Abstract
Exosomes are naturally occurring membranous vesicles secreted by various types of cells. Given their unique and important biological and pharmacological properties, exosomes have been emerging as a promising form of nanomedicine acting via efficient delivery of endogenous and exogenous therapeutics. Here we explore a new concept of utilizing endogenously derived exosomes as artificial controllers of cellular immunity to redirect and activate cytotoxic T cells toward cancer cells for killing. This was achieved through genetically displaying two distinct types of antibodies on exosomal surface. The resulting synthetic multivalent antibodies retargeted exosomes (SMART-Exos), which express monoclonal antibodies specific for T-cell CD3 and cancer cell-associated epidermal growth factor receptor (EGFR), were shown to not only induce cross-linking of T cells and EGFR-expressing breast cancer cells but also elicit potent antitumor immunity both in vitro and in vivo. This proof-of-concept study demonstrates a novel application of exosomes in cancer immunotherapy and may provide a general and versatile approach for the development of a new class of cell-free therapy.
Exosomes are nanosized membrane vehicles secreted by various types of cells and are found in most body fluids.1,2 As endogenous nanocarriers, exosomes play important roles in mediating cell–cell communication.3–5 The membrane of exosome is characterized by a phospholipid bilayer and abundant tetraspanin CD9, which promotes direct membrane fusion with target cells and facilitates cellular delivery of therapeutic agents. Furthermore, CD47 found on exosomes is shown to inhibit clearance of exosomes by circulating monocytes and macrophages.6 Importantly, relative to viral and synthetic nanocarriers which tend to show high immunoreactivity, endogenously derived exosomes are expected to display significantly reduced immunogenicity. Given these unique and pharmacologically important properties, exosomes have been emerging as a new and attractive form of nanomedicine.
A considerable number of preclinical and clinical studies revealed that exosomes with various forms of therapeutic cargos have high potential for the treatment of many human diseases.4,7–15 Multiple types of cells were utilized to produce exosomes for therapeutic development.7–9,16,17 Using different physical and chemical methods, exosomes were packed with exogenous interfering RNAs and chemotherapeutics for drug delivery.6,8,11,18–21 The extensive applications of exosomes in therapeutic delivery raise the question of whether endogenously derived exosomes could be harnessed as artificial cellular immunity controllers to redirect immune effector cells and modulate their immunoreactivity. Such exosome-based nanoagents may possess unique and/or enhanced pharmacological properties for the development of novel therapeutics.
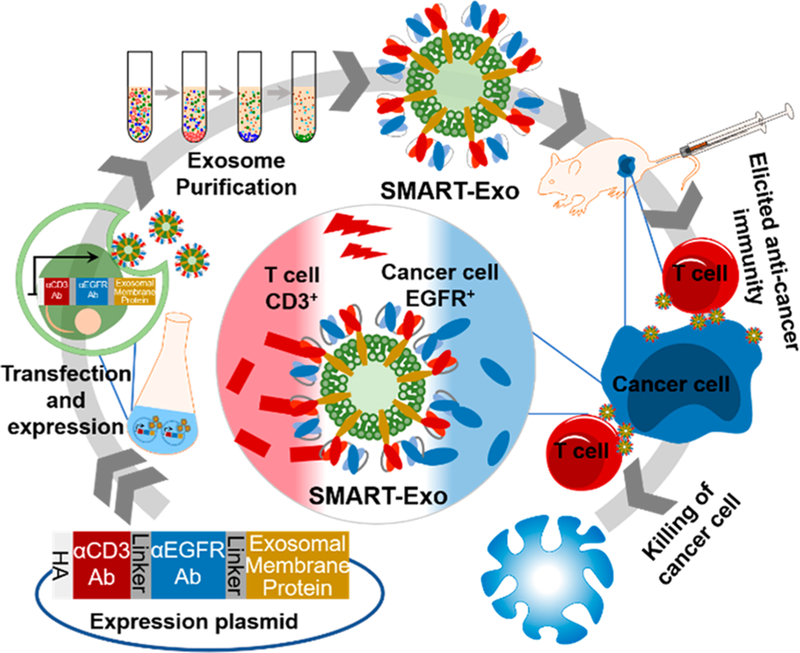
Here we explore this new concept through design, generation, and characterization of an innovative class of exosomes, termed as synthetic multivalent antibodies retargeted exosomes (SMART-Exos) (Figure 1). This was achieved through genetically displaying two distinct types of antibodies on exosomal surface. The generated SMART-Exos simultaneously targeting T-cell surface CD3 and cancer cell-associated epidermal growth factor receptor (EGFR) were shown to bind to both T cells and EGFR-expressing triple negative breast cancer (TNBC) cells. In vitro cytotoxicity studies revealed that the SMART-Exos induce potent and specific killing of EGFR-positive TNBC cells in the presence of nonactivated human peripheral blood mononuclear cells (PBMCs). In vivo efficacy studies using mouse xenograft models demonstrated excellent antitumor activities for the SMART-Exos. As a proof of concept, the SMART-Exos not only demonstrate a new strategy in developing therapeutic exosomes but also may provide a broadly applicable platform technology for next-generation immunotherapeutics.
