Abstract
Altered structural connectivity has been identified as a possible biomarker of autism spectrum disorder (ASD) risk in the developing brain. Core features of ASD include impaired social communication and early language delay. Thus, examining white matter tracts associated with language may lend further insight into early signs of ASD risk and the mechanisms that underlie language impairments associated with the disorder. Evidence of altered structural connectivity has previously been detected in 6-month-old infants at high familial risk for developing ASD. However, as language processing begins in utero, differences in structural connectivity between language regions may be present in the early infant brain shortly after birth. Here we investigated key white matter pathways of the dorsal language network in 6-week-old infants at high (HR) and low (LR) risk for ASD to identify atypicalities in structural connectivity that may predict altered developmental trajectories prior to overt language delays and the onset of ASD symptomatology. Compared to HR infants, LR infants showed higher fractional anisotropy (FA) in the left superior longitudinal fasciculus (SLF); in contrast, in the right SLF, HR infants showed higher FA than LR infants. Additionally, HR infants showed more rightward lateralization of the SLF. Across both groups, measures of FA and lateralization of these pathways at 6 weeks of age were related to later language development at 18 months of age as well as ASD symptomatology at 36 months of age. These findings indicate that early differences in the structure of language pathways may provide an early predictor of future language development and ASD risk.
Keywords: DTI, Language, Autism, Infant, Development, Structural Connectivity
INTRODUCTION
Language development starts in utero based on prenatal exposure to speech. Indeed, by 33–34 weeks of gestation, functional MRI suggests that the fetal brain shows neural activation to speech sounds (see Dunn, Reissland, & Reid, 2015 for review; Jardri et al., 2012; Jardri et al., 2008). At birth, neonates activate a similar network of temporal and frontal regions as observed in adults in response to speech stimuli (Perani et al., 2011; Sato et al., 2012). Based on exposure to the prosodic contours of their native language in utero, infants can discriminate between native and non-native speech sounds by 3 months of age (Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002; see Dehaene-Lambertz, Hertz-Pannier, & Dubois, 2006 for review; Perani et al., 2011). Infancy is also a time of dynamic white matter development as increasing myelination of structural connections between different brain areas supports the growing functional efficiency of neural networks (see Dubois et al., 2014 for review). White matter tracts that connect language regions are present and traceable from early infancy (Dehaene-Lambertz et al., 2006; Dubois et al., 2016; Perani et al., 2011), allowing for the opportunity to examine early alterations in structural connectivity of language tracts in infants at high familial risk for developmental disorders impacting language development, such as autism spectrum disorder (ASD). ASD is characterized by impaired social communication and restrictive behaviors, and many individuals with ASD also experience early language delay and/or language impairment (Landa & Garrett-Mayer, 2006; Leyfer, Tager-Flusberg, Dowd, Tomblin, & Folstein, 2008; Mitchell et al., 2006; Sperdin & Schaer, 2016). Indeed, it is estimated that over half of the children with ASD have persisting language impairments throughout their lifespan (see Sperdin & Schaer, 2016 for review). Prior studies have suggested that these behavioral deficits may in part reflect underlying disruptions in brain connectivity (Joseph et al., 2014; Li, Xue, Ellmore, Frye, & Wong, 2014; Solso et al., 2016; Wolff, Jacob, & Elison, 2017). Here, we aimed to investigate whether language-specific structural alterations can be observed as early as 6 weeks of age in infants at high familial risk for ASD, which, to our knowledge, is the youngest age examined in this population to date.
Infants and toddlers who later go on to develop ASD already show altered structural connectivity in language tracts well before the onset of overt behavioral symptoms and language delay. Previous diffusion tensor imaging (DTI) studies in toddlers with ASD have reported altered developmental trajectory of several white matter tracts during the first 2 years of life. In a study comparing high risk infants who underwent MRI at 6, 12, and 24 months who later did (HR+) or did not (HR-) go on to develop ASD, Wolff et al. (2012) examined the white matter microstructure of many tracts, including the uncinate fasciculus, which is part of the ventral language network. Overall, compared to the HR- group, HR+ infants showed more robust microstructure (as indexed by higher fractional anisotropy, FA) at 6 months extending to 12 months, followed by a blunted developmental trajectory (i.e., lower FA) by 24 months. This altered developmental trajectory was also associated with ASD symptoms and later diagnosis. These findings are consistent with studies in older toddlers with ASD which have reported a slower rate of change with age in various frontal tracts including the arcuate fasciculus and the uncinate fasciculus (Solso et al., 2016), as well as reduced integrity of language tracts by 3 years of age (see Conti et al., 2015 for review). Furthermore, altered structural connectivity, as revealed by differences in white matter microstructure, can be a predictor of later language development (Aeby et al., 2013). Collectively, this evidence suggests that early alterations in white matter development may predict later functional language deficits associated with ASD. However, the timeline for the emergence of these differences remains unknown. Wolff and colleagues (2012; 2017) have examined structural connectivity in infants at high familial risk for ASD as early as 6 months of age, but there have been no studies to date examining earlier time points. In this study, we examined whether differences in the structural connectivity of the dorsal language network can be identified in 6-week-old infants.
In the adult brain, two dorsal white matter pathways connecting canonical language regions – Wernicke’s and Broca’s areas – are associated with language production and syntactic processing (Friederici, 2009, 2011; Glasser & Rilling, 2008). The arcuate fasciculus (AF) connects the superior temporal gyrus (STG) to the precentral gyrus and is thought to play a key role in coupling auditory input and motor output during the early stages of language learning (Friederici, 2012). The superior longitudinal fasciculus (SLF) connects the STG to the inferior frontal gyrus (IFG) pars opercularis and subserves higher-level language processing, such as parsing complex syntactic structures, in the mature brain (Friederici, 2012). Phylogenetically, the AF and SLF are more robust in humans compared to nonhuman primates, suggesting that the dorsal language pathways connecting the temporal and frontal lobes are critical for the human capacity to learn and use language (Friederici, 2012, 2018; Friederici, Chomsky, Berwick, Moro, & Bolhuis, 2017). In adults, both pathways have been shown to support the processing of syntactically complex sentences (Brauer, Anwander, & Friederici, 2011; Brauer, Anwander, Perani, & Friederici, 2013). However, the early development of structural connectivity of these language tracts has only recently been investigated. At birth, the AF has already begun to be myelinated whereas the SLF has not, suggesting that the integration of sensory and motor representations must precede the development of white matter tracts that support more advanced language processing (Perani et al., 2011).
Here we used diffusion tensor imaging (DTI) to characterize the maturation of these white matter tracts during very early development and the extent to which atypicalities associated with ASD risk may already be present in the infant brain. DTI provides a measure of structural connectivity by measuring the diffusion of water molecules in the brain to reveal white matter anatomy (Mori & Zhang, 2006). More specifically, increases in fractional anisotropy (FA) generally indicate increasing myelination and more robust white matter microstructure within the identified tract (Qiu, Mori, & Miller, 2015). Given that much of the prior infant literature on early structural connectivity has focused on differences in FA (Dubois et al., 2016; Elison, Paterson, et al., 2013; Elison, Wolff, et al., 2013; Swanson et al., 2017; Wolff et al., 2012; Wolff, Swanson, et al., 2017), we focused on FA as our primary measure of interest. We also expected to observe differences in laterality based on prior evidence of altered lateralization profiles in older children and youth with ASD (Dubois et al., 2016; Fletcher et al., 2010; Joseph et al., 2014; Wan, Marchina, Norton, & Schlaug, 2012). In this study, we aimed to identify the major dorsal language tracts at 6 weeks of age and relate measures of early structural connectivity to later behavioral measures of language development and ASD symptomatology in order to detect early signs of ASD risk.
