Abstract
Background:
Cyclopentolate is standardly used in ophthalmologic examinations of neonates to facilitate screening for retinopathy of prematurity. Reports of systemic effects have raised concerns of an increased risk of feeding intolerance after the examinations.
Objectives:
The goal of this study was to evaluate systemic concentrations of cyclopentolate after ophthalmic administration, as well as assess changes in weight as an indirect measure of alteration in feeding.
Methods:
Neonatal mice were randomized into three groups to simulate a neonatal model for ophthalmic medication administration. The cyclopentolate group received a one-time administration of tetracaine, cyclopentolate, and phenylephrine ophthalmologic solutions in accordance with the protocol used at the children’s hospital. The placebo group received the same ophthalmic drop administration, except for normal saline in place of cyclopentolate, and the control group received no ophthalmic drops and minimal handling. Daily weights and serum samples to measure systemic concentrations of cyclopentolate post-ophthalmic administration were assessed at baseline and for 7 days following drop administration.
Results:
Analysis of serum levels demonstrated detectability of systemic cyclopentolate after ophthalmic administration as early as 30 min (86 ng/ml), 1 h (60 ng/ml), and 24 h (6.2 ng/ml). There were also differences in weight gained on following ophthalmic administration observed between the cyclopentolate group and placebo group, with the cyclopentolate group weighing significantly less on days 3 and 7 (p = 0.02).
Conclusions:
Results indicate cyclopentolate is absorbed systemically and instillation of cyclopentolate decreases weight gain in neonatal mice compared to placebo. These preclinical findings provide rationale for further studies in neonatal patients.
Keywords: Ophthalmic cyclopentolate, Adverse drug reactions, Feeding intolerance, Retinopathy of prematurity, Neonates, Premature infants, Systemic cyclopentolate, Animal model
Introduction
Retinopathy of prematurity (ROP) is a potentially devastating disease of the retinal vasculature that effects the youngest and smallest of premature infants. Timely screening by the ophthalmologist is a key to diagnosing and preventing severe manifestations of ROP [1]. Current recommendations by the American Academy of Pediatrics, American Academy of Ophthalmology, and American Association for Pediatric Ophthalmology and Strabismus include a dilated fundus examination on all infants with a birth weight <1,500 g or a gestational age of ≤30 weeks [2]. Examinations may also be conducted on infants that weigh between 1,500 and 2,000 g and have an unstable clinical history as deemed by the neonatologist [2], Serial examinations are often required to monitor disease progression [3] and adequate mydriasis is essential to obtaining a reliable examination [4]. This examination, albeit critical, is less than benign. It is considered a painful procedure according to a recent Consensus Statement on Prevention and Management of Pain in the Newborn [5]. Along with being a well-recognized source of discomfort for the infant [6, 7], the medications used provide a platform for the potential of physiologic injury.
A chart review was conducted after several infants were noted to have excessive feeding difficulties following ophthalmologic examination [8]. Significant increases in the development of abdominal distension and large gastric aspirates were found following examinations. 56% of patients (n = 28) experienced at least one gastrointestinal symptom, including abdominal distention, vomiting, bloody stools, loose or watery stools, or gastric aspirates >3 ml in the post-examination period. The study found an increased risk of feeding intolerance afteropthalmologic examination. No conclusion was made, however, as to whether this intolerance was due to adverse systemic effects of mydriatic medications, the physical stress of the examination, or a combination of these factors.
Cyclopentolate is an anticholinergic and antimuscarinic drug that allows for both mydriasis and cycloplegia when instilled in the eye [9], A single drop can quickly pass through the nasolacrimal system as well as systemically through the oropharynx, digestive tract, and skin [10, 11]. Multiple studies have found that mydriatic drops may cause systemic side effects in neonates, ranging from cardiovascular to enteric manifestations [11–24]. Those with lower body weights are especially prone to these sequelae [25], which may include flushing, tachycardia and increases in blood pressure, along with hallucinations, seizure activity, abdominal distension and feeding intolerance [11–24, 26]. Because cyclopentolate readily crosses the blood-brain barrier, physostigmine remains the antidote of the choice [27]. Some preventative methods to decrease medication exposure include punctual occlusion during drop administration and 1–2 min after as well as wiping away any excess drops that may have poured over onto the skin [25, 26].
