Abstract
Cocaine is a highly abused drug and cocaine addiction affects millions of individuals worldwide. Cocaine blocks normal uptake function at the dopamine transporter (DAT), thus increasing extracellular dopamine. Currently, no chemical therapies are available to treat cocaine abuse. Previous works showed that the selective inhibitors of protein kinase Cβ (PKCβ), enzastaurin and ruboxistaurin, attenuate dopamine overflow and locomotion stimulated by another psychostimulant drug, amphetamine. We now test if ruboxistaurin similarly affects cocaine action. Perfusion of 1 μM ruboxistaurin directly into the core of the nucleus accumbens via retrodialysis reduced cocaine-stimulated increases in dopamine overflow, measured using microdialysis sampling, with simultaneous reductions in locomotor behavior. Because cocaine activity is highly regulated by dopamine autoreceptors, we examined whether ruboxistaurin was acting at the level of the D2-autoreceptor. Perfusion of 5 μM raclopride, a selective D2-like receptor antagonist, before addition of ruboxistaurin abrogated the effect of ruboxistaurin on cocaine-stimulated dopamine overflow and hyperlocomotion. Further, ruboxistaurin was inactive against cocaine-stimulated locomotor activity in mice with a genetic deletion in D2 receptors as compared to wild type mice. In contrast, blockade or deletion of dopamine D2 receptors did not abolish the attenuating effect of ruboxistaurin on amphetamine-stimulated activities. Therefore, the inhibition of PKCβ reduces dopamine overflow and locomotor activity stimulated by both cocaine and amphetamine, but the mechanism of action differs for each stimulant. These data suggest that inhibition of PKCβ would serve as a target to reduce the abuse of either amphetamine or cocaine.
Keywords: protein kinase C beta, dopamine transporter, norepinephrine, D2 dopamine receptor knockouts, protein kinase C beta inhibition
Introduction
Amphetamine and cocaine are euphoric stimulants that cause mania, tachycardia, and hedonia by increasing extracellular dopamine.1 Despite the high economic and social costs of stimulant abuse, no therapeutics have been approved to treat abuse of these drugs. Our studies suggest that a novel target for reducing amphetamine abuse would be protein kinase Cβ (PKCβ). We and others have previously shown that inhibition or deletion of PKC, especially the β subtype, reduces amphetamine-stimulated neurochemical effects and locomotion2–5. Amphetamine activates PKC6–7 likely through a flux of intracellular calcium (Ca2+)6, 8. Numerous reports suggest a positive relationship between stimulation of PKC and outward transport through the dopamine transporter (DAT)5–7, 9,10–11. PKC phosphorylates N-terminal serines of DAT; phosphorylation of these sites is reported to be permissive for reversal of the transporter and efflux of dopamine into the extracellular space12–14. PKC inhibitors effectively block amphetamine-stimulated reverse transport, but not influx through the DAT2–4. This result is consistent with the finding that conditions that increase the outward conformation of DAT correlate with increased PKC activity6.
Cocaine also increases extracellular dopamine, but by a mechanism different from that of amphetamine. Cocaine blocks dopamine re-uptake but is not a substrate for DAT and does not evoke reverse transport. As a result, cocaine requires neuronal dopamine release to be effective (See schematic in Figure 6). Evidence suggests that acute cocaine does not directly increase PKC activity15; although, indirect activation of PKC by cocaine via activation of dopamine D2 receptors has been reported.16 If PKCβ inhibitors reduce amphetamine- stimulated dopamine efflux and behaviors by halting a PKCβ-related reversal of the dopamine transporter, then one might expect PKCβ inhibitors to have no immediate effect on cocaine-stimulated dopamine overflow and related hyperactivity.
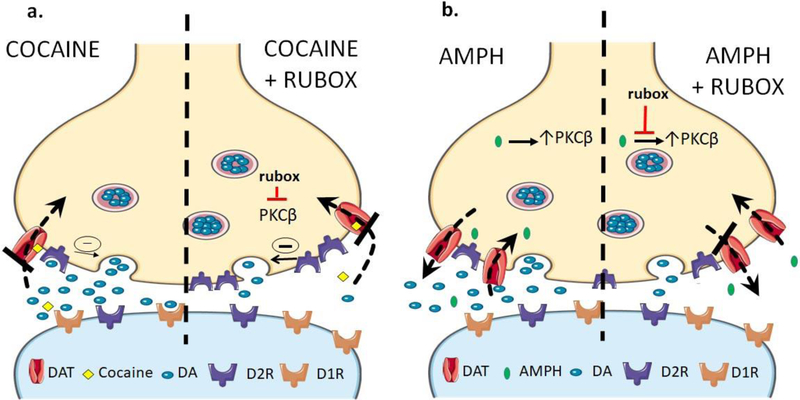
Figure 6: Ruboxistaurin inhibits dopamine overflow elicited by amphetamine (AMPH) and cocaine by two different mechanisms. a. (Cocaine, left side).
Cocaine increases extracellular dopamine by blocking the dopamine transporter64. Because cocaine blocks the reuptake of dopamine at the dopamine transporter that has been released by exocytosis, its effect will be regulated by D2-like autoreceptor activity. PKC activation normally inhibits dopamine autoreceptor activity by reducing surface expression of D2 receptors which will contribute to the concentration of extracellular dopamine24–25, 65. a. (Cocaine + rubox, right side) Ruboxistaurin inhibits PKCβ, increasing the surface expression of D2 autoreceptors by blocking their internalization thus enhancing their effectiveness in inhibiting exocytosis of dopamine. This leads to a reduction in extracellular dopamine (dopamine overflow). b. (AMPH, left side) The mechanism of amphetamine action is dependent on the dopamine transporter. Amphetamine is a substrate for the dopamine transporter and will elicit dopamine efflux through reversal of the transporter66. Amphetamine activity does not depend on dopamine exocytosis and is not significantly regulated by dopamine autoreceptors41–42. Amphetamine that is taken up through the transporter activates PKCβ6–7 which potentiates reversal of the transporter and dopamine efflux5, 9. b. (AMPH + Rubox, right side) Blockade of PKCβ reduces the outward transport of dopamine but does not affect inward transport2–4, 10–11, 54–55. The end result of ruboxistaurin action on activation by either cocaine or amphetamine is a reduction in extracellular dopamine (or norepinephrine) and a reduction in stimulant-induced locomotor activity.
Increases in the levels of extracellular dopamine elicited by cocaine are also modulated by dopamine autoreceptors17–18, particularly in the basal ganglia. Deletion of D2 autoreceptors results in increases in cocaine-stimulated locomotion19 and enhanced acquisition of cocaine- taking behaviors20 whereas activation of D2/D3 subtype dopamine autoreceptors in the ventral tegmental area reduced cocaine-reinstated drug-seeking behavior21–22. Therefore, PKC could affect cocaine function by modulating dopamine autoreceptor function. Supporting this idea, Cubeddu et al23 reported that PKC activation reduced the effectiveness of release-modulating dopamine receptors located on dopamine terminals. The postulate of Cubeddu23 that PKC activation would reduce surface levels of the D2-type autoreceptors was verified in cultured cells24 and in rat striatal synaptosomes25. We found that inhibition or deletion of PKCβ enhanced the D2 autoreceptor-mediated decrease in dopamine release following depolarization in rat striatum and enhanced surface levels of D2-like receptors in mouse striatal synaptosomes25. In other words, inhibition of PKCβ enhanced autoreceptor function and promoted inhibition of dopamine release.
