Abstract
Background
Personalizing medical treatment often requires practitioners to compare multiple treatment options, assess a patient’s unique risk and benefit from each option, and elicit a patient’s preferences around treatment. We integrated these three considerations into a decision modeling framework for the selection of second-line glycemic therapy for type 2 diabetes.
Methods
Based on Multi-Criteria Decision Analysis, we developed a unified treatment decision support tool accounting for three factors; patient preferences, disease outcomes, and medication efficacy and safety profiles. By standardizing and multiplying these three factors, we calculated the ranking score for each medication. This approach was applied to determining second-line glycemic therapy by integrating: (i) treatment efficacy and side-effect data from network meta-analysis of 301 randomized trials (N=219,277); (ii) validated risk equations for type 2 diabetes complications; and (iii) patient preferences around treatment (e.g., to avoid daily glucose testing). Data from participants with type 2 diabetes in the U.S. National Health and Nutrition Examination Survey (NHANES 2003-2014, N=1,107) were used to explore variations in treatment recommendations and associated QALYs given different patient features.
Results
Patients at the highest microvascular disease risk had GLP-1 agonists or basal insulin recommended as top choices, while those wanting to avoid an injected medication or daily glucose testing had SGLT-2 or DPP-4 inhibitors commonly recommended, and those with major cost concerns had sulfonylureas commonly recommended. By converting from the most common sulfonylurea treatment to the model-recommended treatment, NHANES participants were expected to save an average of 0.036 quality-adjusted life-years per person (about a half month) from 10 years of treatment.
Conclusions
Models can help integrate meta-analytic treatment effect estimates with individualized risk calculations and preferences, to aid personalized treatment selection.
Keywords: personalized medicine, type 2 diabetes mellitus, network meta-analysis, shared decision-making
Introduction
“Shared decision-making”—or the joint selection of treatments between practitioners and patients—has been increasingly advocated in type 2 diabetes guidelines.1 Shared decision-making involves incorporating data on treatment effectiveness and side-effects; individual patient risk and potential benefit; and a patient’s specific preferences into treatment decisions. Yet in practice, it is difficult to rigorously and formally calculate the individualized benefit or risk for multiple treatments and disease end-points, particularly during a brief patient encounter.1,2 Shared decision-making is particularly challenging for type 2 diabetes treatment decisions, which often involve choosing among numerous alternative second-line treatments to add to first-line metformin. In fact, primary care providers have identified that second-line diabetes treatment selection is a major source of anxiety and confusion, particularly in regions where specialty endocrinology consultation is limited.3,4 In this context, serious adverse events from type 2 diabetes treatments have become a leading cause of avoidable emergency department visits in the United States.5 Improved decision support for clinicians may assist in choosing treatments that are beneficial and less risky for patients, while also helping patients engage with care decisions and thereby potentially remain more adherent to therapy.6-8
To assist in making treatment selections when there are multiple treatment options, guidelines have suggested incorporation of data from network meta-analyses (NMAs),4 which take the data from all available randomized trials in a field, and assesses the impact of each treatment relative to other available treatments for each of several disease end-points, including treatment-related adverse events.9 To put these results into practice so as to calculate absolute risk reductions across numerous treatment options for an individual patient, relative risk reductions or odds ratios estimated through NMAs must be combined with personalized estimates of a patient’s pre-treatment risk.9-14 Treatment selection decisions can therefore be influenced by a patient’s age, biomarkers, co-morbid conditions, concurrent treatments, and related factors influencing pre-treatment risk for microvascular and macrovascular outcomes as well as treatment-related adverse events. Validated risk equations15 may help make treatment selection more explicit by estimating the absolute risk and benefit from several treatment options given a specific patient’s clinical history, exam features, and biomarkers.16-18
Treatment options are also influenced by patient preferences, given that some patients are very concerned, for example, about weight gain, daily glucose testing, or having to take injected medications; these concerns may substantially influence treatment adherence.1 Patient preferences may complicate treatment decisions, such as when choosing the optimal treatment for a patient with a high risk of hypoglycemia, high cardiovascular risk, limited income to afford a treatment with higher out-of-pocket cost, and difficulty with daily glucose testing.
Here, we developed a pilot modeling approach to select second-line type 2 diabetes treatments, integrating network meta-analytic data for treatment efficacy and side-effect, estimating individualized risk for a U.S. national sample, and simulating a diverse range of possible patient preferences. We hypothesized that our modeling strategy would lead to wide variations in the selection of different second-line treatments for glucose lowering in type 2 diabetes, depending on complex combinations of patient characteristics and preferences.
Methods
Analytic approach
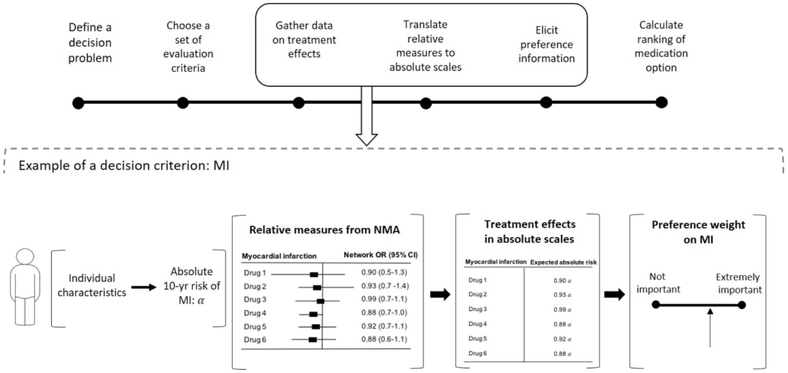
We constructed a unified treatment decision support tool by calculating treatment ranking scores accounting for three factors; patient preferences (e.g. out-of-pocket medication cost and requirement for glucose self-monitoring), disease outcomes (macrovascular and microvascular diseases), and medication efficacy and safety profiles (e.g. Hb1AC reduction and risks of hypoglycemia and adverse events. By standardizing these three factors into 0 to 1 scales, and the multiplying, we calculated the ranking score for each medication. This approach (Figure 1) was applied to identify which treatments would be recommended for a given patient. Using data from participants with type 2 diabetes in the U.S. National Health and Nutrition Examination Survey (NHANES), we explored variations in treatment recommendations and associated QALYs given different patient features
Figure 1.
Conceptual illustration of the decision-making model
Data sources
Data sources and outcome measures are summarized in Table 1. Treatment efficacy and side-effect data were obtained from a random-effects NMA of 301 randomized trials (N = 118,094; Table 2).22 The data included six classes of glucose-lowering medications as potential second-line therapies to add to metformin: sulfonylureas, thiazolidinediones, DPP-4 inhibitors, SGLT-2 inhibitors, basal insulin, and GLP-1 receptor agonists. Treatment effects in terms of reducing or increasing the odds of each outcome measure were summarized in terms of odds ratios or standard mean differences for each medication class, versus placebo. Odds ratios or mean differences that were significant at the P<0.05 level were incorporated into our analysis to ensure that our results are robust (Table 2).
Table 1.
Decision criteria outcomes
| Outcome | Definition | Data Source for Baseline Rate | Data Source for Treatment effects |
|---|---|---|---|
| Myocardial infarction | Fatal or non-fatal myocardial infarction | Validated risk equations (RECODe)15 (Appendix Text 1) |
Network meta-analysis 22 |
| Stroke | Fatal or non-fatal, and hemorrhagic or ischemic stroke | Validated risk equations (RECODe)15 (Appendix Text 1) |
Network meta-analysis 22 |
| Nephropathy | Renal failure/end-stage renal disease | Validated risk equations (RECODe)15(Appendix Text 1) |
Network meta-analysis 22 |
| Retinopathy | Severe vision loss defined as <20/200 visual acuity by Snellen chart | Validated risk equations (RECODe)15 (Appendix Text 1) |
Network meta-analysis 22 |
| Neuropathy | Pressure sensation loss on 10g monofilament testing | Validated risk equations (RECODe)15 (Appendix Text 1) |
Network meta-analysis 22 |
| HbA1c reduction | Hemoglobin A1c (absolute percentage points) | NHANES 2003-2014 52 | Network meta-analysis 22 |
| Whether or not medication is injected | Parenteral drug administration | - | American Diabetes Association28 |
| Body weight change | Body weight gain or loss (in kilograms) | NHANES 2003-2014 52 | Network meta-analysis 22 |
| Daily glucose monitoring | Recommended times per week for glucose self-monitoring | - | American Diabetes Association21 |
| Cost | Medication cost (defined in 2017 U.S. Dollars based on average standardized out-of-pocket price to consumers in the United States) | - | CMS53 |
| Serious treatment-related adverse events other than hypoglycemia | Side effects from medication, including severe nausea, fluid retention (edema), diarrhea, amputation, or rash that were fatal or life-threatening, that resulted in clinically significant or persistent disability, that required or prolonged a hospitalization, or that were judged by the trial investigator to represent a clinically significant hazard or harm | ACCORD24 | Network meta-analysis 22 |
| Hypoglycemia | Low blood glucose | Risk calculation based on age and hemoglobin A1c, using data from U.S. CDC23 (Appendix Table 2) | Network meta-analysis 22 |
ACCORD: Action to Control Cardiovascular Disease Risk in Diabetes; CDC: Centers for Disease Prevention and Control; RECODe: Risk Equations for Complications of Type 2 Diabetes; NHANES: National Health and Nutrition Examination Survey; CMS: Centers for Medicare & Medicaid Service
Table 2. Treatment effects (odds ratios, ORs, or standardized mean differences, SMDs) for each outcome for each glucose-lowering treatment class from a network meta-analysis and supplemental data.22,28, 53-55.
