Abstract
Changes in inflammatory cascades have been implicated in the underlying pathophysiology of psychosis. Translocator protein 18 kDa (TSPO) has been used to assess neuroinflammatory processes in psychotic disorders. Nonetheless, it is unclear whether TSPO, a mitochondrial protein, can be interpreted as a general marker for inflammation in diseases involving psychosis. To address this question, we investigated TSPO signaling in representative mouse models for psychosis with inflammatory disturbances. The maternal immune activation and cuprizone short-term exposure models show different TSPO signaling. Furthermore, we observed similarities and differences in their respective stress pathways including stress hormone signaling and oxidative stress that are functionally interconnected with the inflammatory responses. We propose that more careful studies of TSPO distribution in neuroinflammation and other stress cascades associated with psychotic symptoms will allow us to understand the biological mechanisms underlying psychosis-related behaviors.
Keywords: cuprizone short-term exposure (CSE), maternal immune activation (MIA), inflammatory disturbances, Translocator protein 18 kDa (TSPO), stress cascades, psychosis
1. Introduction
Psychotic conditions, such as schizophrenia and major depressive disorder with psychotic features, are devastating medical conditions that affect certain percentages of the general population (Bogren et al., 2009; Kendler et al., 1996; Kessler et al., 2005; Perälä et al., 2007; van Os et al., 2001). Etiological factors and pathophysiological mediators for schizophrenia and related disorders have been studied using a variety of research approaches. Epidemiological studies consistently report that infection and other immune disturbances may underlie the etiology of the diseases (Brown and Derkits, 2010). Recent advances in human genetics implicated inflammatory molecules in psychotic disorders by both specific candidate molecular approaches as well as group functional pathway analyses (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; Sekar et al., 2016). In addition, examinations of biospecimens from patients including blood and cerebrospinal fluid implicated dynamic changes in inflammatory cascades that are associated with symptoms (Coughlin et al., 2016; Hayes et al., 2014; Miller et al., 2011). Postmortem brain studies also show alterations in the inflammatory responses in these diseases (Fillman et al., 2013; Trepanier et al., 2016).
Given that inflammatory cascades may be changed in patients with psychotic features, both clinical and preclinical studies have addressed how inflammatory changes may affect behaviors relevant to psychosis. Several clinical studies explored the correlation between specific molecular changes in the inflammatory responses and symptoms or treatment responses (Fillman et al., 2016; Mondelli et al., 2015). In preclinical studies, animal models in which inflammatory mechanisms are primarily disturbed have been studied for behaviors relevant to psychosis. For example, administration of polyinosinic:polycytidylic acid [poly(I:C)], a synthetic analogue of double-stranded RNA, to pregnant dams disturbs the inflammatory responses of the offspring during early development and subsequently affects their behavior in adulthood (Meyer and Feldon, 2010). Note that, depending on the timing of poly(I:C) administration, the maternal immune activation (MIA) model may also emphasize behaviors relevant to autistic disorder spectrum (Careaga et al., 2017). Furthermore, a deficiency of schnurri-2, a nuclear factor-κB site-binding protein, induces neuroinflammation and leads to behavioral deficits relevant to schizophrenia (Takao et al., 2013). Our group recently reported that a new animal model exposed to cuprizone, a copper chelator, for one week only in young adulthood shows inflammatory disturbances and subsequent behavioral deficits relevant to psychosis (Kondo et al., 2016; Tezuka et al., 2013).
