0 | Transdermal estradiol 1–2 mg/d with leuprolide acetate 3.75 IM every mo | |||
| Wiepjes et al. 2017 [10] | Belgium, Norway, Italy, Netherlands | 12 mo | 231 | 28 (23–42) | 22.5 (20.5–26.1) | 0 | Estradiol valerate 2–4 mg/d or estradiol patch 50–100 mg twice a wk with cyproterone acetate 50–100 mg/d |
| Fighera et al. 2018 [13] | Brazil | 31 mo [12–40] | 46 | 33.70 ± 10.29 | 25.66 ± 4.16 | 33 | Oral estradiol valerate, 1–4 mg/d, or transdermal 17β estradiol 0.5–2.0 mg/d, or conjugated equine estrogen 0.625–2500 mg/d combined with spironolactone 50–100 mg/d, or cyproterone acetate 50–100 mg/d |
| After GAS only estradiol was used |
Abbreviation: IM, intramuscular.
Mean ± SD.
All 19 studies included trans men receiving CSHT from 12 months to 18 years or trans women receiving CSHT from 12 months to 16 years. Eight studies had a control group [11–13, 17–21], corresponding to natal men for comparisons with trans women and natal women for comparisons with trans men. Before-after studies were assigned good [10, 21, 27] and fair [13, 20, 22–26, 28–31] National Institutes of Health quality assessment scores, whereas all cross-sectional studies were scored as fair [11, 12, 17–19].
Hormone dosages and formulations are described in Tables 1 and 2, stratified by the identity gender. In studies with trans women, the most common CSHT was cyproterone acetate with oral estradiol (valerate, conjugated equine estrogen, or ethinylestradiol) or transdermal estradiol in both cross-sectional studies [12, 13, 17, 19] (Table 1) and before-after studies [10, 21–26, 30] (Table 2). Two studies used oral and parenteral contraceptives as well as oral estrogens [17, 19]. Another two studies used spironolactone as antiandrogen therapy added to estrogens [13, 25], and four studies used GnRH analogs associated with estrogen therapy [22, 23, 26, 30]. Cross-sectional [11, 18] (Table 1) and before-after studies [10, 20, 24, 25, 27–29, 31] (Table 2) with trans men used parenteral testosterone esters or testosterone undecanoate. Three studies also included patients using transdermal testosterone [11, 10, 31], and one study included patients with oral testosterone [25].
Six studies included transgender women [12, 13, 24] or men [11, 18, 24, 28] who had previously undergone GAS. Most before-after studies evaluated exclusively transgender women [10, 21–26, 29, 30] or men [10, 20, 24, 25, 27, 29, 31] without previous CSHT. Other exclusion criteria reported in several studies were use of glucocorticoids or bisphosphonates, renal or hepatic disease, alcohol abuse, bone diseases, or severe comorbidities. DXA assessments were performed with equipment manufactured by Hologic, Norland, or GE medical systems.
Additional clinical data, such as calcium intake, serum vitamin D levels, calcium and vitamin D supplements, smoking habit, alcohol consumption, and physical activity, were reported in some studies (Tables 3 and 4). The reported rate of alcohol consumption varied from 4.6% to 75% depending on the criteria used: >7 drinks per week [10] or casual consumption [26]. In four studies, alcohol abuse was an exclusion criterion [11, 17, 18, 29], and one study adjusted the results for alcohol consumption [10]. Smoking prevalence varied from 12% [11] to 77% [31] and was >50% in four studies [24, 26, 28, 31]. Two studies made adjustments in BMD data for cigarette smoking [10, 12]. Calcium intake was reported in only three cross-sectional studies [12, 17, 19], and areal BMD was adjusted for calcium intake in one study [12]. Vitamin D levels varied from 11.5 ng/mL [31] to 38.5 ng/mL [18] in trans men, and from 16 [21] to 23 ng/mL [12] in trans women. Only one study adjusted bone mass for vitamin D status in trans women [21]. Regarding physical activity, different criteria were used in trans men and trans women. Among those using Baecke’s questionnaire, the scores ranged from 2.68 [12] to 8.9 [20]. Some studies presented BMD results already adjusted for weight or height [11, 17, 19, 20].
Table 3.
