Abstract
The clinical features of 22q11.2 deletion syndrome include virtually every organ of the body. This review will focus on the immune system and the differences related to deletion breakpoints. A hypoplastic thymus was one of the first features described in this syndrome and low T cell counts, as a consequence of thymic hypoplasia, are the most commonly described immunologic feature. These are most prominently seen in early childhood and can be associated with increased persistence of viruses. Later in life, evidence of T cell exhaustion may be seen and secondary deficiencies of antibody function have been described. The relationship of the immunodeficiency to the deletion breakpoints has been understudied due to the infrequent analysis of people carrying smaller deletions. This manuscript will review the immune deficiency in 22q11.2 deletion syndrome and describe differences in the T cell counts related to the deletion breakpoints. Distal, non-TBX1 inclusive deletions, were found to be associated with better T cell counts. Another new finding is the relative preservation of T cell counts in those patients with a 22q11.2 duplication.
Keywords: breakpoints, deletion size, DiGeorge, IgG, low copy number repeats, T cells
1 |. INTRODUCTION
There is a spectrum of immune deficiency recognized in 22q11.2 deletion syndrome (22q11.2del) and some of the more common clinical features related to immune deficiency will be reviewed here. Some patients have completely normal T cells and other infants have no T cells detectable on laboratory studies (Kwan et al., 2014; Ryan et al., 1997). This variability in severity is not conceptually different from that seen with other end organs (Botto et al., 2003; McDonald-McGinn et al., 1999). One of the differences is that there is a linear quantitative scale for T cells, whereas definition of severity in other organs is more qualitative. The explanation for the variability appears to reflect stochastic effects during embryonic development although there are suggestions that background genes may modify the effects of the deletion (Bassett et al., 2017; Driscoll et al., 2006; Lindsay et al., 1999; Lopez-Rivera et al., 2017; Stalmans et al., 2003; Widdershoven et al., 2013). Supporting the concept that features may reflect early binding of TBX1 to target genes are family studies showing differences in members of multiplex kindreds (Digilio, Marino, Giannotti & Dallapiccola, 1997; Driscoll et al., 1995; Yamagishi et al., 1998) (McDonald-McGinn et al., 2001). Several studies have attempted to use clinical features to predict the extent of the immune deficiency. There may be some statistical association of immune deficiency with hypoparathyroidism, however, this has not been observed in other studies and certainly there are no strict predictors of the severity of the immune deficiency in 22q11.2del (Herwadkar, Gennery, Moran, Haeney, & Arkwright, 2010; Sullivan et al., 1998). Thus, current recommendations emphasize that clinical testing for the immunodeficiency should be performed in all patients since there are no robust clinical predictors (Bassett et al., 2011; Habel et al., 2014).
It has been recognized that there is variability in the deletion breakpoints, however, clinical detection of the deletion often does not distinguish specific breakpoints and instead utilizes either PCR detection of a region within the deleted region, multiple ligation probe amplification, or fluorescent in situ hybridization with the probe homologous to a region that is deleted. Thus, our knowledge of the relationship of the deletion breakpoints to the immune deficiency severity is very limited. In addition to reviewing the immune deficiency in 22q11.2del, this manuscript will also present new data on the influence of breakpoints on the immune deficiency. This study of deletion breakpoint size effect on the immune system is of practical value to clinicians managing patients with 22q11.2del and we will review the immunologic context of the condition.
2 |. DYNAMIC CHANGES IN THE IMMUNODEFICIENCY
There are some aspects of the immune deficiency that evolve over time. The direct effect of thymic hypoplasia on peripheral blood T-cell counts is most apparent in early infancy (Chinen, Rosenblatt, Smith, Shearer, & Noroski, 2003; Dar et al., 2015; Kanaya et al., 2006; Lima et al., 2010; Sullivan et al., 1999). The T-cell counts in early infancy are strongly associated with thymic output and functional thymic size (Dar et al., 2015). Thymic size observable on imaging may not accurately reflect the functional thymic size because the thymus can arrest in its descent into the anterior mediastinum leaving small thymic remnants in the neck, not apparent on imaging (Lima et al., 2010). Low T-cell counts are normally corrected physiologically by increased secretion of IL-7 (Tan et al., 2001). IL-7 acts to increase thymic output and increases peripheral proliferation of T cells (Tan et al., 2001; Tchao & Turka, 2012). These combined effects serve to normalize T-cell counts and by adulthood most adults with 22q11.2del will have normal or nearly normal peripheral blood T-cell counts. These T cell counts in adulthood belie the acquisition of some dysfunctional features that compromise the ability of the T cells to contribute to host defense (Piliero, Sanford, McDonald-McGinn, Zackai, & Sullivan, 2004; Zemble et al., 2010). Understanding the dynamic nature of the immune deficiency in 22q11.2del is critical for providing care to patients.
