Abstract
Background:
Few studies have assessed prescription opioid supply preceding death in individuals dying from unintentional prescription opioid overdoses, or described the characteristics of these individuals, particularly among Veterans.
Objectives:
To describe the history of prescription opioid supply preceding prescription opioid overdose death among Veterans.
Methods:
In a national cohort of Veterans who filled ≥1 opioid prescriptions from the Veterans Health Administration (VA) or Medicare Part D during 2008–2013, we identified deaths from unintentional or undetermined-intent prescription opioid overdoses in 2012–2013. We captured opioid prescriptions using both linked VA and Part D data, and VA data only.
Results:
Among 1181 decedents, 643 (54.4%) had prescription opioid supply on the day of death, and 735 (62.2%) within 30 days based on linked data, compared to 40.1% and 46.7%, respectively, using VA data alone. Decedents with prescription opioid supply were significantly older and less likely to have alcohol or illicit drugs as co-occurring substances involved in the overdose. Using linked data, 241 (20.4%) decedents lacked prescription opioid supply within a year of death.
Conclusions:
Many VA patients who die from prescription opioid overdose receive opioid prescriptions outside VA or not at all. It is important to supplement VA with non-VA data to more accurately measure prescription opioid exposure and improve opioid medication safety.
Keywords: Prescription opioids, Veterans, Drug overdose, Poisoning deaths, Medicare Part D
Introduction
Opioid overdose deaths are a public health emergency in the United States. Overdose deaths involving prescription opioid medications (hereafter referred to as opioids) are high relative to poisoning fatalities from other pharmaceuticals, accounting for 17,087 (27%) among 63,632 overall drug overdose deaths in 2016.1,2 As such, prescription opioid overdose remains an important issue despite reductions in opioid prescriptions dispensed,3 and increased overdose fatalities attributed to illicit synthetic opioids and heroin.1
Given the well-documented association between opioid prescribing practices and risks of opioid overdose, much emphasis has been placed on the safe prescribing of opioids in clinical practice.4,5 However, many prior studies of the association between opioid prescribing and overdose deaths have been limited to individuals who had evidence of opioid prescriptions on the exact date of death.6,7 Some prescription opioid overdose victims, however, may not receive opioids via a prescription. Studies from West Virginia, Utah, Florida, and Ohio indicate 25–66% of those who died of opioid overdoses used opioids originally prescribed to someone else.4 Except for studies limited to single states,8–13 prior research has not comprehensively assessed opioid supply prior to opioid overdose death, particularly among Veterans who are at increased risk of opioid use disorders and experience fatal accidental overdoses at nearly twice the rate of the U.S. population of adults.6
In VA, addressing opioid use is challenging because many Veterans receive opioids and other medications outside VA.14–16 Enrollment in multiple health systems may potentiate unsafe medication use and duplicate services because of poorly coordinated care.14 One recent study demonstrated that among dual VA and Medicare enrolled Veterans receiving opioids, 61.7% received their prescriptions from Medicare Part D only and 13.2% received them from both Medicare and VA.15 These findings suggest opioid exposure is potentially underestimated in studies relying on VA data only to ascertain opioid use among Veterans.
Our study aimed to describe the history of opioid supply in the months preceding a fatal unintentional prescription opioid overdose among a national sample of Veterans. We estimated the prevalence of decedents for whom there was opioid supply within one year of death with a focus on the exact day of death, and within 30 days of overdose death. In addition to capturing opioid fills using VA pharmacy claims alone, we utilized combined VA and Medicare data to facilitate more comprehensive capture of opioid prescriptions.
Methods
Data Sources and Study Cohort:
We identified a cohort of all Veterans who filled an opioid from VA or Part D during 2008–2013 using linked national patient data from VA and Centers for Medicare and Medicaid Services (CMS).15 We assessed Veteran demographic characteristics from VA Corporate Data Warehouse and Medicare Beneficiary files, and outpatient prescriptions for opioids from VA Pharmacy Benefits Management Services data and Medicare Part D claims. We obtained death data from the National Death Index (NDI) which is considered the ‘gold standard’ for vital status assessment among national mortality databases.17 The NDI compiles the dates and causes of death from death certificates filed at all state vital statistics offices. NDI data for all Veterans deaths are stored in the Suicide Data Repository (SDR).18 The VA Pittsburgh Healthcare System Institutional Review Board approved this study.