The impact of each medication on each outcome was calculated as independent from the impact of metformin, such that the odds ratios are relative to treatment with metformin plus placebo alone.
| Drug class | Myocardial infarction, OR (95% CI)22 |
Stroke, OR (95% CI)22 |
Hypoglycemia, OR (95% CI)22 |
Adverse events other than hypoglycemia, OR (95% CI)22 |
Hb1A1c, SMD (95% CI)22 |
Body weight, SMD (95% CI)22 |
Cost (2017 USD), mean [min, max]21,56 |
Times per week recommended for glucose self- monitoring, mean [min, max]57 |
|---|---|---|---|---|---|---|---|---|
| Sulfonylurea | 1† | 1† | 5.42 (3.90, 7.54) |
1† | −0.83 (−1.03, −0.64) |
0† | 11 [4,17] |
3 [2,5] |
| Thiazolidinedione | 1† | 1† | 1† | 1† | −0.85 (−1.03, −0.68) |
0† | 160 [5,314] |
4 [3,5] |
| DPP-4 Inhibitor | 1† | 1† | 1† | 1† | −0.68 (−0.87, −0.49) |
0† | 369 [357,382] |
3 [2,5] |
| SGLT-2 Inhibitor | 1†* | 1†* | 1† | 1† | −0.83 (−1.16, −0.50) |
−0.16 (−0.30, −0.01) |
413 [411,415] |
3 [2,5] |
| Basal Insulin | 1† | 1† | 30.96 (3.35, 285.72) |
1† | −0.88 (−1.28, −0.47) |
0† | 302 [203,404] |
11 [7,14] |
| GLP-1 Agonist | 1†* | 1†* | 1† | 1† | −1.05 (−1.32, −0.78) |
−0.37 (−0.62, −0.12) |
633 [500,775] |
3 [2,5] |
Varied in a sensitivity analysis to reflect newer trials published since the network meta-analysis incorporated into the baseline assessment; see main text.
non-significant at the p<0.05 level
Treatment effectiveness and adverse event rates on each class of therapy were calculated for participants aged 18 to 85 in the U.S. National Health and Nutrition Examination Survey (NHANES, 2003-2014) with type 2 diabetes mellitus [based on hemoglobin A1c ≥6.5% (47.5 mmol/mol), fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), or self-reported diabetes treatment] who might be eligible for second-line treatment by virtue of having hemoglobin A1c >7.0% (53mmol/mol) on metformin treatment alone (N=1,107). Missing data (5% of the patient-level features detailed below) were not imputed, and only complete case analyses were performed, using NHANES survey weights to estimate a nationally-representative U.S. sample (characteristics summarized in Appendix Table 1).
Outcomes
Absolute risk increase or decrease for each disease end-point was calculated by multiplying the NMA data (odds ratios for each outcome in Table 1, converted to relative risks based on U.S. outcome incidence rates19,20) by the baseline pre-treatment absolute risk estimate for each outcome. To incorporate changes in biomarkers (change in HbA1c or body weight), the absolute change was estimated by using the standardized mean difference in the biomarker from the NMA, and the baseline patient characteristic from NHANES. For costs, the absolute U.S. dollar increase in cost per 3 months of treatment was calculated. We also included indicator variables for whether the medication was parenteral or oral, and the number of times per week that glucose self-monitoring was recommended by a prior review.21
Absolute pre-treatment risk for each disease end-point was estimated using the Risk Equations for Complications Of type 2 Diabetes (RECODe, see Appendix Text 1),15 which were previously validated in three randomized trial populations and two diverse longitudinal cohort studies in the U.S. ,20 and provide estimates for 10-year risk of myocardial infarction (MI, fatal or non-fatal), stroke (fatal or non-fatal, and hemorrhagic or ischemic), congestive heart failure, retinopathy (severe vision loss defined as <20/200 visual acuity by Snellen chart), neuropathy (pressure sensation loss on 10g monofilament testing), nephropathy (renal failure/end-stage renal disease), and all-cause mortality. The RECODe equations required data on age, sex, race/ethnicity (Black, Latino/Hispanic, and other), current tobacco smoking, systolic blood pressure, history of cardiovascular disease (MI or stroke), current blood pressure-lowering drugs, current statin therapy, current anticoagulant use other than aspirin (e.g., warfarin or non-vitamin K antagonist oral anticoagulant), current glycemic treatment, hemoglobin A1c (HbA1c), total and high-density lipoprotein cholesterol, serum creatinine, and urine microalbumin to creatinine ratio.
Additional outcomes included the risk for hypoglycemia, for which pre-treatment risk on metformin alone (versus the odds on the above therapies) was calculated conditional on patient age and HbA1c, using data from the U.S. Centers for Disease Control and Prevention (CDC; Appendix Table 2).23
Another outcome of interest was treatment-related adverse events other than hypoglycemia, including severe nausea, fluid retention (edema), diarrhea, or rash that were fatal or life-threatening, that resulted in clinically significant or persistent disability, that required or prolonged a hospitalization, or that were judged by trial investigators to represent a clinically significant hazard or harm (coded per the Medical Dictionary for Regulatory Activities). The baseline risk of treatment-related adverse events was estimated as an incidence rate of 0.016 from the metformin-only subset of participants in the standard glycemic therapy arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial.24
Relative decreases and increases in outcome risk across treatment options
The input NMA data used in this assessment (Table 2) indicated no significant reduction in the risk of MI or stroke from the therapies considered (n.b., changed in sensitivity analyses), but significantly increased risk of hypoglycemia from sulfonylureas and basal insulin, and substantial reduction in body weight from GLP-1 agonists and SGLT-2 inhibitors. Costs were highest for GLP-1 agonists, followed by SGLT-2 inhibitors. Glucose self-monitoring requirements were highest for basal insulin.
Treatment features considered for patient preferences
Additional patient-relevant features of each medication were considered, and were selected because they appeared as important to treatment selection on a previously-published U.S. survey.25-27 These included: whether the treatment was parenteral or oral, degree of average body weight gain or loss (in kilograms), change in HbA1c (absolute percentage point reduction), medication cost (in 2017 U.S. Dollars based on average standardized out-of-pocket price to consumers across insurance types), and recommended times per week for glucose self-monitoring (Table 1).28
Risk score calculation
The model involved calculation of a partial value function (Appendix Text 2 and Appendix Figure 1), which transforms absolute ranges of each outcome values that is continuous (disease end-point risk, HbA1c, body weight, cost, and self-monitoring per week) into a 0 to1 scale for each treatment med for outcome i, with the function defined as u(imed).
where B is the best and W is the worst possible absolute risk decrease or increase estimate for each outcome among the treatments, and x is the absolute risk changes expected from the treatment being evaluated for the given individual patient. The modeling strategy used the approach adopted from Multi-Criteria Decision Analysis (MCDA),29-35 which produced a treatment score over N outcomes calculated as:
such that the partial value function enabled different outcomes (reduction in HbA1c, increase in rate of adverse events) to be fairly compared across the range of possible values, and combined these scores into a treatment ranking where preference weights reflected the importance of the outcome to a given individual. The treatment score calculation handles multiple outcomes by assuming additivity of the product of patient preference weight, normalized disability weight, and the partial value function, u(med). The preference weights were on a scale from 0 (not important) to 1 (very important; Appendix Figure 1). To account for relative severity/importance of decision outcomes, normalized disability weights associated with each outcome were incorporated based on prior comprehensive surveys of quality-adjusted disutility (Appendix Table 3), separately from patient preference weights.36-40 For example, if a patient place preference weights of 1 (very important) to both avoiding cardiovascular diseases and cost, the normalized weights would adjust the weights so that the ranking score considers avoiding CVD more seriously than avoiding higher cost with higher disability weights placed on CVD; the ranking score becomes less sensitive to avoiding higher cost as compared to avoiding stroke (Appendix Table 4). We adjust for both the severity of the condition (disability weights) and how much a patient ‘cares’ about the risk of experiencing that condition or not (preference weights, reflecting factors such as risk aversion and discounting), which are conceptually distinct. An automated online decision support tool was also produced for clinical application, where patients could input their preferences around major treatment-related variations.41 Normalized disability weights associated with outcomes were fixed in the calculation, so that patients would only expect to adjust their preference weights (not enter disutility values for each outcome) in the interactive decision support tool.
We assessed how differences among NHANES participant features and potential preferences could change the medication scores, and which treatments were selected as having the highest scores and for what reasons. We varied patient risk profiles, to examine in particular how medication scores differed across patients with lower versus higher baseline HbA1c, cardiovascular disease risk factors, and age, both individually and in concert with different preferences. In each simulated case, we performed Monte Carlo sampling 10,000 times from estimated Gaussian probability distributions around the treatment effect measures in the NMAs to estimate 95% confidence intervals. We selected Gaussian distributions because the NMA data presented means and standard deviations from an estimation procedure that generated a Gaussian outcome estimate.
Sensitivity analyses
We incorporated findings from recent trials not included in the base-case meta-analyses, modifying estimates of the impact of some glucose-lowering medications on cardiovascular outcomes, and including additional secondary outcomes (Table 2). Specifically, we incorporated new evidence of cardiovascular risk reduction among the agents studied in newer trials, with GLP-1 agonists having an odds ratio of 0.87 (0.76, 0.99) for non-fatal stroke, and SGLT-2 inhibitors having an odds ratio of 0.86 (0.77, 0.97) for non-fatal MI.42 We used treatment effect measures for only non-fatal cardiovascular outcomes to ensure that the outcomes are mutually exclusive with all-cause mortality. Because GLP-1 agonists and SGLT-2 inhibitors were both found to significantly lower the risks of heart failure and SGLT-2 inhibitors were found to additionally lower the risk of all-cause mortality, heart failure and all-cause mortality were included in the sensitivity analyses. The odds ratio of all-cause mortality was 0.88 (0.81, 0.94) for GLP-1 agonists.42 The odds ratios of all-cause mortality was 0.80 (0.71, 0.89) and of heart failure was 0.62 (0.52, 0.72) for SGLT-2 inhibitors.42 We included these estimates in the sensitivity analysis and not the baseline modeling, following guidelines for using unaltered meta-analysis data in a baseline modeling assessment,43 and because the newer NMA incorporating these endpoints did not comprehensively consider other medication classes and other disease end-points or side-effects.42
Analyses were performed in R (v. 3.3.3, The R Foundation for Statistical Computing, Vienna), using the code linked to the Appendix for reproduction and extension.
Results
Treatment rankings based on patient features
If patients had no differential preferences around willingness to gain weight, inject medications, perform glucose self-monitoring, avoid treatment-related side-effects or incur higher out-of-pocket drug costs, then their relevant biomarkers and personal features entirely determined their treatment recommendations via the model. Appendix Table 5 provides examples of four different NHANES participants with different features and either high or low cardiovascular risk and high or low microvascular risk. As shown, GLP-1 agonists and basal insulin were recommended as top-ranked medications among people with heightened microvascular risks, due to the input NMA ranking them as most effective relative to the other choices for realized HbA1c reduction. Note that the input NMA did not yet consider cardiovascular risk trials recently revealing potential benefits of GLP-1 agonists and SGLT-2 inhibitors in reducing cardiovascular risks, congestive heart failure exacerbations in specific; these were considered in the sensitivity analysis.
