Abstract
Isomaltulose (α-d-glucopyranosyl-1,6-d-fructofuranose) is an important industrial and raw food material, which can be synthesised from the by-products of sugar cane processing through sucrose isomerization conversion. In this study, we constructed a surface display vector of sucrose isomerase from Pantoeadispersa (pSIase) by a glycosylphosphatidylinositol (GPI)-cell wall protein (CWP) anchor signal sequence and successfully displayed pSIase on the cell surface of Yarrowia lipolytica, thereby increasing the conversion efficiency of isomaltulose. The highest activity of the displayed pSIase reached 2910.3 U/g of cell dry weight. Compared with the free pSIase, the displayed enzyme showed good stability at a broad range of temperatures (20–45 °C). The half-life at 40 °C increased from 62 to 141 min and the deactivation constants (kd) reached 4.91 × 10−3 min−1. Using low-cost cane molasses as the substrate, the isomaltulose conversion rate remained at 85% even after 9 batches were processed, which is a highly desired outcome for industrial use.
Keywords: Sugar cane, Isomaltulose, Sucrose isomerase, Cell surface display
Introduction
Sugar cane is one of the most important crops in the world and a major source of biofuels and sugar production. In Brazil, the first and second generation ethanol produced from sugar cane has a mature commercial market (Maeda et al. 2013). Recently, bioconversion of isomaltulose from cane molasses has drawn wide attention. Isomaltulose (also known as palatinose) is a well-known carbohydrate that releases glucose into blood at a slow rate, thus avoiding a sudden peak and sudden drop in glucose levels (Fleddermann et al. 2016; Hamada 2002). Isomaltulose is often used as an energy bar material for astronauts and special workers due to its more balanced and longer energy supply. More importantly, isomaltulose has accessible carbonyl groups and is preferred as a renewable raw material in the manufacture of bioproducts such as polymers and surfactants over petroleum-based materials (Cartarius et al. 2001).
Both isomaltulose (a-d-glucosylpyranosyl-1,6-d-fructofuranose) and trehalulose (a-d-glucosyl-pyranosyl-1,1-d-fructofuranose) are structural isomers of sucrose. Isomaltulose is 30% as sweet as sucrose and can be readily crystallized for use as a solid additive (Cheetham et al. 1982) whereas trehalulose is extremely water-soluble (Ooshima et al. 1991) and can only be used in highly sweetened foods such as jellies and jams which require highly concentrated sugar solutions. Currently, more than 90% isomaltulose is converted from sucrose using microbial cells and enzymes. Microbial conversion has been accomplished with either free or immobilized cells (Ahn et al. 2003; Kawaguti et al. 2007). However, two key factors limit microbial conversion: (1) biosafety of some strains is unclear. For instance, Klebsiellapneumonia (Aroonnual et al. 2007), Pantoeadispersa (Wu and Birch 2004) and Serratiaplymuthica (Véronèse and Perlot 1999) are considered to be potential pathogens. Therefore, isomaltulose produced by such microbes can not be used in the food industry (2) the isomaltulose conversion ratio is between 60 and 90% and it is not only difficult to separate isomaltulose from the other contaminating microbial metabolites, but the yields and recovery of the desired isomer is also lower (Salvucci et al. 2003; Véronèse and Perlot 1999). Recently, sucrose isomerase (SIase) has been used in industries for the production of isomaltulose. Many SIase have been purified and characterized, including the natural form of enzymes from Erwiniarhapontici (Li et al. 2011), Pantoeadispersa (Wu and Birch 2004) as well as the recombinant enzymes from Enterobacter sp. (Cha et al. 2009), Protaminobacterrubrum (Lee et al. 2008) and Pseudomonasmesoacidophila (Watzlawick and Mattes 2009). Enzymes have remarkable catalytic reactions and substrate specificity compared to microbial conversion as neither the substrate nor the products have to cross the cell membrane barrier. In some cases biocatalytic methods use purified enzyme which are time consuming and expensive. Moreover, the enzyme preparation can only be used once for the desired reaction and is subsequently discarded as waste.
