Abstract
Background
As we transition to value-based care delivery models, risk stratification in total joint arthroplasty is more important than ever. The purpose of this study was to identify patients who would likely require higher level of care and may not be suitable for inclusion in bundled payment models.
Methods
The American College of Surgeons National Surgical Quality Improvement Program database was queried for all patients who underwent primary total joint arthroplasty between 2011 and 2012. Five types of adverse events were assessed: medical complications, surgical complications, readmission, reoperation, and mortality. Univariate and multivariate logistic regression analyses were performed using a large number of demographic and morbidity variables.
Results
A total of 14,185 patients were identified. The 30-day medical complication, surgical complication, readmission, reoperation, and mortality rates were 2.0%, 3.2%, 4.0%, 1.5%, and 0.2%, respectively. Among the different variables assessed, only the American Society of Anesthesiologists (ASA) physical classification system was a significant risk factor for most outcomes assessed. Peripheral vascular disease was the most significant risk factor for medical complications and reoperation (odds ratio, 2.73 and 3.23, respectively). Bleeding disorders were the most significant risk factor for readmission and mortality (odds ratio, 2.03 and 5.86, respectively).
Conclusions
ASA score is a more reliable risk stratification tool than Charlson Comorbidity Index, but it is not sufficient by itself. Patients with higher ASA scores combined with peripheral vascular disease and/or bleeding disorders are at especially high risk of developing postsurgical adverse events and may not be suitable for inclusion in bundled payment models. These data can be used to develop better risk stratification models that are critically needed.
Keywords: Arthroplasty, Hip, Knee, Risk stratification, ASA physical classification system, Charlson Comorbidity Index
Introduction
In a healthcare environment that increasingly prioritizes value-based metrics, risk stratification can help guide preoperative counseling, mitigate potential complications, and allocate perioperative resources appropriately. Orthopaedic surgeons often use the American Society of Anesthesiologists physical status classification (ASA-PSC) or Charlson Comorbidity Index (CCI). The ASA was established in 1941 with the goal of establishing a patient’s degree of systemic illness before an anesthetic procedure. Since its development, the ASA score has increasingly been used as a tool to identify a patient’s perioperative risks, including mortality and adverse outcomes [1]. The CCI was first developed in 1987 as an attempt to more accurately predict the 1-year mortality risk due to severe comorbid conditions [2]. Estimation of the CCI score is more complex than that of the ASA, requiring measurement of several conditions, some of which receive more weight than others. To date, the comparative utility of ASA vs CCI in total joint arthroplasty (TJA) has not been previously reported.
Identifying risk factors for adverse events in TJA has been a major topic of research in recent years. Among these factors are diabetes, heart failure, chronic obstructive pulmonary disease, renal insufficiency, lower extremity arterial calcification, epilepsy, malnutrition, hypothyroidism, obesity, chronic opioid use, young age, and sleep apnea [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. This exhaustive list makes it difficult to guide reliable risk stratification processes. In addition, our state of risk stratification is limited by heterogenous patient populations, sample sizes, study methodologies, range of potential variables, and outcomes assessed.
The purpose of this study was to report on the incidence and risk factors for adverse events in a large population of patients undergoing elective, primary, unilateral TJA. Adverse events were divided into 5 categories: medical complications, surgical complications, readmission, reoperation, and mortality. The comparative utility of ASA and CCI was investigated. Additionally, using a large database, the relative contributions of a wide panel of morbid conditions to adverse events was investigated. Arthroplasty surgeons and patients may benefit from a reliable and efficient risk stratification system to guide preoperative counseling, optimization, and resource allocation.
Material and methods
Data collection
Institutional review board approval was not required. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The database was launched in 2004 with the intent of helping public and private hospitals understand the quality of their surgical programs compared to those from similar hospitals [14]. Demographic and perioperative data are collected prospectively. Patient outcomes are tracked for 30 days after discharge. The data are internally audited to ensure accuracy with reported discrepancy typically around 2% [14].
