Abstract
Proteins have sparked fast growing interest as biological therapeutic agents for several diseases. Antibodies, in particular, carry an enormous potential as drugs owing to their remarkable target specificity and low immunogenicity. Although the market has numerous antibodies directed towards extracellular targets, their use in targeting therapeutically important intracellular targets is limited by their inability to cross cellular membrane. Realizing the potential for antibody therapy in disease treatment, progress has been made in the development of methods to deliver antibodies intracellularly. In this review, we address various platforms for delivery of antibodies, their merits and drawbacks.
Graphical Abstract
1. INTRODUCTION
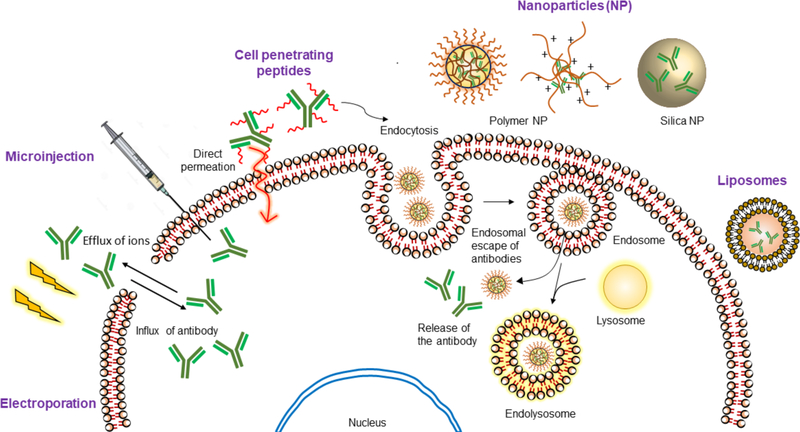
Only 10% of the genome can be targeted by small molecule drugs.1 As a result, protein therapy has emerged as an alternative to small molecules, as witnessed by ~130 FDA-approved biologics in the market.2 Owing to the specificity of proteins in interacting with their targets, the off-target effects of the drug is limited. Proteins have the potential to address key bottlenecks in cancer therapy, in metabolic diseases such as diabetes mellitus-type 1, protein replacement therapy for genetic diseases such as in lysosomal storage disease, anti-viral therapy, diagnosis of bacterial infections and development of vaccines.3 Among proteins, antibodies hold a special place as a therapeutic. An illustrative example of antibodies’ niche involves ‘drugging the undruggable’ targets. Many disease targets, although identified, are considered undruggable; because these targets lack a specific and well-defined binding pocket and are present inside the cells.4 Antibodies hold enormous potential for drugging these targets, as they can be produced against any epitope using the well-established hybridoma technology or phage display.5,6 The cell membrane, however, poses a barrier for the entry of antibodies, thus restricting them for extracellular targets. Intracellular protein delivery, as a general topic, has been a subject of many recent reviews.7,8 In this review, we specifically focus on cytosolic delivery of antibodies. An antibody against an intracellular target in circulation will be non-specifically taken up cells other than targeted cells such as immune cells that present Fc receptor giving rise to off-target effects9. This complication can be partially circumvented by developing smaller fragments of antibody devoid of Fc region such as antigen binding fragment (Fab), single chain variable fragment (ScFv) and nanobodies. However, the shorter antibody mimics are liable towards rapid clearance from the body.10 Furthermore, upon endocytotic uptake of antibody formats, the endosomes are fused with lysosome that degrades the antibody before they reach their targets in the cytoplasm.11 A potential solution to these problems can be achieved by association with a delivery vehicle that protects the antibody from degradation whilst allowing handles for attachment of cell-specific targeting molecules. These vehicles can be chemically modified to escape endosomes or enter cells via non-endocytotic pathways. Aspects of these would be discussed in detail throughout the review. We have organized this review based on the methods (physical vs. chemical approaches) and the materials (polymers, nanoparticles, and liposomes) used for the intracellular delivery of antibodies (Fig. 1).
Fig. 1.
Illustration of different protein delivery systems and their mechanism of cellular entry
2. CLASSICAL METHODS
Microinjection is among the first techniques to be used for delivering antibodies into cells.12,13 For example, the role of IFN-induced protein-Mx in providing protection against influenza was demonstrated through microinjection of an anti-Mx antibody.14 Although this process provides near-quantitative incorporation of the proteins inside cells, the process itself is both harsh and tedious such that it greatly impacts cell viability.15 Electroporation, on the other hand, involves the use of electric field pulses to reversibly permeabilize the cell membrane via creation of transient pores that allow the transport of proteins across the membrane. In-vitro electroporation has been used to introduce antibodies into cytosol of many animal16,17 and plant cells18, whilst a few groups have successfully demonstrated the use of this method for antibody delivery into human cells.19,20 Electroporation allows the entry of antibodies into multiple cells simultaneously, while the microinjection process requires single cell manipulations. However, electroporation was found to be harsh and inefficient, and can only be used in vitro.15,21
2.1. Alternate strategies - Intrabodies
The drawbacks of these delivery methods could potentially be overcome by developing intracellularly expressed antibodies, called intrabodies.22,23,24 Here, cells are transfected with plasmids encoding for antibodies. In this strategy however, the challenges in delivery of antibodies is simply replaced with the challenges in delivering plasmids. Based on their location of action, intrabodies can be of two type-cytosolic and endoplasmic reticulum (ER) intrabodies. The ER provides an oxidizing environment for correct folding of the antibodies following which they can be utilized to study the effects of knockdown in different pathways in ER, be presented on cell surface or secreted by the cell. For targets that are cytosolic or in the nucleus, intrabodies are required to be made in cytoplasm. The reducing intracellular environment and the absence of appropriate chaperone proteins forbid precise folding of the antibody, even if the plasmids were successfully introduced in the cells. The following reviews provide an excellent comprehensive study on production of intrabodies for therapy25,26. Therefore, although delivery of plasmids may be a viable strategy, methods that deliver intact antibodies with structural and functional integrity are attractive. Consequently, cell penetrating peptides conjugated to antibodies (transbody) are argued to be a better option than intrabodies27.
3. CELL PENETRATING PEPTIDES
Protein transduction domain (PTD) or cell penetrating peptides (CPP) comprise 10–30 amino acids, primarily based on cationic lysines and arginines and/or hydrophobic amino acids. These peptides translocate across the cell membrane via different mechanisms.28,29 CPPs have been shown to navigate the membrane in both endocytotic and non-endocytotic pathways (direct cell membrane penetration) depending upon the CPP-cargo combination, the concentration of the cargo and their molecular weights.30 Many CPP-cargo conjugates were able to enter cells at 4 °C invoking direct penetration mechanisms such as pore formation, carpet-like model and inverted micelle formation.31 However, under different circumstances CPPs were shown to enter cells via different endocytosis mechanisms such as clathrin/caveolin mediated, micropinocytosis and caveolin/clathrin independent pathways.32
Although CPPs have been used for delivery of small proteins, utilizing these for antibody delivery is sparse due to a dependence on cargo size33. HIV - transactivator of transcription (HIV-TAT)34 is the earliest protein with known CPP capabilities. The CPP domain of this protein, the so-called Tat-peptide, has been used for antibody delivery.35,36 For example, Tat-conjugated anti-tetanus (Fab’)2 was used to neutralize tetanus toxin in chromaffin cells.37 Similarly, Tat-modified anti-Rev1-Fab was used to combat HIV infection, where the key nuclear export of viral transcripts using Rev-1 was compromised.38 The cytosolic delivery capabilities of the Tat-peptide conjugation were demonstrated by the nuclear localization of anti-Rev1-Fab (Fig. 2a). The nuclear localization itself was facilitated by the fact that Tat-peptide, in addition to the CPP features, also has an embedded nuclear localization signal (NLS) sequence.
Fig. 2.
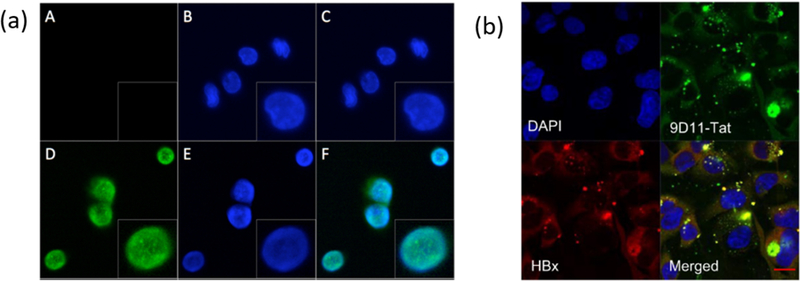
(a) Confocal microscopy for studying the transduction of FabRev1 (A-C) and Tat-conjugated FabRev1 (D-F) into PBMCs. The presence of tat-FabRev1 in nucleus is evident from turquoise color. Blue is from DAPI fluorescence in nucleus, Green is for AF488 conjugated anti-Fab secondary antibody, PBMC-peripheral blood mononuclear cells, reprinted with permission from [38]; Fig.1 (b) Confocal microscopy studying intracellular localization of 9D11-Tat conjugated anti-HBx and HBx in Huh7 cells. 9D11-Tat-anti-HBX was stained with AF488 goat anti-human antibody, HBx was stained with AF594 anti-HBx antibody. Reprinted with permission from [39]
A recombinant fusion of Tat and full-length anti-Hepatitis-B virus X (anti-HBx) was used to inhibit HBx, critical for Hep-B replication, in Huh7 and HepG2 cells39 (Fig. 2b). The effect of utilizing the full-length antibody was demonstrated by the reduction of intracellular concentrations of HBx, because the Fc-domain of the antibody binds to TRIM-21 thus guiding the bound HBx protein for proteasomal degradation. The Fc region of the antibody has essential role in antibody therapy for cancer40. Various monoclonal antibodies targeting tumor cell surface proteins are recognized by Fc-receptors on immune cells such as Natural Killer (NK) cells, monocytes, macrophages and a subset of T-cells9. Following this interaction, the target tumor cells are eliminated via antibody-dependent cellular cytotoxicity.41 The Fc region can also be recognized by complement system that cause complement dependent cytotoxicity42. For the purposes of this review, we will focus on antibody that has intracellular targets as opposed to cell surface proteins for cancer therapy.