Figure 1.
Schematic of the design and generation of αCD3/αEGFR synthetic multivalent antibodies retargeted exosomes (SMART-Exos).
Considering unique and invaluable features of exosomes, we envisioned that functional display of two types of antibodies on exosomal surface could result in an innovative class of therapeutic exosomes with potentially high potency and specificity for recruiting and activating endogenous cytotoxic effector cells toward target cancer cells for destruction. To test this notion, SMART-Exos targeting T-cell CD3 and EGFR were designed and generated. Overexpressed EGFR is frequently found in TNBC and about 90% of TNBC patients show expression of EGFR.22–25
The transmembrane (TM) domain of human platelet-derived growth factor receptor (PDGFR) was exploited as a fusion partner to display antibodies on exosomal surface, which has been widely used in expressing proteins on mammalian cells surfaces.26,27 To ensure coexpression of two types of antibodies on the same exosome and minimize decreased affinities due to potential steric hindrance between two antibody scaffolds, single polypeptides consisting of two single-chain variable fragment (scFv) antibodies targeting CD3 and EGFR were genetically linked to the TM domain of PDGFR. Considering potential effects of the orientation of individual antibodies on physicochemical and biological properties of SMART-Exos, two fusion constructs were designed (denoted as αCD3/αEGFR and αEGFR/αCD3) (Figure S1), in which an anti-human CD3 UCHT1 scFv antibody (VLαCD3-VHαCD3),28 was genetically fused with N- or C-terminus of the anti-human EGFR cetuximab scFv antibody (VHαEGFR-VLαEGFR). The VH and VL for both scFvs were arranged in different orientations to minimize potential mispairing. Flexible (GGGGS)4 linkers were inserted between two scFv antibodies. Additionally, αEGFR and αCD3 scFv antibodies were separately fused with PDGFR TM domain for generation of single antibody-PDGFR fusions as controls (Figure S1). Each fusion contained an N-terminal hemagglutinin (HA) epitope tag.
Expressed SMART-Exos were isolated by differential centrifugation from chemically defined media of Expi293F cells (a suspension-adapted HEK293 cell line) transfected with expression constructs.29 Approximately 100 μg of SMART-Exos (1.3 × 1010 particles) could be generated from 30 mL of cell culture post-transfection. Compared with immune cell- or tumor cell-derived exosomes, which are involved in modulation of immune responses in cancer,30,31 exosomes derived from HEK293 cell line were demonstrated to be immunologically inert,32 providing an excellent source of exosomes for the addition of new functions.