Overdose Death:
We defined fatal prescription opioid overdose as a cause of death using one of the following codes for the underlying cause of death from the International Statistical Classification of Diseases, Tenth Revision (ICD-10): X42, X44, Y12, or Y14 in combination with T40.2, T40.3, or T40.4. These ICD-10 codes, which have been used in death certificates since before the 2015 transition from ICD-9 to ICD-10 coding to report medical diagnoses, capture overdose deaths involving prescription opioids that were declared accidental (X42 and X44) or indeterminate intent (Y12 and Y14). The T codes indicate with greater specificity the type of drug that caused or contributed to an overdose.19 Among these decedents, we further identified ICD-10 codes for co-occurring substances documented in the death certificate, including alcohol (X45), heroin (T40.1), cocaine (T40.5), and other and unspecified drugs (T50.9).20
Usage Patterns of Prescription Opioids:
We assessed the patterns of opioid fills using VA and/or Part D medication data. See Appendix A for a drug list of opioids we included in our analysis. To estimate the last day when decedents were likely to be in possession of opioids, we determined the date when opioids were dispensed and added the total days supplied. We then calculated the time between the last day of opioid supply and the date of death and categorized the proportion of decedents with prescribed opioids on the date of death, and within 30 days of death using VA data only or combined VA and Part D data. Secondarily, we assessed the prevalence of decedents with opioid supply within 180 and 365 days. Because some individuals in the cohort may have had health insurance coverage gaps and/or other sources of insurance for prescription medications, we performed a subgroup analysis focusing only on opioid use among decedents with continuous dual enrollment in VA and Medicare Part D in the 12 months before death. We assumed this subgroup had a relatively low likelihood of having yet a third source of coverage not captured in the study data. Apart from requiring continuous enrollment for the subgroup analysis, we did not apply any exclusion criteria in order to analyze the entire sample of prescription opioid overdose decedents.
Analyses:
In the overall sample of prescription opioid overdose deaths and with combined VA and Part D data, which were the most comprehensive prescription data available, we compared decedent characteristics including demographics (age, sex, race and ethnicity), driving distance to the nearest VA, metropolitan status, and co-occurring substances between those with and without opioid prescriptions preceding death. We examined opioid supply up to one year before death, but limited the primary comparisons to the day of death and 30 days prior because of their proximity to the overdose death. For these comparisons, we used Fisher's exact tests for categorical variables, and analysis of variance or Kruskal-Wallis tests for continuous variables.21 Among decedents with evidence of opioid supply on the day of death, we identified the most prominently prescribed opioids, estimated the prescribed daily opioid dosage in morphine milligram equivalents (MMEs), and the percent with overlapping opioid and benzodiazepine prescriptions. We restricted dosage calculations to Veterans for whom MMEs could be reliably measured by excluding 2% of Veterans only prescribed liquid formulations or buprenorphine/naloxone.
Results
We identified 1181 Veterans who died from an unintentional or undetermined-intent prescription opioid overdose during 2012–2013. Among these, the median age was 54 years, 88.2% were male, and 83.8% were Non-Hispanic White (Table 1). Overall, 134 (11.4%) decedents had alcohol listed in the death certificate as a co-occurring substance used in addition to prescription opioids. There also were 485 (41.1%) deaths for which a co-occurring drug was present but a specific drug was not documented (Table 1).
Table 1.
