Abstract
Human mucin-1 (MUC1) is a highly attractive antigen for the development of anticancer vaccines. However, in human clinical trials of multiple MUC1 based vaccines, despite the generation of anti-MUCl antibodies, the antibodies often failed to exhibit much binding to tumor presumably due to the challenges in inducing protective immune responses in the immunotolerant environment. To design effective MUC1 based vaccines functioning in immunotolerant hosts, vaccine constructs were first synthesized by covalently linking the powerful bacteriophage Qβ carrier with MUC1 glycopeptides containing 20–22 amino acid residues covering one full length of the tandem repeat region of MUC1. However, IgG antibodies elicited by these first generation constructs in tolerant human MUC1 transgenic (Tg) mice did not bind tumor cells strongly. To overcome this, a peptide array has been synthesized. By profiling binding selectivities of antibodies, the long MUC1 glycopeptide was found to contain immunodominant but nonprotective epitopes. Critical insights were obtained into the identity of the key protective epitope. Redesign of the vaccine focusing on the protective epitope led to a new Qβ-MUC1 construct, which was capable of inducing higher levels of anti-MUC1 IgG antibodies in MUC1.Tg mice to react strongly with and kill a wide range of tumor cells compared to the construct containing the gold standard protein carrier, i.e., keyhole limpet hemocyanin. Vaccination with this new Qβ-MUC1 conjugate led to significant protection of MUC1.Tg mice in both metastatic and solid tumor models. The antibodies exhibited remarkable selectivities toward human breast cancer tissues, suggesting its high translational potential.
Graphical Abstract
INTRODUCTION
Vaccination is an appealing strategy to combat cancer, as vaccines can potentially provide long-term protection to the host with few side effects.1,2 A highly attractive anticancer vaccine target is mucin-1 (MUC1), which is a glycoprotein on cancer cell surface. The extracellular domain of MUC1 contains a variable number of 20 amino acid tandem repeats with the sequence of HGVSTAPDTRPAPGSTAPPA.3 The serine (S) and threonine (T) residues in the tandem repeat can be glycosylated. MUC1 is expressed on a variety of tumor cells at significantly higher levels (>100-fold) than those on normal cells, rendering it one of the top ranked tumor antigens by the National Cancer Institute.4
Studies have been carried out on MUC1 based anticancer vaccines. While patients who can generate higher levels of MUC1 antibodies are associated with better prognosis in clinical trials,5,6 no successful MUC1 vaccines are available yet, presumably because anti-MUC1 immunity induced for the full patient population is not sufficient. There is a continual need to develop vaccine constructs to induce higher levels of anti-MUC1 antibodies capable of killing tumor cells.
With the complex structure of MUC1, there are large variations in the structures of MUC1 peptides and glycopeptides utilized as vaccine epitopes.7–9 In terms of peptide backbone sequence alone, epitopes range from a nine amino acid backbone, to 60-mers and to long polypeptides with seven tandem repeat units totaling 140 amino acid residues.7–9 The structures of antigens can profoundly impact immune responses. Some epitopes can be immunogenic yet nonprotective, which can dominate and divert the desired immune responses,10–12 especially in immunotolerant hosts. For vaccine design, it is important to determine the protective epitopes of MUC1 to guide the development of successful vaccines.
In this work, we report new methodologies based on synthetic peptide arrays to decipher the key MUC1 epitopes required for tumor protection. We have discovered that a short glyco-nonapeptide of SAPDT*RPAP (* denotes glycosylation) is the critical protective epitope of MUC1. Utilizing MUC1 epitopes longer than this nonapeptide actually led to inferior antibody responses presumably due to antigen competition. The key MUC1 glyco-nonapeptide has been conjugated with bacteriophage carrier. This new Qβ-MUC1 construct is found to induce high levels of anti-MUC1 IgG antibodies in human MUC1 transgenic mice that mimic human conditions and are immunotolerant toward MUC1. The antibodies elicited exhibited high tumor binding and killing activities, good selectivities in glycopeptide recognition determined by a glycopeptide microarray, and excellent recognition of human breast cancer over normal breast tissues. Furthermore, a head to head comparison was performed against the MUC1 conjugate with the gold standard protein carrier, keyhole limpet hemocyanine (KLH). The new Qβ-MUC1 conjugate produced antibodies with significantly stronger binding with tumor cells and bestowed superior protection of MUC1 transgenic mice from tumor development, highlighting its high translation potential.
RESULTS
First Generation Qβ-MUC1 Constructs Surprisingly Failed To Elicit IgG Antibodies in Immunotolerant MUC1 Transgenic Mice for Strong Tumor Cells Binding, Despite Producing High IgG Titers against the Immunizing MUC1 Structures 1–4.
Our first generation approach in MUC1 vaccine design was to target MUC1 (glyco)peptide epitopes 1–4 (Scheme 1), which bear 20–22 amino acid residues as the backbone to cover one full length of the tandem repeat region. Glycopeptides 2 and 4 contain an N-acetyl galactosamine (GalNAc) moiety linked to the threonine (i.e., the Tn antigen) close to the C-termini. MUC1 (glyco)peptides 1–4 were synthesized and conjugated to bacteriophage Qβ as the carrier through an N-terminal azide group by the copper-catalyzed azide-alkyne cycloaddition (CuAAc) reaction (Qβ-MUC1 conjugates 5–8, Scheme 1). When administered in immunocompetent wild type (WT) C57BL/6 mice by one prime with Complete Freund’s Adjuvant (CFA) and two booster injections with Incomplete Freund’s Adjuvant (IFA), these constructs elicited super strong anti-MUC1 IgG responses‚ with average titers exceeding 1,000,000 evaluated by enzyme-linked immunosorbent assay (ELISA) against MUC1 (glyco)peptides 1–4.13 Furthermore, the antibodies produced could recognize multiple MUC1 expressing tumor cells when analyzed by fluorescence activated cell sorting (FACS) assays.13 With these promising results in hand, we moved onto studying these constructs in human MUC1 transgenic (MUC1.Tg) mice.
Scheme 1.
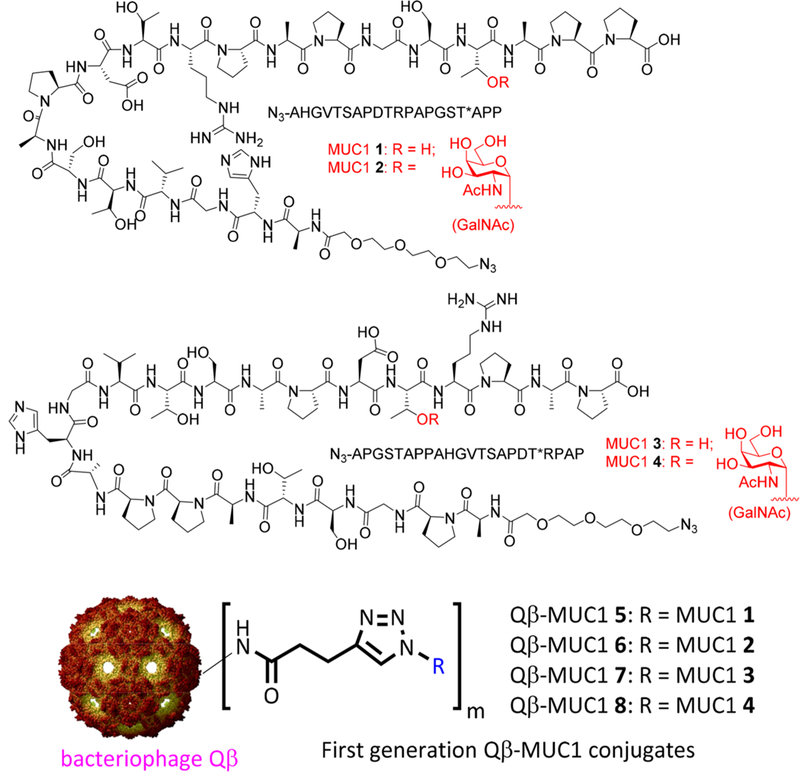
Structures of MUC1 (Glyco)peptides 1−4 and First Generation of Qβ-MUC1 Conjugates 5−8
MUC1.Tg mice are closer models to humans for MUC1 based vaccine evaluation compared to WT mice. This is because human MUC1 is a foreign antigen in WT mice due to the low (34%) sequence homology of mouse mucins and human MUC1.14 As a result, human MUC1 expressing tumors cannot grow in WT mice as they are rejected by the immune system. To mimic the tolerant condition toward MUC1 in humans, we bred human MUC1 transgenic mice (MUC1.Tg) that endogenously express human MUC1 in a developmentally regulated and tissue-specific fashion.15 MUC1 expression levels and patterns as well as MUC1 tolerance by immune cells in MUC1.Tg mice are similar to those in humans. Consistent with their MUC1 tolerance, MUC1.Tg mice cannot reject MUC1-expressing tumor cells.15,16
With MUC1.Tg mice ready, immunization studies of Qβ-MUC1 5–8 were performed following the same protocol as in WT mouse studies (one prime with CFA and two booster injections with IFA, 2 weeks apart). On day 35 after the initial injection, sera were collected from mice and analyzed. ELISA showed that high levels of anti-MUC1 IgG were generated when assayed against the corresponding MUC1 (glyco)-peptides 1–4, with very similar IgG titer numbers as those from WT mice (Figure 1a). FACS analysis was then carried out to test the binding of a panel of MUC1 expressing tumor cells including Ag104-MUC1 fibrosarcoma, B16-MUC1 melanoma, and MCF-7 breast cancer cells, with IgG antibodies in postimmune sera from Qβ-MUC1 5–8 immunized Tg mice. Surprisingly, in spite of the high IgG titers, these sera exhibited much weaker recognition of MUC1 expressing tumor cells (Figure 1b) with the levels of binding less than 3% of those from WT mice, suggesting Qβ-MUC1 5–8 would not be effective in providing protections against tumor development. The low reactivities against tumor cells induced in MUC1.Tg mice are similar to results from clinical studies of MUC1 based vaccines in human patients.17–19
Figure 1.