All patients who underwent total hip or knee arthroplasty (THA/TKA) between 2011 and 2012 were included in this analysis. Emergent and nonelective procedures were excluded. Outside of these 2 years, the NSQIP database did not specify the nature of the arthroplasty procedure (elective vs nonelective), and information needed to calculate the CCI score was not routinely collected. Patients were selected using the Current Procedural Terminology (CPT) codes 27130 (arthroplasty, acetabular, and proximal femoral prosthetic replacement, with or without autograft or allograft) and 27447 (arthroplasty, knee, condyle, and plateau; medial and lateral compartments, with or without patellar resurfacing), leading to the identification of 5251 THAs and 8934 TKAs.
Patient and preoperative measures
Specific patient characteristics, CCI, and ASA were synthetized from the NSQIP database and included in the analysis. Demographic variables included procedure type, age, sex, race, and body mass index (BMI). The ASA score was readily available in the NSQIP database. A modified CCI score, which has been used in previous publications, was calculated based on the available information in the database according to the following formula: peripheral vascular disease (PVD, 1 point), congestive heart failure (1 point), prior myocardial infarction (1 point), diabetes mellitus (1 point), prior transient ischemic attack/stroke (1 point), chronic obstructive pulmonary disease (1 point), renal failure (2 points), hemiplegia (2 points), ascites or esophageal varices (3 points), metastatic cancer (6 points), and age beyond 40 (1 point per decade greater than 40) [15].
Patient outcome measures
The primary outcomes assessed included postoperative complications, readmission, reoperation, and mortality. The NSQIP database only reports events occurring in the first 30 days following surgery. Medical complications consisted of septic shock, coma, cardiac arrest, myocardial infarction, stroke, renal failure, pneumonia, and urinary tract infection. Surgical complications consisted of wound infection, ventilation exceeding 48 hours, reintubation, venous thromboembolism, and return to the operating room. Readmission was defined as a return to the same or different hospital for any reason within 30 days of the initial procedure. Reoperation was defined as any unplanned return to the operating room for a surgical procedure related to either the index or concurrent procedure within 30 days of the initial procedure.
Statistical analysis
Data were imported and analyzed with Stata 15.0 software (StataCorp LLC, College Station, TX). Significant differences in patient characteristics between adverse event and nonadverse event groups were first assessed. Welch’s 2-sample t-test or Wilcoxon’s rank-sum test was used for numerical variables and Pearson’s chi-squared test or Fisher exact test for count data was used for categorical variables. Demographic variables demonstrating a significant P value were included in subsequent multivariable analyses. Assessment of the relationship between significant risk factors and the likelihood of an adverse event was described through a multivariable regression model to yield adjusted odds ratios (OR). P values were reported against a two-sided alpha significance = .05.
Results
Patient characteristics
A total of 5251 THAs and 8934 TKAs were included in the analysis. There were 39.8% males and 60.2% females with a mean age of 66.7 ± 10.5 years and mean BMI of 32.7 ± 7.3. The majority of patients (91.0%) were of white race followed by African Americans (7.3%). Comparison of patients who developed adverse events vs those without adverse events yielded significant differences with regard to age, BMI, ASA, CCI, and a number of morbid conditions. Detailed baseline patient characteristics are described in Table 1.
Table 1.
Baseline characteristics of the study cohorts.