Characteristics of Veterans who died from prescription opioid overdose, overall and stratified by active opioid medications prescribed in VA or Medicare Part D on the day of death and within 30 days of death, 2012–2013.
| Characteristics of Decedentsa | Prescription opioid overdose deaths among Veterans, n (%) |
||||||
|---|---|---|---|---|---|---|---|
| Total | Active opioid prescription on exact day of deathb | Active opioid prescription within 30 days of deathb | |||||
| No | Yes | No | Yes | ||||
| 1181 | 538 (45.6) | 643 (54.4)c | P-value | 446 (37.8) | 735 (62.2)d | P-value | |
| Age in years median (Q1, Q3) | 54 (44,60) | 52 (41,60) | 55 (47,60) | < 0.001 | 51 (40,59) | 55 (47,61) | < 0.001 |
| Age category, years | |||||||
| 18–39 | 218 (18.5) | 121 (22.5) | 97 (15.1) | < 0.001 | 108 (24.2) | 110 (15.0) | < 0.001 |
| 40–64 | 851 (72.2) | 369 (68.6) | 482 (75.2) | 304 (68.2) | 547 (74.6) | ||
| 65+ | 110 (9.3) | 48 (8.9) | 62 (9.7) | 34 (7.6) | 76 (10.4) | ||
| Male sex | 1041 (88.2) | 475 (88.3) | 566 (88.0) | 0.93 | 391 (87.7) | 650 (88.4) | 0.71 |
| Race and ethnicity | |||||||
| Non-Hispanic White | 943 (83.8) | 401 (81.7) | 542 (85.4) | 0.25 | 327 (81.6) | 616 (85.0) | 0.28 |
| Non-Hispanic Black | 103 (9.2) | 50 (10.2) | 53 (8.3) | 40 (10.0) | 63 (8.7) | ||
| Hispanic and Non-Hispanic Other | 80 (7.1) | 40 (8.1) | 40 (6.3) | 34 (8.5) | 46 (6.3) | ||
| Region of residence | |||||||
| Northeast | 159 (13.5) | 85 (15.9) | 74 (11.6) | 0.03 | 69 (15.5) | 90 (12.3) | 0.09 |
| Midwest | 220 (18.7) | 88 (16.4) | 132 (20.6) | 74 (16.7) | 146 (20.0) | ||
| South | 458 (39.0) | 219 (40.9) | 239 (37.3) | 184 (41.4) | 274 (37.4) | ||
| West | 339 (28.8) | 144 (26.9) | 195 (30.5) | 117 (26.4) | 222 (30.3) | ||
| Rurality of residence | |||||||
| Large Metropolitan | 552 (46.9) | 267 (49.8) | 285 (44.5) | 0.30 | 219 (49.3) | 333 (45.5) | 0.60 |
| Small metropolitan | 442 (37.6) | 192 (35.8) | 250 (39.1) | 159 (35.8) | 283 (38.7) | ||
| Micropolitan | 101 (8.6) | 41 (7.7) | 60 (9.4) | 35 (7.9) | 66 (9.0) | ||
| Noncore rural | 81 (6.9) | 36 (6.7) | 45 (7.0) | 31 (7.0) | 50 (6.8) | ||
| Dual enrollment 12 months prior to deathe | 216 (18.3) | 59 (11.0) | 157 (24.4) | < 0.001 | 42 (9.4) | 174 (23.7) | < 0.001 |
| Driving distance to nearest VA, median miles, (Q1,Q3) | 9 (4,19) | 9 (4,18) | 10 (5,20) | 0.10 | 9 (4,18) | 0 (5,20) | 0.29 |
| Documented co-occurring substance used f | |||||||
| Alcohol | 134 (11.4) | 83 (15.4) | 51 (7.9) | < 0.001 | 70 (15.7) | 64 (8.7) | < 0.001 |
| Heroin | 74 (6.3) | 54 (10.0) | 20 (3.1) | < 0.001 | 42 (9.4) | 32 (4.4) | < 0.001 |
| Cocaine | 81 (6.9) | 45 (9.0) | 36 (5.6) | 0.07 | 40 (9.0) | 41 (5.6) | 0.03 |
| Unspecified drugs | 485 (41.1) | 216 (40.2) | 269 (41.8) | 0.55 | 180 (40.4) | 305 (41.5) | 0.71 |
Abbreviations: Q1 = first quartile, Q3 = third quartile.
Missing data: age (n = 2), race (n = 55), region (n = 5), rurality (n = 5), driving distance (n = 2).
We used Fisher's Exact test for categorical variables and ANOVA and Kruskal-Wallis tests for continuous variables.
Measured at or relative to date of death. Numbers represent column percent unless otherwise noted.
Measured using both VA and Medicare data.