Previously, we demonstrated that the PKCβ inhibitors ruboxistaurin and enzastaurin, when delivered directly into the core of the nucleus accumbens, attenuated amphetamine- stimulated dopamine efflux in vivo as measured by microdialysis with liquid chromatography- tandem mass spectrometry (LC-MS/MS)4. These PKCβ inhibitors did not affect basal levels of dopamine nor did they affect uptake by DAT as measured in striatal synaptosomes. In this study, we show that ruboxistaurin attenuates cocaine-stimulated increases in extracellular dopamine, metabolites of dopamine, and locomotion. The effectiveness of the PKCβ inhibitor was significantly reduced when D2-like dopamine receptor activity was blocked by either intra- accumbens raclopride, an antagonist at both D2 and D3 dopamine receptors, or by deletion of D2 receptors, suggesting a D2 receptor- and perhaps D3 receptor-controlled mechanism. These studies demonstrate that ruboxistaurin inhibits the effects of cocaine as well as those of amphetamine, but by a different mechanism.
Results and Discussion
Effect of ruboxistaurin on cocaine-stimulated dopamine overflow and locomotion
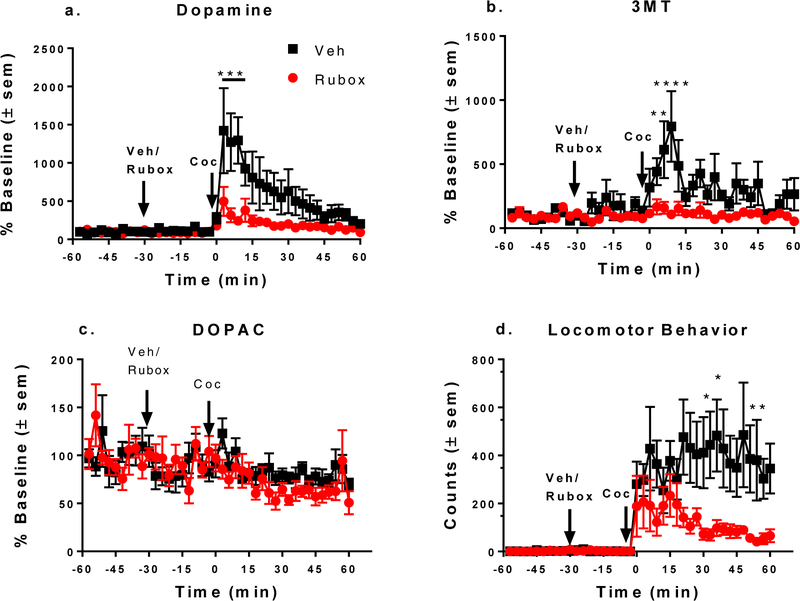
in vivo microdialysis and LC-MS/MS were utilized to measure neurochemical changes in analyte overflow in the core of the nucleus accumbens. Probe placements were determined using histology and are shown in Figure 1. Either vehicle or the selective PKCβ inhibitor, ruboxistaurin, was perfused directly into the core of the nucleus accumbens via retrodialysis 1 h prior to injection of 15 mg/kg cocaine i.p. to test its effect on cocaine-stimulated increases in overflow of dopamine, dopamine metabolites, and locomotion. As shown in Figure 2a, ruboxistaurin significantly decreased cocaine-stimulated overflow of dopamine. In a 2-way repeated measures ANOVA (n = 6 for both groups), there was significance for time (F (39, 390) = 7.951, p<0.0001) and the interaction of ruboxistaurin and time (F (39, 390) = 3.222, p < 0.0001) while ruboxistaurin itself was just shy of significance (F (1, 10) = 4.916, p = 0.05). Similarly, ruboxistaurin significantly decreased 3-methoxytyramine (Figure 2b), which is the initial product of extracellular dopamine metabolism due to postsynaptic catechol-O- methyltransferase (COMT) activity. In 2-way repeated measures ANOVA, there was significance for time (F (39, 390) = 3.103, p < 0.0001), ruboxistaurin (F (1, 10) = 8.5, p = 0.015) and the interaction of ruboxistaurin and time (F (39, 390) = 2.239, p < 0.0001). There was no significant change in dihydroxyphenylacetic acid (DOPAC), a primarily presynaptic dopamine metabolite due to monoamine oxidase activity, in response to ruboxistaurin (Figure 2c). Locomotor activity of the animals was measured simultaneously with microdialysis by the use of a Raturn, a bench- top counter-balanced arm and tethering system. Treatment with ruboxistaurin significantly decreased cocaine-stimulated locomotor behavior as measured simultaneously with collection of the analytes (Figure 2d). In a 2-way repeated measures ANOVA, there was statistical significance for time (F (39, 390) = 7.037, p = 0.0001), ruboxistaurin (F (1, 10) = 9.006, p = 0.013) and interaction between ruboxistaurin and time (F (39, 390) = 2.445, p < 0.0001).
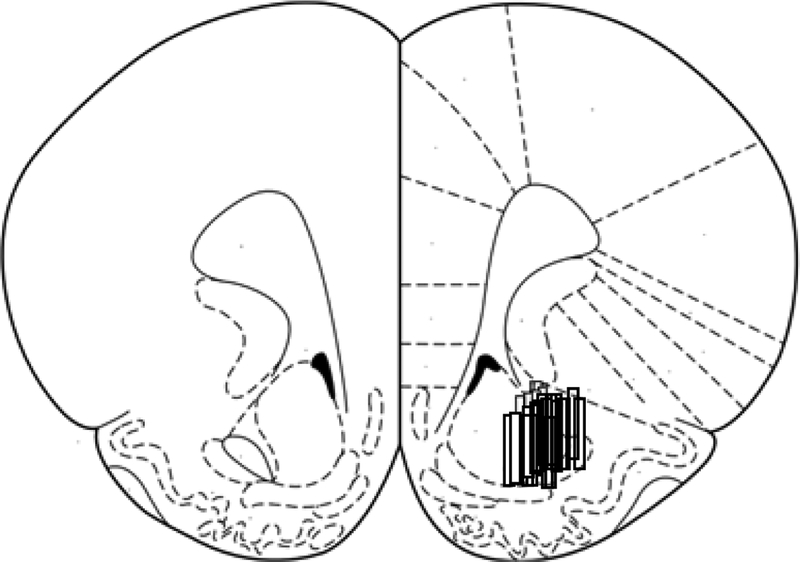
Figure 1:
Microdialysis Probe placements targeting the core of the nucleus accumbens during microdialysis experiments for data in Figures 2–4.
Figure 2: Pretreatment with ruboxistaurin reduces effectiveness of cocaine in stimulating dopamine overflow and locomotor behavior.