| Variable | No adverse event | Any adverse event | P value |
|---|---|---|---|
| Patients (N) | 13,489 | 696 | – |
| Procedure | |||
| Total hip arthroplasty | 5020 (37.2%) | 231 (33.2%) | – |
| Total knee arthroplasty | 8469 (62.8%) | 465 (66.8%) | |
| Laterality | |||
| Unilateral | 13,287 (98.5%) | 683 (98.1%) | .436b |
| Bilateral | 202 (1.5%) | 13 (1.9%) | |
| Demographic characteristics | |||
| Age (y) | 66.6 ± 10.5 | 68.2 ± 10.7 | <.0001a |
| Sex | |||
| Male | 5348 (38.7%) | 294 (42.2%) | .176b |
| Female | 8134 (60.3%) | 402 (57.8%) | |
| Race | |||
| White | 9833 (90.1%) | 479 (91.9%) | .914d |
| Black or African American | 793 (7.3%) | 37 (7.1%) | |
| Asian | 124 (1.2%) | 4 (0.8%) | |
| American Indian | 49 (0.5%) | 1 (0.2%) | |
| Pacific Islander | 15 (0.1%) | 0 (0.0%) | |
| Body mass index, kg/m2 | 32.5 ± 7.3 | 34.1 ± 8.3 | <.0001a |
| Comorbidities | |||
| Diabetes mellitus | 2052 (15.2%) | 152 (21.8%) | <.0001b |
| Dyspnea | 1086 (8.1%) | 74 (10.6%) | .015b |
| Hypertension | 8394 (62.2%) | 477 (68.5%) | .001b |
| Chronic heart failure | 21 (0.2%) | 2 (0.3%) | .313d |
| COPD | 493 (3.7%) | 45 (6.5%) | <.0001b |
| Myocardial infarction | 10 (0.1%) | 2 (0.3%) | .115d |
| Stroke | 95 (0.7%) | 11 (1.6%) | .009b |
| Bleeding disorder | 297 (2.2%) | 25 (3.6%) | .016b |
| Peripheral vascular disease | 71 (0.5%) | 14 (2.0%) | <.0001b |
| Esophageal varices | 3 (0.0%) | 1 (0.1%) | .182d |
| Liver disease | 1 (0.0%) | 1 (0.1%) | .096d |
| Renal disease | 10 (0.1%) | 1 (0.1%) | .425d |
| Metastatic cancer | 14 (0.1%) | 1 (0.1%) | .530d |
| ASA classification | 2.4 ± 0.6 | 2.6 ± 0.6 | <.0001c |
| Charlson comorbidity index | 2.4 ± 1.2 | 2.7 ± 1.3 | <.0001c |
COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists.
Bolded values represents the statistical significance of P values.
Welch’s 2-sample t-test.
Pearson’s chi-squared test.
Wilcoxon’s rank-sum test.
Fisher exact test for count data.
Rates of the adverse events
The rates of medical complications, surgical complications, readmissions, reoperations, and mortality were 2.0%, 3.2%, 4.0%, 1.5%, and 0.2%, respectively.
Risk factors for medical complications
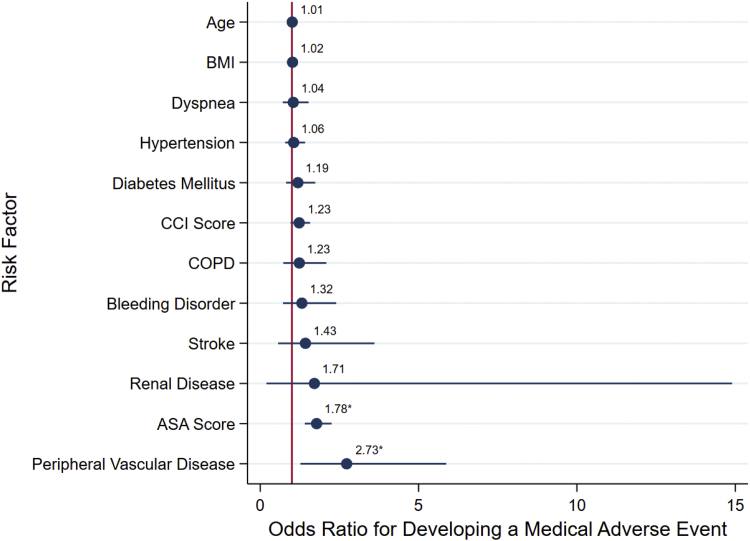
ASA-PSC and PVD were the only significant risk factors for medical complications (OR, 1.78; 95% confidence interval [CI], 1.41-2.25 and OR, 2.73; 95% CI, 1.27-5.87, respectively). Figure 1 summarizes the multivariable model for medical complications.
Figure 1.