Yeast surface display is an extension of the enzyme application technique. One of the most attractive features of surface display is that enzyme molecules are simultaneously synthesized and self-immobilized on the microbial cell surface and the living whole-cell biocatalyst is easily produced by microbial cultivation techniques (Chen et al. 2012; Hiraishi et al. 2012; Ueda and Tanaka 2000). No further work is required to either purify or immobilize the enzymes. Recently, lipase, glucosidase, amylases, cellulases, xylanases and other enzymes have been successfully immobilized on yeast cells and their potential applications have been evaluated (Adachi et al. 2013; Schüürmann et al. 2014; Yue et al. 2008; Yuzbasheva et al. 2015). Inspired by such works we constructed a surface display vector for sucrose isomerase from P. dispersa (pSIase) by glycosylphosphatidylinositol (GPI)-cell wall protein. pSIase is an excellent glycosylated transferase from P.dispersa as it shows a high activity and low Km (40 mM) toward surcose compared to other SIase (Cha et al. 2009; Lee et al. 2008; Wu and Birch 2004, 2005). After 96 h cultivation, the pSIase was successfully displayed on the cell surface of Yarrowialipolytica. This dispalyed pSIase shows a high thermal stability and activity compared to the free pSIase. Using Y.lipolytica harboring displayed pSIase as whole-cell biocatalysts, cane molasses can be efficiently converted to the isomaltulose. Our study suggests that cell surface display is a valuable method in isomaltulose systhesis.
Materials and methods
Materials
pMD19-T vector was purchased from TaKaRa (Japan). The surface display vector pINA1317-CWP110, which contains the C-terminal end of CWP110 from Y.lipolytica, was donated by ZM Chi. The Y.lipolytica yeast strain used for cell surface display was Po1h (genotype: MatA, ura3-302, xpr2-322, axp1-2; phenotype: Ura − , AEP, AXP, Suc + ). In order to produce displayed protein by yeast transformants, the phosphate vitamin B (PPB) medium described by Jolivalt (2005) was used. Sucrose, isomaltulose and trehalulose were supplied by Sangon Inc (Shanghai, China). Cane molasses, the by-product of the sugar cane industry, was obtained from Media Sugar Refinery (Qingdao, China).
Display pSIase on the cell surface of Y. lipolytica
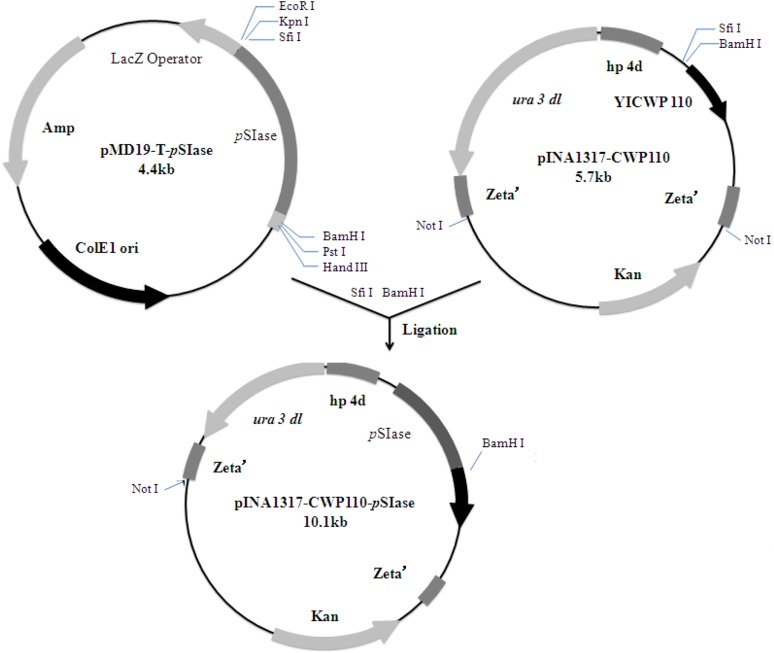
A forward primer S1: 5′-GGCCGTTCTGGCCGCCAGCCCGTTAACTAAGCCATCGA-3′ (underlined bases encode SfiI restriction site) and a reverse primer P15′-GGATCCTCAGTTCAG CTTATAGATCCCGGCTTG-3′ (underlined bases encode BamHI restriction site) were designed to amplify the pSIase gene from P.dispersa (ATCC14589, GenBank accession number AY223549.1) using PCR. Gene amplification and expression were performed as described by Yue et al. (2008). The resulting plasmid carrying pSIase gene was designated as pINA1317-CWP110-pSIase (Fig. 1). The positive transformants carrying pSIase gene were grown in PPB liquid medium for 96 h. The cells of Y.lipolytica Po1h carrying only the yeast cassette without PSIase gene were used as controls.
Fig. 1.