At 30 min of baseline collection, vehicle (Veh) or 1 μM ruboxistaurin (Rubox) was perfused into the core of the nucleus accumbens for the remaining duration of the experiment. A 15 mg/kg i.p. injection of cocaine (Coc) was given 30 min later (at time zero) and fractions were collected for an additional h. Perfusion with ruboxistaurin attenuates cocaine-stimulated overflow of a. dopamine; b. 3-methoxytyramine (3MT); and d. locomotor activity. c. Neither cocaine nor ruboxistaurin affected dihydroxyphenylacetic acid (DOPAC) levels. Results are given as % Baseline or locomotor counts (counts) ± standard error of the mean (sem) (n=6). In post-hoc Bonferroni multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 as compared to ruboxistaurin- treated samples.
Effect of D2/D3 dopamine receptor blockade on ruboxistaurin action
We previously reported similar results for the inhibition of amphetamine-evoked activities by two selective PKCβ inhibitors, ruboxistaurin and enzastaurin4. The effect of the PKC inhibitors is attributed to the inhibition of amphetamine-stimulated phosphorylation which would then reduce reverse transport through the DAT2, 6,26. Activation of PKC by cocaine in the striatum and nucleus accumbens has been demonstrated but the effect is indirect through the activation of dopamine receptors27–28. Acute cocaine had no effect on PKC activity in the medial prefrontal cortex15.
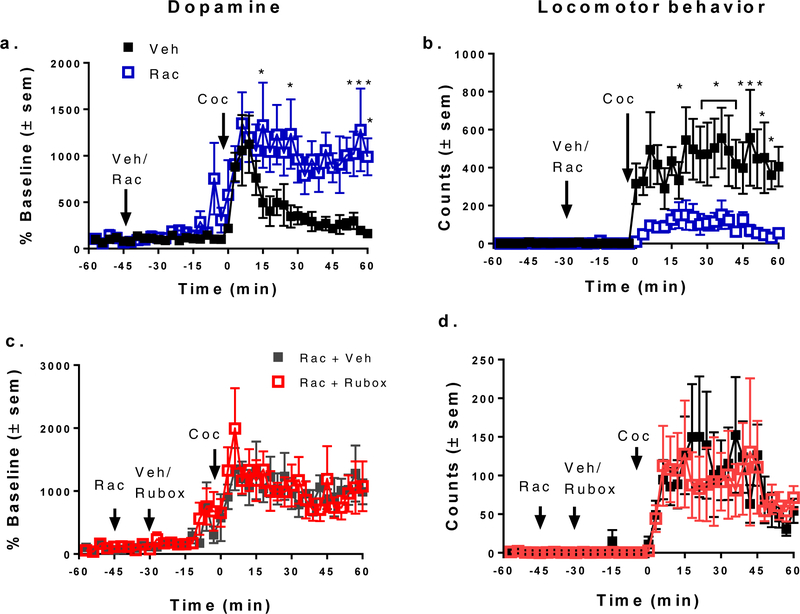
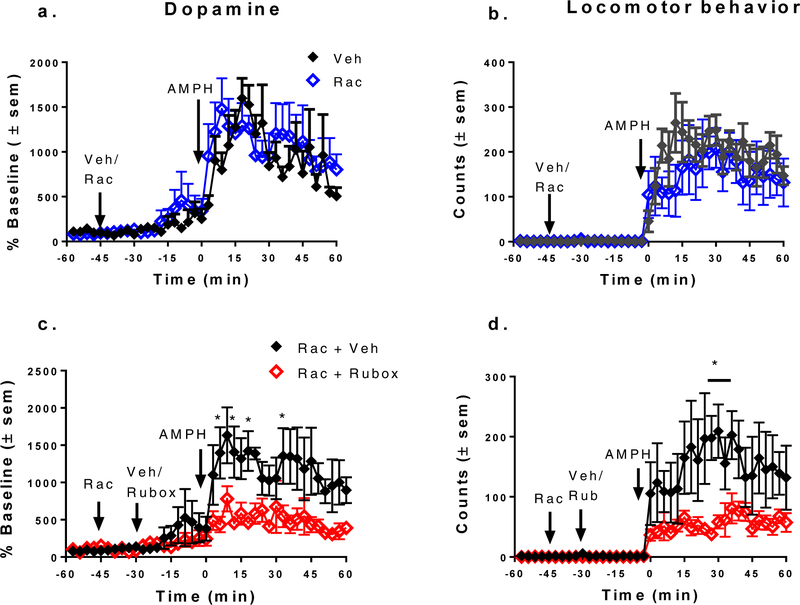
We hypothesized that ruboxistaurin could be inhibiting cocaine activity by enhancing the effectiveness of D2/D3 autoreceptors, as dopamine autoreceptor activation significantly reduces cocaine-stimulated activities19–20, 22, 29. To test this hypothesis, we used the D2/D3 dopamine receptor antagonist, raclopride, to block D2/D3 receptors. Pilot experiments showed that infusion of 5 μM raclopride was effective in increasing cocaine-stimulated dopamine overflow (Figure 3a), which would be expected if dopamine autoreceptors were blocked. In 2way repeated measures ANOVA, there was statistical significance for time (F (39, 312) = 8.466, p < 0.001), raclopride (F (1, 8) = 16.73, p = 0.0035) and the interaction of raclopride and time (F (39, 312) = 2.177, p < 0.0001). Perfusion with raclopride did not enhance the peak response to cocaine, but did extend the duration of dopamine overflow in response to cocaine. In contrast to the effect of raclopride on cocaine-stimulated dopamine overflow, cocaine-stimulated locomotor activity was significantly reduced by raclopride as compared to vehicle (Figure 3b). In a 2-way repeated measures ANOVA, there was statistical difference for time (F (39, 312) = 6.916p < 0.0001), raclopride (F (1, 8) = 20.99, p = 0.0018), and the interaction of raclopride and time (F (39, 312) = 2.965, p < 0.0001). We did not note stereotyped behavior in the rats receiving cocaine and raclopride. Dopamine D2/D3 receptor antagonists reduce cocaine- stimulated locomotor behavior,30–31 likely due to inhibition of postsynaptic D2/D3 receptors32–33 or dopamine heteroreceptors on non-medium spiny cells34.
Figure 3. Pretreatment with raclopride enhances cocaine-stimulated dopamine overflow (a) but inhibits cocaine-stimulated locomotor behavior (b).
In a,b, fifteen min of baseline were collected followed by perfusion of vehicle (Veh) or 5 μM raclopride (Rac) into the core of the nucleus accumbens for the remaining duration of the experiment (90 min). At 1 h (0 time), a 15 mg/kg injection of cocaine (Coc) was given i.p. and fractions were collected for an additional h. a. In post hoc Bonferroni multiple comparison test, * p < 0.05, ***, p < 0.001, n = 5. b. In post hoc Bonferroni multiple comparison test, *p < 0.02, n = 5. In c,d. pretreatment with raclopride blocks inhibition of cocaine-stimulated dopamine overflow (c.) and locomotor activity (d.) by 1 μM ruboxistaurin. Raclopride was perfused after 15 min (−45 min) and either vehicle (Rac + Veh, n=5) or 1 μM ruboxistaurin (Rac + Rubox, n=5) was perfused at −30 min). At 1 h (0 time), a 15 mg/kg i.p., injection of cocaine was given. Results are expressed as % baseline or locomotor counts in 1 h after addition of cocaine ± sem.