The peripheral benzodiazepine receptor/translocator protein 18 kDa (TSPO) has utility as a specific molecular target using positron emission tomography (PET) to probe possible neuroinflammation. TSPO protein is located in the mitochondrial membrane of several types of cells in the brain, in particular astrocytes and microglia (Gut et al., 2015; Rupprecht et al., 2010). Since TSPO is upregulated in some inflammatory conditions in the brain, TSPO was once expected to be a good marker for inflammation using PET-based neuroimaging (Rupprecht et al., 2010). Indeed, in brain inflammatory conditions that are acquired in adulthood, high TSPO PET signals have been reported (Gerhard et al., 2005; Gershen et al., 2015; Oh et al., 2011; Ramlackhansingh et al., 2011; Suridjan et al., 2015). Consistent with these reports, our group successfully visualized high TSPO PET signals in inflammatory conditions such as human immunodeficiency virus-associated dementia and history of sports-related, repeated traumatic brain injury found in National Football League players (Coughlin et al., 2014; Coughlin et al., 2017). Nevertheless, in subjects with psychotic disorders (e.g., those with schizophrenia and first-episode psychosis), as well as subjects with an at-risk mental state for psychosis, the data TSPO imaging are inconsistent across reports (Bloomfield et al., 2016; Collste et al., 2017; Coughlin et al., 2016; Doorduin et al., 2009; Hafizi et al., 2017; Hannestad et al., 2013; Holmes et al., 2016; Kenk et al., 2015; Notter et al., 2017; Setiawan et al., 2015; Takano et al., 2010; van Berckel et al., 2008; van der Doef et al., 2016) (also see Table 1). Although some studies have reported higher TSPO PET signals in subjects in psychotic disorders and in subjects with an at-risk mental state for psychosis compared to controls (Bloomfield et al., 2016; Doorduin et al., 2009; Setiawan et al., 2015; van Berckel et al., 2008), several other groups have observed no change or even lower TSPO PET signals associated with psychosis (Collste et al., 2017; Coughlin et al., 2016; Hafizi et al., 2017; Hannestad et al., 2013; Holmes et al., 2016; Kenk et al., 2015; Notter et al., 2017; Takano et al., 2010; van der Doef et al., 2016). Our group reported that patients with recent onset of schizophrenia displayed an increase in the pro-inflammatory cytokine interleukin-6 (IL-6), whereas there was no difference in the binding of [11C]DPA-713 to TSPO across the brains of these patients compared to matched healthy controls (Coughlin et al., 2016).
Table 1.
Summary of findings from TSPO PET studies in patients with major mental illnesses
| Diseases | Ligands | TSPO levels | References |
|---|---|---|---|
| Psychosis (ultra high risk for psychosis) | [11C]PBR28 | ↑ | [1] |
| Psychosis (first-episode psychosis) | [11C]PBR28 | ↓ | [2] |
| Psychosis (first-episode psychosis) | [18F]FEPPA | → | [3] |
| Schizophrenia | [18F]FEPPA | → | [4] |
| Schizophrenia | [11C]DPA-713 | → | [5], [6] |
| Schizophrenia | [11C]DAA1106 | → | [7] |
| Major depressive disorder | [11C]PBR28 | → | [8] |
| Major depressive disorder | [18F]FEPPA | ↑ | [9] |
[1] Bloomfield et al., 2016, [2] Collste et al., 2017, [3] Hafizi et al., 2017, [4] Kenk et al., 2015, [5] Coughlin et al., 2016, [6] Notter et al., 2017, [7] Takano et al., 2010, [8] Hannestad et al., 2013, [9] Setiawan et al., 2015
Inflammatory responses are functionally interconnected with other stress pathways, such as those involving stress hormones and those associated with oxidative stress (Landek-Salgado et al., 2016). Stress hormones, the hypothalamic-pituitary-adrenal (HPA) axis, and oxidative stress are implicated in the pathophysiology of schizophrenia and related disorders (Coughlin et al., in press; Emiliani et al., 2014; Garner et al., 2005; Hardingham and Do, 2016; Johnson et al., 2013; Niwa et al., 2013; Niwa et al., 2016; Tanaka et al., 2017). Therefore, evaluating the interconnection of multiple stress cascades in animals may provide insight into the biology of schizophrenia and related disorders.
In the present study, we examined two representative animal models of inflammatory disturbances in the context of psychosis: a MIA model and a cuprizone short-term exposure (CSE) model. The MIA model is associated with immune/inflammatory disturbance in early development, whereas in the CSE model the immune disturbance is initially triggered in young adulthood. A recent study using the MIA model reported lower TSPO signal in the prefrontal cortex (PFC) of affected animals compared to controls, which is similar to findings in patients (Notter et al., 2017). Building on this background we conducted TSPO autoradiography in the CSE model. Furthermore, we examined possible changes in cascades related to the HPA axis and oxidative stress in both MIA and CSE models, with the goal of drawing a comprehensive and comparative picture of how stress cascades affect psychosis-related behaviors in these preclinical animal models. To focus on the frontal lobe pathology of psychosis, we studied oxidative stress cascades in the PFC of the CSE and MIA models.
2. Experimental materials and methods
2.1. Animal models
In the CSE model, 8-week-old C57BL/6J male mice were fed either a diet containing 0.2% cuprizone (Sigma-Aldrich, St. Louis, MO), or a control diet consisting of standard chow (Harlan Teklad, Indianapolis, IN) for one week according to our published protocol (Kondo et al., 2016; Tezuka et al., 2013). Experiments were performed on the 7th day of diet exposure. In the MIA model, pregnant C57BL/6J mice were administered poly(I:C) (20 mg/kg, intraperitoneal, Sigma-Aldrich, St. Louis, MO) on embryonic day 9.5 as described previously with minor modifications (Meyer et al., 2005). Experiments were conducted when the offspring reached 8 weeks of age. All experimental procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and the Johns Hopkins University Animal Care and Use Guidelines.