A second aspect of the immunodeficiency is the secondary humoral immune deficiency, an aspect reviewed here but not studied in the cohort descibred below. This is presumed to be secondary to compromised T cell help for B cell development. Nevertheless, it remains unexplained why this aspect of immunodeficiency is most often seen in older children and adults. Described humoral defects include an increased rate of IgA deficiency, compromised differentiation of B cells into the switched memory compartment and decline in immunoglobulin production and function (Derfalvi et al., 2016; Finocchi et al., 2006; McLean-Tooke, Spickett, & Gennery, 2007; Patel et al., 2012; Smith et al., 1998; Zemble et al., 2010). IgM levels appear to decline and this may be a marker for humoral dysfunction in general (Patel et al., 2012). Natural IgM antibodies appear to be diminished in 22q11.2del and these key antibodies have a non-redundant function in the defense against gram negative bacteria (Klocperk, Mejstrikova, Kayserova, Kalina, & Sediva, 2015). In addition, poor responses to vaccines have been described in adults with 22q11.2del (McLean-Tooke et al., 2007). There is still much to learn about the humoral immune deficiency and specifically the evolution of the immunodeficiency in adulthood. While these humoral defects are presumed to be secondary to insufficient T cell help, there has not been an exhaustive study of TBX1 expression or dysregulation of downstream genes in secondary lymphoid organs such as lymph node and spleen. Recently, natural killer cell function and T cell function were shown to be compromised due to haplosufficiency for other genes in the deleted region (Giacomelli et al., 2016; Zheng et al., 2015). Therefore, the humoral defects could be a direct result of the deletion and not due to compromised T cell help.
3 |. DELETION ENDPOINTS AND LYMPHOCYTE COUNTS
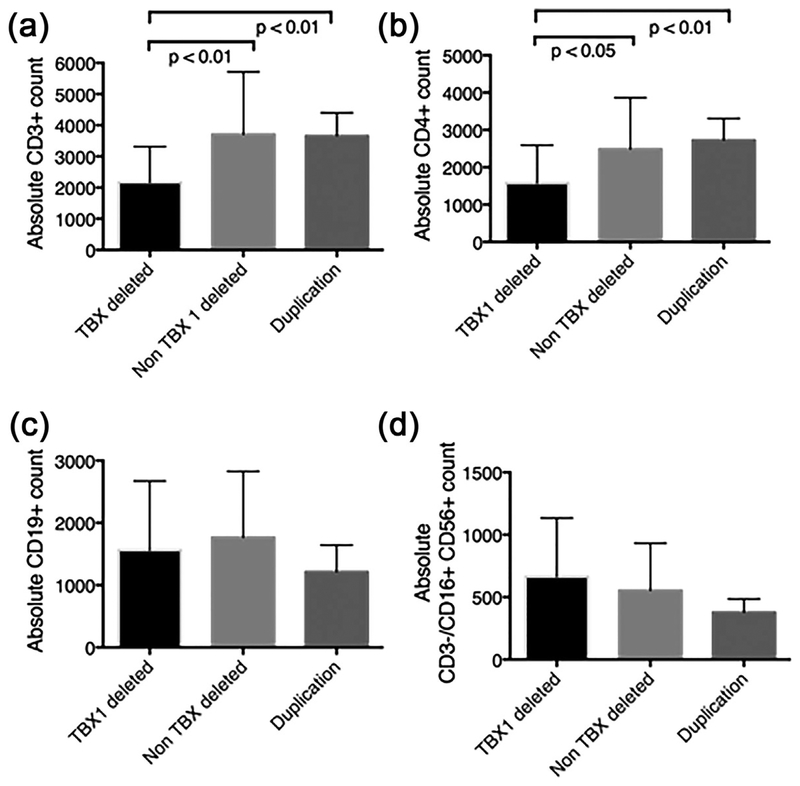
To address the issue of breakpoint contributions to the immune deficiency, we examined our data on 52 infants with deletion testing and a full immunologic evaluation near 1 year of age. Two patients were removed from the analysis due to chylous losses (one patient in each of the deletion cohorts). We compared lymphocyte counts between those with a TBX1 deletion (A–B, A–C, A–D deletions) with those who did not have a TBX1 deletion (B–D, C–D, D–E, D–F deletions) and a set of patients with a 22q11.2 duplication including TBX1. The cohorts consisted of 52 subjects with a deletion that included TBX1, eight patients with a distal deletion that did not include TBX1, and six patients with a duplication that included TBX1. Lymphocyte subset absolute counts for each group were analyzed by t-test, and are shown in Figure1. CD3 counts were significantly lower in the TBX1-deleted cohort compared to the other two cohorts (Figure 2). Similarly, CD4 counts were lower in the TBX1-deleted patients compared to the other two cohorts. CD8 (not shown), CD19, and NK call counts were not different between the three cohorts. The relation of phenotype to deletion breakpoints and TBX1 function has been described in murine models but the T cell phenotype has not been previously reported in clinical cohorts of patients with defined deletions (Jerome & Papaioannou, 2001; Lindsay et al., 1999, 2001; Vitelli et al., 2003). These clinical data provide important information for immune monitoring of patients followed for 22q11.2 deletions and duplications.
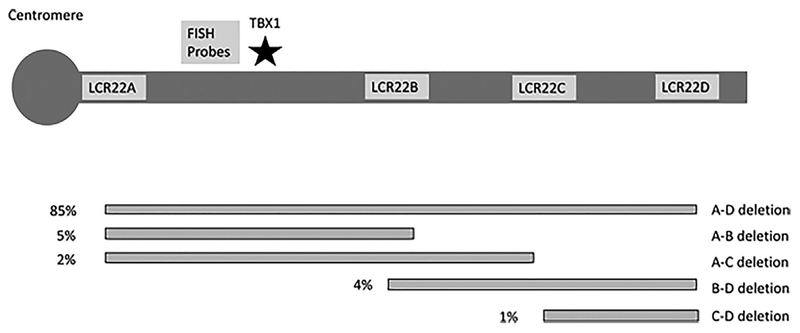
FIGURE 1.
