Abstract
Background
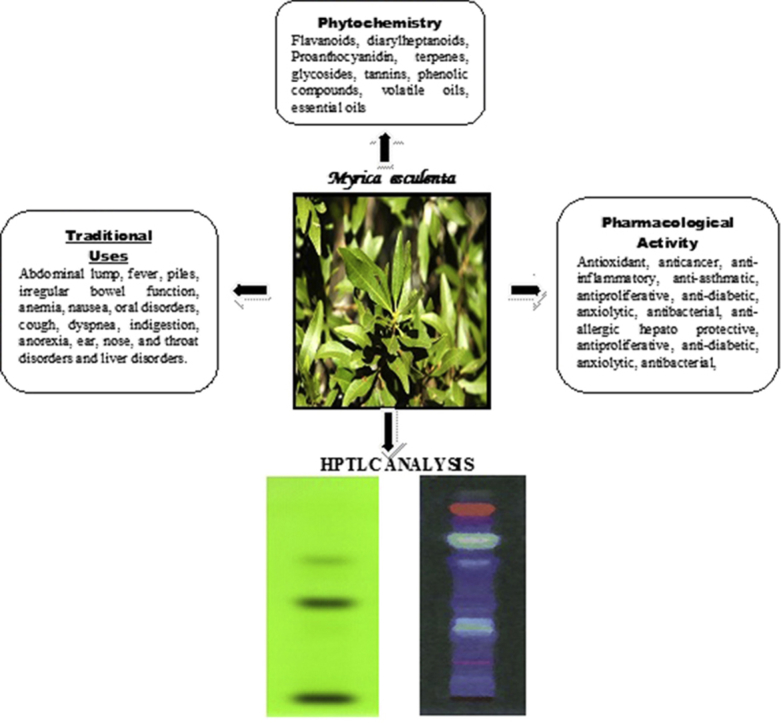
Myrica esculenta (Family: Myricaceae) commonly known as Kaiphala or Katphala is a widely used medicinal plant in Ayurveda. In spite of its numerous medicinal attributes, no published work is available till date on pharmacognostical characterization and HPTLC analysis of its leaves.
Objectives
To investigate the pharmacognostical, physicochemical, and HPTLC profiles of M. esculenta leaves.
Materials and methods
The measures taken for pharmacognostical characterization were organoleptic study, macroscopy, microscopy, powder microscopy, leaf constant, fluorescence analysis, preliminary phytochemical screening and HPTLC spectra profile.
Results
Organoleptic and macroscopic studies found that leaves are lancoelate, thin, spirally arranged, dark green in color, with an astringent taste and acute apex. In transverse section, cuticularised epidermis having polygonal cells were found. Mesophyll cells were differentiated into single layered palisade cells on each surface and 2–3 layered spongy parenchyma, unicellular and uniseriate hollow trichomes, anomocytic stomata and bowl shaped vascular bundle in mid rib portion containing xylem and phloem tissues. Alkaloids, carbohydrates, flavonoids, glycosides, phenolic compounds and tannins were found present. Analysis on the leaf constants, powder microscopy, fluorescence characteristics and physical parameters resulted a valuable data to establish standards for the plant. HPTLC profile provides number of constituents present in the extracts with their respective Retention Factor (Rf).
Conclusion
Present report on pharmacognostical characterization and HPTLC analysis of M. esculenta leaves provides a vital diagnostic tool for identification, authentication and development of quality parameters of the species. Data obtained by present study may be considered as standard for future studies.
Keywords: Myrica esculenta, Microscopy, Macroscopy, Physicochemical, Fluorescence analysis, HPTLC analysis
Graphical abstract
1. Introduction
India has a wide range of traditional medicines from ancient time, based on various medical systems like Unani, Siddha, Homeopathy and mainly Ayurveda. Plants are used as medicine to maintain human health from ages [1] and are also major natural sources of medicinal compounds in current pharmacopoeias [2]. Therefore, there is a need to evaluate phytoconstituents obtained from traditional medicines, based on various phytochemical screening, pharmacological and analytical methods [3]. The very first step in this direction is the herb characterization or detailed pharmacognostic evaluation that gives external appearance, microscopy and physical characteristic of the crude drug [4].
