Abstract
Agrobacterium mediated in planta method was used to transform Indian elite wheat genotype HD2894 with herbicide-tolerant CP4-EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) gene. The apical meristems of germinated seeds were targeted for introgression of transgene. The obtained T1 plants were screened by spraying 1% glyphosate and only positive transformants survived. The presence of transgene was also confirmed by PCR and Southern hybridization. Using this method, 3.07% transformation rate was observed. To identify transgenic lines carrying stably integrated CP4-EPSPS gene, the transgenic populations were screened in T3 generation using 1% glyphosate and lines with 100% survival were considered as homozygous. No significant morpho-physiological variations were observed within the transgenic lines as compared to non-transgenic plants. The present study resulted in herbicide-tolerant transgenic wheat and provides a valuable tool for development of wheat genetic transformation.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1708-6) contains supplementary material, which is available to authorized users.
Keywords: Indian bread wheat, Agrobacterium tumefaciens, In planta transformation, Apical meristem, Herbicide tolerance, CP4-EPSPS transgenic wheat
Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops of the world with an annual production of ~ 757.2 million tons in 2016–17 (FAO 2018). By 2050, world total food production has to be increased by 60–70% to meet the demands of the ever growing population. This target of increased food grain production can be achieved only by enhancing crop productivity by developing genotypes resistant to various biotic and abiotic stresses which negatively affect wheat production (Joshi et al. 2007). Conventional breeding strategies are being successfully employed for wheat improvement throughout the world. However, since the available gene pool is becoming a major limiting factor in most cereals crops, the conventional plant breeding methods alone will not be able to meet the enhanced world food grain demand in the coming years. For the past few decades, recombinant DNA technology has successfully revolutionized the transfer of genes from across living systems to the desired organisms for improved traits (James 2003).
Weeds are one of the major problems in almost all the cereal crops including wheat, causing a significant reduction in grain yield. Farmers invest high amount of money in the form of chemicals or manpower to tackle this problem. The presence of genes encoding herbicides inactivating enzymes have been reported in a number of prokaryotic and eukaryotic organisms. The enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) is known to be involved in the metabolism of aromatic amino acids through the shikimate pathway in plants. EPSPS is the biological target for the herbicide glyphosate, which results in inhibition of the enzyme's catalysis and shuts down the pathway (Geiger and Fuchs 2002; Cobb and Reade 2010). Eventually, this results in the death of the organism due to lack of aromatic amino acids required to survive. However, in few microorganisms as Pseudomonas putida, Agrobacterium CP4, EPSPS genes are not inhibited by glyphosate, It has been proved that the overexpression of CP4-EPSPS gene in transgenics plants leads to herbicide tolerance (Duke 2011). The CP4-EPSPS (Accession no. KJ787649) gene from Agrobacterium tumefaciens strain CP4, reported to be effective in controlling the weeds by providing herbicide tolerance to the targeted crop (Chhapekar et al. 2015), was used in this study.
Genetic transformation forms the core of transgenic development technology; a foolproof protocol for regeneration and genetic transformation is a must for developing wheat transgenics that survive in challenging situations of various abiotic and biotic stresses. But the hexaploid (2n = 6X = 42) complex genomic nature and genotype-specific genetic transformation response further restrict the success of transgenic development in wheat (Han et al. 2011), as it may be a possible reason for any transgene silencing. Although laboratories working on wheat transformation have developed efficient protocols but still that is much less efficient than any other cereal crop used for transformation (Anand et al. 2003). Wheat can be transformed by many different methods ‘such as microinjection, electroporation, PEG (polyethylene glycol)-mediated, silicon carbide-mediated and laser-mediated uptake, but particle bombardment and Agrobacterium-mediated transformation (Cheng et al. 1997) have been proven to be the most successful. Kasirajan et al. (2013) have reported particle bombardment as the most robust method for wheat transformation, but having other disadvantages such as DNA fragmentation and complex genome integration (Hu et al. 2003). Transformation using induced wheat callus requires microbe-free environment and is labor intensive and time consuming. Plant regeneration and callus induction also depend on factors such as genotype, explant and cultural conditions (Han et al. 2011; Bohorova et al. 1995; Kato et al. 1991).
Different plant species including wheat have been successfully transformed using in planta transformation protocol, although with very low transformation efficiency (Supartana et al. 2005; Chugh et al. 2012). Thus, in planta-based wheat transformation protocol can be proven as an alternative method over other methods which require safety procedures, time consumption and sterile conditions (Razzaq et al. 2011).
Genetic transformation forms the core of transgenic development technology and therefore for development of wheat transgenics for various abiotic and biotic stress, a foolproof protocol for transformation and regeneration is must. The genotype-specific genetic transformation response (Han et al. 2011) further restricts the success of transgenic development in wheat. It is essential to have transgenics developed in Indian elite wheat genotypes for the benefit of farmers and the nation as a whole, since varieties other than those popularly grown would not be accepted by farmers and public for consumption. Very few studies report on transformation in Indian elite genotypes of wheat and the results obtained so far are not encouraging. A competent, cost-effective transformation protocol needs to be standardized in Indian elite genotypes of wheat; the same can be followed for developing wheat transgenics against abiotic and biotic stresses. The present study aimed at transforming Indian elite wheat genotype T. aestivum HD2894 by shoot apical meristem-targeted method of in planta transformation using herbicide-tolerance gene (EPSPS) from A. tumefaciens strain CP4. The putative herbicide-tolerant wheat transgenics developed were analyzed for stable integration and expression of transgene. Herbicide-tolerant wheat developed will be a vital tool for farmers to overcome the weed menace. The competent, cost-effective transformation protocol thus standardized in Indian elite genotype of wheat can be followed for developing wheat transgenics for other traits as well.
Materials and methods
Plant material and explant
Indian bread wheat (Triticum aestivum L.) genotype HD2894 (Hybrid Delhi 2894), commonly known as Pusa Wheat109, was used for genetic transformation. The seeds were procured from the Division of Genetics, Indian Agricultural Research Institute (IARI), New Delhi, India. Surface sterilization of healthy seeds was carried out by repeated washing with sterilized distilled water, followed by incubation at 45 °C in a water bath for 90 min to remove fungal and other contamination. The heat-treated seeds were then dipped in 70% ethanol for 1 min and subsequently in 2% sodium hypochlorite solution (NaOCl) for 10 min. The seeds were then thoroughly washed and kept in aseptic dark condition for 2 days. Two days old seedlings were used for genetic transformation.