Includes 3 Veterans who were on buprenorphine/naloxone only.
Includes 4 Veterans who were on buprenorphine/naloxone only.
Veterans continuously enrolled in both VA and Medicare Part D a year before death.
Other categories of co-occurring substances were not reportable due to small sample sizes.
Compared to overdose decedents without opioids supplied within 30 days of death (using both VA and Medicare data), those with opioid supply within this time frame of death were older (median age: 55 vs. 51, P < 0.001) and less likely to have alcohol (8.7% vs. 15.7%, P < 0.001), heroin (4.4% vs. 9.4%, P < 0.001), and cocaine (5.6% vs. 9.0%, P = 0.03) also involved in the overdose (Table 1). There were no statistically significant differences in sex, race/ethnicity, geographic region, rurality, or driving distance to the nearest VA. Analyses based on the day of death yielded substantively similar results except that regional differences were statistically significant and cocaine involvement in overdose death did not differ between those with and without opioids on the exact date of death. Results for comparisons based on the 180 and 365 day time frames are available in Appendix B.
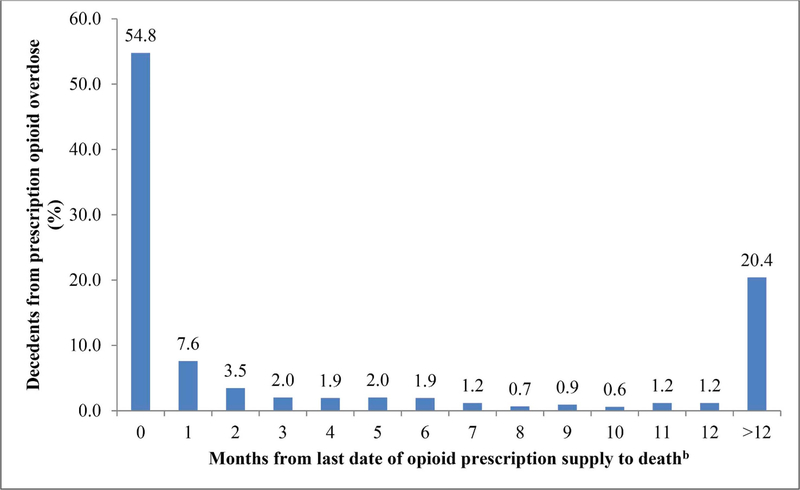
Using combined VA and Part D data, we found 54.4% had opioid supply on the date of their death, and 62.2% within 30 days of death (Table 2). These prevalence estimates were 14.3, and 15.5percentage points lower, respectively, using VA data alone. Using linked VA and Part D claims data, 241 (20.4%) decedents lacked opioid supply within a year of death (Table 2, Fig. 1). In the subgroup of 216 dual VA-Part D enrollees, 72.7% and 80.6% had active opioid supply on the day of death and within 30 days prior to death, respectively, compared with 30.6% and 34.7%, when identified using VA data only.
Table 2.
Prevalence of active opioid medications prescribed prior to death, among a national sample of Veterans who died from prescription opioid overdose, 2012–2013.
| Time frame of active opioid supply relative to day of deatha | Overall Cohort | VA-Part D Dual Enrolleesb | ||
|---|---|---|---|---|
| VA-only data | VA and Part D data | VA-only data | VA and Part D data | |
| (n = 1181) | (n = 1181) | (n = 216) | (n = 216) | |
| On day of death | 473 (40.1%) | 643 (54.4%) | 66 (30.6%) | 157 (72.7%) |
| ≤30 days | 551 (46.7%) | 735 (62.2%) | 75 (34.7%) | 174 (80.6%) |
| ≤180 days | 677 (57.3%) | 872 (73.8%) | 95 (44.0%) | 196 (90.7%) |
| ≤365 days | 755 (63.9%) | 940 (79.6%) | 110 (50.9%) | 201 (93.1%) |
Opioid medications prescribed were based on the prescription release date plus day supplied using VA only or linked VA and Part D data.
Dual enrollment was defined as continuous enrollment in both VA and Medicare Part D 12 months before the date of death.
Fig. 1.