We then evaluated if raclopride infused into the nucleus accumbens before application of 1 μM ruboxistaurin would mitigate the consequences of PKC inhibition on cocaine effects. As opposed to the striking inhibitory effect of ruboxistaurin on cocaine-stimulated dopamine overflow in the absence of raclopride, ruboxistaurin had no effect on cocaine-stimulated increases in extracellular dopamine following pretreatment with raclopride (Figures 3c). This result was also true for the metabolite 3-methoxytyramine (data not shown). These data strongly suggest that D2/D3 receptors must be available to substrates for the PKC3 inhibitor to be effective. While it is possible that the elevation in dopamine induced by raclopride could be masking an inhibitory effect of ruboxistaurin, the evidence presented below in Figures 4 and 5, suggest this is not the explanation. Amphetamine produces a much greater elevation in dopamine than does cocaine, and this elevation is readily reduced by ruboxistaurin (Figure 4). Further, ruboxistaurin had no effect on cocaine-stimulated locomotor behavior in D2 receptor knockout mice, which would mimic D2 receptor blockade (Figure 5d). We demonstrated previously that a lack of PKCβ activity, through either deletion or specific inhibitors, enhanced the D2 autoreceptor inhibition of dopamine release in rat striatum following both chemical and electrical stimulations.25 Activation of PKC, including PKCβ, reduces dopamine autoreceptor function23 and internalizes D2 and D3 dopamine receptors in heterologous cells.24, 35 Inhibition of PKCβ increases the surface content of D2/D3 receptors in striatal synaptosomes which would account for the increased efficacy of the dopamine autoreceptors25 (see schematic and legend in Figure 6). There is another mechanism by which PKCβ could alter dopamine overflow. Activation of dopamine receptors increases the reuptake of dopamine by increasing the surface content of the dopamine transporter through a mechanism dependent on extracellular receptor protein kinase36,37–39. We demonstrated that PKCβ was required for this activation and was upstream of the extracellular receptor protein kinase.40 In any event, the increase in D2/D3 receptors resulting from inhibition of PKCβ would either reduce vesicular exocytosis of dopamine or increase surface levels of dopamine transporter or both.
Figure 4: Pretreatment with raclopride did not change dopamine overflow (a) or locomotion (b) elicited by amphetamine and did not block inhibition by ruboxistaurin (c,d).
In a,b, 15 min of baseline were collected followed by perfusion of 5 μM raclopride (Rac) into the core of the nucleus accumbens for the rest of the experiment (105 min). At 1 h (time 0), a 2 mg/kg injection of amphetamine was given i.p. and fractions were collected for 1 additional h. a. vehicle n=6; raclopride n=6. b. vehicle n=6, raclopride, n=5. In c,d, pretreatment with raclopride did not block inhibition of amphetamine-stimulated dopamine overflow (c) and locomotor activity (d) by 1 μM ruboxistaurin. The experiment was conducted as above, but vehicle (Rac + Veh) or 1 μM ruboxistaurin (Rac + Rubox) was perfused at −30 min (n = 5). At 1 h (0 time), a 2 mg/kg i.p., injection of amphetamine was given. Results are expressed as % baseline or locomotor counts in 1 h ± sem. c. In post-hoc Bonferroni multiple comparison test, *p < 0.05, n = 5. d. In post-hoc Bonferroni multiple comparison test, *p < 0.05. n = 5.
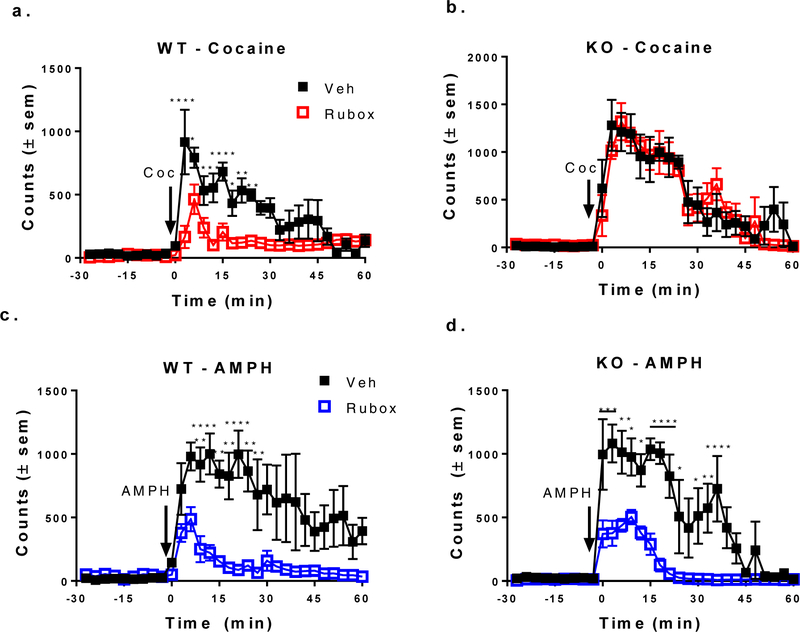
Figure 5: The effects of amphetamine and cocaine administration on wildtype and D2- autoreceptor knockout mice.
Wildtype (WT) and D2-autoreceptor Knockout (KO) C57Bl mice were tested with cocaine (Coc), amphetamine (AMPH), ruboxistaurin (Rubox), and vehicle (Veh) and locomotor behavior was measured in a raturn. The animals were given an injection of 10 pmol ruboxistaurin or vehicle into the core of the nucleus accumbens and allowed to recover after 4 h. 30 min of basal locomotor activity was recorded followed by an injection of 2 mg/kg i.p. of amphetamine or 15 mg/kg i.p. of cocaine. The injection of ruboxistaurin attenuated cocaine-stimulated increases in locomotion in wildtype (a), but not in D2-autoreceptor knockout (b) mice. The injection of ruboxistaurin also attenuated amphetamine-stimulated locomotion in WT (c) mice and KO mice (d). Results are given as locomotor counts ± standard error of the mean (sem) (n=4). In post-hoc Bonferroni multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 WT/D2R-KO mice, n=4.
Effect of raclopride on ruboxistaurin-amphetamine interactions
We next examined whether or not D2/D3 autoreceptors were involved in the inhibition of amphetamine-stimulated activities by ruboxistaurin. Figure 4a shows that pretreatment with 5 μM raclopride had no effect on dopamine overflow activated by amphetamine; moreover, there was no effect of raclopride on amphetamine-stimulated locomotor activity (Figure 4b). Our result is in agreement with prior studies showing that amphetamine-stimulated dopamine overflow is not dependent on dopamine autoreceptors41–42. Dopamine autoreceptors may not strongly influence the reverse-transport function of amphetamine, but increases in extracellular dopamine elicited by amphetamine desensitize dopamine autoreceptor function43–44. Although raclopride, administered locally in the nucleus accumbens, did not block amphetamine- stimulated locomotion in these experiments, D2 receptor antagonists given peripherally do reduce this activity45–46. In the case of amphetamine, as opposed to cocaine, local D2/D3 receptors may not have a significant effect on locomotor activity.