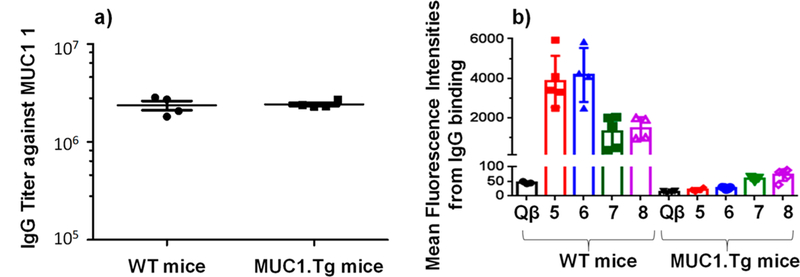
(a) Comparison of postimmune sera from WT and MUC1.Tg mice immunized with Qβ-MUC1 5 showed that the IgG antibody titers were similar when assayed against the immunizing antigen MUC1 1. For clarity, only results from Qβ-MUC1 5 are shown. Qβ-MUC1 6–8 gave similar results. (b) Mean fluorescence intensities of B16-MUC1 cells upon incubation with postimmune sera from immunized mice. Despite similar IgG titers, sera from Tg mice bound B16-MUC1 cells significantly weaker than those from the corresponding WT mice. Similar phenomena were observed with Ag104-MUC1 and MCF-7 cells. Each symbol represents one mouse (n = 3–5 mice for each group).
Synthetic Peptide Array Profiling of Induced Antibodies from WT and Tg Mice Provided Critical Insights into Potential Protective Epitope Structure.
Glycopeptide microarray has been actively explored to probe the specificity in recognition by anti-MUC1 antibodies.20 To gain insights on the weak recognition of tumor cells by antibodies from Tg mice immunized with the first generation Qβ-MUC1 vaccines, we decided to profile the epitopes of the antibodies. A library of 20 MUC1 peptides were synthesized, each of which contained 8 amino acid residues with sequences overlapping by 7 amino acids covering the full length of one MUC1 tandem repeat. These peptides were then conjugated with bovine serum albumin (BSA) as a multivalent platform to afford 20 BSA-MUC1 conjugates 9–28 (Table S1). ELISA wells coated with individual BSA-MUC1 conjugate were then incubated with postimmune sera from Qβ-MUC1 5 immunized MUC1.Tg mice. The relative level of recognition of each MUC1 epitope was quantified through ELISA (right panel Figure S9b). Strong bindings to two main regions around HGVTSAPD and APGSTAPP were observed, which suggest that HGVTSAPD and APGSTAPP are the epitopes dominating antibody responses in Tg mice. The lack of strong tumor cell binding by postimmune sera could be because levels of antibodies against HGVTSAPD or APGSTAPP were not high enough. Alternatively, epitopes other than HGVTSAPD and APGSTAPP may be critical for tumor cell binding.
To gain further insights, epitope profiles of postimmune sera from WT mice were obtained (Figure S9b, left panel) and compared with those from MUC1.Tg mice. The levels of antibody binding to HGVTSAPD and APGSTAPP regions were comparable in WT vs Tg mice. Interestingly, WT mouse sera exhibited much stronger binding to the SAPDTRPAP region. This suggests SAPDTRPAP may be the key epitope required for strong tumor cell recognition with antibodies to HGVTSAPD and APGSTAPP regions not contributing significantly in Tg mice.
Glycosylation can augment the immunogenicity of MUC1 antigen.9 It is of particular interest to perform epitope profiling of the sera from Qβ-MUC1 8, which has Tn glycosylation at SAPDT*RPA region. As shown in Figure 2, significant binding to the SAPDTRPA region was observed in WT vs Tg mice. Interestingly, antibody bindings to HGVTSAPD by the sera from both WT and Tg mice were much reduced. These results confirm that the SAPDTRPA region is likely the protective epitope for tumor cell recognition and that glycosylation does not affect much the protective epitope.
Figure 2.
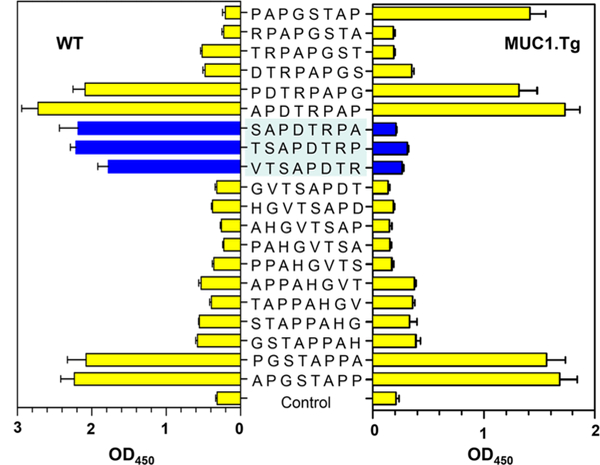
Comparison of epitope profiles of antisera from Qβ-MUC1 8 immunized MUCl.Tg mice (right panel) and WT mice (left panel). Epitope profiles of IgG antibodies were determined through binding with BSA-MUC1 conjugates 9–28 in an ELISA assay. Control wells were coated with BSA only. Significant differences between MUCl.Tg and WT mice in epitope binding were mainly observed in the SAPDTRPA region. The error bars represent standard deviation (SD) of four replicates.
Antibodies Were Elicited against the Free C-Termini of MUC1 Peptides by Qβ-MUC1 5.
During epitope scanning of postimmune sera from immunized mice, free C-termini of MUC1 peptides were found to be important for antibody recognition. As shown in Figure S1, sera from mice immunized with conjugate Qβ-MUC1 5 exhibited strong recognition to APGSTAPP. However, when the C-terminus of this peptide was capped as a methyl ester, binding was much reduced (Figure S1). Similar phenomena were observed for other regions of MUC1, indicating the free C-termini of MUC1 peptides were major epitopes. As the tandem repeat regions of MUC1 do not contain free C-terminus in nature, antibody responses against the free C-terminus of the immunizing antigen should not contribute much to binding ofMUCl expressing tumor cells.
Synthesis and Evaluation of Second Generation of Qβ-MUC1 Conjugates 35–37 with MUC1 Linked from the C-Terminus.
To remove the interference of free C-terminus of MUC1, our second generation vaccines have the MUC1 (glyco)peptide conjugated to Qβ through its C-terminus. The C-terminal coupling of MUC1 glycopeptides to a vaccine carrier has been studied before.21 As antibodies against the SAPDTRPAP region were thought to be important for tumor cell binding, MUC1 peptide sequence 29 was designed with SAPDTRPAP moved closer to the N-terminus (Scheme 2), which would be more accessible to B cell binding. A GalNAc moiety was introduced onto the threonine residue in the SAPDTRPAP region leading to glycopeptide 30 to explore the effect of glycosylation. Furthermore, glycopeptide 31 was also designed, which had the same structure as 30 except for its free N-terminus (Scheme 2).
Scheme 2.
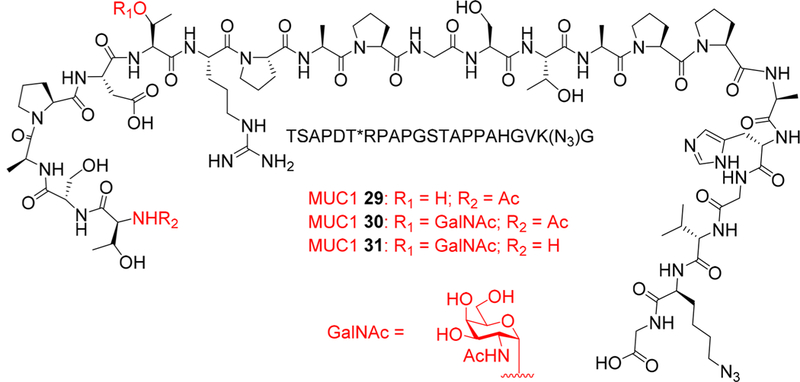
Design of MUC1 (Glyco)peptides 29–31
The synthesis of the MUC1 (glyco)peptides 29–31 was performed through solid-phase peptide synthesis (SPPS) using Fmoc chemistry (Scheme 3). To facilitate bioconjugation, azido lysine was introduced close to the C-terminus with the Nα-Fmoc-Nε-azide-L-lysine (Fmoc-Lys(N3)-OH building block 32). For glycopeptide synthesis, Fmoc-protected GalNAc-threonine 33 (Fmoc-GalNAc-Thr) was used to introduce the Tn antigen.22 After assembly of (glyco)peptides, the N-terminal Fmoc group was removed and was either capped with acetic anhydride (29–30) or left free (for glycopeptide 31). The resulting (glyco)peptides were cleaved from the resins by trifluoroacetic acid (TFA)/triisopropyl silane (TIPS)/H2O. C18 reverse phase HPLC purification produced the desired MUC1 (glyco)peptides 29–31 in 30–40% yields (Figure S2).
Scheme 3.
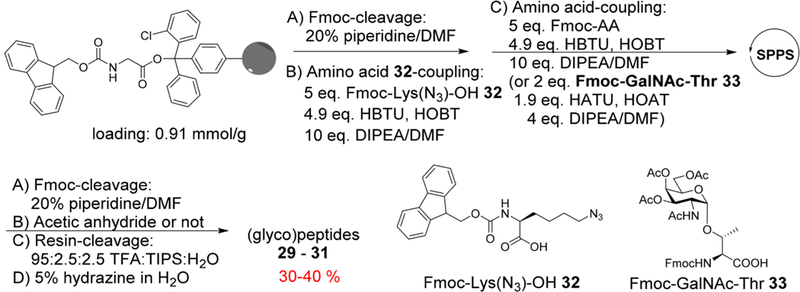
Solid Phase Synthesis of MUC1 (Glyco)peptides 29−31
The ligation ofMUCl 29–31 onto Qβ-VLP was performed with the CuAAC reaction.23 Azide-modified MUC1 29–31 were coupled with the alkyne-functionalized Qβ24 promoted by Cu2+ catalyst and ligand 34 (Scheme 4). The numbers of (glyco)peptides introduced onto each Qβ capsid were determined by mass spectrometry analysis of peak intensities of the modified Qβ conjugates vs those of the unmodified subunits,13 which were 270 on average for these conjugates (Figures S3-S5). The unreacted alkyne groups on Qβ capsids were capped using an excess of 3-azido 1-propanol 38 by a second CuAAC reaction.
Scheme 4.