Distribution of time since last opioid prescription supply and date of overdose death among Veterans, 2012–2013 (n = 1181)a.
a Prescriptions measured using both VA and Medicare data.
b Month 0 denotes presence of opioid medications prescribed from VA and/or Medicare part D on the date of overdose death.
The most prominent opioids prescribed from either VA or Part D among those with opioid supply on the day of death were hydrocodone/acetaminophen, morphine, oxycodone (alone or in combination with acetaminophen), methadone, and tramadol (data not shown). Among Veterans prescribed opioids with measurable MME on the day of death (632 of 643 with opioids), the median and interquartile range (Q1-Q3) opioid dosage was 80 MME (33, 180) using both VA and Part D data compared with 40 MME (0, 112) using VA-only data (Table 3). Among this same cohort, 41.6% were observed to have overlapping opioid and benzodiazepine prescriptions on the date of death using both VA and Part D compared with 32.0% using VA-only data. Among the subgroup of dual VA-Part D enrollees, linked data also captured a greater median opioid dosage on the day of death, 124 MME (45, 285) versus 0 MME (0, 60), and larger proportion with opioid and benzodiazepine overlap, 44.2% versus 22.1%.
Table 3.
Prescribed opioid dose and prevalence of opioid and benzodiazepine overlap among those with an active opioid prescription on the date of death among a national sample of Veterans who died from prescription opioid overdose, 2012–2013.
| Overall Cohorta (n= 632) | Dual Enrolleesa (n =154) | |||
|---|---|---|---|---|
| VA-only data | VA and Part D data | VA-only data | VA and Part D data | |
| Daily MME on the date of death, median (Q1, Q3) | 40 (0,112) | 80 (33,180) | 0 (0, 60) | 124 (45,285) |
| Opioid and benzodiazepine overlap on date of death, n(%) | 202 (32.0) | 263 (41.6) | 34 (22.1) | 68 (44.2) |
AbbreviationsMME = morphine milligram equivalent, SD = standard deviation, Q1 = first quartile, Q3 = third quartile.
Includes 632 of 643 (98%) in overall sample, and 154 of 157 (98%) of dual enrollees after excluding Veterans whose opioid dosage could not be quantified in morphine milligram equivalents (i.e., Veterans only on liquid formulations or suboxone were not counted in these calculations).
Discussion
This national study of Veterans who died from prescription opioid overdoses demonstrates that a meaningful proportion of decedents was not prescribed opioids and did not have opioid supply in the months preceding death. More than one-third of decedents lacked active opioid prescriptions within 30 days of death, and such decedents were younger and more likely to have multiple substances involved in the overdose. Our findings further highlight the critical importance of gathering complete data from all possible sources [e.g., health system claims, electronic health records, prescription drug monitoring programs (PDMPs)]22 to more accurately measure, and more appropriately respond to, the opioid crisis.
Overall, three findings stood out in our analyses. First, many Veterans who die from prescription opioid overdose receive opioid fills outside VA, and monitoring activities that solely rely on VA data will miss a substantial proportion of prescription opioids. While VA-only data identified 40.1% of decedents as having active opioid prescriptions on the exact day of death, 54.4% were identified using linked VA and Medicare data. The latter estimate is consistent with the finding that half (51%) of opioid-involved deaths in North Carolina during 2010 had an active opioid prescriptions on the day of death per PDMP records.23 The combined VA and Medicare data consistently captured at least 14% point more individuals who had opioid supply at all time frames assessed than VA-only data. Further, use of VA data alone under-estimated opioid dosages, and concurrent opioid and benzodiazepine prescriptions which are important risk factors for overdose.
