Abstract
Background
Urolithiasis is the third common disorder of the urinary system affecting 10–15% of the general population. In recent years, search for new antilithiatic drugs from natural sources has assumed greater importance.
Objectives
This study was performed to investigate the anti-urolithiatic activity of methanolic extract of Duranta erecta leaves by in vitro and in vivo analysis.
Materials and methods
The study was designed to determine presence of phytochemicals in D. erecta, its yield in percentage, antioxidant activity against 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and anti-microbial property against few bacteria. In vitro analysis was carried out study anti-urolithiatic property of D. erecta by nucleation assay and synthetic urine assay for inhibition of calcium oxalate and calcium oxalate monohydrate crystals formation. An in vivo experiment was performed on Wistar rats for confirmation of anti-urolithiatic property of D. erecta in animal model.
Results
D. erecta has the presence of primary and secondary metabolites like glycoside, saponins, sterols, flavonoids, phenols, tannins, alkaloids, carbohydrates and proteins. Methanolic extract of D. erecta gave a very good yield (60%). D. erecta proved its antioxidant potential by 93.51% inhibition of DPPH radical at a concentration of 1000 μg/mL where ascorbic showed 94.71% of DPPH radical at the same concentration. In vitro tests like nucleation assay and synthetic urine assay showed that D. erecta inhibits formation of calcium oxalate and calcium oxalate monohydrate crystals. It also showed the anti-microbial property by formation of zone of inhibition against few bacteria. An in vivo experiment on Wistar rat animal model confirmed the anti-urolithiatic property of D. erecta L. leaves extract.
Conclusions
Based on the results, we reported that D. erecta may treat calcium oxalate crystal deposition in the kidney by preventing hyperoxaluria-induced peroxidative damage to the renal tubular membrane surface (lipid peroxidation). It has anti-microbial potential so it may also inhibit the secondary bacterial infection in kidney. Based on the data, it can be concluded that this herb can be used as a potential anti-urolithiasis agent for kidney stone removal.
Keywords: Antiurolithitic, Duranta erecta, Sodium oxalate, Antimicrobial, Urolithiasis
1. Introduction
Urolithiasis or kidney stone is the third common disorder of the urinary system affecting 10–15% of the general population [7]. It originates from tiny crystals that later form a stone which subsequently grows and further builds up on the inner surfaces of the kidney. Kidney stones are named after where they are identified in the urinary system like renal calculi, urinary calculi, urinary tract stone disease, nephrolithiasis, urolithiasis and ureterolithiasis. Urinary calculi, or stones can be eliminated through the urine if they are small by flowing in the ureter from the kidney. But in some cases, the stone may not be able to travel through the ureter, which causes severe pain if untreated and may cause severe medical consequences such as extreme obstruction, hydronephrosis, infection, and hemorrhage in the urinary tract system [14].
In general, stones in the urinary tract are crystalline in nature composed of inorganic and organic crystals amalgamated with proteins. Calcium oxalate (CaOx) stones represent up to 80% of analyzed stones while calcium phosphate and mixed stones account for 15–25%, and 10–15% respectively [3]. Calcium oxalate monohydrate (whewellite) and calcium oxalate dihydrate (weddellite) are two variants of CaOx stones with 78% and 43% occurrence frequency [13]. Several physio-chemical mechanisms are involved in stone formation including CaOx supersaturation and CaOx crystal nucleation, growth and aggregation of the crystals. Circumstances that initiate the calcium oxalate supersaturation lead to the risk of calcium oxalate stones formation. Stones are formed by supersaturation and later nucleation occurs and ions in solution combine with one another into a solid phase. Calcium and oxalate ions can orient themselves on surfaces of other crystals i.e. uric acid and such heterogeneous nuclei may also promote calcium oxalate stones. These phenomena of supersaturation and heterogeneous nucleation are cause of urolithiasis. Surgical procedure, lithotripsy, and laparoscopy for local calculus disruption are used to remove the calculi. However, these procedures may cause risk of acute renal injury that reduces renal function and stone recurrence is observed in many cases with an approximate recurrence rate of 10% at 1st year, 33% at 5th year, and 50% at 10th year. Costly treatment regimen and complicated clinical management of patients indicates an important need to develop suitable alternative therapies [8]. Medicinal plants are an important source of drugs because herbal natural products are effective as well as not as costly as their chemical counter-parts. Few medicinal plants and proprietary composite herbal preparations have been reported as an effective therapeutic option for prevention of recurrence of renal calculi with minimal side effects [10]. In recent years, search for new anti-lithiatic drugs from natural sources has assumed greater importance and this work presents an in vivo study of anti-urolithiatic properties of Duranta erecta.