In examining the effect of raclopride pretreatment on ruboxistaurin action, the results differed between cocaine and amphetamine. Preincubation with raclopride did not alter the inhibition of amphetamine-stimulated dopamine overflow (Figure 4a) or locomotion (Figure 4b) by ruboxistaurin. Infusion of 1 μM ruboxistaurin, given after raclopride, significantly inhibited amphetamine-stimulated dopamine overflow (Figure 4a). In a 2-way repeated measures ANOVA, significance is attained for time (F (39, 312) = 7.389, p < 0.000), ruboxistaurin (F(1, 8) = 53.99, p < 0.0001), and the interaction of ruboxistaurin and time (F(39, 312) = 1.889, p = 0.0017). Similarly, pretreatment with raclopride did not interfere with the inhibition of amphetamine-stimulated locomotor behavior by ruboxistaurin. In a 2-way repeated measures ANOVA, significance is attained for time (F (39, 351) = 7.091, p = 0.0001), ruboxistaurin (F (1, 9) = 11.41, p = 0.0082), and the interaction of ruboxistaurin and time (F (39, 351) = 1.509, p = 0.03). These data demonstrate that the inhibition of amphetamine-stimulated dopamine overflow and locomotion by ruboxistaurin does not require D2/D3 receptors, including D2/D3 autoreceptors.
Effects of ruboxistaurin on cocaine and amphetamine activities in D2 receptor knockout mice
To buttress the finding that D2-like receptors are required for the PKC0 inhibitor to inhibit cocaine but not amphetamine action, we investigated the effect of ruboxistaurin on cocaine- and amphetamine-stimulated locomotor behavior in mice containing a genetic deletion of the D2 receptor47. Despite being constitutive knockouts, D2 receptor knockout (KO) mice have no autoreceptor function41 showing a lack of compensation for that activity by other dopamine receptors.
WT and D2-receptor KO mice were tested for locomotor activation by cocaine or amphetamine in the presence or absence of 10 pmol of ruboxistaurin injected into the nucleus accumbens. As shown in Figure 5, the locomotor results in the D2 receptor-deficient mice recapitulated the data obtained using raclopride in the rat. Ruboxistaurin attenuated locomotion stimulated by cocaine in the wildtype mice as compared to vehicle, but not in the D2 receptor KO mice (Figures 5a and 5b). For the WT mice (n=4 for all groups), in 2-way repeated measures ANOVA, there was a significant effect of time (F (29, 174) = 12.24, p < 0.0001), ruboxistaurin (F (1, 6) = 30.18, p = 0.0015) and interaction between ruboxistaurin and time (F (29, 174) = 4.537, p < 0.0001). On the other hand, as shown in Figures 5c and 5d, ruboxistaurin significantly attenuated amphetamine-stimulated locomotion in both WT (in 2-way RM ANOVA, F (29, 174) = 8.645, p < 0.0001 for time, F (1, 6) = 11.74, p = 0.014 for ruboxistaurin, and F (29, 174) = 4.16, p < 0.0001 for interaction of ruboxistaurin and time) and D2 receptor KO mice (2-way RM ANOVA, F (29, 174) = 17.29, p < 0.0001 for time, F (1, 6) = 18.65, p = 0.005 for ruboxistaurin and F (29, 174) = 5.401, p < 0.0001 for the interaction of ruboxistaurin and time) vs. the vehicle.
Examination of Figures 5a and 5b shows that the locomotor behavior in the D2 receptor KO mouse is actually greater than that of the WT mouse (Supplementary Figure 1). In a 2-way RM ANOVA, statistical significance is shown for time (F (29, 174) = 1.605, p < 0.0001), genotype (F (1, 6) = 9.102, p < 0.05) and the interaction between time and genotype (F (29, 174) = 1.605, p < 0.05). The increase in locomotor behavior in response to cocaine that we see in the mice having a global deletion of D2 receptors resembles the enhanced locomotor response to cocaine in mice having a selective deletion of D2 autoreceptors.19 However, in contrast to our results, reductions in methamphetamine- and cocaine-induced locomotor activity48–49 are reported for the constitutive D2 receptor KO mice. We cannot fully explain the discrepancies in responses at this time. One obvious difference is the methods used for measurement of locomotor behavior. In Neve et al.48, which reported decreased locomotor activity in response to methamphetamine in the D2 receptor KO mice, locomotor activity was measured by a beam- break apparatus after two days of habituation with saline injections. Our mice were habituated overnight, but locomotor behavior in our experiments is measured in a stand-alone animal behavior chamber, raturn, which may measure a different aspect of locomotor behavior.
In our experiments, we are measuring overall changes in locomotor activity to obtain a read-out with which to compare to dopamine overflow in the same animal. It is possible that remaining D3 receptors have an effect on amphetamine-stimulated locomotor activity since D3 receptors seem to contribute to this activity50. Interestingly, no difference in amphetamine- induced dopamine efflux from striatal synaptosomes was found between WT and D2 receptor KO mice41. One might imagine that a change in dopamine transporter content or function would also account for alterations in dopamine efflux or stimulant-induced locomotor behavior in between WT and D2 receptor KO mice. Comparisons of dopamine transporter function in WT versus D2 receptor KO mice have resulted in diverse findings. Some have reported no change51, a decrease52 or an increase in dopamine uptake and transporter function53. It is highly likely that methodological differences account for the disparate results. Therefore, it is difficult to postulate how a change in dopamine transporter function might account for the results in Figure 5.
Conclusion
This study points to the important role of PKCβ in regulating monoamines at different levels. We have made the novel finding that ruboxistaurin, a PKCβ inhibitor, inhibits dopamine overflow and locomotor activity stimulated by either amphetamine or cocaine. Importantly, the mechanism by which ruboxistaurin affects the activity of each stimulant is different (see Figure 6). Use of the D2/D3 receptor inhibitor, raclopride, revealed that ruboxistaurin must be affecting the activity of the dopamine autoreceptor to reduce cocaine-stimulated activities. On the contrary, the regulation of amphetamine-stimulated activities by PKC would appear to simply affect transporter function. Amphetamine that has been transported through the dopamine transporter will activate PKCP and elicit phosphorylation of PKC substrates54 such as the neuronal protein, growth-associated protein-43 (GAP-43)7, 55. The activation of PKC stimulates reverse transport of DAT9, 11 and dopamine overflow is blocked by dopamine transporter blockers. These conclusions were bolstered by the results using wild type and D2 receptor KO mice; ruboxistaurin inhibited cocaine-stimulated behavior in the wild type but not the knockout mice, but was fully able to inhibit amphetamine-stimulated locomotion in both genotypes. Taken together, these studies suggest that inhibition of PKCβ is a viable strategy to limit abuse of either amphetamine or cocaine. A PKC inhibitor synthesized by our group was affective against a motivated behavior stimulated by amphetamine (self-administration) as well as amphetamine-stimulated locomotor behavior56.
Methods and Materials
Materials
All chemicals and reagents were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Artificial cerebral spinal fluid (aCSF) was comprised of 145 mM NaCl, 2.68 mM KCl, 1.01 mM MgSO4, 1.22 mM CaCl2, 1.55 mM Na2HPO4, 0.45 mM NaH2PO4 (salts from Fisher Scientific, Pittsburgh, PA). Vehicle for ruboxistaurin was 0.05% dimethylsulfoxide (DMSO) in aCSF. Cocaine hydrochloride and amphetamine d-sulfate were purchased from the University of Michigan hospital (Ann Arbor, MI). Ruboxistaurin was obtained from NIDA-NIH (Bethesda, MD).
