Abstract
Brain-derived neurotrophic factor (BDNF), traditionally known for promoting neuronal growth and development, is also a modulator of synaptic transmission. In addition to the well-characterized effects at excitatory synapses, BDNF has been shown to acutely suppress inhibitory neurotransmission; however, the underlying mechanisms are unclear. We have previously shown that at inhibitory synapses in layer 2/3 of the somatosensory cortex, BDNF induces the mobilization of endogenous cannabinoids (eCBs) that act retrogradely to suppress GABA release. Here, we hypothesized that in the hippocampus, BDNF acts similarly via eCB signaling to suppress GABAergic transmission. We found that the acute application of BDNF reduced the spontaneous inhibitory postsynaptic currents (sIPSCs) via postsynaptic TrkB receptor activation. The suppressive effects of BDNF required eCB signaling, as this effect on sIPSCs was prevented by a CB1 receptor antagonist. Further, blocking the postsynaptic eCB release prevented the effect of BDNF, whereas eCB reuptake inhibition enhanced the effect of BDNF. These results suggest that BDNF triggers the postsynaptic release of eCBs. To identify the specific eCB release by BDNF, we tested the effects of disrupting the synthesis or degradation of 2-arachidonoylcglycerol (2-AG). Blocking 2-AG synthesis prevented the effect of BDNF and blocking 2-AG degradation enhanced the effect of BDNF. However, there was no change in the effect of BDNF when anandamide degradation was blocked. Collectively, these results suggest that in the hippocampus, BDNF-TrkB signaling induces the postsynaptic release of the endogenous cannabinoid 2-AG, which acts retrogradely on the presynaptic CB1 receptors to suppress GABA release.
Keywords: BDNF, CB1, endocannabinoid, synaptic transmission, TrkB
1 ∣. INTRODUCTION
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin gene family, plays a pivotal role in neuronal survival and differentiation, as well as the formation and maturation of synapses during development (Gottmann, Mittmann, & Lessmann, 2009). BDNF binds to the tropomyosin receptor kinase B (TrkB) receptor as well as the pan-neurotrophin receptor p75. TrkB receptors are expressed throughout the central nervous system with the highest levels of expression in the neocortex and hippocampus (Masana, Wanaka, Kato, Asai, & Tohyama, 1993). At the subcellular level, TrkB receptors are expressed on the postsynaptic dendrites and axon terminals of GABAergic and glutamatergic neurons (Aoki et al., 2000; Cabelli et al., 1996; Drake, Milner, & Patterson, 1999; Fryer et al., 1996; Gomes, Hampton, El-Sabeawy, Sabo, & McAllister, 2006). In addition to its effects on survival and differentiation, BDNF modulates synaptic transmission at both inhibitory and excitatory synapses (Gottmann et al., 2009). Several studies have also shown the involvement of BDNF in long-term potentiation (LTP), a cellular mechanism thought to be required for learning and memory (Lu, Christian, & Lu, 2008; Minichiello, 2009).
BDNF modulates GABAergic neurotransmission, with acute effects that tend to be suppressive, but the mechanisms involved are unclear. In the CA1 region of the hippocampus, BDNF application reduced the amplitude of the evoked inhibitory postsynaptic currents. A reduction in the paired-pulse ratio and a decrease in the coefficient of variation suggested a presynaptic site of action for this effect (Frerking, Malenka, & Nicoll, 1998). In rat hippocampal cultures, BDNF caused a rapid reduction in the postsynaptic GABA receptor number that was responsible for a decrease in mIPSC amplitude (Brunig, Penschuck, Berninger, Benson, & Fritschy, 2001). A study by Mizoguchi et al. (2006) suggested that the effect of BDNF on GABA release was dependent on age. BDNF suppressed mIPSC frequency and amplitude in hippocampal preparations of age P14 and P21 rats but not in P7 rats. A decrease in mIPSC frequency, mIPSC amplitude, and decrease in release probability was also observed in the visual cortex of BDNF heterozygous knockout mice, suggesting that the BDNF may have both presynaptic and postsynaptic effects (Abidin, Eysel, Lessmann, & Mittmann, 2008; Abidin et al., 2006). In the cerebellar granule cells, BDNF decreased both the amplitude and frequency of the spontaneous and miniature postsynaptic currents also suggesting both pre and postsynaptic effects (Cheng & Yeh, 2003). The effect of BDNF was blocked by inclusion of a TrkB antagonist in the postsynaptic cell in the cerebellar granule cells (Cheng & Yeh, 2003). Taken together, these results suggest that the postsynaptic TrkB receptors are often required to initiate the effects of BDNF at GABAergic synapses; however, the diversity of these effects indicates that the effects of BDNF at inhibitory synapses depend on several factors including age, brain area, and cell type.
In the somatosensory cortex, we previously found that BDNF decreases the amplitude of the spontaneous inhibitory postsynaptic currents (sIPSCs), and this effect is initiated by postsynaptic TrkB activation but is expressed presynaptically. This effect was shown to be mediated by the postsynaptic release of endocannabinoids (eCBs) that act in a retrograde manner to suppress GABA release (Lemtiri-Chlieh & Levine, 2010). BDNF triggers the release of eCBs via the activation of the phospholipase C pathway (Zhao & Levine, 2014; Zhao, Yeh, & Levine, 2015). In the hippocampus, BDNF has also been shown to acutely suppress the presynaptic GABA release; however, the underlying mechanisms involved were not identified (Frerking et al., 1998). In the present study, we focused on understanding the mechanism by which BDNF suppresses GABAergic neurotransmission in CA1 hippocampal pyramidal neurons.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Slice preparation
Briefly, Swiss CD-1 mice (postnatal day 14–24) were anesthetized by 3.5% isoflurane inhalation, followed by decapitation. Whole brains were harvested quickly and immersed in ice-cold slicing solution containing (in mM) 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4-H2O, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2-6H2O, 25 d-glucose, 11.6 sodium ascorbate, and 3.1 sodium pyruvate, equilibrated with 95% O2-5% CO2 (pH 7.4, 310 ± 5 mosmol/kg). Sagittal slices (300 μm) containing hippocampus were cut with a Dosaka EM DTK-1000 vibratome (Kyoto, Japan) and transferred to an incubating chamber. Slices were then incubated for 15 min at 34°C in an incubating solution containing (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4-H2O, 25 NaHCO3, 0.5 CaCl2, 3.5 MgCl2-6H2O, 25 d-glucose, 4 sodium lactate, 2 sodium pyruvate, and 0.4 ascorbic acid (pH 7.3, 310 ± 5 mosmol/kg) before being transferred to room temperature. Slices were then individually transferred to a recording chamber (room temperature) fixed to the stage of an Olympus BX51WI upright microscope fitted with a 40× water-immersion objective lens (0.8 NA). All animal procedures were conducted using protocols approved by the University of Connecticut Institutional Animal Care and Use Committee.