Construction of the plasmid pINA1317-YlCWP110-pSIase for surface display on Y. lipolytica cells
Determination of pSIase activity
The fermentation broth (1000 mL) was centrifuged at 9,000 rpm for 5 min at 4 °C, and the cell pellet was rinsed with deionized water and resuspended in 100 mL 50 mM sodium acetate buffer (pH 6.0). The activity of pSIase was measured at 30 °C in 50 mM sodium phosphate buffer (pH 6.0) with 10% sucrose solutions as a substrate. Specifically, 100 μL wet cells solution was mixed with 900 μL substrate solution and incubated for 15 min. The reaction was stopped by boiling the mixture in a 100 °C water bath for 5 min. Resolution and quantification of sucrose, isomaltulose and trehalulose were achieved by High-Performance Liquid Chromatography (HPLC) system equipped with a Thermo Hypersil APS2 NH2 (150 × 4.6 mm) and Refractive Index (RI) detector. The mobile phase was acetonitrile–water (80:20), the flow rate was 0.8 mL/min and the column and detector temperatures were 30 °C. Sucrose, isomaltulose and trehalulose were used as standards (25 mg/L). One unit of pSIase activity is defined as the amount of cell dry weight that produces 1 μmol of isomaltulose per min under the conditions described above.
Thermal characterization of the displayed pSIase
The optimal temperature for displayed pSIase was determined in the temperature range between 20 and 45 °C. Temperature stability of the displayed pSIase was determined by pre-incubating the enzyme for 60 min at different temperatures ranging from 20–45 °C and the residual activity was measured immediately as described above. The deactivation constants (kd) was calculated from the semi-logarithmic plot of residual activity versus time (Eq. 1), where Et is the residual enzyme activity after heat treatment for time, E0 is the initial enzyme activity before heat treatment. The half-lives of the free and displayed pSIase were calculated using Eq. 2.
| 1 |
| 2 |
Pretreatment of cane molasses
The cane molasses an important by-product of the cane sugar industry contains 45.6% (w/w) sucrose, 4.9% (w/w) glucose, 6.3% (w/w) fructose, 2.5% (w/w) other carbohydrates, 4.2% (w/w) crude protein, 0.1% (w/w) crude fat, 8.3% (w/w) ash, 7.9% (w/w) metal ions (e.g., calcium, potassium, sodium, iron, magnesium and copper) and 20.3% (w/w) water. Cane molasses was diluted with distilled water to obtain a total sugar concentration of 200 g/L. In order to promote colloid, protein flocculation and precipitate of the divalent metal ions, its pH was adjusted to 3.5 with 5 M H2S04. Then, 5% (w/v) activated carbon powder was added to the acidic cane molasses for decolorization, followed by incubation at 65 °C for 1 h after which the activated carbon was removed by filtration and the pH adjusted to 6.0–6.5 with 10 M NaOH.
Reusability of the displayed pSIase using cane molasses as substrate
A batch reaction was performed with 30 ml wet cells (approximately 8500 U SIase) mixed with 200 mL pretreated cane molasses and the necessary nutrients for Y.lipolytica (5 g/L yeast extract, 10 g/L tryptone) in a 500 mL flask. The reactions were incubated in a shaking bath at 30 °C until the substrate was completely catalyzed to products. Then, the products were washed with deionized water after each batch. All samples of each batch were analyzed by HPLC.