Myrica esculenta Buch-Ham (Family – Myricaceae) Syn., Myrica nagi Hook., commonly known as Kaiphala or Katphala is an evergreen dioecous tree, found mainly in Punjab, Uttarakhand (Garhwal), Kumaun hills, Khasiya mountain and Sylhet in India at elevations of upto 2100 m. Some different varieties are also found in neighboring countries like Nepal, China etc. It is a shade giving, medium sized tree, having a big crown on it. The bark extract has already been reported to contain steroids, reducing sugars, tannins, glycosides, saponins and volatile oils. The hexane extract of the Katphala roots revealed the presence of friedelin and β–sitosterol [5]. Katphala is widely used in Indian system of medicine and various parts of this botanical are used in conditions like Gulma (abdominal lump), Jvara (fever), Arsha (piles), Grahani (irregular bowel function), Pandu roga (anemia), Hrillasa (nausea), Mukha roga (oral disorders), Kasa (cough), Shvasa (dyspnea), Agnimandhya (indigestion), Aruchi (anorexia) and Kantharoga (ears, nose, and throat disorders) [6]. Stem bark is extensively explored for various pharmacological roles likes free radical-scavenging [7], antioxidant [7], [8], anti-diabetic [9], anxiolytic [10], antibacterial [11], anti-helmintic [12], anti-allergic [13], anti-inflammatory, antimicrobial [14], mast cell stabilizing [15] and anti-asthmatic [16]. The plant has tremendous therapeutic potential with every part of the plant being used medicinally, including leaves.
Earlier reports established pharmacognostical and phytochemical profiles of M. esculenta stem [17], [18]. In spite of its numerous medicinal attributes, no published work is available till date on pharmacognostical characterization and HPTLC analysis of its leaves. Considering this, the present study was undertaken to develop a quality standard for leaves of this plant. In the present study, investigations were attempted to record the organoleptic characters, macroscopy, microscopy, quantitative values such as physical parameters, phytochemical screening and chromatographic profiles of M. esculenta leaves. This would be a milestone in assessing the quality of the crude drug for further development.
2. Materials and methods
2.1. Collection and authentication of plant materials
Leaves of M. esculenta were collected from outskirts of Chail Chowk, Mandi, Himachal Pradesh (H.P.) in November 2016. The plant was identified, authenticated and certified (AGI/2015/1220) by Head, Department of Botany, Abhilashi Group of Institutions, Mandi, Himachal Pradesh.
2.2. Chemicals
Toluene, safranin, chloral hydrate, ethyl acetate, methanol, and all other analytical grade chemicals were purchased from E. Merck Limited India and HiMedia Laboratories, Mumbai, India.
2.3. Organoleptic evaluation
Leaves of M. esculenta were evaluated for their impact on various organs of sense for organoleptic properties. Its color, odor, size (length and width), taste and other diagnostic parameters was observed and noted [19].
2.4. Macroscopic analysis
Macroscopic features of the leaf viz. type of leaf, shape, apex, margin, lamina, base venation and texture were analyzed by standard method [19].
2.5. Microscopical analysis
2.5.1. Study of transverse section
The potato sandwiched specimens were sectioned with the help of a sharp blade. The obtained thin slide was stained by safranin dye (0.1% w/v solution in distilled water). Photographs of different magnification were taken with Nikon lab photo 2 microscope unit.
2.5.2. Powdered drug microscopy
The coarsely powdered leaf was mounted on a glass slide in glycerin and studied under a microscope. Photographs of different magnified cellular structures were taken with Nikon lab photo 2 microscope units.
2.5.3. Analysis of leaf constants
Different leaf constants viz. vein islet number, vein termination number, stomatal index, stomatal number, palisade ratio were analyzed using standard procedures [20].
2.6. Physiochemical analysis
Physiochemical parameters (ash value, moisture content, foreign matter, fluorescence analysis, extractive value) were determined [21], [22].
2.7. Preliminary phytochemical screening
Various extracts of M. esculenta leaves were subjected to phytochemical analysis [23]. A series of identification tests were performed to detect presence of alkaloids, flavonoids, saponins, proteins and amino acids, fixed oils and fats, glycosides, tannins and steroids.
