Abstract
Matrix-induced autologous chondrocyte implantation is a 2-stage surgical procedure used to treat symptomatic, full-thickness chondral lesions of the knee. This third-generation autologous chondrocyte implantation (ACI) technique improves on the limitations of previous methods, including the risk of uneven chondrocyte distribution at the time of implantation and graft hypertrophy. Given the compliant properties of the scaffold, the graft can be easily shaped to treat irregular chondral defects and applied to articular surfaces with multiplanar geometry (e.g., patella, trochlea). Although ACI techniques are ideally suited to treat chondral surface defects, the ACI “sandwich” technique can be used to treat large osteochondral defects with significant bone loss (>8 mm). Historically, this procedure uses autologous bone graft to replace areas of osseous deficiency along with 2 type I/III collagen bilayer membranes to securely contain the cultured chondrocytes within the defect. We present an analogous technique for the treatment of osteochondral lesions of the femoral trochlea using a single matrix-induced ACI scaffold and autologous bone grafting for a segmental osseous defect.
Restorative treatment options for symptomatic, full-thickness chondral and osteochondral lesions of the knee continue to evolve with advancements in our understanding of cartilage biology and surgical techniques. Since the initial description by Brittberg et al.,1 in 1994, autologous chondrocyte implantation (ACI) has gained widespread use, and surgical utilization in the United States has nearly doubled over the past decade. Although the long-term clinical results of first-generation techniques have demonstrated sustained functional improvement, there were significant technical challenges and adverse events related to the requisite use of a periosteal patch over the defect. A large number of patients demonstrated arthrofibrosis and graft hypertrophy, which necessitated additional surgical procedures to address these complications. Ultimately, the use of periosteum was largely abandoned in favor of a bioabsorbable collagen membrane cover in 2007, significantly reducing the rate of graft hypertrophy and the rates of reoperation.2
Third-generation ACI techniques, including matrix-induced autologous chondrocyte implantation (MACI), use cell-loaded membranes to avoid graft-related complications and simplify the surgical technique. The MACI scaffold (Vericel, Cambridge, MA) specifically uses a porcine type I/III collagen membrane seeded with autologous chondrocytes at a density ranging between 500,000 and 1 million cells/cm2. In a recent report of the Superiority of MACI Implant Versus Microfracture Treatment trial, clinical outcomes following the treatment of chondral defects (≥3 cm2) with MACI were clinically superior at 5 years compared with microfracture treatment.3 Additional case series have reported similar mid and long-term results; however, there is a paucity of data investigating clinical outcomes of MACI augmented with bone graft for the treatment of osteochondral lesions.4, 5 In this article, we describe a surgical technique using MACI and autologous bone graft to address a segmental bone defect within the chondral lesion. The surgical indications/contraindications for this technique are summarized in Table 1.
Table 1.
Indications/Contraindications for MACI Transplantation Augmented With Autologous Bone Graft
| Indications | Contraindications |
|---|---|
| Symptomatic, full-thickness osteochondral lesions >2 cm2 | Advanced degenerative changes affecting multiple compartments of the knee |
| Bone deficiency with cavitary defect measuring >8 mm in depth | Uncorrected lower extremity malalignment |
| Femoral condyle, trochlea, patella, or tibial lesions resulting from osteochondritis dissecans, osteonecrosis, post-traumatic osteochondral defects, or localized osteoarthritis | Uncorrected ligamentous instability |
| Young, high-demand patients who are not a candidate for arthroplasty | Meniscal insufficiency |
| BMI <35 | Inflammatory arthritis |
BMI, body mass index; MACI, matrix-induced autologous chondrocyte implantation.
Surgical Technique
Patient Positioning and Bone Marrow Aspirate Concentrate Harvest
The patient is placed in the supine position, and general anesthesia is induced following a regional block (Video 1). The ipsilateral anterior iliac crest is prepped in a sterile fashion, and the overlying periosteum is anesthetized with 1% lidocaine. The trochar is percutaneously inserted through the skin down to the iliac crest. The inner and outer table of the crest is palpated to ensure proper orientation of the trochar as it is manually inserted using a mallet. A total of 60 mL of bone marrow aspirate is obtained and processed using the Arthrex Angel System Centrifuge (Arthrex, Naples, FL).
