Abstract
Inflammatory demyelinating polyneuropathy is a rare but devastating condition. Guillain-Barré syndrome is the most common cause with acute inflammatory demyelinating polyneuropathy being the most common subtype that follows a monophasic course and does not recur. Chronic inflammatory demyelinating polyneuropathy occurs when symptoms persist for greater than 8 weeks. With many proposed etiologies, few reports have described acute inflammatory demyelinating polyneuropathy after total joint arthroplasty. To our knowledge, this is the first case report of chronic inflammatory demyelinating polyneuropathy developing after total hip arthroplasty that was further complicated by dislocation.
Keywords: Guillain-Barré syndrome, Acute inflammatory demyelinating polyneuropathy, Chronic inflammatory demyelinating polyneuropathy, Inflammatory demyelinating polyneuropathy, Total hip arthroplasty, Dislocation
Introduction
The onset of generalized polyneuropathy, characterized by symmetrical sensory symptoms located in the distal parts of the arms and legs that spread toward the center of the body, is a relatively uncommon condition with a prevalence of up to 7% in the general population [1]. These progressive symptoms can induce significant complications. Patients with unconstrained joint replacements, particularly total hip arthroplasty (THA), are an extremely vulnerable patient population, especially in the postoperative period. With over 100 different causes of polyneuropathy [1], we present a case report of a patient who developed an inflammatory demyelinating polyneuropathy after a THA. This case report illustrates important steps in the diagnosis, management, and orthopaedic considerations of this condition.
Case history
A 71-year-old female underwent a right cementless THA via a posterior approach after informed consent (Figs. 1 and 2). Preoperative evaluation revealed medical comorbidities that included hypertension, dyslipidemia, depression, gastroesophageal reflux disease, history of a thoracic disc herniation, and lumbar spinal stenosis. She also had a smoking history of at least 0.5 packs of cigarettes per day for over 50 years. Per protocol, she received 2 grams of Ancef and 1 gram of tranexamic acid within 1 hour of incision. She received a continuous spinal epidural (0.5% bupivicaine at 0.2 mg/kg) with duramorph (4.1 mcg/kg) and light sedation using propofol. Intraoperative stability was excellent through a simulated full range of motion. There were no unexpected intraoperative events or complications. The patient was transferred from the postanesthesia care unit to the joint restoration unit where she received physical therapy twice daily and progressed through therapy milestones without difficulty. She was discharged home with home health physical therapy on postoperative day 4 with warfarin 2 mg oral daily for 4 weeks titrated to a therapeutic international normalized ratio.
Figure 1.
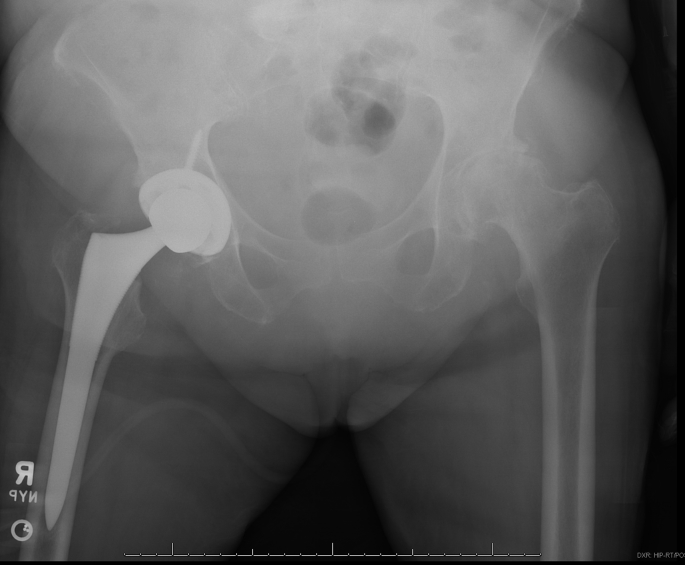
End-stage osteoarthritis of bilateral hips.
Figure 2.