2.8. Preparation of extracts
Firstly, the plant leaves were washed with water to remove dirt and other foreign matters and were separated and shade dried. Dried leaves were then milled to coarse powder and then passed over sieve No. 14. The obtained dried powdered leaves of M. esculenta (500 g) were placed in the tube of Soxhlet apparatus in the form of thimble and kept on heating mental for 6 h for extraction using various solvents as ethyl acetate, methanol and water. The obtained extracts were filtered while hot and dried by evaporation using rotary vacuum evaporator and the final dried extracts samples were kept at low temperature in fridge for further study.
2.9. Development of chromatographic profile by HPTLC of various extracts of M. esculenta
CAMAG-HPTLC system having a sample applicator Linomat 5 was used to obtain HPTLC chromatograms using standard methods [24]. HPTLC profile of the various extracts of M. esculenta was developed using toluene:ethyl acetate (7:3) as mobile phase to confirm the occurrence of different phytoconstituents. The chromatographic development processes were performed in an air-conditioned room where the temperature was maintained at 22 °C and relative humidity was maintained at 55%. Ready to use silica gel-G precoated HPTLC aluminum plates were used for chromatographic separation. The extract (10 μL) was spotted as bands of 10 mm width with the help of the auto sampler fitted with a 100 μL Hamilton syringe. The solvent system was transferred to CAMAG Twin Trough plate development chamber lined with filter paper and pre-saturated with mobile phase (25 mL) for 30 min. The resulted plates were air dried and scanned. Developed spots on the places were scanned at the wavelength of 254 and 366 nm respectively. The retention factor (Rf) value for each spot found on plate was recorded.
3. Results
3.1. Organoleptic and microscopic characters
M. esculenta leaves are dorsiventral with a fairly prominent midrib and thin smooth lamina. They are of dark green color having leathery finish and a somewhat light green color on abaxial surface as compared to adaxial surface. The leaves are of yellowish green color in early ages which become darker with age. Leaves were odorless but having a slight bitter and astringent taste that may be due to presence of tannins.