2.2 ∣. Electrophysiology
Whole cell recordings were obtained from hippocampal CA1 pyramidal neurons. Neurons were visually identified by their morphology and position under infrared differential interference contrast video microscopy. Patch electrodes (6–7 MΩ) were pulled from borosilicate glass capillaries using a Flaming/Brown P-97 micropipette puller (Sutter Instrument, Novato, CA). Pipette internal solution contained (in mM) 120 CsCl, 10.0 HEPES, 10.0 phosphocreatine, 1.0 EGTA, 0.1 CaCl2, 4.0 Na2-ATP, 1.5 MgCl2, 0.4 Na-GTP, and 5.0 QX-314 (pH 7.4, 290 ± 5 mosmol/kg). The recording chamber was continuously perfused at 2.0 ml/min with carboxygenated artificial cerebrospinal fluid (aCSF) consisting of (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4-H2O, 25 NaHCO3, 2 CaCl2·2H2O, 2 MgCl2-6H2O, and 25 d-glucose (pH 7.3, 305 ± 5 mosmol/kg). The chloride equilibrium potential (ECl) was close to 0 mV; thus, the inhibitory postsynaptic currents were recorded as inward currents at a holding potential of −70 mV. The ionotropic glutamate receptor antagonists, 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and 3-[(R)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (CPP; 3 μM), were included in the bath solution in all experiments to isolate the inhibitory activity. In some experiments, miniature inhibitory postsynaptic currents (mIPSCs) were isolated by including tetrodotoxin (TTX; 500 nM) in the bath solution. Electrical currents were filtered at 2.9 kHz and digitized at >6 kHz using a HEKA EPC9 amplifier and ITC-16 digitizer (HEKA Elektronik). Series resistance (Rs) was compensated up to 50% at 100 μs lag. Cells were rejected from the analyses if input resistance (Ri) changed by >15% or fell below 100 MΩ during the experiment.
2.3 ∣. Data analysis
Offline analysis was carried out using Clampfit 10.2 (Molecular Devices) and Prism 7 (GraphPad Software). Group data are reported as mean ± SE. Statistical comparisons were made using one-way ANOVA and Dunnett’s multiple comparison test or Student’s paired t-test.
2.4 ∣. Chemicals
Unless otherwise stated, drugs were obtained from Tocris Biosciences and were delivered by bath perfusion or by intracellular application through a patch pipette, as indicated. Stock solutions of BDNF (PeproTech), CPP (Sigma–Aldrich), and carbachol were dissolved in de-ionized water. Stock solutions of WIN55-212,2 (Cayman Chemical), DNQX (Sigma-Aldrich), D034 (Aobious), K252a, ANA-12, SR141716A, AM404, JZL184, URB597, and THL were dissolved in 100% dimethyl sulfoxide (DMSO). The stock solution of TTX was dissolved in aCSF. The final concentrations of the drugs were obtained by dilution in aCSF from stock solutions on the day of recording. The final concentration of DMSO did not exceed 0.1%, which by itself had no effect on synaptic transmission.
3 ∣. RESULTS
3.1 ∣. BDNF depresses inhibitory transmission via the postsynaptic TrkB receptors
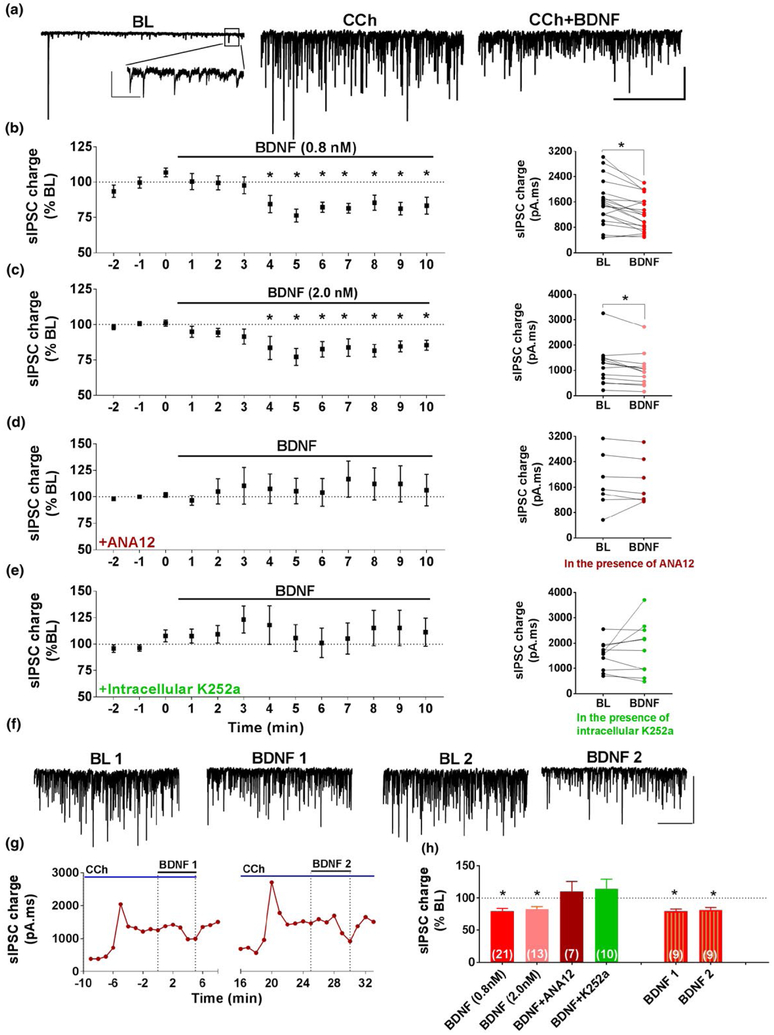
The first set of experiments examined the effect of BDNF on the inhibitory postsynaptic currents recorded from CA1 pyramidal neurons in hippocampal slices. Under basal conditions, bath application of BDNF (0.8 nM) caused a significant decrease in the mean frequency (BL: 9.6 ± 0.9 Hz; BDNF: 8.1 ± 1.2 Hz; n = 6 cells, 2 animals, p = 0.0093) but no effect on mean amplitude (BL: 45.6 ± 7.7 pA; BDNF: 46.8 ± 8.1 pA). In order to increase action potential (AP)-dependent inhibitory synaptic activity in the presence of glutamate receptor antagonists, the muscarinic agonist carbachol (CCh; 10 μM) was used to enhance interneuron firing (Kawaguchi, 1997; Lemtiri-Chlieh & Levine, 2007, 2010; Trettel, Fortin, & Levine, 2004). As shown in the example sweeps in Figure 1a, CCh increased the frequency and amplitude of AP-dependent sIPSCs. Mean sIPSC amplitude increased ~twofold from 67.4 ± 9.1 pA to 132.2 ± 15.0 pA (n = 10 cells, 2 animals, p = 0.0035) and the frequency increased from 7.4 ± 0.6 Hz to 11.8 ± 0.9 Hz (p = 0.0001). BDNF (0.8 nM) applied in the presence of CCh caused a decrease in both mean sIPSC frequency (BL: 12.1 ± 1.2 Hz; BDNF: 10.6 ± 1.1 Hz; n = 21 cells, 8 animals) and amplitude (BL: 119.5 ± 8.2 pA; BDNF: 99.8 ± 7.9 pA).
