Abstract
Background and Purpose
Stroke is one of the leading causes of mortality and long-term disability. Prompt diagnosis and treatment of stroke are crucial for a better outcome. A blood test, which serves as a biomarker in rural areas will help in immediately transferring patients to a hospital for thrombolytic therapy. The aim of the present study was to examine the role of ischemia modified albumin (IMA) as a screening biomarker in acute ischaemic stroke.
Materials and Methods
Serum samples were collected from 50 patients with acute ischaemic stroke within one, 24, 48, 72 and 144 h of time of admission for IMA. We compared patients' 1st-hour value with age- and sex-matched controls by independent sample t test. p value < 0.05 was considered significant.
Results
The serum IMA levels of patients 1st hour (108 ± 8.9) were significantly higher than those of the controls (79 ± 6.3) p < 0.05. The IMA levels showed a steady decline at 1 h (108 ± 8.9), 24 h (94 ± 4.2), 48 h (82 ± 6.1), 72 h (77 ± 5.6) and 144 h (76 ± 3.8) of admission in patients.
Conclusion
We observed that serum IMA was significantly higher in stroke patients as compared to controls. IMA was elevated in the acute phase of stroke and had a gradual graded decline over 1 week. We concluded that IMA may be a sensitive and rapid biomarker for screening of early ischaemic stroke in rural settings.
Key Words: Stroke, Biomarker, Ischemia modified albumin
Introduction
Stroke is one of the leading causes of mortality and long-term disability [1]. Prompt diagnosis and treatment of stroke are crucial for a better outcome. Clinical history and neuroimaging remain the cornerstone in the diagnostic procedures of stroke. Though neuroimaging clinches the diagnosis, a blood test that is sensitive, rapid and cost effective would help to rapidly identify stroke in rural areas, which in turn will help in immediately transferring patients for thrombolytic therapy. In rare cases of stroke mimics, a biomarker may further help in clarifying the diagnosis [2].
Oxidative stress is thought to play an important role in brain damage after stroke [3], wherein significant amounts of oxygen-free radicals are liberated. The N terminal of human serum albumin is unstable and more prone to degradation especially during oxidative stress. There is a decrease in its binding capacity for transitional metals like Cu and Co. This is termed as ischemia modified albumin (IMA). IMA has been reported earlier to be a sensitive marker for myocardial ischemia [4, 5].
However, limited studies are available regarding the role of IMA in acute ischaemic stroke [6]. The aim of the present study was to examine the role of IMA as a biomarker in acute ischaemic stroke.
Materials and Methods
Subjects and Specimen Collection
Fifty patients diagnosed with acute ischaemic stroke were enrolled in the study. Demographic data and risk factors of stroke for each patient was collected. Patients with haemorrhagic stroke (HS) and acute coronary syndrome (ACS) were excluded from the study. Ischaemic stroke was classified according to the Oxfordshire community stroke project (OCSP) [7]. Stroke severity was classified according to National Institute of Health Stroke Scale (NIHSS) [8]. Up to 3mL of blood sample was collected from each patient within 1, 24, 48, 72, and 144 h of the time of admission and centrifuged to obtain the serum. The time of the stroke was noted for each patient. The serum sample was used for analysis for IMA. Fifty age- and sex-matched healthy subjects were included in the study, as controls and a single blood sample was obtained. All the specimens were stored at −20°C until further analysis.
Analysis of IMA in Serum Sample
Albumin Cobalt Binding Assay (IMA)
The assay is based on the theory that cerebral ischemia causes changes in human serum albumin metal binding sites that are demonstrated by reduced exogenous cobalt (II) binding [9]. The concentration of ischemia modified serum albumin can be determined by the addition of a known amount of cobalt (II) to a serum specimen and measurement of the unbound cobalt (II) by colorimetric assay using dithiothreitol (DTT). An inverse relationship thus exists between the level of albumin bound cobalt and the intensity of the colour formation [4]. IMA assay was standardized in the division of Biochemistry of Central Clinical laboratory and a standard curve was prepared in the range 05–50.0 g CoCl2/mL. One IMA unit was defined as “g of free Co (II) in the reaction mixture per ml of serum sample”. Measurement results were reported in IU/L. All patients gave their informed consent and the study was approved by the Institutional Ethical Committee.
Statistical Analysis
SPSS 12 statistical software package was used (SPSS Inc., Chicago, IL, USA). Continuous variables were described as mean and SD. Comparison of means was done between patients and controls by the independent sample t test. Correlation analysis was done between IMA and NIHSS and different stroke subtypes. p value < 0.05 was considered significant.
Results
Sixty-one patients who were clinically diagnosed as stroke were included in the study. Five patients with HS and 6 patients with ACS were excluded from the study after appropriate investigations. Fifty patients were included in the study. The mean age of the patients and controls was 58 ± 5.6 and 56 ± 2.9 years respectively. Male and female ratio was 3.5: 1. Sixteen (32%) were hypertensive and 20 (40%) were diabetic, 8 (16%) were both hypertensive and diabetic and 6 (12%) were normotensive and non-diabetic subjects. Systolic and diastolic blood pressure was significantly raised in the patients when compared to controls (p = 0.04/0.03). The demographic data of patients and controls is shown in Table 1. OCSP classification showed Total anterior circulation Infarct (11), Partial anterior circulation Infarct (13), Lacunar Infarct circulation Infarct (16), Posterior circulation stroke (10). NIHSS scale was 25 ± 15.
