Abstract
Background
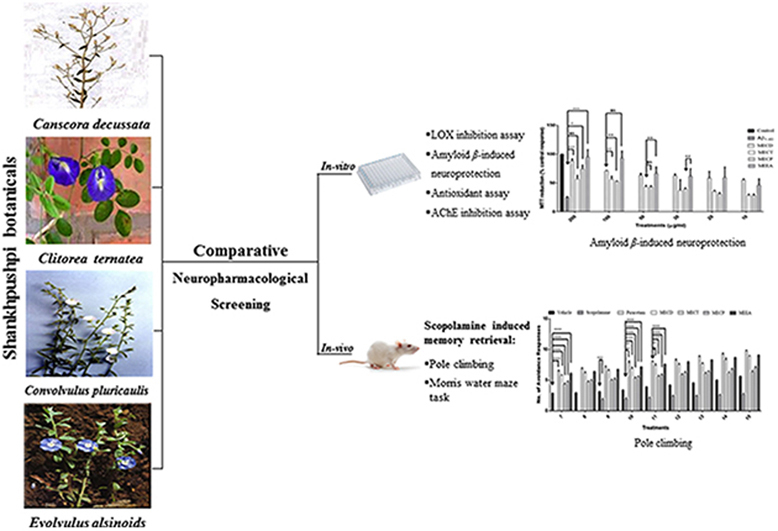
Shankhpushpi is an Ayurvedic drug, widely used for its actions on the central nervous system, especially to improve intellect and boost memory. Four botanicals viz. Canscora decussata Schult. (CD), Clitorea ternatea Linn. (CT), Convolvulus pluricaulis Choisy. (CP) and Evolvulus alsinoides Linn. (EA) are considered as sources of Shankhpushpi by Indian practitioners on the basis of their morphological descriptions given in ancient texts.
Objective
The present study was undertaken to evaluate the neuropharmacological effect of four herbs commonly identified as source of Shankhpushpi.
Materials and methods
Methanol extracts of all four varieties were tested and evaluated in vitro and in vivo for their neuropharmacological effects. Experiments such as protection against β-amyloid induced neurotoxicity on brain cell line (Neuro 2A), antioxidant potential, AchE (acetylcholinesterase enzyme) inhibition, and 5-LOX (lipoxygenase) enzyme inhibition were conducted for in vitro evaluation. For in vivo evaluation, scopolamine (0.3 mg/kg i.p.) induced memory retrieval using pole climbing apparatus and Morris water maze were performed in rat models.
Results
It was found that protective effects of EA and CD against β-amyloid induced neurotoxicity in Neuro 2A cells were significantly higher than CT and CP. EA proved to be superior than other varieties on the basis of antioxidant activity, AchE inhibitory and LOX inhibitory activities. The preventive activity of EA on scopolamine induced memory retrieval in pole climbing and Morris water maze task in rats was found to be higher than that of CD, CT and CP.
Conclusion
EA has remarkable neuropharmacological effect as compared to other three varieties of Shankhpushpi. This effect may be attributed due to the presence of steroids (stigmasterol and betulinic acid), coumarins (scopoletin) and flavonoids (β-carotene and chlorogenic acid). Hence it can be used as a promising lead in development and management of neuronal disorders including Alzheimer's disease.
Keywords: Neuroprotection, Lipoxygenase, Enzyme inhibition, Memory, Shankhpushpi
Graphical abstract
1. Introduction
Shankhpushpi has been recognized in Ayurveda as ‘Medhya Rasayana’– meaning a drug which rejuvenates, maintains and potentiates intellect and memory. On the basis of flower shape and their ability to treat memory related dysfunctions, four botanicals viz. Canscora decussata Schult. (CD) (Gentianaceae), Clitorea ternatea Linn. (CT) (Leguminosae), Convolvulus pluricaulis Choisy. (CP) (Convulvulaceae), and Evolvulus alsinoides Linn. (EA) (Convulvulaceae) are considered as Shankhpushpi by Indian practitioners [1], [2].
Different varieties of Shankhpushpi have proved their curative potential individually in studies related to CNS depression [3], [4], anxiolytic [5], anti-amensic [6], [7], tranquilizing, anti-depressant, anti-stress, neurodegenerative, antioxidant, hypolipidemic, immunomodulatory, analgesic, anti-fungal, anti-bacterial, anti-diabetic, anti-ulcer, anti-catatonic and cardiovascular activity. Several types of alkaloids, terpenoids, phenolics, flavonoids and coumarins are reported as active chemicals responsible for biological effects [8].
Shankhpushpi (in various dosage forms) is one of the widely marketed herbal products in India for boosting memory and intellect [9]. Controversy arises due to synonymous names of different available varieties often leading to selection of plant material with poor neuropharmacological activity, which results a less potent formulation. There are some previous studies as well, related to simultaneous evaluation of nootropic, anxiolytic and CNS-depressant activities on two or three varieties of Shankhpushpi [10], [11], [12], [13], [14], [15]. Moreover, authors have also done similar studies in the past focusing on anxiolytic and memory enhancement activities of different varieties of Shankhpushpi [4], [5], [6], [7]. However, till date none of the studies (including our previous studies) simultaneously evaluated the complete neuropharmacological profile of four varieties, which has been done in the present work by the authors. This helped us to arrive at a conclusion as to which of them is the best in terms of neuropharmacological activity. Various parameters such as in vitro protection against β-amyloid induced neurotoxicity on brain cell line (Neuro 2A), antioxidant potential, AchE inhibition, 5-LOX enzyme inhibition, and in vivo experiments such as scopolamine induced memory retrieval using pole climbing apparatus and Morris water maze task in rats were performed.