Risk of Bias in Cross-Sectional Studies Designed to Assess BMD in Transgender People
| Study | Calcium/Vitamin D Supplements | Calcium Intake | Serum Vitamin D | Smoking | Alcohol Abuse | Physical Activity | Adjustments for BMD | Exclusion Criteria |
|---|---|---|---|---|---|---|---|---|
| Transgender men | ||||||||
| Van Caenegem et al. 2012 [11] | 6% | — | — | 28%; 7–12 pack-y | No | 8.4 ± 1.8 (Baecke’s) | Body weight and height | Illnesses or medications known to affect body composition, hormone levels, or bone metabolism; current or previous use (>2 y) of glucocorticoids, oral contraception, (anti)androgens (except CSHT in FtM), calcium and vitamin D supplements (allowed for FtM, n = 3), insulin, antiepileptic drugs, calcitonin, bisphosphonates, hypogonadism, untreated hyperthyroidism, cystic fibrosis, malabsorption, eating disorders, disorders of collagen or bone metabolism, chronic renal failure, alcohol abuse, autoimmune rheumatoid disease |
| Control natal women | None | — | — | 12%; 3–6 pack-y | No | 8.3 ± 1.5 (Baecke’s) | — | |
| Dan Broulik et al. 2018 [18] | — | — | 19.95 ± 11 ng/mL | 25% | No | — | — | Use of medications known to affect BMD other than calcium, vitamin D, or multivitamins, smoking >10 cigarettes daily, alcohol abuse |
| Control natal women | — | — | 38.5 ± 11.8 ng/mL | 20% | No | — | — | |
| Transgender women | ||||||||
| Reutrakul et al. 1998 [19] 13.9 mo |
— | 0.7 ± 0.2 Glasses of milk per wk | — | 2.3 ± 1.6 pack-y | — | 1.2 ± 0.5 y of physical activity (>3 times per wk) | Body weight | None of the subjects had medical history or risk factors for osteoporosis such as hyperparathyroidism, thyroid disorders, or glucocorticoid usage. |
| 59.8 mo | — | 0.5 ± 0.1 Glasses of milk per wk | — | 4.0 ± 0.9 pack-y | — | 4.3 ± 1.1 y of physical activity (>3 times/wk) | Body weight | |
| Sosa et al. 2003 [17] | None | 773.9 ± 257.9 mg/d | — | 48% | 68% | 36% (active) | Body weight and height | Drugs that might affect bone density, hepatic or renal disorders, alcoholism, Paget disease, gonadectomy, hyperparathyroidism, osteoporotic fracture, HIV infection |
| Control natal men | None | 652.1 ± 265.6 mg/d | — | 40% | 72% | 48% (active) | Body weight and height | |
| Lapauw et al. 2008 [12]a | — | 528 (431–772) mg/d | 23 (14–33) ng/mL | 43.5% | 1.5 (0.8–12) units/wk | 2.91 ± 0.71 (Baecke’s) | A multivariate analysis explored the contributions of muscle strength, physical activity, age, smoking, and calcium intake. Muscle strength predicted cortical bone size. Current smoking was associated with lower BMD at the lumbar spine. Negative association between calcium intake and periosteal or endosteal circumference, and positive association between physical activity and cortical BMC and bone area.b | — |
| Control natal men | — | 544 (423–804) mg/d | 18 (13–25) ng/mL | 17.4% | 9.0 (3.0–16.5) units/wk | 2.68 ± 0.79 (Baecke’s) | — | — |
| Fighera et al. 2018 [13] | — | — | — | — | — | — | — | Other treatment protocol |
Abbreviation: FtM, female to male.
Interquartile range.
Data not shown.
Table 4.
Risk of Bias in Before-After Studies Designed to Assess BMD in Transgender People
| Study | Serum Vitamin D | Smoking | Alcohol Abuse | Physical Activity | Adjustments for BMD | Exclusion Criteria |
|---|---|---|---|---|---|---|
| Transgender men | ||||||
| Van Kesteren et al. 1996 [25] | — | 45.7% | 17.1% (>3 drinks per d) | — | — | Subjects with risk factors for osteoporosis (e.g., hyperparathyroidism or thyroid disorders) were excluded. All subjects were CSHT-naive. |