2.2. Ex vivo autoradiography
Ex vivo autoradiography was performed using the second-generation TSPO radiotracer of [125I]iodo-DPA-713 (Wang et al., 2009), because it is regarded as one of the best TSPO ligands in regard to sensitivity and specificity. Although the disadvantage is that this is affected by a critical genetic polymorphism in humans (Kobayashi et al., 2017), the corresponding polymorphism does not exist in mice (Parente et al., 2016).
Mice were injected intravenously with an average of 9.25 ± 0.55 MBq of [125I]iodo-DPA-713, specific activity 2,200 Ci/mmol, in 200 μl of 10% ethanol in phosphate buffered saline. All mice were sacrificed after 45 min of radiotracer uptake. The brains of the mice were cryosectioned into 20 μm thick horizontal sections according to the atlas of Franklin and Paxinos (Franklin and Paxinos, 2013), and were exposed to Kodak Biomax XR film along with serial dilutions of radiotracer standard. The film was developed using a Kodak X-O-Mat film processor and digitized using an MCID Core densitometry station and software. The regions of interest (ROIs) were set in one mm circles of the same size and shape for each subregion (> 20/subregion). The signals for the ROIs in sections were averaged across each subregion for each mouse section. Radiotracer uptake by region is expressed as fmol radiotracer/mm3 of tissue section.
The specific binding signals of DPA-713 to TSPO were obtained by subtraction of the nonspecific binding from the total binding. The non-specific binding was estimated by evaluating the signals of [125I]iodo-DPA-713 radiotracer when this was co-injected with 20 mg/kg of (R,S)-PK11195.
2.3. Levels of TSPO protein and 3-nitrotyrosine (3-NT)
The PFC (anteroposterior, +2.34 to +1.54 mm from Bregma; mediolateral, −0.50 to +0.50 mm from Bregma; dorsoventral, −0.75 to −3.00 mm from the dura) was collected according to the Mouse Brain Atlas (Franklin and Paxinos, 2013), and protein samples were prepared as previously described (Niwa et al., 2010). The levels of TSPO protein, 3-NT, and β-actin in the lysates were determined using western blotting as previously described (Niwa et al., 2010) with minor modifications. Briefly, TSPO, 3-NT, and β-actin were identified with primary antibodies against TSPO (1:10,000, #Ab109497, Abcam, Cambridge, UK), 3-NT (1:2,000, #06-284, Millipore, Billerica, MA), and β-actin (1:1,000, #4970, Cell Signaling Technology, Danvers, MA) respectively. Horseradish peroxidase-conjugated anti-rabbit IgG (1:2,000, #7074, Cell Signaling Technology, and 1:5,000, NA934, GE Healthcare Bio-Sciences, Piscataway, NJ) was used as the secondary antibody.
2.4. Levels of serum corticosterone
Blood was collected from the inferior vena cava under isoflurane anesthesia between 9 am and 11 am. Serum levels of corticosterone were determined using a commercially available enzyme immune assay kit (Cayman Chemical, Ann Arbor, MI) as described previously (Niwa et al., 2013).
2.5. Levels of protein carbonylation
Levels of protein carbonylation were measured according to our published protocols with minor modifications (Johnson et al., 2013). Quantitative analysis of protein carbonylation levels of the PFC lysates was performed by a dot blot procedure with the OxyBlot Protein Oxidation Detection Kit (Millipore, Billerica, MA).
2.6. Statistical analysis
Data were assessed for normal distribution and homogeneity of variance using the Shapiro-Wilk and F-tests, respectively. Parametric statistical comparisons were performed using a two-tailed unpaired Student’s t-test, except for the comparison of relative carbonylation levels of protein in MIA mice, which didn’t show normal distribution. Thus, the data in Fig. 3B was analyzed with the Mann-Whitney U-test. For the comparison of relative levels of 3-NT protein, one-way analysis of variance was used. An α value of p < 0.05 was considered statistically significant.
Fig. 3. Oxidative stress in CSE and MIA.