2. Materials and methods
2.1. Chemicals and reagents
Eagle’s Minimum Essential Medium (EMEM); Earle's BSS (Balanced Salt Solution); 2, 2-Diphenyl-1-picryl-hydrazyl (DPPH) (Himedia Laboratories Pvt. Ltd., Mumbai, India). Methylthiazol tetrazolium (MTT) (3-[4, 5-dimethyIthiazol-2-yl]-2, 5-diphenyltetra-zolium bromide); β-amyloid (1–40); Acetylthiocholine iodide (ATCI); acetylcholinesterase enzyme (AchE) from human erythrocyte; 5, 5-dithiobis [2-nitrobenzoic acid] (DTNB); galantamine; lipoxidase enzyme and linoleic acid were obtained from Sigma Ltd. (Mumbai, India). Ascorbic acid was procured from Loba Chemie (Mumbai, India). Scopolamine (99.98% pure) was obtained as a gift from Cadila Healthcare Pvt. Ltd. (Goa, India). Scopolamine was dissolved in normal saline for i.p. injection. All the chemicals and reagents used in the experiments were of analytical grade. Pre-coated silica gel 60F254 TLC plates were purchased from Merck (Darmstadt, Germany).
2.2. Plant materials and preparation of extracts
C. decussata (CD) was collected from Ninai ghat (Gujarat, India) and identified by Dr S.C. Agrawal (Department of Botany, CDRI, Lucknow, India). C. ternatea (CT), C. pluricaulis (CP) and E. alsinoides (EA) were collected from the outskirts of Vadodara and identified in the Botany Department of M. S. University of Baroda, Vadodara, Gujarat (India). Voucher specimens of all four plants (No. Pharmacy/CD/09-10/13/NS, Pharmacy/CT/09-10/12/NS, Pharmacy/CP/09-10/11/NS, and Pharmacy/EA/09-10/10/NS) have been deposited in the Herbal Drug Technology Lab, Pharmacy Department of the University. 1000 g shade dried and coarsely powdered whole herb of all the drugs was subjected to Soxhlet extraction with methanol. The solvent was completely removed under reduced pressure using rotary evaporator. The percent yield of methanol extract (ME) were found to be 15.78 ± 0.01, 7.31 ± 0.06, 9.56 ± 0.03 and 10.36 ± 0.07% for CD, CT, CP and EA respectively.
2.3. Characterization of extract
High Performance Thin Layer Chromatography (HPTLC) studies were performed using various solvent systems as reported in our earlier studies [9], [15], [16], [17].
2.4. Experimental procedure
2.4.1. In vitro neuropharmacological studies
2.4.1.1. β-amyloid induced neuroprotection on brain cell line
2.4.1.1.1. Cell culture and stock solutions
Neuro-2a (a neuroblastoma cell line) was obtained from the National Centre for Cell Sciences (NCCS), Pune, India and routinely cultured in EMEM supplemented with 10% (v/v) fetal bovine serum (FBS), gentamicin (50 μg/ml) and amphotericin B (2.5 μg/ml) in 10% CO2/90% humidified air at 37 °C. Neuro-2A was split in 96-well plates at a concentration of 1 × 105 cells per ml and allowed to adhere for 24 h at 37 °C. The toxicity of Aβ on Neuro-2a cells was assessed by MTT assay. Briefly, Neuro-2a cells were exposed to Aβ1–40 (0.1 μM, 0.5 μM, 1 μM, 5 μM, 10 μM and 15 μM) for 24 h and the rate of MTT reduction was recorded [18]. After assessment of toxicity, Neuro-2a with (10 μM) or without Aβ1–40 (dissolved in pyrogen free water) plus different concentrations (10 μM, 20 μM, 30 μM, 50 μM, 100 μM and 200 μM) of methanol extract of Shankhpushpi botanicals viz. MECD, MECT, MECP, MEEA were diluted in DMSO. The final DMSO concentration in each sample was 0.1%, and this concentration did not affect cell growth or death.
2.4.1.1.2. MTT cell viability assay
A MTT conversion assay was used to determine the cell viability under each treatment condition [19]. The MTT assay relies primarily on the mitochondrial metabolic capacity of viable cells and reflects the intracellular redox state. After incubation, the cells were treated with the MTT solution (final concentration: 1 mg/mL) for 3 h. The dark blue formazan crystals formed in intact cells were solubilized with lysis buffer [20% (w/v) sodium dodecyl sulfate in 50% (v/v) aqueous N, N dimethyl formamide with an adjusted pH of 4.5]. The optical density of each well was measured with a 96-well microplate spectrophotometer (Bio-Rad, Hercules, CA, USA, Benchmark) at the test wavelength of 570 nm.
2.4.1.2. Assay of hydroxyl radical scavenging and total antioxidant activity
DPPH radical-scavenging, Ferric-reducing antioxidant power (FRAP) and phosphomolybdenum complex methods were used to evaluate the hydroxyl radical scavenging and total antioxidant capacity of the samples [20]. Five different concentrations (100, 200, 300, 400 and 500 μg/ml) of methanol extract viz., MECD, MECT, MECP and MEEA were used. Vitamin E was used as reference standard for all the three methods.