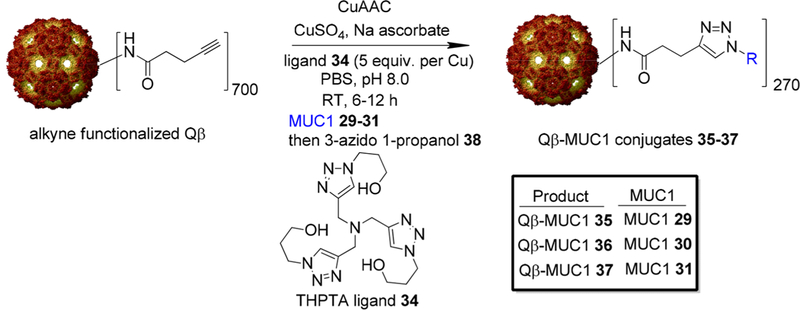
Synthesis of Qβ-MUC1 Conjugates 35−37
With Qβ-MUC1 conjugates 35–37 in hand, immunization of MUC1.Tg mice was performed. As ELISA titers did not reflect well tumor cells binding with the first generation vaccine, the analysis of postimmune sera primarily focused on flow cytometry. Compared to the first generation vaccines 5–8, both Qβ-MUC1 36 and 37 elicited antibodies with significantly higher binding to tumor cells (Figure 3a,b). Similar bindings to tumor cells by sera from 36 and 37 immunization (Figure 3a,b) were observed, suggesting the amino group of N-terminus ofMUC1 (glyco)peptides can be either free or protected as acetamide without affecting much the production of anti-MUC1 antibodies. Thus, connecting MUC1 peptide through the C-terminus could significantly enhance tumor cells binding by the induced antibodies.
Figure 3.
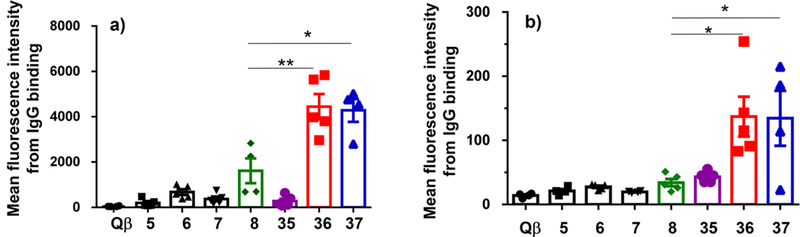
Flow cytometry analysis of anti-MUC1 IgG antibodies by various conjugates showed Qβ-MUC1 36 and 37 elicited antibodies with significantly higher binding to tumor cells. Binding to (a) Ag104-MUC1 cells; (b) B16-MUC1 cells was tested with 1:20 dilution of the corresponding serum. Each symbol represents one mouse (n = 3–5 mice for each group). *p < 0.05, **p < 0.01. The p values were determined through a two-tailed unpaired Student’s t-test using GraphPad Prism.
With enhanced tumor binding, the binding epitopes of mice immunized with the second generation vaccine were mapped using BSA-MUC1 9–28. As shown in Figure 4, significant binding to SAPDTRPA region was observed. Interestingly, while antibody binding to HGVTSAPD was much reduced, there were still significant levels of antibodies recognizing the APGSTAPP region, suggesting APGSTAPP is immunodominant, which may compete with SAPDTRPA for B cell recognition and activation.
Figure 4.
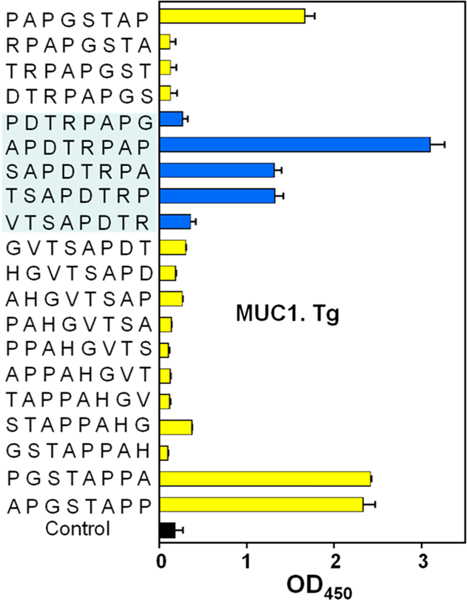
Epitope mapping of sera from Qβ-MUC1 37 immunized MUCl.Tg mice using BSA-MUC1 conjugates 9–28. Major epitopes recognized by the sera are in the APDTRPAP and APGSTAPP regions. The error bars represent standard deviation (SD) of four replicates.
Synthesis of Third Generation Qβ-MUC1 Conjugates 42–43 and KLH-MUC1 Conjugate 44.
To further focus the antibody responses on the desired region, for the third generation immunogen design, MUC1 peptide was shortened to remove both HGVTSAPD and APGSTAPP regions. In addition, in our prior studies of other carbohydrate based vaccines,25 we discovered that the triazole moiety in the linker formed through the CuAAC reaction was detrimental to antibody generation against the desired carbohydrate antigen. Thus, a flexible alkyl amide linker was selected to link MUC1 (glyco)peptides to Qβ carrier.
MUC1 peptide 38 and glycopeptide 39 were synthesized using SPPS starting from the p-nitrophenyl carbonate functionalized Wang resins, which were loaded with Fmoc-1,4-diaminobutane 40 first followed by peptide/glycopeptide elongation (Scheme 5a). After capping the N-terminus, deprotection, and cleavage from the resin, the (glyco)peptides were incubated with adipate bis(4-nitrophenyl) ester 41,26 producing MUC1 (glyco)peptides 38 and 39 (Figure S2).MUC1 (glyco)peptides 38 and 39 were then ligated with Qβ through amide bonds to give Qβ-MUC1 42 and 43 (Scheme 5b, Figures S6-S8).
Scheme 5.
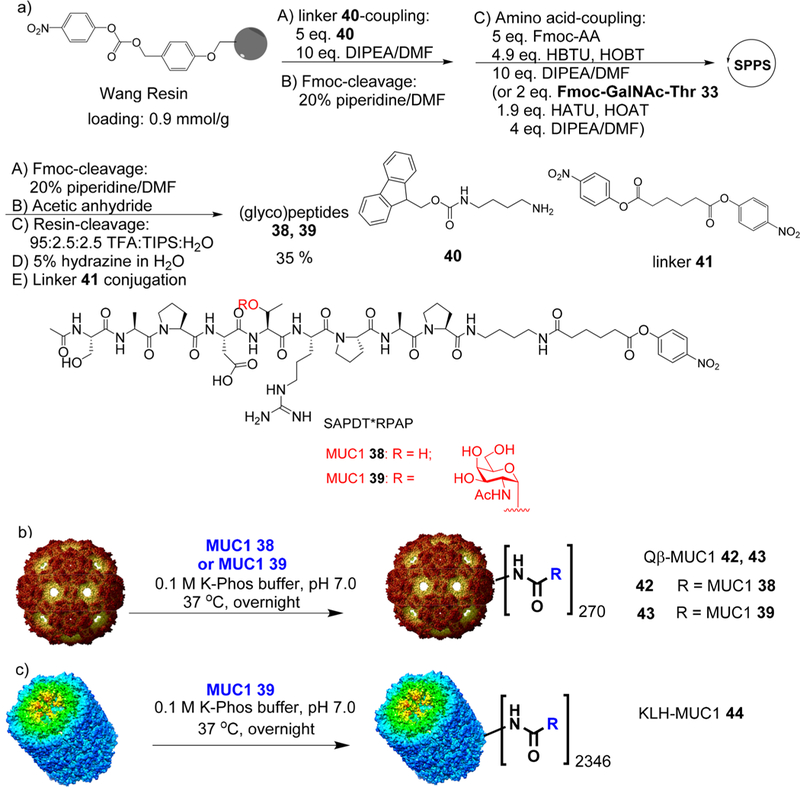
(a) Solid Phase Synthesis of MUC1 (Glyco)peptides 38 and 39; Synthesis of (b) Qβ-MUC1 Conjugates 42 and 43 and (c) KLH-MUC1 Conjugate 44
Keyhole limpet hemocyanin (KLH) has been the most popular protein carrier for carbohydrate based anticancer vaccine design, with multiple KLH conjugates of TACAs including Tn, GM2, GD2, Globo-H, and MUC1 evaluated in clinical trials.18,27–31 KLH-MUC1 conjugates are known to produce anti-MUC1 antibodies in human patients.18,19,32,33 To benchmark the performance of our construct, MUC1 39 was conjugated with KLH to give KLH-MUC1 44 (Scheme 5c), which contained an average of 2346 copies of MUC1-Tn per KLH.
Antibodies Induced by Qβ-MUC1 43 in MUC1.Tg Mice Showed the Strongest Binding to MUC1-Expressing Tumor Cells Compared to Qβ-MUC1 37 and KLH-MUC1 44.
MUC1.Tg mice were immunized with Qβ-MUC1 42 and 43 and KLH-MUC1 44 using CFA/IFA. When analyzed against MUC1 expressing B16-MUC1 cells and MCF-7 cells through flow cytometry, Qβ-MUC1 43 induced IgG antibodies in MUC1.Tg mice capable of binding much stronger with tumor cells than all other Qβ-MUC1 constructs including 37 (Figure 5a,b). This indicates removal of nonessential MUC1 epitopes from the immunogen significantly improved the quality of antibody responses. Furthermore, postimmune antibodies did not exhibit much recognition of a normal cell line MCF-10A (Figure 5c), suggesting good tumor selectivities by the antibodies. Compared to the conjugate 42, Qβ-MUC1 43 immunization induced antibodies in MUC1.Tg mice with much stronger tumor cell binding (Figures 5a,b), suggesting enhancement of the immunogenicity of MUC1 antigen via glycosylation. In addition, compared to KLH-MUC1 44, Qβ-MUC1 43 immunization induced ~3 times the IgG antibody titers (123,800 for Qβ-MUC1 43 vs 45,000 for KLH-MUC1 44) and 2 times higher tumor cell binding than those from KLH-MUC1 44 immunized MUC1.Tg mice (Figures 5a,b, S10, and S11). These results indicate that Qβ is better than KLH for anti-MUC1 antibody induction under the current setting and that Qβ-MUC1 43 is an excellent vaccine candidate for further evaluation.
Figure 5.
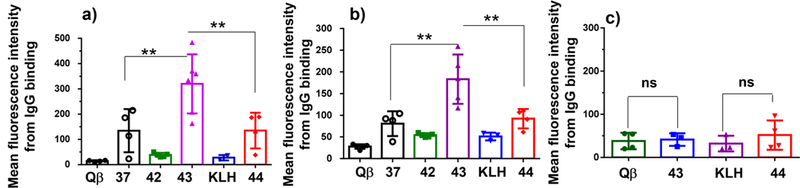
Results of flow cytometry analysis of cell binding by postimmune sera elicited by various conjugates. Mean fluorescence intensities of IgG antibody binding to (a) B16-MUC1 melanoma cells; (b) MCF-7 breast cancer cells; and (c) MCF-10A normal breast endothelial cells. The binding was tested with 1:20 dilution of the sera. Each symbol represents one mouse (n = 3–5 mice for each group). *p < 0.05, **p < 0.01, ***p < 0.001. The p values were determined through a two-tailed unpaired Student’s t-test using GraphPad Prism. ns: not significant.
Glycopeptide Microarray Results Confirmed MUC1-Tn Selectivity in Antibody Recognition.
