Abstract
With the increased use of dual-mobility cups (DMCs) in total-and revision hip arthroplasties, surgeons can expect an increase of known and new complications. During routine follow-up, we observed an asymptomatic patient with an intraprosthetic-dislocation (IPD) and elevated levels of serum metal ions (1.8 ppb of cobalt and 28.0 ppb of chromium). Revision surgery was inevitable. Perioperative metallosis and severe wear of the metal shell and metal femoral head supported the IPD. Literature showed that the modularity of the DMC can result in increased serum metal ions, create excessive wear, and possibly affect implant survival. Our case and review of the literature may form an argument not to consider DMC for primary cases. Furthermore, we advise yearly clinical and radiological follow-up and, on indication, metal ion testing for DMCs.
Keywords: Dual mobility cup, Revision surgery, Metal ions, Total hip arthroplasty, Intraprosthetic dislocation
Introduction
With the increased use of dual mobility (DM) cups in total and revision hip arthroplasties, surgeons can expect an increase in known and new complications. Careful follow-up and awareness of these complications will help avoid further unnecessary problems for our patients. A complication specifically related to the DM cup concept is the intraprosthetic dislocation (IPD). Dissociation between the polyethylene liner and femoral head causes this phenomenon. Several cases of these early and late complications have been reported in literature [1], [2].
The main advantage of using a DM cup is the increased stability, especially after revision surgery. After primary total hip arthroplasty, dislocation rates vary from 0.2% to 7%, whereas after revision surgery, this increases up to 10%-25% [3]. An increased head size and dual articulating surfaces in DM cups improve stability, increase the range of motion, and reduce the possibility of dislocation [4], [5], [6], [7]. With the expanding indication for the use of DM cups and their use in younger and more demanding patients [5], [8], concerns rise about the long-term survival. Owing to the large diameter of articulation and multiple surfaces, an increase in polyethylene wear, fretting corrosion, and release of metal ions may lead to complications and revision surgeries [2], [9], [10], [11].
In this case report, we present a patient with an asymptomatic IPD, with increased serum cobalt and chromium levels. We summarize the current literature about this complication to create awareness and emphasize the need for close patient follow-up. The patient signed informed consent for the use of the data for publication.
Case history
A 75-year-old female patient underwent a left total hip revision in April 2015 because of aseptic loosening and wear 27 years after primary implantation of a Charnley hip prosthesis with Ogee cup (DePuy, Leeds, UK). Through a posterolateral approach, the loose cup was removed, and after impaction bone grafting, a 50/28 Avantage cemented acetabular cup (Biomet, Warsaw, IN) was implanted. A cemented Lubinus SP II (Link, Hamburg, Germany) hip stem was placed after removing the loose stem and cement (Fig. 1). The polyethylene liner and metal head (28 mm with 0 mm offset) were assembled according to manufacturer’s instructions. There were no postoperative complications, and the patient was discharged after 5 days. Outpatient physical and radiological examinations after 3 months and 1 year were normal and showed no limitations. There was no trauma or any feeling of (sub)luxation during the 2 years of follow-up. She had no complaints and only walked with a stick because of weakness in her right leg. However, an anteroposterior (AP) pelvis radiograph showed an eccentric location of the femoral metal head, with signs of an IPD of the DM cup (Fig. 2). Examination of the left hip showed a pain-free range of motion with flexion of 100 degrees, external rotation of 30 degrees, and internal rotation of 30 degrees. Serum cobalt and chromium levels were determined, and both were elevated at 1.9 ppb (reference value: <1 ppb) and 9.7 ppb (reference value: 0.3-1.7 ppb), respectively, with reference to our hospital standard. Our patient declined a revision operation for that time despite our concerns. She had no complaints of the left hip, no systemic signs of cobalt toxicity, no dizziness, no neurological problems, and no hearing problems, and she was mentally competent. We kept her under close control, and after 2 months, the serum chromium level was considerably elevated to 28.0 ppb, with a cobalt level of 1.8 ppb. We reevaluated the situation with our patient and scheduled a cup revision despite the patient having no complaints of her hip area.