D. erecta, also known as ‘golden dewdrops’ is a smooth, unarmed shrub, with straggling and drooping branches and is rich in plant chemicals. This plant is known to contain alkaloids, glycosides and saponin [15]; the whole plant has various applications like fruits are used as febrifuge and treating malaria, intestinal worms while leaves are used for treatment of abscesses. Flowers are believed to be stimulant and whole plant is used as insect repellent, treatment of itches, infertility, fever and pneumonia by the tribal and the in mainstream in Bangladesh. Diuretic properties of D. erecta have also been reported which can be used to expel the kidney stone out of the body [11]. However, to the best of our knowledge and literature survey, no such in vitro or in vivo studies have been reported so far.
In this study, we evaluated the anti-urolithiatic potential of methanolic leaves extract of D. erecta. Primary phytochemical screening and antioxidant activity was determined by in vitro studies which included nucleation and synthetic urine assay followed by in vivo studies in Wistar rats where, various parameters for urolithiasis were analyzed. Fig. 1 illustrates the study design for anti-urolithiatic effect of D. erecta. We have also evaluated the anti-bacterial property of this plant.
Fig. 1.
Schematic representation of study design for anti-urolithiatic effect of D. erecta.
2. Materials and methods
2.1. General experimental procedure
All the chemicals and other solutions used for this study were of analytical grade. All the reagents were prepared fresh before use. Leaves of D. erecta (L) were collected from CSIR – National Chemical Laboratory Colony, Pune. The plant specimen was pressed on paper and left for 24 h. The dried specimen was fixed into herbarium sheet and submitted to Western Regional Centre of Botanical Survey of India, Pune for identification and authentication (No. BSI/WRC/Cert./2015 dated 21/12/15). Healthy and mature plants were selected for the collection of plant leaves. The leaves were washed thoroughly with KMnO4 followed by water. The leaves were shade dried for a week to remove the moisture content.
2.2. Microbial strains and culture conditions
Escherichia coli (NCIM-2065), Pseudomonas desmolyticum (NCIM-2112), Staphylococcus aureus (NCIM-2079) and Bacillus subtilis (NCIM-2010) were obtained from the National Collection of Industrial Micro-organisms and Gene Bank (NCIM), Pune, India, and grown on Nutrient Agar Medium (NAM) for 48 h at 37 °C and preserved at 4 °C for further use.
2.3. Preparation of leaves extract
The leaves extract was prepared by crushing the dried leaves into coarse powder with the use of domestic mixer. Separation of the components from the extracts is made by using percolation method. We used separatory funnel as percolator. It is a conical vessel with a top opening. The bottom has an adjustable closure to allow passage of the fluid at a convenient rate with slight pressure which permits a sharp separation of chemical compounds with minimum mechanical loss [6]. 250 g of dried leaf powder was successfully extracted in 1000 mL of methanol, using a separatory funnel. The methanolic extract was eluted on the next day and concentrated using rotary evaporator (Heidolph, USA). It evaporates the solvent at reduced pressure and temperature. The extract was further concentrated on high vacuum pump and stored in refrigerator at −20 °C until being used.
2.4. Preliminary phytochemical screening
The extract was tested for the presence of phytochemical such as glycosides, saponins, sterols, flavonoids, phenols, tannins, alkaloids, carbohydrates and proteins [16].
2.5. Total phenolic content (TPC)
Folin–Ciocalteu assay was used to estimate the total phenolic content of plant extracts [1]. Various concentrations (0.05–2.5 μM) in 95% methanol and 200 μL of 10% F–C reagent were added to prepare the standard. The reaction was terminated by addition of 800 μL of 700 mM Na2CO3 and incubated in dark for 1 h before absorbance was taken. 200 μL of sample, standard and blank were transferred into a 96-well plate and absorbance was measured at 765 nm on a 96-well plate reader (Biorad). Gallic acid was used as standard and total protein content was estimated in terms of gallic acid equivalence. Blank was prepared using 95% methanol [1].
2.6. Total flavonoid content (TFC)
Total flavonoid content was determined using AlCl3 complexation method using quercetin as standard of concentration 100–500 μg/mL in D/W. 150 μL of 5% NaNO2 was added to the standard solution and incubated for 5 min at room temperature. At the end of 5 min, 250 μL of 2% AlCl3 solution was added to the assay tubes and incubated at room temperature for 5 min. The reaction was terminated by addition of 250 μL of 1 M NaOH. Finally, absorbance was measured at 510 nm on a plate reader. Blank was prepared by replacing AlCl3 with water. The flavonoid concentration (μg/mL) of D. erecta leaves extract was expressed in terms of quercetin equivalence [9].