Cyclopentolate hydrochloride is standardly used at our institution during ROP examinations. Although given as a topical ophthalmic solution, reports of systemic effects in adults and children throughout the past several decades have raised serious concerns. Previous reports have demonstrated systemic detectability of cyclopentolate [23–25]. However, studies that examine systemic levels and effects of cyclopentolate are lacking. Here, we investigated the effects of ophthalmic administration of cyclopentolate on altered feeding assessed by changes in weight in a neonatal mice model.
Methods and Materials
Animals
All animal studies were in accordance with the Association for Research in Vision and Ophthalmology (ARVO) and the research institution. All experiments were performed using C57Bl/6 wild-type mice that were bred in-house. Neonatal mice with a postnatal age of 14–21 days were chosen for this model as this is the age that the mice’s eyes had opened enough for ophthalmic drop administration. The mice were randomized into three groups (cyclopentolate, placebo, and control) to simulate a neonatal model for ophthalmic medication in accordance with the protocol used in the neonatal intensive care unit of our children’s hospital. This protocol consists of tetracaine 0.5%, cyclopentolate 0.5% and phenylephrine 2.5% ophthalmologic solutions for dilation of the eye for examination. The cyclopentolate group received a one-time administration of 3 µl with a fine pipet tip of all three ophthalmic solutions. Cyclopentolate and phenylephrine were instilled 1 min after the tetracaine. The placebo group received the same protocol, with the exception of saline drops in place of the cyclopentolate. The third group of mice was considered the control group and received no ophthalmic drop administration and minimal handling. The study included two arms – one arm in which mice from all three groups were assessed for changes in weight after ophthalmic instillation, and the other arm included mice from the cyclopentolate and placebo groups to assess systemic levels of cyclopentolate.
For the serum level arm, mice received the ophthalmic solutions and were sacrificed to collect blood after 30 min, 1 h, 24 h or 7 days post-instillation (fig. 1). It was necessary to sacrifice the mice to obtain an adequate blood samples for cyclopentolate level detection. For the weight assessment arm, baseline and daily weights were measured in mice selected for weight trends in the cyclopentolate and placebo groups for 7 days after instillation to assess the effects of ophthalmic medication administration on feeding and weight gain. The control group was also weighed over the same time period but did not receive ophthalmic drops.
Fig. 1.
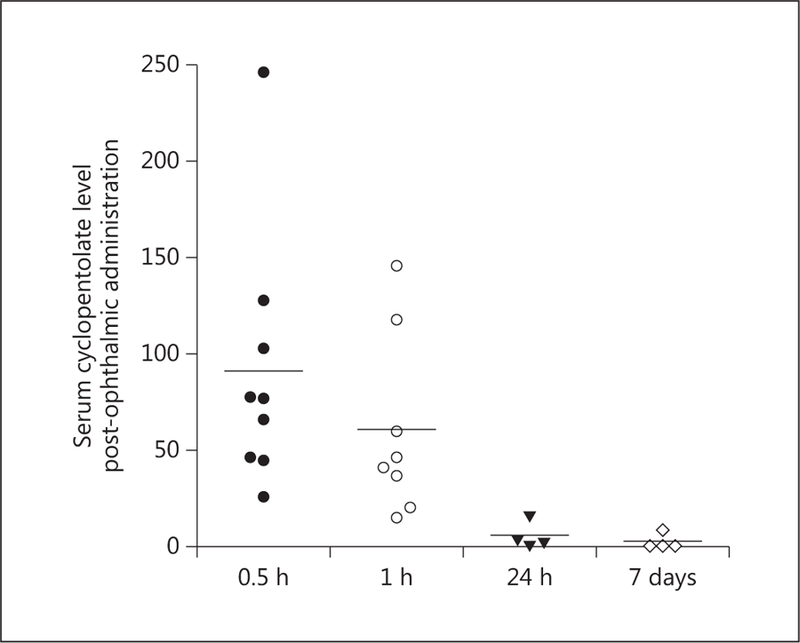
Detection of cyclopentolate in serum post-ophthalmic administration. Cyclopentolate levels were detected in serum samples using LC/MS as described in the Methods and Materials. Samples were collected at 0.5 h, 1 h, 24 h, or 7 days after single-time ophthalmic administration. Results show that cyclopentolate was detected as early as 30–60 min after instillation and then levels gradually decreased over 24 h (n = 4–8).
