Abstract
Black people living with HIV (BPLWH) are less likely to adhere to antiretroviral treatment than are members of other racial/ethnic groups. Data were combined from two studies of BPLWH (n=239) to estimate adherence trajectories using a semiparametric, group-based modeling strategy over three time-points (spanning 6 months). Analyses identified three groups of individuals (high-stable, moderately low-stable, low-decreasing). Multinomial logistic regressions were used to predict trajectory membership with multiple levels of socio-ecological factors (structural, institutional/health system, community, interpersonal/network, individual). Older age was associated with being in the high-stable group, whereas substance use, lower perceived treatment effectiveness, and lower quality healthcare ratings were related to being in the moderately low-stable group. In sum, multiple socio-ecological factors contribute to adherence among BPLWH and thus could be targeted in future intervention efforts.
Keywords: Black/African American, HIV, Antiretroviral therapy, Adherence, Trajectory Analysis
Resumen
Los afroamericanos que viven con VIH tienen menos probabilidades de adherirse al tratamiento antirretroviral que los miembros de otros grupos raciales o étnicos. Los datos de dos estudios de afroamericanos viviendo con VIH (n=239) se combinaron para estimar las trayectorias de adherencia utilizando una estrategia de modelado semiparamétrica basada en grupos de tres puntos de tiempo (en el lapso de 6 meses). Los análisis identificaron tres grupos de individuos (alto-estable, moderadamente bajo-estable, bajo-decreciente). Las regresiones logísticas multinomiales se usaron para predecir la afiliación de la trayectoria con múltiples niveles de factores socio-ecológicos (estructural, institucional/sistema de salud, comunidad, interpersonal/red, individual). Mayor edad se asoció con estar en el grupo alto-estable, mientras que el uso de sustancias, la menor efectividad del tratamiento percibido y las calificaciones de cuidado de salud de menor calidad se relacionaron con estar en el grupo de moderadamente-estable. En resumen, múltiples factores socio-ecológicos contribuyen a la adherencia entre los afroamericanos y, por lo tanto, podrían ser el objetivo en futuros esfuerzos de intervención.
Keywords: Afroamericanos, VIH, terapia antirretroviral, adherencia, análisis de trayectoria
Introduction
Black people living with HIV (BPLWH) in the United States are less likely to be diagnosed, to be engaged in care, to receive and adhere to antiretroviral treatment (ART), and to be virally suppressed than are members of other racial/ethnic groups (1–3). Among men who have sex with men (MSM), Black MSM are the subgroup most disproportionately impacted by HIV. Similarly, Black heterosexual women and men have a higher incidence of new HIV infections than other heterosexuals (4, 5). Use of ART prevents transmission of HIV between serodiscordant sex partners (6–9) and prolongs survival (10). Therefore, access and adherence to ART are vital aspects of HIV prevention and care for BPLWH.
Kaufman and colleagues have suggested that the factors influencing HIV-related health behavior fall along multiple levels of a socio-ecological framework, made up of structural, institutional/healthcare system, community, interpersonal/network, and individual behavior change factors (11–13). This multi-level model aids in the conceptualization and measurement of factors influencing HIV prevention and treatment. Kaufman et al. suggest that these levels run along a spectrum from the most macro or structural-level (e.g., poverty, access to care, cost of services), to the institutional and/or healthcare system-level (e.g., competent supportive providers, culturally congruent care). From the institutional and/or healthcare system-level, factors follow to the community-level (e.g., racism, HIV-related and other forms of stigma, homophobia), and then to the interpersonal or network-level (e.g., relationship factors, social support, social network configuration). Finally, factors flow down to the most micro or individual-level (e.g., mental health, substance use, internalized stigma, medical mistrust, physical health)(11).
Kaufman et al. suggests that many of the factors in this framework are not discrete but represent interrelated relationships among multiple socio-ecological levels and can thus represent multiple levels simultaneously (11). Further these factors, and the complex relationships between them, are likely to fluctuate over time (11). For example, substance use and sexual risk-taking are generally measured as individual-level factors but can, and often do, occur in interpersonal or network contexts (14–16). Medical mistrust among BPLWH is often measured at the individual level, although this factor represents a historically complex and dynamic relationship reflecting tensions among institutions, health systems, medical providers, and Black patients.
With respect to the most macro or structural-level factors influencing HIV-related health behavior, incarceration, poverty, and homelessness disproportionately affect Black communities (17–19). Patients who are low income, uninsured (or Medicaid insured), and have unstable housing are less likely to be retained in care and are less likely to be adherent to ART (17, 18, 20–22). Moreover, institutional and healthcare system factors that include the quality of healthcare and cultural competency of providers may contribute to levels of medical mistrust among Black Americans at the individual-level (23–26); mistrust of HIV treatment efficacy and the healthcare system has been associated with lower ART adherence among BPLWH (27–31). Many Black Americans report beliefs that the US health system is discriminatory and mistrust of medical information about HIV medication efficacy (32–37) and HIV’s origins (i.e., HIV “conspiracy beliefs” that the government created HIV as a form of genocide), which in turn are related to worse ART adherence (28, 29).
In terms of community and interpersonal factors, BPLWH experience high levels of discrimination based on their multiple identities, including serostatus, sexual orientation (for MSM), and race/ethnicity (38–43), and these experiences with discrimination are strongly related to both medical mistrust and ART nonadherence (44–46). These factors are generally measured at the individual-level but are reflective of a dynamic relationship among multiple socio-ecological levels. In addition, stigma at the community-level and experienced from within social networks may be reflected in individual-level internalized stigma around HIV, an established correlate of ART nonadherence (47). Conversely, social support is a strong correlate of better adherence (48), and may serve as a buffer against HIV stigma in the social networks of BPLWH (30).
Depression and use of specific substances such as stimulants (e.g., cocaine, crack, and methamphetamine) are generally measured at the individual-level and serve as major drivers of suboptimal ART adherence (49–53). Use of crack-cocaine and methamphetamine negatively impacts ART adherence and is associated with lower CD4 cell counts and elevated viral load (54–60). Although substance use rates among Black Americans may not differ significantly from those among White Americans, the health, legal, and social consequences of drug use may be significantly greater for racial/ethnic minorities (61). Black Americans report the highest levels of discrimination due to race/ethnicity, poverty, and substance use (62, 63). Risk for substance abuse is high when individuals report negative affect due to multiple types of discrimination reflecting the complex, multi-level interplay between community-level factors, substance use, and mood (64). These discriminatory experiences are likely to increase Black people’s risk for non-adherence by influencing individual-level risk factors such as substance use, as well as through societal and contextual factors that influence the internalization of homonegative attitudes among Black MSM (43, 65–67).