Treatment rankings based on patient preferences
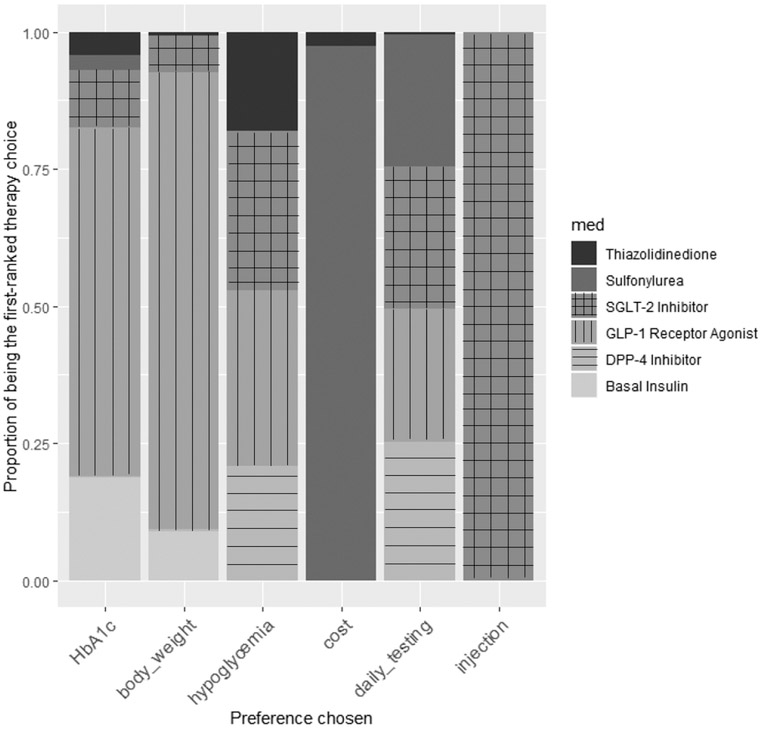
Figure 2 provides an estimate of the frequency with which different treatments were recommended by the MCDA-based method to each participant in the NHANES study if each participant had one particular preference among those available from the NMA (avoid parenteral treatments; weight gain; high medication cost; or glucose self-monitoring), and had no differential preference regarding the other treatment factors. As shown, patients wanting to avoid parenteral treatment and daily glucose testing had SGLT-2 inhibitors or DPP-4 inhibitors recommended most commonly, while those wanting to avoid weight gain were recommended a GLP-1 agonist most commonly, while those with major cost concerns often had sulfonylureas recommended. Notably, participants who had multiple preferences had different treatment rankings based on those combined preferences and their personalized risk/benefit scores. For example, participants with high cost considerations who were also at high risk for hypoglycemia often had thiazolidinediones recommended instead of sulfonylureas (Appendix Table 6).
Figure 2. Therapy recommendations based on preference variations.
The proportion of the NHANES population recommended each medication based on variations in their preferences. The results show the proportion of time that a medication class is the first-ranked therapy from the model, if a patient only selects one decision criterion in the decision-making process. The online decision tool enables multiple decision criteria to be weighted.41 Legend: “HbA1c”: hemoglobin A1c; “body weight”: weight gain; “hypoglycemia”: risk of hypoglycemia; “cost”: out of pocket patient drug cost for 3 months; “daily testing”: desire to avoid daily glucose testing; “injection”: desire to avoid parenteral medications.
Absolute decreases and increases in outcome risk across treatment options
We estimated absolute risk decreases and increases for the NHANES population, when each individual was given the medication ranked as their best option by the model if participants only considered reducing microvascular risk as much as possible in their treatment decision-making. The baseline pre-treatment risk among the NHANES population over 10 years was 10.5% for MI (95% CI: 9.9, 11.0), 4.0% for stroke (95% CI: 3.7, 4.2), 6.6% for nephropathy (95% CI: 6.4, 6.8), 12.5% for neuropathy (95% CI: 12.0, 12.9), and 8.6% for retinopathy (95% CI: 8.3, 8.9).
If reducing microvascular risk was the primary consideration, the most commonly-recommended treatment was GLP-1 agonist, followed by basal insulin, when the decision was based on the input NMA data. Under this scenario, the absolute 10 year-risk of microvascular diseases decreased by 2.1 % (95% CI: −2.2, −2.0), 1.1 % (95% CI: −1.2, −1.1), and 0.9 percentage points (95% CI: −0.9, −0.8) for neuropathy, retinopathy, and nephropathy outcomes, respectively. Absolute HbA1c reduction was −1.1% (−11.5 mmol/mol; 95% CI: −1.3, −0.8). Body weight was reduced by 0.37 kg (95% CI: −0.61, −0.13), and the medication cost incurred was $633 (95% CI: 499, 766) per 3 months.
We compared the estimated the quality-adjusted life-years (QALYs) saved over 10 years (with 1-year increment) among the NHANES population if they converted from the most common second-line treatment of a sulfonylurea to the most-recommended treatment for them by the MCDA-based model if they were preference agnostic. The treatment was given to simulated patients if HbA1c is greater than 6.5%. We estimated that the modeling approach would save at least 0.036 (95% CI: 0.032, 0.040) QALYs per person (about a half month) among NHANES participants for 10 years of treatment by switching to the MCDA-based recommended medication (typically a GLP-1 agonist). Those at high risk of macrovascular and microvascular diseases received the most benefits with incremental QALYs of 0.066 (95% CI: 0.056, 0.076) and 0.057 (0.049, 0.064) per person, respectively (Table 3), typically through selection of a SGLT-2 inhibitor.
Table 3.
Incremental quality-adjusted-life-years converting from the current second-line treatment (e.g., sulfonylurea) to MCDA recommended treatment
| Drug class | NHANES Population, % | Incremental QALYs (95% CI) Base-case network meta- analysis22 |
Incremental QALYs (95% CI) Sensitivity analysis (incorporating results from recent trials42) |
|---|---|---|---|
| All persons | 100.0 | 0.036 (0.032, 0.039) | 0.064 (0.061, 0.067) |
| Age | |||
| 20-39 | 6.9 | 0.009 (0.004, 0.014) | 0.014 (0.009, 0.019) |
| 40-59 | 28.5 | 0.024 (0.020, 0.028) | 0.034 (0.030, 0.037) |
| 60+ | 64.6 | 0.043 (0.038, 0.048) | 0.082 (0.077, 0.087) |
| Sex | |||
| Male | 50.3 | 0.042 (0.037, 0.048) | 0.078 (0.073, 0.083) |
| Female | 49.7 | 0.028 (0.024, 0.033) | 0.049 (0.045, 0.053) |
| Race | |||
| Black | 50.2 | 0.038 (0.033, 0.043) | 0.070 (0.066, 0.074) |
| Non-Black | 48.8 | 0.033 (0.028, 0.037) | 0.058 (0.054, 0.062) |
| Diabetes duration | |||
| < 10 years | 56.6 | 0.034 (0.030, 0.038) | 0.058 (0.054, 0.062) |
| ≥10 years | 43.4 | 0.037 (0.031,0.043) | 0.071 (0.066, 0.076) |
| HbA1c | |||
| < 6.5% | 38.8 | 0.028 (0.021, 0.033) | 0.058 (0.053, 0.063) |
| 6.5% – 7.9% | 40.3 | 0.038 (0.032, 0.044) | 0.069 (0.064, 0.074) |
| 8.0% - 9.4% | 12.5 | 0.040 (0.030, 0.049) | 0.066 (0.057, 0.075) |
| ≥ 9.5% | 8.4 | 0.052 (0.041, 0.063) | 0.063 (0.052, 0.074) |
| 10-year cardiovascular risk (myocardial infarction or stroke) | |||
| < 10% | 41.6 | 0.018 (0.014, 0.021) | 0.030 (0.026, 0.034) |
| 10% – 20% | 36.5 | 0.037 (0.031, 0.044) | 0.071 (0.065, 0.077) |
| ≥ 20% | 21.9 | 0.066 (0.056, 0.076) | 0.118 (0.108, 0.128) |
| 10-year composite Microvascular disease risk | |||
| < 20% | 30.0 | 0.018 (0.014, 0.023) | 0.033 (0.029, 0.037) |
| 20% – 30% | 35.9 | 0.029 (0.024, 0.035) | 0.059 (0.053, 0.065) |
| ≥ 30% | 34.1 | 0.057 (0.049, 0.064) | 0.096 (0.089, 0.107) |
Sensitivity analyses results
When the most optimistic cardiovascular disease odds ratios from a recent meta-analysis (including results from the LEADER, EMPA-REG, CANVAS/CANVAS-R, and SUSTAIN-6 trials) were considered,42,44-46 we found that people with high cardiovascular risk would be treated with SGLT-2 inhibitors more frequently, followed by GLP-1 agonists (Table 4). For people with low cardiovascular risk but high microvascular risk, GLP-1 agonists would be recommended most, followed by SGLT-2 inhibitors.
Table 4.