MATERIALS AND METHODS
Subjects
Infants were assigned to risk cohorts based on family history: High risk (HR) infants had at least one older sibling with a confirmed ASD diagnosis, while low risk (LR) infants had no family history of ASD or any other neurodevelopmental disorders. All infant participants were enrolled prior to 6 weeks of age. Informed consent was obtained from parents/legal guardians of infant participants under protocols approved by the UCLA Institutional Review Board (IRB). Exclusionary criteria for both groups included: 1) genetic syndromes or neurological conditions (e.g., fragile X syndrome, tuberous sclerosis), 2) chronic medical conditions or significant perinatal insult impacting development, 3) severe visual, hearing, or motor impairment, 4) non-English speaking families, and 5) contraindication for MRI.
A total of 34 infants (19 HR, 15 LR) successfully underwent MRI during natural sleep at 6 weeks of age. An additional seven infants (1 HR, 6 LR) did not complete the DTI scan (i.e., woke up before or during the DTI scan). Out of the final sample of 34 infants, 23 infants (15 HR, 8 LR) provided adequate behavioral data tracking language development at 18 months of age, and 19 infants (11 HR, 8 LR) provided data assessing ASD symptomatology at 36 months. Risk-based cohorts were matched on age, gender, race, and family income (Table 1).
Table 1.
Subject Demographics.
|
LR N=15 |
HR N=19 |
P | |
| Sex | .22 | ||
| Female | 4 (27%) | 9 (47%) | |
| Male | 11 (73%) | 10 (53%) | |
| Race | .29 | ||
| White | 12 (80%) | 12 (63%) | |
| Non-white | 3 (20%) | 7 (37%) | |
| Family Income | .73 | ||
| Not Answered | 0 | 2 (11%) | |
| <50 K | 2 (13%) | 3 (16%) | |
| 50–75 K | 2 (13%) | 3 (16%) | |
| 75–100 K | 4 (27%) | 4 (21%) | |
| 100–125 K | 2 (13%) | 2 (11%) | |
| >125 K | 5 (33%) | 5 (26%) | |
| Mean (SD) | Mean (SD) | P | |
| Age at Scan (Weeks) | 6.43 (1.27) | 6.40 (1.03) | .73 |
| CDI Words and Gestures | |||
| Receptive Advantage (18 months) | 168.50 (90.41) | 89.47 (58.17) | .01 |
| ADOS-2 CSS (36 months) | 3.25 (2.82) | 3.55 (2.52) | .40 |
| Gradients remaining [Range] | 30.80 (1.90) [25–32] | 30.68 (1.67) [26–32] | .85 |
MRI Data Acquisition
Imaging data were collected at 6 weeks of age on a Siemens 3T Tim Trio scanner using a 12-channel head coil during natural sleep. Diffusion-weighted data consisted of 32 diffusion weighted directions (b=1000), three scans with no diffusion sensitization (b=0), and six additional directions (b=50): TR=9500ms, TE=87ms, matrix size 256×256, FOV=256mm, 75 slices, 2mm in-plane resolution, with 2mm-thick axial slices. In addition, matched bandwidth T2-weighted high-resolution echo planar images were acquired: TR= 5000ms, TE=34ms, matrix size 128×128, FOV=192mm, 34 slices, 1.5mm in-plane resolution, with 4mm-thick axial slices.
Infants underwent MRI during natural sleep. Parents were instructed to put their infant to sleep using their regular bedtime routine; once asleep, swaddled infants were transferred to the scanner bed. Soft and malleable silicone earplugs as well as MiniMuffs Neonatal Noise Attenuators (Natus Medical Inc., San Carlos, CA) were used as hearing protection; headphones used to convey auditory stimuli during a different scan further dampened scanner noise. Infants were placed on a custom-made bed, which fit inside the head coil, and secured on the scanner bed with a Velcro strap. To minimize movement, a weighted blanket was used and foam pads were positioned around each infant’s head. A study staff member remained in the scan room for the duration of the scan to monitor infants for overt movement, waking, or signs of distress.
Behavioral Measures
Developmental assessments were conducted at two later time points (Table 1). The MacArthur-Bates Communicative Development Inventory (CDI; Fenson et al., 2007) Words and Gestures checklist was completed at 18 months of age. This instrument is a parent-report standardized questionnaire that measures expressive and receptive language skills. Typically developing infants, and even infants who later exhibit language delays, typically show an early receptive advantage (i.e., total number of words comprehended minus words produced) based on the CDI; however, prior research has found that early language profiles are often atypical in HR infants (Nevill et al., 2017). Importantly, several studies showed that HR infants do not show such an advantage; this CDI receptive advantage metric has also been shown to be predictive of ASD outcome in HR infants, such that infants who later develop ASD have a lower receptive advantage score (Ellis Weismer, Lord, & Esler, 2010; Hudry et al., 2014; Hudry et al., 2010). Based on these findings, in this study, we focused on the CDI receptive advantage score.
The Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al., 2012) was administered at 36 months. This is a play-based clinical assessment and gold-standard method for measuring ASD symptom severity. Participants were administered the appropriate ADOS-2 module according to their developmental level (NModule 1=2, NModule 2=17). The calibrated severity score (CSS) was used to compare symptom severity across ADOS modules whereby a higher CSS rating represented greater symptom severity (Gotham, Pickles, & Lord, 2009).
Data Analysis
DTI Data Preprocessing
Diffusion weighted imaging data were first checked for data quality using DTIPrep (Liu et al., 2010), which automatically detects and flags bad gradient diffusion-weighted images. Volumes were then manually inspected and removed based on the presence of residual artifacts. Following this quality control procedure, datasets with fewer than 23 (72%) gradient diffusion-weighted images were excluded due to low signal-to-noise ratio (as in Wolff et al., 2012). Seventeen datasets (9 HR, 8 LR) did not meet this threshold and were removed, resulting in a final sample of 34 infants (19 HR, 15LR). There were no significant differences between the two groups in the average number of gradients remaining following this quality control step (Table 1). DTI data were then further preprocessed and analyzed using FSL’s FDT (FMRIB’s Diffusion Toolbox version 3.0; Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007; Behrens et al., 2003). This included motion correction, eddy current correction, and brain extraction. There were no between-group differences in mean or maximum relative motion, nor in mean or maximum absolute motion (all ps > .05). In preparation for fiber tracking, DTIFit was used to fit the diffusion tensor model at each voxel. Diffusion data were co-registered to each subject’s corresponding matched bandwidth T2-weighted image, which was preprocessed using FSL version 5.0.8 (FMRIB’s Software Library; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2004), then registered to a neonate template brain in standard space (Shi et al., 2011) using 12-parameter affine transformation.