Adherence is thought to be a dynamic process involving a complex interplay among myriad factors over time, some of which may be located at a single socio-ecological level, others representing relationships among multiple levels. The measurement of this dynamic process of adherence over time has been termed adherence trajectory analysis, and although previous work has assessed factors associated with multiple trajectories of adherence (68–73), to date research has not fully elucidated the multiple levels of factors that contribute to different adherence trajectories among BPLWH. The Swiss Cohort Study (73) found four trajectories of self-reported adherence: good, worsening, improving, and poor. Younger age, less education, a change in living conditions, injection drug use initiation, increased alcohol use, depression, greater time since diagnosis, lipodystrophy, and changing care providers were found to be associated with worsening adherence while having a simplified regimen, changed ART class, less time on ART, started another medication (e.g., for opportunistic infections) were found to be associated with improving adherence. Further, a longitudinal analysis of ART adherence found that young Black MSM with high adherence were less likely to report alcohol and/or marijuana use and had higher family acceptance and self-efficacy (74). The current analysis seeks to expand upon these previous adherence trajectory analyses by analyzing factors at multiple levels of a socio-ecological model to better understand the complex and dynamic relationships predicting ART adherence group membership among BPLWH.
Method
Participants
To increase statistical power, we combined two longitudinal (6-month) datasets of HIV-positive African American adults recruited in community settings in Los Angeles, CA (from 2010 to 2015). Specifically, we included data from 246 participants in Project Mednet (75), a longitudinal study of social networks of BPLWH conducted from August 2010 to September 2013, and 108 participants in Project Rise (76), a randomized controlled trial of a culturally congruent adherence intervention for BPLWH conducted from April 2012 to September 2015. Only data from participants in the control group were used from Rise in order to remove from the analysis any intervention effects on adherence trajectories. The dataset omitted 33 duplicate participants who were in both studies, as well as 82 participants missing electronically monitored adherence data at any time point (the main analysis outcome, described below), yielding a final sample size of 239. Participants in both studies conducted a baseline audio computer-assisted self-interview.
Measures
Individual-Level Factors
Socio-Demographic Factors.
Participants were asked to self-report their age at the time of the baseline interview, their current gender identity, their current sexual orientation, and the date of their HIV diagnosis (from which we derived a variable representing length of time since diagnosis). Participant socio-demographic and psychosocial variables are presented in Table I.
Table I.
Characteristics of Study Sample by Adherence Trajectory Group
| Variable | % or Mean (SD) | Corresponding Socio-Ecological Level(s) |
|||
|---|---|---|---|---|---|
| Overall (N=239) |
High-Stable (N=95) |
Moderately Low-Stable (N=83) |
Low- Decreasing (N=61) |
||
| Age (years) | 47.7 (10.0) | 49.7 (11.4) | 47.7 (8.4) | 44.7 (8.9) | Individual |
| Gender identity | Individual | ||||
| Female (non-transgender) | 21 | 23 | 24 | 11 | Individual |
| Male (non-transgender) | 75 | 73 | 72 | 82 | Individual |
| Transgender or other non-binary | 5 | 4 | 4 | 7 | Individual |
| Sexual orientation | Individual | ||||
| Bisexual male | 15 | 13 | 11 | 23 | Individual |
| Bisexual female | <1 | 0 | 0 | 2 | Individual |
| Gay male | 48 | 46 | 48 | 49 | Individual |
| Gay female | 1 | 2 | 1 | 0 | Individual |
| Straight | 33 | 37 | 35 | 25 | Individual |
| Not sure/other | 3 | 2 | 5 | 2 | Individual |
| Time since diagnosis (years) | 14.3 (7.7) | 14.8 (8.1) | 13.3 (6.9) | 14.8 (8.2) | Individual |
| Undetectable viral load | 57 | 75 | 53 | 36 | Individual |
| Most recent CD4 cell count (m/L) | 580 (335) | 633 (288) | 587 (383) | 467 (315) | Individual |
| Perceived ART efficacy rating | 3.23 (0.58) | 3.33 (0.55) | 3.13 (0.64) | 3.20 (0.51) | Individual |
| Depression (past 2 weeks) | 20 | 19 | 21 | 21 | Individual |
| Substance use (past 30 days) | Individual-interpersonal/network | ||||
| Used marijuana | 40 | 36 | 44 | 39 | Individual-interpersonal/network |
| Used stimulant (cocaine/crack/methamphetamine) | 23 | 15 | 30 | 25 | Individual-interpersonal/network |
| Had sex while high | 21 | 12 | 28 | 26 | Individual-interpersonal/network |
| Had sex while drunk | 20 | 16 | 23 | 21 | Individual-interpersonal/network |
| Had 5 or more drinks in sitting | 26 | 22 | 29 | 29 | Individual-interpersonal/network |
| Condomless sex (any with serodiscordant last 3 months) | 20 | 14 | 25 | 21 | Individual-interpersonal/network |
| Medical mistrust, racial/ethnic subscale | 2.28 (0.66) | 2.20 (0.63) | 2.39 (0.65) | 2.26 (0.70) | Individual-institutional/health system |
| Care rating | 8.74 (1.79) | 9.09 (1.44) | 8.29 (2.17) | 8.82 (1.61) | Individual-institutional/health system |
| Doctor rating | 8.87 (1.89) | 9.08 (1.80) | 8.54 (2.07) | 9.00 (1.72) | Individual-institutional/health system |
| Internalized HIV stigma | 2.65 (1.16) | 2.67 (1.22) | 2.64 (1.09) | 2.64 (1.19) | Individual-community |
| Relationship status | Interpersonal/network | ||||
| Single | 72 | 72 | 72 | 74 | Interpersonal/network |
| Steady relationship, not married/other | 18 | 20 | 14 | 18 | Interpersonal/network |
| Married/domestic partnership | 10 | 8 | 13 | 8 | Interpersonal/network |
| Social support | 3.31 (1.12) | 3.41 (1.19) | 3.17 (1.06) | 3.36 (1.08) | Interpersonal/network |
| HIV-related discrimination | 1.29 (2.08) | 1.20 (2.01) | 1.27 (2.02) | 1.46 (2.28) | Community |
| Income <$10,000 annually | 32 | 34 | 30 | 31 | Individual-structural |
| Currently stably housed | 74 | 75 | 73 | 74 | Individual-structural |
| Incarcerated past 3 months | 8 | 5 | 6 | 13 | Individual-structural |
| Education level completed | Individual-structural | ||||
| <High school | 17 | 18 | 16 | 16 | Individual-structural |
| High school diploma or GED | 36 | 35 | 37 | 38 | Individual-structural |
| Some college | 32 | 29 | 36 | 30 | Individual-structural |
| College degree or more | 15 | 18 | 11 | 16 | Individual-structural |
| Current employment status | Individual-structural | ||||
| Full-time (40 or more hours/week) | 5 | 4 | 7 | 3 | Individual-structural |
| Part-time (<40 hours/week) | 3 | 4 | 2 | 3 | Individual-structural |
| Unemployed | 66 | 62 | 65 | 74 | Individual-structural |
| Retired | 12 | 20 | 7 | 5 | Individual-structural |
| Other | 14 | 9 | 18 | 15 | Individual-structural |
| Adherence (% doses per MEMS) | Outcome | ||||
| Time 1 | 64.8 (33.9) | 92.6 (9.9) | 63.5 (24.8) | 23.2 (23.8) | Outcome |
| Time 2 | 65.7 (32.8) | 92.4 (10.4) | 60.2 (24.7) | 26.8 (26.4) | Outcome |
| Time 3 | 62.2 (34.1) | 89.9 (13.7) | 58.4 (23.9) | 16.3 (20.5) | Outcome |
CD4 Cell Count and Undetectable Viral Load.