Individual examples of type 2 diabetes patients from the U.S. National Health and Nutrition Examination Survey (2003-2014, N = 1,107), incorporating results from recent trials on improved cardiovascular disease outcomes from some second-line glycemic therapies 42
| Covariate | Person 1 | Person 2 | Person 3 | Person 4 |
|---|---|---|---|---|
| Sex | Female | Female | Male | Male |
| Age | 47 | 44 | 63 | 66 |
| Race | Hispanic | Black | White | Black |
| SBP (mmHg) | 137 | 95 | 128 | 116 |
| Statin use | No | No | No | No |
| Anticoagulant use | No | No | No | No |
| BP medication use | No | Yes | Yes | Yes |
| Currently smoking | No | Yes | No | No |
| Oral diabetes medication use | No | No | No | No |
| Total cholesterol (mg/dL) | 188 (4.9 mmol/L) |
106 (2.7 mmol/L) |
195 (5.1 mmol/L) |
122 (3.2 mmol/L) |
| HDL cholesterol (mg/dL) | 44 (1.1 mmol/L) |
36 (0.9 mmol/L) |
69 (1.8 mmol/L) |
25 (0.6 mmol/L) |
| Hemoglobin A1c (%) | 7.5 (58.5 mmol/mol) |
9.1 (76.0 mmol/mol) |
8.0 (63.9 mmol/mol) |
11.4 (101.1 mmol/mol) |
| Serum creatinine (mg/DL) | 0.6 (53.0 μmol/L) |
0.7 (61.9 μmol/L) |
1.9 (168.0 μmol/L) |
2.32 (205.1 μmol/L) |
| Urine albumin creatine ratio (mg/g) | 54.2 | 7.6 | 68.8 | 60.0 |
| CVD history | Yes | Yes | No | Yes |
| CVD risk (10-yr risk, %) | Low | High | Low | High |
| Myocardial infarction | 8 | 12 | 7 | 56 |
| Stroke | 2 | 2 | 5 | 34 |
| Microvascular risk (10-yr risk, %) | Low | Low | High | High |
| Nephropathy | 8 | 7 | 23 | 58 |
| Retinopathy | 6 | 5 | 17 | 26 |
| Neuropathy | 7 | 7 | 16 | 23 |
| Drug ranking score (95% CI) based on network meta-analysis22 * | ||||
| 1st, ranking score normalized to 1 [uncertainty scale around being best value of 1] | SGLT-2-i 1.0 [0.83,1.47] |
SGLT-2-i 1.0 [0.83,1.47] |
GLP-1RA 1.0 [0.59,1.56] |
SGLT-2-i 1.0 [0.82,1.56] |
| 2nd | GLP-1RA 0.99 [0.59,1.56] |
GLP-1RA 0.99 [0.59,1.56] |
SGLT-2-i 0.99 [0.83,1.47] |
GLP-1RA 0.99 [0.59,1.66] |
| 3rd | Basal insulin 0.86 [0.33,1.60] |
Basal insulin 0.86 [0.33,1.61] |
Basal insulin 0.86 [0.34,1.61] |
Basal insulin 0.84 [0.35,1.75] |
| 4th | TZD 0.84 [0.59,1.33] |
TZD 0.84 [0.60,1.33] |
TZD 0.84 [0.60,1.34] |
TZD 0.82 [0.58,1.39] |
| 5th | SU 0.83 [0.53,1.39] |
SU 0.83 [0.53,1.39] |
SU 0.83 [0.53,1.39] |
SU 0.80 [0.52,1.45] |
| 6th | DPP-4-i 0.73 [0.49,1.31] |
DPP-4-i 0.73 [0.49,1.31] |
DPP-4-i 0.73 [0.49,1.32] |
DPP-4-i 0.69 [0.49,1.40] |
Preference weights: Only macrovascular and microvascular outcomes were considered important, accounting for the disability weights associated with disease outcomes.36-40
Drug ranking scores were normalized by the best ranking score. Score of 1 refers to the best ranking score, and the scores for other drugs are ranking scores relative the best ranking score. 95% confidence intervals were generated based on the uncertainty around the treatment effect estimates from the network meta-analysis.
TZD: Thiazolidinedione; SU: Sulfonylurea; GLP-1RA; GLP-1 agonist; SGLT-2-I; SGLT-2 inhibitor; DPP-4-I; DPP-4 inhibitor
When secondary outcomes (heart failure and all-cause mortality) were included as decision outcomes, and if reducing the composite of macrovascular (MI, stroke, and heart failure), microvascular, and all-cause mortality risk was the primary consideration, the most commonly recommended treatment was a SGLT-2 inhibitor due to its efficacy in reducing cardiovascular and all-cause mortality risk, followed by a GLP-1 agonist. Under this scenario, the absolute 10 year-risk among NHANES participants decreased by an average of 1.68 % (95% CI: −1.76, −1.59) for MI and by 3.39 % (95% CI: −3.62, −3.16) for heart failure among the NHANES population. The 10-year microvascular disease risk decreased by an average of 1.65 % (95% CI: −1.71, −1.59), 0.94 % (95% CI: −0.98, −0.90), and 0.66% (95% CI: −0.69, −0.62) for neuropathy, retinopathy, and nephropathy, respectively. Absolute HbA1c reduction was −0.8% (−9.09 mmol/mol; 95% CI: −1.2, −0.5) on average. Body weight was reduced by 0.16 kg (95% CI: −0.32, −0.05) on average, and the medication cost incurred was $413 (95% CI: 410, 416) per 3 months per person.
After incorporating secondary outcomes in treatment recommendation, we estimated that the MCDA-based approach would save at least 0.064 (95% CI: 0.061, 0.067) QALYs per person among NHANES participants. Those at high risk of macrovascular and microvascular diseases received the most benefits with incremental QALYs of 0.118 (95% CI: 0.108, 0.128) and 0.096 (0.089, 0.107) per person, respectively (Table 3). In each scenario, the benefits in terms of QALY gains were around twice those accruing from current second-line treatment.
Discussion
We developed a modeling approach to apply a MCDA modeling framework to the assessment of add-on glycemic therapy for type 2 diabetes care as a pilot study. We were able to parameterize complex interrelationship between treatment benefits, side-effects, and patient preferences that may better allow clinicians to implement shared decision-making. This study critically offers a generalizable strategy to incorporate complex quantitative network meta-analytic data that enables comparisons across available treatments; individualized risk/benefit estimates; and patient preferences to aid in personalized treatment selection.
The modeling approach to decision support offers advantages over prior decision support tool frameworks. First, the approach incorporates NMA results to provide objectivequantitative comparisons across all available treatment options.29-33 An advantage of this approach is that the analysis can be rapidly updated as new trials and meta-analyses are released, as we illustrated in sensitivity analyses. Second, the approach incorporates validated individual risk calculations based on patient features, allowing the treatment ranking to be influenced by formal multi-variable risk assessment rather than a single biomarker alone or a loose gestalt of a patient’s clinical profile. Since multiple factors influence a person’s risk for disease endpoints, and therefore their absolute risk reduction from different treatments, it can be complicated to account for all pertinent patient features when selecting a patient’s treatment. Third, the modeling approach can be generalized to several other treatment decisions, not just to the choice of second-line diabetes treatment selection by incorporating treatment efficacy and side-effects comparison data from network meta-analysis and individualized risk/benefit equations for other disease outcomes Hence, the approach may be applicable to a wide range of other problems that require consideration of numerous outcomes and treatment options incorporating patient preferences. The open-sourced code provided with this manuscript permits replication and extension of the approach to other clinical questions for which NMA data are increasingly available. The online decision tool also enables clinical application.41
Our study has limitations inherent to modeling based on secondary data sources. First, most trials of diabetes therapy include only short follow-up periods (ranging between 6 and 79 months),22 which means data from the NMA must be extrapolated to longer time periods. Second, in clinical practice, healthcare costs vary considerably by country and, within countries, by insurance plan. The inclusion of costs presents an important ethical consideration that requires further discussion. On one hand, having higher-risk drugs (e.g., sulfonylureas, a common source of disagreement) by incorporating cost into the weights can be seen as discriminatory against lower-income patients for whom cost is a genuine treatment factor. This raises an ethical issue of ensuring the optimal treatment choice for patients independent of affordability. On the other hand, in most parts of the world, out-of-pocket expenses are a disutility, and even in countries with universal health coverage, health technology agencies select thresholds of benefit per unit cost. Moreover, while this decision support tool provides support for shared decision making between patients and clinicians, from a payer’s perspective, this decision support framework needs to be updated with different cost data and other decision criteria in decision making processes. Third, we consider a broad, but not exhaustive, set of patient preference possibilities in our demonstration analysis; additional patient preferences could be elicited systematically for future iterations, but how to best elicit patient preferences across patients with diverse health literacy and numeracy remains a matter of investigation. Fourth, we intentionally used published and statistically significant NMA data in the baseline assessment and left the addition of recent major cardiovascular risk reduction trials to a sensitivity analysis; this was to ensure that our results are conservative by including only treatment effect measures that are statistically significant (p<0.05), and to follow guidelines for using unaltered meta-analysis data in a baseline modeling assessment,43,47-49 but also to illustrate how new major trials can dramatically alter treatment rankings and be rapidly incorporated into the analysis. We note that in our sensitivity analysis of GLP-1 agonists and SGLT-2 inhibitors, we assumed relative risk reductions in cardiovascular outcomes proportional to baseline risk, though the cardiovascular risk reduction trials themselves were limited to patients with high cardiovascular disease risk. Other limitations include that are inherent with network meta-analysis include that participant characteristics that may affect the relative efficacy of interventions are similar across groups.42 Moreover, because the treatment ranking calculation is performed in relative scale within the available options based on NMA data, a decision maker assigns preferences on a standardized relative scale rather than absolute scales in quantitative terms. Fifth, we compared the estimated the QALYs saved over 10 years among the NHANES population if they converted from the most common second-line treatment of a sulfonylurea to the most-recommended treatment for them by the MCDA-based model if they were preference agnostic. The durability of sulfonylureas may be limited over time, but the ADOPT trial is the only randomized trial to our knowledge that provides evidence of slightly more substantial durability of rosiglitazone than metformin than glyburide, but only as monotherapies rather than as add-on therapy to metformin.50 The GRADE study planned for completion in 2021 may provide the definitive data necessary for addressing this question.51 Lastly, while MCDA-based modeling may help clinicians and patients understand their treatment options, the tool should not yet be implemented in practice as several other aspects of theory must be worked out and tested before such utilization. The tool aggregates many aspects of care that may be disaggregated in future visualizations. Additionally, there are non-quantifiable factors, beyond the considered decision criteria, such as ethical and social factors, which need to be accounted for when making the final treatment decision. Therefore, our modeling strategy can serve as an aid for informed shared decision making, but should not be used as a prescription tool in the current form.
The next logical step for this work is to conduct NMA studies that help distinguish between individual drugs, rather than only between drug classes. An important limitation of all NMAs on glucose-lowering medications to date is that they treat an entire class of drugs together for comparison, without distinguishing within-class, between-drug differences. For example, while hypoglycemia is incorporated in terms of the multiplicative effect by class, any specific side effects that are drug-specific but not generalizable to the class at are not yet included and could be in the future with revised NMA data that includes specific drugs. An additional direction for future research is to further individualize treatment recommendations by NMAs based on individual participant data, which may go beyond the average summary effects presented and incorporate heterogeneous treatment effect estimates based on patient features. A third direction we intend to pursue is to identify how alternative communication strategies for the decision support tool, and alternative strategies for including and eliciting patient and provider preferences, may assist in decision-making. In addition, while we wished to ensure that our results are conservative by including only treatment effect measures from NMA that are statistically significant (p<0.05), in the future we could sample from a broad range of distributions including non-significant ranges. Using a Bayesian approach and propagating uncertainty may help clarify the distributions around drug rankings.