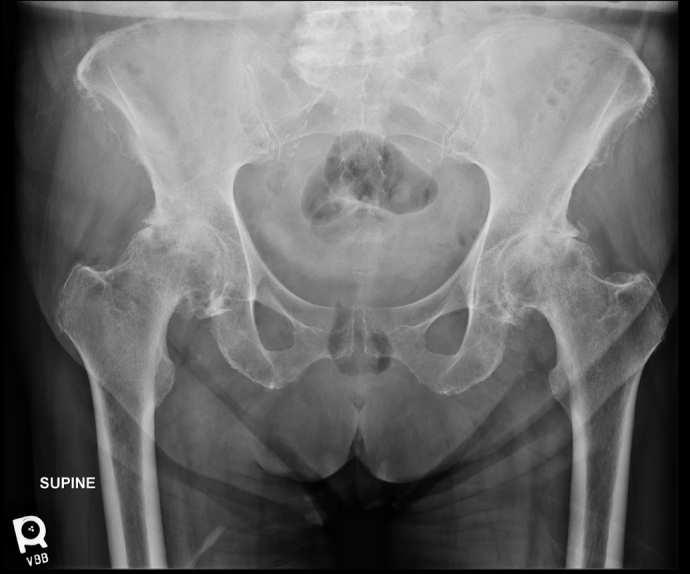
Right total hip arthroplasty.
She was seen for routine follow-up 1 week after discharge, at which time she reported to be doing exceptionally well.
Ten days later (21 days after surgery), the patient was seen in the orthopaedic clinic because of new-onset low back pain, imbalance, and lower extremity weakness. She denied any associated illness, fevers, chills, nausea, vomiting, diarrhea, chest pain, dyspnea, dysphagia, changes in vision, or bowel/bladder irregularities. Her examination at that time demonstrated 5/5-muscle strength in her upper extremities with diminished sensation distal to her wrists (radial more so than median and ulnar distributions), which was unchanged from baseline per the patient. She had intact sensation in all lower extremity cutaneous and dermatomal distributions bilaterally. Bilateral lower extremities had slightly diminished strength graded as 4/5, hyperreflexia throughout, no clonus, and an upward going great toe on Babinski examination. Given these acute changes, she was admitted to the hospital for further evaluation and management.
On the morning of her second hospital day, she experienced a drastic reduction in her strength with diffuse areflexia throughout her upper and lower extremities. A neurologist was consulted, and she underwent further workup including a magnetic resonance imaging (MRI) of her spine, lumbar puncture, and electromyography (EMG).
The MRI of her spine was read as having moderate-to-severe stenosis at the level of her C5-6 with associated myelomalacia and multilevel cervical and lumbar foraminal stenosis. Her EMG had electrodiagnostic evidence suggestive of an acquired demyelinating neuropathy and severe length-dependent axonal sensorimotor polyneuropathy. Her lumbar puncture analysis revealed a cerebrospinal fluid (CSF) protein level of 406 mg/L and a white blood cell (WBC) count of 5 cells/μL consistent with albuminocytologic dissociation, which is an elevation in CSF protein without an accompanying elevation in WBCs.
After obtaining these findings, she was diagnosed with acute inflammatory demyelinating polyneuropathy (AIDP). She was transferred to the neurology intensive care unit where she was started on a 5-day course of intravenous immune globulin (IVIG) on hospital day 3 (24 days after surgery).
Her condition continued to deteriorate over the next few days with worsening flaccid dysarthria and complete absence of motor and sensation in her bilateral lower extremities. In addition, she was found to have a shortened, adducted, and internally rotated right leg on morning rounds. No falls or other acute events were recorded. Subsequent radiographs demonstrated a posterior dislocation of her right prosthetic hip (Fig. 3). On discovery of these findings, she underwent a closed reduction of her right hip at beside without sedation. Her hip was found to have a stable range of motion at 90° of flexion with at least 45° of internal and external rotation. She was placed in an abduction pillow and knee immobilizer.
Figure 3.