Cell penetrating ability of Tat has also been used in enhancing tumor retention of antibodies.43 To target aberrant expression of proteins in cancer, Tat-123I-anti-p21WAF−1/Cip−1 antibody was delivered into MDA-MB-468 breast cancer cells; the antibody was shown to be transported to the nucleus to block p21-mediated G1-S phase arrest, as seen from 35% nuclear radioactivity as compared to 7% for 123I-anti-p21WAF−1/Cip−1 without the Tatpeptide.44,45
In a unique attempt, an anti-DNA antibody46 itself has been shown to exhibit CPP-like features including transport to the nucleus.47 A fusion between mAb3G5, which targets cancer-related MDM2 protein, and anti-DNA-ScFv was delivered into COS-7 cells and melanoma cells. The bispecific antibody inhibited MDM2 in vitro and retarded the growth of tumor in mice. To make the effect of antibody-based inhibition of a target selective, histone-2A-based CPP BR2 was used to deliver the ScFv against a mutated K-Ras to induce apoptosis in cancer cells.48 Some of the other CPPs that have been used for delivering antibodies include transportan49, peptides identified from autoantibodies50 and membrane translocating sequence from Kaposi fibroblast growth factor.51,52
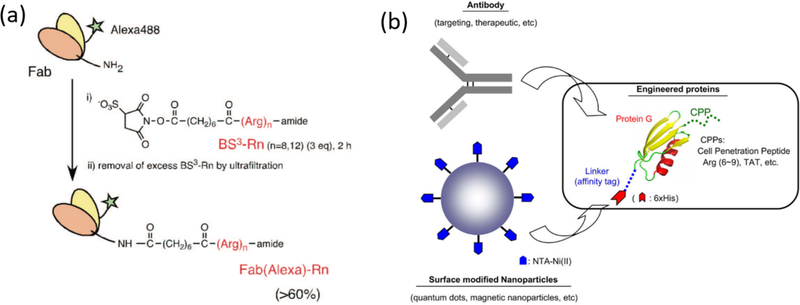
Inspired by naturally occurring CPPs, researchers have designed synthetic guanidinium-rich CPPs to facilitate interaction with cell membrane for internalization of the cargo. In one such example, an oligoarginine was conjugated with the Fab of IgG using their lysine handles (Fig. 3a) and was delivered into HeLa cells.53 Cyclic arginine peptides, on the other hand, were shown to promote non-endocytic cellular uptake54,55 and subsequently used to make cell-permeable nanobodies. The CPP tagged nanobodies were used to re-localize two proteins, polymerase clamp PCNA (proliferating cell nuclear antigen) and p53, to the nucleolus and study the interaction between PCNA and tumor suppressor p53.56 Similarly, a commercially available CPP, Pep-157,58, was used to deliver anti-LAMP and anti-β-actin into mammalian cells (Table 1). Pep-1 was inspired by the lysine rich hydrophobic domain, mimicking the NLS sequence of Simian virus protein, SV40.
Fig. 3.
(a) The lysines on Fab were modified with oligoarginines as CPP, reprinted with permission from [53] (Bioconjug. Chem. 2009, 20 (2), 249–257) Copyright 2009 American Chemical Society; (b) Protein G is modified to contain CPP and hexahistidine tag. The histidine affinity tag complexes with Nickel on nanoparticle while the antibody is bound to protein G, Reprinted with permission from [59]
Table 1.
Summary of antibodies delivered against intracellular targets using various techniques
| Methods | Target | Ref. | Benefits | Drawbacks | |
|---|---|---|---|---|---|
| Classical Methods | Microinjection | Anti-Mx antibody; to study the anti-viral effects of Mx protein | 14 | Quantitative transduction efficiency | Single cell injection, tedious, impacts cell viability |
| Electroporation | Anti-p21-ras antibody (G-protein family encoded by ras oncogenes) | 16 | Entry into multiple cells simultaneously | Requires special instrumentation, impacts cell viability, inefficient, in-vitro technique | |
| Anti-pp60-c-src kinase antibody | 17 | ||||
| Anti-lipoxygenase activity (in plant cells) | 18 | ||||
| Anti-G1-specific cyclin D1 (cell- cycle protein) | 19 | ||||
| Cell Penetrating Peptides | Tat | Anti-Tat antibody | 35 | Protein modified with short peptide sequences, flexibility in electrostatic and covalent complexation with CPPs/generation of fusion proteins, great technique for in-vitro delivery | Delivery efficiency/endoso mal escape is dependent upon the combination of CPP and protein, possibility of altered targeting ability of antibody, lack cell specificity, can seldom be used in-vivo, prone to degradation by proteases |
| Anti-syntaxin 1 (t-SNARE protein) | 36 | ||||
| Anti-tetanus toxin antibody | 37 | ||||
| Anti-HIV-1 Rev antibody | 38 | ||||
| Anti-Hepatitis B Virus X protein | 39 | ||||
| Anti-melanoma antibody NRML- 05 | 43 | ||||
| 123-I-anti mouse antibody, Anti- p21-WAF-1/Cip-1 antibody (cyclin dependent kinase inhibitor) | 44,45 | ||||
| ScFv for EDB domain of fibronectin (in ECM of tumor) | 70 | ||||
| Anti-DNA antibody (3E10) | Bispecific antibody with 3G5 (binds to MDM2 and inhibits interaction with p53) | 47 | |||
| Buforin IIb derived CPP | BR2 fused to anti-Kras ScFv | 48 | |||
| Transportan | Anti-NifS, Anti-biotin antibody | 49 | |||
| Vetocell Peptides | Anti-peroxidase antibody | 50 | |||
| Membrane Translocating Sequence (MTS) | Anti-Akt1-ScFv (serine-threonine kinase protein B), 5D10 (human B-cell lymphoma), S1C5 (mouse B-cell tumor) | 51,52 | |||
| Oligoarginines | Polyclonal IgG | 53 | |||
| Pep-1 | Anti-β-actin, Anti-LAMP-1 | 57, 58 (review) | |||
| Protein A/G | Anti-mouse IgG, Anti- mitochondrial antibody (MTC02) | 59, 60 | |||
| Fc-Binding Peptide | Rabbit/Human IgG | 62 | |||
| Hemagglutinating virus of Japan Envelope | Mouse IgG, Anti-nuclear pore complex, Anti-tubulin | 63 | |||
| M-lycotoxin derived CPP | Anti-His-6-tag-antibody, Anti- glucocorticoid receptor antibody | 67 | |||
| Anti-ATP5A antibody (recognizes α-subunit of mitochondrial ATP synthase) | 68 | ||||
| Collagen-like CPP | Anti-rabbit IgG | 71 | |||
| Nanoparticles | |||||
| Composition | |||||
| Inorganic Nanoparticles (NP) | Non-Porous SiNPs | Anti-phospho-Akt | 78 | Biocompatibility, surface functionalization capabilities, pore volume tunability, inherent rigidity | Delayed and uncontrolled release of payload, toxicity |
| Mesoporous hollow dendritic SiNPs | IgG | 80 | |||
| Mesoporous hollow SiNPs with large surface hole | IgG | 81 | |||
| Rough SiNPs (RSN) | Anti-phospho-Akt | 83 | |||
| Biodegradable SiNPs with poly(disulphide) surface | Cetuximab | 85 | |||
| Polymeric NP/non- covalent complexation |
Poly(N,N- diethylacrylamide) (PDEAAm)-streptavidin | IgG | 88 | Quick formulation, efficient uptake in cells | Toxicity because of cationic species |
| Biotinylated PPAAc | Anti-CD3 antibody | 89 | |||
| Poly (lactic-co-glycolic acid) | Anti-AnnexinA2 (AnxA2) antibody | 91 | |||
| PEI | Anti-Lamin | 93 | |||
| Polybutylcyanoacrylat e nanoparticles | Anti-synuclein | 94 | |||
| Polyion complex based on (PAsp(DET)) | IgG, Anti-nuclear pore complex (NPC), Anti-NPC | 96, 97 | |||
| Guanidinium based amphiphilic polymer | Anti-pPKCθ | 99 | |||
| Polymeric NP/covalent conjugation | PEI | IgG, rabbit anti-body against human S100C | 100 | Tunability in molecular weight, particle size and surface functional groups; longer circulation time | Permanent modification, low endosomal escape, slow release |
| CPD | IgG | 86 | |||
| Amphiphilic polyanhydride nanoparticles |
Anti-TNF-α | 103 | |||
| Liposomal delivery | Cationic PULSin | Mouse-IgG, anti-giantin & anti- nuclear pore complex antibody | 112 | Biocompatible & biodegradable, tunability in surface functionalization, straight-forward formulating steps | Sub-optimal encapsulation efficacy, poor in vivo stability, systemic opsonization & fast clearance, limited scope for incorporation of responsive moieties |
| TFA-DODAPL & DOPE | IgG | 113 | |||
| DOSP & MM27 | Anti-cytokeratin 8 (K8) antibody | 114 | |||
| Stearyl-R8-GALA, DOPE, CHEMS | Mouse anti-nuclear pore complex antibody, IgG | 115 | |||
| DPPC, DOTAP,cholesterol, PEG2000-DSPE | Anti-TuBB-9 antibody (inactivates nuclear Ki-67 protein) | 116 | |||
| DOPC, DOPE, cholesterol, DSPE-PEG3400-NHS modified antibody | Anti-IL6R & Anti-CD44 (for targetting) antibody | 118 | |||
| DSPE-4A (attached with four arginine), DSPE-Hy-PEG2k (attached with benzaldehyde), DOPC, cholesterol | Anti-S100A4 (inactivates apoptotic p53 protein) antibody | 119 |
In addition to directly conjugating CPPs with antibodies, intracellular access was also achieved by conjugating these peptides with protein A/G (derived from Staphylococcus aureus and Streptococcus) which binds strongly to the crystallizable fragment (Fc) of antibodies. This combination was used to deliver a mitochondria-targeting antibody, which was imaged using the nanoparticle bearing the CPP-modified protein A/G (Fig. 3b).59 Similarly, a fusion between protein A and Tat was also used to deliver AF546 conjugated anti-mouse IgG into 3T3 cells that expressed GFP.60 In contrast to proteins, a peptide capable of binding to the IgG-Fc, called FcBP61, was used to deliver human/rabbit IgG into HeLa and 3T3-L1.62 In another example, a fusion protein comprised of a nucleocapsid protein and the ZZ domain of protein A was used to load antibodies into hemagglutinating virus of Japan envelope (HVJ-E) capsid. In this case, the viral capsid itself presumably acted like a CPP. The versatility of this platform was demonstrated by delivering an unspecific mouse IgG1, anti-nuclear pore complex and anti-tubulin.63
Since endocytosis is predominantly the mechanism of uptake for CPP associated cargos, newer membrane disruptive agents derived from viral/bacterial toxins that aid in endosomal escape of the cargo are sought. The endosomal escape properties of CPP was enhanced upon fusion of endosomolytic peptide from influenza virus hemagglutinin-2.64 Even though many fusogenic peptides are employed for improving gene delivery65, optimization of endosomolytic peptides for delivery of biologics is an ongoing challenge.66 Beginning with a cationic membrane lytic M-lycotoxin, a glutamate residue was introduced in the hydrophobic face of the amphiphilic α-helix. The idea here is that the lytic property of the toxin would be only revealed upon protonation of glutamate in endosomal compartments leading to an escape of antibodies from the endosome67 (Fig. 4a). Similarly, cytosolic release of antibody was made possible upon treatment with dimer of Tat-conjugated with tetramethylrhodamine (dfTat) pre-incubated with antibody.68 It was reasoned that endosomal entrapment of Tat could be due to electrostatic interactions with negatively charged proteins/degradation of peptide along the endosomal pathway. The dimer of Tat presumably resists this degradation and is able to deliver to the cytosol.69
Fig. 4.