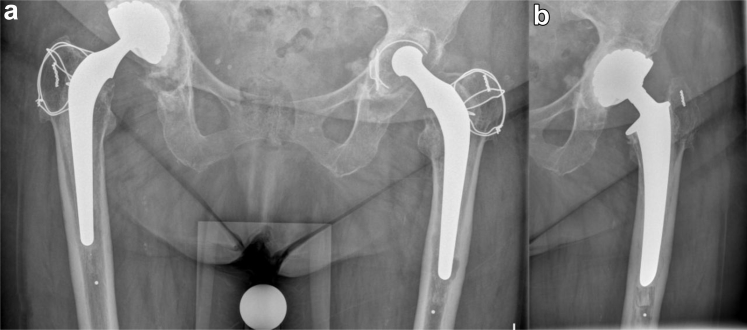
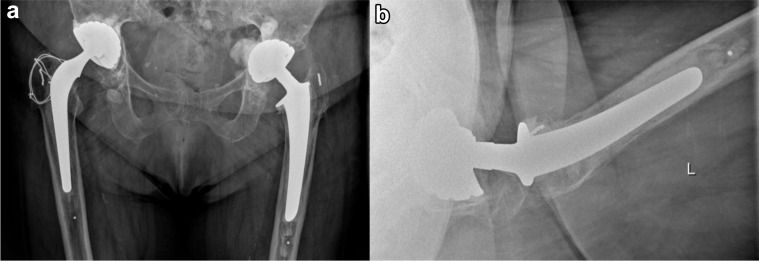
Figure 1.
Anteroposterior radiograph (a) before the first revision of the left total hip prosthesis. The eccentric head of the Charnley prosthesis clearly demonstrates wear. Anteroposterior radiograph (b) after the first total revision and placement of the Avantage cemented dual mobility cup.
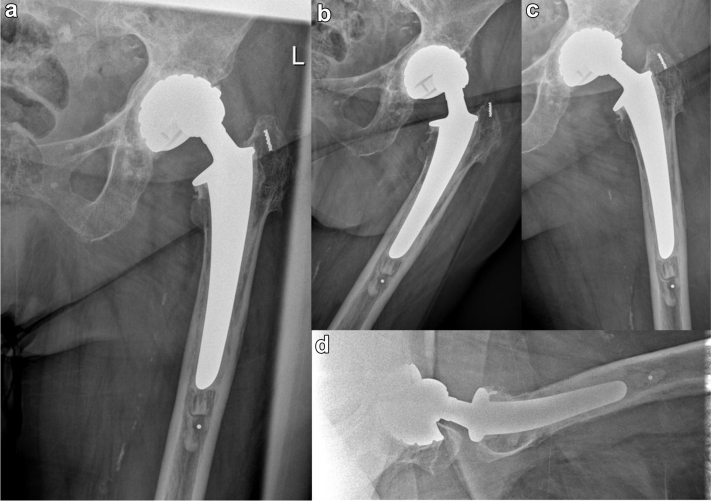
Figure 2.
Regular control shows an eccentric femoral metal head caused by an intraprosthetic dislocation. There is no total dislocated hip, and there is no sign of polyethylene component outside the metal shell (bubble sign). (a) Anteroposterior view. (b) Adduction view. (c) Abduction view. (d) Lateral view.