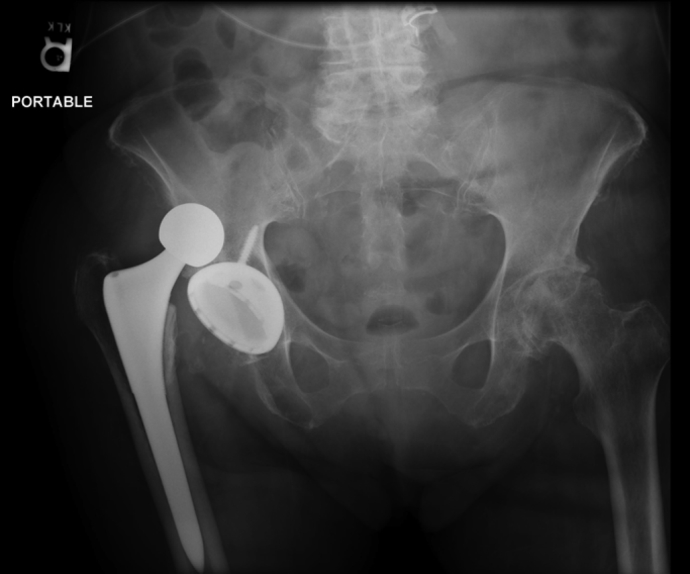
Dislocated right total hip arthroplasty.
The following week brought clinical improvement with resolving distal motor dysfunction and dysarthria until hospital day 19 (37 days after surgery) when she developed worsening of her upper extremity and cranial nerve function. Neurology determined that she likely had a relapse of her AIDP and was therefore restarted on another 3-day course of IVIG. The patient subsequently became dyspneic, developed respiratory distress, and subsequently required intubation.
Her condition continued to lack improvement with her mental status continuing to wax and wane along with responsiveness. In addition, her strength continued to deteriorate. Neurology recommended weekly IVIG infusions to begin 1 week after her last 3-day course. She subsequently developed a deep vein thrombosis in her right axillary vein despite being on Coumadin. After diagnosis, she was switched to therapeutic lovenox.
She received a second EMG examination on hospital day 46 (64 days after surgery). The new EMG showed evidence of a diffuse, severe neurogenic process consistent with progression of her disease process, and ongoing secondary axonal loss. She sustained another deterioration in her mental status the following day and could no longer follow commands, respond to painful stimuli, and had complete absence of her reflexes. At this time, an MRI was ordered which showed significant white matter disease and atrophy. A new electroencephalography was obtained which showed generalized theta slowing indicative of bihemispheric dysfunction.
She continued to remain unresponsive without any other acute conditions for the remainder of her acute hospitalization. She continued to be areflexic with an inability to withdraw from pain. The neurologist determined that she should receive monthly IVIG infusions. She was subsequently discharged to a long-term care facility on hospital day 59 with a diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). She returned to the emergency room (ER) 1 month later with a repeat dislocation that was reduced by the ER physician. She returned to her long-term care facility directly from the ER. Unfortunately, the patient was lost to follow-up and her outcome is not known.
Discussion
There have been few reports of inflammatory demyelinating polyneuropathy associated with orthopaedic surgery [2], [3], [4], [5], [6], [7], [8], [9]. Few studies have highlighted inflammatory demyelinating polyneuropathy after arthroplasty [3], [4], [5]. This is the first report, to our knowledge, that describes a case of CIDP after a THA that was further complicated by postoperative hip dislocation. In this discussion, we will emphasize how orthopaedic surgeons can recognize inflammatory demyelinating polyneuropathy early on as well as highlight special considerations when caring for patients that develop an inflammatory demyelinating polyneuropathy after orthopaedic surgery.
Inflammatory demyelinating polyneuropathy
Guillain-Barré syndrome (GBS) is an acute monophasic immune-mediated polyneuropathy with an overall incidence between 1.1/100,000/year and 1.8/100,000/year with an increase in incidence after the age of 50 years from 1.7/100,000/year to 3.3/100,000/year [10], [11]. The disease process of inflammatory demyelinating polyneuropathy usually starts with symptoms of acroparesthesia (burning, tingling, pricking sensations, or numbness) followed by the predominate feature of symmetric ascending weakness followed by a mild and delayed objective sensory loss and hyporeflexia or areflexia. Most patients initially present with leg weakness (32%) or selective proximal and distal leg weakness (56%) often spreading to the arm, whereas some have onset of weakness in the arms (12%) [12].