2.7. DPPH free radical scavenging activity
The free radical scavenging activity of the extract was evaluated in vitro by 2, 2- diphenyl-1-picrylhydrazyl (DPPH) assay using ascorbic acid as standard. The DPPH solution was prepared by dissolving 25 mg DPPH in 100 mL methanol and stored at 20 °C till further use. The plant extract and the standard were prepared at various concentrations ranging from 25 μg/mL to 1000 μg/mL in methanol. 150 μL of the above prepared DPPH solution was added to 50 μL of various concentrations of plant extract and standard. The reaction mixture was mixed well and incubated in the dark for 30 min at room temperature. The absorbance was measured at 517 nm on a microplate reader (BIORAD xMark microplate spectrophotometer). The blank was prepared using methanol. The scavenging activity was calculated using the following formula based on the percentage of DPPH radical scavenged [12].
| Percentage Inhibition (%) = (Blank(A517) − Sample(A517)) × 100/Blank(A517) |
2.8. Nucleation assay
This assay was chosen for the study of oxalate crystallization because of its satisfactory, simplicity and reproducibility. This assay was used to assess the inhibiting capacity of the plant extract and included the study of crystallization in the form of turbid solution with and without plant extract. Calcium chloride and sodium oxalate solutions were prepared at the final concentrations of 9 mMol/L and 3 mMol/L respectively, in a buffer containing 0.15 M NaCl and 0.05 M Tris at pH 6.5. 250 μL of calcium chloride solution was mixed with 100 mL of extracts at different concentrations. Crystallization took place by addition of 250 mL of sodium oxalate solution. The temperature was maintained at 37 °C. Spectrophotometer (Systronics digital spectrophotometer 166) was used to monitor the OD of the solution after 30 min at 620 nm [2].
The percentage of inhibition was calculated with the help of the following formula:
| Percentage inhibition = AbsCtrl − AbsTest × 100/AbsCtrl |
AbsCtrl represents the turbidity without plant extract while AbsTest represents the turbidity after addition of plant extract.
2.9. Synthetic urine assay
2.9.1. Preparation of synthetic urine
Synthetic urine assay was performed for the study of oxalate crystallization, in order to assess the inhibiting capacity of the plant extract, which included the study of crystallization with and without addition of plant extract. Synthetic urine was prepared by adding NaCl (9 g) in solution A composed of N2C2O4 (2 mM/L) and Solution B of CaCl2 2H2O (10 mM/L). Equal volumes of 50 mL of solutions A and B were mixed and stirred at constant temperature (37 °C) in capped vessels to give final artificial urine. Mixture agitation was maintained to prevent sedimentation.
2.10. Simulation of the sedimentary crystal formation
The crystal developed was monitored by a simple light microscope in a sample drop after mixing solution A and B. A series of experiments were conducted corresponding the physiological concentrations 25, 50, 75 and 100 μg/mL of extract. Hemocytometer counting chamber was used to observe and count the number of crystals in a drop of sample under microscope after 30 min of mixing, for all the concentrations. The percentage of inhibition was calculated with the help of following formula [4].
Control represents the number of calcium oxalate monohydrate crystals without inhibitor. Test represents the number of calcium oxalate monohydrate crystals after addition of inhibitor.
2.11. Antimicrobial activity
Antibacterial activity of methanolic extract of D. erecta was studied by agar well diffusion method (Pharmacopeia of India). The 100 μL (1 × 10−5) stock culture was inoculated onto 25 mL nutrient broth and incubated at 37 °C for 48 h. At the end of incubation period, 10 μL bacterial suspension was spread (L-shaped spreader) onto pre-cooled NAM agar plates and 10 μL, test compound was added to the respective wells (6 mm). 100 μg streptomycin dissolved in 1000 μL DMSO was used as the standard antibiotic, and incubated at above mentioned conditions. At the end of the incubation period, the zone of inhibition (mm) around the well was calculated by using HiAntibiotic Zone Scale-CTM (Himedia).
2.12. Animal study
2.12.1. Animals
Antiurolithiasis activity of methanolic leaves extract of D. erecta was determined against sodium oxalate in Wistar rats. Male Wistar rats weighing around 150 g and 5 weeks old were obtained from National Institute of Bioscience, Pune, after approval from Institutional Animal Ethics Committee (IAEC approval. No. SSBS/IAEC/04-2016) of Symbiosis School of Biomedical Sciences (SSBS), Pune. Rats were housed in standard polyvinyl cages in the animal house facility of SSBS, Pune. They were fed with a balanced diet of standard pellet and maintained under standard laboratory conditions providing 22–24 °C temperature, 60–70% relative humidity, standard light–dark cycle (12 h light, 12 h dark) and water was provided ad libitum. Healthy animals weighing 150–200 g were included in the study and were divided into four groups (A–D) with 6 animals in each group.
-
(1)
Groups A, B, C and D served as normal control, disease control, standard drug treated and D. erecta extract treated animals respectively.
-
(2)
Pre-treatment period: Groups B, C and D received 70 mg/kg sodium oxalate via intraperitoneal injection (IP) for 7 days.