Second, among dual VA-Medicare enrollees, a moderate proportion had no records of active opioid supply - ranging from 27.3% on day of death to 19.4% at 30 days before death. This finding indicates that, even when all likely sources of prescription drug benefits are considered, a nontrivial minority of Veterans who died from overdose lacked evidence of current or recent prescribed opioid supply. While the potential for diversion exists both among individuals with and without evidence of opioid claims, the absence of opioid prescription claims particularly raises questions about cash payments made to procure opioids through legal (such as to circumvent prior authorizations or other prescription limits) or illegal channels (such as family, friends, or drug dealers). All health care professionals, from physicians to pharmacists, can help prevent the diversion of prescription opioids by utilizing the PDMP, and pharmacists may be especially well positioned to educate consumers on the risks of opioids, proper storage and disposal, and unlawfulness of sharing medications.24
Third, with exception of age and involvement of other psychoactive substances, there were limited differences in the characteristics of opioid overdose decedents with and without active opioid supply on the day of death or 30 days prior. Decedents without a supply of prescribed opioids within 30 days of death were younger (24.2% vs. 15.1% aged 18–39 years) than decedents with opioid supply, and had significantly greater proportion of cases where alcohol and illicit drugs were listed as contributing to opioid overdose deaths. Notably, we observed combination exposure to alcohol, heroin, or cocaine almost twice as high among Veterans without prescribed opioid supply than their counterparts with opioid supply 30 days before death. This raises concern that individuals who potentially seek opioids outside the medical system have greater contact with illegal drug supplies, and may be prone to mixing drugs. Although geographic variation exists in the distribution of both overall and prescription opioid overdoses across the US, regional differences in supply of opioids prior to overdose death were inconsistent potentially due to small sample sizes.25
This study has limitations. First, we identified overdoses from a cohort of Veterans who received opioids during 2008–2013 which may underestimate total number opioid overdose deaths among Veterans, and overestimate the prevalence of opioid use prior to overdose because our cohort definition excluded Veterans who were never dispensed an opioid over the six-year period. Second, our study may not represent current prevalence of prior opioid prescription receipt among overdose decedents. In recent years, these measures may have been impacted by widespread implementation and enhancement of PDMPs, VA and CMS initiatives to improve opioid safety,26,27 and changes in drug use and regulatory environments.28,29 Third, our study does not capture opioids received from insurance other than VA or Medicare or those paid for in cash, although we made every effort in subgroup analyses (VA and Medicare dual enrollees) to focus on a population with reduced likelihood of other insurance sources. Fourth, our estimation of days with opioid supply assumes the patient was adherent to prescribed dosages and frequencies. Finally, an inherent limitation of death certificate data is state variation in the completeness and accuracy of investigations into manner of death and primary/contributing drugs.30
Despite these limitations, this study is among the first to characterize opioid prescription histories among a national sample of Veterans who died from prescription opioid overdose. This study emphasizes that efforts to examine the association between opioid prescribing and prescription opioid overdose among Veterans will miss important information when only VA data is used. Further, prevention and intervention efforts aimed at reducing accidental prescription opioid overdose deaths must also actively focus on nonprescription use of opioid medications and multiple drug poisoning involving alcohol and/or illicit substances.
Acknowledgments
Funding/support
VA Health Services Research & Development (HSR&D) I01 HX001765–01. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02–237 and 98–004).
Appendix A. Drug list of opioid medications included in the analysis
| Alfentanil |
|---|
| lphaprodine |
| Buprenorphinea |
| Butorphanol |
| Codeine |
| Dezocine |
| Dihydrocodeine |
| Fentanyl |
| Hydrocodone |
| Hydromorphone |
| Levorphanol |
| Meperidine |
| Methadone |
| Morphine |
| Nalbuphine |
| Oxycodone |
| Oxymorphone |
| Pentazocine |
| Propoxyphene |
| Remifentanil |
| Sufentanil |
| Tapentadolb |
| Tramadolb |
| Phenylephrine/dihydrocodeine |
| Brompheniramine/pseudoephedrine/dihydrocodeine |
| Pyrilam/phenylephedrine/dihydrocodeine |
| Hydrocodone/chlorpherniramine |
This includes all forms of buprenorphine including suboxone. Among 1181 decedents, 14 Veterans had suboxone as the only opioid medication prescribed within one year of death. used to treat pain, and excludes formulations intended for treating opioid use disorder.
We included tramadol and tapentadol because these drugs are opioid receptor agonists and inhibitors of norepinephrine uptake.