Agrobacterium tumefaciens strain and binary vector
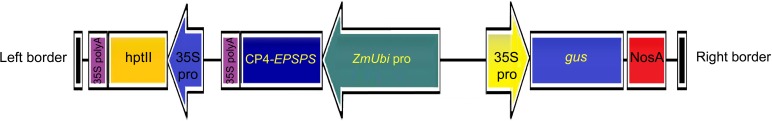
Agrobacterium tumefaciens strain EHA105, harboring binary vector pCAMBIA1301 having codon-modified bacterial CP4-EPSPS gene driven by maize ubiquitin (ubi) promoter (pCAMBIA1301-Ubi-CP4-EPSPS), was used in the present study. The vector was developed by the National Research Centre on Plant Biotechnology, New Delhi, India (Chhapekar et al. 2015) (Fig. 1).
Fig. 1.
Schematic diagram of pCAMBIA 1301-Ubi-C4-EPSPS vector showing the main components between the T-DNA borders
Transformation of wheat using pCAMBIA1301-Ubi-CP4-EPSPS construct
Agrobacterium tumefaciens EHA105 culture having the desired construct was inoculated in 50 ml YEM (yeast extract mannitol) media supplemented with kanamycin (50 mg l−1) and rifampicin (10 mg l−1). The culture was incubated overnight at 28 °C with continuous shaking (100 rpm) and the cells were harvested at log phase (OD600—0.7). Harvested cells were resuspended in 50 ml half-strength MS (Murashige–Skoog) medium supplemented with 100 µM acetosyringone (Murashige and Skoog 1962).
The apical meristem (coleoptile) of two days old wheat seedling (T0) was pierced by a sterile needle up to a depth of about 1 mm, and a minute drop ( ~ 15–20 μl) of Agrobacterium (EHA105) inoculum suspended in half-strength MS media was injected using a 1 ml sterile syringe (Dispovan, India). The inoculated seedlings were transferred to Petri plates containing sterile soilrite and kept under dark condition for 2 days. To eliminate the overgrowth of Agrobacterium, the infected seedlings were dipped in cefotaxime solution @ 250 mg l−1 and incubated for 2h with mild shaking of 100 rpm (BioGentek, India) at 28 °C (Supartana et al. 2006; Razzaq et al. 2011). After 2 h, the seedlings were removed from cefotaxime solution and rinsed with sterile water and then planted into 4-inch plastic pots containing sterile soilrite (Fig. 2). Pots were kept in a controlled growth chamber maintained at 22–25 °C having 16 h photoperiod with 600 µEm−2 s−1 of white light intensity. After 1 month, the plants were transferred into 6-inch pots and kept at the National Phytotron Facility, IARI, New Delhi, India, under controlled conditions. The seeds were collected separately from each plant (T1 lines) after maturity. Subsequently, the T1 plants were subjected to glyphosate screening as standardized in the present study. The transformation efficiency (TE) was calculated by the following formula:
Fig. 2.
Steps in in planta process of Agrobacterium-mediated transformation. a, b Seedlings germinated after 2 days on soaked germination paper. c Injection with Agrobacterium culture (having pCAM1301-Ubi-CP4-EPSPS construct) on shoot tip. d Seedlings kept for co-cultivation in sterile soilrite. e, f Fully grown T0 wheat plants transferred to pots till maturity. g T1 plants obtained after seed germination. h Survived plants after glyphosate spraying; the black marked region represents the WT plants or negative control (HD2894). i Putative transgenics transferred to pots for further germination
Pilot experiment for glyphosate lethal dose
The lethal dose of glyphosate was determined to screen the putative transformants for tolerance to herbicide. Twenty-eight days old non-transgenic (control) HD2894 seedlings were assayed by uniform spraying of commercial glyphosate (Roundup Monsanto, USA) at different concentrations (0.1%, 0.2%, 0.5% and 1%) according to Chappekar et al. (2015) under controlled conditions. We sprayed 2.5% (v/v) Roundup for ≈ 1% glyphosate, as Roundup contains 41% isopropylamine salt of glyphosate. The field-recommended dose of Roundup is 20 l/ha with 1:49 (v/v) dilution rate (2.04% Roundup) and the recommended wheat seedling density in field is ≈ 275 plants/m2. According to the calculation, it is advised to spray 364 μl Roundup/plant. As we used 2.5% (v/v) Roundup in this study, we sprayed ≈ 350 μl Roundup/plant which was more than the recommended dose as per volume × strength. The droplet size for spraying was kept to 400 VMD (volume median diameter) at DV0.5 which means 50% droplets were of above 400 μm (Coarse) in size. A set of 24 plants for each concentration was examined over a period of 15 days. The experiment was carried out in three replicates. The minimum dose of glyphosate which caused 100% plants death was considered as the lethal dose. After identifying the minimum lethal dose concentration of glyphosate, a high stringent screening was followed to avoid non-transformant escape. The seeds (T2 lines) were collected separately from each plant of T1 lines which survived after glyphosate screening. The selected T2 seeds were sown under controlled conditions at the National Phytotron Facility (24 ± 2 °C with 16 h photoperiod) for generation advancement and T3 seeds were harvested after maturing.
Transgene segregation analysis
Genotypic frequencies of wheat transgenic lines harboring a single copy of the transgene were determined through analysis of seedling mortality using Chi-squared test. As wheat is a self-pollinated crop, the heterozygous lines and double insertion of transgenes were identified through mortality rate 3:1 and 15:1, respectively (Passricha et al. 2016). The transgenic lines with 100% plant survival were considered as single homozygous transgenic lines with single copy of transgene. A few selected T3 plants from each T2 plants were screened with 1% glyphosate in phytotron conditions as described before to identify homozygous transgenic populations. The seedlings mortality was analyzed with Chi-square test. The T3 lines only with 100% plant survival were considered as homozygous transgenic lines and taken for further experimentation. To remove the hemizygous lines, higher than the recommended dose of glyphosate was used for screening of glyphosate-resistant wheat lines, as twofold expression of the EPSPS gene helped only homozygous line to survive, not hemizygous lines (James et al. 2002).