Probabilistic Tractography
Probabilistic fiber tracking was carried out in each subject’s native diffusion space using FDT. First, bedpostx was used to generate a Bayesian estimate of the probability distribution of different directions at each voxel. Using anatomically defined regions-of-interest (ROIs) from a neonate atlas (Shi et al., 2011), fiber tracking was performed using probtrackx2, which generated streamlines connecting voxels from an originating seed ROI to voxels in a target ROI; a total of 5,000 samples were drawn from the connectivity distribution from each voxel in the seed ROI. Trajectories were initiated in all voxels with FA > 0.1 and a curvature threshold of 0.2 (minimum angle of +/− 80°) was used. Streamlines were retained only if they passed through the source, target, and waypoint voxels. Each ROI was used as both a source and target region for more accurate delineation of these bidirectional tracts. Regions-of-exclusion (ROEs) were also created to designate regions that streamlines could not pass through. The resulting path distribution map displayed voxels that represented the total number of samples that successfully passed from the seed ROI to the target ROI. For each subject, all tractography outputs were first thresholded to exclude voxels with connectivity values less than 10, then the two seed-to-target tracts were combined into one bidirectional tract for each white matter tract of interest. Tracts were then normalized to standard space and summed to create a normalized tract for each risk group, which was thresholded to include voxels that belonged to a particular tract in at least 15% of the subjects within each group (as in Papinutto et al., 2016).The normalized tract was transformed back to each subject’s native diffusion space and measures of white matter microstructure were extracted and averaged, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). A laterality index, which indicated whether a given tract was more left or right lateralized, was computed for each tract by taking the between-hemisphere difference in FA divided by the sum of the two: (Left FA – Right FA)/(Left FA + Right FA). For each tract, a positive value would indicate leftward lateralization whereas a negative value would indicate rightward lateralization.
Here we examined two tracts that are known to connect language-relevant brain areas and have previously been shown to be present in 2-day-old infants (Perani et al., 2011): the arcuate fasciculus (AF) and superior longitudinal fasciculus (SLF). For the AF, the ROIs were the STG and precentral gyrus; for the SLF, the ROIs were the STG and IFG pars opercularis. For both tracts, ROEs included the contralateral hemisphere and ipsilateral insula.
Statistical Analyses
Given that FA was our primary measure of interest, for each tract (AF, SLF), a mixed analysis of variance (ANOVA) was performed with risk group (HR, LR) and hemisphere (left, right) as fixed factors to examine differences in FA. Next, between-group differences in laterality index were examined by conducting one-way ANOVAs. Since these comparisons were determined a priori, we did not correct for multiple comparisons for these tests. Exploratory post-hoc ANOVAs were performed to examine differences in MD, AD, and RD for each tract. These post-hoc analyses were Bonferroni corrected to control for multiple comparisons. Finally, we examined the strength of association across the whole group between structural connectivity measures, including white matter integrity (FA) and interhemispheric lateralization, and longitudinal behavioral measures, including the CDI receptive advantage (18 months; Pearson’s correlation) and the ADOS-2 CSS (36 months; Spearman’s correlation given that the data were not normally distributed).
RESULTS
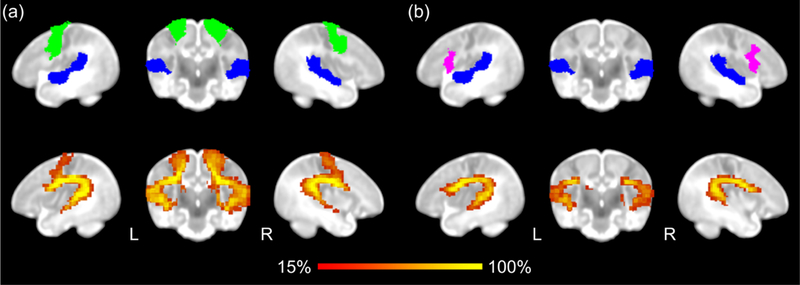
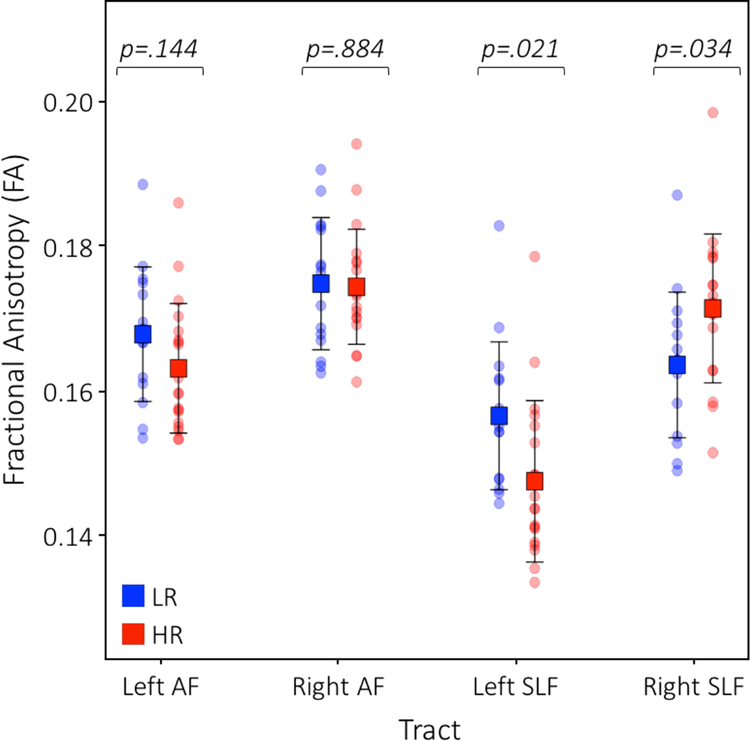
Bidirectional probabilistic tractography performed for each language tract revealed that both dorsal language tracts can be identified by 6 weeks of age (Figure 1). Mean FA values for each tract were computed for each group (Table 2). For the AF, the interaction of risk group by hemisphere was trending (F1,32 = 2.75, p = .107). Follow-up one-way ANOVAs did not reveal significant differences between the HR and LR groups within each hemisphere (Left AF: F1,32 = 2.25, p = .144; Right AF: F1,32 = .02, p = .884; Figure 2). However, right AF FA was significantly higher than left AF FA for both groups (HR: F1,36 = 16.87, p < .001; LR: F1,28 = 4.32, p = .047). For the SLF, the ANOVA testing for differences in FA of the SLF yielded a significant interaction of risk group by hemisphere (F1,32 = 24.23, p < .001; Figure 2). Follow-up one-way ANOVAs showed that LR infants had significantly higher mean FA in the left SLF compared to infants in the HR group (F1,32 = 5.92, p = .021); in contrast, in the right SLF, HR infants showed significantly higher mean FA compared to infants in the LR group (F1,32 = 4.92, p = .034). Furthermore, the right SLF FA was significantly higher than left SLF FA in the HR group (F1,36 = 47.09, p < .001) with a similar pattern trending towards significance in the LR group (F1,28 = 3.61, p = .068). For these analyses, one data point was a mild outlier (i.e., exceeding inner but not outer fence). Importantly, however, all findings remained significant even when excluding this data point, indicating that our results were not due driven by this outlier.
Figure 1. Probabilistic tractography of dorsal language tracts.
(a) Arcuate fasciculus (AF) was traced using seed ROIs from STG (blue) and premotor gyrus (green). (b) Superior longitudinal fasciculus (SLF) was traced using seed ROIs from the STG (blue) and IFG pars opercularis (magenta).
Table 2.
Mean FA and LI values.
| LR N=15 Mean (SD) |
HR N=19 Mean (SD) |
|
|---|---|---|
| Fractional Anisotropy (FA) | ||
| Left AF | .168(.009) | .163(.009) |
| Right AF | .175(.009) | .174(.008) |
| Left SLF | .157(.010) | .147(.011) |
| Right SLF | .164(.010) | .171(.010) |
| Laterality Index (LI) | ||
| AF FA | −.021(.024) | −.034(.021) |
| SLF FA | −.022(.032) | −.075(.032) |
Figure 2. Differences in white matter microstructure by tract and hemisphere.