Multimodal logistic regression analysis showing adjusted odds ratio scatter chart for the associations between development of a medically related adverse event and Charlson Comorbidity Index (CCI), American Society of Anesthesiologists (ASA) physical classification system, or demographic and comorbidity variables shown to be significant. ∗Indicates of significance the risk factor in the multimodal model at the P = .05 level. BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Risk factors for surgical complications
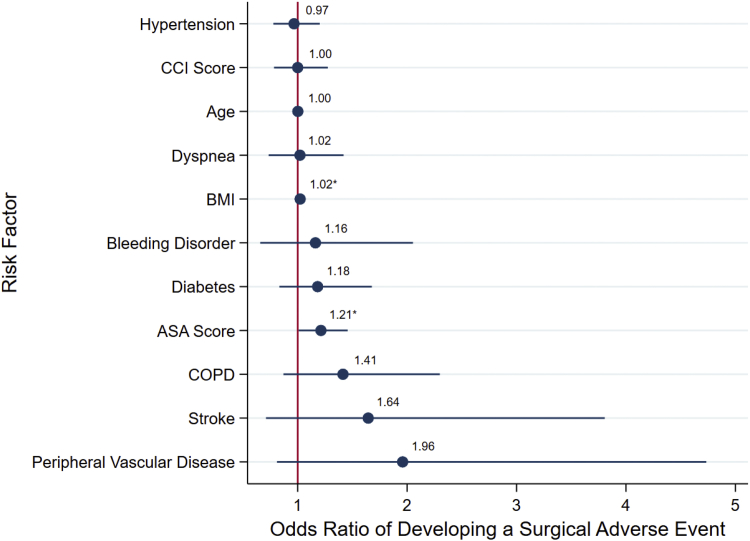
ASA-PSC and BMI were the only significant risk factors for surgical complications (OR, 1.21; 95% CI, 1.01-1.46 and OR, 1.02; 95% CI, 1.01-1.03, respectively). Figure 2 summarizes the multivariable model for surgical complications.
Figure 2.
Multimodal logistic regression analysis showing adjusted odds ratio scatter chart for the associations between development of a surgically related adverse event and CCI, ASA physical classification system, or demographic and comorbidity variables shown to be significant. ∗Indicates of significance the risk factor in the multimodal model at the P = .05 level.
Risk factors for readmissions
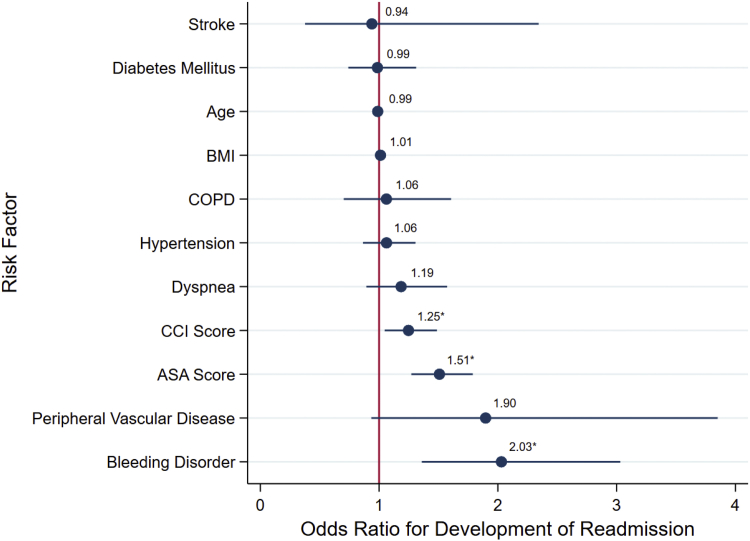
ASA-PSC, CCI, and bleeding disorders were the only significant risk factors for readmission (OR, 1.51; 95% CI, 1.27-1.79; OR, 1.25; 95% CI, 1.05-1.49; and OR, 2.03; 95% CI, 1.36-3.03, respectively). Figure 3 summarizes the multivariable model for readmission.
Figure 3.
Multimodal logistic regression analysis showing adjusted odds ratio scatter chart for the associations between development of readmission and CCI, ASA physical classification system, or demographic and comorbidity variables shown to be significant. ∗Indicates of significance the risk factor in the multimodal model at the P = .05 level.