-
(3)
Treatment period: Group C received 50 mg/kg of standard drug (Potrate MB 6) (suspended in 2% gum acacia) orally. Group D received 50 mg/kg of D. erecta extract orally.
2.13. Serum parameters
On day 7 of treatment, blood was withdrawn by retro-orbital puncture from each rat. The serum was separated by centrifugation at 10,000 rpm at 4 °C using cryocentrifuge machine for the estimation of serum parameter (creatinine, BUN, SGOT and SGPT). Blood related parameters were measured using auto-blood analyzer instrument (Bayer Express Plus, NY, and USA).
2.14. Urine parameters
Urine color, transparency, pH, urine oxalate were checked and microscopic examination was done to observe calcium oxalate crystallization.
2.15. Histopathology
After 7th day of the treatment period, the animals were sacrificed by ethical method. Both kidneys were dissected and one kidney from each rat was placed in 10% formalin solution. The kidney tissues were trimmed longitudinally and routinely processed. Tissue processing was done to dehydrate in ascending grades of alcohol, clearing in xylene and embedded in paraffin wax. Paraffin wax embedded tissue blocks were sections at 5 μm thickness with the Rotary Microtome. All the slides of kidneys were stained with Hematoxylin & Eosin (H & E) stain. The prepared slides were examined under microscope. Fig. 1 represents the study design parameters.
2.16. Statistical analysis
Two way ANOVA followed by Bonferroni's multiple comparison test between the test and standard at different concentrations suggested significant interaction at p < 0.01 for antioxidant activity and one-way ANOVA followed by Dunnett's test. p < 0.01, as compared with vehicle group in in vivo assays. Statistical analyses were performed by using GraphPad PRISM® Version 6.01 (GraphPad software, Inc., California, USA).
3. Results and Discussion
In the present investigation, primary and secondary metabolites present in D. erecta were qualitatively analyzed. Preliminary qualitative phytochemical analysis of D. erecta methanolic leaves extract revealed the presence of glycoside, saponins, sterols, flavonoids, phenols, tannins, alkaloids, carbohydrates and proteins.
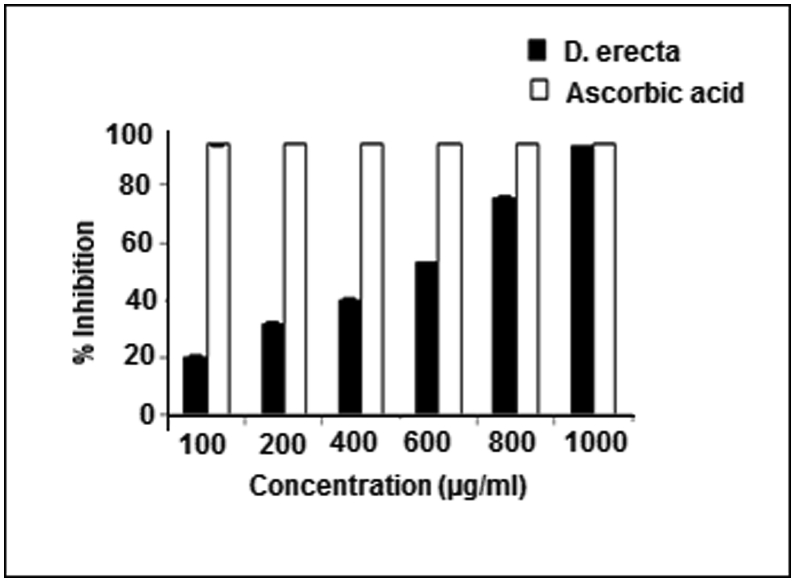
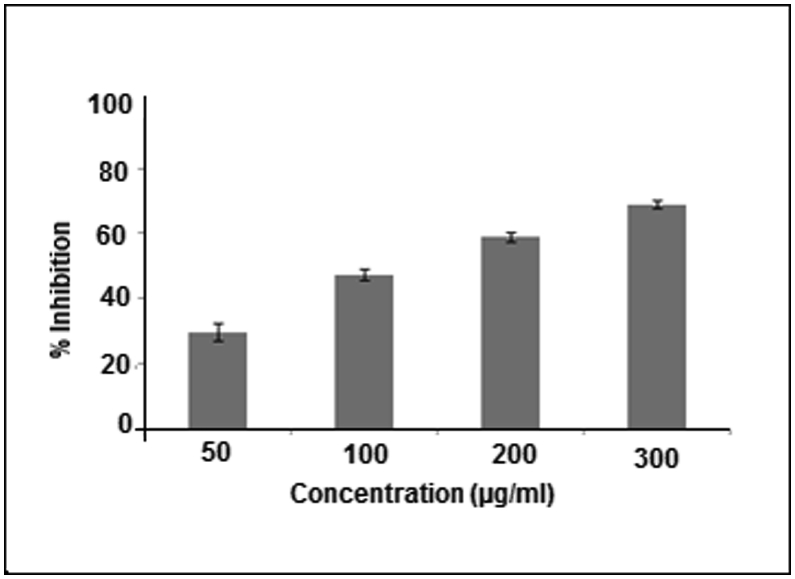
The yield obtained from methanolic extract of D. erecta was 60%. The in vitro antioxidant assay performed on this plant revealed significant antioxidant potential as compared with ascorbic acid. D. erecta methanolic leaves extract inhibited DPPH radical by 93.51% at a concentration of 1000 μg/mL where ascorbic acid inhibited 94.71% of DPPH radical at the same concentration (Fig. 2). The in vitro results revealed that the plant extract has potent anti-urolithiatic ability in both nucleation assays with maximum inhibition of 68.80% at 300 μg/mL