Abstract
Cells must be able to interpret signals they encounter and reliably generate an appropriate response. It has long been known that the dynamics of transcription factor and kinase activation can play a crucial role in selecting an individual cell's response. The study of cellular dynamics has expanded dramatically in the last few years, with dynamics being discovered in novel pathways, new insights being revealed about the importance of dynamics, and technological improvements increasing the throughput and capabilities of single cell measurements. In this review, we highlight the important developments in this field, with a focus on the methods used to make new discoveries. We also include a discussion on improvements in methods for engineering and measuring single cell dynamics and responses. Finally, we will briefly highlight some of the many challenges and avenues of research that are still open.
Keywords: dynamics, decoding, encoding, single cell, signaling, live cell microscopy
Introduction
Specificity requires a cell to be able to recognize heterogeneous signals as inputs and reliably compute heterogenous outputs in response. Cells receive signals that can be derived from the organism itself—autocrine, paracrine, and endocrine signals—from the environment, or from other organisms, for example, during infection. Frequently, several signals are present simultaneously and in rapidly changing amounts and durations. Despite this, cells must be able to reliably differentiate signals and generate a specific response based on the signal identity, intensity (i.e., the amount of signal present), frequency (i.e., the duration the signal is present for), and context (i.e., the other signals present and the cellular state).
Accordingly, cells have evolved myriad mechanisms to receive, transmit, and process information reproducibly in a fluctuating and noisy environment. Cells generally first “encode” the signal they receive, by transmitting information about the signal into the activation of specific signaling pathways. Cells are then able to “decode” this information into phenotypic responses and changes in gene and protein expression. Notably, stochastic fluctuations in the concentration of signaling molecules, the numbers of intracellular signaling proteins, and the composition of the microenvironment can be substantial at the single cell level (1). Therefore, the pathways have evolved to be robust to this unavoidable biological noise. The signaling pathways are also frequently redundant or overlapping; many cellular signaling pathways are able to transmit information from a variety of signals to produce heterogeneous outcomes, while many signals can affect multiple pathways (2–4).
Over the past couple decades, it has become increasingly clear that cells use a variety of signaling architectures to encode detailed information about the signals they encounter as temporal patterns of activation of transcription factors, kinases, calcium ions, and other signaling molecules (5–7). Cells must therefore also possess mechanisms to interpret this dynamic information and translate it into a transcriptional or phenotypic response. One classic example is the discrimination of nerve growth factor (NGF) and epidermal growth factor (EGF) by rat neuronal precursors. NGF stimulation produces sustained activation of the ERK pathway that prompts the cell to differentiate, while EGF stimulation engenders transient activation of the ERK pathway that is decoded as a proliferative cue (Figure 1A) (8–10). Another classic example is the nuclear factor (NF)-κB innate immune signaling pathway, which exhibits oscillatory activation patterns when stimulated with tumor necrosis factor (TNF)-α, but sustained activation when stimulated with lipopolysaccharide (LPS), resulting in different gene expression patterns (11–13).
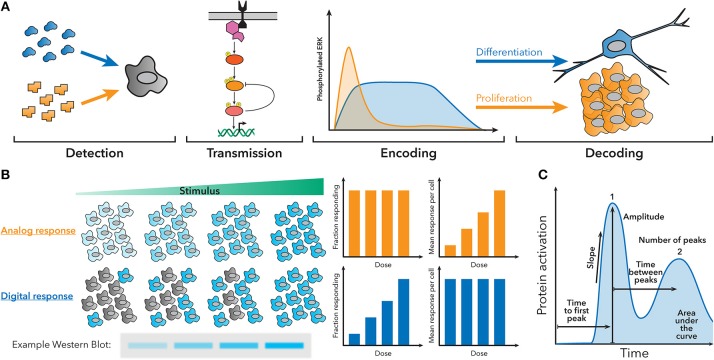
Figure 1.
Fundamentals of dynamic encoding and decoding. (A) Cells can encode information about the signals they encounter as dynamic patterns of signaling pathway activation. These patterns can then be decoded to produce a specific response. For example, NGF creates sustained ERK activation, which leads to differentiation, while EGF creates transient ERK activation, which leads to proliferation. (B) Population-level measurements, such as a western blot, can hide the behavior of single cells in the underlying system. For example, an analog or digital response could produce similar western blots, despite having different amounts of active cells and activity per cell. (C) Examples of some features of dynamic traces that can be used to encode information.
The importance of dynamic decoding of stimulus dose and identity has also been described in several other pathways. For example, p53 is known to use dynamics to differentiate doses of gamma radiation, and between gamma and UV radiation (14, 15). The Msn2 pathway in yeast uses dynamics to differentiate between osmotic and oxidative stress, as well as the severity of glucose starvation (16). Moreover, the Notch pathway has been recently discovered to differentiate between some Delta-like ligands using dynamic patterns (17). Finally, recent studies have described how individual cells interpret multiple, simultaneous stimulants (18, 19). These are only a few examples in a large space; we strongly encourage readers to reference some of the excellent reviews published on cellular encoding and decoding for a more complete overview, especially of classic examples (20–22).
Clearly, dynamic encoding and decoding are widespread in biology, but studying how cells interpret cellular dynamics can be challenging, because population measurements occlude the behavior of individual cells (Figure 1B), and making targeted perturbations in signaling pathways is difficult. In order to understand how individual cells encode and decode dynamics, often multiple different measurements have to be made in the same single cell at multiple timepoints. Features of the signaling dynamic patterns (Figure 1C) can then be correlated to other measurements of cell behavior to understand how the cell is using dynamics. Recently, the portfolio of high-throughput and single-cell technologies has expanded greatly and become accessible to a wider spectrum of labs, allowing signaling dynamics to be studied in myriad systems.
For example, optogenetics has enabled precisely targeted activation of signaling pathways allowing for greater understanding of the role of dynamics in development and cancer signaling (23–26). Advances in microfluidics have enabled new precision in stimulation timing and dosage (19, 27–29), as well as studies of single cell protein secretion or transcriptomics (30–33). Finally, new reporters have created opportunities to make measurements in novel signaling pathways and contexts (29, 34–41). In this review, we will highlight various experimental strategies that have been successfully used to study cellular dynamic decoding, with an emphasis on single cell studies. We will also discuss recent technological developments that have enabled the field to grow rapidly, and end by discussing some potential future avenues of study and technological challenges that still persist.
Dynamic Decoding of Cellular Information
Population-level studies have revealed some of the connections between signaling dynamics and cellular responses (12, 13, 42, 43). However, as discussed previously, population measurements are not necessarily indicative of single cell behavior (Figure 1B). In order to understand how single cells decode dynamic signals into a phenotypic response, it is necessary to make combined measurements of the signaling dynamics and the downstream cellular response in the same single cell. Microscopy has proven to be an invaluable tool for these studies, given the versatility of measurements that it can make, including not only live-cell fluorescence for measuring the signaling dynamics themselves, but also single molecule fluorescence in situ hybridization (smFISH) for measuring gene expression (44), and immunofluorescence or other antibody-based methods for measuring protein expression. In addition, there has been recent work to combine other modalities, such as RNA-seq and microfluidics, with live-cell imaging, expanding the repertoire of possible measurements. Here we present a collection of recent studies that demonstrate effective strategies for probing the connection between dynamics and cellular responses on a single cell level.
Live-Cell Imaging Coupled With Measurements of Physical Phenotypes
The most straightforward way to interrogate how cells decode dynamics is to measure signaling dynamics and clear phenotypic responses, such as cell death, cell migration, or cell division. These measurements are well-adapted to live-cell microscopy, as measurements of cellular dynamics and the phenotypic response can be made using the same measurement modality with few technical limitations.
For example, p53 is a transcription factor with a critical role in regulating cell growth and apoptosis in response to DNA damage (45). Previous population-level studies suggested cells with p53 activation below a specific threshold would initiate growth arrest, while cells above that threshold would undergo apoptosis (46). However, single cell studies using a fluorescent p53 reporter showed that in order to undergo apoptosis, p53 levels in the cell must indeed reach a threshold, but that this threshold increases over time (Figure 2A) (47). Therefore, the decision of apoptosis or cell growth arrest is determined by the dynamics of p53 activation, as opposed to a static threshold. This observation could only have been made using a single cell dynamical approach.