Detection of Cyclopentolate Levels
Blood samples were collected at designated time points, left to coagulate, centrifuged, and serum was collected and frozen immediately at –80 °C. Levels of cyclopentolate in mice sera were analyzed using protein precipitation via ice-cold acetonitrile (containing 0.05% formic acid) followed by LC/MS/MS analysis utilizing an Applied Biosystems API4000 tandem mass spectrometer [LLOQ 0.2–1 ng/ml, ULOQ 1,000 ng/ml; all individual standards were within ±25% bias limits (75–125% accuracy); mean intra-day accuracy ranged from 80 to 112%; mean inter-day accuracy ranged from 89 to 105%; intra-day %CV ranged from 1 to 20%; inter-day %CV ranged from 3 to 13%] [27]. Analysis was completed at Agilux Laboratories (Worcester, Mass., USA), procedure #KN-0001-DB-AA. Levels were calculated based on the standard curve of the cyclopentolate drug itself.
Statistical Analysis
Results were expressed as mean ± SD. Differences among multiple experimental groups were evaluated by one-way ANOVA followed by Bonferroni multiple comparison test, and between two groups using two-sided Student’s t test. Significance was defined as p < 0.05.
Results
Detection of Systemic Levels of Cyclopentolate Post-Ophthalmic Administration
For this arm of the study, 40 mice were included to assess systemic cyclopentolate levels. Serum samples from mice in the cyclopentolate group were evaluated 30 min (n = 8), 1 h (n = 8), 24 h (n = 4), and 7 days (n = 7) postinstillation. Samples from the placebo group (n = 9) were used as a control and assessed for comparison purposes at the same time points. Systemic cyclopentolate levels were detected as early as 30 min after ophthalmic instillation and levels gradually decreased over 24 h. As shown in figure 1, serum levels in the cyclopentolate group reached a mean of 86 ng/ml within 30 min compared to non-detectable levels (<5 ng/ml) in the placebo group. The systemic levels of cyclopentolate gradually decreased to a mean of 60 ng/ml after 1 h then significantly dropped to 6.5 ng/ml at 24 h. After 1 week, levels of cyclopentolate were nearly undetectable in the cyclopentolate group, with a mean concentration of 2.88 ng/ml.
Ophthalmic Cyclopentolate Caused Changes in Weight
For this arm of the study, 27 mice were randomized to the cyclopentolate (n = 9), placebo (n = 9), or control group (n = 7) for daily weight assessment. The three groups had comparable weight at baseline (cyclopentolate group, 6.12 g; placebo group, 6.13 g; control group, 6.23 g). Mice in the control and placebo groups consistently showed a trend of gaining higher weight throughout the study period compared to the cyclopentolate group as shown in figure 2. By day 7, mice in the placebo group were shown to have a significantly higher weight gain compared to the cyclopentolate group (cyclopentolate group, 7.56 g; placebo group, 8.31 g; control group, 8 g; p = 0.021). Of note, mice had a similar average weight at baseline in the three groups and maintained a similar weight within the first 2 days. A statistically significant difference in weight gain was also observed on day 3 (cyclopentolate group, 6.26 g; placebo group, 6.67 g; control group, 6.8 g; p = 0.026).
Fig. 2.