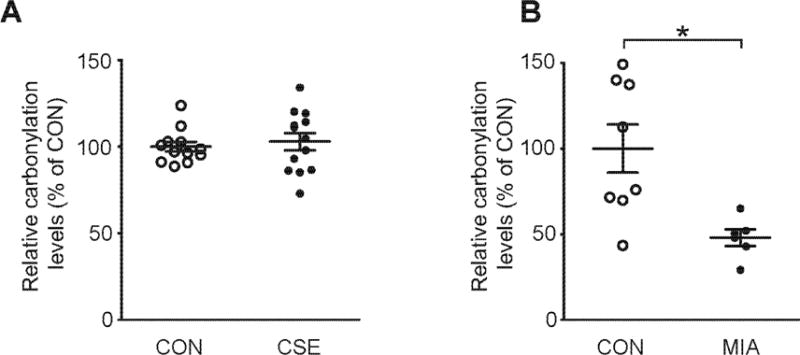
(A) Relative carbonylation levels of protein in the PFC of CSE mice. No significant differences between CON and CSE mice were detected. N=12-13. p=0.6228, as analyzed by Student’s t-test. (B) Relative carbonylation levels of protein in the PFC of MIA mice. MIA mice showed significantly reduction in relative carbonylation levels of protein. N=6-8. *p<0.05, as analyzed by Mann–Whitney U-test (p=0.0118). Data are presented as mean ± S.E.M.
3. Results
3.1. TSPO distribution in CSE
We recently reported that TSPO binding was significantly lower in the MIA model that has inflammatory disturbances from early development, compared to controls (Notter et al., 2017). Here we examined radiotracer binding to the TSPO target in another inflammatory animal model relevant to psychosis, the CSE model, although the initial trigger for immune/inflammatory disturbance is in young adulthood, and not in early development. We observed augmentation of TSPO binding in broad areas of the brain, especially in the PFC, HP, and STR, of CSE mice compared with control (CON) animals (Fig. 1 and Supplemental Fig. 1). No difference of TSPO expression was observed in the PFC between the CSE model and CON animals at least through the biochemical methodology that we used (Supplemental Fig. 2).
Fig. 1. Translocator protein 18kDa (TSPO) disposition in cuprizone short-term exposure (CSE).
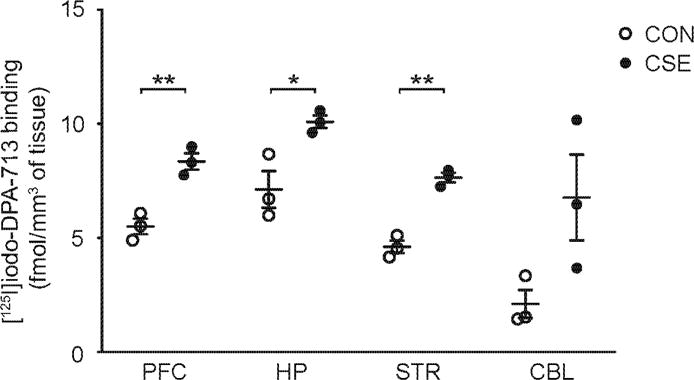
Quantitative analyses of regional [125I]iodo-DPA-713 binding to TSPO: There was a significant increase of [125I]iodo-DPA-713 binding in the prefrontal cortex (PFC), hippocampus (HP) and striatum (STR), but not in the cerebellum (CBL) in CSE mice compared with CON mice. N=3 for each group. *p<0.05, **p<0.01, as analyzed by Student’s t-test (p=0.0045 for PFC, p=0.0250 for HP, p=0.0009 for STR, p=0.0775 for CBL). Data are presented as mean ± S.E.M.
3.2. HPA axis in CSE and MIA
Inflammatory responses are functionally interconnected with other stress pathways, including stress hormones and oxidative stress (Landek-Salgado et al., 2016). To address stress cascades other than inflammatory responses, we also examined changes in the HPA axis. We observed a significant increase in the level of serum corticosterone in both CSE and MIA models compared to baseline (Fig. 2).
Fig. 2. Hypothalamic-pituitary-adrenal (HPA) axis in CSE and maternal immune activation (MIA).
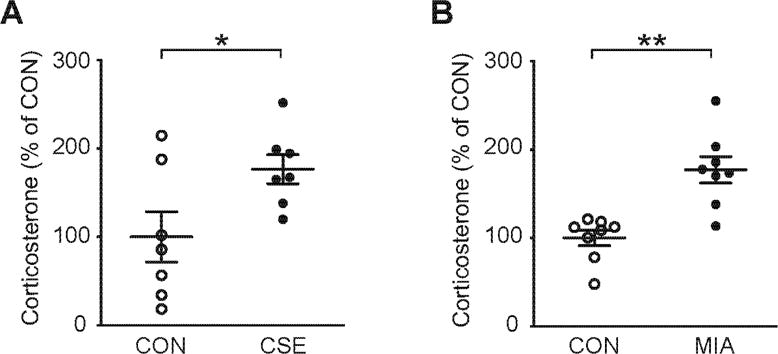
(A) Serum levels of corticosterone in CSE mice. CSE mice showed a significant elevation in levels of serum corticosterone. The serum levels of corticosterone in CON mice were 63.10 ± 17.92 ng/mL. N=7. *p<0.05, as analyzed by Student’s t-test (p=0.0380). (B) Serum corticosterone levels in MIA mice. MIA mice showed a significant elevation in levels of serum corticosterone. The serum levels of corticosterone in CON mice were 128.01 ± 11.29 ng/mL. N=8. **p<0.01, as analyzed by Student’s t-test (p=0.005). Data are presented as mean ± S.E.M.