Figure 2.

Examples of decoding dynamic signaling patterns. (A) Apoptosis due to p53 signaling is not determined by a static threshold, but by a dynamic, increasing threshold. Some cells do not undergo apoptosis, even though they have higher p53 levels than some cells that do undergo apoptosis. Figure adapted from Paek et al. (47). (B) Subpopulations with distinct patterns of NF-κB activity exist in single cells stimulated with LPS. These patterns are correlated with different gene expression patterns for known NF-κB targets. Figure adapted from Lane et al. (32). (C) Basal rates of adipocyte differentiation are low in vivo, despite large pulses of glucocorticoid production daily. However, continuous glucocorticoid inputs of similar total magnitude induce more stabilization of PPARG, indicated in green, and higher differentiation rates. Figure adapted from Bahrami-Nejad et al. (73).
A similar study revealed aspects of TNF signaling that are correlated with apoptosis. TNF signaling initiates a pro-apoptotic cascade, as well as induction of pro-survival genes by NF-κB (48). Using a microfluidic device to precisely control stimulus timing and dosage, Lee et al. showed that short pulses—as short as 1 min—of TNF-α can be more effective at inducing apoptosis than longer pulses. Single-cell measurements of NF-κB activation showed that longer pulses of TNF sustained longer residence times of NF-κB in the nucleus, suggesting that NF-κB dynamics are correlated with the relative balance of pro-apoptotic and pro-survival signaling (49). The pro-apoptotic arm of the pathway is initiated on a slower time-scale than, and is inhibited by, the pro-survival arm of the pathway (48, 50, 51). Therefore, the authors propose a model where the sustained NF-κB activation caused by longer TNF pulses, maintains inhibition of the pro-apoptotic signaling arm, leading to greater relative pro-survival signaling (49).
Live-cell microscopy has also been used to understand how the spatiotemporal dynamics of the mitogen-activated protein kinases (MAPKs) regulate mating processes in single yeast cells. In order to undergo successful mating and fusion, individual yeast cells must remodel their cell walls, arrest the cell cycle, and polarize their growth (52, 53). A Förster resonance energy transfer (FRET) reporter of the activity of two MAPKs, Fus3 and Kss1, coupled with visual observation of cell shape and growth, revealed that elevated Fus3 activity at the sites of polarized growth were required for initiating polarity and fusion between mating cells, showing that the spatiotemporal patterning of Fus3 activity, and not just Fus3 levels, are required for the correct mating phenotype (54).
Reporters of multiple different pathways can also reveal how the context of an immune stimulus can affect how an immune cell will respond (55). Innate immune cells use pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), to detect molecules that are indicative of an infection. A cell line expressing both an NF-κB and a c-Jun N-terminal kinase (JNK) reporter was challenged with increasing levels of immune stimulation, from only LPS to infection with Salmonella typhimirium, allowing for measurements of the cell's signaling response in each case (35). This work showed how an individual cell uses TLR signaling to discriminate similar signals in a variety of different contexts. For example, in a population of cells exposed to S. typhimirium, uninfected cells typically activated only NF-κB, while those cells that were infected with bacteria typically activated both JNK and NF-κB (35).
Multiple live-cell reporters exist that broaden the types of physical phenotypes that can be directly measured using live-cell microscopy. For example, fluorescent probes have been developed that allow for quantitative measurements of single cell kinase activation (34, 37), visualization of various modes of cell death and caspase activation (36, 56), as well as cell cycle progression and measurements of proliferation rate (57). This proliferation reporter was used in conjunction with a FRET-based reporter of ERK activity to show that cells use ERK pulse duration and overall activity to regulate entry into S-phase and cell cycle timing. Subsequent experiments using high content immunofluorescence (HCIF) suggested that the main quantitative factor controlling steady-state proliferation in single cells is ERK output (58).
These experiments are clear examples of ways that we can begin to understand how signaling dynamics generate physical phenotypic responses. Furthermore, the development of new biosensors, improvements in our ability to regulate cellular dynamics (see “Experimental strategies for engineering or modulating dynamic signaling patterns”), and improvements in high-throughput phenotype characterization (59), should allow for even more insight in this area of research.
Live-Cell Imaging Coupled With Measurements of Gene Expression and Transcription
Signaling pathways often exert control over the cell by making changes to the expression of specific target genes. In such cases, dynamic signaling patterns are likely to be decoded as quantitative changes in gene expression. However, unraveling this connection can be more challenging than measuring physical phenotypes because it requires measuring signaling dynamics and gene expression in the same single cell. Notwithstanding this complication, many recent studies have demonstrated experimental strategies to successfully combine measurement modalities to uncover interesting results.
For example, the nuclear abundance of NF-κB varies significantly in both stimulated and unstimulated cells, suggesting that NF-κB transcription might also be highly variable (60). However, smFISH measurements in single cells show that transcript levels for many NF-κB targets vary less than the activity of NF-κB (61). Measurements of a fluorescent NF-κB reporter, combined with end-point smFISH quantification of NF-κB regulated transcripts, showed that dynamic quantities, such as fold change of NF-κB, were better predictors of transcriptional levels than static quantities, such as NF-κB nuclear abundance. Subsequent mathematical modeling revealed a potential mechanism based on an incoherent feed-forward loop generated by transcription factor competition (61). Recent work, also using a smFISH-based strategy, further showed that fold change of NF-κB was an accurate predictor of transcript levels at promoters with both high and low levels of TNF-induced transcription (62).
A similar example involved studying the responses of NF-κB to simultaneous LPS and TNF-α stimulation to understand how cells respond to multiple stimuli. A fluorescent reporter was used to measure NF-κB activation in 3T3 cells across a range of LPS and TNF-α concentrations. For most concentrations, the response could be classified as an LPS-only or TNF-α-only response, but for a number of intermediate concentrations, there was a synergistic response that had characteristics of both TNF- and LPS-stimulated cells. Subsequent smFISH measuring the mRNA of a number of relevant chemokines and cytokines revealed that dual-responding cells had, on average, higher expression of Cxcl10 and Csf3 (18). Similar strategies have also been successfully used in other signaling systems (63–65).
Single-cell dynamic imaging can also reveal new phenomena that can be further studied using other techniques. For example, the HIV virus is known to integrate into the host genome and lay dormant in some T lymphocytes, which presents a major obstacle for treatment with antiretroviral therapy (66). Although these latent reservoirs of virus exhibit stochastic, low level activation, the molecular regulators controlling viral activation are still incompletely understood (67). NF-κB is a known regulator of the HIV long terminal repeat (LTR) promoter; however, a recent study using fluorescent reporters of HIV and NF-κB activation revealed that NF-κB activation is not predictive of levels of viral activation across different clones (68). Instead, using smFISH and chromatin immunoprecipitation (ChIP), the authors found that the chromatin environment regulates transcriptional bursting and can explain clone-to-clone variability. These findings revealed that NF-κB-chromatin interactions are required to explain transcriptional bursting and viral activation (68).
FISH-based strategies have also been used to elucidate the role dynamics can play in vivo during development. For example, it had been shown that myogenesis requires transient, not sustained, activation of Notch, but the mechanism of transient Notch activation was not clear (69). Recently, it was revealed that the Notch pathway also uses dynamics to encode and decode information about the identity of the activating stimulus (17). A creative experimental system, using engineered “sender” cell lines that produce either Dll1 or Dll4 and a “receiver” cell line with a chimeric Notch receptor driving expression of a fluorescent protein allowed measurements of the dynamics of Notch activation in the presence of either ligand. These experiments revealed that Dll1 stimulation leads to pulsatile Notch activation, while Dll4 creates sustained Notch activation, with differences in gene expression as a result. The results were reproduced in an in vivo model by electroporating either Dll1 or Dll4 into one side of the neural crest of a chick embryo and then using hybridization chain reaction (HCR) FISH to stain for MyoD1, a muscle regulatory factor. Their results revealed that MyoD1 is upregulated by Dll1, which creates pulsatile dynamics, while Dll4, which creates sustained dynamics, downregulated MyoD1 (17).