2.4.1.3. Acetylcholinesterase inhibition microplate assay
AchE activity was measured using a 96-well microplate reader based on Ellman's method. The absorbance was measured at 405 nm at every 13 s for 65 s. 25 μL of 0.22 U/ml of AchE enzyme was added and the absorbance was again read at every 13 s for 104 s using 680 XR Microplate Reader S/N 10519 [21]. Percentage inhibition was calculated by comparing the sample to the blank. Ranges of different concentration (50, 100, 150, 200 and 250 μg/ml) of methanol extracts (MECD, MECT, MECP and MEEA) were used. Galantamine was used as reference standard for the study.
2.4.1.4. Lipooxygenase (LOX) enzyme inhibition assay
LOX enzyme inhibition assay was performed using linoleic acid as substrate and lipoxidase as enzyme. Methanol extract of each variety were dissolved separately in 0.25 ml of 2 M borate buffer (pH 9.0) and 0.25 ml of lipoxidase enzyme solution (20,000 U/ml) was added and incubated for 5 min at 25 °C. Further, 1 ml of linoleic acid solution (0.6 mM) was added, mixed well and absorbance was measured at 234 nm. Different concentrations (50, 100, 150, 200 and 250 μg/ml) of the test extracts were plotted to determine the IC50 values. Rutin was used as reference standard [21].
2.4.2. In vivo studies
2.4.2.1. Animals
Male Sprague–Dawley rats (wt. 200–250 g, age 2–3 months) were used for the study. The animals were housed in groups of six in polypropylene cages, under standard laboratory conditions of temperature (25 ± 2 °C), lighting (08:00–20:00 h) and relative humidity (50 ± 5%) and had free access to standard pellet chow (Brooke Bond-Lipton, India) and water. Animals were acclimatized for 7 days before initiation of the study. All the experiments were conducted between 0900 and 1400 h. Experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) of B.R. Nahata College of Pharmacy, Mandsaur (Reg. No. 918/ac/05/CPCSEA).
2.4.2.2. Assessment of nootropic activity by scopolamine induced amnesia
The nootropic activity was assessed using Cook and Weidley's pole climbing apparatus and Morris water maze task. The animals were divided into seven groups (n = 6). Group I received the vehicle (distilled water) only, Group II received scopolamine (0.3 mg/kg i.p) as negative control, Group III received piracetam (100 mg/kg p.o.) as positive control, and Group IV-VII received methanol extracts (400 mg/kg p.o.) of CD, CT, CP and EA.
2.4.2.3. Cook and Weidley's pole climbing apparatus
Briefly, Cook and Weidley's pole climbing apparatus [21] consists of a soundproof transparent experimental chamber with a grid floor and a provision of electric current to be considered as shock zone which was initiated with a buzzer tone. The chamber had a transparent covering lid to which a wooden pole screwed into the inner surface, acted as the shock-free zone (Medicraft Electromedicals Pvt Ltd., India). The condition of stimulus was a foot shock of 0.75 mA given to each rat for a period of 2 s from the electrified grid floor, in order to teach them to avoid electric current by climbing on the wooden pole i.e. the shock free zone. Three trial sessions interspersed with an interval of 10 s were conducted daily. During each of the trials, the rats were allowed to explore the apparatus for 10 s, including 2 s foot shock period. Sensitivity of the rats towards foot shock was measured and only those rats, which were sensitive to the shock and could climb the pole, were included in the experiment. The test drugs and vehicle were administered orally once daily for 14 days before commencement of experiment. All the groups were trained daily for learning. Training trial (TT) was conducted after 7 days of treatment. Twenty-four hours later, on day 8, a retention trial (RT) was conducted and the number of avoidance responses (ARs) in the 10 trial sessions were noted. On day 9 of the experiment, after attaining complete training, all the animals except control, were treated with a single dose of scopolamine (0.3 mg/kg i.p.), 30 min before the administration of the extracts. Training schedule was continued further with daily dosing of test drugs till 15 days, for the complete evaluation of the memory retention in terms of retention trial.
2.4.2.4. Morris water maze task
The Morris water maze was described as forced swimming performance of animals in the presence of hidden platform. The first experimental day was dedicated to four swimming training sessions of 60 s in the presence of the platform. On the 2nd day, the rats were given four trial sessions with the platform in place. The time interval between each trial sessions was 30 min. For eight trial sessions, rats were placed every time in the water facing the pool wall in one of the pool quadrants. The entry point was changed in a different order on daily basis. When a rat located the platform, it was permitted to remain on it for 10 s. If the rat did not locate the platform within 60 s, it was placed on the platform for 10 s. After each trial, the wet animal was returned to a cage, dried with towel, and allowed to dry up under an infrared lamp. During each trial session, the time taken to find the hidden platform (latency) was recorded. After the last training trial session, the platform was removed from the pool and rats were allowed to swim for 60 s to search for it. A record was kept of the number of crossing over the platform position in the pool quadrant where the platform had been previously placed. The test drugs and the vehicle were administered orally once daily for 14 days. On the 14 day, a single dose of scopolamine (0.3 mg/kg i.p.) was administered 45 min before the Morris water maze test began. All the groups, except the vehicle treated, received scopolamine. Then, the rats were exposed to training sessions using Morris water maze for two consecutive days before decapitation. The maximum drug concentration occurs approximately 30 min after scopolamine administration [22].