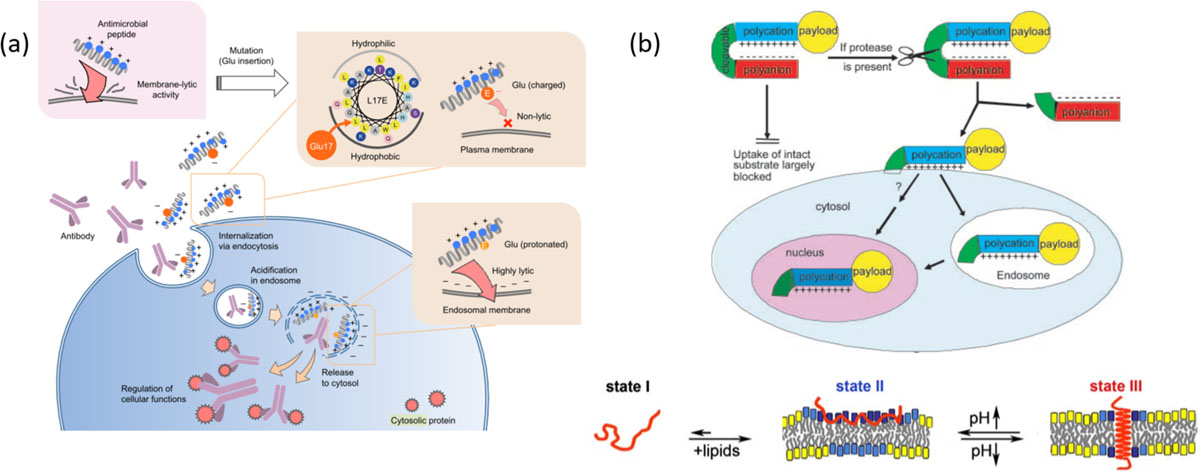
(a) Schematic illustration of design of endosomolytic peptides. The strong lytic activity of a cationic peptide was attenuated by introducing a glutamate residue into hydrophobic face. Protonation of Glu at endosomal pH enables interaction with endosomal membrane followed by membrane perturbation to release the antibodies intracellularly, reprinted with permission from [67]; (b) Activatable cell penetrating peptides or pH low insertion peptides (pHLIP) display their ability to enter cells only upon reaching target site, where protease cleavage reveals CPP in case of ACPP reprinted with permission from [73] (Copyright 2004 National Academy of Sciences, U.S.A) or conformational change observed in peptides due to acidic pH at tumor site, reprinted with permission from [74]
3.1. Limitations and Future Directions:
CPPs are quite attractive for delivering antibodies inside cells, as a simple conjugation of a short peptide can result in a remarkably enhanced cellular uptake. However, these are not without some limitations. These peptides are often conjugated with antibodies using a linker. It has been shown that the nature of the linker (e.g. disulfide37, thioether37, amide53 and Schiff’s base44) can greatly impact their cellular uptake properties. The drawbacks of these chemical conjugation strategies could be circumvented with the fusion protein approaches. However, fusion protein generation is relatively tedious and is not amenable for rapid screening, which in itself provides a significant research opportunity for future development. The report, suggesting that CPP conjugation retards the binding capabilities of the scFv70, is also a cautionary example that shows that CPP-based approaches might not be as generalizable. Despite these drawbacks, the success stories in CPP-based delivery of antibodies suggest that this area does warrant further investigation. As a part of this investigation, there is a surge in interest in the mechanism by which CPPs access the cellular interior. Arguably, the biggest challenge for the CPP-based delivery involves its potential for in vivo translation. For example, CPPs have been shown to exhibit poor stability in vivo. This complication has been addressed by designing a rigid collagen like helix comprised of arginines and delivered FITC-labeled IgG to HeLa cells.71 Also, CPPs cause their appendage to be rapidly taken up by the cells, but this very feature also provides the stumbling block for selectivity in cellular uptake. This complication is being circumvented by developing peptides that are activated to be cell penetrating, upon reaching a specific target. Examples of such strategy include activatable CPPs72,73 and pH-low insertion peptides (pHLIP)74, although these approaches are yet to be used for intracellular delivery of antibodies (Fig. 4b).
4. NANOPARTICLES
4.1. Inorganic Nanoparticles
The use of nanoparticles for delivering antibodies can be broadly classified into two categories, viz., inorganic and polymeric nanoparticles (see Table 1). Recently, use of inorganic nanoparticles to act as an immobilization support for bioactive molecules has gained a lot of attention.75,76 Among these, silica nanoparticles (SiNPs) have additional advantages of biocompatibility, surface functionalization capabilities, and pore volume tunability. In addition, the inherent rigidity of the material offers to protect encapsulated antibodies against harsh species in intra- and extracellular milieu.77
Non-porous SiNPs (~20 nm), surface modified with hydrophobic n-octadecyltrimethoxysilane (n-ODMS) groups, were used to hydrophobically adsorb protein cargos.78 The resultant complexes were shown to cause cellular uptake via energy-dependent endocytotic pathways, such as through clathrin pits and actin filaments. Anti-phospho-Akt was loaded onto the SiNPs and was effectively delivered in cytosol, where cellular apoptosis was used as the evidence for cytosolic protein delivery. On the other hand, mesoporous SiNPs containing 2–50 nm sized voids have been used to non-covalently immobilize larger proteins such as IgG.79 Amine-functionalized hollow dendritic mesoporous silica nano-spheres and surface functionalizable hollow mesoporous silica nanocapsules bearing a singular hole per particle of 25–50 nm, have also been reported with high antibody loading capacity.80,81
Electrostatic complexation between SiNPs of different sizes have been utilized to obtain the so-called rough silica nanoparticles (RSN), which has a raspberry-like shell morphology. The interstitial spaces in the shell were utilized to load antibodies.82 Building on this, RSNs were designed with controlled surface roughness and longer neck space by complexing larger anionic SiNPs as the shell on amine-modified cores.83 These anionic RSNs were loaded with positively charged anti-phospho-Akt via electrostatic complexation, which showed successful release in human breast cancer (MCF-7) cells. In a follow up work, it was found that the enhanced surface roughness and void sizes determine high loading ability, while a hydrophobic octadecyl (C18) functionality plays a key role in better uptake via endocytosis and endo/lysosomal escape of RSNs.84
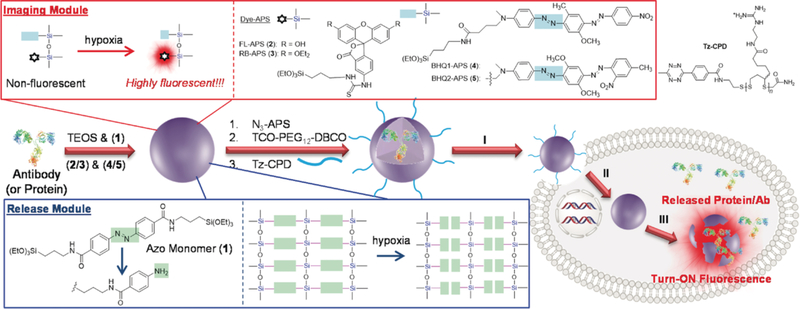
Similarly, a therapeutic antibody Cetuximab was encapsulated in a biodegradable silica nanoquencher (BS-qNP), which was shown to be efficiently taken up by cancer cells and underwent degradation in the presence of the hypoxic environment specific to cancer cells85 (Fig. 5). The exterior of antibody-loaded silica nanoshells was surface functionalized with azo groups, which provide several benefits. First, it acts as a protective sheath for the encapsulated antibody, while also instilling stimuli responsiveness in presence of cytochrome reductase that exists in hypoxic cells causing the BS-qNPs to degrade and concurrently release the native antibody. Additionally, the internal silica nanocapsule was doped with a fluorophore, the fluorescence of which is turned OFF by the azobenzene moiety of the BS-qNP. When the carrier vehicle falls apart, the fluorescence is turned ON, because of the spatial separation between the fluorophore and the quencher components, thus enabling the ability to track the protein release under hypoxia. Also, the poly(disulfide) functionalities on the BS-qNP surface have been implicated in facilitating cellular uptake via an endocytosis-independent, thiol-mediated pathway with minimal cytotoxicity.86 Once inside the cell, disulfide shuffling results in depolymerization of poly(disulfide) moieties (<5 min), exposing the antibody-BS-qNP to hypoxic conditions which then causes reduction-induced cleavage of the azobenzene crosslinkers, leading to Cetuximab release and cellular apoptosis.
Fig. 5.
Scheme showing the preparation of CPD-protein@BS-qNP and its endocytosis-independent cell uptake (step I), endogenous GSH- assisted CPD depolymerization (step II), and hypoxia-triggered intracellular protein release with fluorescence turn-on imaging (step III). The imaging module and release module are highlighted, Reprinted with permission from [85]
4.2. Polymer based nanoparticles
4.2.1. Non-covalent antibody-polymer complexation
Complementary to inorganic nanoparticles, polymer-based nanoparticles offer greater tunability in molecular weights, particle sizes and surface functional groups, which in turn could be used to optimize circulation times and endo/lysosomolytic efficiencies.87 Within the polymer-antibody combinations, approaches can be broadly classified into non-covalent complexation and covalent conjugation.