| Van Kesteren et al. 1998 [24] | — | 52.6% | 15.7% (>3 drinks per wk) | — | — | All subjects were CSHT-naive. |
| Turner et al. 2004 [28] | — | 53.3% | — | — | — | Use of medications known to affect BMD other than calcium and multivitamins, current pregnancy |
| Haraldsen et al. 2007 [29] | — | — | No | — | — | Endocrinological, genetic, neurologic, or major psychiatric comorbidity. All patients were free of any medication, alcohol, or drug abuse. |
| Mueller et al. 2010 [27] | — | — | — | — | — | Previous CSHT, abnormalities in the screening laboratory panel |
| Pelusi et al. 2014 [31]a Testosterone enanthate IM | 11.5 (−0.9–23.9) ng/mL | 33% | 26% (casual) | — | — | Use of medication for hypertension, hyperlipidemia, diabetes, depression, or any psychiatric drugs. All patients were CSHT-naive. |
| Testosterone gel | 14.6 (8–21.2) ng/mL | 77% | 69% (casual) | — | — | |
| Testosterone undecanoate IM | 23.9 (18.6–29.2) ng/mL | 47% | 73% (casual) | — | — | |
| Van Caenegem et al. 2015 [20] | 19 ± 11 ng/mL | 0 (3–4) pack-y | — | 8.9 ± 2.2 (Baecke’s) | Height | Previous CSHT, anorexia, cerebral palsy, refusal, other treatment protocol |
| Wiepjes et al. 2017 [10]b | 54 (31–77) nmol/L | 29.3% | 4.6% (>7 drinks per wk) | — | The gain in lumbar spine and femoral neck did not change after adjustment for increase in body weight (+2 kg), but an attenuation of total femur BMD change was observed. No significant change in BMD was observed after adjustment for alcohol or cigarette consumption and vitamin D supplement use. After 3 and 12 mo, estradiol and testosterone levels were not correlated with BMD change.c | Previous CSHT, psychological vulnerability, insufficient knowledge of the protocol language |
| Transgender women | ||||||
| Van Kesteren et al. 1996 [25] | — | 46.4% | 14.3% (>3 drinks per d) | — | — | Subjects with risk factors for osteoporosis (e.g., hyperparathyroidism or thyroid disorders) were excluded. All patients were CSHT-naive. |
| Van Kesteren et al. 1998 [24] | — | 40% | 15% (>3 drinks per wk) | — | — | All patients were CSHT-naive. |
| Dittrich et al. 2005 [22] | — | — | — | — | — | — |
| Mueller et al. 2005 [23] | — | — | — | — | — | Medications known to affect calcium metabolism (glucocorticoids, anticonvulsants, calcium or vitamin D supplements, calcitonin, bisphosphonates), significant abnormalities in laboratory panel, previous estrogen therapy due to self-medication. |
| Haraldsen et al. 2007 [29] | — | — | No | — | — | Endocrinological, genetic, neurologic, or major psychiatric comorbidity. All subjects were free of any medication, alcohol, or drug abuse. |
| Mueller et al. 2011 [30] | — | — | — | — | — | Previous CSHT, longer periods of illness or immobility, subjects needing treatment change |
| Van Caenegem et al. 2015 [21] | 16 ± 8 ng/mL | 19.1 pack-y | 2 (0–7) drinks per wk | 8.3 ± 1.6 (Baecke’s) | The increase in bone mass did not change after adjustment for age, BMI, fat mass, 25(OH) vitamin D status, PTH, or leptin.c | Previous CSHT, hypergonadotropic hypogonadism, gastric bypass, unwillingness |
| Control natal men | 23 ± 7 ng/mL | 0 (0–8) pack-y | 10 (3–16) drinks per wk | 8.7 ± 1.5 (Baecke’s) | — | |
| Gava et al. 2016 [26] CPA + E |
56.2 ± 27.8 nmol/L | 35% | 75% (casual) | — | — | Previous CSHT, use of medications for hypertension, hyperlipidemia, diabetes, psychiatric history, drug use |
| Leu + E | 47.8 ± 28.3 nmol/L | 65% | 35% (casual) | — | — | |