PCR analysis
Genomic DNA was extracted from leaf tissue of 1-month-old wheat transgenic lines by the modified cetyltrimethylammonium bromide (CTAB) method (Sambrook and Russell 2001). PCR was performed in a 25 µl reaction mixture consisting of 1 × reaction buffer, 2.5 mM MgCl2, 0.25 mM dNTPs, 10 pmol of each primer (Table S1), 1.5 units of Taq DNA polymerase, and 100 ng of plant genomic DNA. The DNA was denatured at 94 °C for 5 min, followed by 35 cycles of amplification (94 °C for 1 min, 59 °C for 1 min and 72 °C for 1 min), final extension at 72 °C for 10 min and finally at 4 °C in a thermocycler (Biorad, USA). The PCR product was fractionated by electrophoresis on a 1% agarose gel and detected by ethidium bromide staining under ultraviolet light.
Southern blot analysis
For Southern hybridization analysis, 25 µg of isolated genomic DNA from selected plants of different transgenic lines was digested with restriction enzyme EcoRI overnight at 37 °C. The digested DNA was separated on 1% agarose gel (Pronastar, Spain) in 1 × TAE buffer. The gel was denatured and neutralized followed by blotting on to nylon membrane (Millipore, USA). The CP4-EPSPS gene labeled with digoxigenin-dUTP was used as the probe. DIG high prime labeling and detection kit (Roche, Germany) was used for Southern blot analysis according to the manufacturer’s instructions.
Real time analysis
The expression level of CP4-EPSPS gene in transgenic lines of T3 progeny was analyzed by qRT-PCR (quantitative Real time PCR). The leaf samples were harvested at the tillering stage from four randomly selected transgenic lines along with non-transgenic control plant of HD2894, quick frozen in liquid nitrogen and stored at − 80 °C for qRT-PCR analysis. Total RNA was extracted from frozen leaf tissues using PureLink™ RNA Mini Kit (Ambion, USA). The cDNA was synthesized with the isolated total RNA ( ~ 1 μg) using SuperScript™ III First-Strand Synthesis System (Invitrogen, USA) and used for qPCR analysis. The qRT-PCR primer sets were designed by IDT Primer Quest software tool (https://eu.idtdna.com/Primerquest/Home/Index) (Table S1) and the reaction mixture was prepared as per Padaria et al. (2013). β-actin gene was used as internal control. The qRT-PCR was carried on LightCycler®480 II System (Roche, Germany). All the reactions were performed in three technical replicates and the fold change values were analyzed by the 2–ΔΔCt equation using Roche Light Cycler 480™ Software v.1.5 (Roche, Germany) (Livak and Schmittgen 2001). The specificity of primer–template binding was confirmed by the melting curve analysis. The Ct value of the transgenic wheat plant having the lowest expression of the EPSPS gene was considered as onefold for comparison.
Chlorophyll content
The chlorophyll contents of transgenic lines at T3 generation and non-transgenic control wheat plants were estimated using the traditional destructive method by organic extraction and based on light absorption in a UV spectrophotometer (Shimadzu, Japan). Three technical replicates were taken for analysis. 5 ml dimethylsulfoxide (DMSO) was added to 50 mg of finely chopped fresh leaf samples. The tubes were covered with aluminum foil and incubated in a water bath at 65 °C for 4 h. The chlorophyll content was determined by measurement of the optical density at 663 nm and 645 nm (Shinano et al. 1996).
Comparative analysis of phenotypic characters
The morphological characteristic data such as leaf length, leaf breadth, and number of spikes of the T3 transgenic lines were recorded and compared with those of non-transgenic control wheat plants. The significant differences between the wild type and T3 transgenic lines were statistically analyzed using OPSTAT software (https://14.139.232.166/opstat/default.asp) and significantly different values were marked by asterisks (*).
Results
Pilot experiment for lethal glyphosate concentration
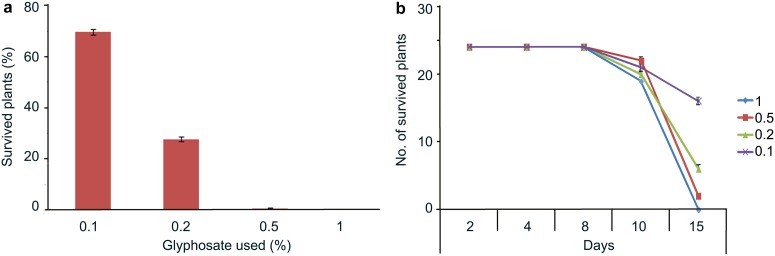
There were no distinct symptoms within the plants sprayed with different glyphosate concentrations up to 4 days. From 8 days onward, clear necrotic symptoms were observed in plants sprayed with 0.5% and 1% glyphosate. The plants sprayed with 0.1 and 0.2% glyphosate showed delayed necrosis. Fifteen days after spraying, 75% of plants sprayed with 0.1% glyphosate were observed to have survived, whereas only 27% of plants sprayed with 0.2% glyphosate survived. Plants sprayed with both concentrations, 0.5% and 1% of glyphosate, were unable to survive (Table 1). 1% glyphosate was used for selection of T1 transgenic wheat lines carrying the CP4-EPSPS gene. On the basis of analyzed data and to keep high stringency conditions, 1% glyphosate was considered as a lethal dose for 28 days old wheat plants (Fig. 3).
Table 1.
Lethal effect of commercial glyphosate on control wheat plants
| Glyphosate concentration | No. of replicates | No. of plants | 15th day No. of plants survived |
|---|---|---|---|
| 0.1% | I | 24 | 16 |
| II | 24 | 18 | |
| III | 24 | 16 | |
| 0.2% | I | 24 | 7 |
| II | 24 | 3 | |
| III | 24 | 10 | |
| 0.5% | I | 24 | 0 |
| II | 24 | 0 | |
| III | 24 | 0 | |
| 1.0% | I | 24 | 0 |
| II | 24 | 0 | |
| III | 24 | 0 |
Fig. 3.