3.2. Macroscopic characters
The leaves are laceolate with an average petiole measuring 0.4–1.5 cm in length. The lamina of leaf is smooth at both the surfaces with entire margin and an acute apex. In some leaves ¼ part of the margin near to apex is serrate. The leaves were 5–10.2 cm long and 2–3.5 cm broad. Phyllotaxy of the leaves is spiral and venation is reticulate pinnate (Fig. 1).
Fig. 1.
Macromorphological features of M. esculenta.
3.3. Microscopic characters
3.3.1. Transverse sections of leave
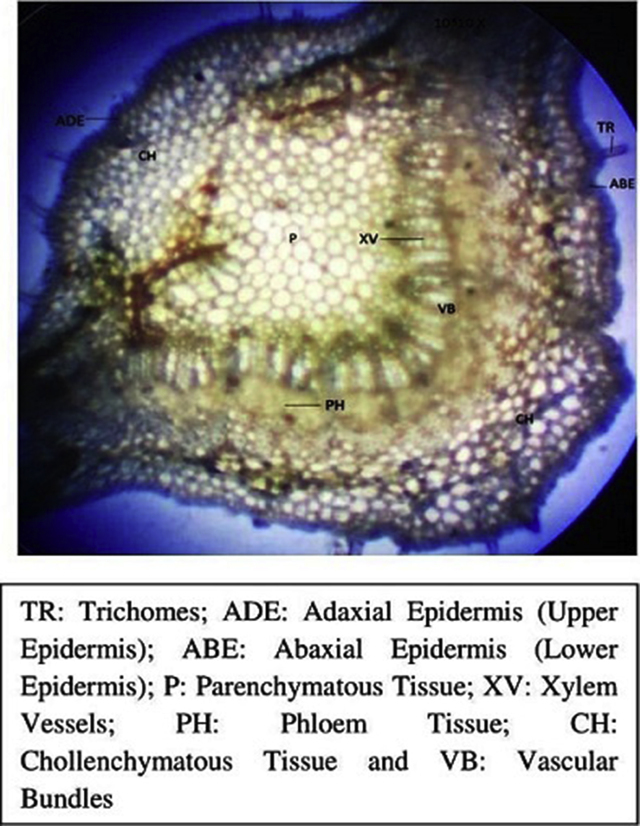
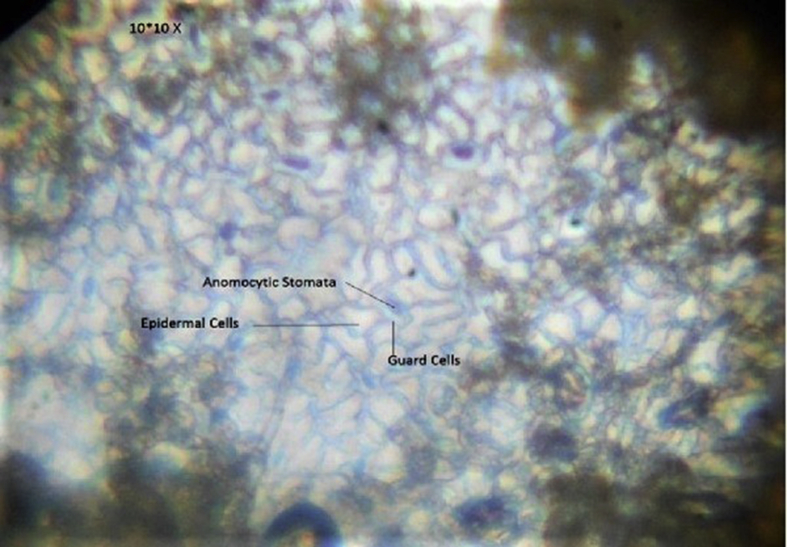
Transverse section is showed in Fig. 2, Fig. 3, Fig. 4. The upper and lower epidermis consisted small single layered thick polygonal cells which appear wavy in outline, covered by a thin cuticle containing mucilage. Both epidermises are abundant in unicellular uniseriate hollow trichomes (Fig. 2). Lamina region is differentiated into regular, long, columnar cells of palisade tissue and 3–4 layers of spongy parenchyma which is extended upto midrib region (Fig. 4). There are 4-6 layers of collenchymatous cells on both sides in the midrib region after epidermis, and the rest are several layers of spherical spongy parenchymatous tissue extended from lamina. The vascular strand embedded in spongy parenchyma of midrib region consist of long compact row of thick walled xylem elements and small nests of phloem tissue, both of which form a bowl shape in the center (Fig. 2). The stomata were of anomocytic type on both of the surfaces. Guard cells are surrounded by limited subsidiary cells which are rarely differentiated from epidermal cells (Fig. 3). Abaxial surface (lower epidermis) contains more stomata as compared to adaxial surface.
Fig. 2.
Transverse section of leaf of M. esculenta viewed at 10*10X
Fig. 3.
Section of lower surface of M. esculenta leaf at 10*10 X view (stomata clearly depicted the structure of an anomocytic).
Fig. 4.
Transverse section of leaf lamina of M. esculenta.
3.3.2. Powdered drug microscopy
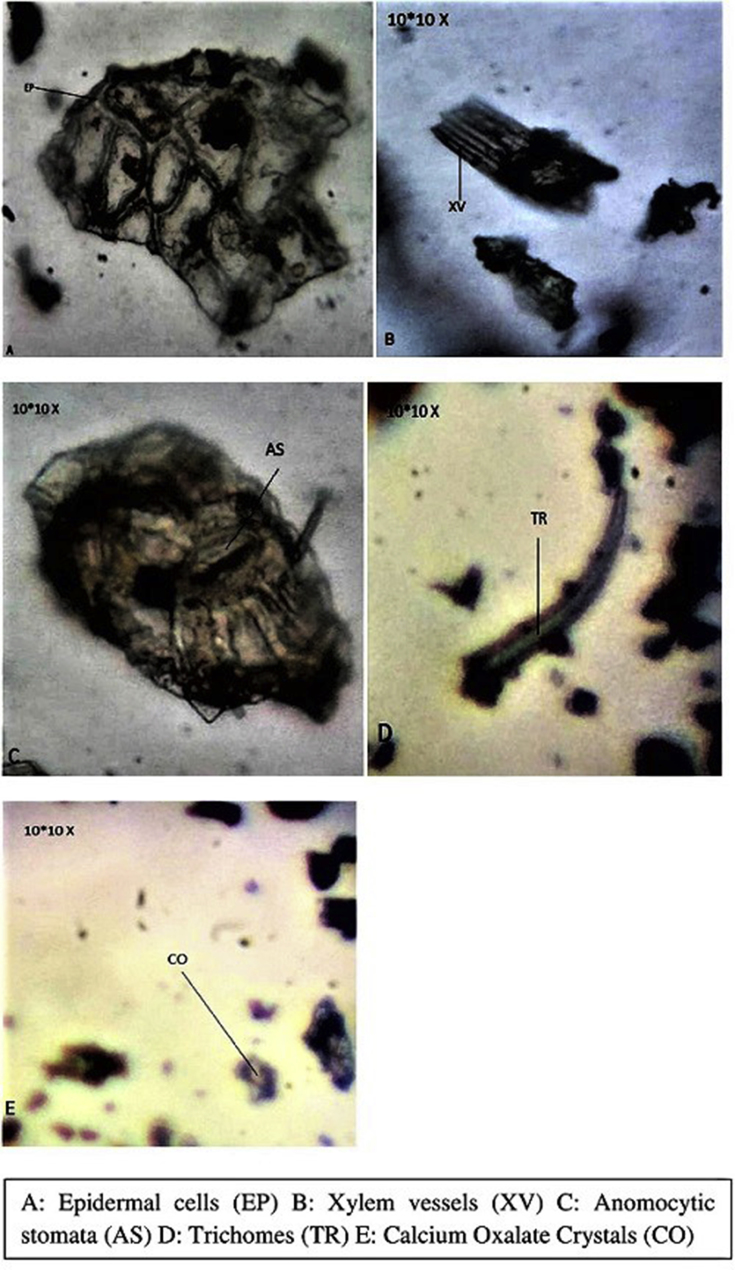