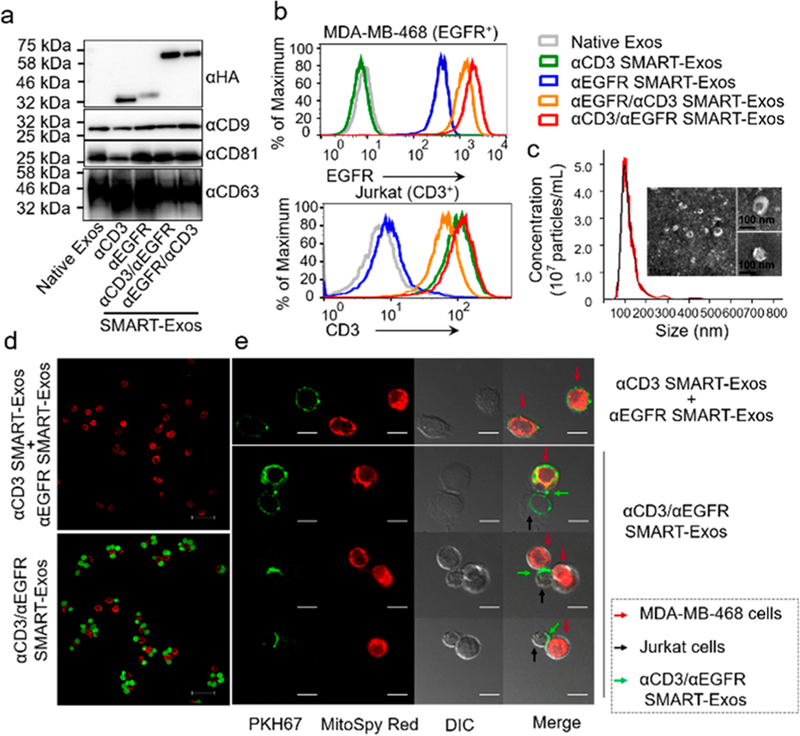
Immunoblot analysis indicated that all scFv antibodies were expressed in exosomes. Moreover, SMART-Exos showed expression of exosomal marker CD9, CD81, and CD63, similar to native exosomes (Figure 2a). Flow cytometric analysis indicated that αCD3/αEGFR SMART-Exos and αEGFR/αCD3 SMART-Exos can tightly bind to both MDA-MB-468 and Jurkat cells (Figure 2b), demonstrating that the dual scFv antibodies on exosomal surface allow to target exosomes to both EGFR- and CD3-expressing cells. These results also suggest no significant mispairing for coexpressed scFvs. Compared with αEGFR/αCD3 SMART-Exos, αCD3/αEGFR SMART-Exos exhibited slightly higher binding affinity to both cell lines and were selected for subsequent in vitro and in vivo studies. None of the SMART-Exos displayed significant binding to MDA-MB-453 cells (CD3− EGFR−) (Figure S2). Nanoparticle tracking analysis (NTA) of αCD3/αEGFR SMART-Exos indicated a size distribution peaking at 100 nm in diameter (Figure 2c), consistent with previous studies.6,8,33,34 The ζ-potential of αCD3/αEGFR SMART-Exos was determined to be −25 ± 4.1 mV, consistent with previous reports.35–39 The αCD3/αEGFR SMART-Exos were visualized by transmission electron microscopy (Figure 2c).
Figure 2.
Characterization of SMART-Exos. (a) Immunoblot analysis of SMART-Exos. (b) Flow cytometric analysis of the binding of SMART-Exos to MDA-MB-468 and Jurkat cells. (c) Size distribution and negative staining transmission electron microscopy (TEM) images of αCD3/αEGFR SMART-Exos. (d) Confocal microscopic analysis of cross-linking of Jurkat (green) and MDA-MB-468 cells (red) induced by αCD3/αEGFR SMART-Exos. A mixture of αCD3 and αEGFR SMART-Exos was used as a control. Scale bars: 50 μm. (e) Confocal imaging of αCD3/αEGFR SMART-Exos (green) participating in cross-linking of MDA-MB-468 (red) and Jurkat (no fluorescent label) cells. A mixture of PKH67-labeled αCD3 and αEGFR SMART-Exos was used as a control. Scale bars: 10 μm.
To demonstrate simultaneous binding of αCD3/αEGFR SMART-Exos to both antigens, SMART-Exos-mediated cross-linking of fluorescently labeled MDA-MB-468 (red) and Jurkat (green) cells was analyzed by confocal microscopy. In contrast to a mixture of αCD3 and αEGFR SMART-Exos, αCD3/αEGFR SMART-Exos induced significant cross-linking of MDA-MB-468 and Jurkat cells (P < 0.001; Figures 2d and S3a). Few Jurkat cells were bound to MDA-MB-453 cells in the presence of αCD3/αEGFR SMART-Exos or a mixture of αCD3 and αEGFR SMART-Exos (Figure S3b,c). Furthermore, PKH67-labeled αCD3/αEGFR SMART-Exos (green) were observed at the interface of MDA-MB-468 (red) and Jurkat cells (no fluorescent label) (Figure 2e). These results demonstrate cell–cell interactions mediated by αCD3/αEGFR SMART-Exos.