Open Surgical Approach and Defect Preparation
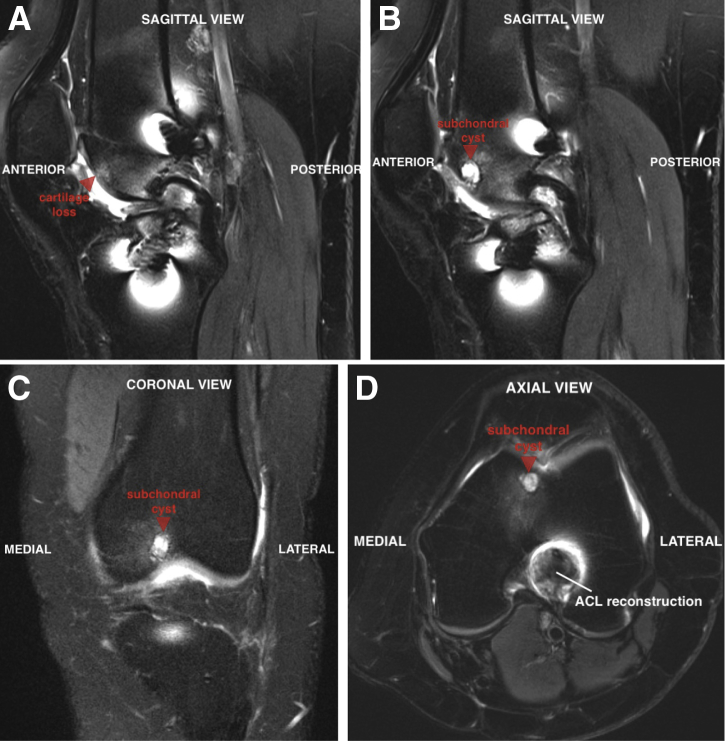
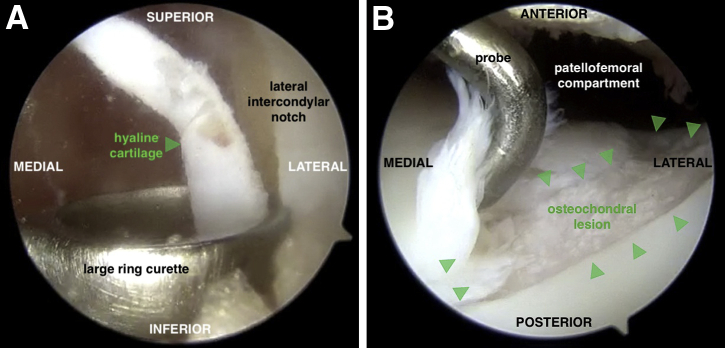
Preoperative magnetic resonance imaging reveals an approximately 6 × 8-mm cyst underlying the central area of the lesion (Fig 1). Given the staged nature of this procedure, an initial diagnostic arthroscopy aids in characterization of the osteochondral lesion with regards to lesion size, depth, and morphology. Using a large ring curette, 200 to 300 mg of hyaline cartilage is harvested from the intercondylar notch along the lateral femoral condyle (Fig 2). The patient is brought back to the operating room approximately 6 weeks later for MACI implantation, at which time we proceed directly with an open surgical approach.
Fig 1.
(A) Preoperative magnetic resonance imaging of the left knee reveals a 1.5-cm region of full-thickness cartilage loss in the femoral trochlea (arrow) on the sagittal view. Underlying the lesion is a 6 × 8-mm subchondral cyst (arrow) that can be seen on sagittal (B), coronal (C), and axial (D) views.
Fig 2.
(A) During the first of this 2-stage procedure, a large ring curette is used to harvest 200 to 300 mg of hyaline cartilage (arrow) from the intercondylar notch along the lateral femoral condyle of the left knee with the knee flexed. An anterolateral portal is used for viewing, and the curette is inserted through the anteromedial portal. (B) A diagnostic arthroscopy is also performed to assess the trochlear lesion (arrows) with regard to size, depth, and morphology. This view is from an anterolateral portal with the knee extended.