In systems where endpoint measurements of gene expression are insufficient, multiple fluorescent reporters can be used to measure signaling and transcriptional output simultaneously. For instance, TNF-α is a known regulatory target of NF-κB, that can subsequently regulate downstream responses through paracrine and autocrine signaling (11, 32, 70). A cell line with reporters for both NF-κB activity and transcription from the TNF-α promoter was used to simultaneously measure the signaling and transcriptional dynamics in real-time. Measurements revealed low correlation between many measures of NF-κB activity and the output from the TNF-α promoter. However, the time-integrated NF-κB activity was well correlated with the total output from the TNF-α promoter, demonstrating that continuous measurements of transcriptional activity can reveal more information than endpoint measurements in some systems (71).
Finally, it is now also possible to measure signaling dynamics and genome-wide transcriptional responses in the same single cell. RNA-seq provided a method to measure the entire transcriptome of a single cell, but it remained unsolved how to connect those data with measurements of transcription factor activation dynamics. Lane et al. used microfluidics to isolate cells for live-cell imaging and single-cell RNA-seq to connect the identity of the cell in both datasets. Their results revealed that distinct patterns of NF-κB signaling in response to the same stimulus correlated to different global transcriptional responses (Figure 2B) (32). The ability to measure global gene expression resultant from heterogeneous dynamics is exceedingly useful, because it allows for phenotypic characterization of single cell dynamics without a need for a priori knowledge of the target genes.
Live-Cell Imaging Coupled With Protein Expression Measurements
Frequently, a cellular response to signals that it receives is to differentially regulate the expression or secretion of proteins. For example, a large part of the immune response is coordination of cytokine and chemokine secretion by immune cells at the site of infection. Therefore, another promising avenue for research in cellular dynamics is to study changes in protein expression and secretion in conjunction with measurements of dynamics. Immunofluorescence can be used to measure intracellular protein expression, similar to measurements of gene expression using smFISH. Alternatively, microfluidic devices or microwell-based assays can be used to measure protein secretion from single cells.
For example, protein quantification can be used to understand how signaling pathways in cells control differentiation. Hormones such as glucocorticoids strongly induce adipogenesis in vivo and in vitro, but basal rates of preadipocyte differentiation are low in living animals, despite large daily spikes in glucocorticoid hormone production (72). This raises the question of how the differentiation pathway in preadipocytes is able to filter daily, pulsatile signals. Live cell imaging of endogenous adipogenic transcription factors CEBPB and PPARG, and staining for markers of fat cell differentiation, revealed that the transcriptional circuit in preadipocytes effectively filters out pulsatile signals, but responds to continuous signals of the same total magnitude (Figure 2C). A model predicted that such a response could be achieved if the system had both fast and slow feedback loops, and further protein and mRNA staining revealed FABP4 as a potential slow-feedback partner in the pathway (73).
Most often, protein expression is measured at the experiment's endpoint, but sometimes more frequent measurements of downstream protein expression changes are required. ERK signaling has been described as both a “persistence detector,” which drives approximately digital expression of target genes based on the duration of ERK activity (74–76), and also as a system where peak amplitude qualitatively regulates gene induction (77). Simultaneously measuring ERK activity and induction of Fra-1, a target of ERK, using live-cell reporters instead revealed that linear integration of ERK activity was the primary determinant of downstream responses (78).
Single-cell protein secretion is more challenging to measure, because of the low amounts of protein secreted and the need for isolating individual cells. One strategy for studying single cell protein secretion is to use total internal reflection microscopy to measure secreted proteins in a microwell by a sandwich immunoassay (79). This approach was used to make concurrent measurements of caspase-1 activation using a FRET reporter and IL-1β secretion in single cells. Further analysis revealed that caspase-1 activation is digital and controls a burst of IL-1β secreted from dead macrophages (33). Alternatively, multiple microfluidic strategies to combine live-cell imaging and antibody-based detection of secreted proteins have been developed (30, 31). Cells are initially captured in single cell wells, where they can be exposed to precise doses and durations of stimuli and imaged using a fluorescent microscope. The media from each cell's well can be sampled and measured with antibodies for secreted proteins at various time points during the experiment. Depending on the device, it is also possible to stain cells using immunofluorescence, allowing for both secreted and intracellular proteins to be measured (30).
Finally, changes in protein expression can also be controlled by chromatin regulators, which impart histone and DNA modifications (80, 81). It is now possible to study how single cells use different chromatin regulators to produce varying dynamics of gene expression. Bintu et al. used a doxycycline-inducible system to recruit individual chromatin regulators to regulate the expression of a fluorescent protein. They showed that epigenetic silencing and reactivation are digital processes in single cells and that different chromatin regulators modulate the fraction of cells silenced. Further, using a stochastic model, they describe the different dynamics for both silencing and reactivation, created by each chromatin regulator (82). How cells use chromatin modifications to process signal information is still poorly understood (83), but studies of single cell chromatin regulation dynamics provide a promising avenue for future research.
Experimental Strategies for Engineering or Modulating Dynamic Signaling Patterns
Studying cellular decoding is more challenging than studying cellular encoding, for technical and biological reasons. The primary biological challenge is that cells have complicated signaling pathways that interact with each other and control heterogeneous outputs. Thus, it is technically difficult to prove that the dynamics are the causative factor in the phenotypic measurement. It has also been difficult to perturb signaling dynamics in ways that would help to establish causality of phenotype, especially in single cells. Here we summarize strategies that have been successfully used to modulate signaling dynamics, as well as significant technical advances that have enabled novel ways of controlling signaling dynamics in single cells.
Engineered Dynamics Using Optogenetics and Other Synthetic Systems
Recent advancements in synthetic biology have opened exciting new ways of precisely and selectively controlling dynamic signaling. One such advancement is the field of optogenetics, which exploits light to control protein function and cell activities with high spatio-temporal resolution (Figure 3A). Optogenetic tools are generally faster and more selective than pharmacological stimulation, and their ability to generate flexible temporal patterns brought us a concept of engineering system identification to study the characteristics of cellular signaling pathway in a more direct manner. Here we introduce a few examples of optogenetic strategies applicable to signaling dynamics, but more detailed information about limitations and other applications have also been recently reviewed (84–86).
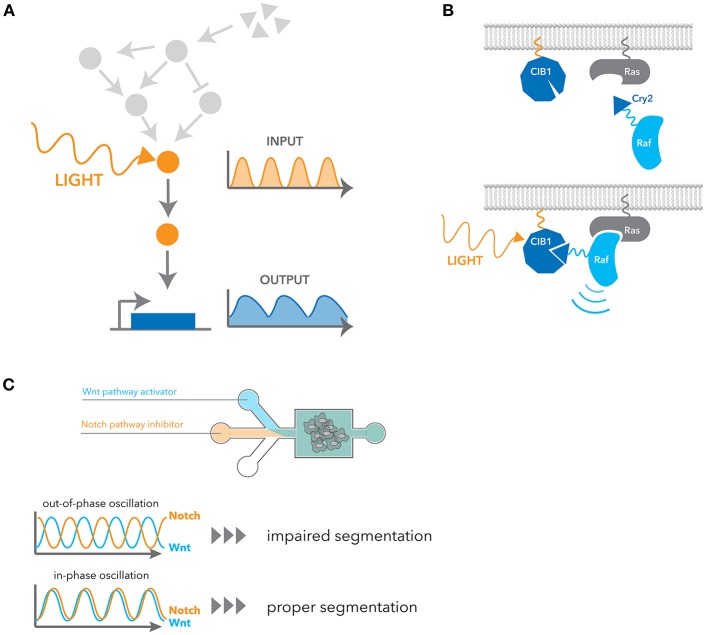
Figure 3.