extract concentration. Incubation of the metastable solutions of calcium chloride and sodium oxalate resulted in the formation of calcium oxalate crystals. The O.D. was monitored at 620 nm after 30 min. The turbidity of solution in the presence of D. erecta extract was lower in comparison to the control, showing that oxalate crystallization was less in the presence of extract. This study proved that % inhibition for calcium oxalate crystal formation was directly proportional to the concentration of the plant extract and minimum inhibition was observed to be of 29.60% at 50 μg/mL, while maximum inhibition of 68.80% was attained at 300 μg/mL of the extract concentration (Fig. 3).
Fig. 2.
DPPH free radical scavenging potential of D. erecta as compared to ascorbic acid.
Fig. 3.
Effect of different concentrations of D. erecta on calcium oxalate crystal inhibition by nucleation assay.
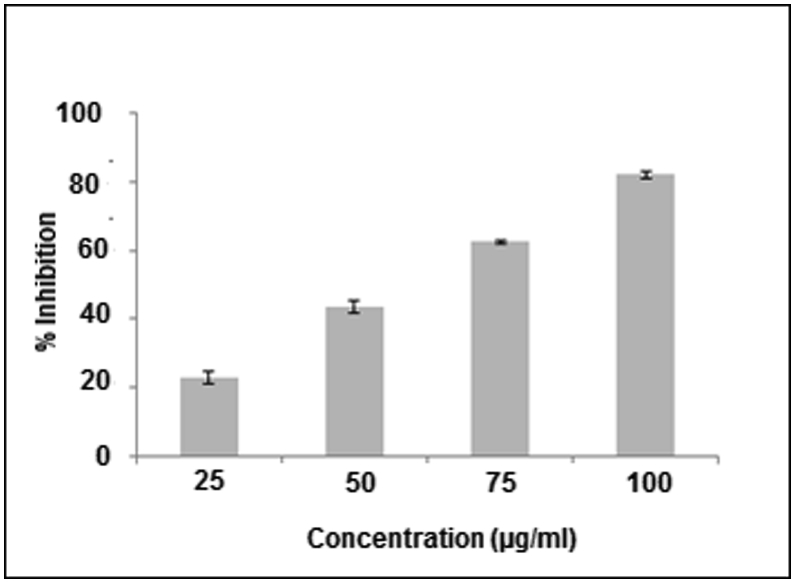
The formation and growth of the calcium oxalate monohydrate crystals from artificial urine at different concentrations were studied. Synthetic urine assay exhibited maximum inhibition of 82.14% at 100 μg/mL extract concentration. It was also inferred from the synthetic urine assay that maximum number of crystals were formed in control while minimum were formed at 100 μg/mL of extract.
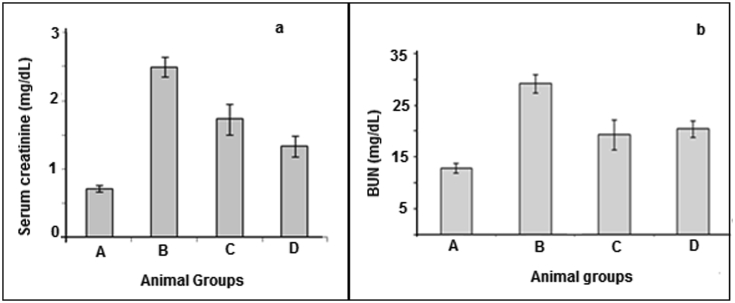
Stone formation was the due to supersaturation of urine with some urinary salts such as calcium oxalate. The number of calcium oxalate monohydrate crystals was found to be maximum in control. Different percentages of plant extract were tested in order to assess the inhibiting potential of plant extract for oxalate crystallization. In the presence of different percentages of plant extract, the size (length and the width) of the crystals were reduced. It was observed that the plant used in this study inhibited the crystal development with maximum number of crystals found at 25 μg/mL extract concentration while at 100 μg/mL concentration of extract, the crystal formation was found minimal. Results show that the decrease in number of crystal as well as % inhibition of the formation of calcium oxalate monohydrate crystals was directly proportional to the increase in percentage of plant extract, with minimum inhibition 23.04% at 25 μg/mL extract while maximum inhibition of 82.14% at 100 μg/mL extract concentration as depicted in Fig. 4. Elevated creatinine level was detected (2.49 ± 0.14 mg/dL) in group B, but the values significantly reduced to 1.73 ± 0.22 mg/dL and 1.33 ± 0.14 mg/dL in group C and group D. Elevated BUN level was detected in 29.15 ± 1.83 group B, but the values significantly reduced to 20.4 ± 1.59 mg/dL and 19.3 ± 2.89 mg/dL in group C and group D. Fig. 5 is representing the effect of D. erecta treatment in Wistar rats.