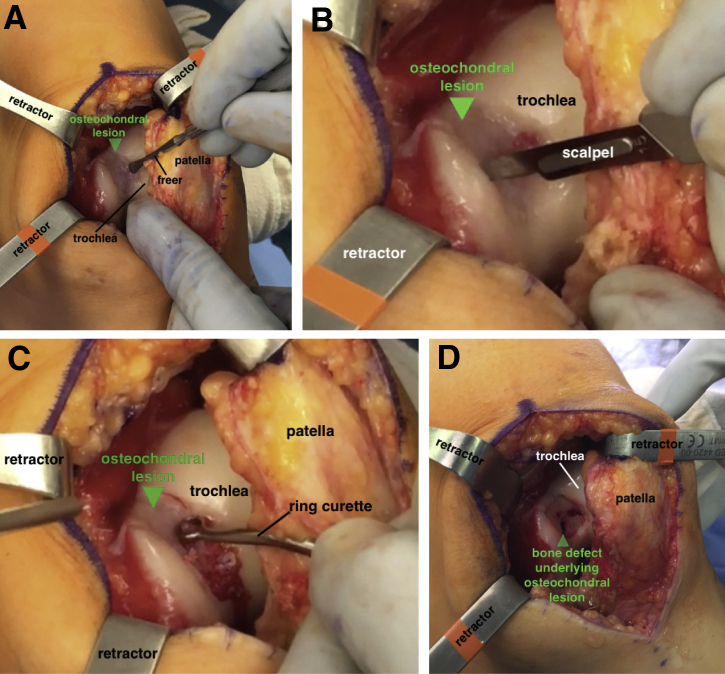
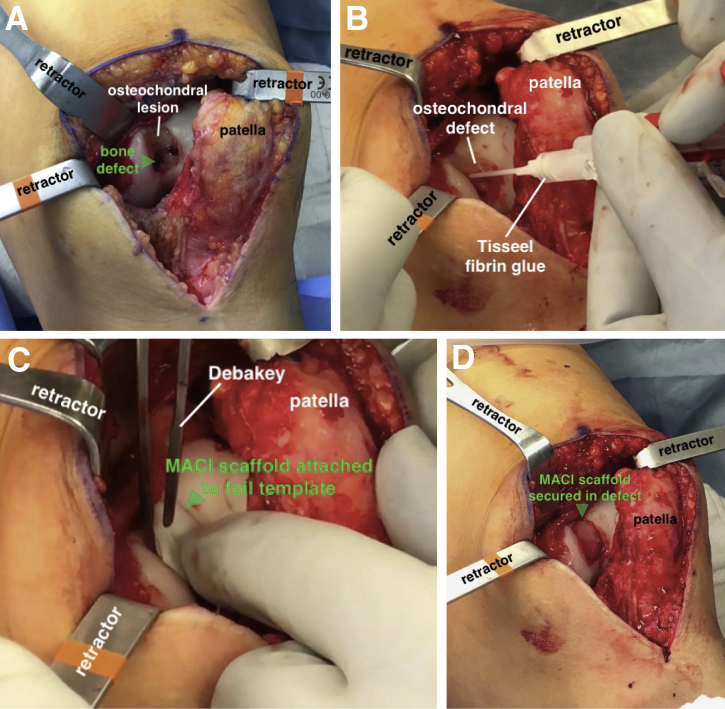
A tourniquet is applied to the proximal thigh and initially inflated to 250 mm Hg. Using a standard anterior midline incision, the skin is sharply incised and a medial parapatellar arthrotomy is performed to provide access to the femoral trochlea. In this case, we identified a large, full-thickness lesion within the central aspect of the trochlea (Fig 3A). Using a 15-blade scalpel, the borders of the lesion are sharply delineated (Fig 3B). A ring curette is used to debride the base of the defect and create stable vertical edges at the periphery of the lesion (Fig 3C). It is important to remove the underlying calcified cartilage layer without penetrating the subchondral bone. The final dimensions of the lesion are measured to be approximately 20 × 25 mm. Although the area of osseous deficiency is readily identifiable in most cases, in this situation, the subchondral bone overlying the segmental 6× 8-mm bone defect was intact but appeared abnormal. A 2.7-mm drill is used to perforate the overlying bone, gradually creating a 6-mm hole to gain access to the underlying bone defect (Fig 3D). Using a curette, the sclerotic base and margins of the cavitary defect are debrided to healthy appearing bone, and a small k-wire may be used to drill the base of the lesion to enhance the blood supply and subsequent healing.
Fig 3.