Engineering approaches for manipulating dynamic signaling patterns. (A) Optogenetic tools can dynamically and selectively activate a pathway in isolation from endogenous receptor signaling contexts. (B) Blue light induces dimerization between the N-terminal CIB1 and Cry2 domain fused to cRaf, leading to the recruitment of cRaf to the membrane. Ras activates cRaf at the membrane, and thus it activates the downstream ERK pathway. (C) Microfluidic devices were used to control the flux of small-molecule inhibitors of the Notch and Wnt signaling pathway. In-phase oscillations of these two pathways led to proper mesoderm segmentation, whereas out-of-phase oscillation impaired segmentation.
One commonly used system is Phy-PIF, an optogenetic system with a fast deactivation rate. Both binding and dissociation are induced by light stimulation; red light induces binding and infrared light induces dissociation (87). OptoSOS adopts this system to activate the Ras-ERK signaling pathway by inducing translocation of SOS to the plasma membrane. It can be used to reproducibly activate the pathway using very short pulses (< 1 min) and high frequencies of activation, enabling one to measure the frequency response of the pathway (88). This system was recently used to show that a mutation in B-Raf in a human cancer line led to slower decay kinetics of the pathway, meaning that a larger space of input frequencies and strengths are interpreted as growth signals in this cell line (23).
Cry2 is another widely used optogenetic system. It has a slower activation and deactivation rate compared to other systems, but it has the unique property that it exhibits both hetero- and homo-dimerization upon blue light stimulation. Cry2 binds to the N-terminal domain of CIB1 in a blue-light dependent manner. Similarly to OptoSOS, cRaf fusion to Cry2 was used to activate the ERK pathway by inducing translocation to the plasma membrane (89) (Figure 3B). Using this system, it was shown that pulsatile ERK activation led to higher cell proliferation than sustained activation. Several genes that are induced better by pulsatile ERK activation were also identified. Photoactivation by recruiting a partner to a membrane comprises many examples such as PKA (90), AKT (26, 91) and TrkA (92). In addition to this heterodimerization between Cry2 and CIB1, Cry2 is known to have a propensity for oligomerization. This property of oligomerization was later improved with a small change in sequences (93, 94). As many signaling events are initiated by homo-oligomerization, particularly receptor signaling, they have been widely applied to many signaling pathways (95–100).
A light-oxygen-voltage-sensing (LOV) domain is a photosensory motif found in many proteins across diverse species. Blue light stimulation induces covalent bond formation between the LOV domain and its flavin cofactor, leading to a partial unfolding between the LOV domain and C-terminal A-helix. LOV domains have been engineered for many applications due to the small size of this domain (~110 amino acids). For example, a light-switchable gene promoter system was developed by fusing a fungal LOV domain and the Gal4 transcription factor lacking the dimerization domain (101). This system was also applied to control temporal patterns of proneural gene Ascl1 expression in neural progenitor cells (24), and the oscillatory and sustained expression of Ascl1 were shown to induce proliferation and differentiation, respectively.
Contrary to the examples above exploiting translocation or recruitment, there are many optogenetic tools that can directly control allostery and fragment complementation. This includes Dronpa-based strategies (25, 102), LOV domain-based proteins utilizing photo-uncaging (103–106), and a number of light-sensitive channels and receptors. For example, Hannanta-Anan and Chow used melanopsin to generate a wave of calcium release (107). By systematically controlling the calcium oscillation amplitude, frequency, and duty cycles, they found downstream NFAT integrates total elevated calcium concentrations due to its slow export rate.
Dimerization can also be induced chemically; the dimerization of FKBP and FRB with rapamycin is a classic example that has been used in a wide variety of applications for many years (108–110). Though many tools are essentially irreversible due to high affinity binding, acute induction of dimerization or translocation has been an effective strategy to investigate causation of signaling events. For instance, Santos et al. constructed the nuclear Cdk1-FKBP and cyclin B1-FRB reporters to test the spatial positive feedback regulation of Cdk1-cyclin B1 (111). The chemical dimerization of these complexes in nuclei triggered cyclin B1 nuclear translocation, which was confirmed by translocation of a fluorescent protein fused to cyclin B1, but not fused to FRB.
Another potential approach to control dynamics is to engineer a fully synthetic version of the pathway in an orthogonal cellular environment. As an example, the ERK pathway was reconstructed in yeast, and this minimal cascade itself was shown to generate ultrasensitivity (112). Recently, a synthetic NF-κB signaling pathway was introduced in yeast to study its oscillatory behavior (113). The amplitude and period of the oscillatory response to α-factor can be experimentally regulated by tuning the level of RelA from an inducible promoter, the stability of the protein, and/or the promoter strength driving the expression of the negative feedback component, IκBα. Synthetic systems provide an easy way to manipulate pathway parameters and circuit structures with small-molecule inputs.
Microfluidics Can Precisely Control Dose and Timing of Stimulus
Fluidic control was a standard approach to dynamically manipulate a stimulation pattern before genetic or synthetic approaches became popular. A simple fluidic setup with a pump has been used for several decades to control input flux, including early studies of glucagon signaling (114) and calcium signaling (115). Microfluidic devices now represent a dramatic improvement, providing us with more precise control to generate virtually any kind of temporal pattern, to study both dynamic encoding and decoding.
NF-κB activation in single cells has been well studied using such devices (116, 117). One study showed that cells respond to TNF-α in a probabilistic manner, meaning that only a fraction of cells activates the NF-κB pathway when TNF-α concentrations are low. Microfluidics-based temporal control of TNF stimulation enabled multiple discrete pulses of TNF-α to be delivered to the media. These experiments showed that variability in the NF-κB response depends not only on pre-existing cellular variability, but also on a previously unknown stochastic element (118).
In terms of dynamic decoding, the filter characteristics of the yeast stress pathway has been extensively studied using microfluidic devices (16, 119, 120). By modulating the amplitude, frequency, and duration of periodic Msn2 nuclear translocation, it was shown that each promoter transcribed by Msn2 has a distinct sensitivity to amplitude and pulse frequency. These differences can differentially regulate at least four classes of genes downstream of Msn2 (121).
Microfluidics have also been used to manipulate cellular phenotypes by controlling signaling dynamics. For example, EGF and NGF stimulation in PC12 cells activate the ERK pathway in a transient and sustained manner, respectively, leading to different cellular outcomes. With a microfluidic device, Ryu et al. inverted the outcomes from each growth factors simply by changing the stimulus patterns (28). As another example, Sonnen et al. observed that the segmentation of the presomitic mesoderm is dependent on relative timing between Wnt and Notch signaling oscillations (29). They used microfluidics to generate either in-phase or out-of-phase oscillations of these two pathways and showed the out-of-phase oscillations impair segmentation (Figure 3C).
Technical Improvements in High Throughput Single Cell Measurements
Many of the examples above show the versatility and capability of microscopy to interrogate relationships between signaling dynamics and downstream phenotypes in single cells. It is becoming clear that signaling dynamics can be decoded to distinct gene expression programs leading to diverse cellular phenotypes; yet most studies were only able to measure a few genes due to technical challenges. Here we will go over some of the recent technical advancements that potentially expand the throughput and accessibility of this measurement modality.
As we saw in the examples above, FISH and immunofluorescence are commonly used techniques for capturing downstream responses. FISH can be implemented in a high-throughput manner, as one can strip or bleach probes and thus iterate detection (122–124). The downside of these techniques is their cost and sensitivity, since many probes are required to bind one species of mRNA and thereby amplify a specific signal. Two recently developed methods utilize different signal amplification schemes, enabling higher sensitivity and gain. The first method is proximity ligation in situ hybridization technology (PLISH) (125). PLISH amplifies a target region by rolling circle amplification after generating closed circle probe oligonucleotides by RNA-templated proximity ligation. The second technique, called click-amplifying FISH (clampFISH), uses non-enzymatic click chemistry to generate closed circle oligonucleotides (126, 127). Both techniques were shown to provide better fluorescence signals in both cell culture and tissue samples (126).