Non-covalent complexation approaches are generally dominated by electrostatics (see below), although there have been isolated efforts to utilize other non-covalent partners such as the biotin-avidin combination.88 A biotinylated poly(propylacrylic acid) (PPAAc) and a biotinylated anti-CD3 antibody was mixed with streptavidin to give rise to a ternary complex.89 These complexes were taken up by Jurkat lymphoma cells via receptor-mediated endocytosis; a diffused fluorescence in cytoplasm after 4 h was attributed to the endolysosomal release, possibly due to the proton-sponge features of the PPAAc moieties.90 Alternately, poly(lactic-co-glycolic acid) (PLGA) based carriers have been used to protect non-covalently encapsulated anti-AnnexinA2 (AnxA2) antibody.91 The slow degrading features of PLGA endowed the material with the ability to release the antibody over 12 days with retained function.
The popularity of charge-based complexation is attributed to its phenomenological simplicity. Polyethyleneimine (PEI) has been widely reported to complex with negatively-charged nucleic acids and deliver them intracellularly by making use of the ‘proton sponge’ effect of protonated amines at endosomal pH.92 Similarly, PEI was used to complex anti-lamin, a nuclear protein; interaction between the negatively charged cellular membrane and positively charged complexes facilitated uptake of antibody in human fibroblasts.93 In a strategy utilizing cell-surface receptors, anti-synuclein complexed polybutylcyanoacrylate nanoparticles were taken up by primary hippocampal cultures via low-density lipoprotein receptor mediated endocytosis.94,95 In an interesting strategy, polyion complex (PIC) micelles were formulated by optimizing ratios between anionized antibody and [N-{N′-(2-aminoethyl)-2-aminoethyl}aspartamide] (PAsp(DET)) based cationic block co-polymer and a homopolymer96,97 (Fig. 6). The anionized antibody was obtained by modification of lysine residues using citraconic anhydride. The protonation of (PAsp(DET)) at endosomal pH caused escape from endosomes, reversal of modification on anionized antibody followed by nuclear envelope targeting. Similarly, polymeric scaffolds, that mimic the cell penetrating features of CPPs, have been approached because of their ease of synthesis and structural tunability.98 In this context, an amphiphilic polymer consisting of phenyl and guanidinium moieties has been used to deliver anti-pPKCθ to human peripheral blood mononuclear cells.99
Fig. 6.
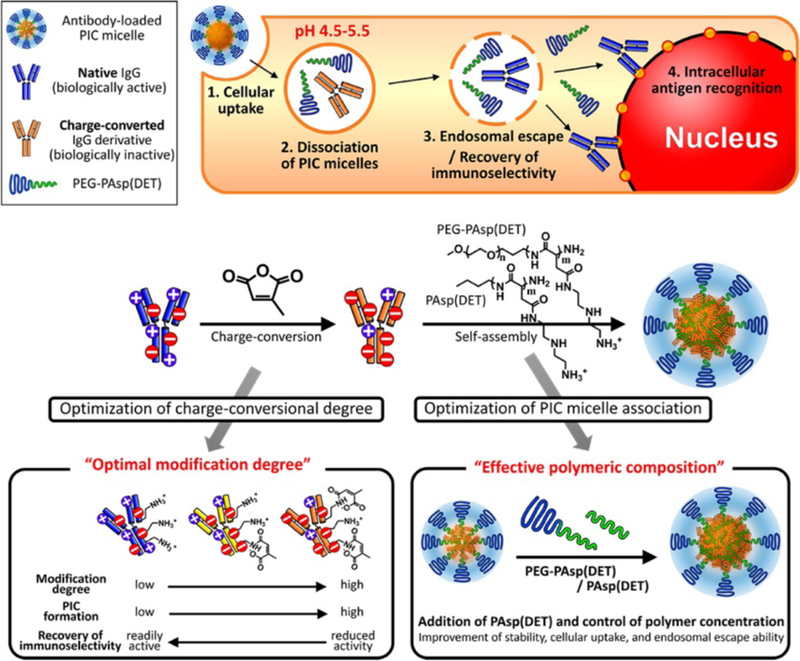
(Top) Pathways for successful intracellular antibody delivery with PIC micelles. (Bottom) Formation of PIC micelles incorporating charge-converted IgG antibody derivatives, PEG-PAsp(DET) and PAsp(DET); Strategies to engineer the micelles with optimal modification degree to maintain bioactivity of antibody, as well as polymer concentration to enhance stability of micelles with high cellular uptake. Reprinted with permission from [97]
4.2.2. Covalent linkage-based antibody-polymer conjugation
Covalent attachment of polymers to form conjugates for intracellular delivery of antibodies is useful since polymeric chains can protect the encapsulated antibody from harsh in vivo conditions.87 The carboxylic acid of the antibody was conjugated to amines of PEI using carbodiimide chemistry and delivered to human fibroblast cells. The conjugates were endocytosed via adsorption-mediated pathways and was localized to cell periphery. However, these studies were performed in serum-free conditions thereby minimizing adsorption of proteins on conjugates, that may pose hindrance to cellular uptake of the antibody.100 Cell penetrating poly(disulfides) (CPD) comprising of guanidium groups and terminated with tetrazine was conjugated to trans-cyclooctyne (TCO) bearing antibody.86,101 The antibody was modified via sulphone chemistry to introduce TCO moiety by reduction of native disulfide linkages. CPD-conjugated antibodies can bypass endocytosis102 and enter cells via thiol-mediated pathways, as seen by confocal microscopy.
Amphiphilic polyanhydride nanoparticles derived from diacids of 1,6-bis-(p-carboxyphenoxy)hexane and 1,8-bis-(p-carboxyphenoxy)-3,6-dioxaoctane and sebacic acid were utilized to demonstrate sustained release of Tetanus antitoxin and anti-TNF-α antibodies in vitro and ex vivo over a period of a month with preservation of biological activity.103 The delivery scaffold is biodegradable, because of the reported surface erosion mechanism and the by-products are speculated to be mildly acidic. An initial burst release of anti-TNF-α was observed with 70–90% total release achieved over a course of 25 days.
4.3. Limitations and Future Directions:
Nanoparticles are an exciting platform for intracellular delivery of antibodies due to the ease of synthesis and tunability in chemistry for optimization. Electrostatic complexation93,96,97, offering faster formulation, can cause toxicity to cells and result in endosomal entrapment especially when positively charged scaffolds are used. On the contrary, covalent conjugation of the antibody with polymeric scaffolds can prevent scaffold-mediated toxicity. Despite numerous examples of covalent conjugation strategies in literature, only a few have been capable of endosomal escape and efficient release of antibody in the cytosol. The released antibody is often linked to remnants of polymeric scaffold, which may pose hindrance towards targeting specific interaction. Our group has developed a protein assisted covalent assembly that undergoes self-immolation under reducing conditions encountered intracellularly to release the protein in its intact form, which has the potential to circumvent these issues.104 Additionally, quick formulation strategies that compete with electrostatic complexation, but are as robust as in covalent conjugation strategies, need to be developed. Unpublished work from our lab has successfully shown a mix-and-go covalent chemistry between protein and polymer, which has been used to deliver β-galactosidase to cells.
5. LIPOSOMAL DELIVERY
Liposome, a lipid based spherical bilayered particle, is considered as an attractive delivery agent due to its biocompatibility, biodegradability and controlled release property.105 Significant research efforts have been made to design liposomes that can be sensitive to stimuli (pH106, redox107, light108, temperature109), have long circulation half-life (PEGylation110) and can even be decorated with ligands/antibodies for specific targeting.111 Understandably, the desirable aspects of this platform has led to commercial development of several therapeutics (Doxil/Caelix-Johnson & Johnson, AmBisome- Gilead, Myocet-Cephalon).105
Within the area of antibody delivery (Table 1), a cationic liposome, PULSin (Polyplus-transfection (Illkirch, France)) was utilized to deliver mouse-IgG, anti-transmembrane golgi protein giantin and anti-nuclear pore complex in HeLa cells.112 In another study, a liposomal formulation was prepared with cationic trifluoroacetylated lipopolyamine (TFA-DODAPL) and neutral dioleoyl phosphatidylethanolamine (DOPE) combination (TFA-DODAPL:DOPE= 2:1, called BioPORTER, Gene Therapy Systems, San Diego, CA) to deliver functional proteins and a fluorescent antibody (FITC-IgG) into cytoplasm of five different cell types.113 Complementary electrostatic charges and hydrophobic interactions governed the lipid-protein/antibody assemblies and their successful internalization into cells. However, highly positively charged bio-macromolecules with low hydrophobic domains could not be delivered successfully. Selection of cationic and/or helper co-lipids were reported to be critical for successful delivery of antibody and lipid compositions were often varied from one target to another. Guanidinium-cholesterol cationic lipid bis(guanidinium)-tren-cholesterol (BGTC) and DOPE could efficiently deliver β-gal with high cellular activity.114 In contrast, BGTC-DOPE and other lipid combinations (DOSP-DOPE and BGTC-MM27, MM27 is a helper lipid based on imidazole) had low transfection efficacy for antibody directed against human cytokeratin 8 (anti-cytokeratin 8, K8). However, only DOSP-MM27 based liposomes were able to transfect 67% of total population of HeLa cells with FITC-anti-K8.
Strategies involving incorporation of cell penetrating peptides in liposome-based formulations have been well reported in literature. However, this approach is often associated with the problem of endosomal entrapment hindering efficient cytosolic delivery. In a recent report, a strategy for ‘high-speed’ intracellular transduction of antibody was developed using octaarginines (R8), a cell penetrating peptide and GALA, a pH sensitive fusogenic lipid (Fig. 7a, b).115 A liposome-based formulation was prepared with DOPE and cholesteryl hemisuccinate decorated with stearyl-R8 and cholesteryl–GALA to deliver mouse anti-NPC and IgG. In comparison to current antibody delivery methods that typically require 4–24 h incubation time, the reported technique can cytosolically translocate antibody in ~99% cells with 30–120 min incubation that includes both uptake and endosomal escape. Similarly, high transfection efficacy (99%) was also observed with a cationic aminolipid liposomes based on lysine for intracellular delivery of mouse anti-F actin antibody.113
Fig. 7.