Participants were asked to self-report their most recent CD4 cell count and whether the result of their most recent viral load test was undetectable. Participants were also asked to provide permission to collect their medical records data on these indicators. For this analysis we used the medical record measurement closest to their baseline assessment. If the medical record was not available, we used self-report of the most recent CD4 cell count and whether the result of their most recent viral load test was undetectable.
Perceived ART Efficacy.
Perceived ART medication efficacy was measured using 8 items adapted from the questions developed by the Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (77). Example items are: “taking HIV medication will keep me healthier longer,” and “I am hopeful that the HIV medications will be effective for me.” Items were scored on a 4-point scale ranging from “strongly disagree” to “strongly agree.” The items displayed adequate internal consistency (Cronbach’s Alpha = 0.75) and the average score was utilized for analysis.
Depression.
Depressive symptom severity during the past two weeks were measured using the 9-item Patient Health Questionnaire (78). Items ask participants to rate the frequency of depressive symptoms such as “little interest or pleasure in doing things” or “feeling down, depressed, or hopeless.” Items were scored on a 4-point scale ranging from “not at all” to “nearly every day.” The items displayed good internal consistency (Cronbach’s Alpha = 0.89). The 4-point scale for each item has range 0–3; these scores were then summed to create a variable with range 0–27. We dichotomized the depression variable according to the recommendation that subjects be considered depressed if the sum was equal to 10 or more (78).
Individual-Interpersonal/Network-Level Factors
Substance Use.
Illicit drug use over the past 30 days was measured by substance using specific items from the Addiction Severity Index (79). Items assess the frequency with which participants used marijuana, heroin, cocaine, crack cocaine, amphetamine, and methamphetamine. For the purposes of the current analyses, stimulant substance use items (i.e., cocaine, crack cocaine, methamphetamine) were combined to form a single simulant substance use score, and both marijuana and stimulant use were dichotomized to indicate any use. All other illicit drug items (other than marijuana) were dropped from the final analyses due to low endorsement of these items among participants. Binge drinking was measured with the question “During the last year, have you had 5 or more drinks on at least one occasion?” This item was also dichotomized to indicate any binge drinking.
Sexual Risk.
Combined substance use and sexual behavior was assessed with two items from the Addiction Severity Index (79): “In the last 30 days, how many times have you had sex when you were high on drugs?,” and “In the last 30 days how many times have you had sex when you were drunk on alcohol?” Number of times participants have had condomless sex with a serodiscordant (HIV-negative) partner in the past three months was assessed for both receptive and penetrative anal and vaginal sex. These items were then combined into one condomless sex with serodiscordant partner item in the final analyses. Because the majority of participants (80%) did not report that they had engaged in these condomless behaviors, they were dichotomized (none versus any).
Individual-Institutional/Health System-Level Factors
Medical Mistrust.
Mistrust of health care organizations on issues related to race-ethnicity was assessed using 4 items from the Medical Mistrust Index (80). Items included questions such as “racial discrimination in a doctor’s office is common,” and “in most hospitals, African Americans and Whites receive the same kind of care as everyone else” (reversed for analysis). Participants rated the extent to which they agreed with these statements on 4-point scale ranging from “strongly agree” to “strongly disagree.” The items displayed adequate internal consistency (Cronbach’s Alpha = 0.76) and the average score was utilized for analysis.
Healthcare and Doctor Ratings.
Two items were used from the Consumer Assessment of Health Plans Study (81): Participants were asked to rate the quality of their HIV medical care for the past 12 months on a scale ranging from 0 (worst medical care possible) to 10 (best medical care possible). Participants were also asked to rate the quality of the HIV doctor they saw most often during the past 12 months on a scale ranging from 0 (worst doctor possible) to 10 (best doctor possible). The healthcare rating item and the doctor rating item were treated separately as single items for analytic purposes.
Individual-Community-Level Factors
Internalized HIV Stigma.
The extent to which participants experienced internalized HIV stigma was measured with 6 items adapted from the AIDS-Related Stigmas Scale (82). Example items include “Being HIV positive makes me feel dirty,” and “I am ashamed that I am HIV positive.” Participants were asked to rate the extent to which they agree with the statement on a 5-point scale with items ranging from “strongly agree” to “strongly disagree.” The items displayed good internal consistency (Cronbach’s Alpha = 0.88) and the average score was utilized for analysis.
Interpersonal/Network-Level Factors
Relationship Status.
Participants were also asked whether they were currently single, in a steady relationship but not married, or currently married or in a domestic partnership. Relationship status was treated as single item for analytic purposes.
Social Support.
Social support was assessed on multiple dimensions using the 19-item Medical Outcomes Study Social Support Survey (83). Items ask participants how often various kinds of support are available to them on a 5-point scale with answers ranging from “none of the time” to “all of the time”. Sample items include “how often is… someone you can count on to listen to you when you talk…available to you?” and “how often is… some to take you to the doctor of you need it… available to you?” The items displayed excellent internal consistency (Cronbach’s Alpha = 0.97) and the average score was utilized for analysis.
Community-Level Factors
HIV-Related Discrimination.
The extent to which participants experienced HIV-related discrimination was measured with the 10 HIV-related items from the Multiple Discrimination Scale(44). Example items include “in the past year, were you ignored, excluded, or avoided by people close to you because you are HIV-positive?” and “In the past year, were you treated with hostility or coldness by strangers because you are HIV-positive?” Participants were asked to answer the questions with either “yes” or “no.” The items displayed good internal consistency (Cronbach’s Alpha = 0.86) and the sum score was utilized for analysis.
Individual-Structural-Level Factors
Several socio-demographic items were measured at the individual-level, but used as proxies for structural-level factors: income (dichotomized as less than $10,000 versus greater than or equal to $10,000 annually), stable housing (with stable housing coded as “rent or own home/apartment” or “publicly subsidized housing” versus unstable housing coded as “residential drug, alcohol or other treatment facility,” “a friend or relative’s home or apartment,” “temporary or transitional housing,” or “homeless: sleeping in a shelter or on the street”); recent incarceration (in the last three months); level of education completed (dichotomized as less than high school/GED versus high school degree/GED or more), and current employment status (dichotomized as unemployed, not working, or retired, versus working full-time or part-time).
Outcome Variable
Adherence.
Adherence was electronically monitored with the Medication Event Monitoring System (MEMS; AARDEX, Inc.), which measures each time the medication bottle is opened. In both studies, data were downloaded at 3 time-points, although the timing differed slightly across studies. In Mednet, MEMS data were downloaded at 2, 4, and 6 months post-baseline and in Rise, MEMS was measured at 1.5, 4.5, and 6 months post-baseline. We used MEMS software to calculate the percentage of doses taken in the past 2-weeks at each of these time-point. In addition, MEMS data were adjusted to account for participants’ self-reported use of the cap not as intended in the past 2 weeks (e.g., bottle opened without removing a dose). These self-report responses were then used to adjust estimates of the percentage of doses taken (84).