Similar to high-throughput FISH approaches, there are methods which attempt to determine the amount of many specific proteins via immunofluorescence over multiple cycles. For example, Lin et al. developed cycIF by bleaching a fluorophore conjugated with a primary antibody with hydrogen peroxide (128). They were able to detect 60 proteins in a tumor tissue sample by repeating the bleaching and staining steps for each protein of interest (129). Another group developed the antibody elution method called 4i which elutes antibodies with a mix of reducing agent, low pH and chaotropic salts, and a blocking buffer (130). This is compatible with indirect immunofluorescence, and 40 proteins were detected with this approach. A similar approach, co-detection by indexing (CODEX), uses oligonucleotide conjugated antibodies (131). Instead of repeating antibody binding steps, this method first carries out the binding process, followed by repeated detection of the barcoded antibodies by incorporating fluorophore-labeled nucleotides by polymerase.
These methods rely on fluorescence detection and thereby the number of detections at a time is limited by the spectral overlap. In contrast to these approaches, imaging mass cytometry and multiplexed ion beam imaging use metal-labeled antibodies (132–134). The signal from the metal isotopes are measured via mass spectrometry, allowing simultaneous detection of more proteins than would be possible by fluorescence. Both methods were able to measure more than 30 proteins from tumor samples (135, 136), and it can be also combined with high-throughput FISH methods (137).
For live-cell image analysis, computational automation is increasingly a requirement due to the amount of data that can readily be acquired. One of the recent breakthroughs in this area involves deep learning. Convolutional neural networks were first applied to classification of histopathologic images for a diagnostic purpose (138–140). In 2016, a software tool called DeepCell employed this type of classification task for automated image segmentation of cells (141). In addition to a higher segmentation accuracy for fluorescent images, this deep learning-based approach also allows us to segment objects using a non-labeled image such as phase-contrast or DIC images (142–144).
Concluding Remarks
The capability and compatibility of single-cell measurement techniques has advanced significantly in the last several years. It is now possible to measure signaling pathway activation and RNA, protein or metabolite levels in single cells, often in real time. The throughput of these measurements is also increasing rapidly, especially with the development of better computational techniques for image analysis and iterative FISH and immunofluorescence approaches. Moreover, our ability to engineer single cell dynamics is also rapidly improving with the development of techniques such as optogenetics. As a result, studies describing how individual cells respond to inputs using dynamic patterns have expanded into new systems and levels of detail.
Practically, these new discoveries may reveal ways to target signaling dynamics in disease contexts, potentially leading to novel treatments (145). Pharmacologically altered signaling dynamics have only been demonstrated in a few studies, but this may change with the development of better tools and knowledge (64, 146). Additionally, better understanding of dynamic cellular responses can help lead the way to cells with functional engineered signaling circuits, which have large potential as possible therapeutics and scientific tools (147).
Nonetheless, this field is still in the early stages and many challenges remain to be addressed. Reporter development remains a challenging problem, and thus reporters currently exist for only a small subset of pathways. Furthermore, most measurements of signaling pathways continue to rely on exogenous reporters, which can differ from the responses seen with endogenous proteins. While work has been done to make it easier to directly measure endogenous proteins, these methods still remain more difficult than using exogenous reporters. Finally, large-scale genetic screens have enabled new levels of understanding in many fields through the ability to search for important effectors across the entire genome. However, using microscopy in conjunction with genome-wide screens is still exceedingly challenging because of the need to connect the measurements made using microscopy to the genetic perturbation in each cell. Thus, there is still much room for improvement in both the techniques available to the study of dynamics, as well as the number of systems that these techniques can be applied to.
Author Contributions
SJ, TK, and MC designed and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank members of the Covert lab for helpful discussions and feedback on the manuscript.
Footnotes
Funding. The authors gratefully acknowledge funding from several sources, including an Allen Discovery Center Award and an Allen Distinguished Investigator Award awarded to MC, the Nakajima Foundation Scholarship awarded to TK, and funding from the National Institutes of Health (NIH) NIGMS Training Grant in Biotechnology (5T32GM008412) awarded to SJ.
References
- 1.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. (2010) 467:167–73. 10.1038/nature09326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huveneers S, Danen EHJ. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. (2009) 122:1059–69. 10.1242/jcs.039446 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. (2011) 34:637–50. 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 4.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. (2011) 12:695–708. 10.1038/ni.2065 [DOI] [PubMed] [Google Scholar]
- 5.Hansen AS, O'Shea EK. Limits on information transduction through amplitude and frequency regulation of transcription factor activity. Elife. (2015) 4:e06559. 10.7554/eLife.06559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selimkhanov J, Taylor B, Yao J, Pilko A, Albeck J, Hoffmann A, et al. Accurate information transmission through dynamic biochemical signaling networks. Science. (2014) 346:1370–3. 10.1126/science.1254933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Gupta S, Schipper DL, Kowalczyk GJ, Mancini AE, Faeder JR, et al. NF-κB dynamics discriminate between TNF doses in single cells. Cell Syst. (2017) 5:638–645.e5. 10.1016/j.cels.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. (1995) 80:179–85. 10.1016/0092-8674(95)90401-8 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TT, Scimeca JC, Filloux C, Peraldi P, Carpentier JL, Van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem. (1993) 268:9803–10. [PubMed] [Google Scholar]
- 10.Santos SDM, Verveer PJ, Bastiaens PIH. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol. (2007) 9:324–30. 10.1038/ncb1543 [DOI] [PubMed] [Google Scholar]
- 11.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science. (2005) 309:1854–7. 10.1126/science.1112304 [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. (2002) 298:1241–5. 10.1126/science.1071914 [DOI] [PubMed] [Google Scholar]
- 13.Nelson DE, Ihekwaba AEC, Elliott M, Johnson JR, Gibney CA, Foreman BE, et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science. (2004) 306:704–8. 10.1126/science.1099962 [DOI] [PubMed] [Google Scholar]
- 14.Batchelor E, Loewer A, Mock C, Lahav G. Stimulus-dependent dynamics of p53 in single cells. Mol Syst Biol. (2011) 7:488. 10.1038/msb.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, et al. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet. (2004) 36:147–50. 10.1038/ng1293 [DOI] [PubMed] [Google Scholar]