Microdialysis
Probes were constructed as previously described57. Briefly, 75/150 μm (i.d./o.d.) fused silica capillaries (Polymicro Technologies, Phoenix, AZ) were glued together with a 2 mm offset. The capillaries were ensheathed in a regenerated cellulose membrane with both ends sealed by polyimide resin (Grace, Deerfield, IL).
Male Sprague-Dawley rats (approximately 2 months old and 250–300 g) were obtained from Harlan (Envigo). Procedures and housing were approved by the University Committee for the Use and Care of Animals at the University of Michigan. Animals were anesthetized with 5% isoflurane and placed in a stereotaxic frame. A burr hole was drilled where the probe was to be inserted (+1.7 A/P, ±1.4 M/L, - 7.5 D/V in reference to bregma) to sample from the nucleus accumbens core58. Probes were implanted bi-laterally and measurements for both probes in each animal were averaged for a single replicate. The probes were lowered 7.5 mm from bregma. Additional burr holes were drilled for anchor screws and dental cement (A-M systems Inc., Sequim, WA) was used to hold the probes in place.
Immediately after probe insertion, animals were allowed to recover and acclimate in the Raturn testing chamber (Bioanalytical Systems, Inc., West Lafayette, IN) for 24 h. Prior to the beginning of the experiment, the probes were attached to inlet and outlet fluid lines. The rat was tethered to the balance arm of the Raturn. The microdialysis probe was perfused with aCSF at a flow rate of 1 μL/min for 1 h before the experiments began. Probe placements were checked with histology (see Figure 1).
Cocaine and Amphetamine Administration
For each microdialysis experiment, 30 min of baseline samples were collected in three- min fractions. Following baseline collection, 1 μM ruboxistaurin or vehicle, (0.05% DMSO in aCSF), was perfused into the core of the nucleus accumbens for the rest of the experiment (90 min). 30 min after administration of ruboxistaurin, an intraperitoneal (i.p.) injection of vehicle (saline), cocaine (15 mg/kg) or amphetamine (2 mg/kg) was given to the rat and fractions were collected for 1 h. In some experiments, the dopamine D2-like receptor antagonist, raclopride (5 μM), was administered by retrodialysis 15 min before administration of ruboxistaurin (1 μM) followed 30 min later by cocaine or amphetamine, as described above. Throughout each experiment, 3 min fractions were collected at a rate of 1 μl/min.
Locomotor Behavior Analysis
While animals were undergoing microdialysis, their movement was recorded using Logitech webcams (Apples, Switzerland) as previously described.4, 59–60 Data are expressed in terms of absolute locomotor activity as calculated by the detection software.
Sample derivatization
Each dialysate or standard sample was derivatized with benzoyl chloride and analyzed as described previously.61 Briefly, samples were sequentially mixed with 100 mM sodium carbonate, benzoyl chloride (2% in acetonitrile, v/v), and internal standard in a 2:1:1:1 volume ratio. Internal standard was comprised of 10 μM 3,4-dihydroxyphenylacetic acid, 3- methoxytyramine, homovanillic acid, and dopamine reacted with 13C6-benzoyl chloride in 100 mM sodium tetraborate which was then diluted 1:100 in DMSO and 1% formic acid (v/v). The samples were analyzed on a Thermo TSQ LCMS.62 Mobile phase A was 10 mM ammonium formate and 0.15% (v/v) formic acid in water. Mobile phase B was acetonitrile. The gradient was: initial, 5% B; 0.1 min, 19% B; 1 min, 26% B; 1.5 min, 75% B; 2.50 min, 100% B; 3.0 min, 100% B; 3.1 min, 5% B; 3.5 min, 5% B; 4.0 min, 0% B. The peak areas of each analyte were divided by the area of the internal standard for quantification.
D2-receptor knockout mice
All procedures were approved by the IACUC at the University of Michigan and followed the Public Health Service guidelines for the humane care and use of experimental animals. Mice were housed in ventilated cages under controlled temperature (~23oC) and photoperiod (12-h light/12-h dark cycle, lights on from 6 a.m. to 6 p.m.), with tap water and laboratory chow (LabDiet, 5L0D) containing 28.5 kcal% protein, 13.5 kcal% fat, and 58.0 kcal% carbohydrate available ad libitum. Mouse Drd2 genomic sequences were targeted with a PGK-neo cassette between SacI (GAGCTC) and Apa I (GATATC) sites.63 The PGK-Neo-bGH polyA cassette replaced exon 8 sequences that encode most of the 6’th and all of the 7’th TM domains, the third extracellular loop, and the intracellular C-terminal domain. A total of 1,221 bp are deleted from the Drd2 gene by the neo cassette insertion. Without normal splicing of exon 7 to exon 8 any transcribed mRNA from the mutant D2dr locus is rapidly degraded and no functional D2 receptor can be produced. The mice used in this study had been backcrossed onto the C57BL/6J genetic background for 30 consecutive generations. They were genotyped with a polymerase chain reaction (PCR) using genomic DNA extracted from the tail tip. Sibling wildtype and homozygous D2 receptor deletion mice derived from mating pairs of heterozygous mice were used in the experiments.
For locomotor experiments, mice were anesthetized for approximately 2 min with 5% isoflurane. The mice were given either an injection of 10 pmol of ruboxistaurin or vehicle (DMSO) in the core of the nucleus accumbens (A/P + 1.7, M/L ± 1.4, D/V – 7.5) in reference to bregma using a stereotaxic instrument and Harvard Apparatus syringe pump at a flow rate of 1 μL/min. The mice were then allowed to recover in the BASi Raturn (West Lafayette, IN) for approximately 4 h with bedding and food provided. Four h after the mice were placed in the Raturn, the baseline locomotor activities of wild type or knockout mice were recorded using a camera and Matlab software for approximately 30 min. After 30 min, control and knockout mice (injected with either ruboxistaurin or vehicle) were given an i.p. injection of 2 mg/kg amphetamine or 15 mg/kg of cocaine. Locomotor counts were subsequently collected for 1 h. The mice were then euthanized with an overdose of sodium pentobarbital.
Statistics
Data were analyzed for statistical significance using Graphpad Prism 7 by a 2-way repeated measures analysis of variance. In the 2-way ANOVA, post hoc comparison was made by comparing the means of control versus drug group at each time point using Bonferroni analysis. Statistical significance was set at p < 0.05.
Supplementary Material
Acknowledgments:
This work was supported by NIH T32 grant DA007268, American University Faculty Research Support Grant (AGZ), HHMI (C.A.C.) RO1 EB003320 (RTK), R01DA011697 (MEG) R01 DK066604 (MJL). We thank Dr. Emily Jutkiewicz for her training in cerebral mouse injections and her consultation on statistics.