Pilot experiment for glyphosate on control plants for minimum lethal dose concentration. a Percent of survived plants vs glyphosate lethal dose. b Number of survived plants after treatment vs number of days
Inoculation of wheat seedlings and transformation efficiency
A total of 65 wheat seedlings (T0) were inoculated with A. tumefaciens strain EHA105 harboring pCAMBIA1301-Ubi-CP4-EPSPS at the coleoptile region using sterile needle. Out of these only 42 seedlings survived. After maturity, the seeds were harvested separately from each plant. A total of 809 T1 seeds were harvested from all T0 plants. The T1 seeds were sown separately, as they were harvested from separate T0 plants. After glyphosate screening (1.0%), only two plants survived. The plants were designated as transgenic Event A and transgenic Event B. Transformation efficiency was calculated and determined as 3.07% (Table 2). Plants were kept under controlled conditions till maturity. A total number of 30 seeds (T2 lines), 16 seeds from Event A and 14 seeds from Event B, were harvested after maturity. The insertion of linear transgene cassette without backbone sequence was confirmed by PCR using primers of selective genes present outside the cassette backbone and we did not find out any amplification for the same (data not shown).
Table 2.
Genetic transformation and final recovery of putative CP4-EPSPS transgenic wheat plants
| No. of seeds co-cultivated | T0 plants survived and grown | Total T1 seeds collected and sown | Plants passed glyphosate selection | Final recovery of putative transgenics (%) |
|---|---|---|---|---|
| 65 | 42 | 809 | 02 | 3.07 |
Transgene segregation analysis
Transgene segregation was analyzed in the seedlings of randomly selected 15 T3 lines from both the events (8 of Event A; and 7 of event B) by glyphosate screening. Based on survival, it was observed that T3 plants of both the events segregated in a normal Mendelian ratio and showed a monogenic ratio of 3:1. The heterozygous and double insertion transgenics were not taken for further experimentation. A total of eight individual lines at T3 generation, four lines of each event A and B, were selected for identification of homozygous lines by screening through herbicide (1% glyphosate)-tolerant test. Out of eight T3 lines, only three T3 lines, two of Event A (L2, L3 and L4) and one of Event B (L1), showed 100% survival ability of the seedlings and were thus identified as homozygous lines.
PCR analysis of putative transgenic plants
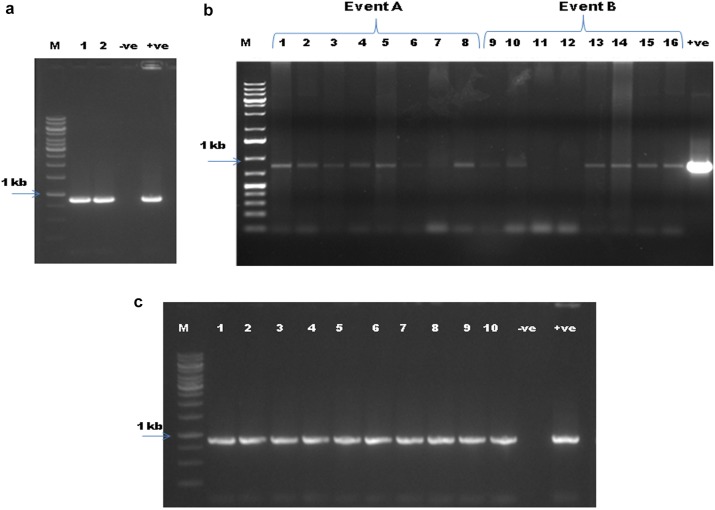
The survived plants at the T1 stage after glyphosate spray (1%) were confirmed by PCR for integration of transgene. Two plants (Event A and Event B) which had survived glyphosate spray were screened primarily through PCR using EPSPS gene specific primers for transgenic events, and both were found to be positive (Fig. 4a). Further, progenies (T2 lines) obtained from T1 plants were screened by PCR. A total of randomly selected 16 T2 plants, 8 from each event, were screened by PCR using EPSPS gene-specific primers. From Event A, one of the samples showed a very faint band and one did not show any amplification. Of them, two plants of Event B did not give any amplification (Fig. 4b), indicating heterozygosity in the T2 population. At T3 generation, ten plants from the identified three homozygous lines (100% plant survival in herbicide-tolerant test) were randomly screened with PCR and all of them showed amplification of the desired band (Fig. 4c).
Fig. 4.
Analysis of transgenic plants for presence of CP4-EPSPS gene. a Screening of T1 transgenic plants using EPSPS primers. b Screening of T2 transgenic plants. c Screening of T3 transgenic plants. Lanes: M marker 1 kb. Numbers 1, 2, 3, etc. represent respective samples, − ve is the negative control, + ve is the positive control
Southern hybridization blot analysis
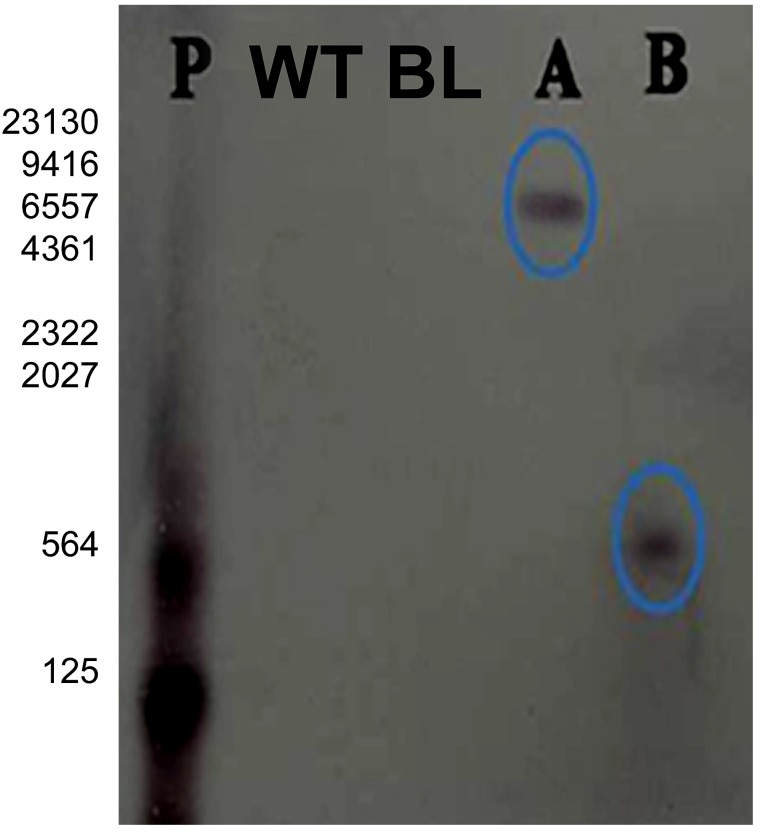
Two plants, one from each event, i.e., Event A and Event B, were randomly selected and examined for the presence of the EPSPS gene. Both the wheat transgenic lines showed a single band on X-ray film, indicating the integration of a single copy of the CP4-EPSPS gene in the segregating locus. No band was observed in the control plant, while the positive control showed a highly dense band at the respective position (Fig. 5).