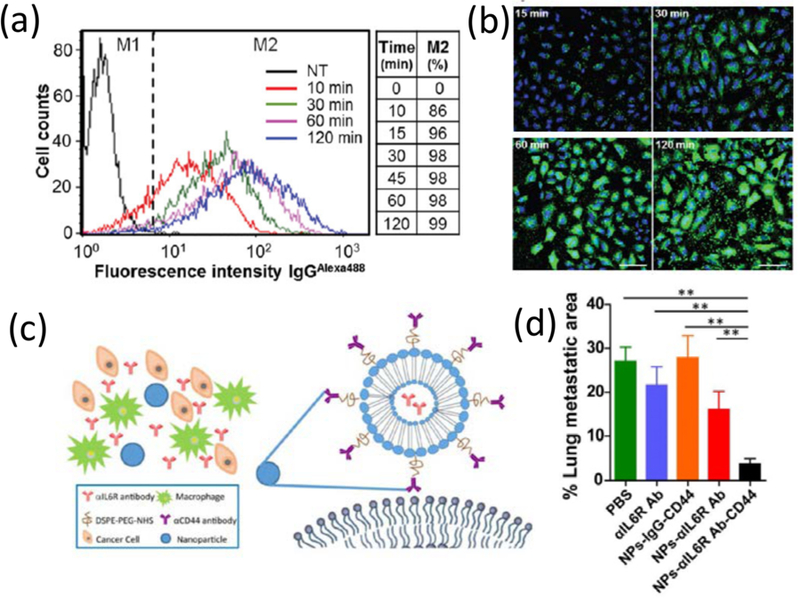
(a, b) Fast antibody delivery with liposomes: time course of cellular uptake of antibodies (IgGAlexa488) via R8-GALA liposomes represented in flow cytometry histograms (a) and confocal microscopy images (b), M1 & M2 corresponds to cell populations with no antibody & with IgG uptake, respectively, M2 % table reflects the percentage of cells with antibody internalization with time. Reprinted with permission from [115]; (c) Schematic diagram of the CD44 antibody decorated and IL6R antibody encapsulated liposomal nanoparticles, (d) Efficacy of CD44/IL6R liposomes in metastasis: % of metastatic foci area in the lung of BALB/c mice after treatment with liposomal nanoparticles and other controls. Reprinted with permission from [118]
In another approach, a liposomal delivery system is developed for photo-controlled targeted delivery of TuBB-9 antibody that inactivates nuclear Ki-67 protein, a bio-marker for proliferating cancer cells.116,117 TuBB-9-FITC construct was encapsulated in liposomes constructed with DPPC, DOTAP, cholesterol, and PEG2000-DSPE. A benzoporphyrin derivative monoacid photosensitizer is utilized for ROS mediated cleavage of endosomes to release the antibody into the cytosol. Specific targeting and inhibition of specific signaling pathways could be coupled together to achieve therapeutic benefit. This strategy is demonstrated in an anti-CD44 antibody decorated liposome formulation (DOPC, DOPE, cholesterol with DSPE-PEG3400-NHS modified anti-CD44 antibody) encapsulated with anti-IL6R antibody to inhibit IL6R-Stat3 signaling and reduce several gene expressions (Stat3, Sox2, VEGFA, MMP-9, CD206)118 (Fig. 7c). The liposome formulation showed efficient CD44+ targeting and anti-tumor metastasis effect in different triple negative and luminal breast cancer mouse models (Fig. 7d). Suppression of critical tumor metastasis factors could also provide effective ways to treat metastatic cancer, where delivery of anti-S100A4, responsible for inactivating apoptotic p53 protein, is reported to show inhibition of metastasis119. A liposome formulation consisting DSPE-4A (attached with 4 arginines) and DSPE-Hy-PEG2k (attached with benzaldehyde) was developed for direct cytosolic entry of vehicles through membrane fusion. Codelivery of doxorubicin was found to be synergistic for suppressing metastasis and improving the function of chemotherapeutic agent.119
5.1. Limitations and Future Directions:
Liposome’s tremendous success as a drug delivery agent stem from its ability to provide versatile guest encapsulation and to encompass a biocompatible tunable composition. The flexibility of liposomal design to incorporate surface functionality, relatively simple preparation methods and encapsulation techniques, in addition to previously approved formulations based on liposomes, have provided some competitive advantage for this platform. Nonetheless, liposome-based systems also suffer from several limitations. Although liposomes can provide home to both hydrophobic (in lipid bilayer) and hydrophilic (inside aqueous pool) guests, encapsulation efficacy for hydrophilic molecules are poor, as there is no driving force for encapsulation inside liposomes’ aqueous core. Other areas that need significant improvements include systemic destabilization of these structurally soft lipids, lack of structurally diverse stimulus-responsive lipids, opsonization via non-specific plasma protein absorption and subsequent macrophage mediated recognition and clearance.120,121
6. CONCLUDING REMARKS
An ideal candidate for intracellular delivery of antibodies is envisioned to fulfill the following criteria: (a) the method must be non-toxic to cells; (b) it is capable of protecting the antibody from degradation by proteases during circulation; (c) the delivery efficiency is high; (d) the method delivers the antibody in active conformation; and (e) have the potential to deliver to a target cell type. Early methods of protein delivery such as electroporation and microinjection can deliver antibodies to cells specifically albeit for in vitro applications but suffer from high cell toxicity and low throughput efficiency. Contrarily cell penetrating peptides have been shown to deliver antibodies intracellularly with improved efficiency; however, CPPs are incapable of providing protection from proteases and lack cell-targeting properties thereby restricting their translation to in vivo therapeutic models. Designs such as activatable CPPs72 and pHLIP74 peptides have shown to enhance cell targeting ability of CPPs. Nonetheless, since the mechanism of uptake in many instances is endocytosis, the delivery efficiency is hampered by endosomal entrapment of the cargo. Nanoparticles, including liposomes, can ameliorate other deficiencies by shielding the antibody from protease degradation and providing a chemical handle for attachment of targeting ligands. Liposomes, however, suffer from low protein encapsulation efficiency thereby demanding a greater dosage for efficient delivery. Polymeric nanoparticles, on the other hand, have improved antibody encapsulation efficiency, but are often associated with high cell toxicity (electrostatic complexation) or deliver antibodies modified with a polymer remains (covalent conjugation). In this regard, it is desired that polymeric nanoparticles retain high protein loading capacity and tracelessly deliver them inside cells, while being non-cytotoxic104. A common theme of delivery agents is associated endosomal entrapment. Therefore, it is pertinent that the focus be now placed on improving endosomal escape of endocytosed particles or newer pathways of internalization that bypass endocytosis such as the thiol-mediated cellular uptake be investigated102.
ACKNOWLEDGEMENT
We thank the NIGMS of the NIH (128181) for the support. KS is partially supported by fellowship from NIH (T32GM108556) as a part of Biotechnology Training Program and KD is partially supported by fellowship from NIH (T32GM008515) as a part of Chemical-Biology Interface at University of Massachusetts, Amherst.
TABLE OF ABBREVIATIONS
- FDA
Food and Drug Administration
- IFN
Interferon
- PTD
Protein Transduction Domains
- CPP
Cell Penetrating Peptides
- HIV
Human Immunodeficiency Virus
- Tat
Transactivator of transcription
- Fab
Antigen binding fragment of an antibody
- NLS
Nuclear Localization Signal
- TRIM
21 Tripartite motif containing protein 21
- PBMCs
Peripheral Blood Mononuclear Cells
- DAPI
4′,6-diamidino-2-phenylindole
- LAMP
Lysosomal associated membrane protein 1
- HVJE
Hemagglutinating virus of Japan Envelope
- pHLIP
pH low insertion peptides
- ACPP
Activatable cell penetrating peptides
- SiNPs
Silica nanoparticles
- n-ODMS
n-octadecyltrimethoxysilane
- RSN
Rough silica nanoparticles
- BS-qNP
Biodegradable- silica nanoquencher
- PLGA
poly(lactic-co-glycolic acid)
- PEI
Polyethylene Imine
- PIC
Polyion complex
- CPD
Cell Penetrating Poly(disulfides)
- TCO
Trans-cyclooctyne
- TNF-α
Tumor Necrosis Factor-α
- PULSin
Polyplus-transfection
- TFA-DODAPL
trifluoroacetylated lipopolyamine
- DOPC
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- BGTC
bis (guanidinium)-tren-cholesterol
- DOSP
dioleyl succinyl paromomycin
- MM27
Imidazole based helper lipid
- DPPC
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- DSPE
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine
- ROS
Reactive Oxygen Species
- ECM
Extracellular matrix
- CHEMS
Cholesteryl hemisuccinate
- R8
Octaarginine
- ER
Endoplasmic reticulum
- DOPE
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
REFERENCES
- (1).Milletti F Cell-Penetrating Peptides: Classes, Origin, and Current Landscape. Drug Discov. Today 2012, 17 (15–16), 850–860. [DOI] [PubMed] [Google Scholar]
- (2).Lagassé HAD; Alexaki A; Simhadri VL; Katagiri NH; Jankowski W; Sauna ZE; Kimchi-Sarfaty C Recent Advances in (Therapeutic Protein) Drug Development. F1000Research 2017, 6, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Leader B; Baca QJ; Golan DE Protein Therapeutics: A Summary and Pharmacological Classification. Nat. Rev. Drug Discov 2008, 7 (1), 21–39. [DOI] [PubMed] [Google Scholar]
- (4).Dang CV; Reddy EP; Shokat KM; Soucek L Drugging the “undruggable” Cancer Targets. Nat. Rev. Cancer 2017, 17 (8), 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Smith GP Filamentous Fusion Phage: Novel Expression Vectors That Display Cloned Antigends on the Virion Surface. Science (80-. ) 1985, 228 (4705), 1315–1317. [DOI] [PubMed] [Google Scholar]
- (6).Rami A; Behdani M; Yardehnavi N; Habibi-Anbouhi M; Kazemi-Lomedasht F An Overview on Application of Phage Display Technique in Immunological Studies. Asian Pac. J. Trop. Biomed 2017, 7 (7), 599–602. [Google Scholar]
- (7).Fu A; Tang R; Hardie J; Farkas ME; Rotello VM Promises and Pitfalls of Intracellular Delivery of Proteins. Bioconjug. Chem 2014, 25 (9), 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Schweizer D; Serno T; Goepferich A Controlled Release of Therapeutic Antibody Formats. Eur. J. Pharm. Biopharm 2014, 88 (2), 291–309. [DOI] [PubMed] [Google Scholar]
- (9).Adcc C; Raúl V; Román G; Murray JC; Weiner LM Antibody-Dependent Cellular Cytotoxicity (ADCC). In Antibody Fc: Linking Adaptive and Innate Immunity; 2014; pp 1–27.