FIGURE 1.
BDNF depresses carbachol (CCh)-induced inhibitory synaptic currents in CA1 pyramidal neurons via the activation of the postsynaptic TrkB receptors. (a) Representative traces of the spontaneous action potential-dependent inhibitory postsynaptic currents (sIPSCs) recorded during baseline (BL), in the presence of carbachol (CCh; 10 μM), and in the presence of CCh + BDNF (0.8 nM). Scale bar: 200 pA, 5 s. Inset shows the events during drug-free BL. Scale bar: 50 pA, 2 s. (b) Left, Group data time course showing the effect of 0.8 nM BDNF on sIPSC charge (n = 21; 8 animals) in the presence of CCh. Right, Data from individual cells during BL and minutes 8–10 of BDNF exposure. (c) Similar layout to (b), showing effect of 2.0 nM BDNF on sIPSC charge (n = 13; 3 animals). (d) Similar layout to (b), showing the lack of effect of 0.8 nM BDNF in the presence of the TrkB receptor antagonist ANA-12 (10 μM; n = 7; 2 animals). (e) Similar layout to (b), showing the lack of effect of 0.8 nM BDNF with a postsynaptic loading of the TrkB inhibitor K252a (200 nM; n = 10; 2 animals). (f) Example traces for two separate exposures to BDNF on the same cell. Scale bar: 200 pA, 5 s. (g) Representative time course showing the effect of repeated exposure to BDNF (0.8 nM) on a single cell. (h) Group data showing the effect of BDNF on CCh-induced inhibitory activity under various conditions, *p < 0.05
Because these sIPSCs are AP-dependent, as opposed to AP-independent miniature IPSCs, changes in the frequency and amplitude of these events cannot be used to determine pre vs postsynaptic locus. Changes in the amplitude of AP-dependent events, for example, can be caused by presynaptic changes in release probability as well as by postsynaptic changes in receptor responsiveness. Changes in event frequency can be caused by changes in AP firing frequency and by changes in presynaptic release probability. Importantly, sIPSC frequency and amplitude are not independent measures, as decreases in amplitude could bring events below detection threshold and result in changes in event frequency. Thus, under these conditions, integrated synaptic charge was used to capture the total change in synaptic current.
As shown in Figure 1b, BDNF decreased sIPSC charge within 3–4 min of BDNF exposure. On average, the mean sIPSC charge decreased significantly to 79.4% ± 4.6% of CCh baseline (BL) after 8–10 mins of BDNF application (n = 21, 8 animals, ANOVA F(20,240) = 84.56, p < 0.0001). Shown at the right are the data from individual cells comparing charge during a 3-min BL to min 8–10 of BDNF application (paired t-test, p = 0.0002). Generally, sIPSC charge returned to baseline values ~ 10 min following BDNF wash out (105.5% ± 4.2% of CCh BL; n = 5, 1 animal). A similar effect was observed with BDNF at 2.0 nM concentration (Figure 1c,h). The mean sIPSC charge decreased significantly to 82.5% ± 3.9% of CCh BL (n = 13, 3 animals, ANOVA F(12,144) = 269.8, p = 0.0006). Shown in the right panel are data from individual cells (paired t-test, p = 0.0074).
Next, we determined if the effect of BDNF on sIPSCs required TrkB receptor activation. As shown in the group time course and individual cells in Figure 1d,h, application of BDNF had no significant effect on sIPSC charge in the presence of the competitive TrkB receptor antagonist ANA-12 (10 μM; 110.3% ± 15.6% of CCh BL; n = 7, 2 animals) suggesting that TrkB receptors are required to initiate the effect of BDNF. To address whether pre or postsynaptic TrkB receptors are involved, we used the Trk tyrosine kinase inhibitor K252a (200 nM) in the patch pipette to selectively block postsynaptic Trk receptor activation (Madara & Levine, 2008). Intracellular application of K252a blocked the effects of BDNF on sIPSC charge as shown in Figure 1e,h (113.9% ± 15.5% of BL; n = 10, 2 animals). Intracellular application of the DMSO vehicle did not block the effect of BDNF (82.3% ± 5.3% of BL; n = 4,1 animal, p < 0.05). These results suggest that the postsynaptic TrkB receptor activation is required for the effect of BDNF on CCh-induced inhibitory synaptic activity.
As evident from the data from individual experiments, the magnitude of the BDNF effect is highly variable across cells. We therefore examined whether the effect of repeated application of BDNF is consistent within individual cells, which is important when employing manipulations predicted to alter the magnitude of the BDNF effect. For these experiments, BDNF was applied for two 5-min exposures separated by 20 min. In each instance, the magnitude of the BDNF effect was normalized to the CCh BL prior to each application. Example traces and an individual time course for repeated BDNF exposure on the same cell are shown in Figure 1f,g. As can be seen in this example, and in the group data in Figure 1h, the effect of a 5-minute exposure to BDNF is reversible and the magnitude of the effect is similar across trials (BDNF 1:79.3% ± 3.3% of CCh BL; BDNF 2:81.2% ± 3.7% of CCh BL; n = 9, 2 animals).