Fig. 5.
Southern analysis of the transgenic plants. Two randomly selected events A and B from T2 generation (A-T1-1-T2-1 and B-T1-1-T2-2). P is positive control; WT represents the wild-type control plant (HD2894), BL represents blank lane. All gDNA samples ( ~ 25 µg) were digested with EcoRI enzyme and probed with DIG-labeled EPSPS gene
Expression analysis of the CP4-EPSPS gene in transgenic plants
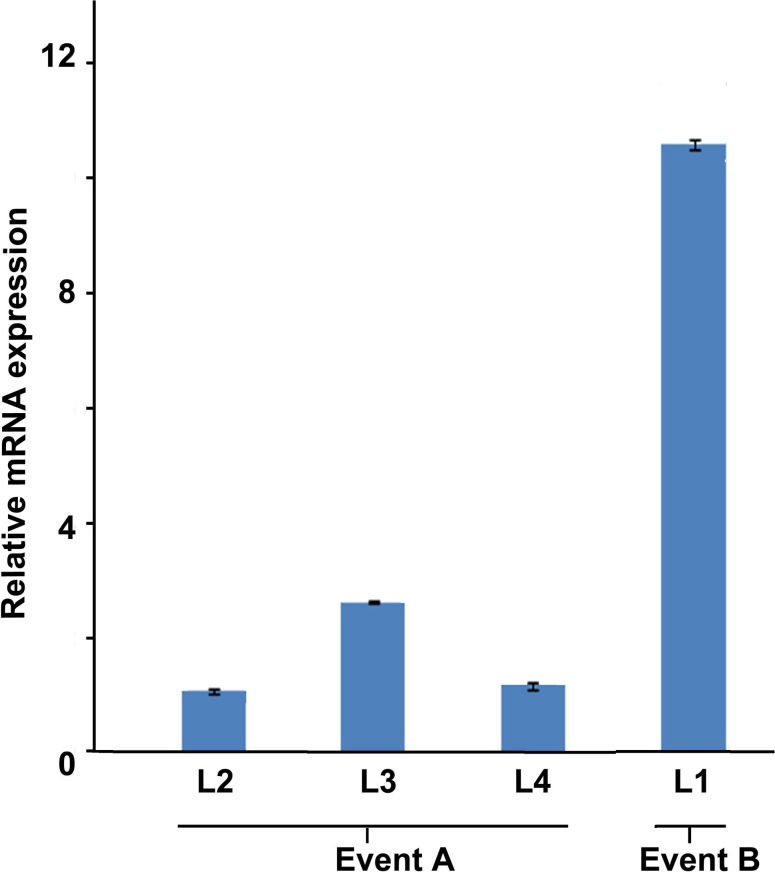
A wide level of CP4-EPSPS transgene expression, ranging from 1.3- to 10.8-fold was recorded at the tillering stage in different independent transgenic lines. The highest level of transgene expression, 10.8-fold, was detected in Event B transgenic line (L1), whereas in Event A transgenic lines (L2, L3 and L4) lower level of transgene expression was detected (Fig. 6).
Fig. 6.
Quantitative real-time PCR analysis of CP4-EPSPS gene expression in randomly selected T3 transgenic lines. Event B L1, and Event A L2, L3 and L4 represent the respective transgenic T3 lines. Three technical replicates were taken for analysis; on the top of the column is the error bar (n = 3)
Chlorophyll content estimation
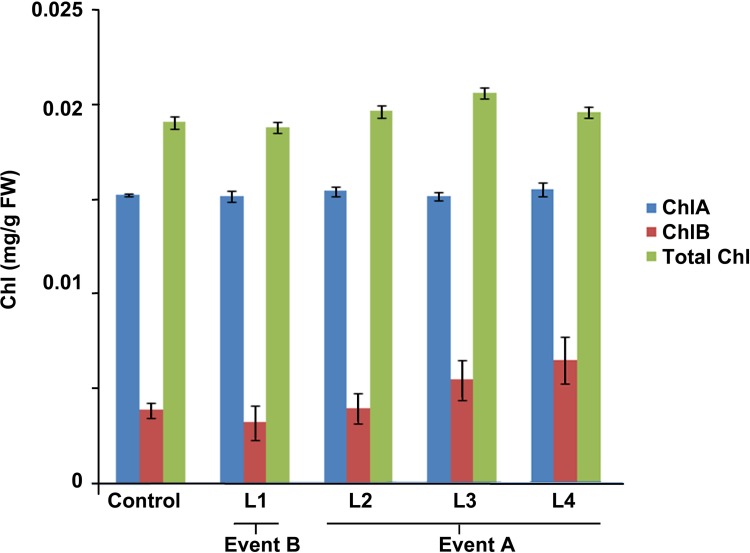
The four identified homozygous T3 transgenic lines were observed to be independent in chlorophyll content and no significant difference was found in non-transgenic control as well as transgenic wheat lines under normal conditions (Yu et al. 2016). The statistical data analysis showed that no difference was observed in transgenic line L1 from Event B as well as transgenic lines L2, L3 and L4 from Event A as compared to the non-transgenic control plant samples (Fig. 7).
Fig. 7.