The etiology of inflammatory demyelinating polyneuropathy is not clearly understood. It is believed to be an immune-mediated process caused by cellular and humoral immune mechanisms against peripheral nerves [13]. GBS is preceded by symptoms of an upper respiratory tract infection or diarrhea in two thirds of cases [14]. Campylobacter jejuni is the most frequently identified organism with reports also identifying other infectious agents such as Epstein-Barr virus, varicella-zoster virus, and Mycoplasma pneumonia [14]. GBS has been reported after both spinal and epidural anesthesia [15], [16]. Epidural morphine has even been proposed as a potential cause [17], [18]. Postsurgical GBS may occur in 1.5%-2.0% of all cases, and the potential triggering factor may be the major surgical stress [19]. Gensicke et al. [13] showed that 9.5% of patients with GBS had undergone surgery within a 6-week period before diagnosis.
The diagnostic criteria for GBS have stood the test of time [20], and the differential diagnosis is wide and depends on the physician recognizing that the problem is an acute peripheral neuropathy and not a brainstem, spinal cord, or conus lesion. Neurophysiological studies help to confirm the presence, pattern, and severity of neuropathy; however, there is no consensus on neurophysiological criteria for classification like there is for clinical diagnostic criteria [21]. In addition to neurophysiological testing, lumbar puncture can aid in the diagnosis. A raised CSF protein concentration without an accompanying elevation in WBCs, termed albuminocytologic dissociation, is present in 75% of patients by the third week, but is only present in up to 50% of patients during the first week of illness [14].
AIDP vs CIDP
GBS and CIDP share many of the same signs and symptoms in the acute phase of the disease. Because treatment strategy and prognosis differ considerably, it is important to distinguish between the 2 as early as possible. GBS generally follows a monophasic course and typically does not recur [14]. There are several subtypes of the disease with the most common subtype being AIDP [21].
With AIDP, weakness reaches a nadir in most by 2 weeks and in 90% of individuals, by 4 weeks that ranges from mild-to-severe flaccid quadriplegia [11], [22]. Respiratory failure can occur in up to 30% of individuals within a few days of onset [12]. Clinical deterioration after initial improvement or stabilization with immunotherapy suggests that the treatment had a transient effect or that CIDP is present [14].
CIDP is classified as symptoms persisting or progressing for 8 weeks or longer [23], [24]. Interestingly, 16% of patients with CIDP have rapidly progressive weakness, with a nadir within 8 weeks from the onset of disease, which is followed by a chronic course and is described as A-CIDP [22]. In 1 study, 5% of patients initially diagnosed with GBS were revealed to have A-CIDP, and all had their nadir within 4 weeks and continued to have active disease exceeding 8 weeks [22].
The most widely used treatments for CIDP consist of IVIg, plasma exchange, and corticosteroids. Long-term prognosis appears to vary according to the time at which therapy begins and the degree of associated axonal loss and up to 80% show improvement in their condition while receiving one of the 3 therapies [24]. A common minimal treatment period is 6 months with approximately 50% of patients remaining stable after IVIg is stopped and no reproducible way to predict which patients will relapse [11]. Despite the use of modern immunomodulatory treatments, significant morbidity and mortality still exist with up to an 8% mortality rate and 38% lack of complete or almost complete recovery at 1 year [25].