Chromosome 22q11.2del breakpoints commonly observed. This schematic diagram demonstrates the most common breakpoints observed in 22q11.2del. The most common deletion is A–D and includes TBX1. The location of TBX1 is shown with a star. The percentages next to each deletion schematic indicate the frequency. In addition, there are rarer deletions not mediated by the low copy number repeats (LCRs) indicated in the figure and larger deletions that extend distal to the region shown in the figure. Duplications are similarly variable but most often lead to duplication of the A–D region
FIGURE 2.
22q11.2 deletions including TBX1 gene region are associated with CD3+ lymphopenia due to decreased CD4+ count. Charts of patients were retrospectively reviewed following approval from the Children’s Hospital of Philadelphia Institutional Review Board. Peripheral blood absolute (a) CD3+ counts, (b) CD4+ counts, (c) CD19 counts, and (d) natural killer CD3−/CD16+ CD56+ counts were determined by flow cytometry. Data were extracted from the patients’ medical records from Immunology visit near 12 months’ of age. 22q11.2 deletion patients with a TBX1 containing deletion (A–B, A–C, A–D deletions, n = 52), were compared to patients with 22q11.2 deletion with deletions not containing TBX1 (B–D, C–D, D–E, D–F deletions, n = 8), and patients with 22q11.2 duplications (n = 11). Bars demonstrate mean and error bars indicate standard deviation. p values refer to an unpaired t-test
4 |. AUTOIMMUNE DISEASE
Autoimmune diseases occur with high frequency in 22q11.2del (Davies, Stiehm, Woo, & Murray, 2001; Davies, Telfer, Cavenagh, Foot & Neat, 2003; Jawad, McDonald-Mcginn, Zackai, & Sullivan, 2001; Kratz et al., 2003). There have been several proposed mechanisms including altered regulatory T cell development in the face of limited thymic tissue, increased responses to self-antigens with homeostatic proliferation, and dysregulation due to lymphopenia (Di et al., 2015; Ferrando-Martinez et al., 2014; McLean-Tooke et al., 2007; Milner, Ward, Keane-Myers, & Paul, 2007; Sullivan, McDonald-McGinn, & Zackai, 2002; Tison et al., 2011). The most common autoimmune disease affecting 22q11.2 patients in childhood is idiopathic thrombocytopenic purpura, and the second most common in juvenile idiopathic arthritis (Bjork, Oskarsdottir, Andersson, & Friman, 2012; Gennery et al., 2002; McLean-Tooke et al., 2007). Platelet size and number are lower at baseline in most patients with the deletion, which may cause confusion with idiopathic thrombocytopenia purpura (Lawrence, McDonald-McGinn, Zackai, & Sullivan, 2003). Adults with 22q11.2 deletion syndrome have a high rate of hypothyroidism but this has not been definitely shown to be secondary to autoimmune destruction (Bassett et al., 2005). Celiac disease may be increased over the frequency in the general population (Digilio et al., 2003). The mechanism underlying the susceptibility to autoimmune disease is probably multifactorial. One study found that low T cells in early childhood were more common in those with subsequent autoimmune disease (Tison et al., 2011).
Therapy for autoimmune disease remains undefined. Standard approaches are most often used although sometimes an effort is made to limit the suppression of T cells. Rituximab has been useful in autoantibody-mediated disorders. There is a growing appreciation that low T cells in early infancy are a marker for subsequent dysregulation, although more study is required.
5 |. ATOPY
Extreme lymphopenia can drive a Th2 skewing, strongly associated with atopy (Khiong et al., 2007; Milner et al., 2007; Wada et al., 2000). Nevertheless, it was only recently appreciated that allergies and Th2 skewing of T cells were identified in patients with 22q11.2del (Morsheimer, Brown Whitehorn, Heimall, & Sullivan, 2017; Staple, Andrews, McDonald-McGinn, Zackai, & Sullivan, 2005; Zemble et al., 2010). Low T cell counts in infancy were associated with an increased risk of atopy, similar to what was described for autoimmune disease (Morsheimer et al., 2017).