- (10).Xenaki KT; Oliveira S; Bergen PMP Van; Henry KA Antibody or Antibody Fragments : Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol 2017, 8, 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chalouni C; Doll S Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res 2018, 37 (20), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Antman KH; Livingston DM Intracellular Neutralization of SV40 Tumor Antigens Following Microinjection of Specific Antibody. Cell 1980, 19 (3), 627–635. [DOI] [PubMed] [Google Scholar]
- (13).Blose SH; Meltzer DI; Feramisco JR 10-Nm Filaments Are Induced To Collapse in Living Cells Microinjected With Monoclonal and Polyclonal Antibodies Against Tubulin. J. Cell Biol 1984, 98 (3), 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Arnheiter H; Haller O Antiviral State against Influenza Virus Neutralized by Microinjection of Antibodies to Interferon-Induced Mx Proteins. EMBO J 1988, 7 (5), 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhang Y; Yu LC Single-Cell Microinjection Technology in Cell Biology. BioEssays 2008, 30 (6), 606–610. [DOI] [PubMed] [Google Scholar]
- (16).Berglund DL; Starkey JR Introduction of Antibody into Viable Cells Using Electroporation. Cytometry 1991, 12 (1), 64–67. [DOI] [PubMed] [Google Scholar]
- (17).Marrero MB; Schieffer B; Paxton WG; Schieffer E; Bernstein KE Electroporation of Pp60c-Src Antibodies Inhibits the Angiotensin II Activation of Phospholipase C-Gamma1 in Rat Aortic Smooth Muscle Cells. J. Biol. Chem 1995, 270 (60), 15734–15738. [DOI] [PubMed] [Google Scholar]
- (18).Maccarrone M; Veldink GA; Vleiegenthart JFG Inhibition of Lipoxygenase Activity in Lentil Protoplasts by Monoclonal Antibodies Introduced into the Cells via Electroporation. Eur. J. Biochem 1992, 205 (3), 995–1001. [DOI] [PubMed] [Google Scholar]
- (19).Lukas J; Bartek J; Strauss M Efficient Transfer of Antibodies into Mammalian Cells by Electroporation. J. Immunol. Methods 1994, 170 (2), 255–259. [DOI] [PubMed] [Google Scholar]
- (20).Morgan DO; Roth RA Acute Insulin Action Requires Insulin Receptor Kinase Activity: Introduction of an Inhibitory Monoclonal Antibody into Mammalian Cells Blocks the Rapid Effects of Insulin. Proc. Natl. Acad. Sci. U. S. A 1987, 84 (1), 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tompers DM; Labosky PA Electroporation of Murine Embryonic Stem Cells: A Step-by-Step Guide. Stem Cells 2004, 22 (3), 243–249. [DOI] [PubMed] [Google Scholar]
- (22).Stocks M Intrabodies as Drug Discovery Tools and Therapeutics. Curr. Opin. Chem. Biol 2005, 9 (4), 359–365. [DOI] [PubMed] [Google Scholar]
- (23).Marschall ALJ; Frenzel A; Schirrmann T; Schüngel M; Dübel S Targeting Antibodies to the Cytoplasm. MAbs 2011, 3 (1), 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kaiser PD; Maier J; Traenkle B; Emele F; Rothbauer U Recent Progress in Generating Intracellular Functional Antibody Fragments to Target and Trace Cellular Components in Living Cells. Biochim. Biophys. Acta - Proteins Proteomics 2014, 1844 (11), 1933–1942. [DOI] [PubMed] [Google Scholar]
- (25).Marschall ALJ; Dübel S Antibodies inside of a Cell Can Change Its Outside : Can Intrabodies Provide a New Therapeutic Paradigm ? CSBJ 2016, 14, 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Marschall ALJ; Dübel S; Böldicke T Specific in Vivo Knockdown of Protein Function by Intrabodies Andrea. MAbs 2015, 7 (6), 1010–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Heng BC; Cao T Making Cell-Permeable Antibodies (Transbody) through Fusion of Protein Transduction Domains (PTD) with Single Chain Variable Fragment (ScFv) Antibodies : Potential Advantages over Antibodies Expressed within the Intracellular Environment (Intrabody). Med. Hypotheses 2005, 64, 1105–1108. [DOI] [PubMed] [Google Scholar]
- (28).Zorko M; Langel Ü Cell-Penetrating Peptides: Mechanism and Kinetics of Cargo Delivery. Adv. Drug Deliv. Rev 2005, 57 (4), 529–545. [DOI] [PubMed] [Google Scholar]
- (29).Guo Z; Peng H; Kang J; Sun D Cell-Penetrating Peptides: Possible Transduction Mechanisms and Therapeutic Applications. Biomed. Reports 2016, 4 (5), 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Mueller J; Kretzschmar I; Volkmer R; Boisguerin P Comparison of Cellular Uptake Using 22 CPPs in 4 Different Cell Lines. Bioconjug. Chem 2008, 19, 2363–2374. [DOI] [PubMed] [Google Scholar]
- (31).Madani F; Lindberg S; Langel Ü; Futaki S; Gräslund A Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. J. Biophys 2011, 2011, 414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Guidotti G; Brambilla L; Rossi D Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci 2017, 38 (4), 406–424. [DOI] [PubMed] [Google Scholar]
- (33).Tunnemann G; Martin RM; Haupt S; Patsch C; Edenhofer F; Cardoso MC Cargo-Dependent Mode of Uptake and Bioavailability of TAT-Containing Proteins and Peptides in Living Cells. FASEB J 2006, 20, 1775–1784. [DOI] [PubMed] [Google Scholar]
- (34).Frankel AD; Pabo CO Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell 1988, 55 (6), 1189–1193. [DOI] [PubMed] [Google Scholar]
- (35).Theisen DM; Pongratz C; Wiegmann K; Rivero F; Krut O; Krönke M Targeting of HIV-1 Tat Traffic and Function by Transduction-Competent Single Chain Antibodies. Vaccine 2006, 24 (16), 3127–3136. [DOI] [PubMed] [Google Scholar]
- (36).Ohara-Imaizumi M; Nishiwaki C; Kikuta T; Kumakura K; Nakamichi Y; Nagamatsu S Site of Docking and Fusion of Insulin Secretory Granules in Live MIN6 Beta Cells Analyzed by TAT-Conjugated Anti-Syntaxin 1 Antibody and Total Internal Reflection Fluorescence Microscopy. J. Biol. Chem 2004, 279 (9), 8403–8408. [DOI] [PubMed] [Google Scholar]
- (37).Stein S; Weiss A; Adermann K; Lazarovici P; Hochman J; Wellho H A Disulfide Conjugate between Anti-Tetanus Antibodies and HIV ( 37 – 72 ) Tat Neutralizes Tetanus Toxin inside Chromaffin Cells. FEBS Lett 1999, 458, 383–386. [DOI] [PubMed] [Google Scholar]
- (38).Zhuang X; Stahl SJ; Watts NR; DiMattia MA; Steven AC; Wingfield PT A Cell-Penetrating Antibody Fragment against HIV-1 Rev Has High Antiviral Activity: Characterization of the Paratope. J. Biol. Chem 2014, 289 (29), 20222–20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhang JF; Xiong HL; Cao JL; Wang SJ; Guo XR; Lin BY; Zhang Y; Zhao JH; Wang Y Bin; Zhang TY; et al. A Cell-Penetrating Whole Molecule Antibody Targeting Intracellular HBx Suppresses Hepatitis B Virus via TRIM21-Dependent Pathway. Theranostics 2018, 8 (2), 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Stavenhagen JB; Gorlatov S; Tuaillon N; Rankin CT; Li H; Burke S; Huang L; Syd J; Bonvini E; Koenig S Fc Optimization of Therapeutic Antibodies Enhances Their Ability to Kill Tumor Cells In Vitro and Controls Tumor Expansion In Vivo via Low-Affinity Activating Fc ; Receptors. Cancer Res 2007, 67 (18), 8882–8891. [DOI] [PubMed] [Google Scholar]
- (41).Scott AM; Wolchok JD; Old LJ Antibody Therapy of Cancer. Nat. Rev. Cancer 2012, 12 (4), 278–287. [DOI] [PubMed] [Google Scholar]
- (42).Rogers LM; Veeramani S; Weiner GJ Complement in Monoclonal Antibody Therapy of Cancer. Immunol Res 2014, 59 (0), 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Anderson DC; Nichols E; Manger R; Woodle D; Barry M; Fritzberg AR Tumor Cell Retention of Antibody Fab Fragments Is Enhanced by an Attached HIV TAT Protein Derived Peptide. Biochem. Biophys. Res. Commun 1993, 194 (2), 876–884. [DOI] [PubMed] [Google Scholar]
- (44).Hu M; Chen P; Wang J; Chan C; Scollard DA; Reilly RM Site-Specific Conjugation of HIV-1 Tat Peptides to IgG: A Potential Route to Construct Radioimmunoconjugates for Targeting Intracellular and Nuclear Epitopes in Cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33 (3), 301–310. [DOI] [PubMed] [Google Scholar]
- (45).Hu M; Chen P; Wang J; Scollard DA; Vallis KA; Reilly RM 123I-Labeled HIV-1 Tat Peptide Radioimmunoconjugates Are Imported into the Nucleus of Human Breast Cancer Cells and Functionally Interact in Vitro and in Vivo with the Cyclin-Dependent Kinase Inhibitor, P21 WAF-1/Cip-1. Eur. J. Nucl. Med. Mol. Imaging 2007, 34 (3), 368–377. [DOI] [PubMed] [Google Scholar]
- (46).Zack DJ; Stempniak M; Wong a L.; Taylor C; Weisbart RH Mechanisms of Cellular Penetration and Nuclear Localization of an Anti-Double Strand DNA Autoantibody. J. Imunol 1996, 157 (5), 2082–2088. [PubMed] [Google Scholar]
- (47).Weisbart RH; Gera JF; Chan G; Hansen JE; Li E; Cloninger C; Levine AJ; Nishimura RN A Cell-Penetrating Bispecific Antibody for Therapeutic Regulation of Intracellular Targets. Mol. Cancer Ther 2012, 11 (10), 2169–2173. [DOI] [PubMed] [Google Scholar]