Risk factors for reoperation
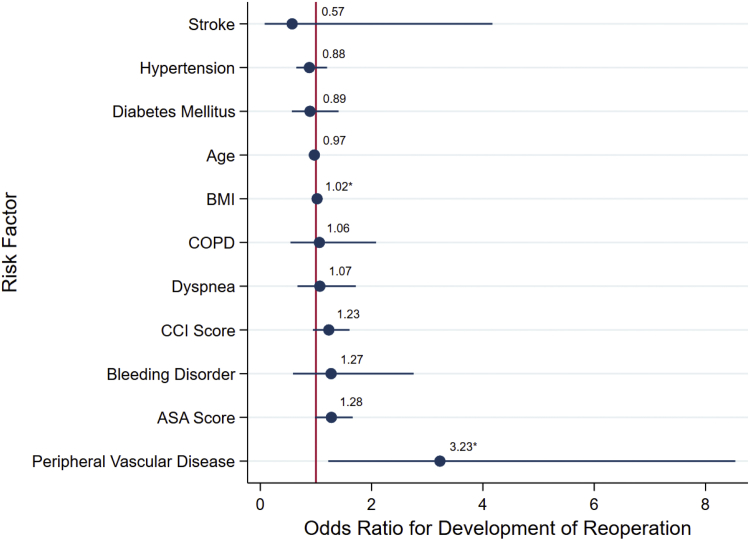
BMI and PVD were the only significant risk factors for reoperation (OR, 1.02; 95% CI, 1.00-1.04 and OR, 3.23; 95% CI, 1.22-8.54, respectively). Figure 4 summarizes the multivariable model for reoperation.
Figure 4.
Multimodal logistic regression analysis showing adjusted odds ratio scatter chart for the associations between development of reoperation and CCI, ASA physical classification system, or demographic and comorbidity variables shown to be significant. ∗Indicates of significance the risk factor in the multimodal model at the P = .05 level.
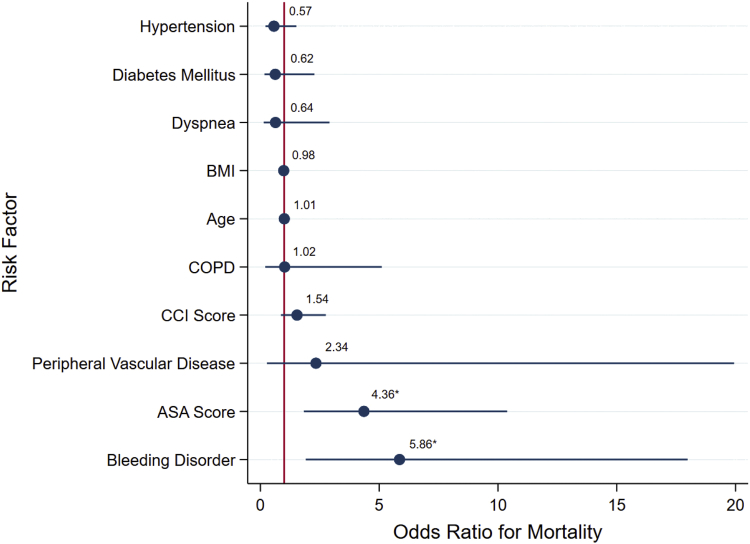
Risk factors for mortality
ASA-PSC and bleeding disorders were the only significant risk factors for mortality (OR, 4.36; 95% CI, 1.83-10.39 and OR, 5.86; 95% CI, 1.91-17.98, respectively). Figure 5 summarizes the multivariable model for mortality.
Figure 5.
Multimodal logistic regression analysis showing adjusted odds ratio scatter chart for the associations between mortality and CCI, ASA physical classification system, or demographic and comorbidity variables shown to be significant. ∗Indicates of significance the risk factor in the multimodal model at the P = .05 level.