3.3. Oxidative stress in CSE and MIA
We next addressed possible involvement of oxidative stress in the two models. We previously reported the preclinical evidence that oxidative stress is linked to neurocognitive deficits relevant to psychotic disease (Johnson et al., 2013). Using the protein carbonylation assay, we did not observe any increase of oxidative stress in the PFC of the CSE model (Fig. 3A). Interestingly, the level of protein carbonylation in the PFC of the MIA animals was significantly reduced compared with the CON animals (Fig. 3B). In contrast, we observed no significant difference in the 3-NT signals among the CSE, MIA, and CON mice (Supplemental Figure 3).
4. Discussion
One of the main goals in the present study was to examine TSPO binding in the CSE model and the connection with the HPA axis and oxidative stress in both the CSE and MIA models as compared to controls. Both CSE and MIA models displayed common signatures in stress cascades, such as an increase in the IL-6 signaling (a representative indicator for inflammation) and the HPA axis (Table 2). Nevertheless, a sharp contrast between these two models exists in the TSPO signals (Table 2).
Table 2.
Comprehensive characterization of cuprizone short-term exposure (CSE) mice and maternal immune activation (MIA) mice
| Stress signals/Behavioral dimensions | Index | Tests | Results CSE | MIA (GD9, 5 mg/kg i.v. or GD9.5, 20 mg/kg i.p.) | |||
|---|---|---|---|---|---|---|---|
| Stress signals | Inflammation | TSPO levels IL-6 levels |
↑ ↑ |
Fig.1 [10] |
↓ ↑ |
[12] [12] |
|
| HPA axis | Serum corticosterone levels | ↑ | Fig. 2A | ↑ | Fig. 2B | ||
| Oxidative stress | Protein carbonylation levels | → | Fig. 3A | ↓ | Fig. 3B | ||
| Behavioral dimensions | Psychosis | Sensitivity to psychostimulants | OF | ↑ | [10] [11] |
↑ | [13] |
| Memory | Spatial learning and memory Recognition memory Spontaneous alternation Context/cue-associated fear learning and memory Avoidance learning |
MWM NORT Y-maze FC AAL |
ND ↓ ↓ Normal ND |
[10] [10] [11] |
Normal ND ↓ ND ↓ |
[14] [15] [13] |
|
| Executive function | Behavioral flexibility | RL | Normal | [11] | Normal | [16] | |
| Social cognition | Sociability/social novelty | SIT | Normal | [11] | ↓ | [12] | |
| Anxiety | Neophobia Hyponeophagia Exploration based conflict |
LDT NSFT OF |
↑ Normal ND |
[11] [11] |
ND ND ↑ |
[16] |
|
| Depression | Behavioral despair | FST/TST | Normal | [11] | ND | ||
| Information processing | Sensorimotor gating | PPI | Normal | [10] | ↓ | [12] | |
| Attention | Latent inhibition | LI | ND | ↓ | [13] | ||
| Motor systems | Motor coordination/learning | Rotarod | Normal | [11] | ND | ||
[10] Tezuka et al. 2013, [11] Kondo et al. 2016, [12] Notter et al. 2017, [13] Meyer et al., 2005, [14] Meyer et al., 2008, [15] Willi et al., 2013, [16] Meyer et al., 2006
GD, gestation day; TSPO, Translocator protein 18kDa; IL-6, Interleukin-6; HPA, Hypothalamic-pituitary-adrenal; OF, Open field; MWM, Morris water maze; ND, not determined; NORT, Novel object recognition test; FC, Fear conditioning; AAL, Active avoidance test; RL, Reversal learning; SIT, Social interaction test; LDT, Light/dark box test; NSFT, Novelty suppressed feeding test; FST, Forced swim test; TST, Tail suspension test; PPI, Prepulse inhibition; LI, Latent inhibition
As described above, TSPO findings in clinical studies in psychotic disorders are not consistent. These differences in data from TSPO brain imaging could be due to disease heterogeneity, analysis during different time points in pathological trajectory, the TSPO gene (rs6971) polymorphism, differences of medications, and differences between each tracer targeting the same molecule (DAA1106, DPA-713, FEPPA, PBR28, and PK11195). We propose that an overarching proposal of upregulated TSPO signaling in psychosis may be avoided. On the other hand, we propose that multiple animal models displaying abnormalities in the inflammatory responses and behavioral deficits relevant to psychosis should be utilized in parallel to decipher different aspects of inflammatory disturbances in patients with psychosis. PK11195 (1st generation TSPO ligand) may be inferior to other 2nd generation TSPO ligands such as DAA1106, DPA-713, FEPPA, and PBR28 in signal-noise ratio and brain penetration (Boutin et al., 2007; Brown et al., 2007; Endres et al., 2009; Rusjan et al., 2011; Wilson et al., 2008; Zhang et al., 2003). Thus, in such comparative studies, the data from 2nd generation TSPO ligands might be useful. A working hypothesis is that TSPO levels in the brain may be downregulated in psychotic conditions that are originally elicited in immune/inflammatory insults in early development (e.g., the MIA model or most of psychotic disorders), whereas the signals may be upregulated in neuroinflammatory conditions. More careful studies of TSPO in neuroinflammatory conditions that are associated with psychotic symptoms are needed to dissect the different mechanistic cascades.