| Wiepjes et al. 2017 [10]b | 34 (22–52) nmol/L | 23.5% | 6.1% (>7 drinks per wk) | — | Gain in lumbar spine BMD did not change after adjustment for weight (+2.4 kg), but an attenuation of total femur and femoral neck BMD was observed. Femoral neck BMD increased more in trans women who used vitamin D supplements compared with those who did not use supplements. No significant change in BMD was observed after adjustment for alcohol and cigarette consumption. After 3 and 12 mo, the estradiol levels was correlated with BMD change.a | Previous CSHT, psychological vulnerability, insufficient knowledge of protocol language |
| Fighera et al. 2018 [13] | — | — | — | — | — | Other treatment protocol |
No study reported use of calcium supplements or calcium intake.
Abbreviations: FtM, female to male; CPA, cyproterone acetate; LEU, leuprolide acetate; PTH, parathyroid hormone.
Median (95% CI).
Interquartile range.
Data not shown.
C. Data Synthesis and Meta-Analyses
A total of 812 trans women and 487 trans men were evaluated in the cross-sectional and before-after studies. Data were analyzed regardless of the dosage or route of hormone therapy, because this information was not provided in most studies. Meta-analysis of T-score and Z-score for BMD considering the various sites and CSHT durations was not possible, because for each site the scores were presented in only one study [11–13, 17, 19, 23, 27]. No study reported data on osteoporotic fractures.
Sixteen studies were performed in European countries. The only study from the United States included 15 trans men [28]. Two studies from Asia and Latin America included 28 and 142 trans women, respectively [13, 19].
D. Transgender Women
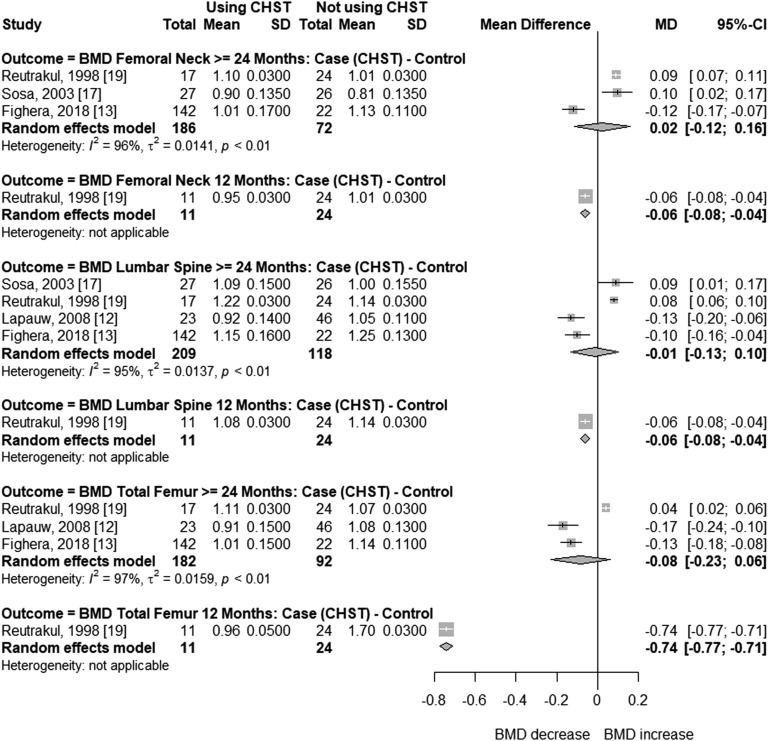
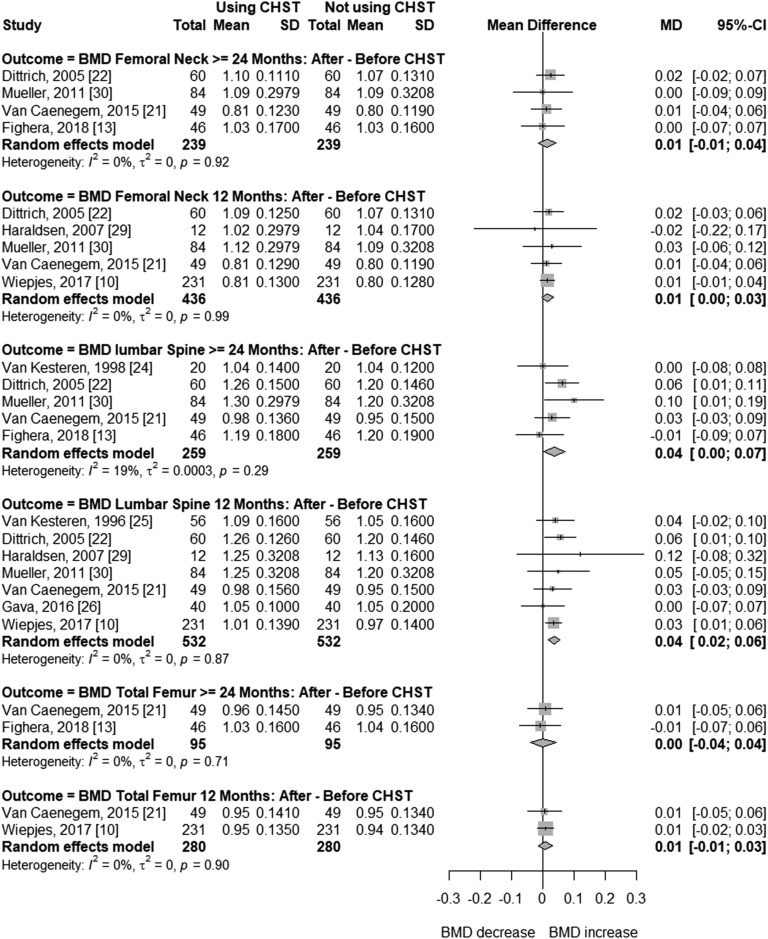
In trans women, the follow-up time in before-after studies ranged from 12 to 45.5 months. In cross-sectional studies, CSHT time varied from 5 to 16 years. In at least two studies, mean age was >40 years [12, 17]. In two studies, mean BMI was slightly higher than 25 kg/m2 (25.3 kg/m2 and 26.0 kg/m2) [13, 17]. Figure 2 presents the meta-analysis of BMD changes at different sites in transgender women receiving CSHT of various durations vs natal men (cross-sectional studies). BMD was not significantly different at the femoral neck (MD = 0.02; 95% CI, –0.12 to 0.16; P = 0.753), total femur (MD = –0.08; 95% CI, –0.23 to 0.06; P = 0.258), or lumbar spine (MD = –0.01; 95% CI, –0.13 to 0.10; P = 0.806) with ≥24 months of CSHT. Each analysis included three or four studies, with high between-study heterogeneity (I2 = 95% to 97%). In two of these studies, high dosages of estrogen or contraceptive pills (up to 4 tablets per day) were used [17, 19]. In the other two, around one-third [13] or all participants [12] had previously undergone GAS procedures. Regarding before-after studies (Fig. 3), BMD values were not significantly different in the total femur after 12 (MD = 0.01; 95% CI, –0.01 to 0.03; P = 0.465) and ≥24 months (MD = 0.00; 95% CI, –0.04 to 0.04; P = 0.950), or in the femoral neck after 12 (MD = 0.01; 95% CI, 0.00 to 0.03; P = 0.121) and ≥24 months of CSHT (MD = 0.01; 95% CI, −0.01 to 0.04; P = 0.315), with no heterogeneity between studies (I2 = 0% for all analyses). In turn, meta-analysis of lumbar spine BMD showed slightly positive mean differences at 12 months (MD = 0.04; 95% CI, 0.02 to 0.06; P = 0.0001; I2 = 0%) and ≥24 months (MD = 0.04; 95% CI, 0.00 to 0.07; P = 0.036; I2 =19%) of hormone therapy (Fig. 3).
Figure 2.
Forest plot showing BMD in cross-sectional studies with transgender women and control natal men.
Figure 3.
Forest plot showing BMD in before-after CSHT studies with transgender women.