In December 2017, a revision of the Avantage cemented cup and head of the left hip was performed through a posterolateral approach. Exploration of the capsule revealed a large amount of dark-gray fluid and signs of metallosis of the surrounding tissues (Fig. 3). The fluid was collected and processed for further research. Tissue around the joint was removed and sent to our pathology department for histological evaluation. After removal of the surrounding tissue, an IPD of the metal head and polyethylene liner was visible. A complete revision of the cup and femoral metal head was performed. The revised metal shell and femoral head both showed macroscopic signs of wear (Fig. 4). There was no macroscopic damage to the highly cross-linked polyethylene (HXLPE) liner, trunnion, or the taper. We had no biomechanical explanation for this situation and no arguments to change our standard care. Therefore, we decided to use our standard 50/28 Avantage cemented cup with a 28-mm metal head (0 mm offset). No complications occurred during and after the surgery. The patient was discharged after 4 days.
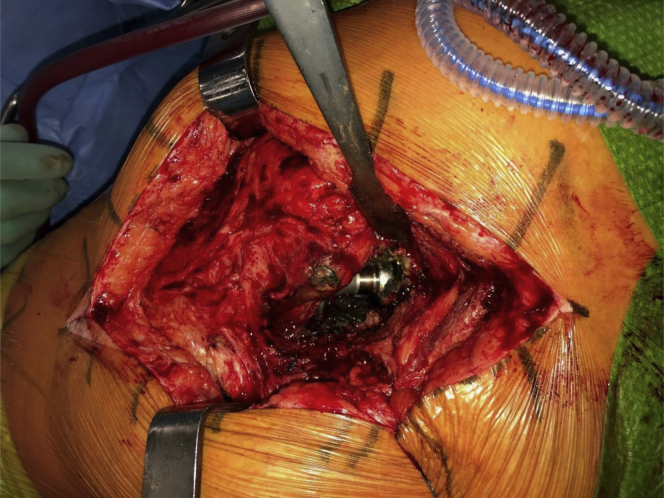
Figure 3.
Perioperative photograph of the revision operation. The black tissue around the prosthesis shows the amount of metallic debris. An intraprosthetic dislocation was found as shown by the dislodged femoral metal head from the high molecular weight cross-linked polyethylene liner.
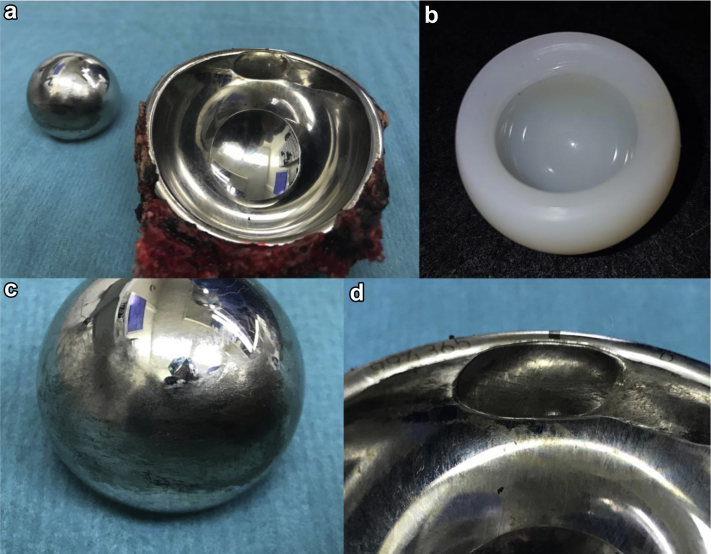
Figure 4.
(a) Metal head and shell with cement immediately after the revision. (b) The undamaged highly cross-linked polyethylene liner. (c) Surface abrasions of the articulating part of the metal head. (d) Close up of the severe wear in the metal shell.