3.2 ∣. Effect of BDNF requires the activation of presynaptic CB1 cannabinoid receptors
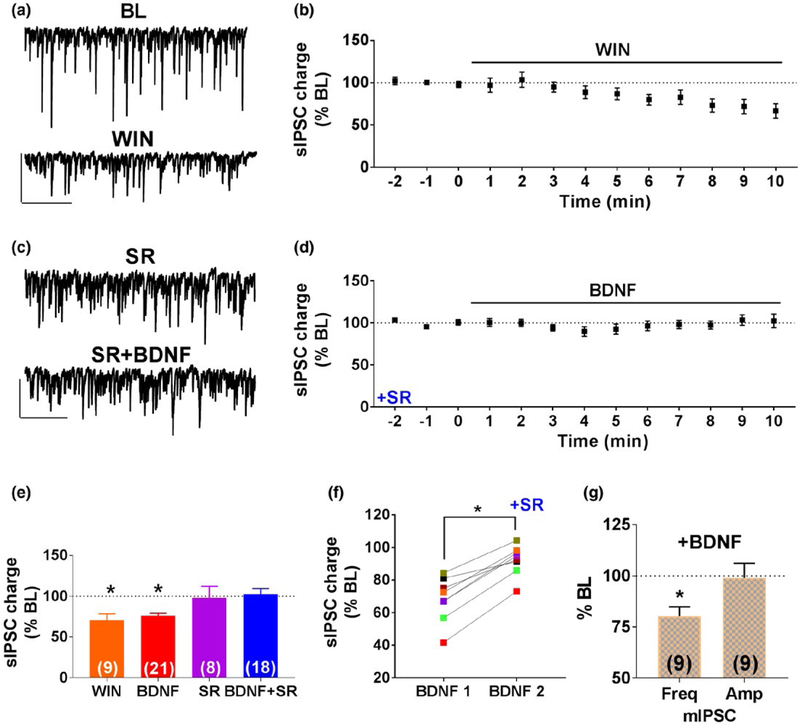
Based on our previous results in the somatosensory cortex (Lemtiri-Chlieh & Levine, 2010), we hypothesized that the effect of BDNF could be mediated by eCB release from the postsynaptic CA1 neurons in the hippocampus. We first examined whether the effect of BDNF was mimicked by the application of a CB1 receptor agonist. As shown in example traces and group time course in Figure 2a,b, acute application of the cannabinoid agonist WIN55,212-2 (WIN; 5 μM) caused a reduction in sIPSC charge to 70.4% ± 8.0% of CCh BL. This effect lasted for the duration of WIN exposure and did not recover to baseline values during the experiment (n = 9; 2 animals, paired t-test, p = 0.0108). This suppressive effect of WIN is similar to the effect of BDNF (Figure 2e). We then used the CB1 receptor inverse agonist, SR141716A (SR; 10 μM), to pharmacologically block CB1 activation. SR alone did not have any effect on CCh-induced sIPSCs within 3 min of SR exposure (100.3% ± 5.4% of CCh BL; n = 8; 2 animals) or after 10 min of SR exposure (98.3% ± 14.0% of CCh BL; Figure 2e). Next, we tested the effects of BDNF in the presence of SR. Representative traces are shown in Figure 2c. The group time course shown in Figure 2d,e illustrates that BDNF failed to suppress the inhibitory activity in the presence of SR (101.2% ± 5.7% of CCh BL; n = 18; 8 animals).
FIGURE 2.
BDNF suppression of inhibitory transmission requires the activation of type 1 cannabinoid (CB1) receptors. (a) Representative traces during BL and in the presence of the cannabinoid receptor agonist WIN55-212,2 (WIN; 5 μM). Scale bar: 200 pA, 2 s. (b) Group time course showing the suppressive effect of WIN on sIPSC charge (n = 9; 2 animals). (c) Representative traces of sIPSCs in the presence of the CB1 receptor antagonist SR141716A (SR; 10 μM) alone and in the presence of SR and BDNF. Scale bar: 200 pA, 2 s. (d) Group time course showing the lack of effect of 0.8 nM BDNF in the presence of SR (n = 18; 5 animals). (e) Group data showing the effect of WIN, SR, and BDNF + SR, on CCh-induced inhibitory activity. Also shown for comparison are the BDNF group data from Figure 1g (red). (f) Data from 8 cells of 2 animals showing the effect of BDNF on sIPSC charge (BDNF 1) and the lack of effect of BDNF in the presence of SR on the same cell (BDNF 2). (g) Group data showing the effects of BDNF on the frequency (freq) and amplitude (amp) of action potential-independent miniature IPSCs (mIPSCs) recorded in the presence of 500 nM tetrodotoxin (n = 9; 3 animals),*p < 0.05
We also used repeated exposures of BDNF on the same cell to further characterize the effect of SR. For these experiments, we used cells that showed a response to the initial BDNF exposure, followed by a second application of BDNF in the presence of SR. Consistent with the results obtained above, BDNF decreased sIPSC charge to 68.2% ± 4.8% of BL in the 1st trial and SR prevented this effect (92.1% ± 3.3% of BL in the 2nd trial) as shown in Figure 2f (n = 8; 2 animals, paired t-test, p = 0.02). Together, these results suggest that CB1 receptors are required for the suppressive effects of BDNF on CCh-induced inhibitory activity.
To isolate whether the effects of BDNF are mediated pre or postsynaptically, we examined the effects of BDNF on the frequency and amplitude of AP-independent mIPSCs recorded in the presence of tetrodotoxin (TTX; 500 nM). Bath application of BDNF caused a significant reduction in mIPSC frequency to 80.3% ± 4.5% of BL, but had no significant effect on average mIPSC amplitude (99.0% ± 7.1% of BL; n = 9; 3 animals; Figure 2g). Taken together, these results suggest that the BDNF reduces the presynaptic release probability, and this effect requires postsynaptic TrkB and presynaptic CB1 receptor activation.