(A) With the patient supine and left knee slightly flexed, the full-thickness lesion in the femoral trochlea is exposed (arrow) through a medial parapatellar arthrotomy. (B) The borders of the lesion (arrow) are sharply delineated using a 15-blade scalpel. (C) A ring curette is used to debride the base of the defect and create stable vertical edges at the periphery of the lesion (arrow). It is important to remove the underlying calcified cartilage layer without penetrating the subchondral bone. (D) A 2.7-mm drill is used to perforate the lesion and gain access to the underlying bone defect (arrow). The sclerotic margins of the defect are debrided with a curette and drilled with a k-wire.
Autologous Bone Graft Harvest: Proximal Tibia
The midline incision is carried distally in line with a previous surgical incision (anterior cruciate ligament reconstruction). The proximal tibia above the pes anserinus is exposed with electrocautery, and a 4.5-mm drill is used to perforate the cortex, creating access to the metaphyseal region of the tibia. A large curved curette is used to harvest an adequate amount of cancellous bone for impaction grafting. This bone is soaked in the bone marrow aspirate concentrate (BMAC) solution for a minimum of 10 to 15 minutes, and the bone void in the proximal tibia is filled with cancellous allograft bone chips.
MACI Implantation
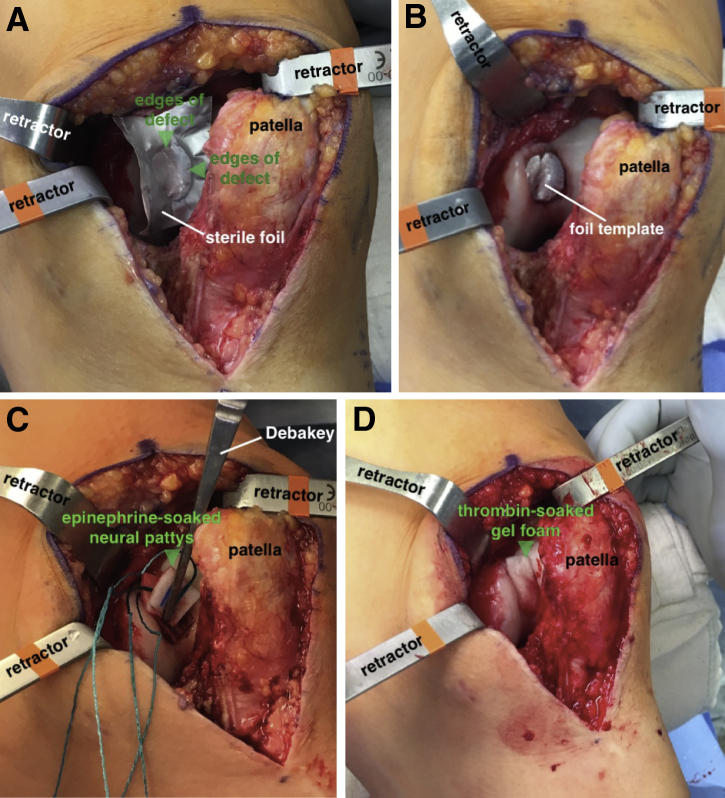
The shape of the defect is templated using a sterile foil wrapper from any commercial suture package. A Freer elevator is used to manually press the foil against the vertical edges of the defect, thereby providing a precise sizing template (Fig 4A). The foil template is cut, and the 12 o'clock position is marked for orientation. The template can be checked for appropriate size and shape by placing it in the defect (Fig 4B). The tourniquet is released, and hemostasis is achieved at the defect site using a combination of epinephrine-soaked neural patties and thrombin-soaked gel foam (Fig 4C and D).
Fig 4.
(A) With the patient supine and left knee slightly flexed, a template is made by placing a sterile foil wrapper from any commercial suture package into the defect and pressing along the edges of the defect (arrows) with a Freer elevator. (B) Once cut, the template can be checked for accurate sizing in the defect, with the 12 o'clock position marked for orientation. After the tourniquet is released, hemostasis at the defect site is achieved with epinephrine-soaked neural patties (C) or thrombin-soaked gel foam (D) (arrows).