Comparison of chlorophyll a, b and total chlorophyll in T3 transgenic and non-transgenic control wheat plant. Event B line L1, and Event A lines L2, L3 and L4 represent transgenic lines. The significant difference values are been shown by asterisk, and the data are the mean of five replicates
Comparative analysis of phenotypic characters
No remarkable variation was observed in the transgenic and non-transgenic control wheat plants with regard to seed weight (100 seeds per gm), seed texture, days taken for germination, days taken for six-leaf emergence, plant height at sixth leaf stage, leaf color, leaf length of sixth leaf, breadth of sixth leaf, days taken for tillering, number of tiller emergence per plant, days taken for emergence of spike, number of spikes, days taken for emergence of spike, spike length and number of seed set/spike (Table 3).
Table 3.
Morphological characteristics observed in transgenic plants as compared to control plants at T3 generation
| General characteristics | Control | Event A—line L3 | Event B—line L1 | |
|---|---|---|---|---|
| Seed morphology | Seed weight (100 seeds in gm) | 2.6 | 2.35 | 2.5 |
| Seed texture | Normal | Normal | Normal | |
| Days taken for germination | 3–4 | 3–5 | 3–5 | |
| Seedling stage | Days taken for six-leaf emergence | 24- 25 | 26–28* | 25–28 |
| Plant height at sixth leaf stage | 28.86 cm | 27.5 cm* | 27.4 cm | |
| Leaf color | Dark green | Dark green | Dark green | |
| Leaf length of sixth leaf | 22.2 cm | 21.2 cm* | 23.7 cm* | |
| Breadth of sixth leaf | 0.52 cm | 0.72 cm* | 0.58 cm* | |
| Tillering | Number of tiller emergence per plant | 0–1 | 0–1 | 0–1 |
| Anthesis | Days taken for emergence of spike | 58–60 | 51–55 | 49–53 |
| Number of spikes | 1–2 | 1–2 | 1–2 | |
| Days taken for emergence of spike | 58–60 days | 51–55 days | 48–60 days | |
| Spike length (in cm) | 7–8 | 7–8 | 7–8 | |
| Number of seed sets/spikes | 25–30 | 25–30 | 22–28 | |
Asterisk (*) represents the significant difference at P < 0.05. Data indicate means ± standard deviation (n = 3)
Discussion
Wheat genotype HD2894 is a high yielding and one of the most widely grown varieties in India. In planta transformation is reported in various crops as an efficient genetic transformation method, but in wheat the information about in planta transformation is limited (Chugh et al. 2012). In the present study, an efficient and simple in planta transformation method for development of herbicide-tolerant Indian wheat genotype (HD2894) has been reported. Here, the wheat seedlings were used as the experimental material, and it was confirmed by PCR that the linear transgene cassette without backbone sequence was efficiently transferred into wheat genome with a relatively higher efficiency compared to other transformation methods. Hamada et al. (2017) reported the transformation efficiency based on PCR (0.87% and 0.70%) and GPF expression (0.17% and 0.35%) in wheat cv. ‘Fielder and Haruyokoi’, respectively, at T1 generation using the biolistic method of transformation (in planta). The protocol standardized in this study gave a transformation efficiency of 3.07% (Table 2) in bread wheat genotype HD2894, which is higher than the reported 1.3% transformation efficiency in wheat (Vasil et al. 1992, 1993). A slightly higher efficiency (4.4%) was reported Hu et al. (2003) in genotype Bobwhite, a genotype well known as highly responsive to transformation. It is well known that the instability of transgene expression and transgenic silencing in plants are often due to multiple copies of transgene being integrated at the same locus, as well as position effects due to random integration. The transgenic plant with a single copy of transgene cassette with desirable expression of transgene is advantageous (Liao et al. 2014). In Southern hybridization analysis, both the wheat transgenic lines A and B generation showed a single band, which confirmed single copy integration of the transgene in the wheat genome and its successful inheritance to the successive progeny. Single copy integration of desired gene by Agrobacterium-mediated genetic transformation in wheat has also been reported by other researchers (Lu and Kang 2008).
In this study, the CP4-EPSPS was used as a herbicide-tolerant gene driven by maize ubiquitin (ubi) promoter to develop transgenic wheat. Studies have shown that maize ubiquitin promoter is able to drive more transgene expression in wheat as compared to other promoters such as the CaMV35S promoter (Mukherjee et al. 2015). Ubiquitin (ubi) promoters are often used with their first untranslated exon and introns; inclusion of these elements results in strong expression of the transgene (Hensel et al. 2011). Maize ubiquitin (ubi) promoter has been extensively used for development of wheat transgenics by various workers (Vickers et al. 2003; Xue et al. 2014). Transgenics having CP4-EPSPS gene are resistant to glyphosate, the herbicide commercially available as Roundup. The CP4-EPSPS gene has been successfully used in transformation of different crops from legumes to major cereals including rice, wheat and maize. To overcome the weed menace, application of glyphosate in agriculture has increased enormously since the introduction of glyphosate-resistant crop plants (Funke et al. 2006; Duke and Powles 2008; Green 2012). Zhou et al. (2003) obtained Roundup Ready transgenic wheat by transforming the herbicide-resistance gene to wheat genotype Bobwhite, which displayed full resistance to the herbicide. In the same year, Hu and co-workers reported glyphosate-resistant transgenic Bobwhite IE C58 wheat using the modified CP4-EPSPS gene. The use of antibiotic and herbicide as selectable markers during plant transformation has been key to the increasing acreage of transgenic crops grown worldwide. As most of the glyphosate bind tightly to the soil, herbicide glyphosate has a very low potential to contaminate the groundwater and is thus considered ecologically safe for human health and environment (Friends of the Earth Europe 2013).
Quantitative real-time PCR could be a powerful tool for the detection of transgene expression as well as determination of transgene copy number and locus structures by overcoming the limitations of Southern analysis (Mason et al. 2002). In the present study, the housekeeping gene β-actin has been used as a reference gene in expression normalization of qRT-PCR analysis for assorting transgenic lines (Padaria et al. 2013, 2014). The transgenic lines are heterozygotes for the CP4-EPSPS transgene cassette and homozygotes for the endogenous gene β-actin in the T1 generation (Mason et al. 2002). So, the number of target genes in the transformed plant is calculated with the value of X0/R0 (the copy number of the target gene (X0)/the copy number of the internal control gene (R0) (Weng et al. 2004). To date, qRT-PCR technology has been applied in a large number of transgenic events to analyze transgene copy number, including in cereals (Li et al. 2004). However, in the present study, qRT-PCR technique was used to only determine the expression of the transgene.