The powder was green in color, with no distinct odor and an astringent taste. It was easy flowable with a fine to coarse texture. Microscopic powder features revealed presence of crystals of calcium oxalate and starch granules; trichomes were found to be hollow unicellular and uniseriate; parts of epidermis in surface view showing straight-walled polygonal epidermal cells, anomocytic stomata and spiral xylem vessels. The guard cells were shrinking elongating the stomata pore (Fig. 5).
Fig. 5.
Powdered characteristics of the leaf of M. esculenta.
3.3.3. Leaf constants
Anomocytic types of stomata were present on the both surfaces of leaves. The stomatal number on the adaxial and abaxial surface was found as 2.14 and 4.25, respectively. The stomatal indexes of adaxial and abaxial surface was found 16.67 and 17.33, respectively. The vein islet and vein termination were calculated as 9-11 and 13-15. The palisade ratio was found to be 2-4.
3.4. Physicochemical evaluation
The results of physicochemical parameters are summarized in Table 1. Sulphated ash value (2.41%) was found lower than the total ash value (2.83%). The acid insoluble ash was found to be 0.52% and water soluble ash value was 0.38%. On further studies it was concluded that the drug contain 8.72% moisture content, foaming index and swelling index were found to be nil, while foreign organic content was reported to be less than 1%. The extractive values for various solvents such as methanol, ethyl acetate and aqueous were found to be 21.2%, 15.7%, 8.72% respectively.
Table 1.
Physicochemical parameters of leaves of M. esculenta.
| Physiochemical parameters | Results (%) | |
|---|---|---|
| Ash value | Total ash | 2.83 |
| Acid insoluble ash | 0.52 | |
| Water soluble ash | 0.38 | |
| Sulphated ash | 2.41 | |
| Extractive value | Methanolic extract | 38.51 |
| Ethyl acetate extract | 21.2 | |
| Aqueous extract | 15.7 | |
| Moisture content | 8.72 | |
| Foreign organic matter | Less that 1% (Presence of petiole stalks with leaves) | |
| Foaming index | Nil | |
| Swelling index | Nil | |
3.5. Preliminary phytochemical screening
Various phytochemical analysis tests supported that the extracts contain alkaloids, carbohydrates, flavonoids, phenolic compounds, tannins and glycosides, recorded in Table 2. The aqueous extract was found to be negative for the presence of alkaloids as compared to methanolic and ethyl acetate extract.
Table 2.