Results
Cell surface display of pSIase
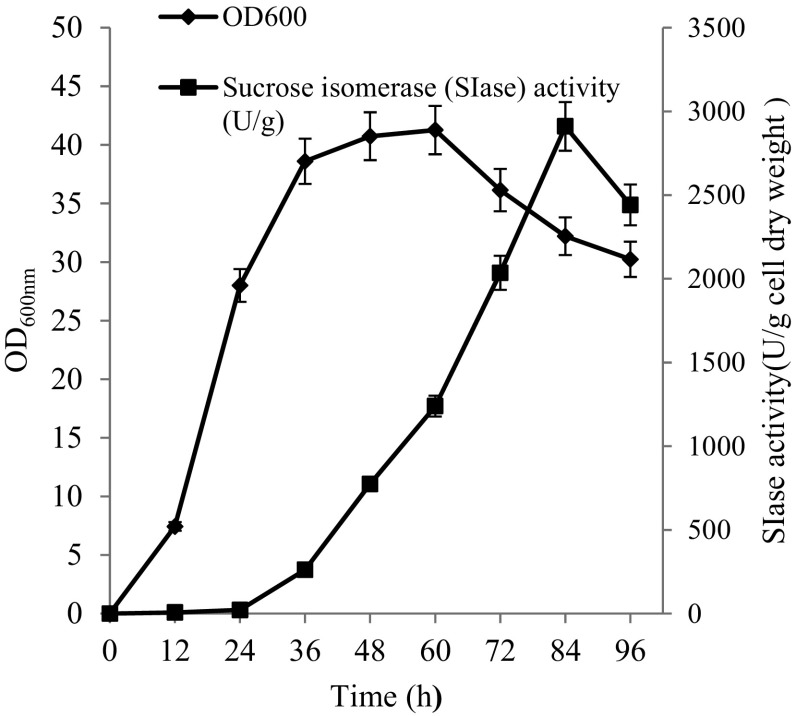
CWP110 is a gene isolated from Y.lipolytica, which encodes GPI-cell wall protein and promotes the immobilization of the targeted protein on the yeast cell surface (Yue et al. 2008). In order to anchor pSIase to the cell surface, the gene encoding pSIase (1731 bp in length without the signal sequence) was fused with the vector pINA1317-CWP110. The constructed pINA1317-CWP110-pSIase was transfered into Y.lipolytica, followed by cultivation in the PPB medium for 96 h. The positive transformants would carry CWP110-pSIase while the control cells would carry only CWP110. The correlation of the culture time with the displayed pSIase activity and cell growth was investigated (Fig. 2). The displayed pSIase activity began to increase at 36 h and reached to a peak value at 82 h. The biomass of the cell reached 17 g dry weight/L and the pSIase activity reached 2910.3 U/g of cell dry weight. Among the tested transformants, the displayed pSIase activity varied significantly due to the differences of the copy numbers within the integrated cassette. The highest SIase activity transformants was then chosen for further cultivation.
Fig. 2.
The growth curve and producing enzyme curve of Y. lipolytica harbing pINA1317-CWP110-pSIase. Data are given as the mean ± SD, n = 3
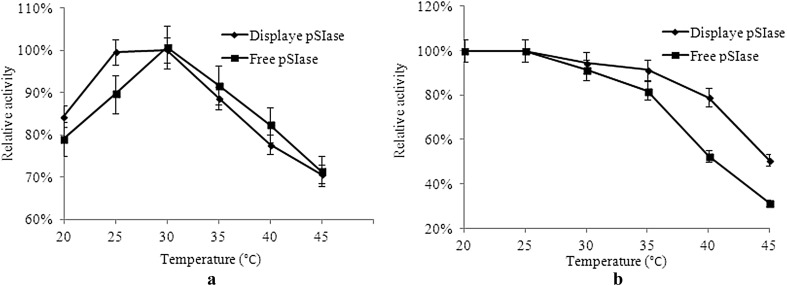
Thermal characterization of the displayed pSIase
The optimal temperature and thermostability of the displayed pSIase were determined. The displayed pSIase showed optimal activity at 30 °C with the performance being the same as the free pSIase (Fig. 3a). However, the thermostability of the displayed pSIase was higher than that of the free enzyme in the range of 25–45 °C. As shown in Fig. 3b, the displayed pSIase retained approximately 52% of its enzyme activity upon incubation at 45 °C for 1 h, whereas the free pSIase lost 71% of its hydrolytic activity at the same condition.
Fig. 3.
Effects of different temperatures on activity a and stability b of displayed and free pSIase, respectively. Data are given as the mean ± SD, n = 3
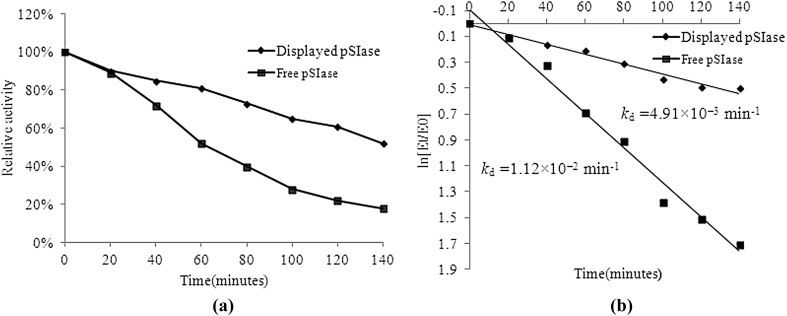
The half-life of the pSIase activity was determined at a constant temperature. As shown in Fig. 4a, the residual enzyme activity of the free pSIase dropped to about 22% of the original after 120 min incubation at 40 °C. In contrast, the displayed pSIase retained approximately 61% of its enzyme activity under the same conditions. The deactivation constants (kd) for the first-order thermal deactivation were calculated using Eq. (1). The kd of the displayed pSIase at 40 °C was 4.91 × 10−3 min−1 in comparison with kd = 1.12 × 10−2 min−1 of free pSIase (Fig. 4 b). The displayed pSIase exhibited a half-life of 141 min, which was higher than that of the free pSIase (62 min). This finding suggests that the pSIase was thermostable when immobilized on the yeast cell wall.