To probe the glycan binding profile of induced antibodies, preimmune and postimmune sera from MUC1.Tg mice immunized with Qβ-MUC1 43, KLH-MUC1 44, or Qβ were screened against a MUC1 glycopeptide microarray.34 This glycopeptide array contained 72 MUC1 glycopeptides with the backbone sequence of one tandem repeat PAHGVTSAPDTRPAPGSTA. Glycans including Tn, T, and cores 1–4 were attached to various locations of the glycopeptides. In addition, mucin-5 (MUC5) glycopeptides13 and glycoproteins including fetuin, transferin, mucins from porcine stomach, and bovine submaxillary glands have been immobilized on the array (Figures S13 and S14). The slides were incubated with individual mouse serum. Following removal of unbound antibodies by thorough washing, a fluorescently labeled antimouse IgG secondary antibody was added to semiquantify the amounts of serum IgG antibodies bound to individual array components.
As can be seen from Figure S14, consistent with higher anti-MUC1 titers from ELISA, Qβ-MUC1 conjugate gave rise to much stronger array bindings on average compared to KLH-MUC1. The apparent dissociation constants of antisera induced by Qβ-MUC1 were 1 order of magnitude lower than those by KLH-MUC1 (Figure S14c). No cross-reactivities were observed to MUC5 glycopeptides or other glycoproteins highlighting MUC1 specificity of antibody responses.
Close examination of microarray data reveals interesting structural dependence of binding. Glycopeptides bearing Tn in its PDTR region were bound much stronger than those lacking glycosylation in this region. For example, glycopeptides 45–47 all contain the same protein backbone and one Tn, but the locations of Tn are different. Glycopeptide 46, which has Tn in its PDTR region, gave the strongest binding to postimmune sera than 45 and 47 (Figure 6a). Glycopeptides 48, 50, and 51 contain multiple Tns in the backbone including a Tn in its PDTR region. They were all recognized well by postimmune sera. Peptide 45 differed from 48 only in the Tn at the second threonine in the peptide backbone in the PDTR region. Yet, sera binding to 48 was more than five time as strong as those to 45. These results collectively suggest that the presence of Tn in the PDTR region is critical for antibody recognition and that induced antibodies have excellent site selectivities toward the PDT*R region contained in the immunizing antigen MUC1 39.
Figure 6.
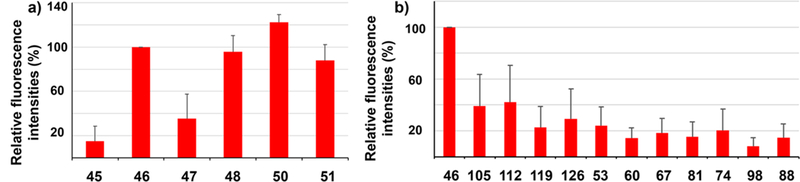
Representative results of MUC1 glycopeptide microarray screening of antisera from Qβ-MUC1 43 immunized mice. (a) Comparison of fluorescence intensities of microarray components containing MUC1 glycopeptides bearing Tn antigen at various locations showed that glycosylation at PDT*R region led to strongest recognition by postimmune sera. Glycopeptide 45, PAHGVT*SAPDTRPAPGSTA; 46, PAHGVTSAPDT*RPAPGSTA; 47, PAHGVTSAPDTRPAPGST*A; 48, PAHGVT*SAPDT*RPAPGSTA; 50, PAHGVTSAPDT*RPAPGST*A; 51, PAHGVT*SAPDT*RPAPGST*A. (b) Comparison of fluorescence intensities of microarray components containing MUC1 glycopeptides bearing various glycans at PAHGVTSAPDT*RPAPGSTA showed that, while Tn gave the strongest recognition, other glycans can be recognized as well. Glycan structures: glycopeptide 46, Tn; 105, C3T1 (for abbreviations and structures, see Figure S14); 112, C3T2; 119, C4T1; 126, C4T2; 53, T; 60, C1T1; 67, C1T2; 81, C2T1he; 74, C2T1te; 98, C2T2he; 88, C2T2te. The error bars represent standard deviation (SD) of eight replicates.
Comparison of PAHGVTSAPDT*RPAPGSTA with varying glycan structures showed that, while the Tn bearing glycopeptide 46 was bound the strongest, glycopeptides with other glycans ranging from disaccharide T to core 4 pentasaccharide could be recognized as well, indicating a wide repertoire of anti-MUC1 antibodies were induced presumably through binding with the Tn core (Figure 6b). As glycosylation of tumor associated MUC1 can be heterogeneous,35–37 the abilities of Qβ-MUC1 43-induced antibodies to recognize multiple glycopeptides bode well for tumor recognition.
Antibodies Induced by Qβ-MUC1 43 Exhibited Good Tumoricidal Activities via Both Complement Mediated Cytotoxicity (CDC) and Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) Mechanisms.
With the strong tumor recognition by sera from Qβ-MUC1 43 immunized mice, their abilities to kill the tumor cells were measured in vitro. Upon incubation of B16-MUC1 cells (Figure 7a) and MCF-7 cells (Figure 7b) with postimmune sera and rabbit complement, significantly higher percentages of tumor cells were killed by Qβ-MUC1 43 immunized sera as compared to cells treated with other sera. Tn glycosylation of MUC1 significantly enhanced the CDC potency of the postimmune sera (43 vs 42).
Figure 7.
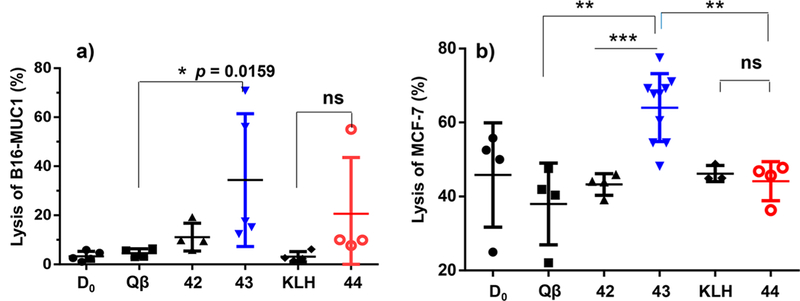
Qβ-MUC1 43 exhibited significantly higher CDC toward tumor cells. CDC toward (a) B16-MUC1 cells or (b) MCF-7 cells was determined by MTS assay. D0 values are the preimmune sera. Each symbol represents one mouse (n = 3−5 mice for each group). *p < 0.05, **p < 0.01, ***p < 0.001. The p values were determined through a two-tailed nonparametric t test using GraphPad Prism. ns: not significant.
ADCC is another important mode of tumor cell killing bestowed by antibodies. An ADCC assay was set up using either natural killer (NK) cells or lymphokine-activated killer (LAK) cells as the effectors cells against MUC1 expressing B16-MUC1 target cells via a chromium release assay.38 As shown in Figure 8, under a variety of target vs effector cell ratios, stronger cytotoxicities were observed from sera of Qβ-MUC1 43 immunized mice in contrast to control sera from Qβ immunized mice.
Figure 8.
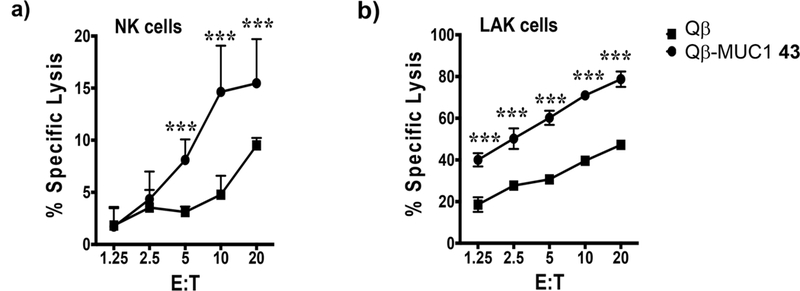
ADCC of B16-MUC1 target cells is increased in the presence of Qβ-MUC1 43 antisera. B16-MUC1 target cells (T) were radiolabeled with 100 μCi of 51Cr and pulsed with 40 μL of Qβ or Qβ-MUC1 43 antisera simultaneously for 2 h at 37 °C. Target cells were washed and plated either with freshly isolated (a) NK or (b) LAK cells (effectors, E) at various E/T ratios. After 16 h, the culture supernatant was harvested, and the specific lysis was analyzed using a gamma counter. Significance was determined by two-way ANOVA with Tukey’s posthoc test. ***p < 0.001.
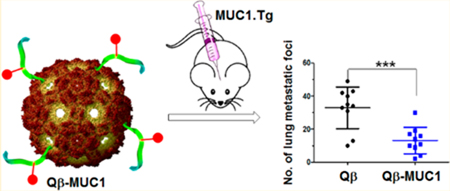
Vaccination of Qβ-MUC1 43 Exhibited Significant Tumor Protection in a Metastasis Model.
With high levels of IgG elicited by Qβ-MUC1 43 and strong tumor binding, we tested tumor protection in a metastasis model as tumor metastasis is a major hurdle to patient survival. MUCl.Tg mice were immunized with Qβ-MUC1 43 or Qβ (the control) with the FDA approved monophosphoryl lipid A (MPLA) as the adjuvant due to its minimal adverse effects on immunized hosts. B16-MUC1 melanoma cells were injected via tail vein, and the numbers of tumor foci in lungs were determined 21 days after tumor inoculation. Excitingly, Qβ-MUC1 43 brought a notable reduction in tumor load (p = 0.0006) vs Qβ control (Figure 9). The efficacy of KLH-MUC1 44 was also evaluated in this model. Immunization with KLH-MUC1 44 exhibited some tumor protection but was significantly less effective than Qβ-MUC1 43 in reducing tumor load (p = 0.037) (Figure 9), confirming that Qβ is superior as a carrier compared to KLH.
Figure 9.