References
- 1.Park TM; Haning WF, Stimulant Use Disorders. Child and Adolescent Psychiatric Clinics 2016, 25 (3), 461–471. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LA; Guptaroy B; Lund D; Shamban S; Gnegy ME, Regulation of amphetamine- stimulated dopamine efflux by protein kinase C beta. J Biol Chem 2005, 280 (12), 10914–9. [DOI] [PubMed] [Google Scholar]
- 3.Chen R; Furman CA; Zhang M; Kim MN; Gereau RW; Leitges M; Gnegy ME, Protein kinase CP is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. Journal of Pharmacology and Experimental Therapeutics 2009, 328 (3), 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zestos AG; Mikelman SR; Kennedy RT; Gnegy ME, PKCP inhibitors attenuate amphetamine-stimulated dopamine efflux. ACS chemical neuroscience 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giambalvo CT, Protein kinase C and dopamine transport—1. Effects of amphetamine in vivo. Neuropharmacology 1992, 31 (12), 1201–1210. [DOI] [PubMed] [Google Scholar]
- 6.Giambalvo CT, Differential effects of amphetamine transport vs. dopamine reverse transport on particulate PKC activity in striatal synaptoneurosomes. Synapse 2003, 49 (2), 125–133. [DOI] [PubMed] [Google Scholar]
- 7.Iwata S; Hewlett GH; Gnegy ME, Amphetamine increases the phosphorylation of neuromodulin and synapsin I in rat striatal synaptosomes. Synapse 1997, 26 (3), 281–91. [DOI] [PubMed] [Google Scholar]
- 8.Gnegy ME; Khoshbouei H; Berg KA; Javitch JA; Clarke WP; Zhang M; Galli A, Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol 2004, 66 (1), 137–43. [DOI] [PubMed] [Google Scholar]
- 9.Cowell RM; Kantor L; Hewlett GK; Frey KA; Gnegy ME, Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. European journal of pharmacology 2000, 389 (1), 59–65. [DOI] [PubMed] [Google Scholar]
- 10.Kantor L; Gnegy M, Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. Journal of Pharmacology and Experimental Therapeutics 1998, 284 (2), 592–598. [PubMed] [Google Scholar]
- 11.Opazo F; Schulz JB; Falkenburger BH, PKC links Gq-coupled receptors to DAT-mediated dopamine release. Journal of neurochemistry 2010, 114 (2), 587–596. [DOI] [PubMed] [Google Scholar]
- 12.Moritz AE; Foster JD; Gorentla BK; Mazei-Robison MS; Yang J-W; Sitte HH; Blakely RD; Vaughan RA, Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. Journal of Biological Chemistry 2013, 288 (1), 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster JD; Pananusorn B; Vaughan RA, Dopamine transporters are phosphorylated on N- terminal serines in rat striatum. J Biol Chem 2002, 277 (28), 25178–86. [DOI] [PubMed] [Google Scholar]
- 14.Khoshbouei H; Sen N; Guptaroy B; Johnson L; Lund D; Gnegy ME; Galli A; Javitch JA, N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol 2004, 2 (3), E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steketee JD; Rowe LA; Chandler LJ, The effects of acute and repeated cocaine injections on protein kinase C activity and isoform levels in dopaminergic brain regions. Neuropharmacology 1998, 37 (3), 339–347. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu J-M; Gainetdinov RR, The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews 2011, 63 (1), 182–217. [DOI] [PubMed] [Google Scholar]
- 17.Sulzer D; Cragg SJ; Rice ME, Striatal dopamine neurotransmission: regulation of release and uptake. Basal ganglia 2016, 6 (3), 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodie MS; Dunwiddie TV, Cocaine effects in the ventral tegmental area: evidence for an indirect dopaminergic mechanism of action. Naunyn-Schmiedeberg’s archives of pharmacology 1990, 342 (6), 660–665. [DOI] [PubMed] [Google Scholar]
- 19.Bello EP; Mateo Y; Gelman DM; Noafn D; Shin JH; Low MJ; Alvarez VA; Lovinger DM; Rubinstein M, Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nature neuroscience 2011, 14 (8), 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holroyd KB; Adrover MF; Fuino RL; Bock R; Kaplan AR; Gremel CM; Rubinstein M; Alvarez VA, Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues. Neuropsychopharmacology 2015, 40 (6), 1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue Y; Steketee JD; Rebec GV; Sun W, Activation of D2-like receptors in rat ventral tegmental area inhibits cocaine-reinstated drug-seeking behavior. European Journal of Neuroscience 33 (7), 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steketee JD, Injection of the protein kinase inhibitor H7 into the A10 dopamine region blocks the acute responses to cocaine: behavioral and in vivo microdialysis studies. Neuropharmacology 1993, 32 (12), 1289–1297. [DOI] [PubMed] [Google Scholar]
- 23.Cubeddu LX; Lovenberg TW; Hoffman IS; Talmaciu RK, Phorbol esters and D2- dopamine receptors. J Pharmacol Exp Ther 1989, 251 (2), 687–93. [PubMed] [Google Scholar]
- 24.Namkung Y; Sibley DR, Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem 2004, 279 (47), 49533–41. [DOI] [PubMed] [Google Scholar]
- 25.Luderman KD; Chen R; Ferris MJ; Jones SR; Gnegy ME, Protein kinase C beta regulates the D 2-Like dopamine autoreceptor. Neuropharmacology 2015, 89, 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giambalvo CT, Protein kinase C and dopamine transport—2. Effects of amphetamine in vitro. Neuropharmacology 1992, 31 (12), 1211–1222. [DOI] [PubMed] [Google Scholar]
- 27.Xue B; Guo M-L; Jin D-Z; Mao L-M; Wang JQ, Cocaine facilitates PKC maturation by upregulating its phosphorylation at the activation loop in rat striatal neurons in vivo. Brain research 1435, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortinski PI; Briand LA; Pierce RC; Schmidt HD, Cocaine-seeking is associated with PKC- dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology 2015, 92, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cubeddu L; Hoffmann I; James M, Frequency-dependent effects of neuronal uptake inhibitors on the autoreceptor-mediated modulation of dopamine and acetylcholine release from the rabbit striatum. Journal of Pharmacology and Experimental Therapeutics 1983, 226 (1), 88–94. [PubMed] [Google Scholar]
- 30.Kita K; Shiratani T; Takenouchi K; Fukuzako H; Takigawa M, Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. European neuropsychopharmacology 1999, 9 (1), 1–7. [DOI] [PubMed] [Google Scholar]
- 31.Prinssen EP; Colpaert FC; Kleven MS; Koek W, Ability of dopamine antagonists to inhibit the locomotor effects of cocaine in sensitized and non-sensitized C57BL/6 mice depends on the challenge dose. Psychopharmacology 2004, 172 (4), 409–414. [DOI] [PubMed] [Google Scholar]
- 32.Ushijima I; Carino M; Horita A, Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacology Biochemistry and Behavior 1995, 52 (4), 737–741. [DOI] [PubMed] [Google Scholar]
- 33.Kharkwal G; Radl D; Lewis R; Borrelli E, Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. Proceedings of the National Academy of Sciences 2016, 113 (41), 11609–11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anzalone A; Lizardi-Ortiz JE; Ramos M; De Mei C; Hopf FW; laccarino C; Halbout B; Jacobsen J; Kinoshita C; Welter M, Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. Journal of Neuroscience 2012, 32 (26), 9023–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho E-Y; Cho D-I; Park JH; Kurose H; Caron MG; Kim K-M, Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Molecular Endocrinology 2007, 21 (9), 2242–2254. [DOI] [PubMed] [Google Scholar]