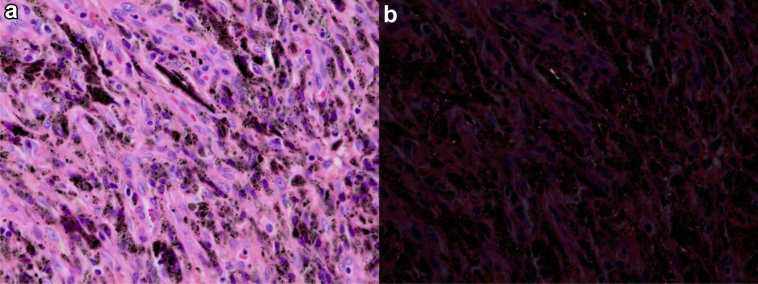
First-day postoperative serum ion control showed an immediate decrease in both cobalt (1.8 ppb-1.3 ppb) and chromium (28 ppb-20.2 ppb) levels. The postoperative radiograph showed no complications (Fig. 5). After 3 months, the serum ion levels of cobalt and chromium further decreased to 1.2 ppb and 8.9 ppb, respectively. This indicates the elevated levels of serum metal ions were mainly due to the articulation of the metal head with the metal shell and supports the IPD as the source for the elevated levels of metal ions. At 1 year examination of the left hip, a pain-free range of motion with flexion of 100 degrees, external rotation of 45 degrees, and internal rotation of 15 degrees was measured. Both cobalt and chromium levels were decreased to 0.7 ppb and 4.5 ppb, respectively. Fluid analyses showed no signs of bacterial growth or infection. Histopathological analyses showed dense lymphohistiocytic aggregates and black birefringent crystalline material, which supports tissue reaction to metal (Fig. 6). According to the aseptic lymphocytic vasculitis-associated lesion score reported in the study by Campbell et al. [12], this lesion scored 8 points on a scale of 10 (3 for synovial lining, 3 for inflammatory infiltrate, and 2 for tissue organization).
Figure 5.
Direct postoperative radiograph after the second revision of the left total hip prosthesis. (a) Anteroposterior view. (b) Lateral view.
Figure 6.
(a) Light micrograph showing typical histologic features of dense lymphohistiocytic aggregates (stain, hematoxylin and eosin, original magnification ×400). (b) Polarized micrograph showing black birefringent crystalline material (original magnification ×400). This received an ALVAL score of 8 (3 for synovial lining, 3 for inflammatory infiltrate, and 2 for tissue organization). ALVAL, aseptic lymphocytic vasculitis-associated lesion.
Discussion
Our literature search was performed by 2 authors: M.C.K. and P.K.B. All articles with the key words “dual mobility” OR “dual mobility cup” OR “modular dual mobility components” AND “metal ion level” OR “metal ions” OR “cobalt level” OR “chromium level” AND “total hip arthroplasty” were screened and selected.
We report a severe complication and failure of a contemporary DM cup in an asymptomatic patient. DM cups are especially used in revision surgery; however, their use in primary hip surgery and in a younger population is increasing worldwide [1], [4], [5], [6], [7], [8], [9], [13]. With the increased use of this relatively new bearing, concerns rise about the possible complications and wear problems.
A complication that can only occur in this type of bearing is IPD. Several studies have described IPDs [2], [14] with incidence rates from 0% to 5.3% [15]. Philippot et al. [16] described 3 types of IPD. Type 1 IPD is a pure dislocation without arthrofibrosis and cup loosening. Type 2 IPD is secondary to blocking the liner due to arthrofibrosis or ectopic ossification, and type 3 IPD is associated with cup loosening. Type 1 dislocations can cause metal wear problems because the femoral head is dislocated from the polyethylene liner and articulates with the metal shell. The latest generation DM cups with HXLPE liners are designed to eliminate the risk of IPD [17]. According to Baitailler et al. [1], IPD is becoming increasingly rare with the contemporary DM cups, and they state in their review that no IPD was reported in recent literature studies.
This case describes an asymptomatic type 1 dislocation in a contemporary DM cup within 2 years after implantation. Our patient was unaware of the problem because she had no complaints of the hip region and a full and painless range of motion. Owing to the rapidly increased serum cobalt and chromium levels in a short time, revision surgery was inevitable. A perioperative sign of metallosis and a dislocated metal head from the liner were visible. No macroscopic damage of the HXLPE liner, taper, or trunnion was observed. The excessive wear of the metal shell (Fig. 4) seems responsible for the highly increased serum chromium level. The stainless steel shell consists for a large amount of chromium and no cobalt according to the manufacturers leaflet (ISO 5832-9:07). The slightly elevated cobalt levels could be due to the abrasions of the metal head. A much higher increase in serum cobalt level would be expected if the metal head taper or trunnion was more involved in the wear process. The decrease in serum cobalt and chromium levels after revision also indicates the metal shell wear as the source of elevated serum metal ion levels.