Orthopaedic considerations
While resources and attention may be focused on the acute medical complications of an inflammatory demyelinating polyneuropathy, certain orthopaedic manifestations may be overlooked and that is where the orthopaedic surgeon can play a role. Dislocation after THA is a significant and common complication. Most occur within the first 3 months after surgery with 50%-70% occurring within the first 5 weeks to 3 months postoperatively [26]. Dislocation rates for primary THA have been reported to be between 0.3% and 10% [26]. Many factors are involved in the risk of dislocation. These include both patient and surgical factors. Diseases that directly cause muscle weakness, particularly abductor weakness, can increase dislocation incidence. Patients with neuromuscular and cognitive disorders have been consistently demonstrated to be at a higher risk for postoperative dislocation [26]. It is imperative to be mindful of this and consideration should be given to prevention of dislocation in these patients through prophylactic knee immobilization or hip abduction bracing during their treatment.
Thromboembolism is one of the most frequent complications after major orthopaedic surgery, and without prophylaxis, deep vein thrombosis (DVT) develops in 50% of patients who undergo elective THA [27]. Patients undergoing THA have rates of symptomatic pulmonary embolus as high as 20% when no prophylaxis is administered, and the risk of fatal pulmonary embolus is between 0.1% and 0.2%, regardless of the chemoprophylactic agent used for prophylaxis [28]. Despite modern prophylaxis, venographically confirmed proximal DVT develops in 2% to 12% of patients [29]. Gaber et al. [30] retrospectively looked at 50 patients with GBS who were prophylactically anticoagulated and found that 6% (3/50) of patients developed a DVT with 2 of those developing a pulmonary embolism. This raises the question for the role of full anticoagulation in these patients.
Summary
Inflammatory demyelinating polyneuropathy is a rare but devastating condition. Few reports exist in the literature on this condition after total joint arthroplasty. The finding of ascending weakness in the postoperative period after arthroplasty should alert the orthopaedist to consider this diagnosis and order appropriate neurologic consultation. It is imperative to work with a dedicated team to make the diagnosis early to optimize outcomes. As it relates to a THA, prophylactic measures including bracing and staff education should be followed to prevent dislocation understanding that flaccid paralysis can lead to higher incidence of dislocation and decreased THA outcomes. Additional measures, both mechanical and pharmacologic measures, should be considered to decrease the incidence of venous thromboembolic disease.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.08.003.
Appendix A. Supplementary data
References
- 1.Hanewinckel R., van Oijen M., Ikram M.A., van Doorn P.A. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31:5. doi: 10.1007/s10654-015-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riebel G.D., Heller J.G., Hopkins L.C. Guillain-Barré syndrome after an operation on the spine. A case report. J Bone Joint Surg Am. 1995;77:1565. doi: 10.2106/00004623-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 3.De Decker V., Pera S.B., Borenstein S., Tombroff M. A case of Guillain-Barré syndrome associated with SIADH, treated by intravenous gammaglobulinsActa Clin Belg. 1996;51:170. doi: 10.1080/17843286.1996.11718507. [DOI] [PubMed] [Google Scholar]
- 4.Olivier N., Laribi H., Pages M., Camu W., Juntas Morales R. Recurrent Guillain-Barré syndrome after surgeryRev Neurol (Paris) 2010;166:644. doi: 10.1016/j.neurol.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Heyworth B.E., Fabricant P.D., Pizzurro M.M., Beksac B., Salvati E.A. Guillain–Barré syndrome mimicking nerve injury after total hip arthroplasty. HSS J. 2011;7:286. doi: 10.1007/s11420-011-9201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendawi T., Zavatsky J.M. Guillain-Barré syndrome after pelvic fracture fixation: a rare cause of postoperative paralysis. Spine. 2015;40:E372. doi: 10.1097/BRS.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 7.Chen E.Y., Stratton C., Mercer B., Hohler A., Tannoury T.Y., Tannoury C. Guillain-Barré syndrome after elective spinal surgery. J Am Acad Orthop Surg. 2017;25:587. doi: 10.5435/JAAOS-D-16-00572. [DOI] [PubMed] [Google Scholar]