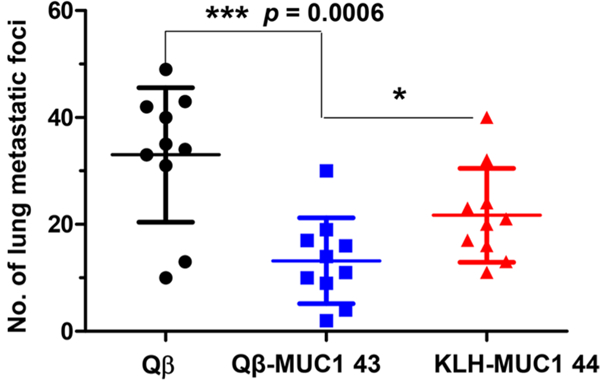
Immunization of Qβ-MUC1 43 significantly protected MUC1.Tg mice from formation of metastatic-like lung tumors. MUC1.Tg mice were immunized respectively with Qβ, Qβ-MUC1 43, or KLH-MUC1 44 on days 0, 14, and 28 plus MPLA as adjuvant, challenged with 1 X 105 B16-MUC1 cells on day 35 via tail vein injection, followed by a 4th immunization, and then given a 5th immunization on day 45. Twenty-one days after tumor inoculation, mice were sacrificed and the number of tumor foci in the lungs were counted. Qβ-MUC1 43 vaccination significantly reduced the number of tumor compared to control animals receiving Qβ or KLH-MUC1 44 immunization. Each symbol represents one mouse (n = 10 mice for each group). *p < 0.05, **p < 0.01, ***p < 0.001. The p values are determined through two-way ANOVA with Bonferroni post-test using GraphPad Prism.
Vaccination of Qβ-MUC1 43 Provided Significant Tumor Protection in a Solid Tumor Model.
With the successful protection of MUC1.Tg mice from metastatic tumors with Qβ-MUC1 43, a solid tumor model was also tested. Solid tumor is known to be difficult to treat, as there can be significant immunosuppression within the tumor microenvironment. Programmed cell death 1 (PD-1) and its ligand (PD-L1) are important inhibitory checkpoint molecules,39 and PD-1 plays a major role in suppressing anti-TACA antibody responses.40 As it is known that anti-PD-1 mAbs can neutralize the functions of PD-1 and aid in the efficacy of vaccines,41 we combined anti-PD-1 mAb with immunization for protection against solid tumor. MUC1.Tg mice were immunized with Qβ-MUC1 43 or Qβ (the control) and MPLA adjuvant. B16-MUC1 melanoma cells were then grafted subcutaneously, which were followed by further immunization and anti-PD-1 mAb administrations. Excitingly, the group receiving Qβ-MUC1 43 immunization had much reduced tumor growth compared to the control group (Figure 10).
Figure 10.
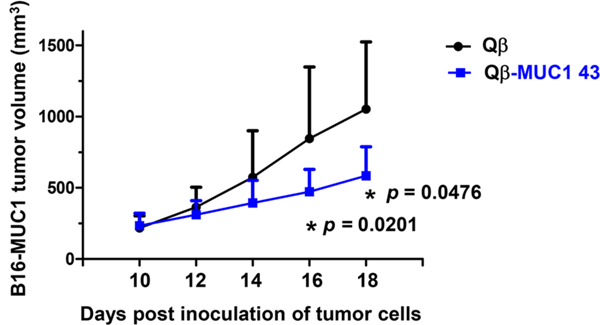
Qβ-MUC1 43 immunization regressed solid tumor growth compared to the control group receiving Qβ. MUC1.Tg mice were immunized subcutaneously with Qß or Qß-MUC1 43 on days −35, −21, and —7 with MPLA, challenged with 5 X 105 B16-MUC1 cells subcutaneously on day 0, followed by a 4th (day 0) and a 5th subcutaneous immunization (day 7), and anti-PD1 injections on days 8 and 11 via intraperitoneal injections. On day 14, the mice were given the sixth immunization with Qβ or Qβ-MUC1 43, followed by anti-PD1 treatments on days 15 and 18 (n = 9 mice for each group). *p < 0.05. The p values are determined through two-way ANOVA with Bonferroni post-test using GraphPad Prism.
High Selectivity in Binding to Human Breast Cancer vs Normal Tissues by Qβ-MUC1 43 Induced Sera in MUC1.Tg Mice.
To test the translational potential of Qβ-MUC1 43 to human patients, we obtained breast cancer tissue microarrays containing cancer tissues immobilized together with normal adjacent breast tissues from the same patient on the array. Sera from Qβ-MUC1 43 immunized MUC1.Tg mice were used to stain the tissue microarrays, which bound strongly with breast cancer tissues (Figure 11a). In stark contrast, little normal breast tissues were stained under the same condition (Figure 11b). This highlights the remarkable selectivity of the antibodies induced in recognition of MUC1 expressing human cancer tissues.
Figure 11.
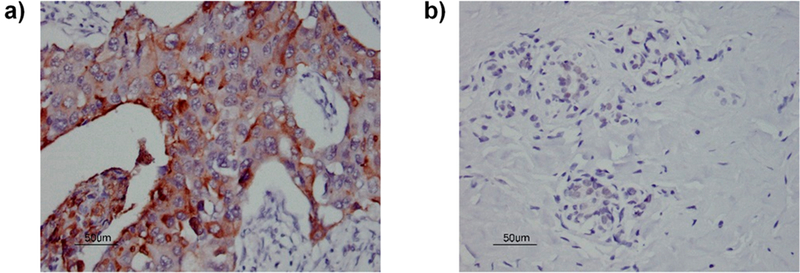
Sera from MUC1.Tg mice immunized with Qβ-MUC1 43 exhibited (a) strong binding to human breast cancer tissues on a tissue microarray while having little reaction with (b) normal breast tissues (1:1000 serum dilution). For other tissue slices, see Figure S15. The brown color in panel (a) was due to antibody binding to tissues. The lack of brown staining in panel (b) indicates little binding of antibodies to normal tissues. Scale bar is 50 μm.
DISCUSSION
MUC1 is a highly attractive target for anticancer immunotherapy development.4,7 Many preclinical evaluations of MUC1 based vaccine constructs have been performed in WT mice.42–50 However, with the low homology between human MUC1 and mouse mucins, WT mice do not capitulate well the tolerant environment in humans toward MUC1. MUC1.Tg mice endogenously express human MUC1 as a selfprotein on normal nontransformed epithelia.15 As a result, MUC1 is centrally tolerated, as the immune cells capable of binding strongly to the natively exposed MUC1 epitopes are largely eliminated due to negative selection.51 The MUC1 immune tolerance in MUC1.Tg mice leads to great difficulties in eliciting effective antibody responses against the protective MUC1 epitopes.52–54 With our first generation vaccine construct Qβ-MUC1 5–8, we observed significantly weaker binding of MUC1 expressing tumor cells by postimmune sera from Tg mice compared to those from WT mice despite similar levels of total antibodies elicited against the immunizing MUC1 structures 1–4. Similar phenomena of low tumor cell binding were observed in clinical studies of MUC1 based vaccines in human patients.17–19 Thus, the protective epitopes from MUC1 need to be better understood to produce IgG antibodies in immunotolerant environment capable of recognizing tumor cells strongly.
MUC1 contains 20–120 copies of the 20 amino acid tandem repeat region HGVSTAPDTRPAPGSTAPPA. With its large size, many possible MUC1 sequences have been evaluated for vaccine design.7–9 However, on cancer cell surface, not all epitopes of MUC1 are exposed for antibody recognition. If such nonprotective epitopes are included in the immunogen, they may compete with the protective ones for antibody production, which reduces the efficacy of tumor recognition. In humans, the most frequent epitopic sequences of naturally occurring anti-MUC1 antibodies were RPAPGS, followed by PPAHGVT and PDTRP.55 Epitopes for vaccine-induced anti-MUC1 antibodies include STAPPAHG, PAPG-STAP, HGVTSA, and APDTRPA.17,18 In MUC1.Tg mice, epitope scanning of sera from mice immunized with Qβ-MUC1 5 (Figure S9) showed main epitopes in HGVTSA and GSTAPPA regions, while serum binding to MUC1 expressing tumor cells was weak. From these results alone, we cannot distinguish whether the low tumor cell binding was due to insufficient antibody generation against HGVTSA/GSTAPPA or if the binding epitopes reside in other regions of MUC1.
The synthetic MUC1 peptide array coupled with the availability of sera from WT and Tg mice immunized with the same MUC1 antigen provided unique opportunities for discovery of protective epitopes with our Qβ carrier. Comparison of WT and Tg mouse sera showed similar affinities to HGVTSA and GSTAPPA regions, and the largest distinctions in peptide binding profile were to the SAPDTR-PAP area. These indicate SAPDTRPAP may be the critical protective epitope of MUC1 for the current vaccine design. While HGVTSA and GSTAPPA are immunodominant, they may not be well exposed on cancer cells, possibly explaining why sera from Tg mice immunized with first generation Qβ-MUC1 constructs did not exhibit strong binding to cancer cells.
The presence of nonessential epitopes in an immunogen can compete with desired epitopes. This is especially the case in immunotolerant environment, as there are low frequencies of the endogenous naive B cells specific to the protective antigens due to negative selection.51 Other B cells may outcompete the low frequency B cells for the limited number of helper T cells for cytokine signals, critical for B cell activation and IgG production.56 In addition, antibodies binding to the nonessential epitopes may sterically block the adjacent protective epitope from being recognized by B cells.25 Thus, to help the immune system focus on the desired protective epitopes, it is desirable to remove the nonessential epitopes from the immunogen. These considerations led to the design of shortened MUC1 peptide SAPDT*RPAP as the antigen. Indeed, the new Qβ-MUC1 immunogen 43 produced IgG antibodies with significant enhancements in binding of MUC1 expressing cancer cells, validating our design.
MUC1 is typically glycosylated.35–37 In WT mice, both MUC1 peptides and the corresponding Tn bearing glycopep-tides were effective in inducing anti-MUC1 antibodies for strong tumor cell binding.13 However, in Tg mice, significant differences were observed between MUC1 peptides and glycopeptides. The glycopeptides elicited IgG antibodies capable of binding much stronger with MUC1 expressing tumor cells (Figures 3 and 5). Furthermore, antibodies against the glycopeptide induced in Tg mice were more effective in killing tumor cells via CDC. Glycosylation can significantly enhance the immunogenicity of MUC1 antigen. This is presumably because glycans can lead to conformational changes of the glycopeptide, rendering it more “foreign” to the immune system.57,58
For vaccine development, it is known that the administration of MUC1 alone cannot elicit sufficient anti-MUC1 antibodies. A variety of carriers has been investigated to conjugate with MUC1 for better potentiation of the humoral responses. These include immunogenic proteins such as KLH18,19,32,33,59 and tetanus toxoid (tt),46,54 nanoparticles,42,50 polymers,48 immunostimulating glycans,49,60 liposomes,61,62 and synthetic carriers.43–45,47 Among these carriers, KLH has been the most popular for TACA-based anticancer vaccines with KLH constructs tested in clinical trials.18,27–31 Compared to KLH, bacteriophage Qβ can have several advantages. Qβ has a highly organized 3D structure63 in contrast to the heterogeneous structure of KLH. Organized display of self-antigens 64,65 such as TACAs can help overcome tolerance and powerfully boost antibody responses. In addition, Qβ contains epitopes for CD4+ helper T cells.66 Although the number of MUC1 specific helper T cells are likely to be significantly reduced in MUC1.Tg mice due to central tolerance, Qβ can provide the requisite stimulatory signals to B cells inducing isotype switching to produce IgG antibodies. Qβ also encapsulates nucleic acid in its interior, which can function as an endogenous adjuvant for immune-potentiation.67 Head-to-head comparison of Qβ-MUC1 with KLH-MUC1 showed that Qβ-MUC1 was able to elicit anti-MUC1 IgG antibodies with much stronger binding with MUC1 expressing tumor cells (Figures 5).