Recently, attention has been given to metal wear and increased serum metal ion levels in patients with DM cups. Increased metal ion production may be due to the different modular components or articulation of the metal head and metal shell after an IPD. Mohammed and Cnudde [18] first described in 2012 about severe metallosis after an IPD in a symptomatic patient. The polyethylene liner had dislodged, and wear in the metal socket by the prosthetic head has led to severe metallosis. A total revision of the cup and stem was performed. Histopathologic examination confirmed metallosis.
In recent literature, only 2 groups reported about serum metal ion levels in patients with DM cups. Matsen Ko et al. [9] investigated serum cobalt and chromium levels in a group of 100 patients with a DM bearing. In 24 patients, an increased serum level of cobalt or chromium was observed. A total of 21 patients had a reported serum cobalt level above the normal range, with 9 of these values being significantly above normal: 1.6 ppb. A total of 3 patients had an increased chromium level, and 1 patient had both metal ions increased. No IPD occurred in their group.
In a prospective study of Nam et al. [10], serum metal ion levels were compared between young patients with DM cups (n = 26) and conventional cups (n = 17). All patients received a titanium cementless stem. The DM cup group showed a significant increased serum cobalt level, of which 4 asymptomatic patients were outside the reference range of 0.03-0.29 ppb).
In our asymptomatic patient, revision surgery was inevitable after observing an IPD and rapidly increasing serum metal ion levels. Physicians need to be aware of the risks and different therapeutic options when performing a revision of a DM cup. In case of an IPD and metallosis, a complete revision of the acetabular shell, liner, and femoral head should be performed [19].
With this case report and short review of current literature, we want to create awareness about the possible problems of DM cups. Asymptomatic complications such as IPD, excessive wear, and/or fretting corrosion can lead to undetected metal wear and eventually increased serum metal ion levels, systemic reactions, local metallosis, bone loss, aseptic lymphocytic vasculitis-associated lesion, and a need for revision surgery. We feel this forms an argument not to consider DM cups for routine primary hip replacement, and placement in younger patients should be performed with caution. Therefore, regular clinical and radiological follow-up and, on indication, metal ion testing of patients with DM cups are advised.
Summary
This case presents an asymptomatic and DMC-specific complication that led to elevated serum metal ion levels, metallosis, and revision surgery. With the increased use of DMC in total and revision hip arthroplasties, we can expect an increase of known and new complications. IPD and the modularity of DMC can cause metal wear and eventually lead to revision surgery. This may form an argument not to consider DMC for primary cases and to use it with caution in younger patients. Awareness of these complications will help avoid unnecessary problems for our patients. Furthermore, we advise regular clinical and radiological follow-up and, on indication, metal ion testing for patients with a DMC.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.12.001.