- 36.Meiergerd SM; Patterson TA; Schenk JO, D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. Journal of neurochemistry 1993, 61 (2), 764–767. [DOI] [PubMed] [Google Scholar]
- 37.Cass WA; Gerhardt GA, Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neuroscience letters 1994, 176 (2), 259–263. [DOI] [PubMed] [Google Scholar]
- 38.Bolan EA; Kivell B; Jaligam V; Oz M; Jayanthi LD; Han Y; Sen N; Urizar E; Gomes I; Devi LA; Ramamoorthy S; Javitch JA; Zapata A; Shippenberg TS, D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol 2007, 71 (5), 1222–32. [DOI] [PubMed] [Google Scholar]
- 39.Mayfield RD; Zahniser NR, Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Molecular Pharmacology 2001, 59 (1), 113–121. [DOI] [PubMed] [Google Scholar]
- 40.Chen R; Daining CP; Sun H; Fraser R; Stokes SL; Leitges M; Gnegy ME, Protein kinase Cβ is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. Journal of neurochemistry 2013, 125 (5), 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L’hirondel M; Cheramy A; Godeheu G; Artaud F; Saiardi A; Borrelli E; Glowinski J, Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor- deficient mice. Brain research 1998, 792 (2), 253–262. [DOI] [PubMed] [Google Scholar]
- 42.Iravani MM; Kruk Z, Effects of Amphetamine on Carrier-Mediated and Electrically Stimulated Dopamine Release in Slices of Rat Caudate Putamen and Nucleus Accumbens. Journal of neurochemistry 1995, 64 (3), 1161–1168. [DOI] [PubMed] [Google Scholar]
- 43.Seutin V; Verbanck P; Massotte L; Dresse A, Acute amphetamine-induced subsensitivity of A10 dopamine autoreceptors in vitro. Brain research 1991, 558 (1), 141–144. [DOI] [PubMed] [Google Scholar]
- 44.Calipari ES; Sun H; Eldeeb K; Luessen DJ; Feng X; Howlett AC; Jones SR; Chen R, Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology 2014, 39 (8), 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharp T; Zetterstrom T; Ljungberg T; Ungerstedt U, Effect of sulpiride on amphetamine- induced behaviour in relation to changes in striatal dopamine release in vivo. European journal of pharmacology 1986, 129 (3), 411–415. [DOI] [PubMed] [Google Scholar]
- 46.Sevak RJ; Owens WA; Koek W; Galli A; Daws LC; France CP, Evidence for D2 receptor mediation of amphetamine-induced normalization of locomotion and dopamine transporter function in hypoinsulinemic rats. Journal of neurochemistry 2007, 101 (1), 151–159. [DOI] [PubMed] [Google Scholar]
- 47.Baik J-H; Picetti R; Saiardi A; Thiriet G; Dierich A; Depaulis A; Le Meur M; Borrelli E, Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 1995, 377 (6548), 424–428. [DOI] [PubMed] [Google Scholar]
- 48.Neve KA; Ford CP; Buck DC; Grandy DK; Neve RL; Phillips TJ, Normalizing dopamine D2 receptor-mediated responses in D2 null mutant mice by virus-mediated receptor restoration: comparing D2L and D2S. Neuroscience 2013, 248, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chausmer AL; Elmer GI; Rubinstein M; Low MJ; Grandy DK; Katz JL, Cocaine- induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology 2002, 163 (1), 54–61. [DOI] [PubMed] [Google Scholar]
- 50.Zhu J; Chen Y; Zhao N; Cao G; Dang Y; Han W; Xu M; Chen T, Distinct roles of dopamine D3 receptors in modulating methamphetamine-induced behavioral sensitization and ultrastructural plasticity in the shell of the nucleus accumbens. Journal of Neuroscience research 2012, 90 (4), 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benoit-Marand M; Borrelli E; Gonon F, Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. Journal of Neuroscience 2001, 21 (23), 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickinson SD; Sabeti J; Larson GA; Giardina K; Rubinstein M; Kelly MA; Grandy DK; Low MJ; Gerhardt GA; Zahniser NR, Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. Journal of neurochemistry 1999, 72 (1), 148–156. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz Y; Schmauss C; Sulzer D, Altered dopamine release and uptake kinetics in mice lacking D2 receptors. Journal of Neuroscience 2002, 22 (18), 8002–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gnegy ME, The effect of phosphorylation on amphetamine-mediated outward transport. European journal of pharmacology 2003, 479 (1), 83–91. [DOI] [PubMed] [Google Scholar]
- 55.Iwata S-I; Hewlett GK; Ferrell ST; Kantor L; Gnegy ME, Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. Journal of Pharmacology and Experimental Therapeutics 1997, 283 (3), 1445–1452. [PubMed] [Google Scholar]
- 56.Carpenter C; Zestos AG; Altshuler R; Sorenson RJ; Guptaroy B; Showalter HD; Kennedy RT; Jutkiewicz E; Gnegy ME, Direct and systemic administration of a CNS-permeant tamoxifen analog reduces amphetamine-induced dopamine release and reinforcing effects. Neuropsychopharmacology 2017, 42 (10), 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Church WH; Justice JB, Rapid sampling and analysis of extracellular dopamine in vivo. Anal. Chem 1987, 59 (5), 712–716. [DOI] [PubMed] [Google Scholar]
- 58.Paxinos G; Watson C, The rat brain in stereotaxic coordinates /George Paxinos, Charles Watson. Elsevier: Amsterdam:, 2007. [Google Scholar]
- 59.Mabrouk OS; Semaan DZ; Mikelman S; Gnegy ME; Kennedy RT, Amphetamine stimulates movement through thalamocortical glutamate release. Journal of neurochemistry 2014, 128 (1), 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mabrouk OS; Dripps IJ; Ramani S; Chang C; Han JL; Rice KC; Jutkiewicz EM, Automated touch screen device for recording complex rodent behaviors. Journal of Neuroscience Methods 2014, 233 (0), 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song P; Mabrouk OS; Hershey ND; Kennedy RT, In Vivo Neurochemical Monitoring Using Benzoyl Chloride Derivatization and Liquid Chromatography-Mass Spectrometry. Anal. Chem 2012, 84 (1), 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zestos AG; Kennedy RT, Microdialysis Coupled with LC-MS/MS for In Vivo Neurochemical Monitoring. The AAPS Journal 2017, 19 (5), 1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly MA; Rubinstein M; Asa SL; Zhang G; Saez C; Bunzow JR; Allen RG; Hnasko R; Ben-Jonathan N; Grandy DK, Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 1997, 19 (1), 103–113. [DOI] [PubMed] [Google Scholar]
- 64.Heikkila RE; Orlansky H; Cohen G, Studies on the distinction between uptake inhibition and release of [3H] dopamine in rat brain tissue slices. Biochemical pharmacology 1975, 24 (8), 847–852. [DOI] [PubMed] [Google Scholar]
- 65.Thibault D; Albert PR; Pineyro G; Trudeau L-É, Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and β-arrestin1-dependent mechanism. Journal of Biological Chemistry 2011, 286 (11), 9174–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glowinski J; Axelrod J, Effect of drugs on the uptake, release, and metabolism of H3- norepinephrine in the rat brain. Journal of Pharmacology and Experimental Therapeutics 1965, 149 (1), 43–49. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.