Discussion
In an era where quality and value are increasingly important and with the increasing use of bundled payment programs, risk stratification for adverse events have gained more importance. Various predictors of adverse events have been used to help identify high-risk patients and provide appropriate perioperative considerations [15]. Although orthopaedic surgeons commonly use ASA or CCI to establish a patient’s morbidity risk, it has been unclear whether any of these measures is superior as a risk stratification tool. Meaningful comparison of these indices may enable arthroplasty surgeons to reliably choose one measure in clinical and research settings. This can yield improved efficiency as all indices use slightly different preoperative characteristics and gathering data for some of them (ie, CCI) can be cost-intensive and time-intensive. In this study, we used the ACS-NSQIP database to identify the risk factors for 5 types of adverse events following TJA: medical complications, surgical complications, readmissions, reoperations, and mortality. ASA, CCI, and all morbid conditions collected in the ACS-NSQIP were analyzed. Identifying major risk factors for adverse events after THA is a fundamental first step for risk stratification.
Our study demonstrated the superiority of ASA-PSC over CCI across all 5 outcomes assessed. In addition to ASA-PSC, PVD was a major risk factor for developing any adverse event, particularly medical complications and reoperations. Bleeding disorders were a major risk factor for readmissions and mortality. To date, there is limited research focused on the effects of PVD or bleeding disorders on TJA outcomes. Cantu Morales et al [3] retrospectively reviewed 900 patients undergoing TKA and found that the presence of lower extremity arterial calcification on preoperative radiographs carried a high risk of having a perioperative cerebrovascular event and should prompt the surgeon for further preoperative cardiac workup. Cancienne et al [16] retrospectively reviewed 4775 patients with bleeding disorders undergoing TKA matched with 427,132 controls using the PearlDiver database. The authors found that patients with either hemophilia or von Willebrand's disease were at significantly higher risk of infection, transfusion, medical complications, and revision after TKA compared to matched controls. Hustedt et al [4] conducted a retrospective review of 4,323,045 patients undergoing TJA using he National Inpatient Sample and found coagulopathy was associated with the highest overall hospital costs.
To our knowledge, there are also no analogous previous studies that compared ASA and CCI in primary TJA. Lakomkin et al [17] retrospectively reviewed 6121 patients undergoing revision THA using the ACS-NSQIP database and found a positive but weak association between CCI and adverse events (OR, 1.12; 95% CI, 1.05-1.20). Ondeck et al [18] retrospectively reviewed 16,495 patients undergoing posterior lumbar fusion using the ACS-NSQIP database. Compared to CCI, the authors found the ASA classification system to be a slightly superior predictor for postoperative adverse events.
This study has some limitations. First, it is a retrospective review of a national database that is subject to coding and data logging errors. Second, our analysis was based on an adjusted CCI. Second, the ACS-NSQIP database does not collect adverse event data beyond 30 days. Despite these limitations, this study addressed important questions regarding the discriminative ability of commonly used comorbidity indices in predicting adverse events following TJA and the major risk factors for different adverse outcomes.
Conclusions
In summary, we demonstrated that the ASA-PSC to be a better predictor for postoperative adverse events than the CCI. This has significant clinical and research implications as ASA-PSC is a simple readily available index that does not require subscription fees or complex computations. In addition, we identified the major contribution of PVD and bleeding disorders to post-TJA complications, readmissions, reoperations, and mortality. Most importantly, our study reiterates the shortfalls of our most commonly used morbidity indices. The limitation of using either ASA or CCI is that each method captures a limited picture of each patient’s risk. For example, none of these methods take into account factors such as psychological distress, physical functioning, surgical indication, and case complexity. Further studies are much needed to develop enhanced risk stratification models.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.10.002.