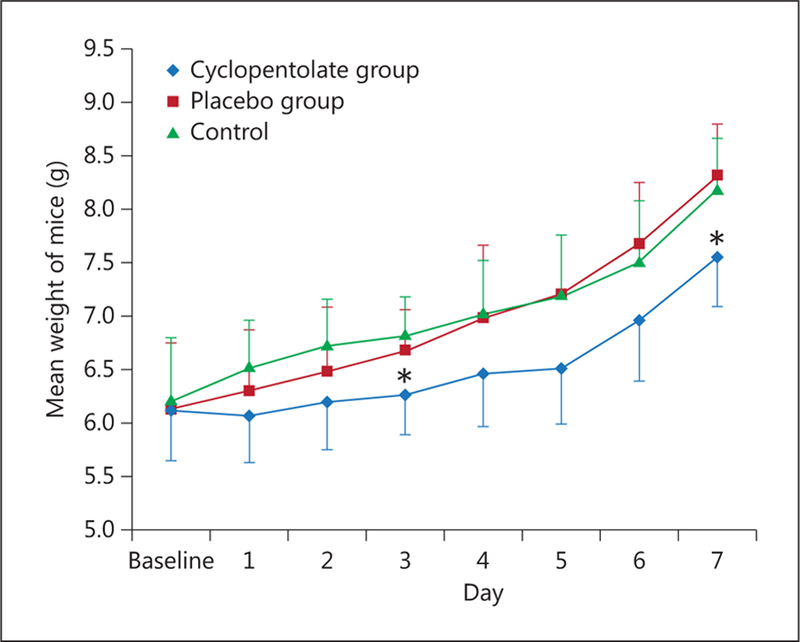
Decreased weight gain in the cyclopentolate group compared to placebo and control groups. Mean weight of mice at baseline and 7 days after ophthalmic administration. Despite similar weights at baseline, mice in the control and placebo groups showed a tendency to have higher weight gain each day following drop administration compared to the cyclopentolate group, with statistically significant differences in weight seen on days 3 and 7 (* p < 0.05, n = 9 per group).
Discussion
ROP is a potentially devastating disease of the retinal vasculature that effects the youngest and smallest of premature infants. One of the greatest impacts seen in the neonatal population from systemic adverse effects of ophthalmic cyclopentolate instillation is post-administration feeding intolerance. The exact mechanism of this effect is unknown, but may be due to intestinal relaxation and delayed gastric emptying secondary to the drug’s anticholinergic activity. Cyclopentolate has also been associated with necrotizing enterocolitis, gastric dilation, paralytic ileus, and a decrease in gastric acid secretion [10, 21–23]. Outside of their retinal disease, most pre-term infants are battling significant morbidity that would likely be worsened by decreased feeding and poor weight gain. As such, it is critical to identify pharmacological risks with the use of cyclopentolate in the human population. The findings of the current study provide the first evidence that ophthalmic cyclopentolate instillation in mice neonates can produce detectable systemic levels of the drug as early as 30–60 min, and also induce significant changes in feeding as indicated by the small yet significant reduction in body weight gain.
Our results in this animal model lend further support to previously reported post-administration feeding intolerance in clinical setting [10, 13–16, 20–23]. Findings from our study show that ophthalmic cyclopentolate administration produces detectable and measurable concentrations in serum samples from neonatal mice. The cyclopentolate levels were detected as early as 30–60 min after ophthalmic administration, indicating rapid systemic absorption. The levels gradually declined to almost placebo level within 24 h. The neonatal mice in this model received one-time defined drop of 3 µl, while the typical dose of cyclopentolate administered to human neonates is approximately 90 µl (3 drops). Even though cyclopentolate is not dosed according to weight in human neonates, the relative dose per body weight for mice may have impacted systemic absorption and detected levels. Of note, the levels detected in mice were higher than what had been reported in human patients (peak range of 6086 ng/ml within 30–60 min in mice versus 3 ng/ml in humans) [23, 24]. possibly due to difference in metabolic rate and pharmacokinetics of the drug in rodents, laboratory testing variations, or an increased amount of ophthalmic medication received or absorbed systemically. Another possibility in this comparison is the risk of the drops leaking into the mouths of the mice during instillation, however the use of a fine pipet limited this from occurring.
Neonatal mice receiving cyclopentolate were found to have decreased weight gain compared to neonates receiving placebo, with the greatest differences on days 3 and 7 post-administration. This suggests a correlation with cyclopentolate, feeding intolerance, and a delayed effect on weight gain. Of note, these results represent the impact of one-time drug administration on changes in weight over a 1-week period. The impact on poor weight gain in the human population may be even greater as patients are examined for ROP at least on a weekly basis and may receive a greater cumulative amount of cyclopentolate.