MCDA is a modeling strategy that may help to address an age-old clinical problem: how best to integrate information regarding treatment benefits and side effects, individualized risk, and patient preferences. The results of this study may help provide a framework to synthesize randomized trial data, personalized risk estimates, and individual patient preferences into a unified platform to support shared decision-making in type 2 diabetes care, and other major medical treatment decisions. The framework to synthesize randomized trial data, personalized risk estimates, and individual patient preferences into a unified platform to support shared decision-making can theoretically be applied to other major medical treatment decisions where there are multiple treatment options without a clear ‘winning’ therapy that is both the most effective and with the least side-effects. This applies to current therapies for heart disease, mental illness, and rheumatological disease, among others.
Acknowledgements
Source of funding: Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K08HL121056, by the National Institute On Minority Health And Health Disparities of the National Institutes of Health under Award Numbers DP2MD010478 and U54MD010724, and by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23DK109200. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Text 1. Risk Equations for Complications of type 2 Diabetes (RECODe)
Risk equations for microvascular and cardiovascular complications of type 2 diabetes were developed using individual participant data from a large intervention study and validated in two randomized trials and two longitudinal cohort studies.1,2 The following table provides the RECODe coefficients.
| MI (fatal or non- fatal) |
Stroke (fatal or non-fatal) |
Neuropathy | Retinopathy | Nephropathy | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 0.04363 | 0.02896 | 0.03022 | 0.02285 | −0.01938 |
| Women | −0.20660 | − 0.00326 | −0.1868 | 0.2264 | −0.01129 |
| Ethnicity | |||||
| Black | −0.11630 | 0.2716 | −0.09448 | −0.1677 | 0.08812 |
| Hispanic or Latino | 0.2338 | ||||
| Clinical features | |||||
| Tobacco smoking, current | 0.2358 | 0.1665 | 0.1483 | ||
| SBP (mmHg) | −0.00514 | 0.01659 | 0.00456 | 0.00824 | 0.00303 |
| History of CVD | 0.9618 | 0.4138 | 0.26672 | 0.1127 | −0.02164 |
| Drug use | |||||
| BP lowering drugs | −0.12480 | 0.1598 | 0.18192 | 0.06393 | −0.07952 |
| Statins | 0.04699 | − 0.18870 | |||
| Anticoagulants | 0.544 | − 0.13870 | 0.03199 | ||
| Oral diabetes drugs | −0.25747 | −0.2349 | −0.1256 | ||
| Biomarkers | |||||
| HbA1c, % | 0.2135 | 0.3365 | 0.18866 | 0.1449 | 0.1369 |
| Total cholesterol, mg/dL | 0.00019 | 0.00171 | 0.00219 | −0.00017 | −0.00111 |
| HDL cholesterol, mg/dL | −0.01358 | − 0.00639 | −0.00539 | 0.00545 | 0.00629 |
| Serum creatine, mg/DL | 0.08027 | 0.5955 | 0.604442 | 0.6947 | 0.8609 |
| Urine albumin:creatine ratio, mg/g | 0.00042 | 0.0003 | 0.0002 | 0.00036 | |
The 10-year risk of an outcome can be computed as 1 – λ^exp (Σ (βx) – mean (Σ (βx))), where β are the equation coefficients and × are the values for each covariate for an individual patient within the cohort under study, λ values are: 0.973 for renal failure or end-stage renal disease (nephropathy), 0.921 for retinopathy, and 0.870 for neuropathy and corresponding values of mean (Σ (βx)) were 0.23 for renal failure/end-stage renal disease (nephropathy), 4.56 for retinopathy, and 4.75 for neuropathy. For CVD outcomes, λ values were 0.93 for fatal or non-fatal MI and 0.98 for fatal or non-fatal stroke, and mean (Σ (βx))) values were 2.92 for fatal or non-fatal MI and 6.96 for fatal or non-fatal stroke.
For example, a 60-year-old non-smoking white man with systolic blood pressure 140 mm Hg, without history of cardiovascular disease, not on any medications, and with HbA1c of 8%, total cholesterol of 190 mg/dL, HDL of 50 mg/dL, serum creatinine 1.1 mg/dL, and urine microalbumin:creatinine ratio of 10 mg/g would have a risk of renal failure/end-stage renal disease of 1–0.973^exp(−0.01938*60 + 0.003027*140 + 0.1369* 8-0.001112*190 + 0.006289* 50 + 0.8609*1.1 + 0.000362*10–0.23) = 0.085 or a 8.5% 10-year risk, where 0.23 is the mean(Σ(βx)).
Text 2. Patient preference and normalized disability weights
A partial value function captures a decision maker’s preferences on a standardized scale.3 It transforms values selected along a slider bar (from least important to most important) to the range 0-1 (lowest preference weight to highest preference weight). Partial value functions can be linear or non-linear depending on a decision maker’s desirability of scale values. For example, in case of serious adverse events, if a decision maker perceives an increase in serious adverse event rates from 0% to 1% is the same as an increase from 1% to 2%, the partial value function is linear. However, if a decision maker is less willing to take an increase from 0% to 1% than 1% to 2%, the partial value function would be concave. In this study, we assumed partial value functions to be linear for each decision criterion.
Patient preference
Patients can determine a set of weights for each decision criterion. For example, the scale of hypoglycemia is 2% to 17% and the scale of if HbA1c is −3.5 to −1.0. If a patient assigns weights of 0.4 to hypoglycemia and 0.8 to HbA1c, the decrease in HbA1c is two times more important than the increase in hypoglycemia for the patient.
In our decision support tool using this model, there were two sets of weights, (1) fixed weights to account for relative severity of disease complications (e.g., myocardial infarction, stroke, etc.) using normalized disability weights associated with each of the outcomes (Appendix Table 3), and (2) patient preference weights to account for features of treatments (e.g., cost, parenteral versus oral, etc.) that a patient could vary from “not important” (weight of 0) to “extremely important” (weight of 1). For example, if a patient place preference weights of 1 (very important) to both avoiding cardiovascular diseases and cost, the normalized weights would adjust the weights so that the ranking score considers avoiding CVD more seriously than avoiding higher cost with higher disability weights placed on CVD; the ranking score becomes less sensitive to avoiding higher cost as compared to avoiding stroke (Appendix Table 4). Normalized disability weights were built in the calculation that patients would be only expected to adjust their preference weights. Medication rankings purely based on patient preferences without normalized disability weights considered are presented in Appendix Table 7.
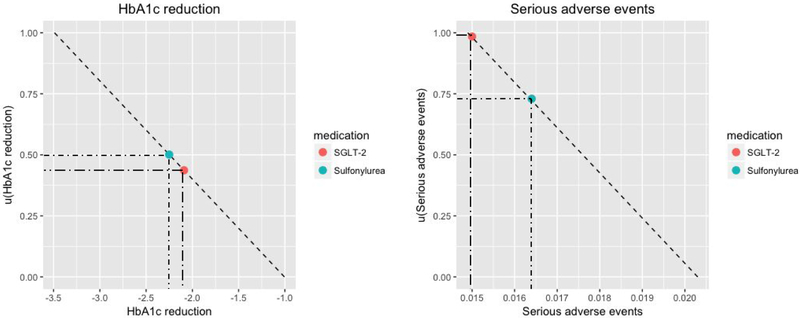
To illustrate how medication ranking is calculated, we present a case with two decision criteria (HbA1c reduction and serious adverse events) and two medication options (Sulfonylurea and SGLT-2 inhibitors). Suppose the absolute scale ranges for HbA1c reduction and serious adverse events are (−3.5, −1.0) and (0.015, 0.020), respectively. Suppose a patient only considers HbA1c reduction and avoiding serious adverse events in the decision-making process for the dual add-on therapy to metformin, with weights 0.7 to HbA1c reduction and 0.3 to avoiding adverse events. Then, the medication ranking score is calculated as a weighted average of the transformed treatment effect measures as shown in Appendix Figure 1.
Text 3. Illustration of converting relative measures into absolute scales
For a patient with following characteristics,
| variable | value |
|---|---|
| Sex | Female |
| Age | 59 |
| Systolic blood pressure (mmHg) | 139 |
| Statin use | 0 |
| Anticoagulant use | 0 |
| BP medication use | 0 |
| Currently smoking | 0 |
| Diabetes | 1 |
| Oral diabetes medication use | 0 |
| Total cholesterol (mg/dL) | 205 (5.3 mmol/L) |
| HDL cholesterol (mg/dL) | 76 (2.0 mmol/L) |
| Hemoglobin A1c (%) | 5.8 (39.9 mmol/mol) |
| Serum creatinine | 0.9 |
| Urine albumin creatinine ratio | 42.8 |
| Black | 0 |
| Hispanic | 0 |
| CVD history | 1 |
The person’s macrovascular and microvascular disease risks can be calculated using RECODe equations:
| Disease | 10-yr risk (%) |
|---|---|
| Stroke | 1.7 |
| Myocardial infarction | 7.2 |
| Nephropathy | 6.2 |
| Retinopathy | 8.9 |
| Neuropathy | 7.6 |
Then the absolute baseline risk for all decision criteria considered would be as following (outcomes using standard mean difference as treatment effectiveness based on the network meta-analysis):
| Outcome | Baseline_risk |
|---|---|
| Adverse_event | 1.6% |
| Hypoglycemia | 1.4% |
| Stroke | 1.7% |
| MI | 7.2% |
| Hba1c | 0 |
| Body_weight | 0 |
| Injection | 0 |
| Daily_glucose_testing | 0 |
| Cost | 0 |
| Nephropathy | 6.2% |
| Retinopathy | 8.9% |
| Neuropathy | 7.6% |
Then the absolute baseline risk after taking metformin as first-line therapy changes to:
| Outcome | Baseline_risk |
|---|---|
| Adverse_event | 1.5% |
| Hypoglycemia | 2.4% |
| Stroke | 1.5% |
| MI | 6.1% |
| Hba1c | −1.01 |
| Body_weight | −0.09 |
| Injection | 0 |
| Daily_glucose_testing | 3 |
| Cost | 10 |
| Nephropathy | 5.2% |
| Retinopathy | 7.8% |
| Neuropathy | 6.6% |
After incorporating treatment efficacy and safety data of different add-on dual therapy medication options (assuming this person gets metformin as first-line therapy), the absolute min/max possible ranges are calculated for each decision criteria. Each second-line medication option would fall within these absolute ranges, and the risk scores will be calculated after transforming these ranges into 0-1 scales:
| Outcome | Best | Worst |
|---|---|---|
| Adverse_event | 1.1% | 2.6% |
| Hypoglycemia | 1.4% | 2.3% |
| Stroke | 0.1% | 4.9% |
| MI | 0.1% | 16.7% |
| Hba1c | −3.0 | −1.4 |
| Body_weight | −1.7 | 1.0 |
| Injection | 0 | 1 |
| Daily_glucose_testing | 5.0 | 17.0 |
| Cost | 15.1 | 776.3 |
| Nephropathy | 4.0% | 5.0% |
| Retinopathy | 5.8% | 7.4% |
| Neuropathy | 4.5% | 6.2% |
Appendix Figure 1. Ranking score calculation
Outcome1:HbA1c reduction
Outcome2: Serious adverse events
Partial value functions transform absolute scales into 0-1 scales. Medication ranking score is calculated as a weighted average of transformed values accounting for patient preference weights and normalized disability weights for each decision criterion
Here, preference weight will be determined by the decision maker through the decision support tool in Appendix Figure 2.