As wheat is a self-pollinating plant, the transformed (T1) wheats plants were allowed to pollinate naturally, and a few T2 seeds were obtained which were further grown to T3 generation. The appearance of T3 transformants generated by the two transgenic events, i.e., Event A and Event B, were similar to that of the non-transformed plants grown simultaneously under the same conditions.
In this study, we standardized an Agrobacterium-mediated meristem targeted in planta transformation method for an Indian elite wheat (genotype HD 2894) using CP4-EPSPS gene. Infecting apical meristem of 2 days old wheat seedlings resulted in a transformation efficiency of 3.07%. The stable integration and expression analysis of the gene and other morpho-physiological data of CP4-EPSPS transgenic lines were determined. The results confirmed that the developed protocol was simple, efficient and cost-effective for in planta-mediated transformation. This developed protocol can be further deployed for other Indian elite wheat genotypes for generating transgenic lines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
List of gene-specific primers used for PCR and qPCR analysis of putative transgenics. (DOCX 11 kb)
Acknowledgements
The authors are thankful to the Project Director NRCPB (National Research Centre on Plant Biotechnology), Pusa Campus, New Delhi, India, for providing facilities to carry out the research work. Funds provided by the ICAR (Indian Council of Agricultural Research) under NICRA (National Innovations in Climate Resilient Agriculture) project are duly acknowledged. Dr. P. A. Kumar, Ex-Project Director NRCPB, is duly thanked for providing the gene construct pCAMBIA 1301-Ubi-C4-EPSPS. The authors are thankful to the Director IARI, Pusa Campus, New Delhi, India, for providing the plant growth chambers at the National Phytotron Facilities.
Author contributions
Concept and design of experiment: JCP; performed the experiments: AT, HV, GS, MB, KB, AP, UC; analyzed the data: AT, HV, GS; manuscript writing and editing: AT, HV, GS, AUS, JCP.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Anand A, Zhou T, Trick HN, Gill BS, Bockus WW, Muthukrishnan S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J Exp Bot. 2003;54:1101–1111. doi: 10.1093/jxb/erg110. [DOI] [PubMed] [Google Scholar]
- Bohorova NE, Ginkel M, Rajaram S, DA Hoisington. Tissue culture response of CIMMYT elite bread wheat genotypes and evaluation of regenerated plants. Cereal Res Commun. 1995;23:243–249. [Google Scholar]
- Cheng M, Fry JE, Pang H, Zhou CM, Hironaka CM, Duncan DR, Conner TW, Wan Y. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol. 1997;115:971–980. doi: 10.1104/pp.115.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhapekar S, Raghavendrarao S, Pavan G, Chopperla R, Singh VK, Phanindra MLV, Dhandapani G, Sreevathsa R, Kumar PA. Transgenic rice expressing a codon-modified synthetic CP4-EPSPS confers tolerance to broad-spectrum herbicide, glyphosate. Plant Cell Rep. 2015;34:721–731. doi: 10.1007/s00299-014-1732-2. [DOI] [PubMed] [Google Scholar]
- Chugh A, Vikrant S, Mahalakshmi A, Khurana A. A novel approach for Agrobacterium-mediated germ line transformation of Indian bread wheat (Triticum aestivum) and pasta wheat (Triticum durum) J phytol. 2012;4:22–29. [Google Scholar]
- Cobb AH, Reade JPH. The inhibition of amino acid biosynthesis. In: Cobb AH, Reade JPH, editors. Herbicides and plant physiology. 2. Hoboken: Wiley-Blackwell; 2010. pp. 126–144. [Google Scholar]
- Duke SO. Glyphosate degradation in glyphosate-resistant and-susceptible crops and weeds. J Agric Food Chem. 2011;59:5835–5841. doi: 10.1021/jf102704x. [DOI] [PubMed] [Google Scholar]
- Duke SO, Powles SB. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci. 2008;64:319–325. doi: 10.1002/ps.1518. [DOI] [PubMed] [Google Scholar]
- FAO (2018) https://www.fao.org/worldfoodsituation/csdb/en/
- Friends of the Earth Europe (2013) https://www.foeeurope.org/sites/default/files/press_releases/foee_5_environmental_impacts_glyphosate.pdf
- Funke T, Han H, Healy-Fried ML, Fischerm M, Schönbrunn E. Molecular basis for the herbicide resistance of Roundup ready crops. Proc Natl Acad Sci USA. 2006;103:13010–13015. doi: 10.1073/pnas.0603638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger DR, Fuchs MA. Inhibitors of aromatic amino acid biosynthesis (glyphosate) In: Boger P, Wakabayashi K, Hirai K, editors. Herbicide classes in development: mode of action, targets, genetic engineering, chemistry. New York: Springer; 2002. pp. 59–85. [Google Scholar]
- Green JM. The benefits of herbicide-resistant crops. Pest Manag Sci. 2012;68:1323–1331. doi: 10.1002/ps.3374. [DOI] [PubMed] [Google Scholar]
- Hamada H, Linghu Q, Nagira Y, Miki R, Taoka N, Imai R. An in planta biolistic method for stable wheat transformation. Sci Rep. 2017;7:11443. doi: 10.1038/s41958-017-11936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Jin XL, Wu FB, Zhang GP. Genotypic differences in callus induction and plant regeneration from mature embryos of barley (Hordeum vulgare L.) J Zhejiang Univ Sci B. 2011;12:399–407. doi: 10.1631/jzus.B1000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel G, Himmelbach A, Chen D, Douchkov K, Kumlehn J. Transgene expression systems in the Triticaceae cereals. J Plant Physiol. 2011;168:30–44. doi: 10.1016/j.jplph.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Hu T, Metz S, Chay C, Zhou HP, Biest N, Chen G, Cheng M, Feng X, Radionenko M, Lu F, Fry J. Agrobacterium-mediated large-scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Rep. 2003;21:1010–1019. doi: 10.1007/s00299-003-0617-6. [DOI] [PubMed] [Google Scholar]
- James C. Global review of commercialized transgenic crops. Curr Sci. 2003;84(3):303–309. [Google Scholar]