Appendix A. Supplementary data
References
- 1.Batailler C., Fary C., Verdier R., Aslanian T., Caton J., Lustig S. The evolution of outcomes and indications for the dual-mobility cup: a systematic review. Int Orthop. 2017;41(3):645. doi: 10.1007/s00264-016-3377-y. [DOI] [PubMed] [Google Scholar]
- 2.De Martino I., D'Apolito R., Waddell B.S., McLawhorn A.S., Sculco P.K., Sculco T.P. Early intraprosthetic dislocation in dual-mobility implants: a systematic review. Arthroplast Today. 2017;3(3):197. doi: 10.1016/j.artd.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel P.D., Potts A., Froimson M.I. The dislocating hip arthroplasty: prevention and treatment. J Arthroplasty. 2007;22(4 Suppl 1):86. doi: 10.1016/j.arth.2006.12.111. [DOI] [PubMed] [Google Scholar]
- 4.Civinini R., Carulli C., Matassi F., Nistri L., Innocenti M. A dual-mobility cup reduces risk of dislocation in isolated acetabular revisions. Clin Orthop Relat Res. 2012;470(12):3542. doi: 10.1007/s11999-012-2428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epinette J.A., Beracassat R., Tracol P., Pagazani G., Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323. doi: 10.1016/j.arth.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Heffernan C., Banerjee S., Nevelos J. Does dual-mobility cup geometry affect posterior horizontal dislocation distance? Clin Orthop Relat Res. 2014;472(5):1535. doi: 10.1007/s11999-014-3469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langlais F.L., Ropars M., Gaucher F., Musset T., Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop Relat Res. 2008;466(2):389. doi: 10.1007/s11999-007-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epinette J.A., Harwin S.F., Rowan F.E. Early experience with dual mobility acetabular systems featuring highly cross-linked polyethylene liners for primary hip arthroplasty in patients under fifty five years of age: an international multi-centre preliminary study. Int Orthop. 2017;41(3):543. doi: 10.1007/s00264-016-3367-0. [DOI] [PubMed] [Google Scholar]
- 9.Matsen Ko L.J., Pollag K.E., Yoo J.Y., Sharkey P.F. Serum metal ion levels following total hip arthroplasty with modular dual mobility components. J Arthroplasty. 2016;31(1):186. doi: 10.1016/j.arth.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Nam D., Salih R., Brown K.M., Nunley R.M., Barrack R.L. Metal ion levels in young, active patients receiving a modular, dual mobility total hip arthroplasty. J Arthroplasty. 2017;32(5):1581. doi: 10.1016/j.arth.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Laura A.D., Hothi H., Battisti C. Wear of dual-mobility cups: a review article. Int Orthop. 2017;41(3):625. doi: 10.1007/s00264-016-3326-9. [DOI] [PubMed] [Google Scholar]
- 12.Campbell P., Ebramzadeh E., Nelson S., Takamura K., De Smet K., Amstutz H.C. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloemheuvel E.M., van Steenbergen L.N., Swierstra B.A. Dual mobility cups in primary total hip arthroplasties: trend over time in use, patient characteristics, and mid-term revision in 3,038 cases in the Dutch Arthroplasty Register (2007-2016) Acta Orthop. 2018;19:1. doi: 10.1080/17453674.2018.1542210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banka T.R., Ast M.P., Parks M.L. Early intraprosthetic dislocation in a revision dual-mobility hip prosthesis. Orthopedics. 2014;37(4):e395. doi: 10.3928/01477447-20140401-63. [DOI] [PubMed] [Google Scholar]
- 15.Fabry C., Langlois J., Hamadouche M., Bader R. Intra-prosthetic dislocation of dual-mobility cups after total hip arthroplasty: potential causes from a clinical and biomechanical perspective. Int Orthop. 2016;40(5):901. doi: 10.1007/s00264-015-3000-7. [DOI] [PubMed] [Google Scholar]
- 16.Philippot R., Boyer B., Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965. doi: 10.1007/s11999-012-2639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslanian T. All dual mobility cups are not the same. Int Orthop. 2017;41(3):573. doi: 10.1007/s00264-016-3380-3. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed R., Cnudde P. Severe metallosis owing to intraprosthetic dislocation in a failed dual-mobility cup primary total hip arthroplasty. J Arthroplasty. 2012;27(3):493.e1. doi: 10.1016/j.arth.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P., Dubory A., Potage D., Roubineau F., Flouzat Lachaniette C.H. Dual-mobility arthroplasty failure: a rationale review of causes and technical considerations for revision. Int Orthop. 2017;41(3):481. doi: 10.1007/s00264-016-3328-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.