There was a significant interaction of risk group by hemisphere in the SLF (F1, 64 = 10.84, p = .002). LR infants, in blue, showed higher FA in the left SLF whereas HR infants, in red, showed elevated FA in the right SLF.
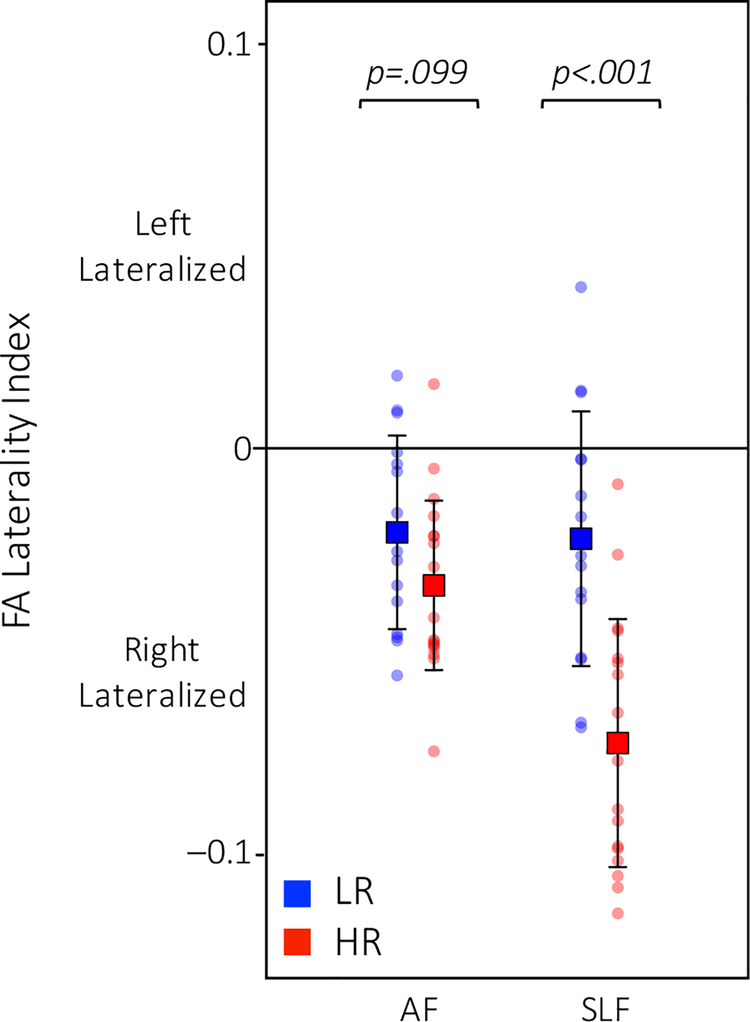
To examine between-group differences in laterality, we computed a laterality index for each tract whereby negative values indicated more robust connectivity (i.e., higher FA values) in the right hemisphere and positive values indicated stronger connectivity in the left hemisphere. Mean laterality index values for each tract were computed for each group (Table 2). HR infants showed more rightward lateralization than the LR infants in the SLF (F1, 32 = 23.51, p < .001; Figure 3). There was a similar pattern of laterality in the AF with HR infants trending towards more rightward lateralization than the LR infants (F1, 32 = 2.90, p = .099; Figure 3).
Figure 3. Differences in laterality.
In the SLF, HR infants, in red, showed more rightward lateralization than the LR infants, in blue (trending in the AF).
Exploratory post-hoc analyses were also conducted to examine between-group differences in MD, AD, and RD of each tract. Mean MD, AD, and RD values were first computed for each group (Table S1 and Figure S1). Post-hoc ANOVAs yielded an interaction of risk group by hemisphere only for RD of the SLF (F1,32 = 4.62, p = .039). As this finding did not survive correction for multiple comparisons (Bonferroni-corrected for the three diffusion measures, p < .0167; Table S2), no further analyses were conducted.
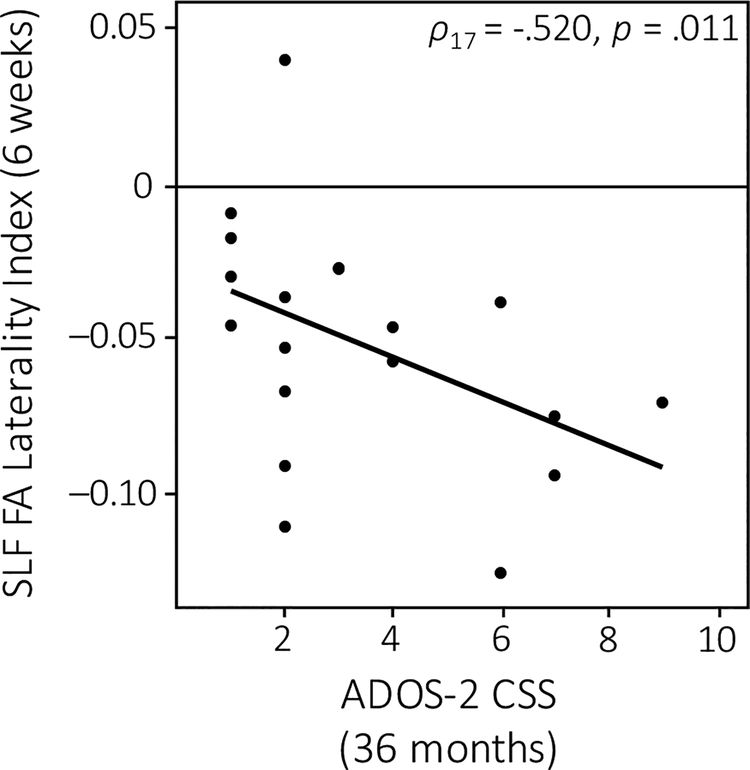
Lastly, we addressed the question of how these early differences in structural connectivity at 6 weeks of age might relate to later language development, as indexed by behavioral measures tracking language development as well as ASD symptomatology. Across the whole group, infants who displayed more robust white matter integrity (i.e., higher FA) of the left AF (r21 = .418, p = .024; Figure 4a) and left SLF (r21 = .437, p = .019; Figure 4b) showed higher CDI receptive advantage scores at 18 months of age. Furthermore, early measures of SLF laterality correlated with ASD symptom severity; infants who exhibited more rightward lateralization of the SLF at 6 weeks of age had higher levels of social communicative impairments as indexed by the ADOS-2 CSS at 36 months (ρ17 = −.520, p = .011; Figure 5).
Figure 4. Correlations between early white matter microstructure and later language development.
Infants whose left language tracts were more robust (i.e., higher FA) at 6 weeks of age showed higher (i.e., normative) receptive advantage scores at 18 months of age. This was true for both the left AF (a) and left SLF (b).
Figure 5. Correlation between early interhemispheric asymmetry and later ASD symptomatology.
Infants who displayed more rightward lateralization of the SLF at 6 weeks of age had higher (i.e., more severe) ADOS-2 CSS at 36 months.