Statistical Analysis
Descriptive statistics were first computed for all variables. A procedure written for SAS software (Proc TRAJ) was then used to identify clusters of individuals with similar progressions of adherence over time, by forming developmental trajectories estimated from the longitudinal data based on a semiparametric, group-based modeling strategy, and then assessing membership probabilities estimated in each group for every participant (85). The Bayesian Information Criterion (BIC) was relied on for model selection as described by Jones and Nagin (86). We then developed bivariate and multivariate multinomial logistic regression models to predict trajectory membership with the structural, institutional/health system, community, interpersonal/network, and individual-level factors. We compared pairs of trajectories for each predictor variable and developed a final multivariate model from predictors found to be significantly associated (p<0.05) with membership in any pair of trajectories. To avoid issues with multicollinearity, we dropped any predictors correlated with others at r >.50, retaining the item with the stronger bivariate associations (87, 88). Adherence was measured as percentage of doses taken (of those prescribed) per MEMS cap reading. Undetectable viral load and most recent CD4 count were associated with trajectory group membership but were dropped from the final multivariate model as these outcomes are highly associated with adherence.
Results
Adherence Modeling
Trajectory Analysis:
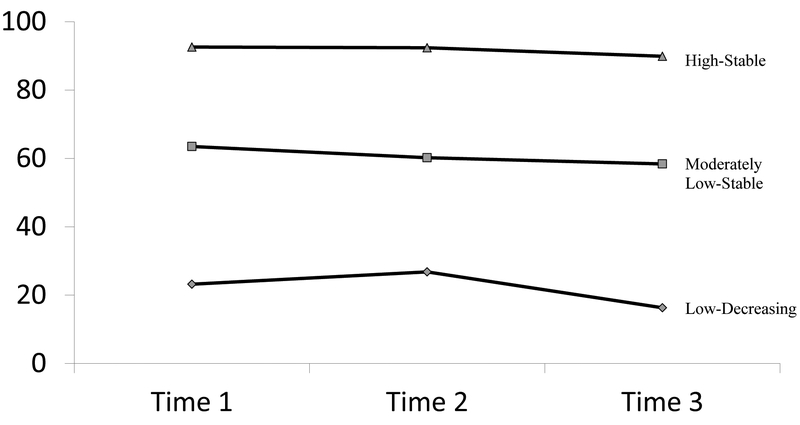
Adherence progressions fit a three-group model over the three time-points (see Figure 1). As shown in Table I, 40.0% (n=95) of participants were classified as having “high-stable adherence,” with an average of 92.6% (SD = 9.9) of doses taken at Time 1, 92.4% (SD = 10.4) of doses taken at Time 2, and 89.9% (SD = 13.7) of doses taken at Time 3. A total of 35.0% (n=83) of participants were classified as “moderate-low-stable adherence,” with an average of 63.5% (SD = 24.8) of doses taken at Time 1, 60.2% (SD = 24.7) of doses taken at Time 2, and 58.4% (SD = 23.9) of doses taken at Time 3. The remaining 25.0% (n=61) of participants were classified as “low-decreasing adherence,” with 23.2% (SD = 23.8) of doses taken at Time 1, 26.8% (SD = 26.4) of doses taken at Time 2, and 16.3% (SD = 20.5) of doses taken at Time 3.
Figure 1.
Adherence Trajectories by Time
Bivariate Analysis.
A separate bivariate model was run for each possible comparison group (Table II). Bivariate results are presented by model below:
Table II.
Bivariate Analyses of Predictors by Adherence Trajectory Group
| Variable | Odds Ratio (95% Confidence Interval) | Corresponding Socio-ecological Level |
||
|---|---|---|---|---|
| High-Stable vs. Low-Decreasing |
Moderately Low- Stable vs. Low- Decreasing |
High-Stable vs. Moderately Low- Stable |
||
| Age (years) | 1.05 (1.02-1.09)** | 1.03 (1.00-1.06)+ | 1.02 (0.99-1.06) | Individual |
| Female (biological and MTF transgender) | 1.70 (0.77-3.76) | 1.72 (0.76-3.89) | 0.99 (0.51-1.91) | Individual |
| Heterosexual orientation | 1.76 (0.86-3.61) | 1.61 (0.77-3.37) | 1.09 (0.59-2.03) | Individual |
| Time since diagnosis (years) | 1.00 (0.96-1.05) | 0.98 (0.93-1.02) | 1.03 (0.99-1.07) | Individual |
| Undetectable viral load | 5.31 (2.63-10.70)*** | 2.06 (1.04-4.08)* | 2.57 (1.36-4.85)** | Individual |
| Most recent CD4 cell count (m/10L) | 1.02 (1.00-1.03)*** | 1.01 (1.00-1.02)+ | 1.00 (0.99-1.01) | Individual |
| Perceived ART efficacy rating | 1.54 (0.85-2.78) | 0.83 (0.48-1.46) | 1.85 (1.09-3.15)* | Individual |
| Depression (past 2 weeks) | 0.90 (0.40-2.01) | 0.99 (0.43-2.24) | 0.91 (0.44-1.91) | Individual |
| Substance use (past 30 days) | Individual-interpersonal/network | |||
| Used marijuana | 0.85 (0.44-1.66) | 1.21 (0.61-2.38) | 0.70 (0.38-1.30) | Individual-interpersonal/network |
| Used stimulant (cocaine/crack/methamphetamine) | 0.53 (0.24-1.21) | 1.31 (0.61-2.79) | 0.41 (0.20-0.86)* | Individual-interpersonal/network |
| Hads sex while high | 0.36 (0.15-0.85)* | 1.00 (0.47-2.15) | 0.36 (0.16-0.80)* | Individual-interpersonal/network |
| Had sex while drunk | 0.68 (0.30-1.57) | 1.05 (0.47-2.36) | 0.65 (0.30-1.39) | Individual-interpersonal/network |
| Had 5 or more drinks in sitting | 0.67 (0.31-1.43) | 1.02 (0.48-2.16) | 0.66 (0.33-1.33) | Individual-interpersonal/network |
| Condomless sex (any with serodiscordant last 3 months) | 0.58 (0.21-1.58) | 1.22 (0.48-3.11) | 0.48 (0.19-1.17) | Individual-interpersonal/network |
| Medical mistrust, racial/ethnic subscale | 0.86 (0.52-1.41) | 1.34 (0.80-2.24) | 0.64 (0.40-1.02)+ | Individual-institutional/health system |
| Care rating | 1.11 (0.89-1.37) | 0.84 (0.69-1.01)+ | 1.32 (1.09-1.59)** | Individual-institutional/health system |
| Doctor rating | 1.01 (0.84-1.23) | 0.86 (0.72-1.03) | 1.18 (1.00-1.39)+ | Individual-institutional/health system |
| Internalized HIV stigma | 1.02 (0.77-1.34) | 1.00 (0.75-1.33) | 1.02 (0.79-1.32) | Individual-community |
| Married/domestic partnership | 1.02 (0.32-3.28) | 1.69 (0.55-5.16) | 0.60 (0.23-1.58) | Interpersonal/network |
| Social support | 1.04 (0.78-1.40) | 0.86 (0.64-1.16) | 1.22 (0.93-1.59) | Interpersonal/network |
| HIV-related discrimination | 0.95 (0.81-1.11) | 0.97 (0.83-1.13) | 0.98 (0.85-1.14) | Community |
| Income <$10,000 annually | 0.98 (0.49-1.93) | 1.21 (0.60-2.47) | 0.80 (0.43-1.51) | Individual-structural |
| Currently stably housed | 1.01 (0.48-2.12) | 0.93 (0.44-1.99) | 1.09 (0.55-2.14) | Individual-structural |
| Incarcerated past 3 months | 0.37 (0.11-1.18)+ | 0.42 (0.13-1.35) | 0.88 (0.25-3.17) | Individual-structural |
| Education < high school | 1.06 (0.45-2.52) | 0.88 (0.36-2.19) | 1.20 (0.54-2.66) | Individual-structural |