6 |. SUMMARY
The 22q11.2del syndrome is associated with a broad range of end organ effects. Prolonged viral infections have long been known to be associated with compromised T cells. Secondary consequences related to T cell lymphopenia include an increased risk of atopy and autoimmune disease. A key aspect of the immunodeficiency is the relationship of CD4 + lymphopenia to deletion breakpoints including the TBX1 region, a previously unappreciated association.
ACKNOWLEDGMENTS
The authors would like to acknowledge the patients and families, the Bioinformatics Department at The Children’s Hospital of Philadelphia, the Division of Genetics, and the outstanding nurses and caregivers.
REFERENCES
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, & Gatzoulis MA (2005). Clinical features of 78 adults with 22q11 deletion syndrome. American Journal of Medical Genetics Part A, 138(4), 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Lowther C, Merico D, Costain G, Chow EWC, van Amelsvoort T, & Marshall CR International 22q DSB, Behavior C (2017). Rare genome-wide copy number variation and expression of schizophrenia in 22q11.2 deletion syndrome. American Journal of Psychiatry, 174(11), 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, … Vorstman J (2011). Practical guidelines for managing patients with 22q11.2 deletion syndrome. The Journal of Pediatrics, 159(2), 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork AH, Oskarsdottir S, Andersson BA, & Friman V (2012). Antibody deficiency in adults with 22q11.2 deletion syndrome. American Journal of Medical Genetics Part A, 158(8), 1934–1940. [DOI] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, & Campbell RM … (2003). A population-based study of the 22q11.2 deletion: Phenotype, incidence, and contribution to major birth defects in the population. Pediatrics, 112(1 Pt 1), 101–107. [DOI] [PubMed] [Google Scholar]
- Chinen J, Rosenblatt HM, Smith EO, Shearer WT, & Noroski LM (2003). Long-term assessment of T-cell populations in DiGeorge syndrome. Journal of Allergy and Clinical Immunology, 111(3), 573–579. [DOI] [PubMed] [Google Scholar]
- Dar N, Gothelf D, Korn D, Frisch A, Weizman A, Michaelovsky E, … Somech R (2015). Thymic and bone marrow output in individuals with 22q11.2 deletion syndrome. Pediatric Research, 77(4), 579–585. [DOI] [PubMed] [Google Scholar]
- Davies JK, Telfer P, Cavenagh JD, Foot N, & Neat M (2003). Autoimmune cytopenias in the 22q11.2 deletion syndrome. Clinical and Laboratory Haematology, 25(3), 195–197. [DOI] [PubMed] [Google Scholar]
- Davies K, Stiehm ER, Woo P, & Murray KJ (2001). Juvenile idiopathic polyarticular arthritis and IgA deficiency in the 22q11 deletion syndrome. Journal of Rheumatology, 28(10), 2326–2334. [PubMed] [Google Scholar]
- Derfalvi B, Maurer K, McDonald McGinn DM, Zackai E, Meng W, Luning Prak ET, & Sullivan KE (2016). B cell development in chromosome 22q11.2 deletion syndrome. Clinical Immunology, 163, 1–9. [DOI] [PubMed] [Google Scholar]
- Di Cesare S, Puliafito P, Ariganello P, Marcovecchio GE, Mandolesi M, Capolino R, … Cancrini C (2015). Autoimmunity and regulatory T cells in 22q11.2 deletion syndrome patients. Pediatric Allergy and Immunology, 26(6), 591–594. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Giannotti A, Castro M, Colistro F, Ferretti F, Marino B, & Dallapiccola B (2003). Screening for celiac disease in patients with deletion 22q11.2 (DiGeorge/velo-cardio-facial syndrome). American Journal of Medical Genetics Part A, 121A(3), 286–288. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Marino B, Giannotti A, & Dallapiccola B (1997). Familial deletions of chromosome 22q11 [letter; comment]. American Journal of Medical Genetics, 73(1), 95–96. [PubMed] [Google Scholar]