DISCUSSION
To our knowledge, this is the first study to examine structural connectivity of language networks in 6-week-old infants at high familial risk for developing ASD. Whereas Perani and colleagues (2011) were not able to detect the SLF in 2-day-old typically developing neonates, here we demonstrate that both the SLF and AF are present and traceable by 6 weeks of age. We found that HR infants already showed altered structural connectivity in the dorsal language network by this age. LR infants showed more robust connectivity of the left SLF; in contrast, HR infants demonstrated the opposite pattern with stronger connectivity in the right SLF. In examining laterality differences between the two groups, while both groups showed evidence of weak rightward lateralization, HR infants showed more rightward lateralization of the SLF compared to the LR group. Furthermore, white matter integrity (FA) of both the SLF and AF was associated with measures of language development at 18 months of age. Lastly, interhemispheric asymmetry of the SLF was associated with ASD symptomatology at 36 months of age.
Our finding that 6-week-old LR infants display stronger connectivity in the left SLF compared to the HR group is consistent with prior work on older typically developing infants as well as adults (see Dubois et al., 2014 for review; Dubois et al., 2016). Though both HR and LR infants showed evidence for weak rightward lateralization, within the left hemisphere, LR infants showed higher SLF FA compared with HR infants. In typical development, more robust structural connectivity in the left hemisphere may lay the groundwork for later leftward lateralization of functional language networks during speech processing (Dehaene-Lambertz et al., 2002; Dehaene-Lambertz et al., 2010). Indeed, beginning in the second year of life, functional connectivity of left hemisphere primary language regions is known to increase (Emerson, Gao, & Lin, 2016). White matter development can help to support functional connectivity by enabling language networks to efficiently process speech. Since the increase in myelination of the SLF during development correlates with an increasing ability to process complex sentences (see Friederici, 2017 for review), an earlier transition toward a leftward structural asymmetry may prove important for subsequent language development.
Altered structural connectivity has been found in many brain regions in infants later diagnosed with ASD. Wolff and colleagues (2012; 2017) previously showed that HR infants who later develop ASD exhibit altered developmental trajectories across many white matter fiber tracts throughout the brain from 6–24 months of age compared with HR infants who do not go on to develop ASD. It is important to note that these studies utilized a different sampling strategy compared to the present study in that they did not include a LR group in their analyses. Nevertheless, our results provide further evidence that infants at high risk for ASD display differences in structural connectivity. In addition, our findings demonstrate that atypical development of white matter tracts, specifically of language tracts, starts very early in infancy and is manifested by 6 weeks of age. Furthermore, in the HR group, more robust connectivity in the right hemisphere as well as evidence for rightward lateralization of the dorsal language network is consistent with prior findings in older children and adolescents with ASD, who show less robust structural connectivity between left hemisphere language regions (Joseph et al., 2014; Knaus et al., 2010; Wan et al., 2012). To the extent that the atypical structural connectivity we observed in 6-week-old infants at high risk for ASD antecedes language acquisition and any overt manifestation of language impairment, our findings indicate that the altered language lateralization profiles observed in older individuals with ASD in prior studies do not merely reflect a history of delayed language development and/or aberrant language processing.
Altered interhemispheric laterality in the early infant brain observed in the present study is also consistent with evidence from prior work using functional neuroimaging methods showing atypical functional connectivity and language processing across development in ASD. Imaging studies using fMRI have shown that while typically developing infants initially show bilateral activity in response to language stimuli that shifts to left-lateralized activity over the course of development (see Skeide & Friederici, 2016 for review), infants and toddlers later diagnosed with ASD show atypical right temporal cortex activity (Dinstein et al., 2011), left temporal region hypoactivity (Eyler, Pierce, & Courchesne, 2012), and right-lateralized activation to speech sounds during language processing (Lombardo et al., 2015; Redcay & Courchesne, 2008; Redcay, Haist, & Courchesne, 2008). This trend toward an atypical rightward lateralization in ASD holds across development; adolescents and adults with ASD, particularly those with language impairment, show reduced functional lateralization of language and greater right hemisphere activation to language stimuli (Fitzgerald et al., 2017; Herringshaw, Ammons, DeRamus, & Kana, 2016; Kleinhans, Muller, Cohen, & Courchesne, 2008; Knaus et al., 2010; Lange et al., 2010; Sperdin & Schaer, 2016). Alterations in the lateralization and function of language networks have been hypothesized to be due to the failure of left hemisphere regions, such as the STG, to properly respond to language stimuli (Eyler et al., 2012; Seery, Vogel-Farley, Tager-Flusberg, & Nelson, 2013). The present findings suggest that altered structural lateralization beginning very early in infancy could have cascading effects leading to the manifestation of atypical language acquisition and altered functional lateralization.
Increasing myelination during development improves the efficiency of functional brain networks by insulating axons that deliver information from one region to another (van der Knaap et al., 1991). Thus, hemispheric differences of the dorsal language tracts observed in this study suggest that language networks are differentially organized in HR infants very early on. However, neuronal activity induced by stimulation can also influence the degree of white matter myelination such that tracts connecting regions that respond with greater activity are also more likely to be myelinated to facilitate information transfer. Accordingly, differences in white matter pathways may also reflect altered processing of language input. Since language development and use require the integration of information across many brain regions, altered structural connectivity may reflect inefficient integration of multisensory information (e.g., visual, motor, auditory), which is critical for language acquisition. Indeed, toddlers who later develop ASD show decreased local as well as global efficiency particularly in the temporal lobe (Lewis et al., 2014; Lewis et al., 2017).
Early alterations in white matter connections between language regions may be a useful predictor of early language acquisition and ASD symptomatology. In order to examine this possibility, we examined the relationship between early structural connectivity measures and clinically relevant language profiles associated with ASD. More specifically, we related white matter integrity of the dorsal language network with measures of receptive advantage at 18 months of age, which has previously been shown to be significantly reduced for infants with an ASD outcome (Ellis Weismer et al., 2010; Hudry et al., 2014; Hudry et al., 2010). Higher FA of both the AF and SLF was positively correlated with later language development such that infants who showed more robust dorsal tracts at 6 weeks of age had higher receptive advantage scores at 18 months of age. Our results are consistent with a study examining structural connectivity of temporal regions in preterm infants (Aeby et al., 2013), showing that early microstructure metrics in the neonate brain were associated with receptive and expressive language at 2 years of age. Furthermore, in our study, interhemispheric asymmetry strongly correlated with later ASD symptomatology; infants who showed more rightward asymmetry in their dorsal tracts at 6 weeks of age had more severe ADOS-2 CSS at 36 months of age. Taken together, these findings suggest that early measures of structural connectivity may provide insight into subsequent language development, as well as ASD risk.
In line with prior work on structural connectivity in language-relevant tracts in HR infants (Wolff et al., 2012; Wolff, Swanson, et al., 2017) and in toddlers with ASD (Ben Bashat et al., 2007; Conti et al., 2017; Solso et al., 2016; Weinstein et al., 2011; Xiao et al., 2014; Zhang et al., 2018), our primary focus was on FA, a measure that provides an overall index of white matter integrity. Exploratory analyses using MD, AD, and RD failed to yield robust differences between the two risk groups. While these other white matter microstructure indices can further inform our understanding of structural connectivity, prior studies in older children and adults with ASD using such metrics are relatively few and have yielded often inconsistent findings, perhaps reflecting differences in sample characteristics and tractography approaches (see Travers et al., 2012 for review). However, in light of a growing body of work which examines these diffusivity measures in typically developing infants (e.g., Dubois et al., 2016; Gao et al., 2009; Sadeghi et al., 2013; Swanson et al., 2017), future studies in infants at high familial risk for ASD should include a broad array of white matter microstructure metrics in order to better characterize atypical development of structural connectivity in this population.