Table 1.
Baseline demographic and biochemical parameters of the study subjects
| Variable | Patients | Controls | p value |
|---|---|---|---|
| Age, years | 58±5.6 | 56±2.9 | 0.8 |
| Gender, M/F | 39/11 | 30/20 | NA |
| SBP, mm Hg | 152±14 | 118±7.2 | <0.04 |
| DBP, mm Hg | 93±7.5 | 76±8.6 | <0.03 |
| Hypertension, % | 32 | Nil | NA |
| Diabetes, % | 20 | Nil | NA |
| Fasting plasma glucose, mg/dL | 136±12 | 85±6.1 | <0.03 |
| Total cholesterol, mg/dL | 189±16 | 172±8.2 | 0.91 |
| HDL-cholesterol, mg/dL | 43±7 | 46±5.6 | 0.83 |
| LDL-cholesterol, mg/dL | 102±11 | 99±8.9 | 0.72 |
| Triglycerides, mg/dL | 160±11.5 | 144±7.2 | <0.04 |
| IMA, IU/L | 108±8.9* | 79±6.3 | <0.02 |
1st h IMA mean value.
p value <0.05 is significant.
IMA, ischaemia modified albumin; SBP, systolic blood pressure; DBP, diastolic blood pressure.
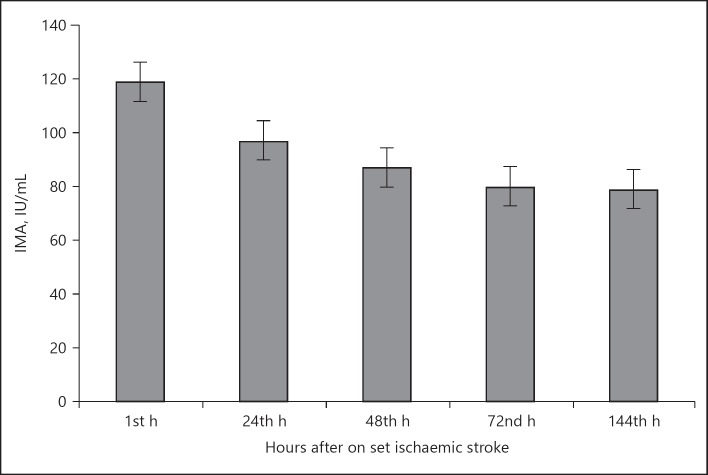
Table 1 shows the biochemical parameters of patients and controls. The fasting plasma glucose was significantly elevated in patients (136 ± 12) than controls (85 ± 6.1; p < 0.05). Increased levels of serum triglycerides were observed in patients (160 ± 11.5) than controls (144 ± 7.2; p = 0.03). The IMA levels of 1st h serum sample of patients (108 ± 8.9) were significantly higher than those of the controls (79 ± 6.3 IU/L) p < 0.02. We did not note any significance between IMA levels of 4th, 24th, 48th, 72nd, 144th h and control subjects. The IMA levels showed a steady decline at 1 h (108 ± 8.9 IU/L), 24 h (94 ± 4.2 IU/L), 48 h (82 ± 6.1 IU/L), 72 h (77 ± 5.6 IU/L) and 144 h (76 ± 3.8 IU/L) of admission in patients. The time of collection of samples and the time of stroke are shown in Figure 1. The remaining biochemical parameters did not show any difference between patients and controls. There was no statistical difference of IMA levels between the different stroke subtypes (OCSP) or the stroke severity (NIHSS). The overall sensitivity of IMA for the diagnosis of acute ischaemic stroke was 85.71% (95% CI 72.76–94.06) and the overall specificity was 84.31% (95% CI 71.4–92.98).
Fig. 1.
Serum ischemia modified albumin (IMA) levels of patients after 1st, 24th, 48th, 72nd, and 168th h after onset of ischaemic stroke. 1st - < 8 h of stroke; 24th - 24–36 h of stroke; 48th - 36–48 h of stroke; 72nd - 48–72 h of stroke; 168th - 72–144th h of stroke.