Fig. 4.
Effect of different percentages of D. erecta extract on calcium oxalate monohydrate crystal inhibition in synthetic urine assay.
Fig. 5.
(a): Serum creatinine values and (b) BUN values which are expressed as mean S.E.M., (n = 6).
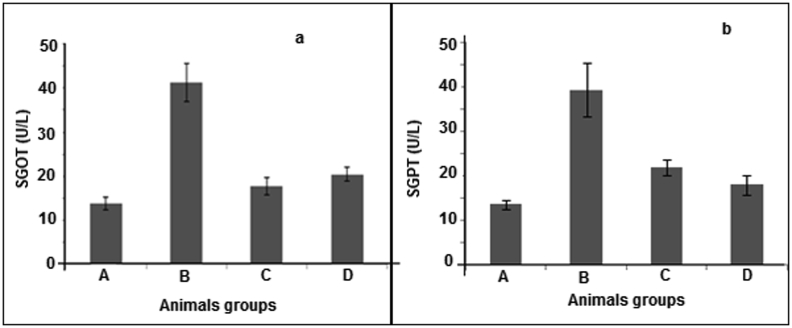
Elevated SGOT level was detected as 41.26 ± 4.30 unit/L in group B while group C and D were in normal range i.e. 17.75 ± 1.97 and 20.4 ± 1.59 respectively. Values of SGPT in B, C and D treated groups were 39.3 ± 0.9, 17.93 ± 2.23 and 21.81 ± 1.88 respectively as illustrated in Fig. 6. In vivo evaluation of group B revealed an elevated level of serum creatinine and BUN level which indicated a marked renal damage; however in group A (vehicle group), C (Potrate MB 6 treated) and D (D. erecta treated) serum creatinine and BUN was in normal range.
Fig. 6.
The SGOT and SGPT values of rats after treatment with D. erecta, are expressed as mean S.E.M., (n = 6).
Renal damage caused due to urolithiasis is associated with liver damages which were confirmed by SGOT and SGPT test. In this study, Potrate MB 6 was used as a standard drug. There was an increase in urinary oxalate after sodium oxalate administration while a decrease in oxalate levels was observed in C and D groups. This effect may be due to the inhibition of formation of oxalate by the D. erecta treatment.
The kidney sections derived from Group B when observed microscopically showed polymorphic irregular crystals deposited in the tubules which lead to dilation of the proximal tubules and also interstitial inflammation that might be due to oxalate. Treatment with the Potrate MB 6 and D. erecta reduced the number and size of calcium oxalate crystals deposited in different parts of the renal tubules and also saved the tubules and calyces from damaging. Although the exact mechanism of action couldn't be determined in this analysis, it could be possibly through an antioxidant, nephroprotective property of D. erecta extract. Further studies are going on to predict the possible mechanism of its anti-urolithiatic action.
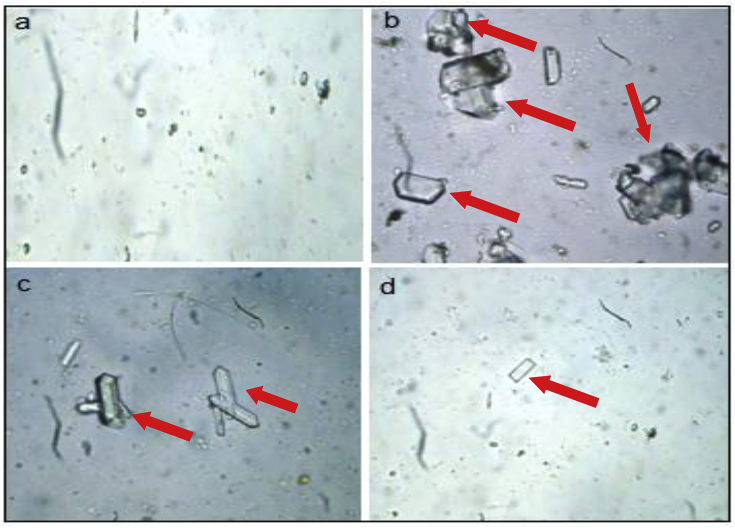
Urine color was detected pale yellow in all the grouped animals. Transparency of urine in group B induced group was found be opaque but the transparency of group A, C and D was clear. Microscopic view of urine samples from group A didn't show any granular debris nor crystals of calcium oxalate (Fig. 7a). On the other hand, microscopic view of urine samples of group ‘B’ depicted higher amount of granular debris and calcium oxalate crystals (red arrows) were observed, the crystals varied from large to medium size comparative to citrate and D. erecta treated group (Fig. 7b). For group C and D (treated animals), urine samples depicted lesser amount of granular debris (red arrows). Calcium oxalate crystals (red arrows) were of smaller size in comparison to sodium oxalate induced group (Fig. 7c and d).