Fig. 4.
Thermal deactivation curves (a) and thermal deactivation constants (b) of displayed pSIase and free pSIase. The thermal inactivation at 40 °C of enzyme was determined up to 0 to 140 min. The residual activities after heat treatment were measured immediately. The optimal enzyme activity was taken as 100%
The effect of reaction temperatures on products
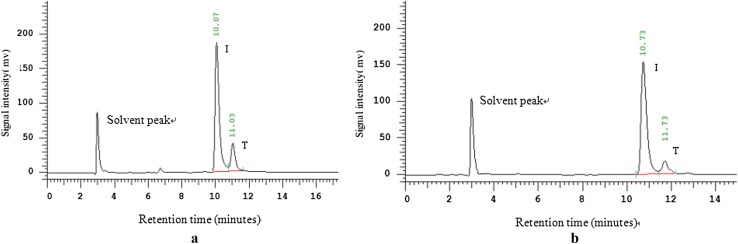
To date there is still no consistent conclusion reported on the effect of temperature on enzyme products. Wu and Birch reported that low temperatures promoted the release of isomaltulose but suppressed trehalulose formation (Wu and Birch 2005), while others observed the opposite phenomena. For example, the maximum production of SIase from S.plymuthica was obtained at 30 °C for isomaltulose and at 25 °C for trehalulose (Véronèse and Perlot 1999). Using the displayed pSIase, we determined the effect of reaction temperatures on the ratio of the products. As shown in Fig. 5, with the decrease of reaction temperatures, the ratio of isomaltulose increased from 84 to 93%. We speculated that the increased products ratio was due to the thermal isomeric effect. Sugar isomerization is a two-step reaction mechanism. First, the enzyme binds sugar to form an enzyme-sugar complex, followed by cleaved at the α-1,2 bond (fructosyl moiety) and produces a second enzyme form (enzyme-glucose complex). Then, the free fructose is isomerized at the active site and pSIase catalyzes the enzymatic rearrangement of the α-1,2 linkage between glucose and fructose to an α-1,6 linkage (producing isomaltulose) or α-1,4 linkage (producing trehalulose). Isomaltulose formation requires rotation of the fructose residue through 180° (Cheetham 1984). Upon changes in the reaction temperature, enzyme molecules reduced the activation energy by changing the structure rigidity and flexibility to ensure the progress of the reaction. As a result, the structural constraints of active site of the enzyme will be changed correspondingly, which leads to the disturbance of the rotation of the fructose and allows the formation of kinetically or thermodynamically preferred products, thereby forming the different sucrose isomer.
Fig. 5.
HPLC analysis of products by displayed pSIase. Trehalulose (T), Isomaltulose (I). The effect of reaction temperatures on products was tested by 10 ml wet cells solution harboring pSIase were mixed with 100 ml 20% (m/v) sucrose solutions as a substrate at 40 °C (a) and 30 °C (b) for 2 h
Isomaltulose synthesis by a whole-cell biocatalyst with pSIase
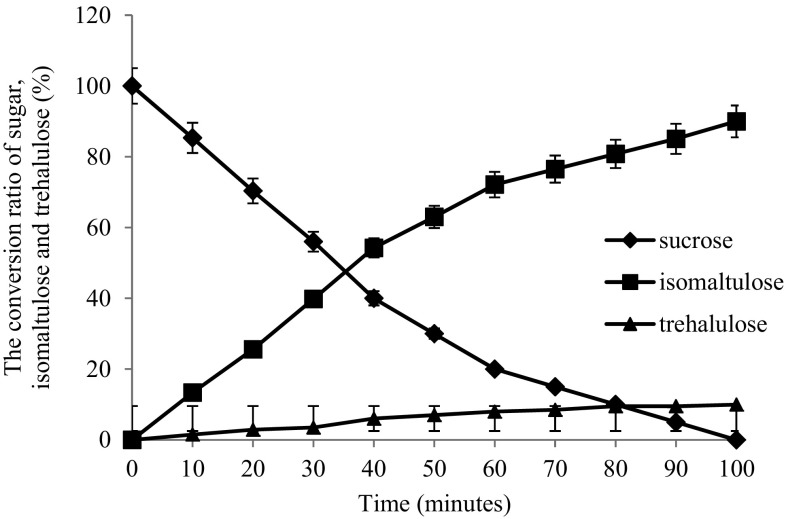
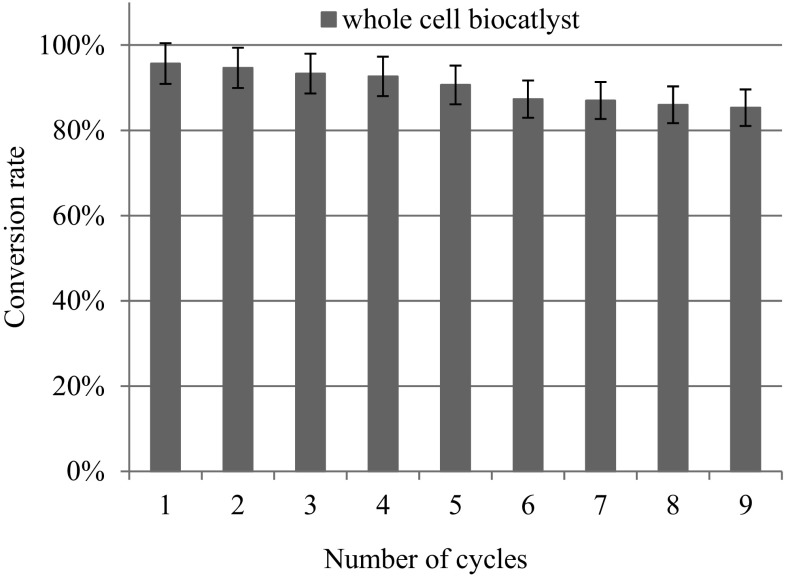