In the long run, the mechanism of increased TSPO binding in the CSE model should be defined not merely by protein levels of TSPO protein but also by protein conformation and many other biological factors. The use of micro-PET, in which the Bmax/Kd values in vivo are to be available by reflecting pharmacokinetics, will provide us with more mechanistic insight in future studies. Furthermore, understanding the effects of antipsychotic drugs on TSPO binding and stress cascades would also be important.
The differences in the stress signals may originate from the timing of the original immune/inflammatory insults. The acute nature of the CSE model induces the increase in TSPO signals by massive activation of glial cells. In contrast, in the MIA model, the immune/inflammatory response occurs in early development, which is likely to alter the homeostatic set point inside the brain as a compensatory mechanism (McEwen et al., 2015). This may lead to reduction in protein carbonylation (oxidative stress) and suppression of glial activation associated with a decrease in TSPO binding. In contrast, it is interesting that such a compensatory mechanism did not seem to affect the levels of corticosterone that were commonly upregulated in both CSE and MIA models.
Interconnection of stress cascades among inflammation, HPA axis, and oxidative stress are important. For each paradigm, corresponding peripheral biomarkers may be utilized in clinical settings, including blood levels of C-reactive protein, cytokines, cortisol, and glutathione. We believe that this is a study with two animal models that aims to contribute to this long-term goal.
Supplementary Material
Acknowledgments
We thank Mr. Daniel J. Wood and Ms. Yukiko Lema for critical reading of the manuscript and organizing the figures, respectively. This work was supported by the National Institute of Health [MH-094268 Silvio O. Conte center (A.S., A.K., and M.G.P.), MH-092443 (A.S.), MH-105660 (A.S.), MH-091230 (A.K.), K99MH-094408 (M.N.), DA-040127 (A.S. and M.N), AT-008547 (A.K.)], NARSAD (A.S., A.K., and M.N.), Stanley (A.S.), S-R/RUSK (A.S.), JST PRESTO JPMJPR14M6 (M.N.), and DoD W81XWH-14-1-0620 (M.G.P.). This study was also supported in part by Mitsubishi Tanabe Pharma Corporation.