CDC is an important mechanism of antibody cytotoxicities against cancers. Effective CDC depends partly on the class/ subclass of antibodies and their complement-fixing abilities. MUC1 is a large protein, that can extend up to 5000 A from the cell surface. The long distance for the activated complement component to travel from the antibody binding site to tumor cell surface can pose a significant challenge for CDC. In clinical studies, Livingston and co-workers reported no CDC toward MCF-7 breast cancer cells from patients immunized with KLH-MUC1 construct.68 This result is recapitulated in our study, as sera from MUC1.Tg mice immunized with KLH-MUC1 failed to kill more MCF-7 cells in CDC assays compared to control sera from KLH immunized mice (Figure 7b). The low CDC activities may be overcome by higher levels of desired anti-MUC1 antibodies. Indeed, sera from Qβ-MUC1 43 immunized Tg mice induced more potent killing of MCF-7 cells in CDC assay compared to those from Qβ or KLH-MUC1 mice (Figure 7b). The CDC activities were not limited to MCF-7 cells as significant CDC was observed against B16-MUC1 as well. Stronger tumor cell binding, enhanced tumoricidal activities, and significantly better tumor protection by Qβ-MUC1 immunized mice suggest Qβ is a superior carrier compared to KLH.
A key requirement for a successful antitumor vaccine is that the induced antibodies have high selectivities toward tumor cells while sparing normal cells. IgG antibodies elicited by Qβ-MUC1 43 had good binding selectivities to breast cancer tissues by immuno-histostaining human breast cancer tissues over normal breast tissues on a tissue microarray (Figure 11). It was consistent with the FACS analysis of antibodies induced by Qβ-MUC1 43, which reacted well with many tumor-associated MUC1-expressing cancer cells while exhibiting little binding to normal cells (Figure 5c). The excellent tumor selectivity may be partly attributed to higher MUC1 density on tumor cells, and different glycan structures on MUC1 from normal vs cancer cells, as MUC1 on normal cells, bear much longer carbohydrate chains-potentially shielding peptide chains for immune recognition.35–37
The current study focuses on the discovery of a protective epitope from MUC1, a prototypical tumor-associated antigen. The peptide epitope array coupled with comparison of postimmune sera from WT and Tg mice yielded critical insights on the sequence of the protective epitope, which provided extremely valuable guidance toward the design of effective immunogen for successful tumor protections. Similar to MUC1, there are other tumor-associated antigens such as mucin-16 (CA125)69 and HER2/neu,70 which are immunoto-lerant in humans but not in wild type mice. Thus, the WT and Tg mouse epitope profiling strategy developed here for MUC1 can be potentially applied to a range of anticancer vaccines.
A limitation of the current study is that, while MUC1.Tg mice utilized are transgenic for human MUC1, they are not transgenic for human leukocyte antigens. High titers of IgG antibodies were induced by Qβ-MUC1 43 in MUC1.Tg mice, suggesting that helper T cells were activated by vaccination, resulting in antibody isotype switching to IgGs. T cell responses to Qβ-MUC1 43 in humans may vary vs those in mice due to different epitope preferences for major histocompatibility complexes. However, Qβ constructs with peptide antigens have been tested in human trials, which have induced robust activation of Qβ specific helper T cells and IgG antibodies.66 Thus, while further studies are needed, it is probable that Qβ-MUC1 43 can translate well in humans.
In conclusion, due to the immunotolerance of MUC1 in humans, it is challenging to develop MUC1-based immunogen as an effective anticancer vaccine. With the large tandem repeat sequences in MUC1, there are many possibilities in MUC1-based immunogen design. While our first generation constructs bearing one full tandem repeat region generated potent IgG antibodies for tumor cells binding in wild type mice, antibodies induced by the same construct in immunotolerant MUC1.Tg mice only bound weakly with tumor cells. To overcome this challenge in vaccine design, a library of MUC1 octapeptides were synthesized covering all possible sequences of the tandem repeat regions. Sera from immunized WT and MUC1.Tg mice were screened based on these epitopes, which provided critical insights into the protective epitope sequence.
Centering on the crucial protective epitope and removing immunodominant yet nonprotective epitope led to the design of Qβ-MUC1 43. This new construct was able to elicit powerful IgG antibody responses, which caused tumor cell death by CDC and ADCC in vitro. Furthermore, vaccination of MUC1.Tg mice brought significant tumor reductions in both metastatic and solid tumor models. The postimmune sera exhibited superb selectivities toward tumor over normal tissues, laying the ground for translating Qβ-MUC1 43 into clinics as a promising anticancer candidate.
METHODS
Mouse Immunization.
MUC1.Tg mice were generated by breeding MUC1.Tg male mice containing a 10.6 kb genomic Sac II fragment of the human MUC1 gene with C57BL/6 wild type female mice with approximately 50% yield of MUC1.Tg mice. MUC1.Tg mice aged 6–10 weeks were maintained in the University Laboratory Animal Resources facility of Michigan State University and used for studies. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Michigan State University. While the majority of mice utilized were female, comparison of immunological responses showed that male and female MUC1.Tg mice did not give significant differences in immunological results.
In all studies except for the cancer immunotherapy studies, MUC1.Tg mice were subcutaneously injected under the scruff on day 0 with 0.1 mL of various Qβ-MUC1 vaccines as emulsions in Complete Freund’s Adjuvant according to the manufacturer’s instructions. Boosters were given subcutaneously under the scruff on days 14 and 28 mixed with Incomplete Freund’s Adjuvant. For cancer immunotherapy studies, MPLA was used as the adjuvant for immunization. The identity of the adjuvant does not affect the immune responses much (Figure S12). All MUC1 vaccine conjugates administered have the same amounts of GalNAc (1.9 μg). Sera samples were collected on days 0 (before immunization), 7, and 35. The final bleeding was done by cardiac bleed.
Cancer Immunotherapy Studies.
For the lung metastasis model, pathogen-free MUC1.Tg female mice aged 6–10 weeks were subcutaneously injected under the scruff on day 0 with 0.2 mL of Qβ-MUC1 43, KLH-MUC1 44, or Qβ in PBS mixed with MPLA (20 μL, 1 mg mL−1 in DMSO). Boosters were given subcutaneously under the scruff on days 14 and 28 mixed with MPLA. On day 35, vaccinated mice were challenged by intravenous injection of 1 X 105 B16-MUC1 cells per mouse, followed by fourth immunization of conjugates mixed with MPLA. On day 45, the mice were given a 5th immunization of vaccines mixed with MPLA. On day 56, pulmonary metastases were enumerated by intratracheal injection of black ink (50% in PBS). Black-ink-injected lungs were washed in Feket’s solution (300 mL of 70% EtOH, 30 mL of 37% formaldehyde, and 5 mL of glacial acetic acid) and then placed in fresh Feket’s solution overnight. White tumor nodules against a black lung background were then counted.
For the solid tumor model, pathogen-free MUC1.Tg female mice aged 6–10 weeks were immunized with Qβ or Qβ-MUC1 43 on days −35, −21, and −7 plus MPLA as an adjuvant, challenged with 5 X 105 B16-MUC1 cells on day 0 by subcutaneous inoculation, followed by a 4th immunization and then given a 5th immunization on day 7, followed by anti-PD1 (100 μg per mouse, clone: RMP1–14, BioXcell) treatment on days 8 and 11 via intraperitoneal injection. On day 14, the mice were given a 6th immunization of Qβ or Qβ-MUC1, followed by anti-PD1 (100 μg per mouse) treatment on days 15 and 18. Tumor volume was determined by the formula: volume (mm3) = 1/2 (length X width X height).71
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the National Cancer Institute, National Institutes of Health (Grant R01 CA225105), Michigan State University Foundation Strategic Partnership Grant program, the Deutsche Forschungsgemeinschaft, DFG (WE 4751/2–1), Fonds der Chemischen Industrie (Liebig fellowship to UW, Li 184/01), Ministerium fur Kultur und Wissenschaft des Landes Nordrhein-Westfalen, and the Bundesministerium fur Bildung und Forschung for financial support of our work. We would like to thank Profs. O. J. Finn (Univ. of Pittsburgh) and S. J. Gendler (Mayo Clinic) for kindly providing us the cells as well as the MUC1.Tg mice, and Prof. H. Chang (Michigan State Univ.) for histological analysis of the tissue microarray
Footnotes
ASSOCIATED CONTENT
Ⓢ Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b08473.
Detailed experimental procedures and Supplementary Figures S1-S15 (PDF)
Notes
The authors declare the following competing financial interest(s): X.H. is the founder of Iaso Therapeutics, which is dedicated to the development of next generation carbohydrate and glyco-conjugate based anti-cancer vaccines.