3.3 ∣. Effect of BDNF is mediated by postsynaptic endocannabinoid release
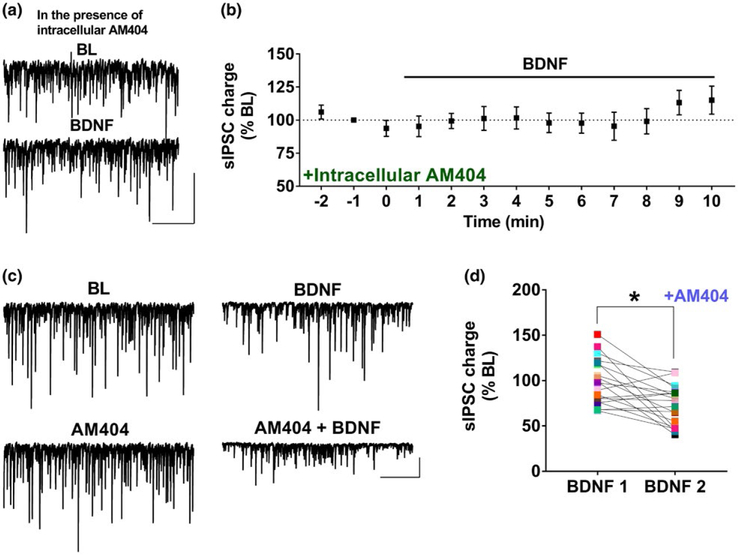
Because the effect of BDNF requires the activation of the postsynaptic TrkB receptors and presynaptic CB1 receptors, we hypothesized that endocannabinoids are released from the postsynaptic CA1 neurons as a retrograde messenger. It has previously been established that intracellular loading of the membrane-impermeable eCB transport inhibitor AM404 can be used to block eCB release (Lemtiri-Chlieh & Levine, 2010; Maglio, Noriega-Prieto, Maraver, & Fernandez de Sevilla, 2018; Ronesi, Gerdeman, & Lovinger, 2004; Yeh, Selvam, & Levine, 2017; Zhao et al., 2015). As shown in the representative traces in Figure 3a, we found that including AM404 (5 μM) in the patch pipette disrupted the effect of BDNF. The group time course is shown in Figure 3b (109.2% ± 9.2% of CCh BL; n = 8; 1 animal). These results suggest that endocannabinoids are released from the postsynaptic cell in response to BDNF.
FIGURE 3.
Effect of BDNF is mediated by endocannabinoid release. (a) Example traces during BL and BDNF in the continuous presence of intracellular AM404 (5 μM). Scale bar: 200 pA, 2 s. (b) Group time course showing the lack of effect of 0.8 nM BDNF with a postsynaptic intracellular loading of AM404 (5 μM; n = 8; 1 animal). (c) Example traces of BDNF on first exposure and BDNF in the presence of bath-applied AM404 (25 μM) on second exposure on the same cell. Scale bar: 200 pA, 5 s. (d) Data from individual cells showing the effect of BDNF alone on sIPSC charge and the effect of BDNF in the presence of bath-applied AM404 in the same cell (n = 19; 5 animals),*p < 0.05
AM404 can also be applied extracellularly to block the reuptake of eCBs, and bath application of AM404 in vitro has been shown to enhance the magnitude of eCB-mediated plasticity, including depolarization-induced suppression of inhibition (DSI) and long-term depression (LTD) (Beltramo et al., 1997; Du, Kwon, & Kim, 2013; Fortin, Trettel, & Levine, 2004; Sheinin, Talani, Davis, & Lovinger, 2008; Trettel et al., 2004; Wilson & Nicoll, 2001). Because of the variability in the magnitude of the BDNF effect across cells, we examined the effect of bath-applied AM404 using the within-cell experimental design. Representative traces from a single experiment are shown in Figure 3c, illustrating a greatly enhanced effect of BDNF in the presence of AM404, while AM404 itself did not affect baseline activity. Data for all experiments are shown in Figure 3d. BDNF in the presence of AM404 significantly depressed sIPSC charge to 73.1% ± 4.9% of BL in the 2nd trial compared to BDNF alone (98.5% ± 5.8% of BL; n = 19; 5 animals, paired t-test, p = 0.0003). In this cohort of cells, there were several cells that did not show a decrease to the first BDNF application; however, most of those cells did show a decrease to BDNF in the presence of AM404. These results suggest that BDNF triggers the release of eCBs that in turn mediate the effect on inhibitory synaptic activity.
3.4 ∣. Effect of BDNF is mediated by 2-AG
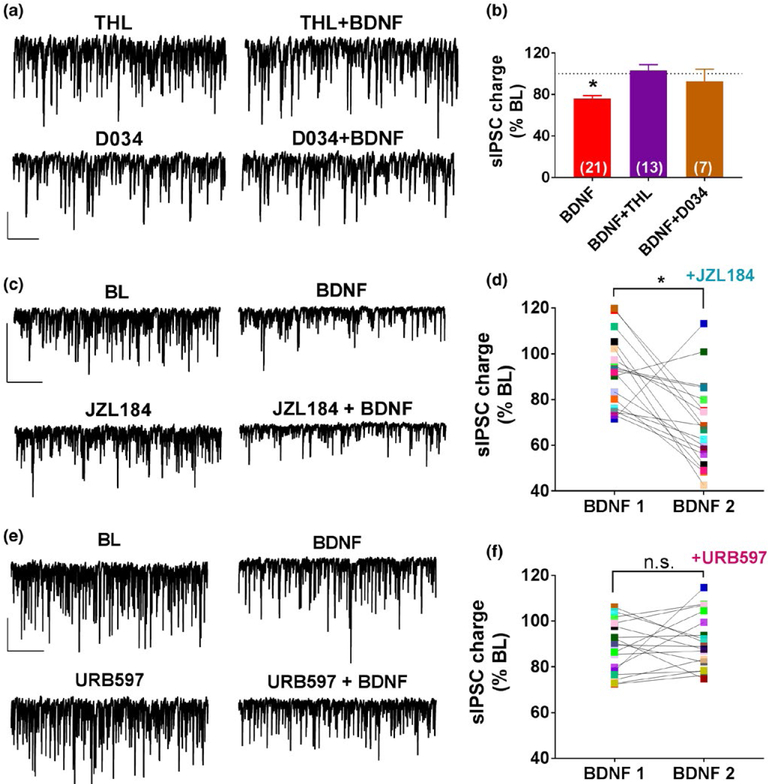
We have previously shown that, at inhibitory synapses of the somatosensory cortex, BDNF causes release of 2-AG that acts retrogradely on the presynaptic terminals to suppress GABA release (Lemtiri-Chlieh & Levine, 2010). We therefore investigated whether the eCB released by BDNF at hippocampal inhibitory synapses is also 2-AG. To address this, we blocked the synthesis of 2-AG using two different diacylglycerol (DAG) lipase inhibitors, tetrahydrolipostatin (THL; 25 μM) and D034 (1 μM) (Baggelaar et al., 2015; Liu et al., 2016). Representative traces of the effect of BDNF in the presence of THL or D034 are shown in Figure 4a. The group data show that the bath application of THL or D034 completely prevented the effect of BDNF (Figure 4b; THL: 103% ± 5% of BL; n = 13; 2 animals; D034: 92.6% ± 11% of BL; n = 7; 2 animals). We further explored the involvement of 2-AG using the monoacylglycerol lipase (MAGL) inhibitor JZL184 to increase 2-AG levels by preventing breakdown (Kiritoshi, Ji, & Neugebauer, 2016; Pan et al., 2009; Schlosburg et al., 2010; Wang et al., 2016). As shown in Figure 4c,d, BDNF in the presence of JZL184 depressed sIPSC charge to 69.3% ± 4.4% of BL, a significantly enhanced effect compared to BDNF alone (91.8% ± 3.6% of BL) (n = 18; 5 animals, paired t-test, p = 0.0125). These results suggest the BDNF causes the release of 2-AG to suppress the spontaneous inhibitory activity.