Results of phytochemical screening of different extracts of M. esculenta leaves extracts.
| Constituent | Leaves extracts |
||
|---|---|---|---|
| Aqueous extract | Ethyl acetate extract | Methanolic extract | |
| Alkaloids | −ve | +ve | +ve |
| Carbohydrates | +ve | +ve | +ve |
| Proteins & amino acids | −ve | −ve | −ve |
| Fixed oils & fats | −ve | −ve | −ve |
| Flavonoids | +ve | +ve | +ve |
| Phenolic compounds | +ve | +ve | +ve |
| Tannins | +ve | +ve | +ve |
| Glycosides | +ve | +ve | +ve |
| Saponins | −ve | −ve | −ve |
| Steroids | −ve | −ve | −ve |
+ve- Present, −ve- Absent.
3.6. Fluorescence analysis
The fluorescence characters of powdered drug impart a valuable role in the determination of quality and purity of the drug materials. The powdered drugs when subjected to ultraviolet light and visible light in the presence of various chemical reagents, exhibit characteristic fluorescence [20]. Fluorescence report of M. esculenta powdered leaf is tabulated in Table 3.
Table 3.
Fluorescence characteristics of leaves extracts.
| S. no. | Treatment | Visible light | UV Light |
|
|---|---|---|---|---|
| 254 nm (Short wavelength) | 366 nm (Long wavelength) | |||
| 1 | Leaf powder | Green | Green | Light Green |
| 2 | Leaf powder rubbed on filter paper | Light Green | Light Yellow | Light Green |
| 3 | Leaf powder + 1N NaOH | Reddish Brown | Black | Brown |
| 4 | Leaf powder + 1N HCl | Light Green | Light Yellow | Light Green |
| 5 | Leaf powder + 1N HNO3 | Light Yellowish Green | Light Yellow | Light Yellow |
| 6 | Leaf powder + 1N H2SO4 | Light Green | Light Green | Light Green |
| 7 | Methanolic extract of Leaf powder | Dark Green | Black | Green |
| 8 | Ethyl acetate Extract of Leaf powder | Green | Light Green | Light Green |
| 9 | Aqueous extract of leaf powder | Greenish Brown | No fluorescence | Brown |
3.7. Development of chromatographic HPTLC profile of different extracts of M. esculenta leaves
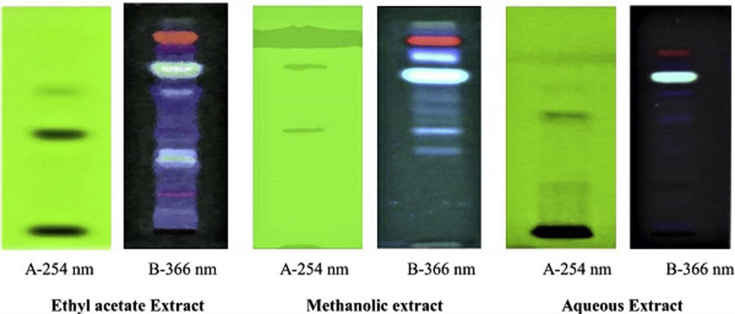
Qualitative HPTLC of ethyl acetate extract, methanolic extract and aqueous extract was carried out by using toluene:ethyl acetate (7:3) solvent system to assure the presence of various phytochemicals. HPTLC chromatogram of ethyl acetate extract (Fig. 6) showed a total of 3 spots at different Rf value at 254 nm, whereas 7 spots were observed at 366 nm (Fig. 6). Methanolic extract showed 2 spots having different Rf values at 254 nm while 5 spots at 366 nm (Fig. 6). Least spots were observed in aqueous extract. There were a total of 2 spots at 254 nm and 3 spots at 366 nm having different Rf values (Fig. 6).
Fig. 6.
Visualization of ethyl acetate, methanolic and aqueous extract of M. esculenta leaves at 254 and 366 nm.