This study presents some limitations that warrant discussion. First, though the risk groups were matched based on socioeconomic status, which has been associated with differences in language development (see Hackman & Farah, 2009; Hackman, Farah, & Meaney, 2010 for review) and autism diagnosis (Durkin et al., 2017), we were not able to match based on maternal education. Importantly, however, maternal education was not related to any of the DTI metrics for which we observed significant between-group differences (Table S3), suggesting that our results were not driven by differences in level of maternal education. Our findings are also based on a modest sample of infants for whom outcome measures were not readily available; as such, our findings should be replicated in a larger sample and directly related to developmental outcomes. Indeed, our goal was to examine whether white matter microstructure of language tracts is associated with subsequent development, independently of possible clinical implications which would be best addressed in a much larger sample with variable outcomes. Our findings indicate that the relationship between white matter microstructure and ADOS-2 CSS only accounts for 27% of the variance in the population. While this is in line with previously published work relating structural connectivity to behavioral measures in similar populations (e.g., Solso et al., 2016), the significant amount of unexplained variance clearly highlights that many other variables, including genetic as well as environmental/experiential factors, ultimately contribute to the developmental trajectories observed in this population. Future research should aim to examine the complex interplay between these contributing factors. In addition, while this study focused only on the dorsal language tracts, several ventral tracts have also been shown to play a supporting role in language processing in the adult brain (Dehaene-Lambertz, 2017). Despite mixed findings on the developmental trajectories of these tracts in early childhood (Dubois et al., 2016; Elison, Wolff, et al., 2013; Solso et al., 2016; Wolff et al., 2012), future research should examine the role of these ventral tracts in early language development in at-risk populations.
Given that our study focused on infants at high familial risk for ASD prior to the onset of overt behavioral symptoms, some of these infants may ultimately receive an ASD diagnosis, but this group is also expected to exhibit a range of heterogeneous outcomes which may include other neurodevelopmental, learning, or language disorders (Miller et al., 2016; Zwaigenbaum et al., 2007). Indeed, some of these infants may go on to experience difficulties in the language domain, in line with prior reports that HR siblings of children with ASD show higher rates of language delay, slower language acquisition, and lower language scores as compared to LR infants (Franchini et al., 2018; Gamliel, Yirmiya, & Sigman, 2007; Iverson & Wozniak, 2007; Miller et al., 2015; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011). Interestingly, a recent study examining high risk infants with ASD and infants with early language delay, Swanson and colleagues (2017) reported associations between subcortical brain volume (including the thalamus, amygdala, and caudate) and later language skills, indicating that other language learning-related brain structures may also be affected by risk status. The results from the present study may therefore serve as an early predictor of atypical language development reflecting a more general susceptibility to language impairment common to other developmental disorders. Indeed, the mechanisms underlying disordered language acquisition may not be unique to ASD but instead shared across a range of neurodevelopmental disorders. Findings from the imaging genetics literature further support this notion since common variants on several genes associated with language impairment, including CNTNAP2, have been linked to delayed language development and altered structural connectivity in both typical and atypical development (Alarcón et al., 2008; Eicher & Gruen, 2015; Geschwind, 2011; von Hohenberg et al., 2013). Accordingly, future studies should also consider genetic risk when linking early brain development to language outcome.
In summary, the results of the present study confirm that white matter tracts connecting dorsal language regions in the brain can be readily identified in 6-week-old infants using DTI during natural sleep. Already by this age, early differences in these language tracts can be detected: HR infants show altered structural connectivity in the SLF compared to their LR counterparts and early atypical laterality profiles are related to behavioral indices of language development. Importantly, our findings also suggest that early differences in language-relevant white matter pathways are associated with future language development and ASD symptomatology in infants at risk for developmental disorders. As such, future large scale studies are warranted to further evaluate the validity of white matter microstructure metrics in predicting language outcome and ASD diagnosis.
Supplementary Material
RESEARCH HIGHLIGHTS.
Compared to infants at high risk for ASD, low risk infants show more robust connectivity in the left hemisphere superior longitudinal fasciculus at 6 weeks of age.
High risk infants show increased rightward lateralization in the dorsal language network compared to their low risk counterparts.
Differences in white matter microstructure and laterality of dorsal language tracts are associated with later language development and ASD symptomatology.
These findings indicate that altered white matter integrity and atypical lateralization of the developing dorsal language pathways may lead to altered language processing in ASD.
Acknowledgements:
This work was supported by the National Institute of Child Health and Human Development grant P50 HD055784 (to SYB), National Institute on Drug Abuse grant T90 DA022768 (to JL), and National Institute of Child Health and Human Development National Research Service Award F31 HD088102 (to JL). We are grateful for the generous support from the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the families who generously gave their time to participate in this study.
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
REFERENCES
- Aeby A, De Tiege X, Creuzil M, David P, Baleriaux D, Van Overmeire B, … Van Bogaert P (2013). Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. Neuroimage, 78, 145–151. doi: 10.1016/j.neuroimage.2013.03.076 [DOI] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, … Geschwind DH (2008). Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet, 82(1), 150–159. doi: 10.1016/j.ajhg.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, & Woolrich MW (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage, 34(1), 144–155. doi: 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, … Smith SM (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med, 50(5), 1077–1088. doi: 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, … Ben Sira L (2007). Accelerated maturation of white matter in young children with autism: A high b value DWI study. Neuroimage, 37(1), 40–47. doi: 10.1016/j.neuroimage.2007.04.060 [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, & Friederici AD (2011). Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex, 21(2), 459–466. doi: 10.1093/cercor/bhq108 [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Perani D, & Friederici AD (2013). Dorsal and ventral pathways in language development. Brain Lang, 127(2), 289–295. doi: 10.1016/j.bandl.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Conti E, Calderoni S, Marchi V, Muratori F, Cioni G, & Guzzetta A (2015). The first 1000 days of the autistic brain: a systematic review of diffusion imaging studies. Front Hum Neurosci, 9, 159. doi: 10.3389/fnhum.2015.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Mitra J, Calderoni S, Pannek K, Shen KK, Pagnozzi A, … Guzzetta A (2017). Network over-connectivity differentiates autism spectrum disorder from other developmental disorders in toddlers: A diffusion MRI study. Hum Brain Mapp, 38(5), 2333–2344. doi: 10.1002/hbm.23520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G (2017). The human infant brain: A neural architecture able to learn language. Psychon Bull Rev, 24(1), 48–55. doi: 10.3758/s13423-016-1156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, & Hertz-Pannier L (2002). Functional neuroimaging of speech perception in infants. Science, 298, 2013–2015. doi: 10.1126/science.1077066 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, & Dubois J (2006). Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci, 29(7), 367–373. doi: 10.1016/j.tins.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, & Dehaene S (2010). Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang, 114(2), 53–65. doi: 10.1016/j.bandl.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, & Courchesne E (2011). Disrupted neural synchronization in toddlers with autism. Neuron, 70(6), 1218–1225. doi: 10.1016/j.neuron.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Huppi PS, & Hertz-Pannier L (2014). The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. doi: 10.1016/j.neuroscience.2013.12.044 [DOI] [PubMed] [Google Scholar]