An additional limitation of this study is that feeding intolerance was assessed indirectly by measuring weight gain. Accurate assessment of direct food intake in breastfeeding mice, as well as humans, is challenging. In controlled clinical trials of feeding intolerance in premature neonates, average daily weight gain has been used as a secondary endpoint [28–33].
In summary, advances in neonatal intensive care have increased survival of extremely low birth weight infants and the incidence of ROP [reviewed in 21] and therefore improving the screening procedure for these infants is crucial. Our studies using mice neonates provided preliminary evidence that a single administration of ophthalmic cyclopentolate resulted in detectable level of the drug in the blood that positively correlated with delay in weight gain. These preclinical findings provide rationale for further studies in neonatal patients. The next step of this research is to establish the link in humans, and evaluate the potential preventative or treatment measures to avoid this adverse effect.
Acknowledgements
The authors are grateful for the technical expertise and input by Vinod Valentine, MD. This work was supported by grants from EY-022408, JDRF (2-2008-149) and Vision Discovery Institute to ABE, and Translational Research Initiative Grant from College of Pharmacy, University of Georgia, to K.B.M., S.G., and A.B.E.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Hartnett EM, Penn JS: Mechanisms and management of retinopathy of prematurity. N Engl J Med 2012;367:2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Section of Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus: Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006; 117:572–576. [DOI] [PubMed] [Google Scholar]
- 3.Quiram PA, Capone A: Current understanding and management of retinopathy of prematurity. Curr Opin Ophthalmol 2007; 18: 228–234. [DOI] [PubMed] [Google Scholar]
- 4.Vicente GV, Bahri M, Palafoutas JJ, Wang H, Mehta N: A randomized controlled trial to determine the lowest effective dose for adequate mydriasis in premature infants. J AAPOS 2012;16:365–369. [DOI] [PubMed] [Google Scholar]
- 5.Belda S, Pallas CR, De la Cruz J, Tejada P: Screening for retinopathy of prematurity: is it painful? Biol Neonate 2004;86:195–200. [DOI] [PubMed] [Google Scholar]
- 6.Samra HA, McGrath JM: Pain management during retinopathy of prematurity eye examinations: a systematic review. Adv Neonatal Care 2009;9:99–110. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey E, McCreery K: Local anaesthetic eye drops for prevention of pain in preterm infants undergoing screening for retinopathy of prematurity. Cochrane Database Syst Rev 2011;9:CD007645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermansen MC, Sullivan LS: Feeding intolerance following ophthalmologic examination. Am J Dis Child 1985;139:367–369. [DOI] [PubMed] [Google Scholar]
- 9.Cyclogyl® (package insert) Fort Worth, Alcon Laboratories Inc, 2004. [Google Scholar]
- 10.Gray C: Systemic toxicity with topical ophthalmic medications in children. Paediatr Perinat Drug Ther 2006;7:23–29. [Google Scholar]
- 11.Lim DL, Batilando M, Rajadurai VS: Transient paralytic ileus following the use of cyclopentolate-phenylephrine eye drops during screening for retinopathy of prematurity. J Paediatr Child Health 2003;39:318–320. [DOI] [PubMed] [Google Scholar]
- 12.Merritt JC, Kraybill EN: Effect of mydriatics on blood pressure in premature infants. J Pediatr Ophthalmol Strabismus 1981;18:42–46. [DOI] [PubMed] [Google Scholar]
- 13.Sindel BD, Baker MD, Maisels MJ, Weinstein J: A comparison of the pupillary and cardiovascular effects of various mydriatic agents in preterm infants. J Pediatr Ophthalmol Strabismus 1986;23:273–276. [DOI] [PubMed] [Google Scholar]