A typical conversion curve of sucrose to isomaltulose and trehalulose using the whole-cells biocatalyst under optimal conditions (30 °C) was determined (Fig. 6). The conversion velocity of sucrose was boosted during the initial l40 min and the conversion peaked at approximately 100 min. The isomaltulose concentration reached 184.8 g/L, indicating that the isomaltulose yield was 92.4% when the sucrose was almost completely consumed. From the perspective of industrial catalysts, continued reusability of an enzyme is very important. To address this issue, we used the pretreated cane molasses as a substrate and the batch catalysis reaction of sucrose was repeated to determine the practical reusability of the whole-cell biocatalyst. The displayed pSIase showed an outstanding operational stability, the isomaltulose conversion rate still remained more than 85% of original even after 9 batches catalysis (Fig. 7).
Fig. 6.
The conversion curve of sucrose. 10 g wet Y. lipolytica harboring pSIase were mixed with 100 ml 20% (m/v) sucrose solutions under 30 °C
Fig. 7.
Number of cycles of the whole cell biocatalyst
Conclusions
In this study, we demonstrated how to display a pSIaseb on the Y.lipolytica and its reusability on continuous production of isomaltulose. Compared with the traditional microbiological conversions, displayed pSIase showed three advantages: First, the sucrose isomerization can be achieved at high substrate concentration, which greatly enhances the production efficiency of isomaltulose. Second, there are almost no microbial metabolites except for the small amounts of monosaccharides in the enzymatic products; simpler product purification and cost effective downstream processing are convenient for industrial production. Thirdly, the displayed enzyme was anchored in a matrix, in this case, the cell envelope stabilizes the enzyme and prevents it from proteolytic degradation resulting in continuous higher activities and stability.
Although surface display shows great properties and applications, there are still some uncertainties affecting its universality. First, the amount of enzyme produced is limited due to the unavailability of membrane area. In general, the number of displayed enzymes is between 103 and 104 (Feldhaus et al. 2004; Kuroda et al. 2009). Second, some catalysis reactions need endogenous coenzymes for enzymatic reactions, which limit the kind of displayed enzymes. Therefore, if we want to develop a commercial displayed enzyme technology, the types, structures and applications of enzyme should be considered.
Acknowledgements
This study was supported by the grants from Central Public interset Scientific Institution Basal Research Funds, CAFS (no. 2017GH07) and 2017 green manufacturing projects of china (no. Z135060009002).
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Yuan Zheng, Email: zhengyuan@ysfri.ac.cn.
Zhipeng Wang, Email: spirit87@163.com.
Xiaofeng Ji, Email: Jixf@ysfri.ac.cn.