Fig. 7.
Microscopic view of urine samples of rats of (a) Group A, (b) Group B, (c) Group C, (d) Group D.
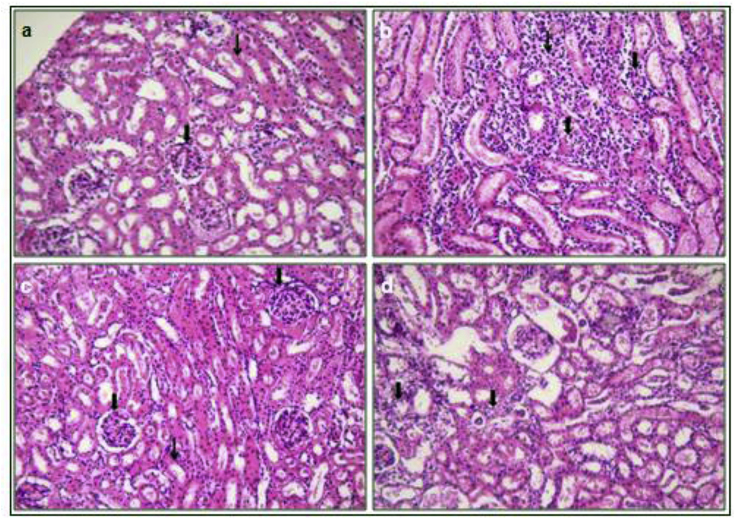
H & E staining of kidney tissues from rats of vehicle group showed normal histology with regular size tubules with single epithelial lining along the margin and did not reveal any lesion of pathological significance. The arrows in the picture indicate glomerulus (large arrow) and tubule (small arrow) (Fig. 8). Rats of sodium oxalate treated group showed degeneration of epithelial lining, focal mild degeneration of tubules of medulla, focal mild tubular atrophy of cortex and multifocal minimal intertubular lymphocytic infiltration. The arrows in the picture indicate degeneration of tubules (large arrow), lymphocytic infiltration (small arrow). Group C-citrate treated group rats did not reveal any lesion of pathological significance suggesting attenuation of lesions, showing normal histology (Fig. 8c). D. erecta treated group D showed lesions of lesser severity with less distribution suggesting decrement of adverse effect caused due to sodium oxalate (Fig. 8d).
Fig. 8.
H & E staining of kidney tissue samples of group A, B, C, D (Represented by a, b, c, d respectively).
The antimicrobial activity of methanolic extract D. erecta was tested against both gram positive (G+ve) and gram negative (G−ve) bacteria Fig. 9. The highest zone of inhibition was recorded with E. coli (25 mm) and P. desmolyticum (17 mm). However, zone of inhibition of gram positive bacteria, B. subtilis and S. aureus was recorded 17 mm and 16 mm respectively as compared with standard antibiotic streptomycin. Damage of urinary tract leads to bacterial attack and a stone nucleus might develop, leading to a full stone in the urinary tract. At this point, some extracts that show antimicrobial properties can be considered anti-lithogenic by protecting urinary tract from bacterial infection [5] proved that plant extracts which have antimicrobial activity can act as better anti-urolothiasis medicine.
Fig. 9.
Antimicrobial activity D. erecta against gram positive and gram negative bacteria.
4. Discussion
The presence of primary and secondary metabolites like glycoside, saponins, sterols, flavonoids, phenols, tannins, alkaloids, carbohydrates and proteins in D. erecta has been reported to have huge potential of medicinal properties. Methanolic extract of D. erecta has given very good yield (60%). DPPH radicals are widely used in the model system to investigate the scavenging activity of various natural products. The plant has proven to be potentially active antioxidant by the results of DPPH scavenging activity in this study. DPPH radicals are widely used in the model system to investigate the scavenging activity of various natural products. The in vitro results revealed that the plant extract has potent anti-urolithiatic ability in both nucleation assay and synthetic urine assay. Results has proved that the decrease in number of crystal as well as % inhibition of the formation of calcium oxalate and calcium oxalate monohydrate crystals was directly proportional to the increase in concentration of plant extract. Based in vivo experiment, it can be concluded that D. erecta may treat calcium oxalate crystal deposition in the kidney by preventing hyperoxaluria-induced peroxidative damage to the renal tubular membrane surface (lipid peroxidation), which in turn can prevent calcium oxalate crystal attachment and subsequent development of kidney stones. Antimicrobial property of D. erecta enhances its anti-urolithiatic activity by prevention of secondary bacterial infection at the site of damaged urinary tubules.