- 16.Hao N, O'Shea EK. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat Struct Mol Biol. (2012) 19:31–9. 10.1038/nsmb.2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandagopal N, Santat LA, LeBon L, Sprinzak D, Bronner ME, Elowitz MB. Dynamic ligand discrimination in the Notch signaling pathway. Cell. (2018) 172:869–880.e19. 10.1016/j.cell.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutschow MV, Mason JC, Lane KM, Maayan I, Hughey JJ, Bajar BT, et al. Combinatorial processing of bacterial and host-derived innate immune stimuli at the single-cell level. Mol Biol Cell. (2019) 30:282–92. 10.1091/mbc.E18-07-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellogg RA, Tian C, Etzrodt M, Tay S. Cellular decision making by non-integrative processing of TLR inputs. Cell Rep. (2017) 19:125–35. 10.1016/j.celrep.2017.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behar M, Hoffmann A. Understanding the temporal codes of intra-cellular signals. Curr Opin Genet Dev. (2010) 20:684–93. 10.1016/j.gde.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. (2010) 11:414–26. 10.1038/nrm2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purvis JE, Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell. (2013) 152:945–56. 10.1016/j.cell.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bugaj LJ, Sabnis AJ, Mitchell A, Garbarino JE, Toettcher JE, Bivona TG, et al. Cancer mutations and targeted drugs can disrupt dynamic signal encoding by the Ras-Erk pathway. Science. (2018) 361:eaao3048. 10.1126/science.aao3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. (2013) 342:1203–8. 10.1126/science.1242366 [DOI] [PubMed] [Google Scholar]
- 25.Zhou XX, Fan LZ, Li P, Shen K, Lin MZ. Optical control of cell signaling by single-chain photoswitchable kinases. Science. (2017) 355:836–42. 10.1126/science.aah3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Wang J, Chen J, Qi Y, Nan D, Jin L, et al. Optogenetic control of epithelial-mesenchymal transition in cancer cells. Sci Rep. (2018) 8:14098. 10.1038/s41598-018-32539-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellogg RA, Gómez-Sjöberg R, Leyrat AA, Tay S. High-throughput microfluidic single-cell analysis pipeline for studies of signaling dynamics. Nat Protoc. (2014) 9:1713–26. 10.1038/nprot.2014.120 [DOI] [PubMed] [Google Scholar]
- 28.Ryu H, Chung M, Dobrzyński M, Fey D, Blum Y, Sik Lee S, et al. Frequency modulation of ERK activation dynamics rewires cell fate. Mol Syst Biol. (2015) 11:838. 10.15252/msb.20156458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnen KF, Lauschke VM, Uraji J, Falk HJ, Petersen Y, Funk MC, et al. Modulation of phase shift between Wnt and Notch signaling oscillations controls mesoderm segmentation. Cell. (2018) 172:1079–1090.e12. 10.1016/j.cell.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junkin M, Kaestli AJ, Cheng Z, Jordi C, Albayrak C, Hoffmann A, et al. High-content quantification of single-cell immune dynamics. Cell Rep. (2016) 15:411–22. 10.1016/j.celrep.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaestli AJ, Junkin M, Tay S. Integrated platform for cell culture and dynamic quantification of cell secretion. Lab Chip. (2017) 17:4124–33. 10.1039/C7LC00839B [DOI] [PubMed] [Google Scholar]
- 32.Lane K, Van Valen D, DeFelice MM, Macklin DN, Kudo T, Jaimovich A, et al. Measuring signaling and RNA-seq in the same cell links gene expression to dynamic patterns of NF-κB activation. Cell Syst. (2017) 4:458–469.e5. 10.1016/j.cels.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Yamaguchi Y, Shirasaki Y, Shikada K, Yamagishi M, Hoshino K, et al. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep. (2014) 8:974–82. 10.1016/j.celrep.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 34.Kudo T, Jeknić S, Macklin DN, Akhter S, Hughey JJ, Regot S, et al. Live-cell measurements of kinase activity in single cells using translocation reporters. Nat Protoc. (2018) 13:155–69. 10.1038/nprot.2017.128 [DOI] [PubMed] [Google Scholar]
- 35.Lane K, Andres-Terre M, Kudo T, Monack DM, Covert MW. Escalating threat levels of bacterial infection can be discriminated by distinct MAPK and NF-κB signaling dynamics in single host cells. Cell Syst. (2019) 8:183–96. 10.1016/j.cels.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 36.Murai S, Yamaguchi Y, Shirasaki Y, Yamagishi M, Shindo R, Hildebrand JM, et al. A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs. Nat Commun. (2018) 9:4457. 10.1038/s41467-018-06985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW. High-sensitivity measurements of multiple kinase activities in live single cells. Cell. (2014) 157:1724–34. 10.1016/j.cell.2014.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart-Ornstein J, Lahav G. Dynamics of CDKN1A in single cells defined by an endogenous fluorescent tagging toolkit. Cell Rep. (2016) 14:1800–11. 10.1016/j.celrep.2016.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strasen J, Sarma U, Jentsch M, Bohn S, Sheng C, Horbelt D, et al. Cell-specific responses to the cytokine TGFβ are determined by variability in protein levels. Mol Syst Biol. (2018) 14:e7733. 10.15252/msb.20177733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yissachar N, Fischler TS, Cohen AA, Reich-Zeliger S, Russ D, Shifrut E, et al. Dynamic response diversity of NFAT isoforms in individual living cells. Mol Cell. (2013) 49:322–30. 10.1016/j.molcel.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, Huang H, Zhang L, Wu R, Chung C-I, Zhang S-Q, et al. Visualizing dynamics of cell signaling in vivo with a phase separation-based kinase reporter. Mol Cell. (2018) 69:334–346.e4. 10.1016/j.molcel.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai TY-C, Theriot JA, Ferrell JE, Jr. Changes in oscillatory dynamics in the cell cycle of early Xenopus laevis embryos. PLoS Biol. (2014) 12:e1001788. 10.1371/journal.pbio.1001788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. (2005) 309:1857–61. 10.1126/science.1113319 [DOI] [PubMed] [Google Scholar]
- 44.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. (2008) 5:877–9. 10.1038/nmeth.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. (2008) 7:979–87. 10.1038/nrd2656 [DOI] [PubMed] [Google Scholar]
- 46.Kracikova M, Akiri G, George A, Sachidanandam R, Aaronson SA. A threshold mechanism mediates p53 cell fate decision between growth arrest and apoptosis. Cell Death Differ. (2013) 20:576–88. 10.1038/cdd.2012.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paek AL, Liu JC, Loewer A, Forrester WC, Lahav G. Cell-to-cell variation in p53 dynamics leads to fractional killing. Cell. (2016) 165:631–42. 10.1016/j.cell.2016.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. (2003) 114:181–90. 10.1016/S0092-8674(03)00521-X [DOI] [PubMed] [Google Scholar]
- 49.Lee REC, Qasaimeh MA, Xia X, Juncker D, Gaudet S. NF-κB signalling and cell fate decisions in response to a short pulse of tumour necrosis factor. Sci Rep. (2016) 6:39519. 10.1038/srep39519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. Identification of direct genomic targets downstream of the nuclear factor-κB transcription factor mediating tumor necrosis factor signaling. J Biol Chem. (2005) 280:17435–48. 10.1074/jbc.M500437200 [DOI] [PubMed] [Google Scholar]
- 51.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. (2003) 10:45–65. 10.1038/sj.cdd.4401189 [DOI] [PubMed] [Google Scholar]
- 52.Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. (2007) 1773:1311–40. 10.1016/j.bbamcr.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merlini L, Dudin O, Martin SG. Mate and fuse: how yeast cells do it. Open Biol. (2013) 3:130008. 10.1098/rsob.130008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conlon P, Gelin-Licht R, Ganesan A, Zhang J, Levchenko A. Single-cell dynamics and variability of MAPK activity in a yeast differentiation pathway. Proc Natl Acad Sci USA. (2016) 113:E5896–E5905. 10.1073/pnas.1610081113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. (2009) 6:10–21. 10.1016/j.chom.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spencer SL, Sorger PK. Measuring and modeling apoptosis in single cells. Cell. (2011) 144:926–39. 10.1016/j.cell.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. (2008) 132:487–98. 10.1016/j.cell.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 58.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. (2013) 49:249–61. 10.1016/j.molcel.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccinini F, Balassa T, Szkalisity A, Molnar C, Paavolainen L, Kujala K, et al. Advanced cell classifier: user-friendly machine-learning-based software for discovering phenotypes in high-content imaging data. Cell Syst. (2017) 4:651–655.e5. 10.1016/j.cels.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 60.Cheong R, Rhee A, Wang CJ, Nemenman I, Levchenko A. Information transduction capacity of noisy biochemical signaling networks. Science. (2011) 334:354–8. 10.1126/science.1204553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee REC, Walker SR, Savery K, Frank DA, Gaudet S. Fold change of nuclear NF-κB determines TNF-induced transcription in single cells. Mol Cell. (2014) 53:867–79. 10.1016/j.molcel.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong VC, Mathew S, Ramji R, Gaudet S, Miller-Jensen K. Fold-change detection of NF-κB at target genes with different transcript outputs. Biophys J. (2019) 116:709–24. 