| Employed (full-time or part-time) | 1.27 (0.36-4.43) | 1.45 (0.41-5.09) | 0.87 (0.31-2.45) | Individual-structural |
| Adherence (% doses per MEMS) | Outcome | |||
| Time 1 | 1.17 (1.13-1.21)*** | 1.07 (1.05-1.09)*** | 1.09 (1.06-1.12)*** | Outcome |
| Time 2 | 1.14 (1.10-1.17)*** | 1.04 (1.02-1.06)*** | 1.09 (1.07-1.12)*** | Outcome |
| Time 3 | 1.13 (1.10-1.16)*** | 1.05 (1.03-1.06)*** | 1.08 (1.06-1.11)*** | Outcome |
p<.10,
p<.05,
p<.01,
p<.001
High-Stable vs. Low-Decreasing Adherence Groups:
In terms of individual-level factors, the results of the bivariate analyses indicated significant differences in age between the high-stable and low-decreasing adherence groups (OR 1.05, 95% CI 1.02–1.09, p<.01) with younger participants significantly more likely to be in the low-decreasing group than in the high-stable group. Having an undetectable viral load was significantly associated with being in the high-stable adherence group over the low-decreasing group (OR 5.31, CI 2.63–10.70, p<.001). Having a higher most recent CD4 cell count (m/10L) was significantly associated with being in the high-stable adherence group over the low-decreasing group (OR 1.02, CI 1.00–1.03, p<.001).
In terms of individual-interpersonal/network level factors, those who reported having recently had sex while high were less likely to be in the high-stable group than in the low-decreasing adherence (OR 0.36, CI 0.15–0.85, p<.05).
Moderately Low-Stable vs. Low-Decreasing Adherence Groups:
One individual-level factor, having an undetectable viral load, was more highly associated with being in the moderately low-stable group than the low-decreasing group (OR 2.06, CI 1.04–4.08, p<.05).
High-Stable vs. Moderately Low-Stable Adherence Groups:
In terms of individual-level factors, having an undetectable viral load had a greater association with being in the high-stable group than the moderately low-stable group (OR 2.57, CI 1.36–4.85, p<.01). Higher ratings of ART efficacy were also associated with being in the high-stable adherence group over the moderately low-stable group (OR 1.85, 1.09–3.15, p<.05).
With respect to individual-interpersonal/network level factors, those who reported having recently used stimulants (e.g., cocaine, crack, methamphetamine) were less likely to be in the high-stable and more likely to be in the moderately low-stable adherence group (OR 0.41, CI 0.20–0.86, p<.05). Those who reported having recently had sex while high were less likely to be in the high-stable group than in the moderately low-stable adherence group (OR 0.36, CI 0.16–0.80, p<.05).
In terms of individual-institutional/health system factors, participants who rated their healthcare as worse overall were more likely to be in moderately low-stable adherence group than in the high-stable adherence (OR 1.32, CI 1.09–1.59, p<.01) group.
Multivariate Analysis:
Based on the bivariate results, we built a multivariate binary logistic model including the predictor variables found to be significantly (p <.05) related to adherence group membership (see Table III). Although significantly associated with adherence group membership, undetectable viral load and CD4 cell count were not included as these were viewed as individual-level outcomes rather than predictors of adherence. A significant model was achieved (Wald χ2(10) = 28.56, p = 0.002) with significant individual-level and individual-interpersonal/network-level factors. Specifically, being older was related to a higher probability of being in the high-stable group than in the low-decreasing adherence group (OR 1.05, CI 1.02–1.09, p<.01). Higher ART efficacy perceptions were related to a higher probability of being in the high-stable adherence group than in the moderately low-stable group (OR 1.81, CI 1.04–3.13, p<.05). Using stimulants was related to a lower probability of being in the high-stable group than in the moderately low-stable adherence group (OR 0.39, CI 0.18–0.83, p<.05). Higher overall healthcare ratings were related to a higher probability of being in the high-stable than in the moderately low-stable group (OR 1.30, CI 1.07–1.56, p<.01) and a higher probability of being in the moderately low-stable group than in the low-decreasing adherence group (OR 0.82, CI 0.67–1.00, p<.05).
Table III.
Multivariate Analyses of Predictors by Adherence Trajectory Group
| Variable | Odds Ratio (95% Confidence Interval) | Corresponding Socio-ecological Level |
||
|---|---|---|---|---|
| High-Stable vs. Low-Decreasing |
Moderately Low- Stable vs. Low- Decreasing |
High-Stable vs. Moderately Low- Stable |
||
| Age (years) | 1.05 (1.02 – 1.09)** | 1.03 (1.00 – 1.07)+ | 1.02 (0.99 – 1.05) | Individual |
| Perceived ART efficacy rating | 1.66 (0.91 – 3.06) | 0.92 (0.52 – 1.65) | 1.81 (1.04 – 3.13)* | Individual |
| Used stimulant past 30 days (cocaine/crack/methamphetamine) | 0.55 (0.24 – 1.27) | 1.41 (0.65 – 3.05) | 0.39 (0.18 – 0.83)* | Individual-interpersonal/network |
| Care rating | 1.06 (0.85 – 1.32) | 0.82 (0.67 – 1.00)* | 1.30 (1.07 – 1.56)** | Individual-interpersonal/network |
p<.10
p<.05
p<.01
Discussion
This study sought to delineate the longitudinal trajectories of ART adherence among BPLWH across three assessments that took place over six months, and to examine the multi-level socio-ecological factors associated with membership in each trajectory group. Participants fell into one of three ART adherence trajectory groups: high-stable, moderately low-stable, and low-decreasing, with the majority (75%) in either the high- or low- two stable adherence categories. Although prior research has examined adherence trajectories (68–73), this study extends previous work by examining such trajectories among BPLWH who generally show low adherence and viral suppression rates (89), using electronically monitored adherence data.