E. Transgender Men
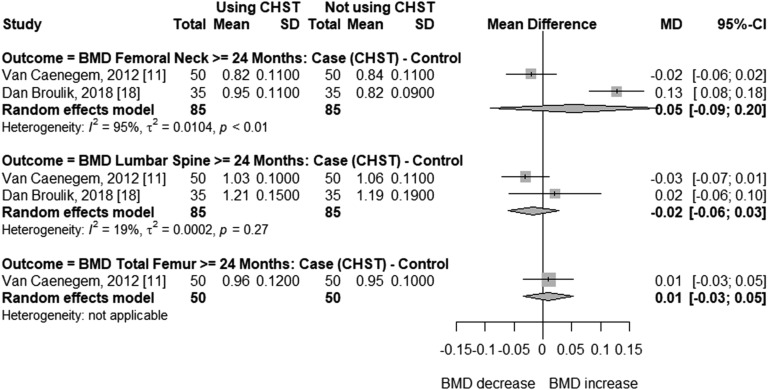
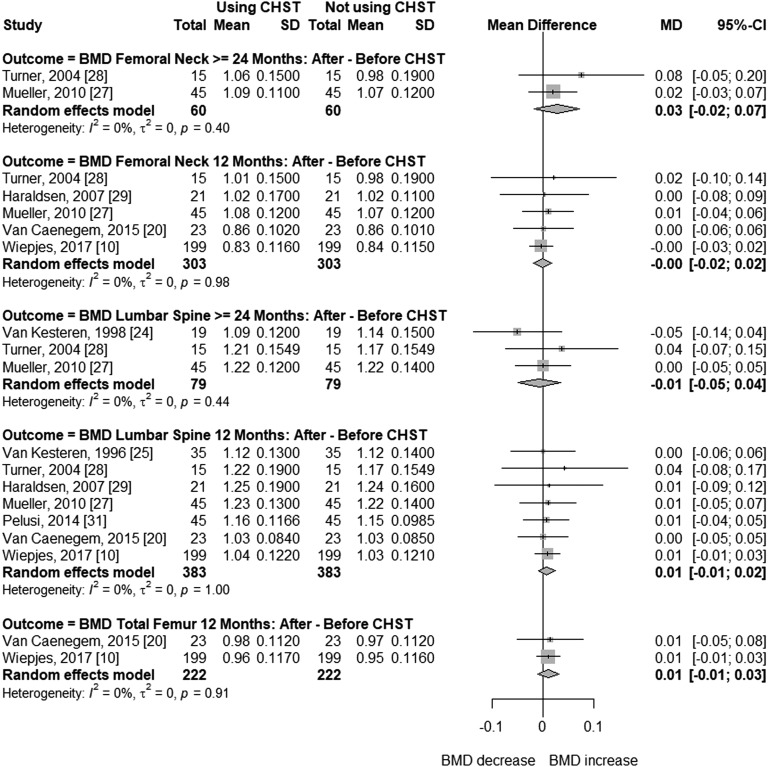
In cross-sectional studies with trans men, age ranged from 37 to 47 years, and duration of CSHT ranged from 9.9 to 18 years. Compared with cross-sectional studies, participants of before-after studies were younger (24 to 37 years) and had shorter time of CSHT use (12 to 38 months). Mean BMI was normal in most studies, with the highest being 25.67 kg/m2 [18]. Meta-analysis of cross-sectional studies with trans men (Fig. 4) receiving CSHT for ≥24 months showed that BMD was not significantly different compared with natal women at the femoral neck (MD = 0.05; 95% CI, –0.09 to 0.20; P = 0.468) or lumbar spine (MD = –0.02; 95% CI, –0.06 to 0.03; P = 0.460). Only two studies were included in these analyses, with 85 trans men and 85 controls. High (I2 = 95%, femoral neck BMD) and low (I2 = 19%, lumbar spine BMD) heterogeneity was found between these studies (Fig. 4). The meta-analysis of before-after studies (Fig. 5) shows that femoral neck BMD did not differ significantly in trans men before or during androgen treatment of 12 (MD = –0.00; 95% CI, –0.02 to 0.02; P = 0.952) or ≥24 months (MD = 0.03; 95% CI, –0.02 to 0.07; P = 0.226). Similar results were found at the total femur after 12 months of CSHT (MD = 0.01; 95% CI, –0.01 to 0.03; P = 0.342), and at the lumbar spine after 12 (MD = –0.01; 95% CI, −0.01 to 0.02; P = 0.378) or ≥24 months (MD = –0.01; 95% CI, –0.05 to 0.04; P = 0.759), with no heterogeneity between the studies at ≥24 or 12 months.
Figure 4.
Forest plot showing BMD in cross-sectional studies with transgender men and control natal women.
Figure 5.
Forest plot showing BMD in before-after CSHT studies with transgender men.
A sensitivity analysis excluding before-after studies with transgender women [12, 13, 17, 19] and men [11, 18, 28] having previous CSHT did not change the results obtained (data not shown).
3. Discussion
In this meta-analysis including 19 cross-sectional and before-after studies, with a total of 487 trans men and 812 trans women, hormone therapy had a neutral effect on BMD at all sites evaluated, except for the lumbar spine of trans women, where a modest but significant increase in bone mass was detected. Even though all the studies considered were observational, including mostly small samples, the evidence from this meta-analysis indicates that BMD is preserved in transgender people during CSHT.
Until now, only one meta-analysis of the effects of CSHT on BMD in transgender people has been published [15]. That search was conducted for a period ending in April 2015; 13 studies were selected, with 392 trans women aged 14.9 to 43 years and 247 trans men aged 15 to 33.1 years. The authors concluded that hormone therapy did not appear to be associated with significant changes in BMD in trans men, whereas in trans women an increase in BMD was observed in the lumbar spine. Fracture data were not reported. Since then, new articles have been published [10, 13, 18, 26], some of them with larger samples and longer follow-up periods, which were included in the present updated meta-analysis.
Estrogen is considered to be a principal regulator of skeletal homeostasis in both men and women. It is involved in the synthesis of various cytokines and growth factors, with direct effects on osteocytes and osteoblasts and suppressive action on the activation of osteoclasts either directly or via osteoblasts and T-cells. Based on in vitro studies, several genes have been proposed over the years as possible targets of the antiresorptive effect of estrogens on bone. Indeed, gene transcription of many cytokines, such as IL-1β, IL-6, IL-7, tumor necrosis factor, macrophage colony-stimulating factor, RANKL, osteoprotegerin, T and B lymphocytes, macrophages, and dendritic cells, may be regulated by estrogen binding to its receptor [8, 32]. The available evidence also indicates that the estrogen receptor in bone cells plays a critical role in the accrual of cortical bone mainly by inducing the periosteal expansion and endosteal perimeter [32]. Although our meta-analysis of cross-sectional studies indicated that CSHT does not affect BMD, most studies had a small sample, with large variation in mean age (24.1 to 43.0 years). In addition, analysis of the influence of factors that could affect BMD, such as physical activity and vitamin D status, was limited by the lack of this information in many studies or because definition criteria for these factors were different between studies. All these limitations are reflected in the results of the cross-sectional studies, which presented high heterogeneity and low strength of evidence. Indeed, the two studies reporting an unfavorable impact of estrogen therapy on BMD in trans women did not describe exclusion criteria and may therefore have included subjects with health impairments [12, 13]. In turn, the studies with favorable results regarding estrogen therapy used high dosages in formulations such as oral and injectable contraceptives [17, 19].