Jun Sheng, Phone: +86-532-85833961, Email: shengjun@ysfri.ac.cn.
References
- Adachi N, Takahashi C, Ono-Murota N, Yamaguchi R, Tanaka T, Kondo A. Direct L- lysine production from cellobiose by Corynebacteriumglutamicum displaying beta-glucosidase on its cell surface. Appl Microbiol Biotechnol. 2013;97(16):7165–7172. doi: 10.1007/s00253-013-5009-4. [DOI] [PubMed] [Google Scholar]
- Ahn SJ, Yoo JH, Lee HC, Kim SY, Noh BS, Kim JH, Lee JK. Enhanced conversion of sucrose to isomaltulose by a mutant of Erwiniarhapontici. Biotechnol Lett. 2003;25:1179–1183. doi: 10.1023/A:1024554728773. [DOI] [PubMed] [Google Scholar]
- Aroonnual A, Nihira T, Seki T, Panbangred W. Role of several key residues in the catalytic activity of sucrose isomerase from Klebsiellapneumoniae NK33-98-8. Enzyme Microb Technol. 2007;40:1221–1227. doi: 10.1016/j.enzmictec.2006.09.011. [DOI] [Google Scholar]
- Cartarius R, Krause T, Vogel H. Degradable surfactants via heterogeneously catalyzed reductive amination of isomaltulose-examination of catalyst deactivation in a continuous gradient free reaction vessel. Chem Eng Technol. 2001;24:55A–59A. [Google Scholar]
- Cha J, Jung JH, Park SE, Cho MH, Seo DH, Ha SJ, Yoon JW, Lee OH, Kim YC, Park CS. Molecular cloning and functional characterizationof a sucrose isomerase (isomaltulose synthase) gene from Enterobacter sp. FMB-1. J Appl Microbiol. 2009;107:1119–1130. doi: 10.1111/j.1365-2672.2009.04295.x. [DOI] [PubMed] [Google Scholar]
- Cheetham PS. The extraction and mechanism of novel isomaltulose synthesizing enzyme from Erwiniarhapontici. Biochem J. 1984;220(1):213–220. doi: 10.1042/bj2200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham PSJ, Imber CE, Isherwood J. The formation of isomaltulose by immobilized Erwiniarhapontici. Nature. 1982;299:628–631. doi: 10.1038/299628a0. [DOI] [Google Scholar]
- Chen YP, Hwang IE, Lin CJ, Wang HJ, Tseng CP. Enhancing the stability of xylanase from Cellulomonas fimi by cell-surface display on Escherichia coli. J Appl Microbiol. 2012;112(3):455–463. doi: 10.1111/j.1365-2672.2012.05232.x. [DOI] [PubMed] [Google Scholar]
- Feldhaus M, Siegel R. Flow cytometric screening of yeast surface display libraries. Methods Mol Biol. 2004;263:311–332. doi: 10.1385/1-59259-773-4:311. [DOI] [PubMed] [Google Scholar]
- Fleddermann M, Rauh-Pfeiffer A, Demmelmair H, Holdt L, Teupser D, Koletzko B (2016) Effects of a Follow-on formula containing isomaltulose (PalatinoseTM) on metabolic response, acceptance, tolerance and safety ininfants: a randomized-controlled trial. PLoS One 11(3):e151614 [DOI] [PMC free article] [PubMed]
- Hamada S. Role of sweeteners in the etiology and prevention of dentalcaries. Pure Appl Chem. 2002;74:1293–1300. doi: 10.1351/pac200274071293. [DOI] [Google Scholar]
- Hiraishi T, Yamashita K, Sakono M, Nakanishi J, Tan LT, Sudesh K, Abe H, Maeda M. Display of functionally active PHB depolymerase on Escherichia coli cell surface. Macromol Biosci. 2012;12(2):218–224. doi: 10.1002/mabi.201100273. [DOI] [PubMed] [Google Scholar]
- Jolivalt C, Madzak C, Brault A, Caminade E, Malosse C, Mougin C. Expression of laccase lllb from the white-rot fungus Trametes versicolor in the yeast Yarrowialipolytica for environmental applications. Appl Micro biol Biotechnol. 2005;66(4):450–456. doi: 10.1007/s00253-004-1717-0. [DOI] [PubMed] [Google Scholar]
- Kawaguti HY, Sato HH. Palatinose production by free and Ca-alginate gel immobilized cells of Erwinia sp. Biochem Eng J. 2007;36:202–208. doi: 10.1016/j.bej.2007.02.017. [DOI] [Google Scholar]
- Kuroda K, Matsui K, Higuchi S, Kotaka A, Sahara H, Hata Y, Ueda M. Enhancement of display efficiency in yeast display system by vector engineering and gene disruption. Appl Microbiol Biotechnol. 2009;82(4):713–719. doi: 10.1007/s00253-008-1808-4. [DOI] [PubMed] [Google Scholar]