4. Discussion
M. esculenta is used extensively as an ancient traditional medicine for the treatment of wide range of diseases [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. But there are no pharmacopeial standards for the correct identification and authentication of leaf of the plant. Thus, in the present study, the leaf part of M. esculenta was analyzed for its pharmacognostic features. M. esculenta is an evergreen tree having leaves of green color, lanceolate having a smooth, leathery lamina with acute apex [5]. The TS examination and powder microscopy showed a prominent dorsiventral leaf features with presence of calcium oxalate crystals. There are uniseriate hollow trichomes and anomocytic stomata were present on epidermis making these features as important diagnostic characters. Further leaf constant can also be used for identification of leaf and analyzing purity and adulterations.
Physicochemical evaluations provide various important parameters like moisture content, ash values and extractive values for various solvents. The ash values determined in the present study may be useful in stabilizing standards of purity and quality. The low total ash value indicate the low amount of inorganic salts of carbonates, phosphates, silicates of sodium, potassium, calcium and magnesium. The acid insoluble ash was very low that support the fact that a very small amount of the inorganic component is insoluble in acid and this is a diagnostic tool. The moisture content of the fresh leaves was 8.72% showing the leaf dried easily after plucking. The extractive values gives an idea about the nature of the chemical constituents present in the plant and is useful for the estimation of specific constituents soluble in that particular solvent used for the extraction as well as the determination of exhausted materials. The methanol and ethyl acetate soluble extractives were higher than that of water. Thus, these are a good choice as solvent for extraction of leaves of M. esculenta. The fluorescence study showed characteristic fluorescence under visible light and UV light (short and long wave length), and this may be useful in the detection of adulterants.
Preliminary phytochemical screening was done to check the presence of active constituents of the leaves. It reveals the presence of alkaloids, carbohydrates, flavonoids, phenolic compounds, tannins and glycosides. The HPTLC chromatogram of various extracts may be useful in the confirmation of presence of number of constituents along with the respective Rf value.
5. Study limitations and future directions
Limitation of present study is the HPTLC carried out is without use of standard/active compound. Since present report is focused mainly on pharmacognostic evaluation and preliminary phytochemical screening; here HPTLC was performed to confirm the number of phytoconstituents present in different extracts and to support the phytochemical screening. M. esculenta leaves are known to contain very important phytoconstituents, therefore by using standards, different constituents can be identified and even isolated in future studies. Data obtained by present study may be considered as standard for future studies.
6. Conclusion
Macro-microscopic studies revealed that leaves are lancoelate, thin, spirally arranged, dark green in color, astringent taste, acute apex, cuticularised epidermis having polygonal cells, single layered palisade mesophyll cells on each surface, 2–3 layered spongy parenchyma, unicellular and uniseriate hollow trichomes, anomocytic stomata and bowl shaped vascular bundle in mid rib portion. Alkaloids, carbohydrates, flavonoids, glycosides, phenolic compounds and tannins were found present. HPTLC provides number of constituents present in the extracts with their respective Rf value. Present pharmacognostical assay of M. esculenta leaves including data on leaf constants, powder microscopy, fluorescence characteristics and physical parameters provides a vital diagnostic tool for identification, authentication, and detection of adulterants of M. esculenta leaves, along with development of quality parameters of the species.
Sources of funding
Abhilashi University, Mandi.
Conflict of interest
None.