FIGURE 4.
BDNF causes the release of 2-arachidonoylglycerol (2-AG). (a) Representative traces during baseline and BDNF exposure in the presence of the DAG lipase inhibitor tetrahydrolipostatin (5 μM) or D034 (1 μM). Scale bar: 200 pA, 2 s. (b) Group data showing the lack of effect of BDNF in the presence of THL (n = 13; 2 animals) or D034 (n = 7; 2 animals). BDNF group data from Figure 1g are included for comparison. (c) Representative traces from a single cell showing effect of BDNF alone followed by BDNF in the presence of the monoacylglycerol lipase (MAGL) inhibitor JZL184 (500 nM). Scale bar: 200 pA, 5 s (d) Data from individual cells showing the effect of BDNF alone and in the presence of JZL184 (n = 18; 5 animals). (e) Representative traces from a single cell showing the effect of BDNF alone followed by BDNF in the presence of the fatty acid amide hydrolase (FAAH) inhibitor URB597 (2 μM). Scale bar: 200 pA, 5 s (f) Data from individual cells showing the effect of BDNF alone and in the presence of URB597 (n = 17; 3 animals),*p < 0.05
The potential contributing role of BDNF-induced anandamide release was explored by blocking anandamide degradation with the fatty acid amide hydrolase (FAAH) inhibitor URB597 (2 μM). Example traces of BDNF alone and BDNF in the presence of URB597 are shown in Figure 4e. BDNF caused reduction in charge to 88.2% ± 2.7% of BL in the 1st trial and in the presence of URB597, BDNF similarly decreased the charge to 91.0% ± 2.9% of BL in the 2nd trial as shown in Figure 4f (n = 17; 3 animals). These results suggest that blocking anandamide degradation does not alter the effects of BDNF.
4 ∣. DISCUSSION
BDNF and eCBs are potent neuromodulators that are highly expressed throughout the forebrain, including the hippocampus, and play critical roles in many behavioral and physiological processes. Interestingly, disruption of either BDNF or eCB signaling is associated with an overlapping set of neurologic and psychiatric diseases, including anxiety, depression, seizure disorders, and schizophrenia (Hill & Patel, 2013; Lupica, Hu, Devinsky, & Hoffman, 2017; McNamara & Scharfman, 2012). As a result, these systems are of high interest for the development of novel therapeutics (Autry & Monteggia, 2012; Patel, Hill, Cheer, Wotjak, & Holmes, 2017).
The present results indicate that the BDNF-induced suppression of inhibitory transmission at CA1 pyramidal cell synapses results from the activation of the postsynaptic TrkB receptors, which causes the mobilization of 2-AG that acts retrogradely on the presynaptic CB1 receptors. On average, BDNF (0.8 nM) caused a ~25% decrease in CCh-induced inhibitory activity, although the magnitude of the effect was variable across cells. This is consistent with the results obtained in other studies on the effect of BDNF on GABAergic transmission (Frerking et al., 1998; Tanaka, Saito, & Matsuki, 1997). Although CCh itself can enhance eCB release under some conditions (Kim, Isokawa, Ledent, & Alger, 2002), we found in the present study that blocking CB1 receptors with SR had no effect on CCh-induced inhibitory synaptic currents.
The effect of BDNF on inhibitory transmission was triggered by the postsynaptic activation of TrkB receptors as loading the postsynaptic neuron with the Trk tyrosine kinase inhibitor K252a was sufficient to prevent the BDNF effect. We should also note that K252a is membrane-permeable drug and therefore could diffuse outside the cell to block the presynaptic TrkB receptors. Previous work in our lab, however, found that intracellular K252a prevented the postsynaptic effect of BDNF at glutamatergic synapses but did not block the presynaptic effect on mEPSC frequency (Madara & Levine, 2008). The effect of BDNF in the present study was also blocked by a CB1 receptor antagonist and mimicked by a CB1 receptor agonist, suggesting that the activation of CB1 receptors is required for BDNF suppression of GABAergic transmission. Further, we found that the effect of BDNF was disrupted by blocking 2-AG synthesis and enhanced by blocking 2-AG degradation.
Presynaptic effects of BDNF at inhibitory synapses have been identified in multiple brain regions. In an earlier study on the CA1 pyramidal neurons of the hippocampus, BDNF caused a decrease in the evoked inhibitory response and an increase in the paired-pulse ratio suggesting a presynaptic mechanism (Frerking et al., 1998). Chronic application of BDNF on solitary neurons cultured from rat visual cortex caused a reduction in mIPSC frequency but not in mIPSC amplitude also suggesting a presynaptic effect (Palizvan et al., 2004). We have also previously shown that the effect of BDNF at layer 2/3 inhibitory synapses of the somatosensory cortex is expressed presynaptically, as indicated by the changes in the paired-pulse ratio, the coefficient of variation, and the frequency of mIPSCs (Lemtiri-Chlieh & Levine, 2010). Further, several studies have presented evidence of synaptic localization of CB1 and TrkB receptors—both are highly expressed in the cortex and hippocampus (Cabelli et al., 1996; Egertova, Cravatt, & Elphick, 2003; Fryer et al., 1996; Marsicano & Lutz, 1999; Matsuda, Bonner, & Lolait, 1993; Miller & Pitts, 2000; Tsou, Brown, Sanudo-Pena, Mackie, & Walker, 1998). The present study provides evidence for presynaptic effects of BDNF at CA1 synapses that are also mediated by eCBs, which may be generalized to inhibitory synapses throughout the nervous system.