Trajectories were generally flat, suggesting that there is a tendency towards consistency within different adherence groups irrespective of particular group membership. However, this finding should be interpreted with caution, as participants were only followed for a 6-month period of time. Factors at multiple socio-ecological levels were associated with ART adherence. In particular, individual-level factors associated with suboptimal adherence included being of younger age and perceiving ART to be less efficacious. These findings suggest that increasing trust in the efficacy of ART should be a focus of future adherence interventions and that interventions should be specifically developed for younger BPLWH.
With regard to individual-interpersonal/network-level factors, use of stimulants was predictive of trajectory group membership. Previous work has shown that the use of stimulants is highly associated with decreased odds of ART adherence and persistence, elevated viral load, and elevated risk for HIV transmission (60, 90, 91). The BPLWH in the current study were significantly more likely to be in the low adherence group if they reported stimulant use. Recent research has shown evidence that cognitive behavioral therapy (CBT) and combined CBT plus medication assisted treatment (MAT) may be effective in treating stimulant use and improving ART adherence among people living with HIV (90, 92, 93). Future research efforts could tailor and evaluate these treatments for BPLWH stimulant users specifically.
Another individual-interpersonal/network-level factor, overall perceptions of healthcare, was also significantly predictive of trajectory group membership. Previous negative encounters with healthcare institutions and providers have helped to explain the poorer outcomes of HIV care among BPLWH (94). The quality of relationships with healthcare providers has also been shown to affect ART adherence (95–97). These findings underscore the need for BPLWH to have access to high quality HIV services and providers who can foster strong relationships and provide a safe space where BPLWH can receive culturally competent, non-judgmental HIV care services.
The current study has several limitations. The sample size was relatively small and there was a limited number of assessments for a trajectory analysis, and thus some variables may not have been significant due to a lack of statistical power. In addition, some important structural and healthcare variables such as access to care were not measured and may be barriers to adherence. Although methods and research staff were mostly consistent, data points were combined over two different studies over a five-year timespan, and the length of time between assessment time-points varied slightly. Given the idiosyncrasies in these data as well as the need to drop certain predictors due to multicollinearity, the reproducibility of these findings is uncertain. Future work should involve the replication and extension of these findings with a larger sample size, with a greater number of assessment points over a longer time-span, and include additional social-ecological predictors (11).
Overall, findings from the current study suggest that there are several key factors located within and across multiple socio-ecological levels that contribute to ART adherence group membership among BPLWH. Several interventions have shown promise of increasing engagement in HIV care and improving ART adherence (76, 98–100). However, most interventions have not been tailored to address the specific needs of BPLWH, for example, by addressing key factors such as perceptions of HIV medication efficacy and mistrust of HIV-related healthcare services interventions may further improve overall adherence rates among BPLWH. Further, given our findings regarding the effect of stimulant use on adherence trajectory group membership, cognitive behavioral and other evidence-based approaches that focus on increasing coping skills, regulating emotions, and reducing stimulant use also need to be tailored for, and targeted to BPLWH.
Acknowledgments
The authors would like to thank the participants as well as Brian Risley, Kieta Mutepfa, and the APLA Treatment Education Community Advisory Board. We would also like to thank Sean Lawrence, Nikki Rachal, and Kelsey Nogg, who helped to conduct the study.
Funding Sources: This work was supported by grants R01MD003964, R01MD006058, R01NR017334, P30MH058107, and R03DA042660, from the National Institutes of Health.
References
- 1.Crepaz N Racial and ethnic disparities in sustained viral suppression and transmission risk potential among persons receiving HIV care—United States, 2014. MMWR Morb Mortal Wkly Rep. 2018;67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham W HIV racial disparities: Comment on “The influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007”. Arch Intern Med. 2012;172(20):1599–600. [DOI] [PubMed] [Google Scholar]
- 3.Simoni JM, Huh D, Wilson IB, Shen J, Goggin K, Reynolds NR, et al. Racial/ethnic disparities in ART adherence in the United States: Findings from the MACH14 study. J Acquir Immune Defic Syndr. 2012;60(5):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV in the United States: At a glance. [Available from: http://www.cdc.gov/hiv/statistics/basics/ataglance.html.]
- 5.Centers for Disease Control and Prevention. New HIV infections drop 18 percent in six years. 2017. [Available from: https://www.cdc.gov/nchhstp/newsroom/2017/croi-hiv-incidence-press-release.html.]
- 6.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. Aids. 2009;23(11):1397–404. [DOI] [PubMed] [Google Scholar]
- 7.Castilla J, Del Romero J, Hernando V, Marincovich B, García S, Rodríguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40(1):96–101. [DOI] [PubMed] [Google Scholar]
- 8.Del Romero J, Castilla J, Hernando V, Rodríguez C, García S. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ. 2010;340:c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: A prospective cohort analysis. Lancet HIV. 2010;375(9731):2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman MR, Cornish F, Zimmerman RS, Johnson BT. Health behavior change models for HIV prevention and AIDS care: Practical recommendations for a multi-level approach. J Acquir Immune Defic Syndr. 2014;66(Suppl 3):S250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson BT, Redding CA, DiClemente RJ, Mustanski BS, Dodge B, Sheeran P, et al. A network-individual-resource model for HIV prevention. AIDS Behav. 2010;14(2):204–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosby RA, Salazar LF, DiClemente RJ. Ecological approaches in the new public health. Health behavior theory for public health: Principles, foundations, and applications. 2011:231–51. [Google Scholar]
- 14.Hermanstyne K Links between substance use behavior and sexual risk behavior patterns within social networks of high-risk Black MSM in 6 US cities. Primary Paper: No Ancillary Study: No Date soundbite disseminated to 061 Pubs Team: 22 April 2014. [Google Scholar]
- 15.Schneider JA, Cornwell B, Ostrow D, Michaels S, Schumm P, Laumann EO, et al. Network mixing and network influences most linked to HIV infection and risk behavior in the HIV epidemic among Black men who have sex with men. Am J Public Health. 2013;103(1):e28–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galea S, Nandi A, Vlahov D. The social epidemiology of substance use. Epidemiol Rev. 2004;26(1):36–52. [DOI] [PubMed] [Google Scholar]
- 17.Rumptz MH, Tobias C, Rajabiun S, Bradford J, Cabral H, Young R, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDs. 2007;21(S1):S-30–S-9. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281(24):2305–15. [DOI] [PubMed] [Google Scholar]