- Driscoll DA, Boland T, Emanuel BS, Kirschner RE, LaRossa D, Manson J, … Mitchell LE (2006). Evaluation of potential modifiers of the palatal phenotype in the 22q11.2 deletion syndrome. The Cleft Palate-Craniofacial Journal, 43(4), 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Li M, Chien P, Capuano S, Zackai EH, McDonald-McGinn DM, … Budarf ML (1995). Familial 22q11 deletions: Phenotypic variability and determination of deletion boundaries by FISH. American Journal of Human Genetics, 57, A163. [Google Scholar]
- Ferrando-Martinez S, Lorente R, Gurbindo D, De Jose MI, Leal M, Munoz-Fernandez MA, & Correa-Rocha R (2014). Low thymic output, peripheral homeostasis deregulation, and hastened regulatory T cells differentiation in children with 22q11.2 deletion syndrome. Jornal De Pediatria, 164(4), 882–889. [DOI] [PubMed] [Google Scholar]
- Finocchi A, Di Cesare S, Romiti ML, Capponi C, Rossi P, Carsetti R, & Cancrini C (2006). Humoral immune responses and CD27+ B cells in children with DiGeorge syndrome (22q11.2 deletion syndrome). Pediatric Allergy and Immunology, 17(5), 382–388. [DOI] [PubMed] [Google Scholar]
- Gennery AR, Barge D, O’Sullivan JJ, Flood TJ, Abinun M, & Cant AJ (2002). Antibody deficiency and autoimmunity in 22q11.2 deletion syndrome. Archives of Disease in Childhood, 86(6), 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli M, Kumar R, Soresina A, Tamassia N, Lorenzini T, Moratto D, … Badolato R (2016). Reduction of CRKL expression in patients with partial DiGeorge syndrome is associated with impairment of T-cell functions. Journal of Allergy and Clinical Immunology, 138(1), 229–240. [DOI] [PubMed] [Google Scholar]
- Habel A, Herriot R, Kumararatne D, Allgrove J, Baker K, Baxendale H, … Tsai-Goodman B (2014). Towards a safety net for management of 22q11.2 deletion syndrome: Guidelines for our times. European Journal of Pediatrics, 173(6), 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwadkar A, Gennery AR, Moran AS, Haeney MR, & Arkwright PD (2010). Association between hypoparathyroidism and defective T cell immunity in 22q11.2 deletion syndrome. Journal of Clinical Pathology, 63(2), 151–155. [DOI] [PubMed] [Google Scholar]
- Jawad AF, McDonald-Mcginn DM, Zackai E, & Sullivan KE (2001). Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). The Journal of Pediatrics, 139(5), 715–723. [DOI] [PubMed] [Google Scholar]
- Jerome LA, & Papaioannou VE (2001). DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nature Genetics, 27(3), 286–291. [DOI] [PubMed] [Google Scholar]
- Kanaya Y, Ohga S, Ikeda K, Furuno K, Ohno T, Takada H, … Hara T (2006). Maturational alterations of peripheral T cell subsets and cytokine gene expression in 22q11.2 deletion syndrome. Clinical and Experimental Immunology, 144(1), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiong K, Murakami M, Kitabayashi C, Ueda N, Sawa S, Sakamoto A, … Hirano T (2007). Homeostatically proliferating CD4 T cells are involved in the pathogenesis of an Omenn syndrome murine model. The Journal of Clinical Investigation, 117(5), 1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocperk A, Mejstrikova E, Kayserova J, Kalina T, & Sediva A (2015). Low marginal zone-like B lymphocytes and natural antibodies characterize skewed B-lymphocyte subpopulations in del22q11 DiGeorge patients. Clinical Immunology, 161(2), 144–149. [DOI] [PubMed] [Google Scholar]