Appendix B. Characteristics of Veterans who died from prescription opioid overdose, overall and stratified by active opioid medications prescribed in VA or Medicare Part D within 180 days of death, 2012–2013
| Characteristics of Decedents a | Active opioid prescription within 180 days of death, n (%) b | Active opioid prescription within 365 days of death, n (%) b | ||||
|---|---|---|---|---|---|---|
| No | Yes | P-value | No | Yes | P-value | |
| 309 (26.2) | 872 (73.8) c | 241 (20.4) | 940 (79.6) d | |||
| Age in years median (Q1,Q3) | 51 (41,58) | 55 (46,61) | < 0.001 | 51 (43,58) | 55 (45,60) | 0.001 |
| Age category, years | ||||||
| 18–39 | 73 (23.6) | 145 (16.7) | 0.01 | 54 (22.4) | 164 (17.5) | 0.05 |
| 40–64 | 215 (69.6) | 636 (73.1) | 171 (71.0) | 680 (72.5) | ||
| 65 + | 21 (6.8) | 89 (10.2) | 16 (6.0) | 94 (10.0) | ||
| Male sex | 271 (87.7) | 770 (88.3) | 0.76 | 208 (86.3) | 833 (88.6) | 0.32 |
| Race and ethnicity | ||||||
| Non-Hispanic White | 217 (81.0) | 726 (84.6) | 0.33 | 162 (79.4) | 781 (84.7) | 0.17 |
| Non-Hispanic Black | 28 (10.5) | 75 (8.7) | 24 (11.8) | 79 (8.6) | ||
| Hispanic and Non-Hispanic Other | 23 (8.6) | 57 (6.6) | 18 (8.9) | 62 (6.7) | ||
| Region of residence | ||||||
| Northeast | 47 (15.3) | 112 (12.9) | 0.53 | 35 (14.6) | 124 (13.3) | 0.31 |
| Midwest | 53 (17.2) | 167 (19.2) | 40 (16.7) | 180 (19.2) | ||
| South | 125 (40.6) | 333 (38.4) | 104 (43.3) | 354 (37.8) | ||
| West | 83 (27.0) | 256 (29.5) | 61 (25.4) | 278 (29.7) | ||
| Rurality of residence | ||||||
| Large Metropolitan | 158 (51.3) | 394 (45.4) | 0.18 | 121 (50.4) | 431 (46.1) | 0.55 |
| Small metropolitan | 110 (35.7) | 332 (38.3) | 87 (36.3) | 355 (37.9) | ||
| Micropolitan | 19 (6.2) | 82 (9.5) | 16 (6.7) | 85 (9.1) | ||
| Noncore rural | 21 (6.8) | 60 (6.9) | 16 (6.7) | 65 (6.9) | ||
| Dual enrollment 12 months prior to death e | 20 (6.5) | 196 (22.5) | < 0.001 | 15 (6.2) | 201 (21.4) | < 0.001 |
| Driving distance to nearest VA, median miles, (Q1,Q3) | 9 (5,19) | 9 (4,19) | 0.76 | 8 (4,19) | 9.5 (5,19) | 0.68 |
| Documented co-occurring substance used f | ||||||
| Alcohol | 49 (15.9) | 85 (9.8) | 0.01 | 41 (17.0) | 93 (9.9) | 0.003 |
| Heroin | 29 (9.4) | 45 (5.2) | 0.01 | 23 (9.5) | 51 (5.4) | 0.02 |
| Cocaine | 25 (8.1) | 56 (6.4) | 0.36 | Small sample suppressed | ||
| Unspecified drugs | 124 (40.1) | 361 (41.4) | 0.74 | 90 (37.3) | 395 (42.0) | 0.21 |
Abbreviations: Q1 = first quartile, Q3 = third quartile.
Missing data: age (n = 2), race (n = 55), region (n = 5), rurality (n = 5), driving distance (n = 2).
We used Fisher's Exact test for categorical variables and ANOVA and Kruskal-Wallis tests for continuous variables.
Measured at or relative to date of death. Numbers represent column percent unless otherwise noted.
Measured using both VA and Medicare data.
Includes 6 Veterans who were on buprenorphine/naloxone only.
Includes 14 Veterans who were on buprenorphine/naloxone only.
Veterans continuously enrolled in both VA and Medicare Part D a year before death.
Other categories of co-occurring substances were not reportable due to small sample sizes.