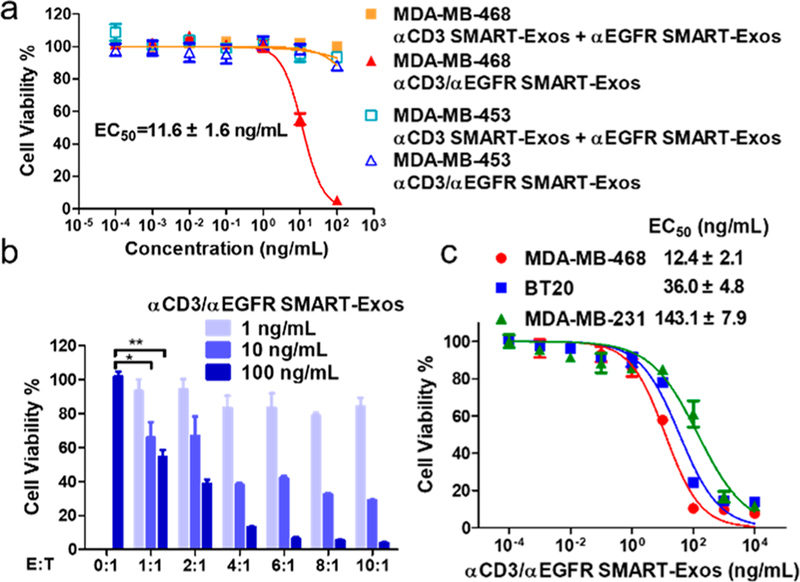
In vitro cytotoxicity assays were next performed. In the presence of human PBMCs, αCD3/αEGFR SMART-Exos exhibited highly potent and specific cytotoxicity against MDAMB-468 cells with an EC50 of 11.6 ± 1.6 ng mL−1 and significantly decreased cytotoxicity for MDA-MB-453 cells (EC50 > 1000 ng mL−1) (Figure 3a). The αCD3/αEGFR SMART-Exos could induce significant cytotoxicity for target cells with an E:T ratio as low as 1. When the E:T ratios were increased to 4 and above, comparable maximal killings were achieved at same concentrations of SMART-Exos (Figure 3b). Additionally, cytotoxicity of αCD3/αEGFR SMART-Exos were determined for TNBC cell lines with various levels of EGFR expression (Figure S4), including MDA-MB-468 (EGFR+++), BT20 (EGFR++), and MDA-MB-231 (EGFR+) cells. The αCD3/αEGFR SMART-Exos showed potent cytotoxicity for MDA-MB-468, BT20, and MDA-MB-231 cells with EC50 values in a range of 12–143 ng mL−1, positively correlating with levels of EGFR expression (Figures 3c and S5). These results demonstrate remarkable potency and specificity of αCD3/αEGFR SMART-Exos for inducing immune attack of EGFR-positive TNBC cells.
Figure 3.
In vitro cytotoxicity of αCD3/αEGFR SMART-Exos. (a) Cytotoxicity of αCD3/αEGFR SMART-Exos for MDA-MB-468 (EGFR+) or MDA-MB-453 (EGFR−) cells. (b) Dose-dependent cytotoxicity of αCD3/αEGFR SMART-Exos for MDA-MB-468 cells at various E:T ratios. * P < 0.05 or ** P < 0.01. (c) Dose-dependent cytotoxicity of αCD3/αEGFR SMART-Exos for three TNBC cell lines. Human PBMCs (effector cells) were incubated with TNBC cells (target cells) at an E:T ratio of 10 (a) and (c) or various E:T ratios (b) in the presence of αCD3/αEGFR SMART-Exos. A mixture of αCD3 and αEGFR SMART-Exos was used as a control in (a).