- 8.Rashid A., Kurra S., Lavelle W. Guillain-Barré syndrome after revision lumbar surgery: a case report. Cureus. 2017;9:e1393. doi: 10.7759/cureus.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahai N., Hwang K.S., Emami A. Guillain-Barré syndrome following elective spine surgery. Eur Spine J. 2017;26:6. doi: 10.1007/s00586-016-4577-2. [DOI] [PubMed] [Google Scholar]
- 10.McGrogan A., Madle G.C., Seaman H.E., de Vries C.S. The epidemiology of Guillain-Barré syndrome worldwide. Neuroepidemiology. 2009;32:150. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 11.Dimachkie M., Saperstein D. Acquired immune demyelinating neuropathies. Contin (Minneap Minn) 2014;20:1241. doi: 10.1212/01.CON.0000455883.91426.12. [DOI] [PubMed] [Google Scholar]
- 12.Dimachkie M.M., Barohn R.J. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31:491. doi: 10.1016/j.ncl.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gensicke H., Datta A.N., Dill P., Schindler C., Fischer D. Increased incidence of Guillain-Barré syndrome after surgery. Eur J Neurol. 2012;19:1239. doi: 10.1111/j.1468-1331.2012.03730.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuki N., Hartung H. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 15.Sayin R., Kati I., Günes M. Guillain-Barre syndrome following spinal anaesthesia. J Coll Physicians Surg Pak. 2013;23:440. [PubMed] [Google Scholar]
- 16.Mangar D., Sprenker C., Karlnoski R., Puri S., Decker D., Camporesi E. Rapid onset of Guillain-Barré syndrome after an obstetric epidural block. A A Case Rep. 2013;1:19. doi: 10.1097/ACC.0b013e318291d378. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg S.K., Stacey B.R. Postoperative Guillain-Barré syndrome, arachnoiditis, and epidural analgesia. Reg Anesth. 1996;21:486. [PubMed] [Google Scholar]
- 18.Collier C.B. Postoperative paraplegia: an unusual case. Anaesth Intensive Care. 1994;22:293. doi: 10.1177/0310057X9402200311. [DOI] [PubMed] [Google Scholar]
- 19.Koc M., Ozalp N., Zulfikaroglu B. Major surgery with Guillain-Barre syndrome: a case report. J Int Med Res. 2002;30:601. doi: 10.1177/147323000203000609. [DOI] [PubMed] [Google Scholar]
- 20.Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27:S21. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 21.Hughes R.A., Cornblath D.R. Guillain-Barré syndrome. Lancet. 2005;366:1653. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 22.Ruts L., Drenthen J., Jacobs B.C., Van Doorn P.A., others Distinguishing acute-onset CIDP from fluctuating Guillain-Barré syndrome: a prospective study. Neurology. 2010;74:1680. doi: 10.1212/WNL.0b013e3181e07d14. [DOI] [PubMed] [Google Scholar]
- 23.Saperstein D.S., Katz J.S., Amato A.A., Barohn R.J. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve. 2001;24:311. doi: 10.1002/1097-4598(200103)24:3<311::aid-mus1001>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Köller H., Kieseier B., Jander S., Hartung H. Chronic inlammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 25.Rees J.H., Thompson R.D., Smeeton N.C., Hughes R.A.C. Epidemiological study of Guillain-Barré syndrome in South East England. J Neurol Neurosurg Psychiatry. 1998;64:74. doi: 10.1136/jnnp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner B.C. Instability after total hip arthroplasty. World J Orthop. 2012;3:122. doi: 10.5312/wjo.v3.i8.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haake D.A., Berkman S.A. Venous thromboembolic disease after hip surgery: risk factors, prophylaxis, and diagnosis. Clin Orthop. 1989;242:212. [PubMed] [Google Scholar]
- 28.Sheth N.P., Lieberman J.R., Della Valle C.J. DVT prophylaxis in total joint reconstruction. Orthop Clin North Am. 2010;41:273. doi: 10.1016/j.ocl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Della Valle C.J., Steiger D.J., Di Cesare P.E. Thromboembolism after hip and knee arthroplasty: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6:327. doi: 10.5435/00124635-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Gaber T.A., Kirker S.G., Jenner J.R. Current practice of prophylactic anticoagulation in Guillain-Barré syndrome. Clin Rehabil. 2002;16:190. doi: 10.1191/0269215502cr475oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.