Figure 2. User interface of the online decision support tool 4
(A)Risk calculator tab for absolute pre-treatment risk of each outcome
(B) Patient preference elicitation tab with individualized treatment rankings
Table 1. NHANES population characteristics (2003-2014)
Profiles of patients with type 2 diabetes mellitus in the National Health and Nutrition Examination Survey (NHANES, N = 35,034, 2003-2014) were used to explore sensitivity of treatment rankings to different patient features and preferences
Analyses were restricted to the subset (N=1,107) that might be eligible for second-line treatment by virtue of having hemoglobin A1C ≥7% (53 mmol/mol), on metformin treatment alone. Missing data (60.3% of the patient-level features detailed below) were not imputed, and only complete case analyses were performed, using NHANES survey weights to estimate a nationally-representative sample
| Characteristics [Mean or % (sd)] | NHANES (2003-2014, N=1,107) |
|---|---|
| Demographics | |
| Age, years | 62.4 (13.4) |
| Women | 49.7% (1.5) |
| Ethnicity | |
| Black | 50.2% (1.5) |
| Hispanic or Latino | 13.4% (1.0) |
| Clinical features | |
| Tobacco smoking, current | 17.3% (1.1) |
| SBP (mmHg) | 130.1 (19.5) |
| History of CVD | 20.6% (1.2) |
| Drug use | |
| BP lowering drugs | 66.2% (1.4) |
| Statins | 9.3% (0.8) |
| Anticoagulants | 1.4% (0.3) |
| Oral diabetes drugs (metformin) | 27.1% (1.3) |
| Biomarkers | |
| HbA1c, (%) | 7.2 (1.6) [55 (12) mmol/mol] |
| Total cholesterol, mg/dL | 182.1 (41.8) [4.72 (1.1) mmol/L] |
| HDL cholesterol, mg/dL | 49.2 (14.5) [1.5 (0.4) mmol/L] |
| Serum creatinine, mg/dL | 1.0 (0.4) [88.4 (35.4) umol/L] |
| Urine albumin:creatinine ratio, mg/g | 23.2 (26.2) |
Table 2. Baseline risk for symptomatic hypoglycemia
Incidence per 1,000 diabetic adults5
| Age Group | |||||||
|---|---|---|---|---|---|---|---|
| 18–44 | 45–64 | 65–74 | 75+ | ||||
| Rate | Std Error | Rate | Std Error | Rate | Std Error | Rate | Std Error |
| 14.4 | 1.1 | 10.0 | 0.5 | 15.1 | 0.9 | 27.6 | 1.8 |
Incidence conditioned on baseline hemoglobin A1c.6
| Baseline HbA1c (vs ≤5.6%) | OR (95% CI) |
|---|---|
| 5.7% - 6.4% | 1.12 (0.81-1.54) |
| 6.5%-7.0% | 0.99 (0.77-1.28) |
Table 3. Disutility weights for disease outcomes
By disease outcome,
| Disease | Disutility weight | Sources |
|---|---|---|
| MI | 0.422 (1-2 days) 0.056 (3-28 days) |
7,8 |
| Stroke | 0.284 | 7,8 |
| Heart Failure | 0.097 | 9 |
| Neuropathy | 0.133 | 10 |
| Nephropathy | 0.104 | 10 |
| Retinopathy | 0.085 | 10 |
| Obesity | 0.040 | 11 |
In addition to preference weights assigned by the patient/clinicians, disutility weights were assigned and fixed weights to disease outcomes normalized by the weight of MI (the highest disutility weight). Disutility weight of obesity was applied to non-clinical outcomes; body weight gain, injection, daily glucose testing, and cost to account for relative severity/importance of outcomes. In microsimulations, the average time-weighted disability value was used to estimate QALYs lost due to MI.
Table 4. Individual example with and without normalized disability weights
A preference weight of 1 was assigned to cost and cardiovascular diseases (myocardial infarction and stroke). The rest of the outcomes were not considered important in this example.
| Covariate | Person 1 High consideration for cost and CVD (weight of 1) Without normalized disability weight |
Person1 High consideration for cost and CVD (weight of 1) With normalized disability weight |
|---|---|---|
| Sex | Male | Male |
| Age | 63 | 63 |
| Race | Hispanic | Hispanic |
| SBP (mmHg) | 134.7 | 134.7 |
| Statin use | No | No |
| Anticoagulant use | No | No |
| BP medication use | Yes | Yes |
| Currently smoking | No | No |
| Oral diabetes medication use | Yes | Yes |
| Total cholesterol (mg/dL) | 181 (4.8 mmol/L) | 181 (4.8 mmol/L) |
| HDL cholesterol (mg/dL) | 61 (1.4 mmol/L) | 61 (1.4 mmol/L) |
| Hemoglobin A1c (%) | 6.1 (51.5 mmol/mol) | 6.1 (51.5 mmol/mol) |
| Serum creatinine (mg/DL) | 0.63 (53.0 μmol/L) | 0.63 (53.0 μmol/L) |
| Urine albumin creatinine ratio (mg/g) | 301.6 | 301.6 |
| CVD history | No | No |
| CVD risk (10-yr risk, %) | ||
| Myocardial infarction | 4 | 4 |
| Stroke | 1 | 1 |
| Microvascular risk (10-yr risk, %) | ||
| Nephropathy | 5 | 5 |
| Retinopathy | 6 | 6 |
| Neuropathy | 6 | 6 |
| Drug ranking score (95% CI) based on network meta-analysis12* | ||
| 1st | SU 1.0 [0.71,1.28] |
SGLT-2-i 1.0 [0.77, 1.23] |
| 2nd | TZD 0.90 [0.56, 1.27] |
SU 0.81 [0.37, 1.25] |
| 3rd | SGLT-2-i 0.87 [0.72,1.02] |
TZD 0.79 [0.41,1.18] |
| 4th | Basal insulin 0.81 [0.31, 1.29] |
GLP-1 0.78 [0.73, 1.26] |
| 5th | DPP-4 0.77 [0.45, 1.02] |
Basal insulin 0.78 [0.16, 1.37] |
| 6th | GLP-1 0.62 [0.26, 0.99] |
DPP-4 0.77 [0.36, 1.17] |
Drug ranking scores were normalized by the best ranking score. Score of 1 refers to the best ranking score, and the scores for other drugs are ranking scores relative the best ranking score. 95% confidence intervals were generated based on the uncertainty around the treatment effect estimates from the network meta-analysis.
TZD: Thiazolidinedione; SU: Sulfonylurea; GLP-1RA; GLP-1 agonist; SGLT-2-I; SGLT-2 inhibitor; DPP-4-I; DPP-4 inhibitor
Table 5. Individual example (Base-case)
Preference weights: the composite outcome of macrovascular and microvascular endpoints were considered important, accounting for the disability weights associated with disease outcomes (Appendix Table 3), and using the base-case network meta-analysis for input data (not accounting for recent trials with reduced cardiovascular disease from SGLT-2 or GLP-1 agents). The rest of the outcomes were not considered in this example.
| variable | Person 1 | Person 2 | Person 3 | Person 4 |
|---|---|---|---|---|
| Sex | Female | Female | Male | Male |
| Age | 47 | 44 | 63 | 66 |
| Race | Hispanic | Black | White | Black |
| SBP (mmHg) | 137 | 95 | 128 | 116 |
| Statin use | No | No | No | No |
| Anticoagulant use | No | No | No | No |
| BP medication use | No | Yes | Yes | Yes |
| Currently smoking | No | Yes | No | No |
| Oral diabetes medication use | No | No | No | No |
| Total cholesterol (mg/dL) | 188 (4.9 mmol/L) | 106 (2.7 mmol/L) | 195 (5.1 mmol/L) | 122 (3.2 mmol/L) |
| HDL cholesterol (mg/dL) | 44 (1.1 mmol/L) | 36 (0.9 mmol/L) | 69 (1.8 mmol/L) | 25 (0.6 mmol/L) |
| Hemoglobin A1c (%) | 7.5 (58.5 mmol/mol) | 9.1 (76.0 mmol/mol) | 8.0 (63.9 mmol/mol) | 11.4 (101.1 mmol/mol) |
| Serum creatinine (mg/DL) | 0.6 (53.0 μmol/L) | 0.7 (61.9 μmol/L) | 1.9 (168.0 μmol/L) | 2.32 (205.1 μmol/L) |
| Urine albumin creatinine ratio (mg/g) | 54.2 | 7.6 | 68.8 | 60.0 |
| CVD history | Yes | Yes | No | Yes |
| CVD risk (10-yr risk, %) | Low | High | Low | High |
| Myocardial infarction | 8 | 12 | 7 | 56 |
| Stroke | 2 | 2 | 5 | 34 |
| Microvascular risk (10-yr risk, %) | Low | Low | High | High |
| Nephropathy | 8 | 7 | 23 | 58 |
| Retinopathy | 6 | 5 | 17 | 26 |
| Neuropathy | 7 | 7 | 16 | 23 |
| Drug ranking score (95% CI) based on network meta-analysis12* | ||||
| 1st | GLP-1RA 1.0 [0.48,1.69] |
GLP-1RA 1.0 [0.48,1.71] |
GLP-1RA 1.0 [0.47,1.70] |
GLP-1RA 1.0 [0.52,1.98] |
| 2nd | Basal insulin 0.89 [0.48,1.51] |
Basal insulin 0.89 [0.48,1.52] |
Basal insulin 0.89 [0.49,1.52] |
Basal insulin 0.86 [0.48,1.69] |
| 3rd | TZD 0.87 [0.73,1.26] |
TZD 0.87 [0.73,1.26] |
TZD 0.87 [0.73,1.26] |
TZD 0.84 [0.71,1.32] |
| 4th | SGLT-2-i 0.85 [0.62,1.35] |
SGLT-2-i 0.85 [0.62,1.35] |
SGLT-2-i 0.85 [0.62,1.35] |
SGLT-2-i 0.82 [0.59,1.44] |
| 5th | SU 0.85 [0.69,1.28] |
SU 0.85 [0.69,1.28] |
SU 0.85 [0.69,1.28] |
SU 0.82 [0.68,1.34] |
| 6th | DPP-4-I 0.75 [0.57,1.28] |
DPP-4-I 0.75 [0.56,1.29] |
DPP-4-I 0.75 [0.57,1.29] |
DPP-4-I 0.70 [0.55,1.41] |
Drug ranking scores were normalized by the best ranking score. Score of 1 refers to the best ranking score, and the scores for other drugs are ranking scores relative the best ranking score. 95% confidence intervals were generated based on the uncertainty around the treatment effect estimates from the network meta-analysis. TZD: Thiazolidinedione; SU: Sulfonylurea; GLP-1RA; GLP-1 agonist; SGLT-2-I; SGLT-2 inhibitor; DPP-4-I; DPP-4 inhibitor
Table 6. Individual example with differences in preference weights
Preference weights: the composite macrovascular and microvascular endpoints were considered important in this example, accounting for the disability weights associated with disease outcomes (Appendix Table 3), and using the base-case network meta-analysis for input data (not accounting for recent trials with reduced cardiovascular disease from SGLT-2 or GLP-1 agents). A preference weight of 1 was assigned to injection and daily glucose testing. The rest of the outcomes were not considered important in this example.