- (48).Lim KJ; Sung BH; Shin JR; Lee YW; Kim DJ; Yang KS; Kim SC A Cancer Specific Cell-Penetrating Peptide, BR2, for the Efficient Delivery of an ScFv into Cancer Cells. PLoS One 2013, 8 (6), e66084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Pooga M; Kut C; Kihlmark M; Hällbrink M; Fernaeus S; Raid R; Land T; Hallberg E; Bartfai T; Langel U Cellular Translocation of Proteins by Transportan. FASEB J 2001, 15 (8), 1451–1453. [DOI] [PubMed] [Google Scholar]
- (50).de Coupade C; Fittipaldi A; Chagnas V; Michel M; Carlier S; Tasciotti E; Darmon A; Ravel D; Kearsey J; Giacca M; et al. Novel Human-Derived Cell-Penetrating Peptides for Specific Subcellular Delivery of Therapeutic Biomolecules. Biochem. J 2005, 390 (2), 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Shin I; Edl J; Biswas S; Lin PC; Mernaugh R; Arteaga CL Proapoptotic Activity of Cell-Permeable Anti-Akt Single-Chain Antibodies. Cancer Res 2005, 65 (7), 2815–2824. [DOI] [PubMed] [Google Scholar]
- (52).Zhao Y; Lou D; Burkett J; Kohler H Chemical Engineering of Cell Penetrating Antibodies. J. Immunol. Methods 2001, 254 (1–2), 137–145. [DOI] [PubMed] [Google Scholar]
- (53).Takayama K; Tadokoro A; Pujals S; Nakase I; Giralt E; Futaki S Novel System to Achieve One-Pot Modification of Cargo Molecules with Oligoarginine Vectors for Intracellular Delivery. Bioconjug. Chem 2009, 20 (2), 249–257. [DOI] [PubMed] [Google Scholar]
- (54).Lättig-Tünnemann G; Prinz M; Hoffmann D; Behlke J; Palm-Apergi C; Morano I; Herce HD; Cardoso MC Backbone Rigidity and Static Presentation of Guanidinium Groups Increases Cellular Uptake of Arginine-Rich Cell-Penetrating Peptides. Nat. Commun 2011, 2, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Nischan N; Herce HD; Natale F; Bohlke N; Budisa N; Cardoso MC; Hackenberger CPR Covalent Attachment of Cyclic TAT Peptides to GFP Results in Protein Delivery into Live Cells with Immediate Bioavailability **. Angew. Chemie - Int. Ed 2015, 54, 1950–1953. [DOI] [PubMed] [Google Scholar]
- (56).Herce HD; Schumacher D; Schneider AFL; Ludwig AK; Mann FA; Fillies M; Kasper M; Reinke S; Krause E; Leonhardt H; et al. Cell-Permeable Nanobodies for Targeted Immunolabelling and Antigen Manipulation in Living Cells. Nat. Chem 2017, 9 (8), 762–771. [DOI] [PubMed] [Google Scholar]
- (57).Morris MC; Depollier J; Mery J; Heitz F; Divita G A Peptide Carrier for the Delivery of Biologically Active Proteins into Mammalian Cells. Nat. Biotechnol 2001, 19 (12), 1173–1176. [DOI] [PubMed] [Google Scholar]
- (58).Gros E; Deshayes S; Morris MC; Aldrian-Herrada G; Depollier J; Heitz F; Divita G A Non-Covalent Peptide-Based Strategy for Protein and Peptide Nucleic Acid Transduction. Biochim. Biophys. Acta - Biomembr 2006, 1758 (3), 384–393. [DOI] [PubMed] [Google Scholar]
- (59).Lim YT; Cho MY; Lee JM; Chung SJ; Chung BH Simultaneous Intracellular Delivery of Targeting Antibodies and Functional Nanoparticles with Engineered Protein G System. Biomaterials 2009, 30 (6), 1197–1204. [DOI] [PubMed] [Google Scholar]
- (60).Mie M; Takahashi F; Funabashi H; Yanagida Y; Aizawa M; Kobatake E Intracellular Delivery of Antibodies Using TAT Fusion Protein A. Biochem. Biophys. Res. Commun 2003, 310 (3), 730–734. [DOI] [PubMed] [Google Scholar]
- (61).Jung Y; Kang HJ; Lee JM; Jung SO; Yun WS; Chung SJ; Chung BH Controlled Antibody Immobilization onto Immunoanalytical Platforms by Synthetic Peptide. Anal. Biochem 2008, 374 (1), 99–105. [DOI] [PubMed] [Google Scholar]
- (62).Kang HJ; Choe W; Kim BM; Chung SJ IgG Fc-Binding Peptide (FcBP)-Tat Conjugate as a Smart Antibody Carrier into Live Cells. Macromol. Res 2015, 23 (9), 876–881. [Google Scholar]
- (63).Kondo Y; Fushikida K; Fujieda T; Sakai K; Miyata K; Kato F; Kato M Efficient Delivery of Antibody into Living Cells Using a Novel HVJ Envelope Vector System. J. Immunol. Methods 2008, 332 (1–2), 10–17. [DOI] [PubMed] [Google Scholar]
- (64).Liou JS; Liu BR; Martin AL; Huang YW; Chiang HJ; Lee HJ Protein Transduction in Human Cells Is Enhanced by Cell-Penetrating Peptides Fused with an Endosomolytic HA2 Sequence. Peptides 2012, 37 (2), 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ahmad A; Ranjan S; Zhang W; Zou J; Pyykkö I; Kinnunen PKJ Novel Endosomolytic Peptides for Enhancing Gene Delivery in Nanoparticles. Biochim. Biophys. Acta - Biomembr 2015, 1848 (2), 544–553. [DOI] [PubMed] [Google Scholar]
- (66).Lönn P; Kacsinta AD; Cui XS; Hamil AS; Kaulich M; Gogoi K; Dowdy SF Enhancing Endosomal Escape for Intracellular Delivery of Macromolecular Biologic Therapeutics. Sci. Rep 2016, 6, 32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Akishiba M; Takeuchi T; Kawaguchi Y; Sakamoto K; Yu HH; Nakase I; Takatani-Nakase T; Madani F; Gräslund A; Futaki S Cytosolic Antibody Delivery by Lipid-Sensitive Endosomolytic Peptide. Nat. Chem 2017, 9 (8), 751–761. [DOI] [PubMed] [Google Scholar]
- (68).Erazo-Oliveras A; Najjar K; Dayani L; Wang TY; Johnson GA; Pellois JP Protein Delivery into Live Cells by Incubation with an Endosomolytic Agent. Nat. Methods 2014, 11 (8), 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Allen JK; Brock DJ; Kondow-McConaghy HM; Pellois JP Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from DfTAT and Its Analogs. Biomolecules 2018, 8 (3), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Niesner U; Halin C; Lozzi L; Günthert M; Neri P; Wunderli-Allenspach H; Zardi L; Neri D Quantitation of the Tumor-Targeting Properties of Antibody Fragments Conjugated to Cell-Permeating HIV-1 TAT Peptides. Bioconjug. Chem 2002, 13 (4), 729–736. [DOI] [PubMed] [Google Scholar]
- (71).Masuda R; Yamamoto K; Koide T Cellular Uptake of IgG Using Collagen-Like Cell-Penetrating Peptides. Biol Pharma Bull 2016, 39 (1), 130–134. [DOI] [PubMed] [Google Scholar]
- (72).Olson ES; Jiang T; Aguilera TA; Nguyen QT; Ellies LG; Scadeng M; Tsien RY Activatable Cell Penetrating Peptides Linked to Nanoparticles as Dual Probes for in Vivo Fluorescence and MR Imaging of Proteases. Proc. Natl. Acad. Sci 2010, 107 (9), 4311–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Jiang T; Olson ES; Nguyen QT; Roy M; Jennings PA; Tsien RY Tumor Imaging by Means of Proteolytic Activation of Cell-Penetrating Peptides. Proc. Natl. Acad. Sci 2004, 101 (51), 17867–17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Andreev OA; Karabadzhak AG; Weerakkody D; Andreev GO; Engelman DM; Reshetnyak YK PH (Low) Insertion Peptide (PHLIP) Inserts across a Lipid Bilayer as a Helix and Exits by a Different Path. Proc. Natl. Acad. Sci 2010, 107 (9), 4081–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Sousa F; Castro P; Fonte P; Kennedy PJ; Neves-Petersen MT; Sarmento B Nanoparticles for the Delivery of Therapeutic Antibodies: Dogma or Promising Strategy? Expert Opin. Drug Deliv 2017, 14 (10), 1163–1176. [DOI] [PubMed] [Google Scholar]
- (76).Scaletti F; Hardie J; Lee Y-W; Luther DC; Ray M; Rotello VM Protein Delivery into Cells Using Inorganic Nanoparticle–protein Supramolecular Assemblies. Chem. Soc. Rev 2018, 47, 3421–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Tang L; Cheng J Nonporous Silica Nanoparticles for Nanomedicine Application. Nano Today 2013, 8 (3), 290–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Bale SS; Kwon SJ; Shah D. a; Banerjee A; Dordick JS; Kane RS Nanoparticle-Mediated Cytoplasmic Delivery of Proteins to Target Cellular Machinery. ACS Nano 2010, 4 (3), 1493–1500. [DOI] [PubMed] [Google Scholar]
- (79).Wang Y; Zhao Q; Han N; Bai L; Li J; Liu J; Che E; Hu L; Zhang Q; Jiang T; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomedicine Nanotechnology, Biol. Med 2015, 11 (2), 313–327. [DOI] [PubMed] [Google Scholar]
- (80).Meka AK; Abbaraju PL; Song H; Xu C; Zhang J; Zhang H; Yu M; Yu C A Vesicle Supra- Assembly Approach to Synthesize Amine-Functionalized Hollow Dendritic Mesoporous Silica Nanospheres for Protein Delivery. Small 2016, 12 (37), 5169–5177. [DOI] [PubMed] [Google Scholar]
- (81).Lim J-S; Lee K; Choi J-N; Hwang Y-K; Yun M-Y; Kim H-J; Won YS; Kim S-J; Kwon H; Huh S Intracellular Protein Delivery by Hollow Mesoporous Silica Capsules with a Large Surface Hole. Nanotechnology 2012, 23 (8), 085101. [DOI] [PubMed] [Google Scholar]
- (82).Niu Y; Yu M; Hartono SB; Yang J; Xu H; Zhang H; Zhang J; Zou J; Dexter A; Gu W; et al. Nanoparticles Mimicking Viral Surface Topography for Enhanced Cellular Delivery. Adv. Mater 2013, 25 (43), 6233–6237. [DOI] [PubMed] [Google Scholar]
- (83).Niu Y; Yu M; Zhang J; Yang Y; Xu C; Yeh M; Taran E; Hou JJC; Gray PP; Yu C Synthesis of Silica Nanoparticles with Controllable Surface Roughness for Therapeutic Protein Delivery. J. Mater. Chem. B 2015, 3 (43), 8477–8485. [DOI] [PubMed] [Google Scholar]
- (84).Niu Y; Yu M; Meka A; Liu Y; Zhang J; Yang Y; Yu C Understanding the Contribution of Surface Roughness and Hydrophobic Modification of Silica Nanoparticles to Enhanced Therapeutic Protein Delivery. J. Mater. Chem. B 2016, 4 (2), 212–219. [DOI] [PubMed] [Google Scholar]