REFERENCES
- (1).Gilboa E The Promise of Cancer Vaccines. Nat. Rev. Cancer 2004, 4, 401. [DOI] [PubMed] [Google Scholar]
- (2).Melero I; Gaudernack G; Gerritsen W; Huber C; Parmiani G; Scholl S; Thatcher N; Wagstaff J; Zielinski C; Faulkner I; Mellstedt H Therapeutic Vaccines for Cancer: an Overview of Clinical Trials. Nat. Rev. Clin. Oncol. 2014, 11, 509. [DOI] [PubMed] [Google Scholar]
- (3).Apostolopoulos V; Hu XF; Pouniotis DS; Xing PX MUC1: a Molecule of Many Talents. Curr. Trends Immunol. 2004, 12, 629. [Google Scholar]
- (4).Cheever M; Allison J; Ferris A; Finn O; Hastings B; Hecht T; Mellman I; Prindiville S; Viner J; Weiner L; Matrisian L The Prioritization of Cancer Antigens: a National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hirasawa Y; Kohno N; Yokoyama A; Kondo K; Hiwada K; Miyake M Natural Autoantibody to MUC1 is a Prognostic Indicator for Non-small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2000, 161, 589. [DOI] [PubMed] [Google Scholar]
- (6).Hamanaka Y; Suehiro Y; Fukui M; Shikichi K; Imai K; Hinoda Y Circulating Anti-MUC1 IgG Antibodies as a Favorable Prognostic Factor for Pancreatic Cancer. Int. J. Cancer 2003, 103, 97. [DOI] [PubMed] [Google Scholar]
- (7).Hossain MK; Wall KA Immunological Evaluation of Recent MUC1 Glycopeptide Cancer Vaccines. Vaccines 2016, 4, 25 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gaidzik N; Westerlind U; Kunz H The Development of Synthetic Antitumour Vaccines from Mucin Glycopeptide Antigens. Chem. Soc. Rev. 2013, 42, 4421. [DOI] [PubMed] [Google Scholar]
- (9).von Mensdorff-Pouilly S; Moreno M; Verheijen RHM Natural and Induced Humoral Responses to MUC1. Cancers 2011, 3, 3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wrightsman RA; Dawson BD; Fouts DL; Manning JE Identification of Immunodominant Epitopes in Trypanosoma Cruzi Trypomastigote Surface Antigen-1 Protein that Mask Protective Epitopes. J. Immunol. 1994, 153, 3148. [PubMed] [Google Scholar]
- (11).Guo H; Zhou EM; Sun ZF; Meng XJ Immunodominant Epitopes Mapped by Synthetic Peptides on the Capsid Protein of Avian Hepatitis E Virus are Non-protective. Viral Immunol. 2008, 21, 61. [DOI] [PubMed] [Google Scholar]
- (12).Novotny LA; Bakaletz LO The Fourth Surface-Exposed Region of the Outer Membrane Protein P5-Homologous Adhesin of Nontypable Haemophilus influenzae Is an Immunodominant But Nonprotective Decoying Epitope. J. Immunol. 2003, 171, 1978. [DOI] [PubMed] [Google Scholar]
- (13).Yin Z; Wu X; Kaczanowska K; Sungsuwan S; Comellas-Aragones M; Pett C; Yu J; Baniel C; Westerlind U; Finn MG; Huang X Antitumor Humoral and T Cell Responses by Mucin-1 Conjugates of Bacteriophage in Wild-type Mice. ACS Chem. Biol. 2018, 13, 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Spicer AP; Parry G; Patton S; Gendler SJ Molecular Cloning and Analysis of the Mouse Homologue of the Tumor-associated Mucin, MUC1, Reveals Conservation of Potential O-Glycosylation Sites, Transmembrane, and Cytoplasmic Domains and a Loss of Minisatellite-like Polymorphism. J. Biol. Chem. 1991, 266, 15099. [PubMed] [Google Scholar]
- (15).Rowse GJ; Tempero RM; VanLith ML; Hollingsworth MA; Gendler SJ Tolerance and Immunity to MUC1 in a Human MUC1 Transgenic Murine Model. Cancer Res. 1998, 58, 315. [PubMed] [Google Scholar]
- (16).Tempero RM; VanLith ML; Morikane K; Rowse GJ; Gendler SJ; Hollingsworth MA CD4+ Lymphocytes Provide MUC1-specific Tumor Immunity in vivo that is Undetectable in vitro and is Absent in MUC1 Transgenic Mice. J. Immunol. 1998, 161, 5500. [PubMed] [Google Scholar]
- (17).Karanikas V; Hwang L; Pearson J; Ong C; Apostolopoulos V; Vaughan H; Xing P; Jamieson G; Pietersz G; Tait B; Broadbent R; Thynne G; McKenzie I Antibody and T Cell Responses of Patients with Adenocarcinoma Immunized with Mannan-MUC1 Fusion Protein. J. Clin. Invest. 1997, 100, 2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Adluri S; Gilewski T; Zhang S; Ramnath V; Ragupathi G; Livingston PO Specificity Analysis of Sera from Breast Cancer Patients Vaccinated with MUC1-KLH plus QS-21. Br. J. Cancer 1999, 79, 1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Gilewski T; Adluri S; Ragupathi G; Zhang S; Yao TJ; Panageas KS; Moynahan M; Houghton A; Norton L; Livingston PO Vaccination of High-risk Breast Cancer Patients with Mucin-1 (MUC1) Keyhole Limpet Hemocyanin Conjugate plus QS-21. Clin. Cancer Res. 2000, 6, 1693. [PubMed] [Google Scholar]
- (20).Westerlind U; Schroder H; Hobel A; Gaidzik N; Kaiser A; Niemeyer CM; Schmitt E; Waldmann H; Kunz H Synthetic Vaccines Consisting of Tumor-associated MUC1 Glycopeptide Antigens and a T-cell epitope for the Induction of a Highly Specific Humoral Immune Response. Angew. Chem., Int. Ed. 2009, 48, 8263. [DOI] [PubMed] [Google Scholar]
- (21).Glaffig M; Stergiou N; Hartmann S; Schmitt E; Kunz H A Synthetic MUC1 Anticancer Vaccine Containing Mannose Ligands for Targeting Macrophages and Dendritic Cells. ChemMedChem 2018, 13, 25. [DOI] [PubMed] [Google Scholar]
- (22).Paulsen H; Adermann K Synthese von O-Glycopeptid-Sequenzen des N-Terminus von Interleukin-2. Liebigs Ann. Chem. 1989, 1989, 751. [Google Scholar]
- (23).Hong V; Presolski SI; Ma C; Finn MG Analysis and Optimization of Copper-catalyzed Azide-alkyne Cycloaddition for Bioconjugation. Angew. Chem., Int. Ed. 2009, 48, 9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yin Z; Comellas-Aragones M; Chowdhury S; Bentley P; Kaczanowska K; BenMohamed L; Gildersleeve JC; Finn MG; Huang X Boosting Immunity to Small Tumor-associated Carbohydrates with Bacteriophage Q^ Capsids. ACS Chem. Biol. 2013, 8, 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yin Z; Wright WS; McKay C; Baniel C; Kaczanowska K; Bentley P; Gildersleeve JC; Finn MG; BenMohamed L; Huang X Significant Impact of Immunogen Design on the Diversity of Antibodies Generated by Carbohydrate-based Anti-cancer Vaccine. ACS Chem. Biol. 2015, 10, 2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wu X; Ling C-C; Bundle D R A New Homobifunctional p-Nitro Phenyl Ester Coupling Reagent for the Preparation of Neoglycoproteins. Org. Lett. 2004, 6, 4407. [DOI] [PubMed] [Google Scholar]
- (27).Eggermont AMM; Suciu S; Rutkowski P; Marsden J; Santinami M; Corrie P; Aamdal S; Ascierto PA; Patel PM; Kruit WH; Bastholt L; Borgognoni L; Bernengo MG; Davidson N; Polders L; Praet M; Spatz A Adjuvant Ganglioside GM2-KLH/QS-21 Vaccination Versus Observation after Resection of Primary Tumor > 1.5 mm in Patients with Stage II Melanoma: Results of the EORTC 18961 Randomized Phase III Trial. J. Clin. Oncol. 2013, 31, 3831. [DOI] [PubMed] [Google Scholar]
- (28).Miles D; Roche H; Martin M; Perren TJ; Cameron DA; Glaspy J; Dodwell D; Parker J; Mayordomo J; Tres A; Murray JL; Ibrahim NK; Group TS Phase III Multicenter Clinical Trial of the Sialyl-Tn (STn)-Keyhole Limpet Hemocyanin (KLH) Vaccine for Metastatic Breast Cancer. Oncologist 2011, 16, 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhu J; Wan Q; Lee D; Yang G; Spassova M; Ouerfelli O; Ragupathi G; Damani P; Livingston P; Danishefsky S From Synthesis to Biologics: Preclinical Data on a Chemistry Derived Anticancer Vaccine. J. Am. Chem. Soc. 2009, 131, 9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sabbatini PJ; Kudryashov V; Ragupathi G; Danishefsky SJ; Livingston PO; Bornmann W; Spassova M; Zatorski A; Spriggs D; Aghajanian C; Soignet S; Peyton M; O’Flaherty C; Curtin J; Lloyd KO Immunization of Ovarian Cancer Patients with a Synthetic Lewis(y)-protein Conjugate Vaccine: a Phase 1 Trial. Int. J. Cancer 2000, 87, 79. [PubMed] [Google Scholar]
- (31).Danishefsky SJ; Allen JR From the Laboratory to the Clinic: a Retrospective on Fully Synthetic Carbohydrate-based Anticancer Vaccines. Angew. Chem., Int. Ed. 2000, 39, 836. [DOI] [PubMed] [Google Scholar]
- (32).Sabbatini PJ; Ragupathi G; Hood C; Aghajanian CA; Juretzka M; Iasonos A; Hensley ML; Spassova MK; Ouerfelli O; Spriggs DR; Tew WP; Konner J; Clausen H; Abu Rustum N; Dansihefsky SJ; Livingston PO Pilot Study of a Heptavalent Vaccine-Keyhole Limpet Hemocyanin Conjugate plus QS21 in Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer. Clin. Cancer Res. 2007, 13, 4170. [DOI] [PubMed] [Google Scholar]
- (33).Musselli C; Livingston PO; Ragupathi G Keyhole Limpet Hemocyanin Conjugate Vaccines Against Cancer: The Memorial Sloan Kettering Experience. J. Cancer Res. Clin. Oncol. 2001, 127, R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pett C; Cai H; Liu J; Palitzsch B; Schorlemer M; Hartmann S; Stergiou N; Lu M; Kunz H; Schmitt E; Westerlind U Microarray Analysis of Antibodies Induced with Synthetic Antitumor Vaccines: Specificity against Diverse Mucin Core Structures. Chem. - Eur. J. 2017, 23, 3875. [DOI] [PubMed] [Google Scholar]
- (35).Storr SJ; Royle L; Chapman CJ; Hamid UM; Robertson JF; Murray A; Dwek RA; Rudd PM The O-linked Glycosylation of Secretory/shed MUC1 from an Advanced Breast Cancer Patient’s Serum. Glycobiology 2008, 18, 456. [DOI] [PubMed] [Google Scholar]