| Covariate | Person 1 No consideration for hypoglycemia (w=0) High consideration for cost (w=1) |
Person1 Consideration for hypoglycemia (w=0.5) High consideration for cost (w=1) |
|---|---|---|
| Sex | Female | Female |
| Age | 47 | 47 |
| Race | Hispanic | Hispanic |
| SBP (mmHg) | 137 | 137 |
| Statin use | No | No |
| Anticoagulant use | No | No |
| BP medication use | No | No |
| Currently smoking | No | No |
| Oral diabetes medication use | No | No |
| Total cholesterol (mg/dL) | 188 (4.9 mmol/L) | 188 (4.9 mmol/L) |
| HDL cholesterol (mg/dL) | 44 (1.1 mmol/L) | 44 (1.1 mmol/L) |
| Hemoglobin A1c (%) | 7.5 (58.5 mmol/mol) | 7.5 (58.5 mmol/mol) |
| Serum creatinine (mg/DL) | 0.6 (53.0 μmol/L) | 0.6 (53.0 μmol/L) |
| Urine albumin creatinine ratio (mg/g) | 54.2 | 54.2 |
| CVD history | Yes | Yes |
| CVD risk (10-yr risk, %) | Low | Low |
| Myocardial infarction | 8 | 8 |
| Stroke | 2 | 2 |
| Microvascular risk (10-yr risk, %) | Low | Low |
| Nephropathy | 8 | 8 |
| Retinopathy | 6 | 6 |
| Neuropathy | 7 | 7 |
| Drug ranking score (95% CI) based on network meta-analysis12* | ||
| 1st | TZD 1.0 [0.89,1.28] |
SU 1.0 [0.95, 1.21] |
| 2nd | SU 0.98 [0.93, 1.37] |
TZD 0.97 [0.85, 1.25] |
| 3rd | SGLT-2-i 0.93 [0.81,1.25] |
SGLT-2-i 0.84 [0.81,1.08] |
| 4th | DPP-4-i 0.91 [0.77, 1.31] |
DPP-4-i 0.81 [0.73, 1.11] |
| 5th | GLP-1RA 0.82 [0.79, 1.32] |
GLP-1RA 0.63 [0.52, 1.01] |
| 6th | Basal insulin 0.49 [0.18, 1.63] |
Basal insulin 0.56 [0.32, 1.57] |
Drug ranking scores were normalized by the best ranking score. Score of 1 refers to the best ranking score, and the scores for other drugs are ranking scores relative the best ranking score. 95% confidence intervals were generated based on the uncertainty around the treatment effect estimates from the network meta-analysis.
TZD: Thiazolidinedione; SU: Sulfonylurea; GLP-1RA; GLP-1 agonist; SGLT-2-I; SGLT-2 inhibitor; DPP-4-I; DPP-4 inhibitor
Table 7. Individual example with different sets on weights in ranking calculation
-
i)Ranking calculation with normalized disability weights only, and without patient preference weights (patients would consider each outcome equally)
Covariate Person 1 Sex Male Age 63 Race Hispanic SBP (mmHg) 134.7 Statin use No Anticoagulant use No BP medication use Yes Currently smoking No Oral diabetes medication use Yes Total cholesterol (mg/dL) 181 (4.8 mmol/L) HDL cholesterol (mg/dL) 61 (1.4 mmol/L) Hemoglobin A1c (%) 6.1 (51.5 mmol/mol) Serum creatinine (mg/DL) 0.63 (53.0 μmol/L) Urine albumin creatinine ratio (mg/g) 301.6 CVD history No CVD risk (10-yr risk, %) Myocardial infarction 4 Stroke 1 Microvascular risk (10-yr risk, %) Nephropathy 5 Retinopathy 6 Neuropathy 6 Drug ranking score (95% CI) based on network meta-analysis 1st SGLT-2-i
1.0 [0.81,1.37]2nd GLP-1RA
0.94 [0.593, 1.39]3rd TZD
0.87 [0.65,1.27]4th SU
0.86 [0.62, 1.31]5th DPP-4
0.77 [0.55, 1.23]6th Basal insulin
0.76 [0.27, 1.39] -
ii)Ranking calculation with patient preference weights only (patients would consider each outcome equally), without normalized disability weights assigned to decision criteria.Preference weight of 1 (very important) was assigned to each outcome at a time with the rest of outcomes set to 0 (not important)
rank No
preferenceAdverse
eventHypoglyce
miaStroke MI HbA1c Body
weightInjection Daily
glucose
testingCost Nephro
pathyRetino
pathyNeuro
pathy1st SGLT-2-i
1.00
[0.78,1.37]SGLT-2-i*
1.00
[0.81,1.17]GLP-1*
1.00
[0.97,1.02]GLP-1
1.00
[0.90,1.10]SGLT-2-i
1.00
[0.90,1.26]GLP-1
1.00
[0.78,1.37]GLP-1
1.00
[0.73,1.27]SGLT-2-i
1.00
[1.00,1.00]GLP-1*
1.00
[1.00,1.00]SU
1.00
[0.99,1.01]GLP-1
1.00
[0.53,1.45]GLP-1
1.00
[0.53,1.45]GLP-1
1.00
[0.53,1.45]2nd TZD
0.99
[0.83, 1.28]TZD*
1.00
[0.73, 1.27]SGLT-2-i*
1.00
[0.98, 1.02]Basal
insulin*
0.89
[0.00, 1.72]GLP-1*
0.64
[0.00, 1.30]Basal
insulin
0.70
[0.05, 1.35]SGLT-2-i
0.76
[0.58,0.94]TZD
1.00
[1.00,1.00]SGLT-2-i*
1.00
[0.91, 1.08]TZD
0.80
[0.61, 0.99]Basal
insulin
0.71
[0.05, 1.33]Basal
insulin
0.71
[0.05, 1.33]Basal
insulin
0.71
[0.05, 1.33]3rd SU
0.98
[0.85,1.27]SU*
1.00
[0.65,1.27]DPP-4*
1.00
[0.98, 1.02]SGLT-2-i*
0.89
[0.73, 1.05]Basal
insulin*
0.64
[0.18, 1.11]TZD
0.65
[0.37,0.93]DPP-4*
0.58
[0.35, 0.82]SU
1.00
[1.00,1.00]DPP-4*
1.00
[0.91, 1.08]Basal
insulin
0.61
[0.48,0.74]TZD
0.66
[0.38,0.93]TZD
0.66
[0.38,0.93]TZD
0.66
[0.38,0.93]4th GLP-1
0.96
[0.71, 1.29]GLP-1*
1.00
[0.62, 1.31]TZD*
1.00
[0.98, 1.02]DPP-4*
0.89
[0.13, 1.65]DPP-4*
0.64
[0.42, 0.86]SGLT2-i*
0.61
[0.03, 1.19]TZD*
0.58
[0.36, 0.82]DPP-4
1.00
[1.00,1.00]SU*
1.00
[0.91, 1.08]DPP-4
0.52
[0.51, 0.54]SGLT2-i*
0.62
[0.04, 1.18]SGLT2-i*
0.62
[0.04, 1.18]SGLT2-i*
0.62
[0.04, 1.18]5th DPP-4
0.85
[0.71, 1.20]DPP-4*
1.00
[0.60,1.40]SU
0.77
[0.73,0.80]TZD*
0.89
[0.53,1.25]TZD*
0.64
[0.27,1.02]SU*
0.61
[0.32, 0.91]SU
0.58
[0.42,0.74]GLP-1
0.00
[0.00,0.00]TZD
0.91
[0.82,0.99]SGLT2-i
0.47
[0.46, 0.47]SU*
0.62
[0.32, 0.92]SU*
0.62
[0.32, 0.92]SU*
0.62
[0.32, 0.92]6th Basal
insulin
0.65
[0.23, 1.20]Basal
insulin*
1.00
[0.00, 2.00]Basal
insulin
0.04
[0.00, 0.08]SU*
0.89
[0.55, 1.23]SU*
0.64
[0.16, 1.13]DPP-4
0.35
[0.00,0.70]Basal
insulin
0.58
[0.00, 1.17]Basal
insulin
0.00
[0.00,0.00]Basal
insulin
0.32
[0.00, 0.64]GLP-1
0.18
[0.00, 0.35]DPP-4
0.36
[0.00,0.71]DPP-4
0.36
[0.00,0.71]DPP-4
0.36
[0.00,0.71]
References
- 1.Basu S, Sussman JB, Berkowitz SA, et al. Validation of Risk Equations for Complications of Type 2 Diabetes Mellitus (RECODe) using Individual Participant Data from Diverse Longitudinal Cohorts in the United States. Diabetes care. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017;5(10):788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thokala P, Devlin N, Marsh K, et al. Multiple Criteria Decision Analysis for Health Care Decision Making--An Introduction: Report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19(1):1–13. [DOI] [PubMed] [Google Scholar]
- 4.Choi SB, S. Decision Support Tool for Dual Add-on Therapy to Metformin. https://sungchoi.shinyapps.io/code/. Accessed Mar 19, 2018.