Interestingly, some observational studies found a higher prevalence of low bone mass in trans women. Although some of these studies did not present BMD values and were not included in the present meta-analyses, they reported a prevalence of osteoporosis of about 25% in trans women with long-term CSHT [14, 33]. More recently, the results of another study by our group [13] were in line with these earlier studies, showing a prevalence of 18.3% of low bone mass in trans women after long-term CSHT, whereas no cases were observed in male or female controls [13]. Also, Lapauw et al. [12] found a prevalence of 35% of low bone mass after a mean of 96 months of estrogen therapy. The studies reporting osteoporosis or low bone mass prevalence >25% included trans women with previous GAS followed for 5 [12, 14] to 6.3 years [33] after the procedure. In our experience, hormone therapy is sometimes irregular, involving poor adherence, which may affect BMD, especially after GAS.
It should be noted that studies evaluating bone mass status in trans women used male reference values in DXA analysis, because all subjects experienced normal pubertal development, with the usual effects on bone size and geometry. This may have influenced the results of studies, overestimating the prevalence of low bone mass in transgender women. In addition, some studies used GnRH analogs associated with CSHT to suppress endogenous hormones [22, 23, 26, 30]; however, the results reported in these studies were similar to those obtained with other CSHT protocols. In this sense, research with trans women and longer follow-ups, with standardized estrogen therapy and larger samples, is needed to obtain more robust evidence on the impact of CSHT on bone mass in the long term.
Regarding the effects of CSHT in trans men, the present results show that testosterone therapy does not affect bone mass, with preservation of BMD ≤3 years after the start of CSHT in before-after studies and after 9.9 to 18 years of testosterone treatment in cross-sectional studies. In the case of these latter studies, it is important to highlight that only two could be included in the meta-analysis [11, 18]. Furthermore, there was high heterogeneity between these two studies, which also differed regarding femoral neck BMD. In this sense, whereas Broulik et al. [18] found higher BMD at the femoral neck and similar lumbar spine BMD in transgender men compared with female controls, Van Caenegem et al. [11] reported no significant differences in BMD in the femoral neck or lumbar spine. Thus, if analyzed individually, both studies show preservation of BMD. These data seem to reflect the anabolic effect of testosterone, which acts directly on androgen receptors or indirectly through aromatization to estradiol. In fact, despite testosterone being the predominant sex steroid in natal men, bioavailable estradiol levels are better correlated with male BMD than testosterone [34]. In a study comparing the addition of an aromatase (letrozole) or 5α-reductase (dutasteride) inhibitor to testosterone therapy, Meriggiola et al. [35] have shown that bone mass was significantly affected by the inhibition of aromatization, whereas the 5α reductase group produced results that were similar to those of testosterone therapy alone. That study was not included in the present meta-analysis because of the small sample size in each group (n = 5). In addition, recently published data [10] showed a larger increase in BMD in trans men at postmenopausal age compared with other age groups, possibly because estradiol levels were low at baseline and increased with testosterone aromatization.
Animal studies have also helped elucidate the role played by testosterone in bone health. In male mice, estrogen receptor deletion in osteoblasts causes a delay in cortical bone mass accrual during puberty. However, in contrast to female mice, this effect is transient; a few months later, male mice develop normal bone mass, suggesting that androgen action via androgen receptor has a compensatory effect. Interestingly, androgen receptor deletion in osteoblasts and osteocytes has no effect on cortical bone, suggesting an indirect action of androgens. Androgens may also exert anabolic actions via paracrine mechanisms by acting on muscle fibroblasts [32, 36]. In fact, muscle mass is one of the main triggers of periosteal apposition, leading to larger periosteal circumference [37]. It is important to keep in mind that DXA does not provide information on bone volume and that men have larger bones than women, which gives them greater resistance even with similar densities. Volume changes associated with the treatment would not be detected by DXA. However, the use of peripheral quantitative computed tomography, a technique that allows assessment of bone size, has shown increased volumetric BMD in transgender men [11, 20], with larger endosteal and periosteal bone circumference [11] after androgen therapy. Also, in animal models, estrogens stimulate, rather than inhibit, periosteal apposition. Low estrogen concentrations may decrease the mechanostat set point, which could indirectly increase bone sensitivity to androgens [32]. In transgender men treated with testosterone, studies show an increase in lean body mass [11, 20, 27, 31, 38] and strength [11, 20], which in turn may be associated with bone mass maintenance. These data may, at least in part, provide a mechanistic basis for the evidence generated by this meta-analysis regarding the impact of CSHT on preserving bone mass in transgender men.
In our meta-analysis, most studies evaluated eutrophic or overweight people. However, none had a mean BMI consistent with obesity, which prevented an analysis of the influence of obesity on bone density in trans people. The excess of adipose tissue has been traditionally considered a protector against fractures, because obesity is associated with higher BMD and the soft tissue padding has a protective effect against fall injuries [39]. Nevertheless, obesity seems to affect bone health through different mechanisms, such as changes in bone-regulating hormones such as leptin and adiponectin [40], increased oxidative stress, and compromised bone quality, possibly leading to structural bone damage and increased risk of fracture [41, 42]. In addition, the influence of high BMI on fracture risk varies according to skeletal site and is partially independent from BMD [41, 43].