2.5. Data analysis
All the analyses were carried out in triplicate and the results were expressed as mean ± SEM. Regression analysis was used to calculate IC50, defined as the concentration of inhibitor necessary for 50% inhibition of the enzyme reaction. For animal studies, the data were expressed as mean ± SEM and analyzed by one-way ANOVA followed by Bonferroni multiple comparison post-test. P and F values and degrees of freedom were calculated. The data was analyzed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, California).
3. Results
3.1. Characterization of extract
HPTLC studies revealed the presence of various phytochemicals shown in Table 1, as reported and published in our earlier studies and may be utilized for phytochemical differentiation among four varieties of Shankhpushpi [9], [15], [16], [17].
Table 1.
Characterization of each methanol extract of Shankhpushpi botanicals viz. MECD, MECT, MECP and MEEA respectively.
| MECD | MECT | MECP | MEEA |
|---|---|---|---|
| Stigmasterol | Stigmasterol | Stigmasterol | Stigmasterol |
| Ursolic acid | Lupeol | Scopoletin | Betulinic acid |
| Mangiferin | Ursolic acid | Scopoletin | |
| Scopoletin | Scopoletin | β-carotene | |
| β-carotene | β-carotene | Chlorogenic acid | |
| Rutin |
3.2. β-amyloid induced neuroprotection on brain cell line
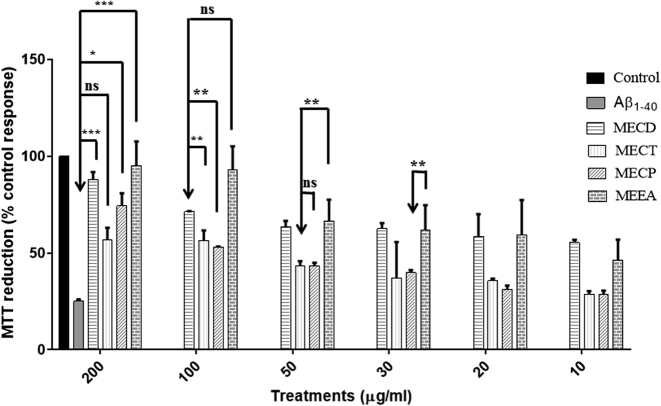
The toxicity of Aβ1–40 on Neuro-2a cells was assessed by MTT assay. It was confirmed that when Neuro-2a cells were exposed to Aβ1–40 (0.1 μM, 0.5 μM, 1 μM, 5 μM, 10 μM and 15 μM) for 24 h, the rate of MTT reduction was decreased in a concentration-dependent manner (Fig. 1). Therefore, a fixed concentration of Aβ1–40, 2.5 μM, was used for the determination of Aβ1–40-induced Neuro-2a cells damage. Neuro-2a cells were incubated in culture medium containing 2.5 μM Aβ1–40 with or without test extracts viz. MECD, MECT, MECP, MEEA. As shown in Fig. 2, MEEA and MECD significantly protected Neuro-2a cells from the cytotoxic effect of Aβ1–40. After 24 h exposure to 2.5 μM Aβ1–40 alone, the degree of reduction of MTT by Neuro-2a cells was decreased and MEEA and MECD significantly increased cell viability. Further analysis revealed that the protective effects of MEEA and MECD were significantly better than MECT and MECP (Fig. 2).
Fig. 1.
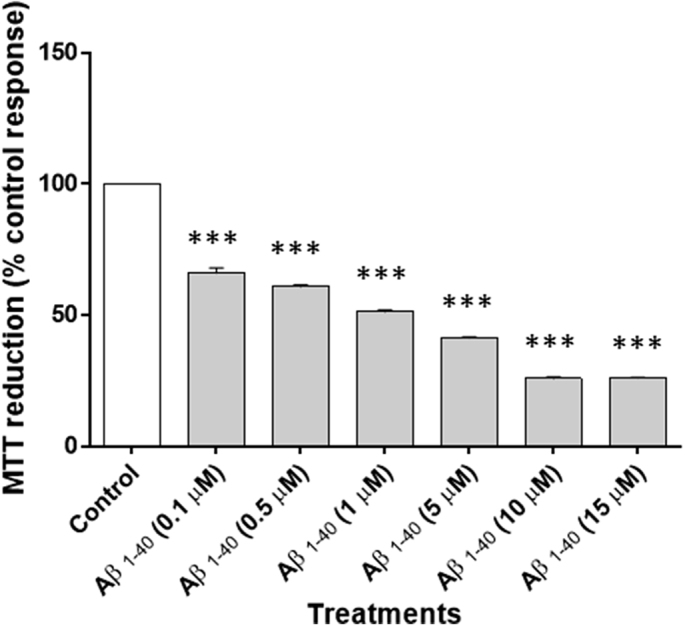
Characterization of Aβ1–40-induced neuronal death in Neuro-2a cells. Cell viability at 24 h was determined by measuring the ability to reduce MTT as a percentage of the ability of control cells. Results are the mean ± SEM. (n = 3), ***p < 0.001 compared with control group without Aβ1–40.
Fig. 2.