- Kratz CP, Niehues T, Lyding S, Heusch A, Janssen G, & Gobel U (2003). Evans syndrome in a patient with chromosome 22q11.2 deletion syndrome: A case report. Pediatric Hematology and Oncology, 20(2), 167–172. [DOI] [PubMed] [Google Scholar]
- Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, … Bonagura VR (2014). Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA, 312(7), 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence S, McDonald-McGinn DM, Zackai E, & Sullivan KE (2003). Thrombocytopenia in patients with chromosome 22q11.2 deletion syndrome. Jornal De Pediatria, 143(2), 277–278. [DOI] [PubMed] [Google Scholar]
- Lima K, Abrahamsen TG, Foelling I, Natvig S, Ryder LP, & Olaussen RW (2010). Low thymic output in the 22q11.2 deletion syndrome measured by CCR9+CD45RA+ T cell counts and T cell receptor rearrangement excision circles. Clinical and Experimental Immunology, 161(1), 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EA, Botta A, Jurecic V, Carattini-Rivera S, Cheah YC, Rosenblatt HM, … Baldini A (1999). Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature, 401(6751), 379–383. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, … Baldini A (2001). Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature, 410(6824), 97–101. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivera E, Liu YP, Verbitsky M, Anderson BR, Capone VP, Otto EA, … Sanna-Cherchi S (2017). Genetic drivers of kidney defects in the DiGeorge syndrome. New England Journal of Medicine, 376(8), 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Kirschner R, Goldmuntz E,Sullivan K, Eicher P, Gerdes M, … Zackai EH (1999). The philadelphia story: The 22q11.2 deletion: Report on 250 patients. Genetic Counseling, 10, 11–24. [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, & Zackai EH 2001. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: Cast a wide FISHing net! Genetics in medicine: official. Journal of the American College of Medical Genetics, 3(1), 23–29. [DOI] [PubMed] [Google Scholar]
- McLean-Tooke A, Spickett GP, & Gennery AR (2007). Immunodeficiency and autoimmunity in 22q11.2 deletion syndrome. Scandinavian Journal of Immunology, 66(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Milner JD, Ward JM, Keane-Myers A, & Paul WE (2007). Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proceedings of the National Academy of Sciences of the United States of America, 104(2), 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsheimer MM, Brown Whitehorn T, Heimall J, & Sullivan K (2017). The immune deficiency of chromosome 22q11.2 deletion syndrome. American Journal of Medical Genetics Part A, 173(9), 2366–2372. [DOI] [PubMed] [Google Scholar]
- Patel K, Akhter J, Kobrynski L, Benjamin Gathmann MA, Davis O, & Sullivan KE International DiGeorge Syndrome Immunodeficiency C (2012). Immunoglobulin deficiencies: The B-lymphocyte side of DiGeorge syndrome. The Journal of Pediatrics, 161(5), 950–953. [DOI] [PubMed] [Google Scholar]
- Piliero LM, Sanford AN, McDonald-McGinn DM, Zackai EH, & Sullivan KE (2004). T-cell homeostasis in humans with thymic hypoplasia due to chromosome 22q11.2 deletion syndrome. Blood, 103(3), 1020–1025. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, … Scambler PJ (1997). Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. Journal of Medical Genetics, 34, 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Driscoll DA, Emanuel BS, McDonald-McGinn DM, Zackai EH, & Sullivan KE (1998). Increased prevalence of immunoglobulin A deficiency in patients with the chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clinical and Diagnostic Laboratory Immunology, 5(3), 415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans I, Lambrechts D, De Smet F, Jansen S, Wang J, Maity S, … Carmeliet P (2003). VEGF: A modifier of the del22q11 (DiGeorge) syndrome? Nature Medicine, 9(2), 173–182. [DOI] [PubMed] [Google Scholar]