- Lee HC, Kim JH, Kim SY, Lee JK. Isomaltose production by modification of the fructose binding site on the basis of the predicted structure of sucrose isomerase from “Protaminobacterrubrum”. Appl Environ Microbiol. 2008;74:5183–5194. doi: 10.1128/AEM.00181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cai H, Qing Y, Ren B, Xu H, Zhu H, Yao J. Cloning and characterization of a sucrose isomerase from Erwiniarhapontici NX-5 for isomaltulose hyperproduction. Appl Biochem Biotechnol. 2011;163:52–63. doi: 10.1007/s12010-010-9015-z. [DOI] [PubMed] [Google Scholar]
- Maeda RN, Barcelos CA, Santa Anna LM, Pereira N. Cellulase production by Penicilliumfuniculosum and its application in the hydrolysis of sugar cane bagasse for second generation ethanol production by fed batch operation. J Biotechnol. 2013;163:38–44. doi: 10.1016/j.jbiotec.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Ooshima T, Izumitani A, Minami T, Fujiwara T, Nakajima Y, Hamada S. Trehalulose does not induce dental caries in rats infected with mutans Streptococci. Caries Res. 1991;25:277–282. doi: 10.1159/000261376. [DOI] [PubMed] [Google Scholar]
- Salvucci ME. Distinct sucrose isomerases catalyze trehalulose synthesis in whiteflies, Bemisiaargentifolii, and Erwiniarhapontici. Comp Biochem Physiol B. 2003;135:385–395. doi: 10.1016/S1096-4959(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Schüürmann J, Quehl P, Festel G, Jose J. Bacterial whole-cell biocatalysts by surface display of enzymes: toward industrial application. Appl Microbiol Biotechnol. 2014;98:8031–8046. doi: 10.1007/s00253-014-5897-y. [DOI] [PubMed] [Google Scholar]
- Ueda M, Tanaka A. Genetic immobilization of proteins on the yeast cell surface. Biotechnol Adv. 2000;18:121–140. doi: 10.1016/S0734-9750(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Veronese T, Perlot P. Proposition for the biochemical mechanism occurring inthesucrose isomerase active site. FEBS Lett. 1998;441(3):348–352. doi: 10.1016/S0014-5793(98)01582-8. [DOI] [PubMed] [Google Scholar]
- Veronese T, Perlot P. Mechanism of sucrose conversion by the sucrose isomerase of Serratiaplymuthica ATCC 15928. Enzyme Microb Technol. 1999;24:263–269. doi: 10.1016/S0141-0229(98)00115-X. [DOI] [Google Scholar]
- Watzlawick H, Mattes R. Gene cloning, protein characterization and alteration of product selectivity for the trehalulose hydrolase and trehalulose synthase from “Pseudomonasmesoacidophila” MX-45. Appl Environ Microbiol. 2009;75:7026–7036. doi: 10.1128/AEM.01781-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Birch RG. Characterization of Pantoeadispersa UQ68J: producer of a highly efficient sucrose isomerase for isomaltulose biosynthesis. J Appl Microbiol. 2004;97:93–103. doi: 10.1111/j.1365-2672.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- Wu L, Birch RG. Characterization of the highly efficient sucrose isomerase from UQ-68J and cloning of the sucrose isomerase gene. Appl Environ Microbiol. 2005;71(3):1581–1590. doi: 10.1128/AEM.71.3.1581-1590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue LX, Chi ZM, Wang L, Liu J, Madzak C, Li J, Wang XH. Construction of a new plasmid for surface display on cells of Yarrowialipolytica. J Microbiol Methods. 2008;72:116–123. doi: 10.1016/j.mimet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Yuzbasheva EY, Yuzbashev TV, Perkovskaya NI, Mostova EB, Vybornaya TV, Sukhozhenko AV, Toropygin IY, Sineoky SP. Cell surface display of Yarrowialipolytica lipase Lip2p using the cell wall protein YlPir1p, its characterization and application as a whole-cell biocatalyst. Appl Biochem Biotechnol. 2015;75:3888–3900. doi: 10.1007/s12010-015-1557-7. [DOI] [PubMed] [Google Scholar]