Effect of methanol extract of various Shankhpushpi botanicals on Aβ1–40-induced Neuro 2A cell death. Neuro 2A cells were incubated in culture medium containing 10 μM Aβ1–40 with or without the following extracts: MECD, MECT, MECP and MEEA. Cell viability at 24 h was determined by measuring the ability to reduce MTT as a percentage of the ability of control cells. The results are the mean ± SEM (n = 3). One-way ANOVA followed by Bonferroni multiple comparison test [***p < 0.001, **p < 0.01, *p < 0.05; F = 26.59, df (5, 30) = 35].
3.3. Assay of hydroxyl radical scavenging and total antioxidant activity
Radical scavenging effects and total antioxidant activity of methanol extracts (MECD, MECT, MECP and MEEA) are represented in Table 2. Results revealed that MEEA possesses highest radical scavenging effect as compared to others, with IC50 value of 18.21 ± 0.51 in DPPH method. MEEA also possessed higher antioxidant potential with IC50 value of 20.38 ± 0.37 by FRAP (ferric-reducing antioxidant power) and 20.63 ± 0.30 μg/ml by phosphomolybdenum complex method. The order of activity was found to be MEEA>MECD>MECP>MECT in all the three methods.
Table 2.
Comparative antioxidant and hydroxyl radical scavenging activity of methanol extract of various Shankhpushpi botanicals.
| Treatments | IC50 value (μg/ml) |
||
|---|---|---|---|
| DPPH | FRAP | Phosphomolybdenum | |
| Vitamin E | 13.62 ± 2.03 | 12.39 ± 0.17 | 4.41 ± 0.08 |
| MECD | 19.15 ± 0.35ns | 21.65 ± 0.15*** | 21.62 ± 0.40*** |
| MECT | 36.09 ± 2.10*** | 66.71 ± 1.28*** | 24.64 ± 1.73*** |
| MECP | 25.46 ± 0.08** | 26.94 ± 0.14*** | 22.11 ± 0.41*** |
| MEEA | 18.21 ± 0.51ns | 20.38 ± 0.37*** | 20.63 ± 0.30*** |
All values are mean ± SEM (n = 6). One-way ANOVA followed by Bonferroni post-tests regardless of all p-values. nsp > 0.05, **p < 0.01, ***p < 0.001 compared to Vitamin E for all three separate experiment.
3.4. Acetylcholinesterase inhibition microplate assay
MEEA showed appreciably higher activity than other three varieties and inhibited AchE with an IC50 value of 65.67 ± 11.32 μg/ml (Table 3). Galantamine was used as a positive control, showed inhibition with an IC50 value of 4.68 ± 0.16 μg/ml. The order of activity was found to be MEEA>MECD>MECP>MECT.
Table 3.
Comparative acetylcholinesterase (AchE) and lipoxygenase (LOX) inhibitory activity of methanol extract of various Shankhpushpi botanicals.
| Treatments | IC50 value (μg/ml) |
|
|---|---|---|
| AchE | LOX | |
| RutinA | Not applicable | 4.21 ± 0.17 |
| GalantamineB | 4.68 ± 0.16 | Not applicable |
| MECD | 65.88 ± 3.78* | 28.39 ± 1.24*** |
| MECT | 132.40 ± 18.96*** | 114.35 ± 0.99*** |
| MECP | 107.99 ± 7.57** | 81.76 ± 2.07*** |
| MEEA | 65.67 ± 11.32* | 19.04 ± 0.78*** |
Where ‘A’ is used as positive control for LOX and ‘B’ is used as positive control for AchE. All values are mean ± SEM (n = 6). One-way ANOVA followed by Bonferroni post-tests regardless of all p-values. ∗p < 0.05, **p < 0.01, ***p < 0.001 compared to galantamine for AchE and rutin for LOX.
3.5. Lipooxygenase (LOX) enzyme inhibition assay
Among all the tested methanol extracts, MEEA showed highest activity and inhibited LOX enzyme with the IC50 value of 19.04 ± 0.78 μg/ml (Table 3). Rutin was used as a positive control, showed inhibition with an IC50 value of 4.21 ± 0.17 μg/ml. The order of activity was found to be MEEA>MECD>MECP>MECT.
3.6. Assessment of nootropic activity by scopolamine induced amnesia
Inhibitory avoidance test conducted by administration of scopolamine induced amnesia resulted in reduction in the number of avoidance responses. However, continued treatment of MEEA produced better retention and recovery as compared to the extracts of other three varieties.
3.6.1. Cook and Weidley's pole climbing apparatus
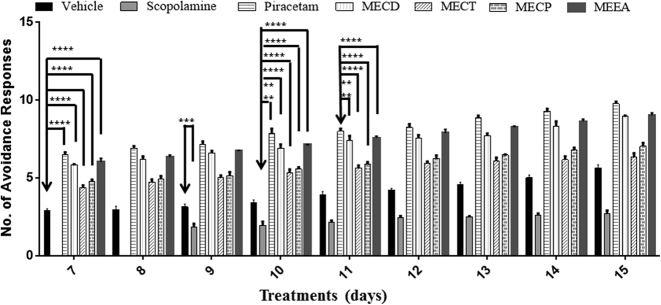
There was a significant increase in memory retention and retrieval in MEEA treated groups. The order of activity was found to be MEEA>MECD>MECP>MECT. A one-way ANOVA followed by Bonferroni's multiple comparison post-test, conducted on the avoidance response of rats when they were administered a single dose of scopolamine on 9th day followed by trials on 10th, 11th, 12th, 13th, 14th and 15th day demonstrated that the Shankhpushpi extract treated groups exhibited a significant [****p < 0.0001, ***p < 0.001, **p < 0.01; F = 114.1; df (4, 40) = 44] increase in the number of ARs as compared to the scopolamine and vehicle treated groups (Fig. 3). The order of activity was found to be MEEA>MECD>MECP>MECT.