10.1016/j.bpj.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frick CL, Yarka C, Nunns H, Goentoro L. Sensing relative signal in the Tgf-β/Smad pathway. Proc Natl Acad Sci USA. (2017) 114:E2975–E2982. 10.1073/pnas.1611428114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. (2012) 336:1440–4. 10.1126/science.1218351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang HW, Chung M, Kudo T, Meyer T. Competing memories of mitogen and p53 signalling control cell-cycle entry. Nature. (2017) 549:404–8. 10.1038/nature23880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Margolis DM, Garcia JV, Hazuda DJ, Haynes BF. Latency reversal and viral clearance to cure HIV-1. Science. (2016) 353:aaf6517. 10.1126/science.aaf6517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. (2013) 155:540–51. 10.1016/j.cell.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong VC, Bass VL, Elise Bullock M, Chavali AK, Lee REC, Mothes W, et al. NF-κB-chromatin interactions drive diverse phenotypes by modulating transcriptional noise. Cell Rep. (2018) 22:585–99. 10.1016/j.celrep.2017.12.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rios AC, Serralbo O, Salgado D, Marcelle C. Neural crest regulates myogenesis through the transient activation of NOTCH. Nature. (2011) 473:532–5. 10.1038/nature09970 [DOI] [PubMed] [Google Scholar]
- 70.Lee TK, Denny EM, Sanghvi JC, Gaston JE, Maynard ND, Hughey JJ, et al. A noisy paracrine signal determines the cellular NF-κB response to lipopolysaccharide. Sci Signal. (2009) 2:ra65. 10.1126/scisignal.2000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sung M-H, Li N, Lao Q, Gottschalk RA, Hager GL, Fraser IDC. Switching of the relative dominance between feedback mechanisms in lipopolysaccharide-induced NF-κB signaling. Sci Signal. (2014) 7:ra6. 10.1126/scisignal.2004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. (2008) 453:783–7. 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- 73.Bahrami-Nejad Z, Zhao ML, Tholen S, Hunerdosse D, Tkach KE, van Schie S, et al. A transcriptional circuit filters oscillating circadian hormonal inputs to regulate fat cell differentiation. Cell Metab. (2018) 27:854–868.e8. 10.1016/j.cmet.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mackeigan JP, Murphy LO, Dimitri CA, Blenis J. Graded mitogen-activated protein kinase activity precedes switch-like c-Fos induction in mammalian cells. Mol Cell Biol. (2005) 25:4676–82. 10.1128/MCB.25.11.4676-4682.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphy LO, Smith S, Chen R-H, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. (2002) 4:556–64. 10.1038/ncb822 [DOI] [PubMed] [Google Scholar]
- 76.Nakakuki T, Birtwistle MR, Saeki Y, Yumoto N, Ide K, Nagashima T, et al. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell. (2010) 141:884–96. 10.1016/j.cell.2010.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. (2012) 18:1503–10. 10.1038/nm.2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gillies TE, Pargett M, Minguet M, Davies AE, Albeck JG. Linear integration of ERK activity predominates over persistence detection in Fra-1 regulation. Cell Syst. (2017) 5:549–563.e5. 10.1016/j.cels.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shirasaki Y, Yamagishi M, Suzuki N, Izawa K, Nakahara A, Mizuno J, et al. Real-time single-cell imaging of protein secretion. Sci Rep. (2014) 4:4736. 10.1038/srep04736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kouzarides T. Chromatin modifications and their function. Cell. (2007) 128:693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 81.Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. (2011) 12:7–18. 10.1038/nrg2905 [DOI] [PubMed] [Google Scholar]
- 82.Bintu L, Yong J, Antebi YE, McCue K, Kazuki Y, Uno N, et al. Dynamics of epigenetic regulation at the single-cell level. Science. (2016) 351:720–4. 10.1126/science.aab2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol. (2013) 14:211–24. 10.1038/nrm3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Repina NA, Rosenbloom A, Mukherjee A, Schaffer DV, Kane RS. At light speed: advances in optogenetic systems for regulating cell signaling and behavior. Annu Rev Chem Biomol Eng. (2017) 8:13–39. 10.1146/annurev-chembioeng-060816-101254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rost BR, Schneider-Warme F, Schmitz D, Hegemann P. Optogenetic tools for subcellular applications in neuroscience. Neuron. (2017) 96:572–603. 10.1016/j.neuron.2017.09.047 [DOI] [PubMed] [Google Scholar]
- 86.Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. (2015) 33:92–100. 10.1016/j.tibtech.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. (2009) 461:997–1001. 10.1038/nature08446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell. (2013) 155:1422–34. 10.1016/j.cell.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aoki K, Kumagai Y, Sakurai A, Komatsu N, Fujita Y, Shionyu C, et al. Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Mol Cell. (2013) 52:529–40. 10.1016/j.molcel.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 90.O'Banion CP, Priestman MA, Hughes RM, Herring LE, Capuzzi SJ, Lawrence DS. Design and profiling of a subcellular targeted optogenetic cAMP-dependent protein kinase. Cell Chem Biol. (2018) 25:100–109.e8. 10.1016/j.chembiol.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katsura Y, Kubota H, Kunida K, Kanno A, Kuroda S, Ozawa T. An optogenetic system for interrogating the temporal dynamics of Akt. Sci Rep. (2015) 5:14589. 10.1038/srep14589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duan L, Hope JM, Guo S, Ong Q, François A, Kaplan L, et al. Optical activation of TrkA signaling. ACS Synth Biol. (2018) 7:1685–93. 10.1021/acssynbio.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park H, Kim NY, Lee S, Kim N, Kim J, Heo WD. Optogenetic protein clustering through fluorescent protein tagging and extension of CRY2. Nat Commun. (2017) 8:30. 10.1038/s41467-017-00060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, et al. An optimized optogenetic clustering tool for probing protein interaction and function. Nat Commun. (2014) 5:4925. 10.1038/ncomms5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alapin JM, Dines M, Vassiliev M, Tamir T, Ram A, Locke C, et al. Activation of EphB2 forward signaling enhances memory consolidation. Cell Rep. (2018) 23:2014–25. 10.1016/j.celrep.2018.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Endo M, Hattori M, Toriyabe H, Ohno H, Kamiguchi H, Iino Y, et al. Optogenetic activation of axon guidance receptors controls direction of neurite outgrowth. Sci Rep. (2016) 6:23976. 10.1038/srep23976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fischer A, Warscheid B, Weber W, Radziwill G. Optogenetic clustering of CNK1 reveals mechanistic insights in RAF and AKT signalling controlling cell fate decisions. Sci Rep. (2016) 6:38155. 10.1038/srep38155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim N, Park H, Woo D, Do Heo W. Building light-inducible receptor tyrosine kinases. In: Vriz S, Ozawa T. editors. Optogenetics: Light-driven Actuators and Light-emitting Sensors in Cell Biology. London: The Royal Society of Chemistry; (2018). p. 181–96. [Google Scholar]
- 99.Kyung T, Lee S, Kim JE, Cho T, Park H, Jeong Y-M, et al. Optogenetic control of endogenous Ca2+ channels in vivo. Nat Biotechnol. (2015) 33:1092–6. 10.1038/nbt.3350 [DOI] [PubMed] [Google Scholar]
- 100.Wang L, Cooper JA. Optogenetic control of the Dab1 signaling pathway. Sci Rep. (2017) 7:43760. 10.1038/srep43760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods. (2012) 9:266–9. 10.1038/nmeth.1892 [DOI] [PubMed] [Google Scholar]
- 102.Zhou XX, Chung HK, Lam AJ, Lin MZ. Optical control of protein activity by fluorescent protein domains. Science. (2012) 338:810–4. 10.1126/science.1226854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cosentino C, Alberio L, Gazzarrini S, Aquila M, Romano E, Cermenati S, et al. Engineering of a light-gated potassium channel. Science. (2015) 348:707–10. 10.1126/science.aaa2787 [DOI] [PubMed] [Google Scholar]