- Dubois J, Poupon C, Thirion B, Simonnet H, Kulikova S, Leroy F, … Dehaene-Lambertz G (2016). Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cereb Cortex, 26(5), 2283–2298. doi: 10.1093/cercor/bhv082 [DOI] [PubMed] [Google Scholar]
- Dunn K, Reissland N, & Reid VM (2015). The functional foetal brain: A systematic preview of methodological factors in reporting foetal visual and auditory capacity. Dev Cogn Neurosci, 13, 43–52. doi: 10.1016/j.dcn.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Baio J, Christensen D, Daniels J, Fitzgerald R, … Yeargin-Allsopp M (2017). Autism Spectrum Disorder Among US Children (2002–2010): Socioeconomic, Racial, and Ethnic Disparities. Am J Public Health, 107(11), 1818–1826. doi: 10.2105/AJPH.2017.304032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher JD, & Gruen JR (2015). Language impairment and dyslexia genes influence language skills in children with autism spectrum disorders. Autism Res, 8(2), 229–234. doi: 10.1002/aur.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, … Piven J (2013). White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry, 170(8), 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, … Network I (2013). Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev Sci, 16(2), 186–197. doi: 10.1111/desc.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Weismer S, Lord C, & Esler A (2010). Early language patterns of toddlers on the autism spectrum compared to toddlers with developmental delay. J Autism Dev Disord, 40(10), 1259–1273. doi: 10.1007/s10803-010-0983-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Gao W, & Lin W (2016). Longitudinal Study of the Emerging Functional Connectivity Asymmetry of Primary Language Regions during Infancy. J Neurosci, 36(42), 10883–10892. doi: 10.1523/JNEUROSCI.3980-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, & Courchesne E (2012). A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain, 135(Pt 3), 949–960. doi: 10.1093/brain/awr364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, & Bates E (2007). MacArthur-Bates Communicative Development Inventories: User’s guide and technical manual (2nd ed.) Baltimore, MD: Brookes. [Google Scholar]
- Fitzgerald J, Leemans A, Kehoe E, O’Hanlon E, Gallagher L, & McGrath J (2017). Abnormal Fronto-Parietal White Matter Organisation in the Superior Longitudinal Fasciculus Branches in Autism Spectrum Disorders. Eur J Neurosci. doi: 10.1111/ejn.13655 [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, … Lainhart JE (2010). Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage, 51(3), 1117–1125. doi: 10.1016/j.neuroimage.2010.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M, Duku E, Armstrong V, Brian J, Bryson SE, Garon N, … Smith IM (2018). Variability in Verbal and Nonverbal Communication in Infants at Risk for Autism Spectrum Disorder: Predictors and Outcomes. J Autism Dev Disord. doi: 10.1007/s10803-018-3607-9 [DOI] [PubMed] [Google Scholar]
- Friederici AD (2009). Pathways to language: fiber tracts in the human brain. Trends Cogn Sci, 13(4), 175–181. doi: 10.1016/j.tics.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: from structure to function. Physiol Rev, 91(4), 1357–1392. doi: 10.1152/physrev.00006.2011 [DOI] [PubMed] [Google Scholar]
- Friederici AD (2012). Language development and the ontogeny of the dorsal pathway. Front Evol Neurosci, 4, 3. doi: 10.3389/fnevo.2012.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2017). Evolution of the neural language network. Psychon Bull Rev, 24(1), 41–47. doi: 10.3758/s13423-016-1090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2018). The neural basis for human syntax: Broca’s area and beyond. Current Opinion in Behavioral Sciences, 21, 88–92. doi: 10.1016/j.cobeha.2018.03.004 [DOI] [Google Scholar]
- Friederici AD, Chomsky N, Berwick RC, Moro A, & Bolhuis JJ (2017). Language, mind and brain. Nature Human Behaviour, 1(10), 713–722. doi: 10.1038/s41562-017-0184-4 [DOI] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, & Sigman M (2007). The development of young siblings of children with autism from 4 to 54 months. J Autism Dev Disord, 37(1), 171–183. doi: 10.1007/s10803-006-0341-5 [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, & Gilmore JH (2009). Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol, 30(2), 290–296. doi: 10.3174/ajnr.A1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH (2011). Genetics of autism spectrum disorders. Trends Cogn Sci, 15(9), 409–416. doi: 10.1016/j.tics.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, & Rilling JK (2008). DTI tractography of the human brain’s language pathways. Cereb Cortex, 18(11), 2471–2482. doi: 10.1093/cercor/bhn011 [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord, 39(5), 693–705. doi: 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends Cogn Sci, 13(2), 65–73. doi: 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci, 11(9), 651–659. doi: 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringshaw AJ, Ammons CJ, DeRamus TP, & Kana RK (2016). Hemispheric differences in language processing in autism spectrum disorders: A meta-analysis of neuroimaging studies. Autism Res, 9(10), 1046–1057. doi: 10.1002/aur.1599 [DOI] [PubMed] [Google Scholar]
- Hudry K, Chandler S, Bedford R, Pasco G, Gliga T, Elsabbagh M, … Charman T (2014). Early language profiles in infants at high-risk for autism spectrum disorders. J Autism Dev Disord, 44(1), 154–167. doi: 10.1007/s10803-013-1861-4 [DOI] [PubMed] [Google Scholar]
- Hudry K, Leadbitter K, Temple K, Slonims V, McConachie H, Aldred C, … Consortium P (2010). Preschoolers with autism show greater impairment in receptive compared with expressive language abilities. Int J Lang Commun Disord, 45(6), 681–690. doi: 10.3109/13682820903461493 [DOI] [PubMed] [Google Scholar]
- Iverson JM, & Wozniak RH (2007). Variation in vocal-motor development in infant siblings of children with autism. J Autism Dev Disord, 37(1), 158–170. doi: 10.1007/s10803-006-0339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R, Houfflin-Debarge V, Delion P, Pruvo JP, Thomas P, & Pins D (2012). Assessing fetal response to maternal speech using a noninvasive functional brain imaging technique. Int J Dev Neurosci, 30(2), 159–161. doi: 10.1016/j.ijdevneu.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Jardri R, Pins D, Houfflin-Debarge V, Chaffiotte C, Rocourt N, Pruvo JP, … Thomas P (2008). Fetal cortical activation to sound at 33 weeks of gestation: a functional MRI study. Neuroimage, 42(1), 10–18. doi: 10.1016/j.neuroimage.2008.04.247 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, & Smith SM (2012). Fsl. Neuroimage, 62(2), 782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Joseph RM, Fricker Z, Fenoglio A, Lindgren KA, Knaus TA, & Tager-Flusberg H (2014). Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav, 8(1), 60–72. doi: 10.1007/s11682-013-9245-0 [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Muller RA, Cohen DN, & Courchesne E (2008). Atypical functional lateralization of language in autism spectrum disorders. Brain Res, 1221, 115–125. doi: 10.1016/j.brainres.2008.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, & Tager-Flusberg H (2010). Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang, 112(2), 113–120. doi: 10.1016/j.bandl.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, & Garrett-Mayer E (2006). Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry, 47(6), 629–638. doi: 10.1111/j.1469-7610.2006.01531.x [DOI] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, … Lainhart JE (2010). Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res, 3(6), 350–358. doi: 10.1002/aur.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Evans AC, Pruett JR, Botteron K, Zwaigenbaum L, Estes A, … Piven J (2014). Network inefficiencies in autism spectrum disorder at 24 months. Transl Psychiatry, 4, e388. doi: 10.1038/tp.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Evans AC, Pruett JR Jr., Botteron KN, McKinstry RC, Zwaigenbaum L, … Infant Brain Imaging Study, N. (2017). The Emergence of Network Inefficiencies in Infants With Autism Spectrum Disorder. Biol Psychiatry, 82(3), 176–185. doi: 10.1016/j.biopsych.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Tager-Flusberg H, Dowd M, Tomblin JB, & Folstein SE (2008). Overlap between autism and specific language impairment: comparison of Autism Diagnostic Interview and Autism Diagnostic Observation Schedule scores. Autism Res, 1(5), 284–296. doi: 10.1002/aur.43 [DOI] [PubMed] [Google Scholar]