Fig. 3.
Effect of methanol extract of various Shankhpushpi botanicals on nootropic activity by scopolamine induced amnesia in rats using Cook and Weidley's pole climbing apparatus. All values are mean ± SEM (n = 6). One-way ANOVA followed by Bonferroni multiple comparison tests [***p < 0.0001, ***p < 0.001, **p < 0.01; F = 114.1; df (4, 40) = 44].
3.6.2. Morris water maze task
The data in Table 4 shows the comparative improvement in memory-dependent learning by treatment with methanolic extracts. MEEA revealed comparative significant higher increase in latency time in scopolamine treated rats in comparison with others and the corresponding control [****p < 0.0001; ***p < 0.0005; **p < 0.005; *p < 0.05; F = 14.42; df (6, 35) = 41]. All the extract treated groups exhibited decrease in the latency time as compared to the corresponding scopolamine treated rats. These rats showed a significant decrease in number of crossings over the platform position as compared to the corresponding control. The order of activity was found to be MEEA>MECD>MECP>MECT.
Table 4.
Effect of methanol extract of various Shankhpushpi botanicals on scopolamine induced amnesia by Morris water maize test.
| Treatment# (mg/kg) |
Escape Latency |
|
|---|---|---|
| 60 Secondsa | Crossing the platform in 7 trialsb | |
| Vehicle | 32.89 ± 6.74**** | 5.66 ± 0.33**** |
| Scopolamine | 48.52 ± 0.25 | 1.66 ± 0.33 |
| Piracetam | 19.22 ± 0.21**** | 6.33 ± 0.33**** |
| MECD | 26.03 ± 0.07**** | 4.66 ± 0.33**** |
| MECT | 36.05 ± 0.12* | 2.33 ± 0.33ns |
| MECP | 30.02 ± 0.09*** | 2.66 ± 0.33ns |
| MEEA | 23.08 ± 0.02**** | 5.33 ± 0.33**** |
The test drugs and the vehicle were administered orally once daily for 14 days. On the 14 day, a single dose of scopolamine (0.3 mg/kg i.p.) was administered 45 min before the moris water maze began. All the groups, except the vehicle treated, received scopolamine. One-way ANOVA followed by Bonferroni multiple comparison tests. All values are mean ± SEM (n = 6). [****p < 0.0001; ***p < 0.0005;**p < 0.005; *p < 0.05; F = 14.42; df (6, 35) = 41].
4. Discussion
Synonymous use of the word ‘Shankhpushpi’ for different varieties creates a controversy regarding their identity which often results in the use of less potent variety in herbal formulations [23]. The present work provides comparative neuropharmacological evaluation, which was needed to identify most suitable memory enhancer among them. Although all plants are individually tested and found active in various parameters involving in vitro and in vivo experiments, still, they differ in their pharmacological effectiveness.
Many of the phytochemicals present in Shankhpushpi are reported individually to possess activities related to antioxidant action, memory dysfunction and CNS stimulation [24], [25]. Presence and absence of such phytochemicals in different varieties of Shankhpushpi creates confusion as to which is the most potent variety [8].
It was reported that β-amyloid peptides have been used to initiate neurotoxicity in Neuro-2a (a neuroblastoma cell lines) cultured cells [26]. The toxic effects of β-amyloid peptides as found in our studies (Fig. 2) agree with previously reported data in neuronal cells [27]. MTT assay was reported to measure protective effects of plant extracts and compounds on cell viability. In order to determine and compare the effect of four varieties against Aβ1–40 induced neurotoxicity, MTT based cell protection assay was performed. The order of cell protection against Aβ1–40 induced neurotoxicity damage was found to be MEEA>MECD>MECP>MECT (Fig. 2). These data support the neuroprotective activity of tested drugs by reducing β-amyloid deposition in the brain to protect from memory dysfunction.
It has been established earlier that brain aging is mainly associated with free radical action [28]. In the present study, comparative free radical scavenging and total antioxidant capacities of four Shankhpushpi botanicals were measured by using the DPPH, FRAP and phosphomolybdenum method reaction. The order of activity was found to be MEEA>MECD>MECP>MECT in all the three methods performed. These data supports neuroprotective activity of tested drugs, underlying mechanism being free radical scavenging and antioxidant action on the brain cells, providing protective action against memory dysfunction.
Disturbance of cholinergic neurotransmission in the brain contributes to the salient cognitive decline and progression of memory dysfunction [29], [30]. Present study depicts that all the four Shankhpushpi botanicals inhibited AchE significantly and protected the loss of neurotransmitter AchE, which is directly responsible for cognitive function. The order of activity was found to be MEEA>MECD>MECP>MECT.
It was reported earlier that 5-lipooxygenase is a key enzyme in the biosynthesis of leukotriene, which has been postulated to play an important role in the pathophysiology of aging-associated neurodegenerative disorders [31]. Present study revealed that all the four Shankhpushpi botanicals inhibited 5-LOX significantly, which is directly responsible for neurodegenerative disorders. The order of activity was found to be MEEA>MECD>MECP>MECT.