Discussion
We estimated the IMA levels in 50 stroke patients and compared to age- and sex-matched controls. We excluded ACS, as there is evidence that IMA levels raise in coronary ischemia. We excluded HS from the study, as prognosis and outcome are different from ischaemic stroke. We found serum IMA was significantly higher in stroke patients as compared to controls. This significance was only for the first hour, which corresponded to patients having stroke within 8 h. Subsequent IMA levels did not show any difference between control subjects. Our study did not find any difference in the different stroke subtypes and IMA levels probably indicating that IMA levels increase in stroke irrespective of the size and area involved. There was no difference in the IMA levels and the stroke severity. However, we did not have a follow up NIHSS of the patients to know the prognostic value of IMA. To demonstrate the prognostic utility of a biomarker, a larger sample and prospective design are required. However, a cross sectional study with a smaller sample like our study is enough for demonstrating its usefulness as a diagnostic test [10]. Our results are in agreement with the earlier studies that IMA level increases in stroke patients, particularly in ischaemic stroke patients [11]. We also evaluated serial IMA levels over 1 week. Our study showed that IMA was elevated in the acute phase of stroke and had a gradual graded decline over 1 week. Stroke is characterized by lack of blood circulation and oxygen to the affected area. Reactive oxygen species have been implicated in brain injury after stroke. The cascade of inflammatory and oxidative stress post stroke is responsible for the clinical deterioration of the patient. Reduced oxygen supply to brain causes localized acidosis and the generation of free radicals. Ions like copper and zinc, which are normally bound to proteins in the plasma are released from protein-binding sites and circulate in the free form [12]. The N-terminus of albumin, which normally binds transition metals, however, is susceptible to biochemical alteration [8]. It is postulated that albumin acts as a “sacrificial” antioxidant to reduce injury during reperfusion [13]. The altered form is referred to as IMA. Following a period of ischemia, a reduction in the ability of albumin to bind cobalt is apparent and hence the levels of IMA increase. Studies have demonstrated that IMA is a marker of ischemia, and oxidative stress originates as a consequence of tissue hypoxia [14, 15, 16]. We believe that with the institution of therapy, the oxidative stress decreases, subsequently decreasing the IMA values as seen in our study.
Stroke remains the biggest cause of death worldwide and hence it is essential to recognize the symptoms early. As thrombolytic therapy represents the gold standard for acute ischaemic stroke treatment, it is essential that we have a biomarker at the primary care level to make some diagnostic decision so that the patient reaches the hospital in the window period. Biomarkers definitely will add further information in the diagnosis, thereby complementing the early admission of the patient to specialized stroke care.
In conclusion, our study demonstrated that IMA may be a sensitive, rapid and cost effective biomarker for screening of early ischaemic stroke, particularly in the rural settings. Further, well-designed validation studies are required for the development of blood biomarkers to improve the care of patients with ischaemic stroke.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Author Contribution
B.M. and K.R. were responsible for article drafting, conception and design and analysis and interpretation of data; B.M. and K.R. were responsible for article drafting and data collection; V.K. and K.R. were involved in biochemical analysis; B.M. did the statistical analysis; B.M., K.R., and V.K. gave the final approval of the article.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;119:489–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Kim MH, Kang SY, Kim MC, Lee WI. Plasma biomarkers in the diagnosis of acute ischemic stroke. Ann Clin Lab Sci. 2010;40:336–341. [PubMed] [Google Scholar]
- 3.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 4.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–315. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 5.Sinha MK, Roy D, Gaze DC, Collinson PO, Kaski JC. Role of IMA, a new biochemical marker of myocardial ischeamia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004;21:29–34. doi: 10.1136/emj.2003.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayak AR, Kashyap RS, Kabra D, Purohit HJ, Taori GM, Daginawala HF. Prognostic significance of ischemia-modified albumin in acute ischemic stroke patients: a preliminary study. Ann Neurosci. 2011;18:5–7. doi: 10.5214/ans.0972.7531.1118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Health National of Neurological Disorders and Stroke. Stroke Scale. www.ninds.nih.gov/doctors/NIH_Stroke_Scale. [Google Scholar]
- 9.Chan B, Dodsworth N, Woodrow J, Tucker A, Harris R. Site-specific N-terminal auto-degradation of human serum albumin. Eur J Biochem. 1995;227:524–528. doi: 10.1111/j.1432-1033.1995.tb20419.x. [DOI] [PubMed] [Google Scholar]
- 10.Hlatky MA. Exercise testing to predict outcome in patients with angina. J Gen Intern Med. 1999;14:63–65. doi: 10.1046/j.1525-1497.1999.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin levels in cerebrovascular accidents. Am J Emerg Med. 2008;26:874–878. doi: 10.1016/j.ajem.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 12.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Correa J, Stoppani AO. Inactivation of yeast glutathione reductase by Fenton systems: effect of metal chelators, catecholamines and thiol compounds. Free Radic Res. 1997;27:543–555. doi: 10.3109/10715769709097858. [DOI] [PubMed] [Google Scholar]
- 14.Lippi G, Montagnana M. Ischemia-modified albumin in ischemic disorders. Ann Thorac Cardiovasc Surg. 2009;15:137. [PubMed] [Google Scholar]
- 15.Senes M, Kazan N, Coskun O, Zengi O, Inan L, Yücel D. Oxidative and nitrosative stress in acute ischaemic stroke. Ann Clin Biochem. 2007;44:43–47. doi: 10.1258/000456307779596057. [DOI] [PubMed] [Google Scholar]
- 16.Can M, Demirtas S, Polat O, Yildiz A. Evaluation of effects of ischaemia on the albumin cobalt binding (ACB) assay in patients exposed to trauma. Emerg Med J. 2006;23:537–539. doi: 10.1136/emj.2005.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]