Acknowledgement
The author thanks Dr. R.K. Abhilashi, Chancellor Abhilashi University for providing excellent research facilities. Mr. Gaurav is gratefully acknowledged for helping in collection of plant material.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Upadhya V., Hegde H.V., Bhat S., Hurkadale P.J., Kholkute S.D., Hegde G.R. Ethno medicinal plants used to treat bone fracture from North-Central Western Ghats of India. J Ethnopharmacol. 2012;142:557–562. doi: 10.1016/j.jep.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 2.Kingston D.G. Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod. 2011;74:496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil Vikas V., Patil R. Vijay. Ficus bengalensis Linn.-An overview. Int J Pharm Bio Sci. 2010;1(2):1–11. [Google Scholar]
- 4.Rakholiya K., Chanda S. Pharmacognostic, physicochemical and phytochemical investigation of Mangifera indica L. var. Kesar leaf. Asian Pac J Trop Biomed. 2012;2(Suppl. 2):S680–S684. [Google Scholar]
- 5.Paranjpe P. 3rd ed. Chaukhamba Sanskrit Pratishthan; New Delhi: 2012. Indian medicinal plants; p. 128. [Google Scholar]
- 6.1st ed. vol. III. Government of India, Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homeopathy; New Delhi: 1999. pp. 92–93. (The ayurvedic pharmacopoeia of India, part I). [Google Scholar]
- 7.Chen J., Wang Y., Wu D., Wu Z. Preliminary study on antioxidative and radical scavenging activities of extracts from Myrica esculenta Buch-Ham. Bark. Chem Ind For Prod. 2007:1–7. [Google Scholar]
- 8.Rana R.K., Patel R.K. Antioxidant activity of bark of Myrica nagi. Int J Pharm Sci Rev Res. 2014;28(1):99–101. [Google Scholar]
- 9.Amalraj T., Ignacimuthu S. Antidiabetic effect of Myrica nagi on diabetic rats. Uttar Pradesh J Zool. 1997;17(3):200–202. [Google Scholar]
- 10.Khan M.Y., Sagrawat H., Upmanyu N., Siddique S. Anxiolytic properties of M. nagi bark extract. Pharm Biol. 2008;46(10–11):757–761. [Google Scholar]
- 11.Suryawanshi J.S., Karande K.M., Udugade B.V. Antibacterial activity of bark and fruits of M. nagi. Ind J Nat Prod. 2009;25(3):21–23. Res 2012;26(23):2266-9. [Google Scholar]
- 12.Jain V.K., Jain B. Antihelmintic activity of ethanolic extract of bark of Myrica esculenta. J Pharm Sci. 2010;1(11):129–131. [Google Scholar]
- 13.Patel K.G., Rao N.J., Gajera V., Bhatt P., Patel K., Gandhi T. Anti-allergic activity of stem bark of Myrica esculenta Buch.-Ham. (Myricaceae) J Young Pharm. 2010;2(1):74–78. doi: 10.4103/0975-1483.62219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel T., Dudhpejiya A., Sheath N. Anti inflammatory activity of Myrica nagi Linn. Bark. Anc Sci Life. 2011;30(4):100–103. [PMC free article] [PubMed] [Google Scholar]
- 15.Patel T., Rajshekar C., Parmar R. Mast cell stabilizing activity of Myrica nagi bark. J Pharmacogn Phytother. 2011;3(8):114–117. [Google Scholar]
- 16.Patel T., Shah S. Antiasthmatic activity of aqueous extract of M. nagi bark. J Curr Pharm Res. 2012;10(1):34–39. [Google Scholar]
- 17.Singh J., Lan V.K., Trivedi V.P. Pharmacognostic evaluation of Katphala (the bark of Myrica esculenta Buch–Ham) Anc Sci Life. 1986;6(2):85–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava B., Sharma V.C., Pant P., Pandey N.K., Jadhav A.D. Evaluation for substitution of stem bark with small branches of Myrica esculenta for medicinal use e a comparative phytochemical study. J Ayu Int Med. 2016;7:218–223. doi: 10.1016/j.jaim.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amponsah I.K., Mensah A.Y., Otoo A., Mensah M.L.K., Jonathan J. Pharmacognotic standardisation of Hilleria latifolia (Lam.) H. Watt. (Phytolaccaceae) Asian Pac J Trop Biomed. 2014;4:9410–9946. [Google Scholar]
- 20.Kumar D., Kumar K., Kumar S., Kumar T., Kumar A., Prakash O. Pharmacognostic evaluation of leaf and root bark of Holoptelea integrifolia Roxb. Asian Pac J Trop Biomed. 2012;2:169–175. doi: 10.1016/S2221-1691(12)60036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbar S., Hanif U., Ali J., Ishtiaq S. Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac J Trop Biomed. 2014;4(5):410–415. doi: 10.12980/APJTB.4.2014C1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najafi S., Deokule S.S. Pharmacognostic study of Tylophora dalzelli Hook.F. J Med Plant Res. 2010;4(5):403–406. [Google Scholar]
- 23.Menpara D., Chanda S. Phytochemical and pharmacognostic evaluation of leaves of Pongamia pinnata L. (Fabaceae) Phcog Commun. 2014;4(2):3–7. [Google Scholar]
- 24.Sethi P.D. Student 1st ed. vol. X. CBS Publishers and Distributers; New Delhi: 1996. (High performance thin layer chromatography). [Google Scholar]