We further confirmed our findings from in vitro studies by adopting various animal protocols reported earlier for evaluation of nootropic activity. We assessed nootropic activity by Cook and Weidley's pole climbing apparatus and Morris water maze task on scopolamine induced amnesia model. It was seen that animals receiving only scopolamine on day 9 (Cook and Weidley's pole climbing apparatus) and day 14 (Morris water maze task) showed a considerable loss of memory and amnesia produced was persistent. There is substantial evidence that muscarinic receptor blockade by drugs like scopolamine results into disruptions of various tasks related to performance of memory [32], [33]. Reduction in the number of avoidance responses is an evidence of amnesia during inhibitory avoidance test conducted after administration of scopolamine. However, it is evident in our experiments that continued treatment with methanol extracts of four Shankhpushpi botanicals individually produced better retention and recovery. The order of activity in both the performed in vivo task was found to be MEEA>MECD>MECP>MECT, which is similar to the in vitro findings.
Thus, anti-amensic effects of Shankhpushpi on scopolamine induced amnesia by utilizing Cook and Weidley's pole climbing apparatus and Morris water maze task were successfully demonstrated through the study. EA was proven to be most potent for memory and related disorders in many of previous studies [7], [12], [34]. EA possessed higher activity than other as an evidence from all the experiments performed in present studies.
5. Conclusion
The findings validate that the traditional use of Shankhpushpi for its neuropharmacological action and provide great relevance for its use in the prevention and therapies of memory and CNS related dysfunctions. The comparative multiple beneficial effects of E. alsinoides over other Shankhpushpi botanicals makes it a promising agent for controlled clinical trials to establish its safety and efficacy as an antioxidant, NSAID-like prophylactic agent, NMDA down-regulator and acetylcholinesterase enhancer for protection against AD and possibly other neurodegenerative age-related diseases. The results of neuropharmacological activity suggest that E. alsinoides should be used as the true source of Shankhpushpi while the other three plants can be used as its substitutes.
Sources of funding
One of the authors, Neeraj K. Sethiya acknowledges the financial support of University Grants Commission, New Delhi, Govt. of India, for providing Junior Research Fellowship (F. No. 10-01/2008).
Conflict of interest
None.
Acknowledgement
The authors would like to express their sincere thanks to the Director, B.R. Nahata Smriti Sansthan, Contract Research Centre, Mandsaur (M.P.), India for granting permission to carry out the in vivo studies. We would also like to thank Cadila Healthcare Pvt. Ltd, Goa, India for providing a gift sample of scopolamine.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Sethiya N.K., Trivedi A., Patel M.B., Mishra S.H. Comparative pharmacognostical investigation on four ethnobotanical traditionally used as Shankhpushpi in India. J Adv Pharm Technol Res. 2010;1:388–395. doi: 10.4103/0110-5558.76437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramani S.P., Venkatasubramanian P., Kukkupuni S.K., Patwardhan B. Plant-based Rasayana drugs from Ayurveda. Chin J Integr Med. 2011;17(2):88–94. doi: 10.1007/s11655-011-0659-5. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006;20(12):1023–1035. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 4.Sethiya N.K., Nahata A., Dixit V.K. Anxiolytic activity of Canscora decussata in albino rats. J Complement Integr Med. 2010;7(1):19. [Google Scholar]
- 5.Nahata A., Patil U.K., Dixit V.K. Anxiolytic activity of Evolvulus alsinoides and Convulvulus pluricaulis in rodents. Pharm Biol. 2009;47(5):444–451. [Google Scholar]
- 6.Nahata A., Patil U.K., Dixit V.K. Effect of Convulvulus pluricaulis Choisy. on learning behaviour and memory enhancement activity in rodents. Nat Prod Res. 2008;22(16):1472–1482. doi: 10.1080/14786410802214199. [DOI] [PubMed] [Google Scholar]
- 7.Nahata A., Patil U.K., Dixit V.K. Effect of Evolvulus alsinoides Linn. on learning behavior and memory enhancement activity in rodents. Phytother Res. 2010;24:486–493. doi: 10.1002/ptr.2932. [DOI] [PubMed] [Google Scholar]
- 8.Sethiya N.K., Nahata A., Mishra S.H., Dixit V.K. An update on Shankhpushpi, a cognition boosting Ayurvedic medicine. Zhong Xi Yi Jie He Xue Bao. 2009;7:1001–1022. doi: 10.3736/jcim20091101. [DOI] [PubMed] [Google Scholar]
- 9.Sethiya N.K., Mishra S.H. Simultaneous HPTLC analysis of ursolic acid, betulinic acid, stigmasterol and lupeol for the identification of four medicinal plants commonly available in the Indian market as Shankhpushpi. J Chromatogr Sci. 2015;53(5):816–823. doi: 10.1093/chromsci/bmu111. [DOI] [PubMed] [Google Scholar]
- 10.Malik J., Karan M., Vasisht K. Nootropic, anxiolytic and CNS-depressant studies on different plant sources of Shankhpushpi. Pharm Biol. 2011;49(12):1234–1242. doi: 10.3109/13880209.2011.584539. [DOI] [PubMed] [Google Scholar]