BDNF-induced mobilization of eCBs most likely involves 2-AG, as blocking DAG lipase to prevent 2-AG synthesis disrupted the effect of BDNF, and blocking MAG lipase to increase 2-AG levels enhanced the effect of BDNF. Although the specific signaling mechanisms for BDNF-induced 2-AG release have not been identified, it is likely that the postsynaptic PLCⴴ signaling is involved. TrkB receptor activation and subsequent PLCⴴ signaling can generate diacylglycerol (DAG) which can then be converted to 2-AG by DAG lipase. This signaling pathway is similar to 2-AG mobilization in response to metabotropic glutamate receptor (mGluR) or muscarinic receptor activation. Our previous work at layer 2/3 inhibitory synapses in the somatosensory cortex supports the conclusion that BDNF-induced eCB release requires PLCⴴ signaling and is independent of mGluR activation (Zhao & Levine, 2014). Similarly, in the cerebellar Purkinje cells, BDNF modulates the GABA receptor function via TrkB receptor-PLCⴴ signaling cascade (Cheng & Yeh, 2005). Further, elevated calcium is not necessary for mGluR-induced (Hashimotodani et al., 2005; Maejima et al., 2005; Ohno-Shosaku, Hashimotodani, Maejima, & Kano, 2005) or CCh-induced eCB release (Kano, Ohno-Shosaku, Hashimotodani, Uchigashima, & Watanabe, 2009). However, BDNF requires elevated calcium through calcium intracellular stores or calcium influx to mediate its effects (Amaral & Pozzo-Miller, 2012). It has been shown that at inhibitory synapses of the somatosensory cortex, intracellular calcium is required for BDNF to trigger the release of eCBs (Lemtiri-Chlieh & Levine, 2010). It is thus possible that different signaling mechanisms could be involved in mGluR-induced, CCh-induced, and BDNF-induced 2-AG release.
There is a growing body of evidence supporting the interactions between BDNF and eCB signaling. In the current study, we have shown that BDNF induces eCB release to suppress GABAergic neurotransmission in the hippocampus, similar to previous results in the somatosensory cortex. We have also shown that endogenous BDNF, released in response to theta-burst stimulation, triggers the release of eCBs that mediate inhibitory long-term depression (iLTD) at layer 2/3 inhibitory synapses of the somatosensory cortex (Zhao et al., 2015). In addition, BDNF induces eCB release at layer 5 excitatory synapses of the somatosensory cortex, where eCB signaling acts to limit the direct potentiation of glutamate release triggered by BDNF (Yeh et al., 2017). BDNF also appears to increase eCB production in ventral tegmental area dopamine neurons to facilitate DSI and inhibitory LTD (Zhong et al., 2015). BDNF can inhibit CB1 receptor function in the striatum and this effect is mediated by cholesterol metabolism (De Chiara et al., 2010). On the other hand, BDNF has been shown to mediate CB1 receptor-dependent protection against excitotoxicity (Khaspekov et al., 2004; Marsicano et al., 2003). CB1 receptor signaling results in striatal neuroprotection from excitotoxicity via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin complex 1 pathway, which, in turn, induces BDNF expression through BDNF gene promoter IV (Blazquez et al., 2015). In the visual cortex, BDNF opposes eCB-mediated heterosynaptic LTD by inducing homosynaptic LTP (Huang, Yasuda, Sarihi, & Tsumoto, 2008). In addition, eCBs released by AP bursts of layer 5 pyramidal neurons of the barrel cortex are crucial for the induction of BDNF-mediated LTP at excitatory synapses (Maglio et al., 2018). Understanding the mechanistic basis of these varied interactions will provide insights into the physiological roles of BDNF and eCBs in regulating synaptic transmission.
Acknowledgments
Funding information
National Institute of Mental Health, Grant/Award Number: R01 MH094896
REFERENCES
- Abidin I, Eysel UT, Lessmann V, & Mittmann T (2008). Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. The Journal of Physiology, 586(7), 1885–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidin I, Kohler T, Weiler E, Zoidl G, Eysel UT, Lessmann V, & Mittmann T (2006). Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. The European Journal of Neuroscience, 24(12), 3519–3531. [DOI] [PubMed] [Google Scholar]
- Amaral MD, & Pozzo-Miller L (2012). Intracellular Ca2+ stores and Ca2+ influx are both required for BDNF to rapidly increase quantal vesicular transmitter release. Neural Plasticity, 2012, 203536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, & Black IB (2000). Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. Journal of Neuroscience Research, 59(3), 454–463. [DOI] [PubMed] [Google Scholar]
- Autry AE, & Monteggia LM (2012). Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological Reviews, 64(2), 238–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggelaar MP, Chameau PJP, Kantae V, Hummel J, Hsu KL, Janssen F, … van der Stelt M (2015). A highly selective, reversible inhibitor identified by comparative chemoproteomics modulates diacylglycerol lipase activity in neurons. Journal of the American Chemical Society, 137(27), 8851–8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, & Piomelli D (1997). Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science (New York, NY), 277(5329), 1094–1097. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Chiarlone A, Bellocchio L, Resel E, Pruunsild P, Garcia-Rincon D, … Guzman M (2015). The CB(1) cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death and Differentiation, 22(10), 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, & Fritschy JM (2001). BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. The European Journal of Neuroscience, 13(7), 1320–1328. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Allendoerfer KL, Radeke MJ, Welcher AA, Feinstein SC, Shatz CJ (1996). Changing patterns of expression and subcellular localization of TrkB in the developing visual system. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 16(24), 7965–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, & Yeh HH (2003). Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABA(A) receptor-mediated responses via postsynaptic mechanisms. The Journal of Physiology, 548(Pt 3), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, & Yeh HH (2005). PLCgamma signaling underlies BDNF potentiation of Purkinje cell responses to GABA. Journal of Neuroscience Research, 79(5), 616–627. [DOI] [PubMed] [Google Scholar]
- De Chiara V, Angelucci F, Rossi S, Musella A, Cavasinni F, Cantarella C, … Centonze D (2010). Brain-derived neurotrophic factor controls cannabinoid CB1 receptor function in the striatum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(24), 8127–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Milner TA, & Patterson SL (1999). Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. Journal of Neuroscience, 19(18), 8009–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Kwon IK, & Kim J (2013). Neuregulin-1 impairs the long-term depression of hippocampal inhibitory synapses by facilitating the degradation of endocannabinoid 2-AG. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(38), 15022–15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, & Elphick MR (2003). Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: Evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience, 119(2), 481–496. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, & Levine ES (2004). Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. Journal of Neurophysiology, 92(4), 2105–2112. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, & Nicoll RA (1998). Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. Journal of Neurophysiology, 80(6), 3383–3386. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, & Kromer LF (1996). Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. The Journal of Comparative Neurology, 374(1), 21–40. [DOI] [PubMed] [Google Scholar]
- Gomes RA, Hampton C, El-Sabeawy F, Sabo SL, & McAllister AK (2006). The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(44), 11487–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, & Lessmann V (2009). BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Experimental Brain Research, 199(3–4), 203–234. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, … Kano M (2005). Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron, 45(2), 257–268. [DOI] [PubMed] [Google Scholar]