Regarding limitations, the current study only included observational studies, most of which had small samples, with differences in participant age and CSHT duration. Also, CSHT protocols were not uniform and used different hormone types, dosages, and routes of administration. Some studies included people using CSHT associated with GnRH agonists and various hormonal antagonists. Additional limitations are related to bone geometry assessment. Because DXA results are areal, volumetric changes cannot be detected, and BMD data were collected with different equipment, so data are not comparable between studies and were not adjusted for variables known to affect bone tissue, such as vitamin D levels and physical activity. However, until long-term fracture and follow-up data from well-controlled longitudinal trials on bone health in transgender populations become available, these studies are the best available evidence on the impact of CSHT on bone mass.
In conclusion, long-term CSHT had a neutral effect on BMD in transgender men. In transgender women, no changes in femoral BMD were found, and an increase in lumbar spine BMD was observed after 12 and ≥24 months of CSHT. Even though all the studies considered were observational, including mostly small samples and diverse hormone protocols, the evidence from the present meta-analyses indicates that BMD is preserved in transgender individuals during CSHT.
Acknowledgments
Financial Support: This work was supported by grants from the Brazilian National Institute of Hormones and Women’s Health/Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq Institutos Nacionais de Ciéncia e Tecnologia (INCT) 465482/2014-7, to P.M.S.], Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS INCT 17/2551-0000519-8, to P.M.S.), and Fundo de Apoio à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA 2014-0608, to P.M.S.). The funding sources had no influence in the writing or decision to submit the article for publication.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMD
bone mineral density
- BMI
body mass index
- CSHT
cross-sex hormone therapy
- DXA
dual-energy X-ray absorptiometry
- GAS
gender-affirming surgery
- MD
mean difference
References and Notes
- 1. Dekker MJ, Wierckx K, Van Caenegem E, Klaver M, Kreukels BP, Elaut E, Fisher AD, van Trotsenburg MA, Schreiner T, den Heijer M, T’Sjoen G. A European Network for the Investigation of Gender Incongruence: endocrine part. J Sex Med. 2016;13(6):994–999. [DOI] [PubMed] [Google Scholar]
- 2. ICD-11 for mortality and morbidity statistics. December 2018. https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f411470068. Accessed 7 February 2019.
- 3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013: 451–460. [Google Scholar]
- 4. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. [DOI] [PubMed] [Google Scholar]
- 5. Costa R, Colizzi M. The effect of cross-sex hormonal treatment on gender dysphoria individuals’ mental health: a systematic review. Neuropsychiatr Dis Treat. 2016;12:1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valashany BT, Janghorbani M. Quality of life of men and women with gender identity disorder. Health Qual Life Outcomes. 2018;16(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neu CM, Rauch F, Manz F, Schoenau E. Modeling of cross-sectional bone size, mass and geometry at the proximal radius: a study of normal bone development using peripheral quantitative computed tomography. Osteoporos Int. 2001;12(7):538–547. [DOI] [PubMed] [Google Scholar]
- 8. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Caenegem E, TʼSjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–466. [DOI] [PubMed] [Google Scholar]
- 10. Wiepjes CM, Vlot MC, Klaver M, Nota NM, de Blok CJM, de Jongh RT, Lips P, Heijboer AC, Fisher AD, Schreiner T, T’Sjoen G, den Heijer M. Bone mineral density increases in trans persons after 1 year of hormonal treatment: a multicenter prospective observational study. J Bone Miner Res. 2017;32(6):1252–1260. [DOI] [PubMed] [Google Scholar]
- 11. Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T’Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab. 2012;97(7):2503–2511. [DOI] [PubMed] [Google Scholar]
- 12. Lapauw B, Taes Y, Simoens S, Van Caenegem E, Weyers S, Goemaere S, Toye K, Kaufman JM, T’Sjoen GG. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone. 2008;43(6):1016–1021. [DOI] [PubMed] [Google Scholar]
- 13. Fighera TM, da Silva E, Lindenau JD, Spritzer PM. Impact of cross-sex hormone therapy on bone mineral density and body composition in transwomen. Clin Endocrinol (Oxf). 2018;88(6):856–862. [DOI] [PubMed] [Google Scholar]
- 14. T’Sjoen G, Weyers S, Taes Y, Lapauw B, Toye K, Goemaere S, Kaufman JM. Prevalence of low bone mass in relation to estrogen treatment and body composition in male-to-female transsexual persons. J Clin Densitom. 2009;12(3):306–313. [DOI] [PubMed] [Google Scholar]
- 15. Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ, Murad MH. Effect of sex steroids on bone health of transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102(11):3904–3913. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22(1):128, author reply 128. [DOI] [PubMed] [Google Scholar]