- 5.Centers for Disease Control and Prevention. Emergency Department Visit Rates for Hypoglycemia as First-Listed Diagnosis per 1,000 Diabetic Adults Aged 18 Years or Older, United States, 2006–2009. 2012; https://www.cdc.gov/diabetes/statistics/hypoglycemia/fig5.htm. Accessed Nov 11, 2017.
- 6.McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive Treatment and Severe Hypoglycemia Among Adults With Type 2 Diabetes. JAMA internal medicine. 2016;176(7):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell GE, Marrero WJ, Lavieri MS, Sussman JB, Hayward RA. Data-driven Markov Decision Process approximations for personalized hypertension treatment planning. MDM Policy & Practice. 2016;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128(21):2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2010 (GBD 2010) Disability Weights. http://ghdx.healthdata.org/record/global-burden-disease-study-2010-gbd-2010-disability-weights. Accessed Feb 22, 2016.
- 11.Franks P, Hanmer J, Fryback DG. Relative disutilities of 47 risk factors and conditions assessed with seven preference-based health status measures in a national U.S. sample: toward consistency in cost-effectiveness analyses. Med Care. 2006;44(5):478–485. [DOI] [PubMed] [Google Scholar]
- 12.Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis. JAMA : the journal of the American Medical Association. 2016;316(3):313–324. [DOI] [PubMed] [Google Scholar]
Footnotes
Conflicts of interests: All authors have no potential conflicts to disclose.
References
- 1.Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, et al. Shared decision making in endocrinology: present and future directions. The lancet Diabetes & endocrinology. 2016;4(8):706–716. [DOI] [PubMed] [Google Scholar]
- 2.Smith SA, Shah ND, Bryant SC, et al. Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system. Mayo Clin Proc. 2008;83(7):747–757. [DOI] [PubMed] [Google Scholar]
- 3.Cefalu WT, Boulton AJM, Tamborlane WV, et al. Status of Diabetes Care: New Challenges, New Concepts, New MeasuresFocusing on the Future! Diabetes care. 2015;38(7):1177–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic Therapy for Type 2 Diabetes: Synopsis of the 2017 American Diabetes Association Standards of Medical Care in Diabetes. Annals of internal medicine. 2017;166(8):572–578. [DOI] [PubMed] [Google Scholar]
- 5.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013–2014. JAMA : the journal of the American Medical Association. 2016;316(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laiteerapong N, Cooper JM, Skandari MR, et al. Individualized Glycemic Control for U.S. Adults With Type 2 Diabetes: A Cost-Effectiveness Analysis. Annals of internal medicine. 2018;168(3):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson MJ, Nathan AG, Huang ES. Personalized decision support in type 2 diabetes mellitus: current evidence and future directions. Curr Diab Rep. 2013;13(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yudkin JS, Kavanagh J, McCormack JP. Guidelines for treating risk factors should include tools for shared decision making. Bmj. 2016;353:i3147. [DOI] [PubMed] [Google Scholar]
- 9.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. Bmj. 2013;346:f2914. [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. [DOI] [PubMed] [Google Scholar]
- 11.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–437. [DOI] [PubMed] [Google Scholar]
- 12.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. [DOI] [PubMed] [Google Scholar]
- 13.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–428. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628–630. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol. 2017;5(10):788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwyn G, Stiel M, Durand MA, Boivin J. The design of patient decision support interventions: addressing the theory-practice gap. J Eval Clin Pract. 2011;17(4):565–574. [DOI] [PubMed] [Google Scholar]
- 17.Bowen DJ, Allen JD, Vu T, Johnson RE, Fryer-Edwards K, Hart A Jr. Theoretical foundations for interventions designed to promote informed decision making for cancer screening. Ann Behav Med. 2006;32(3):202–210. [DOI] [PubMed] [Google Scholar]
- 18.Durand MA, Stiel M, Boivin J, Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ Couns. 2008;71(1):125–135. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z Converting Odds Ratio to Relative Risk in Cohort Studies with Partial Data Information. Journal of Statistical Software. 2013;55(5). [Google Scholar]
- 20.Basu S, Sussman JB, Berkowitz SA, et al. Validation of Risk Equations for Complications of Type 2 Diabetes Mellitus (RECODe) using Individual Participant Data from Diverse Longitudinal Cohorts in the United States. Diabetes care. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes A. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S55–S64. [DOI] [PubMed] [Google Scholar]
- 22.Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis. JAMA : the journal of the American Medical Association. 2016;316(3):313–324. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Emergency Department Visit Rates for Hypoglycemia as First-Listed Diagnosis per 1,000 Diabetic Adults Aged 18 Years or Older, United States, 2006–2009. 2012; https://www.cdc.gov/diabetes/statistics/hypoglycemia/fig5.htm. Accessed Nov 11, 2017.
- 24.Group AS, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. The New England journal of medicine. 2010;362(17):1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant RW, Wexler DJ, Watson AJ, Lester WT, Nathan DM. How doctors choose medications to treat type 2 diabetes: A national survey of generalists and specialists. Diabetes. 2007;56:A307–A307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31(10):877–892. [DOI] [PubMed] [Google Scholar]
- 27.Gelhorn HL, Poon JL, Davies EW, Paczkowski R, Curtis SE, Boye KS. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naive type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes A. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S73–S85. [DOI] [PubMed] [Google Scholar]
- 29.Mussen F, Salek S, Walker S. A quantitative approach to benefit-risk assessment of medicines-part 1: The development of a new model using multi-criteria decision analysis. Pharmacoepidem Dr S. 2007;16:S2–S15. [DOI] [PubMed] [Google Scholar]
- 30.Rajala DW. Decisions with Multiple Objectives - Preferences and Value Trade-Offs - Keeney,Rl, Raiffa,H. Ieee T Syst Man Cyb. 1979;9(7):403–403. [Google Scholar]
- 31.Coolen F Multiple criterion decision analysis: An integrated approach. J Oper Res Soc. 2003;54(9):1019–1019. [Google Scholar]
- 32.van Valkenhoef G, Tervonen T, Zhao J, de Brock B, Hillege HL, Postmus D. Multicriteria benefit-risk assessment using network meta-analysis. Journal of clinical epidemiology. 2012;65(4):394–403. [DOI] [PubMed] [Google Scholar]
- 33.Naci H, van Valkenhoef G, Higgins JP, Fleurence R, Ades AE. Evidence-based prescribing: combining network meta-analysis with multicriteria decision analysis to choose among multiple drugs. Circulation Cardiovascular quality and outcomes. 2014;7(5):787–792. [DOI] [PubMed] [Google Scholar]
- 34.Marsh K, M IJ, Thokala P, et al. Multiple Criteria Decision Analysis for Health Care Decision Making--Emerging Good Practices: Report 2 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19(2):125–137. [DOI] [PubMed] [Google Scholar]
- 35.Tervonen T, Naci H, van Valkenhoef G, et al. Applying Multiple Criteria Decision Analysis to Comparative Benefit-Risk Assessment: Choosing among Statins in Primary Prevention. Medical decision making : an international journal of the Society for Medical Decision Making. 2015;35(7):859–871. [DOI] [PubMed] [Google Scholar]
- 36.Franks P, Hanmer J, Fryback DG. Relative disutilities of 47 risk factors and conditions assessed with seven preference-based health status measures in a national U.S. sample: toward consistency in cost-effectiveness analyses. Med Care. 2006;44(5):478–485. [DOI] [PubMed] [Google Scholar]
- 37.Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2010 (GBD 2010) Disability Weights. http://ghdx.healthdata.org/record/global-burden-disease-study-2010-gbd-2010-disability-weights. Accessed Feb 22, 2016.
- 38.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schell GJ, Marrero WJ, Lavieri MS, Sussman JB, Hayward RA. Data-Driven Markov Decision Process Approximations for Personalized Hypertension Treatment Planning. MDM Policy & Practice. 2016;1(1):238146831667421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128(21):2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi SB S Decision Support Tool for Dual Add-on Therapy to Metformin. https://sungchoi.shinyapps.io/code/. Accessed Mar 19, 2018.
- 42.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA. 2018;319(15):1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 45.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 46.Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2016;374(11):1094. [DOI] [PubMed] [Google Scholar]
- 47.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Medical decision making : an international journal of the Society for Medical Decision Making. 2007;27(5):696–713. [DOI] [PubMed] [Google Scholar]
- 48.Harrison M, Rigby D, Vass C, Flynn T, Louviere J, Payne K. Risk as an attribute in discrete choice experiments: a systematic review of the literature. Patient. 2014;7(2):151–170. [DOI] [PubMed] [Google Scholar]
- 49.Tervonen T, Gelhorn H, Sri Bhashyam S, et al. MCDA swing weighting and discrete choice experiments for elicitation of patient benefit-risk preferences: a critical assessment. Pharmacoepidemiol Drug Saf. 2017;26(12):1483–1491. [DOI] [PubMed] [Google Scholar]
- 50.Viberti G, Lachin J, Holman R, et al. A Diabetes Outcome Progression Trial (ADOPT): baseline characteristics of Type 2 diabetic patients in North America and Europe. Diabet Med. 2006;23(12):1289–1294. [DOI] [PubMed] [Google Scholar]
- 51.Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Continuous NHANES web tutorial. 2013; http://www.cdc.gov/nchs/tutorials/Nhanes/index_continuous.htm. Accessed Sep 11, 2014.
- 53.Centers for Medicare & Medicaid Services. Pharmacy pricing: national average drug acquisition cost. 2018; https://www.medicaid.gov/medicaid/prescription-drugs/pharmacy-pricing/index.html. Accessed Feb 5, 2018.
- 54.Lee G, Oh SW, Hwang SS, et al. Comparative effectiveness of oral antidiabetic drugs in preventing cardiovascular mortality and morbidity: A network meta-analysis. PloS one. 2017;12(5):e0177646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MAYO Clinic. Diabetes Medication Choice Decision Aid. https://diabetesdecisionaid.mayoclinic.org/index.php/site/compare?PHPSESSID=7l6ep2a37li4s2rk6ft25dnjf7. Accessed Dec 23, 2017.
- 56.Centers for Medicare & Medicaid Services. Pharmacy pricing: national average drug acquisition cost. 2018; https://www.medicaid.gov/medicaid/prescription-drugs/pharmacy-pricing/index.html. Accessed Jun 2, 2018.
- 57.MAYO Clinic. Diabetes Medication Choice Decision Aid. https://diabetesdecisionaid.mayoclinic.org/index.php/site/compare?PHPSESSID=7l6ep2a37li4s2rk6ft25dnjf7. Accessed Jun 2, 2018.