- James VA, Avart C, Worland B, Snape JW, Vain P. The relationship between homozygous and hemizygous transgene expression levels over generations in populations of transgenic rice plants. Theor Appl Genet. 2002;104:553–561. doi: 10.1007/s001220100745. [DOI] [PubMed] [Google Scholar]
- Joshi AK, Ortiz-Ferrara G, Crossa J, Singh G, Sharma RC, Chand R, Parsad R. Combining superior agronomic performance and terminal heat tolerance with resistance to spot blotch (Bipolaris sorokiniana) of wheat in the warm humid Gangetic Plains of South Asia. Field Crops Res. 2007;103:53–61. doi: 10.1016/j.fcr.2007.04.010. [DOI] [Google Scholar]
- Kasirajan L, Kovilpillai B, Bansal KC. Optimization of genetic transformation protocol mediated by biolistic method in some elite genotypes of wheat (Triticum aestivum L.) Afr J Biotechnol. 2013;6:531–538. [Google Scholar]
- Kato K, Chowdhury SH, Harada S. Effect of culture condition on plant regeneration capacity of mature embryo derived callus in wheat (Triticum aestivum L.) Wheat Inf Serv. 1991;72:95–97. [Google Scholar]
- Li ZW, Hansen JL, Liu Y, Zemetra RS, Berger PH. Using real-time PCR to determine transgene copy number in wheat. Plant Mol Biol Rep. 2004;22(2):179–188. doi: 10.1007/BF02772725. [DOI] [Google Scholar]
- Liao P, Wang H, Wang M, Hsiao A, Bach TJ, Chye M. Transgenic tobacco overexpressing Brassica juncea HMG CoA synthase 1 shows increased plant growth, pod size and seed yield. PLoS One. 2014;9(5):e98264. doi: 10.1371/journal.pone.0098264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu C, Kang J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 2008;27:273–278. doi: 10.1007/s00299-007-0454-0. [DOI] [PubMed] [Google Scholar]
- Mason G, Provero P, Vaira A, Accotto GP. Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotechnol. 2002;2:20. doi: 10.1186/1472-6750-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Stasolla C, Brule-Babel A, Ayele BT. Isolation and characterization of rubisco small subunit gene promoter from common wheat. Plant Signal Behav. 2015;10(2):e989033. doi: 10.4161/15592324.2014.989033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog FA. Revised medium for rapid growth and bioassays with tobacco cultures. Plant Physiol. 1962;159:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Padaria JC, Bhatt D, Biswas K, Singh G, Raipuria R. In-silico prediction of an uncharacterized protein generated from heat responsive SSH library in wheat (Triticum aestivum L.) Plant Omics. 2013;6:150–156. [Google Scholar]
- Padaria JC, Vishwakarma H, Biswas K, Jasrotia RS, Singh GP. Cloning and in-silico characterization of heat responsive pAPX gene from heat tolerant Indian cv Raj3765. BMC Res Notes. 2014;7:149. doi: 10.1186/1756-0500-7-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passricha N, Saifi S, Khatodia S, Tuteja N. Assessing zygosity in progeny of transgenic plants: current methods and perspectives. J Biol Methods. 2016;3:e46. doi: 10.14440/jbm.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq A, Hafiz IA, Mahmood I, Hussain A. Development of in planta transformation protocol for wheat. Afr J Biotechnol. 2011;10:740–750. [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Shinano T, Lei TT, Kawamukai T, Inoue MT, Koike T, Tadano T. Dimethylsulfoxide method for the extraction of chlorophylls a and b from the leaves of wheat, field bean, dwarf bamboo and oak. Phytosynthetica. 1996;32(3):409–415. [Google Scholar]
- Supartana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M. Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng. 2005;100:391–397. doi: 10.1263/jbb.100.391. [DOI] [PubMed] [Google Scholar]
- Supartana P, Shimizu T, Nogawa M, Shioiri H, Nakajima T, Haramoto M, Nozue M, Kojima M. Development of simple and efficient in planta method of transformation for wheat (Triticum aestivum L.), using Agrobacterium tumefaciens. J Biosci Bioeng. 2006;102:162–170. doi: 10.1263/jbb.102.162. [DOI] [PubMed] [Google Scholar]
- Vasil V, Castillo AM, Fromm ME, Vasil I. Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Nat Biotechnol. 1992;10:667–674. doi: 10.1038/nbt0692-667. [DOI] [Google Scholar]
- Vasil V, Srivastava V, Castillo AM, Fromm ME, Vasil I. Rapid production of transgenic wheat plants by direct bombardment of cultured immature embryos. Nat Biotechnol. 1993;11:1553–1558. doi: 10.1038/nbt1293-1553. [DOI] [Google Scholar]
- Vickers CE, Xue GP, Gresshoff PM. A synthetic xylanase as a novel reporter in plants. Plant Cell Rep. 2003;22:135–140. doi: 10.1007/s00299-003-0667-9. [DOI] [PubMed] [Google Scholar]
- Weng H, Pan A, Yang P, Zhang C, Liu Z, Zhang D. Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay with HMG I/Y as an endogenous reference gene. Plant Mol Biol Rep. 2004;22(3):289–300. doi: 10.1007/BF02773139. [DOI] [Google Scholar]
- Xue G, Sadat S, Drenth J, Mclyntyre L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation in heat shock protein genes. J Exp Bot. 2014;65(2):539–557. doi: 10.1093/jxb/ert399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Xu Z, Gou J, Wang Y, Abernathy B, Fu J, Chen X, Zhou Y, Chen M, Ye X, Ma Y. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Sci Rep. 2016;7:44050. doi: 10.1038/srep44050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Berg JD, Blank SE, Chay CA, Chen G, Eskelsen SR, Fry JE, Hoi S, Hu T, Isakson PJ, Lawton MB, Metz SG, Rempel CB, Ryerson DK, Sansone AP, Shook AL, Starke RJ, Tichota JM, Valenti SA. Field efficacy assessment of transgenic roundup ready wheat. Crop Sci. 2003;43:1072–1075. doi: 10.2135/cropsci2003.1072. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of gene-specific primers used for PCR and qPCR analysis of putative transgenics. (DOCX 11 kb)