- 17. Sosa M, Jódar E, Arbelo E, Domínguez C, Saavedra P, Torres A, Salido E, de Tejada MJ, Hernández D. Bone mass, bone turnover, vitamin D, and estrogen receptor gene polymorphisms in male to female transsexuals: effects of estrogenic treatment on bone metabolism of the male. J Clin Densitom. 2003;6(3):297–304. [DOI] [PubMed] [Google Scholar]
- 18. Broulik PD, Urbánek V, Libanský P. Eighteen-year effect of androgen therapy on bone mineral density in trans(gender) men. Horm Metab Res. 2018;50(2):133–137. [DOI] [PubMed] [Google Scholar]
- 19. Reutrakul S, Ongphiphadhanakul B, Piaseu N, Krittiyawong S, Chanprasertyothin S, Bunnag P, Rajatanavin R. The effects of oestrogen exposure on bone mass in male to female transsexuals. Clin Endocrinol (Oxf). 1998;49(6):811–814. [DOI] [PubMed] [Google Scholar]
- 20. Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Lapauw B, Kaufman JM, T’Sjoen G. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (ENIGI). Eur J Endocrinol. 2015;172(2):163–171. [DOI] [PubMed] [Google Scholar]
- 21. Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Kaufman JM, T’Sjoen G. Preservation of volumetric bone density and geometry in trans women during cross-sex hormonal therapy: a prospective observational study. Osteoporos Int. 2015;26(1):35–47. [DOI] [PubMed] [Google Scholar]
- 22. Dittrich R, Binder H, Cupisti S, Hoffmann I, Beckmann MW, Mueller A. Endocrine treatment of male-to-female transsexuals using gonadotropin-releasing hormone agonist. Exp Clin Endocrinol Diabetes. 2005;113(10):586–592. [DOI] [PubMed] [Google Scholar]
- 23. Mueller A, Dittrich R, Binder H, Kuehnel W, Maltaris T, Hoffmann I, Beckmann MW. High dose estrogen treatment increases bone mineral density in male-to-female transsexuals receiving gonadotropin-releasing hormone agonist in the absence of testosterone. Eur J Endocrinol. 2005;153(1):107–113. [DOI] [PubMed] [Google Scholar]
- 24. van Kesteren P, Lips P, Gooren LJG, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf). 1998;48(3):347–354. [DOI] [PubMed] [Google Scholar]
- 25. van Kesteren P, Lips P, Deville W, Popp-Snijders C, Asscheman H, Megens J, Gooren L. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab. 1996;81(6):2227–2232. [DOI] [PubMed] [Google Scholar]
- 26. Gava G, Cerpolini S, Martelli V, Battista G, Seracchioli R, Meriggiola MC. Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness. Clin Endocrinol (Oxf). 2016;85(2):239–246. [DOI] [PubMed] [Google Scholar]
- 27. Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, Cupisti S, Beckmann MW, Dittrich R. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7(9):3190–3198. [DOI] [PubMed] [Google Scholar]
- 28. Turner A, Chen TC, Barber TW, Malabanan AO, Holick MF, Tangpricha V. Testosterone increases bone mineral density in female-to-male transsexuals: a case series of 15 subjects. Clin Endocrinol (Oxf). 2004;61(5):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav. 2007;52(3):334–343. [DOI] [PubMed] [Google Scholar]
- 30. Mueller A, Zollver H, Kronawitter D, Oppelt PG, Claassen T, Hoffmann I, Beckmann MW, Dittrich R. Body composition and bone mineral density in male-to-female transsexuals during cross-sex hormone therapy using gonadotrophin-releasing hormone agonist. Exp Clin Endocrinol Diabetes. 2011;119(2):95–100. [DOI] [PubMed] [Google Scholar]
- 31. Pelusi C, Costantino A, Martelli V, Lambertini M, Bazzocchi A, Ponti F, Battista G, Venturoli S, Meriggiola MC. Effects of three different testosterone formulations in female-to-male transsexual persons. J Sex Med. 2014;11(12):3002–3011. [DOI] [PubMed] [Google Scholar]
- 32. Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T’Sjoen G. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641–2651. [DOI] [PubMed] [Google Scholar]
- 34. Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, Kalhorn T, Perrone AM, Ghi T, Pelusi C, Pelusi G. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med. 2008;5(10):2442–2453. [DOI] [PubMed] [Google Scholar]
- 36. Cauley JA. Estrogen and bone health. Steroids. 2015;99(Pt A):11–15. [DOI] [PubMed] [Google Scholar]
- 37. Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081–1101. [DOI] [PubMed] [Google Scholar]
- 38. Klaver M, de Blok CJM, Wiepjes CM, Nota NM, Dekker MJHJ, de Mutsert R, Schreiner T, Fisher AD, T’Sjoen G, den Heijer M. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178(2):163–171. [DOI] [PubMed] [Google Scholar]
- 39. Compston J. Obesity and fractures in postmenopausal women. Curr Opin Rheumatol. 2015;27(4):414–419. [DOI] [PubMed] [Google Scholar]
- 40. Mpalaris V, Anagnostis P, Anastasilakis AD, Goulis DG, Doumas A, Iakovou I. Serum leptin, adiponectin and ghrelin concentrations in post-menopausal women: is there an association with bone mineral density? Maturitas. 2016;88:32–36. [DOI] [PubMed] [Google Scholar]
- 41. Prieto-Alhambra D, Premaor MO, Fina Avilés F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C, Nogués X, Compston JE, Díez-Pérez A. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27(2):294–300. [DOI] [PubMed] [Google Scholar]
- 42. Taes YE, Lapauw B, Vanbillemont G, Bogaert V, De Bacquer D, Zmierczak H, Goemaere S, Kaufman JM. Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab. 2009;94(7):2325–2331. [DOI] [PubMed] [Google Scholar]
- 43. Nunes Cavalcante Castro BA, Torres Dos Reis Neto E, Szejnfeld VL, Szejnfeld J, Marvulle V, de Medeiros Pinheiro M. Could obesity be considered as risk factor for non-vertebral low-impact fractures? Adv Rheumatol. 2018;58(1):42. [DOI] [PubMed] [Google Scholar]