5. Conclusion
Our results indicate that D. erecta may treat calcium oxalate crystal deposition in the kidney by preventing hyperoxaluria-induced peroxidative damage to the renal tubular membrane surface (lipid peroxidation). It has antimicrobial potential so it may also inhibit the secondary bacterial infection in kidney. Based on the data, it can be concluded that this herb can be used as a potential anti-urolithiasis agent for kidney stone removal.
Sources of funding
Council of Scientific & Industrial Research, New Delhi and CSIR-National Chemical Laboratory, Pune. Authors would like to acknowledge the CSIR Network Project CSC-0133 (FUNHEALTH).
Conflict of interest
None.
Acknowledgement
Authors are thankful to Director of SSBS, Pune for their help. Authors are also thankful to Ms. Jacika Kanwar and Ms. Chaitali Rana for their technical help.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Ainsworth E.A., Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 2.Atmani F., Khan S.R. Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in-vitro. Int Braz J Urol. 2000;85:621–625. doi: 10.1046/j.1464-410x.2000.00485.x. [DOI] [PubMed] [Google Scholar]
- 3.Awari M.D., Mute V., Babhale S.P., Chaudhar P.S. Antilithiatic effect of Achyranthes aspera Linn. leaves extract on ethylene glycol induced nephrolithiasis. J Pharma Res. 2009;2:994–997. [Google Scholar]
- 4.Beghalia M., Ghalem S., Allali H., Belouatek A., Marouf A. Inhibition of calcium oxalate monohydrate crystal growth using Algerian medicinal plants. J Med Plant Res. 2008;2:66–70. [Google Scholar]
- 5.Bouabdelli F., Djelloul A., Kaid-Omar Z., Semmoud A., Addou A. Antimicrobial activity of 22 plants used in urolithiasis medicine in Western Algeria. Asian Pac J Trop Dis. 2012;2:S530–S555. [Google Scholar]
- 6.Fessenden J., Fessenden Joan S., Feist Patty. 3rd ed. Brooks Cole Publishing; Pacific Grove, California: 2001. Organic laboratory techniques; p. 59. [Google Scholar]
- 7.Kamboj P., Aggarwal M., Puri S., Singla S.K. Effect of aqueous extract of Tribulus terrestris on oxalate-induced oxidative stress in rats. Indian J Nephrol. 2011;21:154–159. doi: 10.4103/0971-4065.83727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar M.S. Kidney stones. BMJ. 2004;328:1420–1424. doi: 10.1136/bmj.328.7453.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pękal A., Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Method. 2014;7:1776–1782. [Google Scholar]
- 10.Prasad K.V., Sujatha D., Bharathi K. Herbal drugs in urolithiasis – a review. Pharmacogn Rev. 2007;1:175–179. [Google Scholar]
- 11.Rahmatullah M., Jahan R., Azam F.M.S., Hossan S., Mollik M.A.H., Rahman T. Folk medicinal uses of Verbenaceae family plants in Bangladesh. Afr J Tradit Complement Altern Med. 2011;8:53–65. doi: 10.4314/ajtcam.v8i5S.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao R.R., Tiwari A.K., Reddy P.P., Babu K.S., Ali A.Z., Madhusudana K. New furanoflavanoids, intestinal α-glucosidase inhibitory and free-radical (DPPH) scavenging, activity from antihyperglycemic root extract of Derris indica (Lam.) Bioorg Med Chem. 2009;17:5170–5175. doi: 10.1016/j.bmc.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 13.Rao P.N., Glenn M.P., John P.K. Springer–Verlag London Ltd; 2011. Urinary tract stone disease. [Google Scholar]
- 14.Ringold S., Glass T.J., Glass R.M. Kidney stones. JAMA. 2005;293:1158. doi: 10.1001/jama.293.9.1158. [DOI] [PubMed] [Google Scholar]
- 15.Shahat A.A., Nazif N.M., Abousetta L.M., Ibrahim N.A., Cos P., Miert S.V. Phytochemical investigation and antioxidant activity of Duranta repens. Phytother Res. 2005;19:1071–1073. doi: 10.1002/ptr.1766. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. IPS. 2011;1:98–106. [Google Scholar]