- 19.Kingdon MJ, Storholm ED, Halkitis PN, Jones DC, Moeller RW, Siconolfi D, et al. Targeting HIV prevention messaging to a new generation of gay, bisexual, and other young men who have sex with men. J Health Commun. 2013;18(3):325–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: Outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;12(suppl 1):S40–S8. [DOI] [PubMed] [Google Scholar]
- 21.Tobias C, Cunningham WE, Cunningham CO, Pounds MB. Making the connection: The importance of engagement and retention in HIV medical care. AIDS Patient Care STDs. 2007;21(suppl 1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham WE, Andersen RM, Katz MH, Stein MD, Turner BJ, Crystal S, et al. The impact of competing subsistence needs and barrier on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37(12):1270–81. [DOI] [PubMed] [Google Scholar]
- 23.Landrine H, Klonoff EA. Cultural diversity and health psychology In: Baum A, Singer J, Revenson T, editors. Handbook of health psychology. Mahway, NJ: Erlbaum; 2001. p. 855–95. [Google Scholar]
- 24.Brandon DT, Isaac LA, LaVeist TA. The legacy of Tuskegee and trust in medical care: Is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc. 2005;97(7):951–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong K, McMurphy S, Dean LT, Micco E, Putt M, Halbert CH, et al. Differences in the patterns of health care system distrust between Blacks and Whites. J Gen Intern Med. 2008;23(6):827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97(7):1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogart LM, Bird ST, Walt LC, Delahanty DL, Figler JL. Association of stereotypes about physicians to health care satisfaction, help-seeking behavior, and adherence to treatment. Soc Sci Med. 2004;58(6):1049–58. [DOI] [PubMed] [Google Scholar]
- 28.Bogart LM, Wagner G, Galvan FH, Banks D. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among African American men with HIV. J Acquir Immune Defic Syndr. 2010;53(5):648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogart LM, Wagner GJ, Green HD, Mutchler MG, Klein DJ, McDavitt B, et al. Medical mistrust among social network members may contribute to antiretroviral treatment nonadherence in African Americans living with HIV. Soc Sci Med. 2016;164:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogart LM, Wagner GJ, Green HD, Mutchler MG, Klein DJ, McDavitt B. Social network characteristics moderate the association between stigmatizing attributions about HIV and non-adherence among Black Americans living with HIV: A longitudinal assessment. Ann Behav Med. 2015;49(6):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dale SK, Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Medical mistrust is related to lower longitudinal medication adherence among African-American males with HIV. J Health Psychol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen FM, Fryer GEJ, Phillips RLJ, Wilson E, Pathman DE. Patients’ beliefs about racism, preferences for physician race, and satisfaction with care. Ann Fam Med. 2005;3(2):138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausmann LR, Jeong K, Bost JE, Ibrahim SA. Perceived discrimination in health care and health status in a racially diverse sample. Med Care. 2008;46(9):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(suppl 1):146–61. [DOI] [PubMed] [Google Scholar]
- 35.Lillie-Blanton M, Brodie M, Rowland D, Altman D, McIntosh M. Race, ethnicity, and the health care system: Public perceptions and experiences. Med Care Res Rev. 2000;57(suppl 1):218–35. [DOI] [PubMed] [Google Scholar]
- 36.Schrimshaw EW, Siegel K, Lekas HM. Changes in attitudes toward antiviral medication: A comparison of women living with HIV/AIDS in the pre-HAART and HAART eras. AIDS Behav. 2005;9(3):267–79. [DOI] [PubMed] [Google Scholar]
- 37.Siegel K, Karus D, Schrimshaw EW. Racial differences in attitudes toward protease inhibitors among older HIV-infected men. AIDS Care. 2000;12(4):423–34. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary A, Fisher HH, Purcell DW, Spikes PS, Gomez CA. Correlates of risk patterns and race/ethnicity among HIV-positive men who have sex with men. AIDS Behav. 2007;11(5):706–15. [DOI] [PubMed] [Google Scholar]
- 39.Foster PP, Gaskin SW. Older African Americans’ management of HIV/AIDS stigma. AIDS Care. 2009;21(10):1306–12. [DOI] [PubMed] [Google Scholar]
- 40.Jerome RC, Halkitis PN. Stigmatization, stress, and the search for belonging in Black men who have sex with men who use methamphetamine. J Black Psychol. 2009;35(3):343–65. [Google Scholar]
- 41.Graham LF, Braithwaite K, Spikes P, Stephens CF, Edu UF. Exploring the mental health of Black men who have sex with men. Community Ment Health J. 2009;45(4):272–84. [DOI] [PubMed] [Google Scholar]
- 42.Malebranche DJ, Fields EL, Bryant LO, Harper SR. Masculine socialization and sexual risk behaviors among Black men who have sex with men. Men Masculinities. 2009;12(1):90–112. [Google Scholar]
- 43.Peterson JL, Jones KT. HIV prevention for Black men who have sex with men in the United States. Am J Public Health. 2009;99(6):976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Longitudinal relationships between antiretroviral treatment adherence and discrimination due to HIV-serostatus, race, and sexual orientation among African-American men with HIV. Ann Behav Med. 2010;40(2):184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayles JN, Wong MD, Cunningham WE. The inability to take medications openly at home: Does it help explain gender disparities in HAART use? J Women’s Health 2006;15(2):173–81. [DOI] [PubMed] [Google Scholar]
- 46.Galvan FH, Bogart LM, Klein DJ, Wagner GJ, Chen YT. Medical mistrust as a key mediator in the association between perceived discrimination and adherence to antiretroviral therapy among HIV-positive Latino men. J Behav Med. 2017;40(5):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV‐related stigma on treatment adherence: Systematic review and meta‐synthesis. J Int AIDS Soc. 2013;16(3S2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blashill AJ, Bedoya CA, Mayer KH, O’Cleirigh C, Pinkston MM, Remmert JE, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav. 2015;19(6):981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalichman SC, Grebler T. Stress and poverty predictors of treatment adherence among people with low-literacy living with HIV/AIDS. Psychosom Med. 2010;72(8):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mugavero MJ, Raper JL, Reif S, Whetten K, Leserman J, Thielman NM, et al. Overload: The impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multi-site HIV cohort study. Psychosom Med. 2009;71(9):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner GJ, Bogart LM, Galvan FH, Banks D, Klein DJ. Discrimination as a key mediator of the relationship between posttraumatic stress and HIV treatment adherence among African American men. J Behav Med. 2012;35(1):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magidson JF, Blashill AJ, Safren SA, Wagner GJ. Depressive symptoms, lifestyle structure, and ART adherence among HIV-infected individuals: A longitudinal mediation analysis. AIDS Behav. 2015;19(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected Black women. J Community Health. 2004;29(2):117–27. [DOI] [PubMed] [Google Scholar]
- 55.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50(1):93–9. [DOI] [PubMed] [Google Scholar]
- 56.Duncan R, Shapshak P, Page JB, Chiappelli F, McCoy CB, Messiah SE. Crack cocaine: Effect modifier of RNA viral load and CD4 count in HIV-infected African American women. Front Biosci. 2007;12:1488–95. [DOI] [PubMed] [Google Scholar]