- 104.Gehrig S, Macpherson JA, Driscoll PC, Symon A, Martin SR, MacRae JI, et al. An engineered photoswitchable mammalian pyruvate kinase. FEBS J. (2017) 284:2955–80. 10.1111/febs.14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oakes PW, Wagner E, Brand CA, Probst D, Linke M, Schwarz US, et al. Optogenetic control of RhoA reveals zyxin-mediated elasticity of stress fibres. Nat Commun. (2017) 8:15817. 10.1038/ncomms15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. (2009) 461:104–8. 10.1038/nature08241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hannanta-Anan P, Chow BY. Optogenetic control of calcium oscillation waveform defines NFAT as an integrator of calcium load. Cell Syst. (2016) 2:283–8. 10.1016/j.cels.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castellano F, Montcourrier P, Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. (2000) 113:2955–61. Available online at: http://jcs.biologists.org/content/113/17/2955 [DOI] [PubMed] [Google Scholar]
- 109.DeRose R, Miyamoto T, Inoue T. Manipulating signaling at will: chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflügers Arch. (2013) 465:409–17. 10.1007/s00424-012-1208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods. (2005) 2:415–8. 10.1038/nmeth763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santos SDM, Wollman R, Meyer T, Ferrell JE, Jr. Spatial positive feedback at the onset of mitosis. Cell. (2012) 149:1500–13. 10.1016/j.cell.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Shaughnessy EC, Palani S, Collins JJ, Sarkar CA. Tunable signal processing in synthetic MAP kinase cascades. Cell. (2011) 144:119–31. 10.1016/j.cell.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Z-B, Wang Q-Y, Ke Y-X, Liu S-Y, Ju J-Q, Lim WA, et al. Design of tunable oscillatory dynamics in a synthetic NF-κB signaling circuit. Cell Syst. (2017) 5:460–470.e5. 10.1016/j.cels.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 114.Weigle DS, Koerker DJ, Goodner CJ. Pulsatile glucagon delivery enhances glucose production by perifused rat hepatocytes. Am J Physiol Endoc M. (1984) 247:E564–E568. 10.1152/ajpendo.1984.247.4.E564 [DOI] [PubMed] [Google Scholar]
- 115.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. (1998) 392:933–6. 10.1038/31960 [DOI] [PubMed] [Google Scholar]
- 116.Heltberg M, Kellogg RA, Krishna S, Tay S, Jensen MH. Noise induces hopping between NF-κB entrainment modes. Cell Syst. (2016) 3:532–539.e3. 10.1016/j.cels.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kellogg RA, Tay S. Noise facilitates transcriptional control under dynamic inputs. Cell. (2015) 160:381–92. 10.1016/j.cell.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 118.Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-κB dynamics reveal digital activation and analogue information processing. Nature. (2010) 466:267–71. 10.1038/nature09145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hansen AS, O'Shea EK. Promoter decoding of transcription factor dynamics involves a trade-off between noise and control of gene expression. Mol Syst Biol. (2013) 9:704. 10.1038/msb.2013.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hansen AS, O'Shea EK. cis Determinants of promoter threshold and activation timescale. Cell Rep. (2015) 12:1226–33. 10.1016/j.celrep.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 121.Hansen AS, O'Shea EK. Encoding four gene expression programs in the activation dynamics of a single transcription factor. Curr Biol. (2016) 26:R269–71. 10.1016/j.cub.2016.02.058 [DOI] [PubMed] [Google Scholar]
- 122.Battich N, Stoeger T, Pelkmans L. Control of transcript variability in single mammalian cells. Cell. (2015) 163:1596–610. 10.1016/j.cell.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 123.Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. (2015) 348:eaaa6090. 10.1126/science.aaa6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lubeck E, Coskun AF, Zhiyentayev T, Ahmad M, Cai L. Single-cell in situ RNA profiling by sequential hybridization. Nat Methods. (2014) 11:360–1. 10.1038/nmeth.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagendran M, Riordan DP, Harbury PB, Desai TJ. Automated cell-type classification in intact tissues by single-cell molecular profiling. Elife. (2018) 7:e30510. 10.7554/eLife.30510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. (2018) 359:1118–23. 10.1126/science.aam6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rouhanifard SH, Mellis IA, Dunagin M, Bayatpour S, Jiang CL, Dardani I, et al. ClampFISH detects individual nucleic acid molecules using click chemistry–based amplification. Nat Biotechnol. (2018) 37:84–9. 10.1038/nbt.4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin J-R, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun. (2015) 6:8390. 10.1038/ncomms9390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin J-R, Izar B, Mei S, Wang S, Shah P, Yapp C, et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. Elife. (2018) 7:e31657. 10.7554/eLife.31657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gut G, Herrmann MD, Pelkmans L. Multiplexed protein maps link subcellular organization to cellular states. Science. (2018) 361:eaar7042. 10.1126/science.aar7042 [DOI] [PubMed] [Google Scholar]
- 131.Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. (2018) 174:968–81. 10.1016/j.cell.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. (2014) 20:436–42. 10.1038/nm.3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bodenmiller B, Zunder ER, Finck R, Chen TJ, Savig ES, Bruggner RV. Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat Biotechnol. (2012) 30:858–67. 10.1038/nbt.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bodenmiller B. Multiplexed epitope-based tissue imaging for discovery and healthcare applications. Cell Syst. (2016) 2:225–38. 10.1016/j.cels.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 135.Giesen C, Wang HAO, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. (2014) 11:417–22. 10.1038/nmeth.2869 [DOI] [PubMed] [Google Scholar]
- 136.Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. (2018) 174:1373–87. 10.1016/j.cell.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schulz D, Zanotelli VRT, Fischer JR, Schapiro D, Engler S, Lun X-K, et al. Simultaneous multiplexed imaging of mRNA and proteins with subcellular resolution in breast cancer tissue samples by mass cytometry. Cell Syst. (2018) 6:25–36. 10.1016/j.cels.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen W, Zhou M, Yang F, Yang C, Tian J. Multi-scale convolutional neural networks for lung nodule classification. In: Ourselin S, Alexander D, Westin CF, Cardoso M. editors. Information Processing in Medical Imaging. Cham: Springer; (2015). p. 588–99. [DOI] [PubMed] [Google Scholar]
- 139.Wang H, Cruz-Roa A, Basavanhally A, Gilmore H, Shih N, Feldman M, et al. Mitosis detection in breast cancer pathology images by combining handcrafted and convolutional neural network features. J Med Imaging. (2014) 1:034003. 10.1117/1.JMI.1.3.034003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen X, Xu Y, Kee Wong DW, Wong TY, Liu J. Glaucoma detection based on deep convolutional neural network. Conf Proc IEEE Eng Med Biol Soc. (2015) 2015:715–8. 10.1109/EMBC.2015.7318462 [DOI] [PubMed] [Google Scholar]
- 141.Van Valen DA, Kudo T, Lane KM, Macklin DN, Quach NT, DeFelice MM, et al. Deep learning automates the quantitative analysis of individual cells in live-cell imaging experiments. PLoS Comput Biol. (2016) 12:e1005177. 10.1371/journal.pcbi.1005177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Christiansen EM, Yang SJ, Ando DM, Javaherian A, Skibinski G, Lipnick S, et al. In silico labeling: predicting fluorescent labels in unlabeled images. Cell. (2018) 173:792–803.e19. 10.1016/j.cell.2018.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Falk T, Mai D, Bensch R, Çiçek Ö, Abdulkadir A, Marrakchi Y, et al. U-Net: deep learning for cell counting, detection, and morphometry. Nat Methods. (2019) 16:67–70. 10.1038/s41592-018-0261-2 [DOI] [PubMed] [Google Scholar]
- 144.Sadanandan SK, Ranefall P, Le Guyader S, Wählby C. Automated training of deep convolutional neural networks for cell segmentation. Sci Rep. (2017) 7:7860. 10.1038/s41598-017-07599-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Behar M, Barken D, Werner SL, Hoffmann A. The dynamics of signaling as a pharmacological target. Cell. (2013) 155:448–61. 10.1016/j.cell.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sung M-H, Bagain L, Chen Z, Karpova T, Yang X, Silvin C, et al. Dynamic effect of bortezomib on nuclear factor- κB activity and gene expression in tumor cells. Mol Pharmacol. (2008) 74:1215–22. 10.1124/mol.108.049114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Bio. (2010) 11:393–403. 10.1038/nrm2904 [DOI] [PMC free article] [PubMed] [Google Scholar]