- 11.Sethiya N.K., Thakore S.G., Mishra S.H. Comparative evaluation on commercial sources of indigenous medicine Shankhpushpi for anti-stress potential – A preliminary study. Pharmacologyonline. 2009;2:460–467. [Google Scholar]
- 12.Kothiyal P., Rawat M.S.M. Comparative nootropic effect of Evolvulus alsinoides and Convolvulus pluricaulis. Int J Pharma Bio Sci. 2011;2(1):616–621. [Google Scholar]
- 13.Pawar S.A., Dhuley J.N., Naik S.R. Neuropharmacology of an extract derived from Convolvulus microphyllus. Pharm Biol. 2001;39(4):253–258. [Google Scholar]
- 14.Siddiqui N.A., Ahmad N., Musthaq N., Chattopadhyaya I., Kumria R., Gupta S. Neuropharmacological profile of extracts of aerial parts of Convolvulus pluricaulis Choisy in mice model. Open Neurol J. 2014;8:11–14. doi: 10.2174/1874205X01408010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethiya N.K., Raja M.K.M.M., Mishra S.H. Antioxidant markers based thin layer chromotography-2-diphenyl-1-picrylhydrazyl differentiation on four commercialized botanical sources of Shankhpushpi (Medhya Rasayana): a preliminary assessment. J Adv Pharm Technol Res. 2013;4:25–30. doi: 10.4103/2231-4040.107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethiya N.K., Trivedi A., Mishra S.H. Rapid validated high performance thin layer chromatography method for simultaneous estimation of mangiferin and scopoletin in Canscora decussata (South Indian Shankhpushpi) extract. Rev Bras Farmacogn. 2015;25(3):193–198. [Google Scholar]
- 17.Sethiya N.K., Nahata A., Dixit V.K. Comparative thin layer chromatographic investigations on sources of Shankhpushpi. Phcog J. 2009;1:224–226. [Google Scholar]
- 18.LePage K.T., Dickey R.W., Gerwick W.H., Jester E.L., Murray T.F. On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol. 2005;17:27–50. doi: 10.1615/critrevneurobiol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Sethiya N.K., Trivedi A., Mishra S.H. The total antioxidant content and radical scavenging investigation on 17 phytochemical from dietary plant sources used globally as functional food. Biomed Prev Nutr. 2014;4(3):439–444. [Google Scholar]
- 21.Sethiya N.K., Mishra S.H. Investigation of mangiferin, as a promising natural polyphenol xanthone on multiple targets of Alzheimer's disease. J Biol Act Prod Nat. 2014;4(2):111–119. [Google Scholar]
- 22.Ali E.H.A., Arafa N.M.S. Comparative protective action of curcumin, memantine and diclofenac against scopolamine-induced memory dysfunction. Fitoterapia. 2011;82:601–608. doi: 10.1016/j.fitote.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Dixit V.K. Controversial ayurvedic herbs. J Adv Pharm Technol Res. 2011;2(2):78–80. doi: 10.4103/2231-4040.82948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sethiya N.K., Dixit V.K. vol. 1. LAP Lambert Academic Publishing; Germany: 2012. p. 116. (Investigation on South Indian Shankhpushpi). [Google Scholar]
- 25.Sethiya N.K., Dube B., Mishra S.H. vol. 1. LAP Lambert Academic Publishing; Germany: 2012. p. 75. (Herbs in metal health). [Google Scholar]
- 26.Hoi C.P., Ho Y.P., Baum L., Chow A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother Res. 2010;24(10):1538–1542. doi: 10.1002/ptr.3178. [DOI] [PubMed] [Google Scholar]
- 27.Shih P.H., Wu C.H., Yeh C.T., Yen G.C. Protective effects of anthocyanins against amyloid β-peptide-induced damage in neuro-2A cells. J Agric Food Chem. 2011;59(5):1683–1689. doi: 10.1021/jf103822h. [DOI] [PubMed] [Google Scholar]
- 28.Perrig W.J., Perrig P., Stähelin H.B. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45(6):718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 29.Vinutha B., Prashanth D., Salma K., Sreeja S.L., Pratiti D., Padmaja R. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2007;109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Kumar V., Khanna V.K., Seth P.K., Singh P.N., Bhattacharya S.K. Brain neurotransmitter receptor binding and nootropic studies on Indian Hypericum perforatum Linn. Phytother Res. 2002;16(3):210–216. doi: 10.1002/ptr.1101. [DOI] [PubMed] [Google Scholar]
- 31.Czapski G.A., Czubowicz K., Strosznajder J.B., Strosznajder R.P. The Lipoxygenases: their regulation and implication in Alzheimer's disease. Neurochem Res. 2016;41:243–257. doi: 10.1007/s11064-015-1776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melamed J.L., de Jesus F.M., Maior R.S., Barros M. Scopolamine induces deficits in spontaneous object-location recognition and fear-learning in marmoset monkeys. Front Pharmacol. 2017;8:395. doi: 10.3389/fphar.2017.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIntyre C.K., Ragozzino M.E., Gold P.E. Intra-amygdala infusions of scopolamine impair performance on a conditioned place preference task but not a spatial radial maze task. Behav Brain Res. 1998;95:219–226. doi: 10.1016/s0166-4328(97)00161-7. [DOI] [PubMed] [Google Scholar]
- 34.Sethiya N.K., Keluskar P., Ingle S., Mishra S.H. Antimalarial activity of Evolvulus alsinoides Linn.-an in vitro Plasmodium falciparum specific lactate dehydrogenase enzyme inhibition assay. Asian Pac J Trop Dis. 2014;4(6):489–491. [Google Scholar]