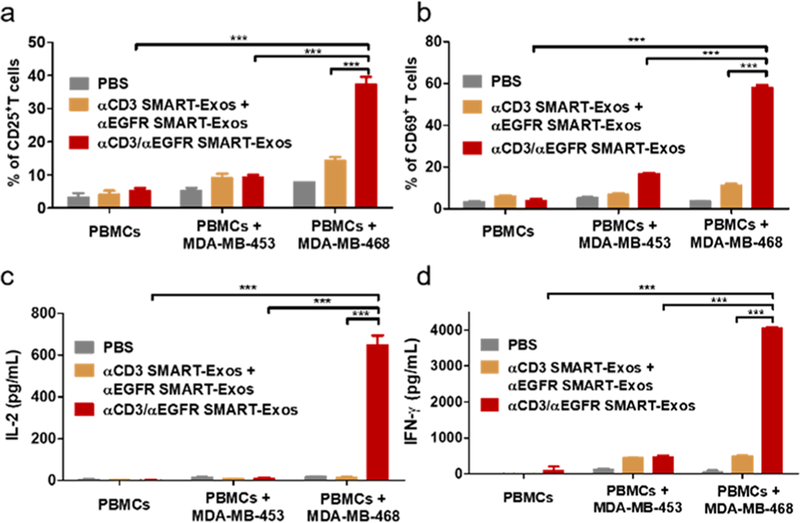
Next, T-cell activation was characterized (Figures 4 and S6). It was shown that T cells can be potently activated by αCD3/αEGFR SMART-Exos and the SMART-Exos-mediated T-cell activation is dependent on EGFR-expressing MDA-MB-468 cells (Figure 4). On the basis of T-cell activation markers CD25 and CD69, it was found that αCD3/αEGFR SMART-Exos can result in dose-dependent activation of T cells in the presence of EGFR-positive MDA-MB-468 cells (Figure S6). These results demonstrate potent activation of human T cells by αCD3/αEGFR SMART-Exos in a target cell-dependent manner.
Figure 4.
T-cell activation induced by αCD3/αEGFR SMART-Exos. Human PBMCs were incubated with αCD3/αEGFR SMART-Exos or a mixture of αCD3 and αEGFR SMART-Exos in the absence or presence of MDA-MB-453 or MDA-MB-468 cells. The percentages of CD25+ (a) and CD69+ (b) T cells were analyzed by flow cytometry. The levels of secreted IL-2 (c) and IFN-γ (d) cytokines were determined by ELISA. *** P < 0.001.
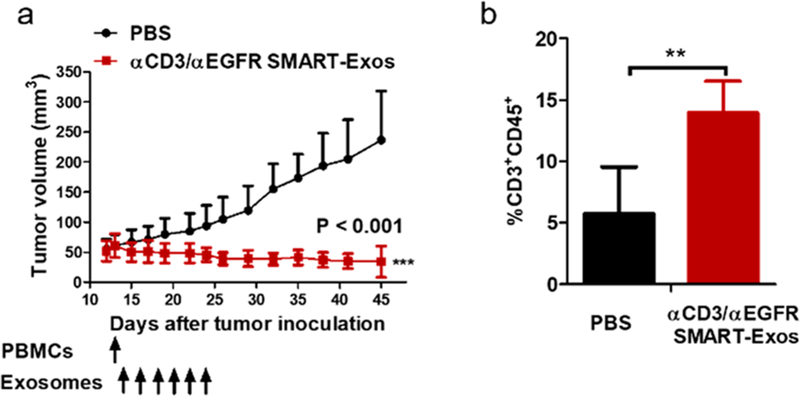
A pharmacokinetic study was performed for αCD3/αEGFR SMART-Exos in mice. The intravenously administered αCD3/αEGFR SMART-Exos exhibited a characteristic two-phase pharmacokinetic behavior with an elimination half-life of 172.5 ± 42.0 min (Figure S7), comparable to the half-lives determined for exosomes from different cell types.40–42 To evaluate in vivo antitumor activity of αCD3/αEGFR SMART-Exos, human TNBC xenograft mouse models were established using MDA-MB-468 cells. Shortly after treatment was initiated, tumor shrinkage was observed in the treatment group, whereas the PBS-treated mice displayed steady increase of tumor size. After treatment was stopped, no significant tumor regrowth was observed in the treatment group (P < 0.001) (Figure 5a). These results demonstrate excellent in vivo efficacy of the SMART-Exos for established tumors in mice. Moreover, no overt toxicity or loss of body weight was observed for mice in PBS- or SMART-Exos-treated groups (Figure S8). Flow cytometric analysis indicated that in contrast to tumors from PBS-treated mice that show low levels of intratumoral T cells, significant T-cell infiltrations were observed in SMART-Exos-treated animals (Figures 5b and S9), suggesting specific recruitment of cytotoxic T cell to the microenvironment of tumors by the administered SMART-Exos.
Figure 5.