Abbreviations
- CBL
cerebellum
- CON
control
- CSE
cuprizone short-term exposure
- HP
hippocampus
- HPA
hypothalamic-pituitary-adrenal
- MIA
maternal immune activation
- PET
positron emission tomography
- PFC
prefrontal cortex
- poly(I
C), polyinosinic:polycytidylic acid
- ROIs
regions of interest
- STR
striatum
- TSPO
Translocator protein 18 kDa
- 3-NT
3-nitrotyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry. 2016;173(1):44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogren M, Mattisson C, Isberg PE, Nettelbladt P. How common are psychotic and bipolar disorders? A 50-year follow-up of the Lundby population. Nordic journal of psychiatry. 2009;63(4):336–346. doi: 10.1080/08039480903009118. [DOI] [PubMed] [Google Scholar]
- Boutin H, Chauveau F, Thominiaux C, Gregoire MC, James ML, Trebossen R, Hantraye P, Dolle F, Tavitian B, Kassiou M. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48(4):573–581. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, Imaizumi M, Hong J, Pike VW, Innis RB. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. 2007;48(12):2072–2079. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Murai T, Bauman MD. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol Psychiatry. 2017;81(5):391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collste K, Plaven-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband S, Erhardt S, Halldin C, Flyckt L, Farde L, Cervenka S. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry. 2017 doi: 10.1038/mp.2016.247. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Hayes LN, Tanaka T, Xiao M, Yolken RH, Worley P, Leweke FM, Sawa A. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr Res. doi: 10.1016/j.schres.2016.10.040. in press. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6:e777. doi: 10.1038/tp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV, Gage KL, Stathis M, Rais R, Rojas C, McGlothan JL, Watkins CC, Sacktor N, Guilarte TR, Zhou Y, Sawa A, Slusher BS, Caffo B, Kassiou M, Endres CJ, Pomper MG. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol. 2014;20(3):219–232. doi: 10.1007/s13365-014-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Minn I, Bienko N, Ambinder EB, Xu X, Peters ME, Dougherty JW, Vranesic M, Koo SM, Ahn HH, Lee M, Cottrell C, Sair HI, Sawa A, Munro CA, Nowinski CJ, Dannals RF, Lyketsos CG, Kassiou M, Smith G, Caffo B, Mori S, Guilarte TR, Pomper MG. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA neurology. 2017;74(1):67–74. doi: 10.1001/jamaneurol.2016.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Emiliani FE, Sedlak TW, Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr Opin Psychiatry. 2014;27(3):185–190. doi: 10.1097/YCO.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres CJ, Pomper MG, James M, Uzuner O, Hammoud DA, Watkins CC, Reynolds A, Hilton J, Dannals RF, Kassiou M. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J Nucl Med. 2009;50(8):1276–1282. doi: 10.2967/jnumed.109.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, Weickert CS. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016;21(8):1090–1098. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. Paxinos and Franklin’s The mouse brain in stereotaxic coordinates. Fourth. Academic Press, an imprint of Elsevier; Amsterdam: 2013. [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58(5):417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. NeuroImage. 2005;24(2):591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Gershen LD, Zanotti-Fregonara P, Dustin IH, Liow JS, Hirvonen J, Kreisl WC, Jenko KJ, Inati SK, Fujita M, Morse CL, Brouwer C, Hong JS, Pike VW, Zoghbi SS, Innis RB, Theodore WH. Neuroinflammation in Temporal Lobe Epilepsy Measured Using Positron Emission Tomographic Imaging of Translocator Protein. JAMA neurology. 2015;72(8):882–888. doi: 10.1001/jamaneurol.2015.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut P, Zweckstetter M, Banati RB. Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol Metab. 2015;26(7):349–356. doi: 10.1016/j.tem.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, Suridjan I, Wilson AA, Meyer JH, Remington G, Houle S, Rusjan PM, Mizrahi R. Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [18F]FEPPA. Am J Psychiatry. 2017;174(2):118–124. doi: 10.1176/appi.ajp.2016.16020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee JY, O’Connor KC, Pelletier D, Carson RE. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C]PBR28 PET study. Brain, behavior, and immunity. 2013;33:131–138. doi: 10.1016/j.bbi.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17(2):125–134. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM, Leweke FM, Yolken RH, Sawa A. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull. 2014;40(5):963–972. doi: 10.1093/schbul/sbu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672–1679. doi: 10.1038/mp.2016.180. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, Emiliani F, Sawa A, Gallagher M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci U S A. 2013;110(30):12462–12467. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996;53(11):1022–1031. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S, Mizrahi R. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull. 2015;41(1):85–93. doi: 10.1093/schbul/sbu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Demler O, Falloon IR, Gagnon E, Guyer M, Howes MJ, Kendler KS, Shi L, Walters E, Wu EQ. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R) Biol Psychiatry. 2005;58(8):668–676. doi: 10.1016/j.biopsych.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Jiang T, Telu S, Zoghbi SS, Gunn RN, Rabiner EA, Owen DR, Guo Q, Pike VW, Innis RB, Fujita M. 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-(R)-PK11195. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17699223. 271678X17699223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo MA, Fukudome D, Smith DR, Gallagher M, Kamiya A, Sawa A. Dimensional assessment of behavioral changes in the cuprizone short-term exposure model for psychosis. Neurosci Res. 2016;107:70–74. doi: 10.1016/j.neures.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry. 2016;21(1):10–28. doi: 10.1038/mp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29(6):913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain, behavior, and immunity. 2008;22(4):469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, Marques TR, Zunszain PA, Morgan C, Murray RM, Pariante CM, Dazzan P. Cortisol and Inflammatory Biomarkers Predict Poor Treatment Response in First Episode Psychosis. Schizophr Bull. 2015;41(5):1162–1170. doi: 10.1093/schbul/sbv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S, Ozaki N, Nabeshima T, Sawa A. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339(6117):335–339. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, Hiyama H, Huang B, Kohda K, Noda Y, O’Donnell P, Nakajima K, Sawa A, Nabeshima T. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65(4):480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Lee RS, Tanaka T, Okada K, Kano S, Sawa A. A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Hum Mol Genet. 2016;25(7):1370–1381. doi: 10.1093/hmg/ddw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A, Meyer U. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry. 2017 doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- Oh U, Fujita M, Ikonomidou VN, Evangelou IE, Matsuura E, Harberts E, Fujimura Y, Richert ND, Ohayon J, Pike VW, Zhang Y, Zoghbi SS, Innis RB, Jacobson S. Translocator protein PET imaging for glial activation in multiple sclerosis. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2011;6(3):354–361. doi: 10.1007/s11481-010-9243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente A, Feltes PK, Vallez Garcia D, Sijbesma JW, Moriguchi Jeckel CM, Dierckx RA, de Vries EF, Doorduin J. Pharmacokinetic Analysis of 11C-PBR28 in the Rat Model of Herpes Encephalitis: Comparison with (R)-11C-PK11195. J Nucl Med. 2016;57(5):785–791. doi: 10.2967/jnumed.115.165019. [DOI] [PubMed] [Google Scholar]
- Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, Harkanen T, Koskinen S, Lonnqvist J. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64(1):19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Annals of neurology. 2011;70(3):374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S, Mizrahi R. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(8):1807–1816. doi: 10.1038/jcbfm.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Schizophrenia Working Group of the Psychiatric Genomics, C. Daly MJ, Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72(3):268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suridjan I, Pollock BG, Verhoeff NP, Voineskos AN, Chow T, Rusjan PM, Lobaugh NJ, Houle S, Mulsant BH, Mizrahi R. In-vivo imaging of grey and white matter neuroinflammation in Alzheimer’s disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry. 2015;20(12):1579–1587. doi: 10.1038/mp.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y, Suhara T. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol. 2010;13(7):943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, Koshimizu H, Umemori J, Toyama K, Nakamura HK, Kuroiwa M, Maeda J, Atsuzawa K, Esaki K, Yamaguchi S, Furuya S, Takagi T, Walton NM, Hayashi N, Suzuki H, Higuchi M, Usuda N, Suhara T, Nishi A, Matsumoto M, Ishii S, Miyakawa T. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology. 2013;38(8):1409–1425. doi: 10.1038/npp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Matsuda T, Hayes LN, Yang S, Rodriguez K, Severance EG, Yolken RH, Sawa A, Eaton WW. Infection and inflammation in schizophrenia and bipolar disorder. Neurosci Res. 2017;115:59–63. doi: 10.1016/j.neures.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K, Fukunari A, Miwa K, Ohzeki H, Kano S, Yasumatsu H, Sawa A, Kajii Y. Cuprizone short-term exposure: astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol Dis. 2013;59:63–68. doi: 10.1016/j.nbd.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21(8):1009–1026. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, de Haan L, Eriksson J, Lammertsma AA, Kahn RS, van Berckel BN. In vivo (R)-[(11)C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr. 2016;2:16031. doi: 10.1038/npjschz.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Hanssen M, Bijl RV, Vollebergh W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry. 2001;58(7):663–668. doi: 10.1001/archpsyc.58.7.663. [DOI] [PubMed] [Google Scholar]
- Wang H, Pullambhatla M, Guilarte TR, Mease RC, Pomper MG. Synthesis of [(125)I]iodoDPA-713: a new probe for imaging inflammation. Biochemical and biophysical research communications. 2009;389(1):80–83. doi: 10.1016/j.bbrc.2009.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi R, Harmeier A, Giovanoli S, Meyer U. Altered GSK3beta signaling in an infection-based mouse model of developmental neuropsychiatric disease. Neuropharmacology. 2013;73:56–65. doi: 10.1016/j.neuropharm.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, Houle S, Vasdev N. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nuclear medicine and biology. 2008;35(3):305–314. doi: 10.1016/j.nucmedbio.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang MR, Kida T, Noguchi J, Furutsuka K, Maeda J, Suhara T, Suzuki K. [(11)C]DAA1106: radiosynthesis and in vivo binding to peripheral benzodiazepine receptors in mouse brain. Nuclear medicine and biology. 2003;30(5):513–519. doi: 10.1016/s0969-8051(03)00016-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