- (36).Hanisch FG; Stadie TR; Deutzmann F; Peter-Katalinic J MUC1 Glycoforms in Breast Cancer - Cell Line T47D as a Model for Carcinoma-associated Alterations of O-Glycosylation. Eur. J. Biochem. 1996, 236, 318. [DOI] [PubMed] [Google Scholar]
- (37).Beatty P; Hanisch FG; Stolz DB; Finn OJ; Ciborowski P Biochemical Characterization of the Soluble Form of Tumor Antigen MUC1 Isolated from Sera and Ascites Fluid of Breast and Pancreatic Cancer Patients. Clin. Cancer Res. 2001, 7, 781s. [PubMed] [Google Scholar]
- (38).Das R; Bassiri H; P, G.; Wiener S; Banerjee PP; Zhong MC; Veillette A; Orange JS; Nichols KE The Adaptor Molecule SAP Plays Essential Roles during Invariant NKT Cell Cytotoxicity and Lytic Synapse Formation. Blood 2013, 121, 3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Pardoll DM The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Leyva MA; Littrell CA; Yin Z; Huang X; Haas KM PD-1 Suppresses B Cell Responses to Tn Antigen and Protection against Tn-expressing Tumors. Cancer Immunol. Res. 2016, 4, 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kuai R; Ochyl LJ; Bahjat KS; Schwendeman A; Moon JJ Designer Vaccine Nanodiscs for Personalized Cancer. Nat. Mater. 2017, 16, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sungsuwan S; Yin Z; Huang X Lipopeptide Coated Iron Oxide Nanoparticles as Potential Glyco-conjugate Based Synthetic Anti-cancer Vaccines. ACS Appl Mater. Interfaces 2015, 7, 17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wilkinson BL; Day S; Malins LR; Apostolopoulos V; Payne RJ Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer. Angew. Chem. Int. Ed. 2011, 50, 1635. [DOI] [PubMed] [Google Scholar]
- (44).Liu Y-F; Sun Z-Y; Chen P-G; Huang Z-H; Gao Y; Shi L; Zhao Y-F; Chen Y-X; Li Y-M Glycopeptide Nanoconjugates Based on Multilayer Self-assembly as an Antitumor Vaccine. Bioconjugate Chem. 2015, 26, 1439. [DOI] [PubMed] [Google Scholar]
- (45).Geraci C; Consoli GM; Granata G; Galante E; Palmigiano A; Pappalardo M; Di Puma SD; Spadaro A First Self-adjuvant Multicomponent Potential Vaccine Candidates by Tethering of Four or Eight MUC1 Antigenic Immunodominant PDTRP Units on a Calixarene Platform: Synthesis and Biological Evaluation. Bioconjugate Chem. 2013, 24, 1710. [DOI] [PubMed] [Google Scholar]
- (46).Hoffmann-Roder A; Kaiser A; Wagner S; Gaidzik N; Kowalczyk D; Westerlind U; Gerlitzki B; Schmitt E; Kunz H Synthetic Antitumor Vaccines from Tetanus Toxoid Conjugates of MUC1 Glycopeptides with the Thomsen-Friedenreich Antigen and a Fluorine-substituted Analogue. Angew. Chem. Int. Ed. 2010, 49, 8498. [DOI] [PubMed] [Google Scholar]
- (47).Huang Z-H; Shi L; Ma J-W; Sun Z-Y; Cai H; Chen YX; Zhao Y-F; Li Y-M A Totally Synthetic, Self-assembling, Adjuvant-free MUC1 Glycopeptide Vaccine for Cancer Therapy. J. Am. Chem. Soc. 2012, 134, 8730. [DOI] [PubMed] [Google Scholar]
- (48).Glaffig M; Palitzsch B; Stergiou N; Schull C; Strassburger D; Schmitt E; Frey H; Kunz H Enhanced Immunogenicity of Multivalent MUC1 Glycopeptide Antitumour Vaccines Based on Hyperbranched Polymers. Org. Biomol Chem. 2015, 13, 10150. [DOI] [PubMed] [Google Scholar]
- (49).Karmakar P; Lee K; Sarkar S; Wall KA; Sucheck SJ Synthesis of a Liposomal MUC1 Glycopeptide-based Immunotherapeutic and Evaluation of the Effect of L-Rhamnose Targeting on Cellular Immune Responses. Bioconjugate Chem. 2016, 27, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Newman KD; Sosnowski DL; Kwon GS; Samuel J Delivery of MUC1 Mucin Peptide by Poly(D,L-Lactic-co-Glycolic Acid) Microspheres Induces Type 1 T Helper Immune Responses. J. Pharm. Sci 1998, 87, 1421. [DOI] [PubMed] [Google Scholar]
- (51).Melchers F Checkpoints that Control B Cell Development. J. Clin. Invest. 2015, 125, 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Soares MM; Mehta V; Finn OJ Three Different Vaccines based on the 140-Amino Acid MUC1 Peptide with Seven Tandemly Repeated Tumor-specific Epitopes Elicit Distinct Immune Effector Mechanisms in Wild-type versus MUC1-Transgenic Mice with Different Potential for Tumor Rejection. J. Immunol. 2001, 166, 6555. [DOI] [PubMed] [Google Scholar]
- (53).Acres B; Apostolopoulos V; Balloul J-M; Wreschner D; Xing P-X; Ali-Hadji D; Bizouarne N; Kieny MP; McKenzie IF MUC1-specific Immune Responses in Human MUC1 Transgenic Mice Immunized with Various Human MUC1 Vaccines. Cancer Immunol. Immunother. 2000, 48, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Stergiou N; Glaffig M; Jonuleit H; Schmitt E; Kunz H Immunization with a Synthetic Human MUC1 Glycopeptide Vaccine against Tumor-associated MUC1 Breaks Tolerance in Human MUC1 Transgenic Mice. ChemMedChem 2017, 12, 1424. [DOI] [PubMed] [Google Scholar]
- (55).von Mensdorff-Pouilly S; Petrakou E; Kenemans P; van Uffelen K; Verstraeten AA; Snijdewint FGM; van Kamp GJ; Schol DJ; Reis CA; Price MR; Livingston PO; Hilgers J Reactivity of Natural and Induced Human Antibodies to MUC1 Mucin with MUC1 Peptides and N-Acetylgalactosamine (GalNAc) Peptides. Int. J. Cancer 2000, 86, 702. [DOI] [PubMed] [Google Scholar]
- (56).Pobre K; Tashani M; Ridda I; Rashid H; Wong M; Booy R Carrier Priming or Suppression: Understanding Carrier PrimingEnhancement of Anti-polysaccharide Antibody Response to Conjugate Vaccines. Vaccine 2014, 32, 1423. [DOI] [PubMed] [Google Scholar]
- (57).Kinarsky L; Suryanarayanan G; Prakash O; Paulsen J; Clausen H; Hanisch FA; Hollingsworth MA; Sherman S Conformational Studies on the MUC1 Tandem Repeat Glycopeptides: Implication for the Enzymatic O-Glycosylation of the Mucin Protein Core. Glycobiology 2003, 13, 929. [DOI] [PubMed] [Google Scholar]
- (58).Ryan SO; Turner MS; Gariepy J; Finn OJ Tumor Antigen Epitopes Interpreted by the Immune System as Self or Abnormal-self Differentially Affect Cancer Vaccine Responses. Cancer Res. 2010, 70, 5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Thie H; Toleikis L; Li J; von Wasielewski R; Bastert G; Schirrmann T; Esteves IT; Behrens CK; Fournes B; Fournier N; de Romeuf C; Hust M; Dubel S Rise and Fall of an Anti-MUC1 Specific Antibody. PLoS One 2011, 6, No e15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Vassilaros S; Tsibanis A; Tsikkinis A; Pietersz GA; McKenzie IF; Apostolopoulos V Up to 15-year Clinical Follow-up of a Pilot Phase III Immunotherapy Study in Stage II Breast Cancer Patients using Oxidized Mannan-MUC1. Immunotherapy 2013, 5, 1177. [DOI] [PubMed] [Google Scholar]
- (61).Lakshminarayanan V; Supekar NT; Wei J; McCurry DB; Dueck AC; Kosiorek HE; Trivedi PP; Bradley JM; Madsen CS; Pathangey LB; Hoelzinger DB; Wolfert MA; Boons GJ; Cohen PA; Gendler SJ MUC1 Vaccines, Comprised of Glycosylated or Non-Glycosylated Peptides or Tumor-Derived MUC1, Can Circumvent Immunoediting to Control Tumor Growth in MUC1 Transgenic Mice. PLoS One 2016, 11, e0145920 and references cited therein . [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Lakshminarayanan V; Thompson P; Wolfert MA; Buskas T; Bradley JM; Pathangey LB; Madsen CS; Cohen PA; Gendler SJ; Boons G-J Immune Recognition of Tumor-associated Mucin MUC1 is Achieved by a Fully Synthetic Aberrantly Glycosylated MUC1 Tripartite Vaccine. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Golmohammadi R; Fridborg K; Bundule M; Valegard K; Liljas L The Crystal Structure of Bacteriophage Qß at 3.5A Resolution. Structure 1996, 4, 543. [DOI] [PubMed] [Google Scholar]
- (64).Bachmann MF; Jennings GT Vaccine Delivery: a Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787. [DOI] [PubMed] [Google Scholar]
- (65).Bachmann MF; Rohrer UH; Kundig TM; Burki K; Hengartner H; Zinkernagel RM The Influence of Antigen Organization on B Cell Responsiveness. Science 1993, 262, 1448. [DOI] [PubMed] [Google Scholar]
- (66).Braun M; Jandus C; Maurer P; Hammann-Haenni A; Schwarz K; Bachmann MF; Speiser DE; Romero P Virus-like Particles Induce Robust Human T-helper Cell Responses. Eur. J. Immunol. 2012, 42, 330. [DOI] [PubMed] [Google Scholar]
- (67).Jegerlehner A; Maurer P; Bessa J; Hinton HJ; Kopf M; Bachmann MF TLR9 Signaling in B Cells Determines Class Switch Recombination to IgG2a. J. Immunol. 2007, 178, 2415. [DOI] [PubMed] [Google Scholar]
- (68).Ragupathi G; Liu GX; Musselli C; Powell S; Lloyd K; Livingston PO Antibodies against Tumor Cell Glycolipids and Proteins, but Not Mucins, Mediate Complement-Dependent Cytotoxicity. J. Immunol. 2005, 174, 5706. [DOI] [PubMed] [Google Scholar]
- (69).Felder M; Kapur A; Gonzalez-Bosquet J; Horibata S; Heintz J; Albrecht R; Fass L; Kaur J; Hu K; Shojaei H; Whelan RJ; Patankar MS MUC16 (CA125): Tumor Biomarker to Cancer Therapy, a Work in Progress. Mol. Cancer 2014, 13, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Molina R; Auge JM; Escudero JM; Filella X; Zanon G; Pahisa J; Farrus B; Munoz M; Velasco M Evaluation of Tumor Markers (HER-2/neu Oncoprotein, CEA, and CA 15.3) in Patients with Locoregional Breast Cancer: Prognostic Value. Tumor Biol. 2010, 31, 171. [DOI] [PubMed] [Google Scholar]
- (71).Tomayko MM; Reynolds CP Determination of Subcutaneous Tumor Size in Athymic (Nude) Mice. Cancer Chemother. Pharmacol. 1989, 24, 148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