In vivo evaluation of αCD3/αEGFR SMART-Exos. (a) In vivo efficacy of SMART-Exos. MDA-MB-468 cells were s.c. inoculated into the flank of female immunodeficient NSG mice (n = 5). Nonactivated human PBMCs were i.p. injected into the mice. One day post-PBMCs injection, mice were i.v. injected with PBS or αCD3/αEGFR SMART-Exos (10 mg kg−1; 2.8 × 1010 particles per mouse) every other day for a total of six times. Data are shown as mean ± SD. *** P < 0.001. (b) In vivo T-cell infiltration induced by αCD3/αEGFR SMART-Exos. (n = 5; ** P < 0.01).
This study shows, for the first time, functional display of two distinct types of monoclonal antibodies on exosome surface for inducing antitumor immunity in a controlled and directed fashion. By selectively recruiting cytotoxic T cells to cancer cells, the spherical and multivalent dual-targeted SMART-Exos may promote the formation of immunological synapses and enhance the activation of immune effector cells. Notably, the SMART-Exos can possibly be loaded with a variety of therapeutic cargos for selective delivery to target cells to enhance efficacy. Moreover, through functionally displaying two or more types of monoclonal antibodies and/or effector proteins, SMART-Exos may provide a general and versatile platform technology for the development of a new class of exosome-based therapeutics. Compared with conventional nanoparticles, exosomes are expected to possess high biocompatibility, increased serum stability and efficiency for therapeutic delivery, and low immunogenicity. But in-depth characterization of exosomal composition may be required for developing therapeutic exosomes with improved efficacy and reduced side effects. In conclusion, the SMART-Exos characterized by genetically encoded, surface-displayed monoclonal antibodies exhibit excellent activity and specificity in eliciting potent anticancer immunity against EGFR-positive TNBC cells both in vitro and in vivo. Future studies include biodistribution and toxicity, in vivo mechanism(s) of action, and generation of SMART-Exos targeting other immune effector cells and/or disease-associated antigens.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by University of Southern California School of Pharmacy Start-Up Fund for New Faculty, University of Southern California Ming Hsieh Institute for Engineering Medicine for Cancer, American Association of Pharmaceutical Scientists (AAPS) Foundation New Investigator Grant (to Y.Z.), P30CA014089 to the USC Norris Comprehensive Cancer Center, and P30DK048522 to the USC Research Center for Liver Diseases.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b10047.
Additional experimental materials and methods and supplementary table and figures (PDF)
The authors declare the following competing financial interest(s): The authors have filed a patent application on this work.
REFERENCES
- (1).Thery C; Zitvogel L; Amigorena S Exosomes: composition, biogenesis and function. Nat. Rev. Immunol 2002, 2 (8), 569–79. [DOI] [PubMed] [Google Scholar]
- (2).Yanez-Mo M; Siljander PR; Andreu Z; Zavec AB; Borras FE; Buzas EI; Buzas K; Casal E; Cappello F; Carvalho J; Colas E; Cordeiro-da Silva A; Fais S; Falcon-Perez JM; Ghobrial IM; Giebel B; Gimona M; Graner M; Gursel I; Gursel M; Heegaard NH; Hendrix A; Kierulf P; Kokubun K; Kosanovic M; Kralj-Iglic V; Kramer-Albers EM; Laitinen S; Lasser C; Lener T; Ligeti E; Line A; Lipps G; Llorente A; Lotvall J; Mancek-Keber M; Marcilla A; Mittelbrunn M; Nazarenko I; Nolte-’t Hoen EN; Nyman TA; O’Driscoll L; Olivan M; Oliveira C; Pallinger E; Del Portillo HA; Reventos J; Rigau M; Rohde E; Sammar M; Sanchez-Madrid F; Santarem N; Schallmoser K; Ostenfeld MS; Stoorvogel W; Stukelj R; Van der Grein SG; Vasconcelos MH; Wauben MH; De Wever O Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jiang XC; Gao JQ Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm 2017, 521 (1–2), 167–175. [DOI] [PubMed] [Google Scholar]
- (4).Johnsen KB; Gudbergsson JM; Skov MN; Pilgaard L; Moos T; Duroux M A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta, Rev. Cancer 2014, 1846 (1), 75–87. [DOI] [PubMed] [Google Scholar]