References
- 1.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. [DOI] [PubMed] [Google Scholar]
- 2.Seth P, Rudd RA, Noonan RK, Haegerich TM. Quantifying the epidemic of prescription opioid overdose deaths. Am J Public Health. 2018;108(4):500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA. J Pain Res. 2017;10:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulozzi LJ, Baldwin G, Franklin G, et al. CDC grand rounds: prescription drug overdoses - a U.S. Epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(01):10–13. [PubMed] [Google Scholar]
- 5.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. [DOI] [PubMed] [Google Scholar]
- 6.Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the department of veterans Affairs health system. Med Care. 2011;49(4):393–396. [DOI] [PubMed] [Google Scholar]
- 7.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350 h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 20082009. J Gen Intern Med. 2013;28(4):522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt CT, McCall KL, Cattabriga G, Tu C, Smalley EK, Nichols SD. Using controlled substance receipt patterns to predict prescription overdose death. Pharmacology. 2017;101(3–4):140–147. [DOI] [PubMed] [Google Scholar]
- 11.Paulozzi LJ, Kilbourne EM, Shah NG, et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13(1):87–95. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch A, Proescholdbell SK, Bronson W, Dasgupta N. Prescription histories and dose strengths associated with overdose deaths. Pain Med. 2014;15(7):1187–1195. [DOI] [PubMed] [Google Scholar]
- 13.Paulozzi LJ, Logan JE, Hall AJ, McKinstry E, Kaplan JA, Crosby AE. A comparison of drug overdose deaths involving methadone and other opioid analgesics in West Virginia. Addiction. 2009;104(9):1541–1548. [DOI] [PubMed] [Google Scholar]
- 14.Stroupe KT, Smith BM, Bailey L, et al. Medication acquisition by veterans dually eligible for Veterans Affairs and Medicare Part D pharmacy benefits. Am J Health Syst Pharm. 2017;74(3):140–150. [DOI] [PubMed] [Google Scholar]
- 15.Gellad WF, Thorpe JM, Zhao X, et al. Impact of dual use of department of veterans Affairs and Medicare Part D drug benefits on potentially unsafe opioid use. Am J Public Health. 2018;108(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–468. [DOI] [PubMed] [Google Scholar]
- 18.Center of Excellence for Suicide Prevention. Joint department of veterans Affairs (VA) and department of defense (DoD) Suicide data repository – national death Index (NDI). http://vaww.virec.research.va.gov/Mortality/Overview.htm; December 2014.
- 19.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–659. [DOI] [PubMed] [Google Scholar]
- 20.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidemiol Drug Saf. 2008;17(10):997–1005. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons JD, Chakraborti S. Nonparametric Statistical Inference. Berlin, Heidelberg: Springer; 2011. [Google Scholar]
- 22.Gellad WF, Commentary on Daubresse, et al. An epidemic of outdated data. Addiction. 2017;112(6):1054–1055 2017. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17(1):85–98. [DOI] [PubMed] [Google Scholar]
- 24.Compton WM, Jones CM, Stein JB, Wargo EM. Promising roles for pharmacists in addressing the U.S. opioid crisis. Res Soc Adm Pharm. 2017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minegishi T, Frakt A. Reducing long-term opioid use in the veterans health administration. J Gen Intern Med. 2018;33(6):781–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Government Accountability Office. Prescription Opioids: Medicare Needs to Expand Oversight Efforts to Reduce Risk of Harm. GAO-18-15 Washington, DC: 2017; 2017 GAO-18–15. [Google Scholar]
- 28.Ruhm CJ. Deaths of Despair or Drug Problems. vol. 2018 Cambridge, MA: The National Bureau of Economic Research; January 2018:24188. [Google Scholar]
- 29.Covvey JR. Recent developments toward the safer use of opioids, with a focus on hydrocodone. Res Soc Adm Pharm. 2015;11(6):901–908. [DOI] [PubMed] [Google Scholar]
- 30.Warner MP LJ, Nolte KB, Davis GG, Nelson LS. State variation in certifying manner of death and drugs involved in drug intoxication deaths. Acad Forensic Pathol. 2013(2):231–237. [Google Scholar]