- Staple L, Andrews T, McDonald-McGinn D, Zackai E, & Sullivan KE (2005). Allergies in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) and patients with chronic granulomatous disease. Pediatric Allergy and Immunology, 16(3), 226–230. [DOI] [PubMed] [Google Scholar]
- Sullivan KE, Jawad AF, Randall P, Driscoll DA, Emanuel BS, McDonald-McGinn DM, & Zackai EH (1998). Lack of correlation between impaired T cell production, immunodeficiency and other phenotypic features in chromosome 22q11.2 deletions syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clinical Immunology and Immunopathology, 84, 141–146. [DOI] [PubMed] [Google Scholar]
- Sullivan KE, McDonald-McGinn D, Driscoll DA, Emanuel BS, Zackai EH, & Jawad AF (1999). Longitudinal analysis of lymphocyte function and numbers in the first year of life in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clinical and Diagnostic Laboratory Immunology, 6(6), 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, McDonald-McGinn D, & Zackai EH (2002). CD4(+) CD25(+) T-cell production in healthy humans and in patients with thymic hypoplasia. Clinical and Diagnostic Laboratory Immunology, 9(5), 1129–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, & Surh CD (2001). IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchao NK, & Turka LA (2012). Lymphodepletion and homeostatic proliferation: Implications for transplantation. American Journal of Transplantation, 12(5), 1079–1090. [DOI] [PubMed] [Google Scholar]
- Tison BE, Nicholas SK, Abramson SL, Hanson IC, Paul ME, Seeborg FO, … Chinen J (2011). Autoimmunity in a cohort of 130 pediatric patients with partial DiGeorge syndrome. Journal of Allergy and Clinical Immunology, 128(5), 1115–1117. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Viola A, Morishima M, Pramparo T, Baldini A, & Lindsay E (2003). TBX1 is required for inner ear morphogenesis. Human Molecular Genetics, 12(16), 2041–2048. [DOI] [PubMed] [Google Scholar]
- Wada T, Takei K, Kudo M, Shimura S, Kasahara Y, Koizumi S, … Yachie A (2000). Characterization of immune function and analysis of RAG gene mutations in Omenn syndrome and related disorders. Clinical and Experimental Immunology, 119(1), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdershoven JC, Bowser M, Sheridan MB, McDonald-McGinn DM, Zackai EH, Solot CB, … Emanuel BS (2013). A candidate gene approach to identify modifiers of the palatal phenotype in 22q11.2 deletion syndrome patients. International Journal of Pediatric Otorhinolaryngology, 77(1), 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H, Ishii C, Maeda J, Kojima Y, Matsuoka R, Kimura M, … Matsuo N (1998). Phenotypic discordance in monozygotic twins with 22q11.2 deletion. American Journal of Medical Genetics, 78(4), 319–321. [PubMed] [Google Scholar]
- Zemble R, Luning Prak E, McDonald K, McDonald-McGinn D, Zackai E, & Sullivan K (2010). Secondary immunologic consequences in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clinical Immunology, 136(3), 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Noroski LM, Hanson IC, Chen Y, Lee ME, Huang Y, … Liu D. 2015. Molecular mechanisms of functional natural killer deficiency in patients with partial DiGeorge syndrome. Journal of Allergy and Clinical Immunology 135(5):1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]