- (5).Raposo G; Stoorvogel W Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol 2013, 200 (4), 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kamerkar S; LeBleu VS; Sugimoto H; Yang S; Ruivo CF; Melo SA; Lee JJ; Kalluri R Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546 (7659), 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ferguson SW; Nguyen J Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J. Controlled Release 2016, 228, 179–90. [DOI] [PubMed] [Google Scholar]
- (8).Alvarez-Erviti L; Seow Y; Yin H; Betts C; Lakhal S; Wood MJ Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol 2011, 29 (4), 341–5. [DOI] [PubMed] [Google Scholar]
- (9).Cooper JM; Wiklander PB; Nordin JZ; Al-Shawi R; Wood MJ; Vithlani M; Schapira AH; Simons JP; El-Andaloussi S; Alvarez-Erviti L Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord 2014, 29 (12), 1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Oh K; Kim SR; Kim DK; Seo MW; Lee C; Lee HM; Oh JE; Choi EY; Lee DS; Gho YS; Park KS In vivo Differentiation of Therapeutic Insulin-Producing Cells from Bone Marrow Cells via Extracellular Vesicle-Mimetic Nanovesicles. ACS Nano 2015, 9 (12), 11718–27. [DOI] [PubMed] [Google Scholar]
- (11).Ohno S; Takanashi M; Sudo K; Ueda S; Ishikawa A; Matsuyama N; Fujita K; Mizutani T; Ohgi T; Ochiya T; Gotoh N; Kuroda M Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther 2013, 21 (1), 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ren J; He W; Zheng L; Duan H From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater. Sci 2016, 4 (6), 910–21. [DOI] [PubMed] [Google Scholar]
- (13).Fais S; O’Driscoll L; Borras FE; Buzas E; Camussi G; Cappello F; Carvalho J; Cordeiro da Silva A; Del Portillo H; El Andaloussi S; Ficko Trcek T; Furlan R; Hendrix A; Gursel I; Kralj-Iglic V; Kaeffer B; Kosanovic M; Lekka ME; Lipps G; Logozzi M; Marcilla A; Sammar M; Llorente A; Nazarenko I; Oliveira C; Pocsfalvi G; Rajendran L; Raposo G; Rohde E; Siljander P; van Niel G; Vasconcelos MH; Yanez-Mo M; Yliperttula ML; Zarovni N; Zavec AB; Giebel B Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nano-medicine. ACS Nano 2016, 10 (4), 3886–99. [DOI] [PubMed] [Google Scholar]
- (14).Gyorgy B; Hung ME; Breakefield XO; Leonard JN Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol 2015, 55, 439–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Natasha G; Gundogan B; Tan A; Farhatnia Y; Wu W; Rajadas J; Seifalian AM Exosomes as immunotheranostic nanoparticles. Clin. Ther 2014, 36 (6), 820–829. [DOI] [PubMed] [Google Scholar]
- (16).Rani S; Ryan AE; Griffin MD; Ritter T Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther 2015, 23 (5), 812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yeo RW; Lai RC; Zhang B; Tan SS; Yin Y; Teh BJ; Lim SK Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Delivery Rev 2013, 65 (3), 336–41. [DOI] [PubMed] [Google Scholar]
- (18).El Andaloussi S; Lakhal S; Mager I; Wood MJ Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Delivery Rev 2013, 65 (3), 391–7. [DOI] [PubMed] [Google Scholar]
- (19).Munoz JL; Bliss SA; Greco SJ; Ramkissoon SH; Ligon KL; Rameshwar P Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multi-forme Cells Conferred Chemosensitivity. Mol. Ther.–Nucleic Acids 2013, 2, No. e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sun D; Zhuang X; Xiang X; Liu Y; Zhang S; Liu C; Barnes S; Grizzle W; Miller D; Zhang HG A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther 2010, 18 (9), 1606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tian Y; Li S; Song J; Ji T; Zhu M; Anderson GJ; Wei J; Nie G A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35 (7), 2383–90. [DOI] [PubMed] [Google Scholar]
- (22).Changavi AA; Shashikala A; Ramji AS Epidermal Growth Factor Receptor Expression in Triple Negative and Nontriple Negative Breast Carcinomas. Journal of laboratory physicians 2015, 7 (2), 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Costa R; Shah AN; Santa-Maria CA; Cruz MR; Mahalingam D; Carneiro BA; Chae YK; Cristofanilli M; Gradishar WJ; Giles FJ Targeting Epidermal Growth Factor Receptor in triple negative breast cancer: New discoveries and practical insights for drug development. Cancer Treat. Rev 2017, 53, 111–119. [DOI] [PubMed] [Google Scholar]