- 57.Carrico AW, Johnson MO, Morin SF, Remien RH, Riley ED, Hecht FM, et al. Stimulant use is associated with immune activation and depleted tryptophan among HIV-positive persons on anti-retroviral therapy. Brain Behav Immun. 2008;22(8):1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient–provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21(5):575–82. [DOI] [PubMed] [Google Scholar]
- 59.Reback C, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15(6):775–85. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez A, Barinas J, O’Cleirigh C. Substance use: Impact on adherence and HIV medical treatment. Curr HIV/AIDS Rep. 2011;8(4):223. [DOI] [PubMed] [Google Scholar]
- 61.Iguchi MY, Bell J, Ramchand R, Fain T. How criminal system racial disparities may translate into health disparities. J Health Care Poor Underserved. 2005;16(4 suppl B):48–56. [DOI] [PubMed] [Google Scholar]
- 62.Minior T, Galea S, Stuber J, Ahern J, Ompad D. Racial differences in discrimination experiences and responses among minority substance users. Ethn Dis. 2003;13(4):521–7. [PubMed] [Google Scholar]
- 63.Ramchand R, Pacula RL, Iguchi MY. Racial differences in marijuana-users’ risk of arrest in the United States. Drug Alcohol Dep. 2006;84(3):264–72. [DOI] [PubMed] [Google Scholar]
- 64.McCabe SE, Bostwick WB, Hughes TL, West BT, Boyd CJ. The relationship between discrimination and substance use disorders among lesbian, gay, and bisexual adults in the United States. Am J Public Health. 2010;100(10):1946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amola O, Grimmett MA. Sexual identity, mental health, HIV risk behaviors, and internalized homophobia among Black men who have sex with men. J Couns Dev. 2015;93(2):236–46. [Google Scholar]
- 66.Quinn K, Dickson-Gomez J. Homonegativity, religiosity, and the intersecting identities of young Black men who have sex with men. AIDS Behav. 2016;20(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinn K, Dickson-Gomez J, DiFranceisco W, Kelly JA, Lawrence JS. Correlates of internalized homonegativity among Black men who have sex with men. AIDS Educ Prev. 2015;27(3):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lazo M, Gange SJ, Wilson TE, Anastos K, Ostrow DG, Witt MD, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: Longitudinal study of men and women. Clin Infect Dis. 2007;45(10):1377–85. [DOI] [PubMed] [Google Scholar]
- 69.Levine AJ, Hinkin CH, Castellon SA, Mason KI, Lam MN, Perkins A, et al. Variations in patterns of highly active antiretroviral therapy (HAART) adherence. AIDS Behav. 2005;9(3):355–62. [DOI] [PubMed] [Google Scholar]
- 70.Kleeberger CA, Buechner J, Palella F, Detels R, Riddler S, Godfrey R, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18(4):683–8. [DOI] [PubMed] [Google Scholar]
- 71.Mannheimer S, Friedland G, Matts J, Child C, Chesney M, Terry Beirn Community Programs for Clinical Research on AIDS. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for Human Immunodeficiency Virus—infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–21. [DOI] [PubMed] [Google Scholar]
- 72.Carrieri P, Cailleton V, Le VM, Spire B, Dellamonica P, Bouvet E, et al. The dynamic of adherence to highly active antiretroviral therapy: Results from the French National APROCO cohort. J Acquir Immune Defic Syndr. 2001;28(3):232–9. [DOI] [PubMed] [Google Scholar]
- 73.Glass TR, Battegay M, Cavassini M, De Geest S, Furrer H, Vernazza PL, et al. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2010;54(2):197–203. [DOI] [PubMed] [Google Scholar]
- 74.Voisin DR, Quinn K, Kim DH, Schneider J. A longitudinal analysis of antiretroviral adherence among young Black men who have sex with men. J Adolesc Health. 2017;60(4):411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoover MA, Green HD, Bogart LM, Wagner GJ, Mutchler MG, Galvan FH, et al. Do people know I’m poz?: Factors associated with knowledge of serostatus among HIV-positive African Americans’ social network members. AIDS and Behavior. 2016;20(1):137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogart LM, Mutchler MG, McDavitt B, Klein DJ, Cunningham WE, Goggin KJ, et al. A randomized controlled trial of Rise, a community-based culturally congruent adherence intervention for Black Americans living with HIV. Ann Behav Med. 2017;(51)6:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chesney MA, Ickovics J, Chambers D, Gifford A, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12(3):255–66. [DOI] [PubMed] [Google Scholar]
- 78.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9):509–15. [Google Scholar]
- 79.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index: Reliability and validity in three centers. J Nerv Ment Dis. 1985. [DOI] [PubMed] [Google Scholar]
- 80.LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. 2009;44(6):2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee Hargraves J, Hays RD, Cleary PD. Psychometric properties of the Consumer Assessment of Health Plans Study (CAHPS®) 2.0 adult core survey. Health Serv Res. 2003;38(6p1):1509–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalichman SC, Simbayi L. Traditional beliefs about the cause of AIDS and AIDS-related stigma in South Africa. AIDS Care. 2004;16(5):572–80. [DOI] [PubMed] [Google Scholar]
- 83.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 84.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. Aids. 2000;14(4):357–66. [DOI] [PubMed] [Google Scholar]
- 85.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–93. [Google Scholar]
- 86.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35(4):542–71. [Google Scholar]
- 87.Booth GD, Niccolucci MJ, Schuster EG. Identifying proxy sets in multiple linear regression: An aid to better coefficient interpretation. Research paper INT (USA). 1994. [Google Scholar]
- 88.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 89.Wilson IB, Bangsberg DR, Shen J, al. e, editors. Heterogeneity among studies in rates of declines of antiretroviral therapy (ART) adherence over time: Findings from MACH14 (# 62221). 5th International Conference on HIV Treatment Adherence, Miami, FL; 2010. [Google Scholar]
- 90.Carrico AW, Johnson MO, Colfax GN, Moskowitz JT. Affective correlates of stimulant use and adherence to anti-retroviral therapy among HIV-positive methamphetamine users. AIDS Behav. 2010;14(4):769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction. 2008;103(8):1242–57. [DOI] [PubMed] [Google Scholar]
- 92.McElhiney MC, Rabkin JG, Rabkin R, Nunes EV. Provigil (modafinil) plus cognitive behavioral therapy for methamphetamine use in HIV+ gay men: A pilot study. Am J Drug Alcohol Abuse. 2009;35(1):34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. 2007;69(8):785–92. [DOI] [PubMed] [Google Scholar]
- 94.Maulsby C, Millett G, Lindsey K, Kelley R, Johnson K, Montoya D, et al. HIV among Black men who have sex with men (MSM) in the United States: A review of the literature. AIDS Behav. 2014;18(1):10–25. [DOI] [PubMed] [Google Scholar]
- 95.Bakken S, Holzemer WL, Brown M-A, Powell-Cope GM, Turner JG, Inouye J, et al. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care STDs. 2000;14(4):189–97. [DOI] [PubMed] [Google Scholar]
- 96.Beach MC, Keruly J, Moore RD. Is the quality of the patient‐provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21(6):661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ingersoll KS, Heckman CJ. Patient–clinician relationships and treatment system effects on HIV medication adherence. AIDS Behav. 2005;9(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence‐enhancing interventions for highly active antiretroviral therapy in HIV‐infected patients–a systematic review. HIV Med. 2013;14(10):583–95. [DOI] [PubMed] [Google Scholar]
- 99.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: A research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–97. [DOI] [PubMed] [Google Scholar]