Appendix A. Supplementary data
References
- 1.Hopkins T.J., Raghunathan K., Barbeito A. Associations between ASA Physical Status and postoperative mortality at 48 h: a contemporary dataset analysis compared to a historical cohort. Perioper Med (Lond) 2016;5:29. doi: 10.1186/s13741-016-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 3.Cantu Morales D., de Beer J., Petruccelli D., Kabali C., Winemaker M. Lower extremity arterial calcification on preoperative knee radiographs as a predictor of postoperative cardiovascular events after primary total knee arthroplasty. J Arthroplasty. 2018;33(4):1181. doi: 10.1016/j.arth.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Hustedt J.W., Goltzer O., Bohl D.D., Fraser J.F., Lara N.J., Spangehl M.J. Calculating the cost and risk of comorbidities in total joint arthroplasty in the United States. J Arthroplasty. 2017;32(2):355. doi: 10.1016/j.arth.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh P.K., Chen A.F., Rasouli M.R., Post Z.D., Orozco F.R., Ong A.C. Complications and mortality in chronic renal failure patients undergoing total joint arthroplasty: a comparison between dialysis and renal transplant patients. J Arthroplasty. 2016;31(2):465. doi: 10.1016/j.arth.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Webb M.L., Golinvaux N.S., Ibe I.K., Bovonratwet P., Ellman M.S., Grauer J.N. Comparison of perioperative adverse event rates after total knee arthroplasty in patients with diabetes: insulin dependence makes a difference. J Arthroplasty. 2017;32(10):2947. doi: 10.1016/j.arth.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Curtis G.L., Newman J.M., George J., Klika A.K., Barsoum W.K., Higuera C.A. Perioperative outcomes and complications in patients with heart failure following total knee arthroplasty. J Arthroplasty. 2018;33(1):36. doi: 10.1016/j.arth.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Buller L.T., Rosas S., Sabeh K.G., Roche M.W., McLawhorn A.S., Barsoum W.K. Hypothyroidism increases 90-day complications and costs following primary total knee arthroplasty. J Arthroplasty. 2018;33(4):1003. doi: 10.1016/j.arth.2017.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohl D.D., Shen M.R., Kayupov E., Della Valle C.J. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after total joint arthroplasty. J Arthroplasty. 2016;31(1):15. doi: 10.1016/j.arth.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Alvi H.M., Mednick R.E., Krishnan V., Kwasny M.J., Beal M.D., Manning D.W. The effect of BMI on 30 day outcomes following total joint arthroplasty. J Arthroplasty. 2015;30(7):1113. doi: 10.1016/j.arth.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 11.Rozell J.C., Courtney P.M., Dattilo J.R., Wu C.H., Lee G.C. Preoperative opiate use independently predicts narcotic consumption and complications after total joint arthroplasty. J Arthroplasty. 2017;32(9):2658. doi: 10.1016/j.arth.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Aujla R.S., Esler C.N. Total knee arthroplasty for osteoarthritis in patients less than fifty-five years of age: a systematic review. J Arthroplasty. 2017;32(8):2598. doi: 10.1016/j.arth.2017.02.069. [DOI] [PubMed] [Google Scholar]
- 13.Naqvi S.Y., Rabiei A.H., Maltenfort M.G. Perioperative complications in patients with sleep apnea undergoing total joint arthroplasty. J Arthroplasty. 2017;32(9):2680. doi: 10.1016/j.arth.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 14.User Guide for the 2014 ACS NSQIP Participant Use Data File . 2015. American College of Surgeons National Surgical Quality Improvement Program. https://www.facs.org/∼/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2014.ashx. [accessed 27.10. 2018] [Google Scholar]
- 15.Soffin E.M., YaDeau J.T. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62. doi: 10.1093/bja/aew362. [DOI] [PubMed] [Google Scholar]
- 16.Cancienne J.M., Werner B.C., Browne J.A. Complications after TKA in patients with hemophilia or von Willebrand's disease. J Arthroplasty. 2015;30(12):2285. doi: 10.1016/j.arth.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Lakomkin N., Goz V., Lajam C.M., Iorio R., Bosco J.A. Higher modified Charlson index scores are associated with increased incidence of complications, transfusion events, and length of stay following revision hip arthroplasty. J Arthroplasty. 2017;32(4):1121. doi: 10.1016/j.arth.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Ondeck N.T., Bohl D.D., Bovonratwet P. Discriminative ability of commonly used indices to predict adverse outcomes after poster lumbar fusion: a comparison of demographics, ASA, the modified Charlson Comorbidity Index, and the modified Frailty Index. Spine J. 2018;18(1):44. doi: 10.1016/j.spinee.2017.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.