- (24).Nakai K; Hung MC; Yamaguchi H A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res 2016, 6 (8), 1609–1623. [PMC free article] [PubMed] [Google Scholar]
- (25).Park HS; Jang MH; Kim EJ; Kim HJ; Lee HJ; Kim YJ; Kim JH; Kang E; Kim SW; Kim IA; Park SY High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod. Pathol 2014, 27 (9), 1212–22. [DOI] [PubMed] [Google Scholar]
- (26).Beerli RR; Bauer M; Buser RB; Gwerder M; Muntwiler S; Maurer P; Saudan P; Bachmann MF Isolation of human monoclonal antibodies by mammalian cell display. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (38), 14336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ho M; Nagata S; Pastan I Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc. Natl. Acad. Sci. U. S. A 2006, 103 (25), 9637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhu Z; Carter P Identification of heavy chain residues in a humanized anti-CD3 antibody important for efficient antigen binding and T cell activation. J. Immunol 1995, 155 (4), 1903–1910. [PubMed] [Google Scholar]
- (29).Livshits MA; Khomyakova E; Evtushenko EG; Lazarev VN; Kulemin NA; Semina SE; Generozov EV; Govorun VM Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep 2015, 5, 17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Robbins PD; Morelli AE Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol 2014, 14 (3), 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Thery C; Ostrowski M; Segura E Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol 2009, 9 (8), 581–93. [DOI] [PubMed] [Google Scholar]
- (32).Zhu X; Badawi M; Pomeroy S; Sutaria DS; Xie Z; Baek A; Jiang J; Elgamal OA; Mo X; Perle K; Chalmers J; Schmittgen TD; Phelps MA Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6 (1), 1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gangalum RK; Atanasov IC; Zhou ZH; Bhat SP AlphaB-Crystallin is found in detergent-resistant membrane micro-domains and is secreted via exosomes from human retinal pigment epithelial cells. J. Biol. Chem 2011, 286 (5), 3261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Katsuda T; Tsuchiya R; Kosaka N; Yoshioka Y; Takagaki K; Oki K; Takeshita F; Sakai Y; Kuroda M; Ochiya T Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep 2013, 3, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Soares Martins T; Catita J; Martins Rosa I; da Cruz e Silva OAB; Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One 2018, 13 (6), No. e0198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Nguyen DB; Ly TB; Wesseling MC; Hittinger M; Torge A; Devitt A; Perrie Y; Bernhardt I Characterization of Microvesicles Released from Human Red Blood Cells. Cell. Physiol. Biochem 2016, 38 (3), 1085–1099. [DOI] [PubMed] [Google Scholar]
- (37).Jin Y; Lee JS; Min S; Park HJ; Kang TJ; Cho SW Bioengineered Extracellular Membranous Nanovesicles for Efficient Small-Interfering RNA Delivery: Versatile Platforms for Stem Cell Engineering and In vivo Delivery. Adv. Funct. Mater 2016, 26 (32), 5804–5817. [Google Scholar]
- (38).Kim MS; Haney MJ; Zhao Y; Mahajan V; Deygen I; Klyachko NL; Inskoe E; Piroyan A; Sokolsky M; Okolie O; Hingtgen SD; Kabanov AV; Batrakova EV Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12 (3), 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Stremersch S; Vandenbroucke RE; Van Wonterghem E; Hendrix A; De Smedt SC; Raemdonck K Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J. Controlled Release 2016, 232, 51–61. [DOI] [PubMed] [Google Scholar]
- (40).Takahashi Y; Nishikawa M; Shinotsuka H; Matsui Y; Ohara S; Imai T; Takakura Y Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol 2013, 165 (2), 77–84. [DOI] [PubMed] [Google Scholar]
- (41).Lai CP; Mardini O; Ericsson M; Prabhakar S; Maguire C; Chen JW; Tannous BA; Breakefield XO Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8 (1), 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Morishita M; Takahashi Y; Nishikawa M; Sano K; Kato K; Yamashita T; Imai T; Saji H; Takakura Y Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci 2015, 104 (2), 705–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.