- Hill MN, & Patel S (2013). Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biology of Mood & Anxiety Disorders, 3(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yasuda H, Sarihi A, STsumoto T (2008). Roles of endocannabinoids in heterosynaptic long-term depression of excitatory synaptic transmission in visual cortex of young mice. Journal of Neuroscience, 28(28), 7074–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, & Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiological Reviews, 89(1), 309–380. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y (1997). Selective cholinergic modulation of cortical GABAergic cell subtypes. Journal of Neurophysiology, 78(3), 1743–1747. [DOI] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, & Lutz B (2004). Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. The European Journal of Neuroscience, 19(7), 1691–1698. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, & Alger BE (2002). Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. Journal of Neuroscience, 22(23), 10182–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritoshi T, Ji G, & Neugebauer V (2016). Rescue of Impaired mGluR5-Driven Endocannabinoid Signaling Restores Prefrontal Cortical Output to Inhibit Pain in Arthritic Rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(3), 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, & Levine ES (2007). Lack of depolarization-induced suppression of inhibition (DSI) in layer 2/3 interneurons that receive cannabinoid-sensitive inhibitory inputs. Journal of Neurophysiology, 98(5), 2517–2524. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, & Levine ES (2010). BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. Journal of Neurophysiology, 104(4), 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen Y, Vickstrom CR, Li Y, Viader A, Cravatt BF, & Liu Q (2016). Coordinated regulation of endocannabinoid-mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase. Scientific Reports, 6:35829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, & Lu B (2008). BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiology of Learning and Memory, 89(3), 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Hu Y, Devinsky O, & Hoffman AF (2017). Cannabinoids as hippocampal network administrators. Neuropharmacology, 124, 25–37. [DOI] [PubMed] [Google Scholar]
- Madara JC, & Levine ES (2008). Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. Journal of Neurophysiology, 100(6), 3175–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, … Kano M (2005). Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(29), 6826–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglio LE, Noriega-Prieto JA, Maraver MJ, & Fernandez de Sevilla D (2018). Endocannabinoid-dependent long-term potentiation of synaptic transmission at rat barrel cortex. Cerebral Cortex, 28(5), 1568–1581. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Lutz B (2003). CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science, 302(5642), 84–88. [DOI] [PubMed] [Google Scholar]
- Marsicano G, & Lutz B (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. The European Journal of Neuroscience, 11(12), 4213–4225. [DOI] [PubMed] [Google Scholar]
- Masana Y, Wanaka A, Kato H, Asai T, & Tohyama M (1993). Localization of trkB mRNA in postnatal brain development. Journal of Neuroscience Research, 35(5), 468–479. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, & Lolait SJ (1993). Localization of cannabinoid receptor mRNA in rat brain. The Journal of Comparative Neurology, 327(4), 535–550. [DOI] [PubMed] [Google Scholar]
- McNamara JO, & Scharfman HE (2012). Temporal lobe epilepsy and the BDNF receptor, TrkB In Noebels JL, Avoli M, Rogawski MA, Olsen RW, & Delgado-Escueta AV (Eds.), Jasper’s basic mechanisms of the epilepsies. Bethesda, MD: Rogawski Michael A, Delgado-Escueta Antonio V, Noebels Jeffrey L, Avoli Massimo and Olsen Richard W. [Google Scholar]
- Miller MW, & Pitts FA (2000). Neurotrophin receptors in the somatosensory cortex of the mature rat: Co-localization of p75, trk, isoforms and c-neu. Brain Research, 852(2), 355–366. [DOI] [PubMed] [Google Scholar]
- Minichiello L (2009). TrkB signalling pathways in LTP and learning. Nature Reviews Neuroscience, 10(12), 850–860. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Kitamura A, Wake H, Ishibashi H, Watanabe M, Nishimaki T, & Nabekura J (2006). BDNF occludes GABA receptor-mediated inhibition of GABA release in rat hippocampal CA1 pyramidal neurons. The European Journal of Neuroscience, 24(8), 2135–2144. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Maejima T, & Kano M (2005). Calcium signaling and synaptic modulation: Regulation of endocannabinoid-mediated synaptic modulation by calcium. Cell Calcium, 38(3–4), 369–374. [DOI] [PubMed] [Google Scholar]
- Palizvan MR, Sohya K, Kohara K, Maruyama A, Yasuda H, Kimura F, &Tsumoto T (2004). Brain-derived neurotrophic factor increases inhibitory synapses, revealed in solitary neurons cultured from rat visual cortex. Neuroscience, 126(4), 955–966. [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, & Liu QS (2009). Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. The Journal of Pharmacology and Experimental Therapeutics, 331(2), 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hill MN, Cheer JF, Wotjak CT, & Holmes A (2017). The endocannabinoid system as a target for novel anxiolytic drugs. Neuroscience and Biobehavioral Reviews, 76(Pt A), 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, & Lovinger DM (2004). Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(7), 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, … Cravatt BF (2010). Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature Neuroscience, 13(9), 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin A, Talani G, Davis MI, & Lovinger DM (2008). Endocannabinoid- and mGluR5-dependent short-term synaptic depression in an isolated neuron/bouton preparation from the hippocampal CA1 region. Journal of Neurophysiology, 100(2), 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, & Matsuki N (1997). Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17(9), 2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, & Levine ES (2004). Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. Journal of Physiology, 556(Pt 1), 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, & Walker JM (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience, 83(2), 393–411. [DOI] [PubMed] [Google Scholar]
- Wang W, Trieu BH, Palmer LC, Jia Y, Pham DT, Jung KM, … Lynch G (2016). A primary cortical input to hippocampus expresses a pathway-specific and Endocannabinoid-Dependent Form of Long-Term Potentiation. eNeuro, 3(4). 10.1523/ENEURO.0160-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, & Nicoll RA (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature, 410(6828), 588–592. [DOI] [PubMed] [Google Scholar]
- Yeh ML, Selvam R, & Levine ES (2017). BDNF-induced endocannabinoid release modulates neocortical glutamatergic neurotransmission. Synapse (New York, NY), 71(5). e21962 10.1002/syn.21962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, & Levine ES (2014). BDNF-endocannabinoid interactions at neocortical inhibitory synapses require phospholipase C signaling. Journal of Neurophysiology, 111(5), 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yeh ML, & Levine ES (2015). Role for endogenous BDNF in endocannabinoid-mediated long-term depression at neocortical inhibitory synapses, eNeuro, 2(2). 10.1523/ENEURO.0029-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Liu Y, Hu Y, Wang T, Zhao YP, & Liu QS (2015). BDNF interacts with endocannabinoids to regulate cocaine-induced synaptic plasticity in mouse midbrain dopamine neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(10), 4469–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]