- (85).Yuan P; Zhang H; Qian L; Mao X; Du S; Yu C; Peng B; Yao SQ Intracellular Delivery of Functional Native Antibodies under Hypoxic Conditions by Using a Biodegradable Silica Nanoquencher. Angew. Chemie - Int. Ed 2017, 56 (41), 12481–12485. [DOI] [PubMed] [Google Scholar]
- (86).Fu J; Yu C; Li L; Yao SQ Intracellular Delivery of Functional Proteins and Native Drugs by Cell-Penetrating Poly(Disulfide)S. J. Am. Chem. Soc 2015, 137 (37), 12153–12160. [DOI] [PubMed] [Google Scholar]
- (87).Liechty WB, Kryscio DR, Slaughter BV and Peppas NA Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng 2010, 1, 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Ding Z; Fong RB; Long CJ; Stayton PS; Hoffman AS Size-Dependent Control of the Binding of Biotinylated Proteins to Streptavidin Using a Polymer Shield. Nature 2001, 411 (6833), 59–62. [DOI] [PubMed] [Google Scholar]
- (89).Lackey CA; Press OW; Hoffman AS; Stayton PS A Biomimetic PH-Responsive Polymer Directs Endosomal Release and Intracellular Delivery of an Endocytosed Antibody Complex. Bioconjug. Chem 2002, 13 (5), 996–1001. [DOI] [PubMed] [Google Scholar]
- (90).Murthy N; Robichaud JR; Tirrell DA; Stayton PS; Hoffman AS The Design and Synthesis of Polymers for Eukaryotic Membrane Disruption. J. Control. Release 1999, 61 (1–2), 137–143. [DOI] [PubMed] [Google Scholar]
- (91).Gdowski A; Ranjan A; Mukerjee A; Vishwanatha J Development of Biodegradable Nanocarriers Loaded with a Monoclonal Antibody. Int. J. Mol. Sci 2015, 16 (2), 3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Neuberg P; Kichler A Chapter Nine - Recent Developments in Nucleic Acid Delivery with Polyethylenimines. In Nonviral Vectors for Gene Therapy; Huang L, Liu D, Wagner EB T.-A. in G., Eds.; Academic Press, 2014; Vol. 88, pp 263–288. [DOI] [PubMed] [Google Scholar]
- (93).Didenko VV; Ngo H; Baskin DS Polyethyleneimine as a Transmembrane Carrier of Fluorescently Labeled Proteins and Antibodies. Anal. Biochem 2005, 344 (2), 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Hasadsri L; Kreuter J; Hattori H; Iwasaki T; George JM Functional Protein Delivery into Neurons Using Polymeric Nanoparticles. J. Biol. Chem 2009, 284 (11), 6972–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Kreuter J Nanoparticulate Systems for Brain Delivery of Drugs. Adv. Drug Deliv. Rev 2001, 47, 65–81. [DOI] [PubMed] [Google Scholar]
- (96).Lee Y; Ishii T; Kim HJ; Nishiyama N; Hayakawa Y; Itaka K; Kataoka K Efficient Delivery of Bioactive Antibodies into the Cytoplasm of Living Cells by Charge-Conversional Polyion Complex Micelles. Angew. Chemie - Int. Ed 2010, 49 (14), 2552–2555. [DOI] [PubMed] [Google Scholar]
- (97).Kim A; Miura Y; Ishii T; Mutaf OF; Nishiyama N; Cabral H; Kataoka K Intracellular Delivery of Charge-Converted Monoclonal Antibodies by Combinatorial Design of Block/Homo Polyion Complex Micelles. Biomacromolecules 2016, 17 (2), 446–453. [DOI] [PubMed] [Google Scholar]
- (98).Sgolastra F; Deronde BM; Sarapas JM; Som A; Tew GN Designing Mimics of Membrane Active Proteins. Acc. Chem. Res 2013, 46 (12), 2977–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Ozay EI; Gonzalez-Perez G; Torres JA; Vijayaraghavan J; Lawlor R; Sherman HL; Garrigan DT; Burnside AS; Osborne BA; Tew GN; et al. Intracellular Delivery of Anti-PPKCθ (Thr538) via Protein Transduction Domain Mimics for Immunomodulation. Mol. Ther 2016, 24 (12), 2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Futami J; Kitazoe M; Maeda T; Nukui E; Sakaguchi M; Kosaka J; Miyazaki M; Kosaka M; Tada H; Seno M; et al. Intracellular Delivery of Proteins into Mammalian Living Cells by Polyethylenimine-Cationization. J. Biosci. Bioeng 2005, 99 (2), 95–103. [DOI] [PubMed] [Google Scholar]
- (101).Qian L; Fu J; Yuan P; Du S; Huang W; Li L; Yao SQ Intracellular Delivery of Native Proteins Facilitated by Cell-Penetrating Poly (Disulfide)S. Angew. Chemie - Int. Ed 2018, 57, 1532–1536. [DOI] [PubMed] [Google Scholar]
- (102).Du S; Liew SS; Li L; Yao SQ Bypassing Endocytosis: Direct Cytosolic Delivery of Proteins. J. Am. Chem. Soc 2018, 140, 15986–15996. [DOI] [PubMed] [Google Scholar]
- (103).Carrillo-Conde BR; Darling RJ; Seiler SJ; Ramer-Tait AE; Wannemuehler MJ; Narasimhan B Sustained Release and Stabilization of Therapeutic Antibodies Using Amphiphilic Polyanhydride Nanoparticles. Chem. Eng. Sci 2015, 125, 98–107. [Google Scholar]
- (104).Dutta K; Hu D; Zhao B; Ribbe AE; Zhuang J; Thayumanavan S Templated Self-Assembly of a Covalent Polymer Network for Intracellular Protein Delivery and Traceless Release. J. Am. Chem. Soc 2017, 139 (16), 5676–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Allen TM; Cullis PR Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev 2013, 65 (1), 36–48. [DOI] [PubMed] [Google Scholar]
- (106).Karanth H; Murthy RSR PH-Sensitive Liposomes-Principle and Application in Cancer Therapy. J. Pharm. Pharmacol 2007, 59 (4), 469–483. [DOI] [PubMed] [Google Scholar]
- (107).Pezzoli D; Tallarita E; Rosini E; Candiani G Characterization and Investigation of Redox-Sensitive Liposomes for Gene Delivery. In Non-Viral Gene Delivery Vectors: Methods and Protocols; Candiani G, Ed.; Springer New York: New York, NY, 2016; pp 217–233. [DOI] [PubMed] [Google Scholar]
- (108).Yavlovich A; Smith B; Gupta K; Blumenthal R; Puri A Light-Sensitive Lipid-Based Nanoparticles for Drug Delivery: Design Principles and Future Considerations for Biological Applications. Mol. Membr. Biol 2010, 27 (7), 364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Kono K; Ozawa T; Yoshida T; Ozaki F; Ishizaka Y; Maruyama K; Kojima C; Harada A; Aoshima S Highly Temperature-Sensitive Liposomes Based on a Thermosensitive Block Copolymer for Tumor-Specific Chemotherapy. Biomaterials 2010, 31 (27), 7096–7105. [DOI] [PubMed] [Google Scholar]
- (110).Milla P; Dosio F; Cattel L PEGylation of Proteins and Liposomes: A Powerful and Flexible Strategy to Improve the Drug Delivery. Curr. Drug Metab 2012, 13 (1), 105–119. [DOI] [PubMed] [Google Scholar]
- (111).Deshpande PP; Biswas S; Torchilin VP Current Trends in the Use of Liposomes for Tumor Targeting. Nanomedicine (Lond) 2013, 8 (9), 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Weill CO; Biri S; Erbacher P Cationic Lipid-Mediated Intracellular Delivery of Antibodies into Live Cells. Biotechniques 2008, 44 (7), 7–11. [DOI] [PubMed] [Google Scholar]
- (113).Zelphati O; Wang Y; Kitada S; Reed JC; Felgner PL; Corbeil J Intracellular Delivery of Proteins with a New Lipid-Mediated Delivery System. J. Biol. Chem 2001, 276 (37), 35103–35110. [DOI] [PubMed] [Google Scholar]
- (114).Chatin B; Mével M; Devallière J; Dallet L; Haudebourg T; Peuziat P; Colombani T; Berchel M; Lambert O; Edelman A; et al. Liposome-Based Formulation for Intracellular Delivery of Functional Proteins. Mol. Ther. - Nucleic Acids 2015, 4 (June), e244. [DOI] [PubMed] [Google Scholar]
- (115).Yamada Y; Perez SMV; Tabata M; Abe J; Yasuzaki Y; Harashima H Efficient and High-Speed Transduction of an Antibody into Living Cells Using a Multifunctional Nanocarrier System to Control Intracellular Trafficking. J. Pharm. Sci 2015, 104 (9), 2845–2854. [DOI] [PubMed] [Google Scholar]
- (116).Wang S; Hüttmann G; Zhang Z; Vogel A; Birngruber R; Tangutoori S; Hasan T; Rahmanzadeh R Light-Controlled Delivery of Monoclonal Antibodies for Targeted Photoinactivation of Ki-67. Mol. Pharm 2015, 12 (9), 3272–3281. [DOI] [PubMed] [Google Scholar]
- (117).Rahmanzadeh R; Rai P; Celli JP; Rizvi I; Baron-Lühr B; Gerdes J; Hasan T Ki-67 as a Molecular Target for Therapy in an in Vitro Three-Dimensional Model for Ovarian Cancer. Cancer Res 2010, 70 (22), 9234–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Guo C; Chen Y; Gao W; Chang A; Ye Y; Shen W; Luo Y; Yang S; Sun P; Xiang R; et al. Liposomal Nanoparticles Carrying Anti-IL6R Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models. Theranostics 2017, 7 (3), 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Deng H; Song K; Zhao X; Li Y; Wang F; Zhang J; Dong A; Qin Z Tumor Microenvironment Activated Membrane Fusogenic Liposome with Speedy Antibody and Doxorubicin Delivery for Synergistic Treatment of Metastatic Tumors. ACS Appl. Mater. Interfaces 2017, 9 (11), 9315–9326. [DOI] [PubMed] [Google Scholar]
- (120).Akbarzadeh A; Rezaei-Sadabady R; Davaran S; Joo SW; Zarghami N; Hanifehpour Y; Samiei M; Kouhi M; Nejati-Koshki K Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett 2013, 8 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Sercombe L; Veerati T; Moheimani F; Wu SY; Sood AK; Hua S Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol 2015, 6 (DEC), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]