- Li H, Xue Z, Ellmore TM, Frye RE, & Wong ST (2014). Network-based analysis reveals stronger local diffusion-based connectivity and different correlations with oral language skills in brains of children with high functioning autism spectrum disorders. Hum Brain Mapp, 35(2), 396–413. doi: 10.1002/hbm.22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, & Styner M (2010). Quality Control of Diffusion Weighted Images. Proc SPIE Int Soc Opt Eng, 7628. doi: 10.1117/12.844748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C, Solso S, … Courchesne E (2015). Different functional neural substrates for good and poor language outcome in autism. Neuron, 86(2), 567–577. doi: 10.1016/j.neuron.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule 2nd Edition Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill M, Phelps Hanzel E, Hutman T, … Ozonoff S (2016). School-age outcomes of infants at risk for autism spectrum disorder. Autism Res, 9(6), 632–642. doi: 10.1002/aur.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Young GS, Hutman T, Johnson S, Schwichtenberg AJ, & Ozonoff S (2015). Early pragmatic language difficulties in siblings of children with autism: implications for DSM-5 social communication disorder? J Child Psychol Psychiatry, 56(7), 774–781. doi: 10.1111/jcpp.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, & Bryson S (2006). Early language and communication development of infants later diagnosed with autism spectrum disorder. Developmental and Behavioral Pediatrics, 27(2), S69–S78. [DOI] [PubMed] [Google Scholar]
- Mori S, & Zhang J (2006). Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 51(5), 527–539. doi: 10.1016/j.neuron.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Nevill R, Hedley D, Uljarevic M, Sahin E, Zadek J, Butter E, & Mulick JA (2017). Language profiles in young children with autism spectrum disorder: A community sample using multiple assessment instruments. Autism. doi: 10.1177/1362361317726245 [DOI] [PubMed] [Google Scholar]
- Papinutto N, Galantucci S, Mandelli ML, Gesierich B, Jovicich J, Caverzasi E, … Gorno-Tempini ML (2016). Structural connectivity of the human anterior temporal lobe: A diffusion magnetic resonance imaging study. Hum Brain Mapp, 37(6), 2210–2222. doi: 10.1002/hbm.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, & Klin A (2011). Out of the mouths of babes: vocal production in infant siblings of children with ASD. J Child Psychol Psychiatry, 52(5), 588–598. doi: 10.1111/j.1469-7610.2010.02332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, … Friederici AD (2011). Neural language networks at birth. Proceedings of the National Academy of Sciences, 108(38), 16056–16061. doi: 10.1073/pnas.1102991108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Mori S, & Miller MI (2015). Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol, 66, 853–876. doi: 10.1146/annurev-psych-010814-015340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, & Courchesne E (2008). Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry, 64(7), 589–598. doi: 10.1016/j.biopsych.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Haist F, & Courchesne E (2008). Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci, 11(2), 237–252. doi: 10.1111/j.1467-7687.2008.00674.x [DOI] [PubMed] [Google Scholar]
- Sadeghi N, Prastawa M, Fletcher PT, Wolff J, Gilmore JH, & Gerig G (2013). Regional characterization of longitudinal DT-MRI to study white matter maturation of the early developing brain. Neuroimage, 68, 236–247. doi: 10.1016/j.neuroimage.2012.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hirabayashi Y, Tsubokura H, Kanai M, Ashida T, Konishi I, … Maki A (2012). Cerebral hemodynamics in newborn infants exposed to speech sounds: a whole-head optical topography study. Hum Brain Mapp, 33(9), 2092–2103. doi: 10.1002/hbm.21350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery AM, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2013). Atypical lateralization of ERP response to native and non-native speech in infants at risk for autism spectrum disorder. Dev Cogn Neurosci, 5, 10–24. doi: 10.1016/j.dcn.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, & Shen D (2011). Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One, 6(4), e18746. doi: 10.1371/10.1371/journal.pone.0018746.g001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide MA, & Friederici AD (2016). The ontogeny of the cortical language network. Nat Rev Neurosci, 17(5), 323–332. doi: 10.1038/nrn.2016.23 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23 Suppl 1, S208–219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Solso S, Xu R, Proudfoot J, Hagler DJ Jr., Campbell K, Venkatraman V, … Courchesne E (2016). Diffusion Tensor Imaging Provides Evidence of Possible Axonal Overconnectivity in Frontal Lobes in Autism Spectrum Disorder Toddlers. Biol Psychiatry, 79(8), 676–684. doi: 10.1016/j.biopsych.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperdin HF, & Schaer M (2016). Aberrant Development of Speech Processing in Young Children with Autism: New Insights from Neuroimaging Biomarkers. Front Neurosci, 10, 393. doi: 10.3389/fnins.2016.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MR, Wolff JJ, Elison JT, Gu H, Hazlett HC, Botteron K, … Network I (2017). Splenium development and early spoken language in human infants. Dev Sci, 20(2). doi: 10.1111/desc.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, … Alexander AL (2012). Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res, 5(5), 289–313. doi: 10.1002/aur.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Valk J, Bakker CJ, Schooneveld M, Faber JAJ, Willemse J, & Gooskens RHJM (1991). Myelination as an expression of the functional maturity of the brain. Dev Med Child Neurol, 33, 849–857. [DOI] [PubMed] [Google Scholar]
- von Hohenberg C, Wigand MC, Kubicki M, Leicht G, Giegling I, Karch S, … Mulert C (2013). CNTNAP2 polymorphisms and structural brain connectivity: a diffusion-tensor imaging study. J Psychiatr Res, 47(10), 1349–1356. doi: 10.1016/j.jpsychires.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Marchina S, Norton A, & Schlaug G (2012). Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann N Y Acad Sci, 1252, 332–337. doi: 10.1111/j.1749-6632.2012.06446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, … Ben Bashat D (2011). Abnormal white matter integrity in young children with autism. Hum Brain Mapp, 32(4), 534–543. doi: 10.1002/hbm.21042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, … Piven J (2012). Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry, 169, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Jacob S, & Elison JT (2017). The journey to autism: Insights from neuroimaging studies of infants and toddlers. Development and Psychopathology, 1–17. doi: 10.1017/s0954579417000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Swanson MR, Elison JT, Gerig G, Pruett JR Jr., Styner MA, … Network I (2017). Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism, 8, 8. doi: 10.1186/s13229-017-0126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Qiu T, Ke X, Xiao X, Xiao T, Liang F, … Liu Y (2014). Autism spectrum disorder as early neurodevelopmental disorder: evidence from the brain imaging abnormalities in 2–3 years old toddlers. J Autism Dev Disord, 44(7), 1633–1640. doi: 10.1007/s10803-014-2033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li K, Zhang C, Qi X, Zheng N, & Wang G (2018). Arcuate Fasciculus in Autism Spectrum Disorder Toddlers with Language Regression. Open Med (Wars), 13, 90–95. doi: 10.1515/med-2018-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, … Sigman M (2007). Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J Autism Dev Disord, 37(3), 466–480. doi: 10.1007/s10803-006-0179-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.