- 14.Rosales T, Isenberg S, Leake R, Everett S: Systemic effects of mydriatics in low weight infants. J Pediatr Ophthalmol Strabismus 1981; 18:42–44. [DOI] [PubMed] [Google Scholar]
- 15.Adcock EW: Cyclopentolate (Cyclogyl®) toxicity in pediatric patients. J Pediatr 1971; 79: 127–129. [DOI] [PubMed] [Google Scholar]
- 16.Awan KJ: Systemic toxicity of cyclopentolate hydrochloride in adults following topical ocular instillation. Ann Ophthalmol 1976;8:695–698. [PubMed] [Google Scholar]
- 17.Laws DW, Morton C, Weindling M, Clark D: Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol 1996; 80: 425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaga M, Kaila T, Salminen L, Ylitalo P: Systemic and ocular absorption and antagonist activity of topically applied cyclopentolate in man. Pharmacol Toxicol 1998; 82:19–22. [DOI] [PubMed] [Google Scholar]
- 19.Demayo AL, Reidenberg MM: Grand mal seizure in a child 30 min after Cyclogyl® (cyclopentolate hydrochloride) and 10% Neo-Synephrine® (phenylephrine hydrochloride) eye drops were instilled. Pediatrics 2004; 113:e499–e500. [DOI] [PubMed] [Google Scholar]
- 20.Derinoz O, Er A: Inability to walk, disequilibrium, incoherent speech, disorientation following the instillation of 1% cyclopentolate eye drops. Pediatr Emerg Care 2012; 28: 5960. [DOI] [PubMed] [Google Scholar]
- 21.Raghuveer TS, Bloom BT: A paradigm shift in the prevention of retinopathy of prematurity. Neonatology 2011;100:116–129. [DOI] [PubMed] [Google Scholar]
- 22.Kaila T, Huupponen R, Salminen L, Iisalo E: Systemic absorption of ophthalmic cyclopentolate. Am J Ophthalmol 1989;107: 562–564. [DOI] [PubMed] [Google Scholar]
- 23.Lahdes K, Huupponen R, Kaila T, Ali-Melkkilä T, Salminen L, Saari M: Systemic absorption of ocular cyclopentolate in children. Ger J Ophthalmol 1992;1:16–18. [PubMed] [Google Scholar]
- 24.Lahdes K, Huupponen R, Kaila T, Monti D, Saettone MF, Salminen L: Plasma concentrations and ocular effects of cyclopentolate after ocular application of three formulations. Br J Clin Pharmacol 1993;35:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pooniya V, Pandey N: Systemic toxicity of topical cyclopentolate eyedrops in a child. Eye (Lond) 2012;26:1391–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derinoz O, Emeksiz HC: Use of physostigmine for cyclopentolate overdose in an infant. Pediatrics 2012;130:e703–e705. [DOI] [PubMed] [Google Scholar]
- 27.Brown DV, Heller F, Barkin R: Anticholinergic syndrome after anesthesia: a case report and review. Am J Ther 2004; 11: 144–153. [DOI] [PubMed] [Google Scholar]
- 28.Agilux Laboratories Analytical Procedure #KN-0001-DB-AA.
- 29.Bauer CR, Trottier MCT, Stern L: Systemic cyclopentolate (Cyclogyl®) toxicity in the newborn infant. J Pediatr 1973;82:501–505. [DOI] [PubMed] [Google Scholar]
- 30.Sarici SU, Yurdakok M, Unal S: Acute gastric dilation complicating the use of mydriatics in a preterm newborn. Pediatr Radiol 2001;31: 581–583. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg SJ, Abrams C, Hyman PE: Effects of cyclopentolate eyedrops on gastric secretory function in pre-term infants. Ophthalmology 1985; 92: 698–700. [DOI] [PubMed] [Google Scholar]
- 32.Mansi Y, Abdelaziz N, Ezzeldin Z, Ibrahim R: Randomized controlled trial of a high dose of oral erythromycin for the treatment of feeding intolerance in preterm infants. Neonatology 2011;100:290–294. [DOI] [PubMed] [Google Scholar]
- 33.Ng YY, Su PH, Chen JY, Quek YW, Hu JM, Lee IC, et al. : Efficacy of intermediate-dose oral erythromycin on very low birth weight infants with feeding intolerance. Pediatr Neonatol 2012;53:34–40. [DOI] [PubMed] [Google Scholar]