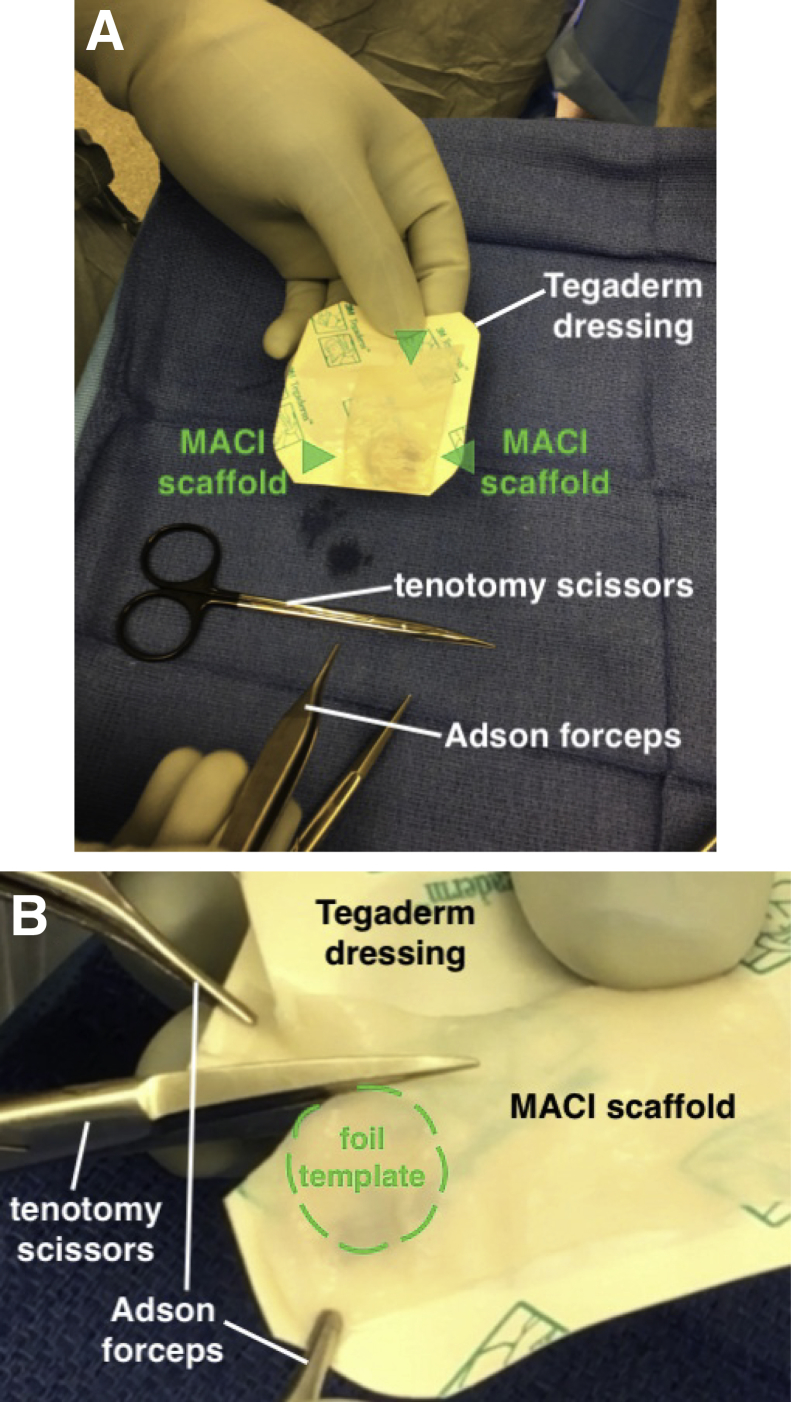
The MACI scaffold is removed from the container and placed cell side up on a Tegaderm dressing to provide a stable platform for graft preparation (Fig 5A). For orientation purposes, it is important to ensure that the notch in the scaffold is in the lower left corner to ensure that the cellular side of the graft is facing up. After noting the appropriate orientation of the foil template, it is placed between the scaffold and Tegaderm dressing so that it is clearly visible. Tenotomy scissors are used to cut the graft according to the underlying margins of the foil template (Fig 5B).
Fig 5.
(A) The MACI scaffold (arrows) is placed on a Tegaderm dressing to provide a stable platform for graft preparation. When the notch is in the lower left corner of the scaffold, the cellular side is facing up. It is imperative to note proper orientation before cutting the graft. (B) The template is placed between the Tegaderm and the scaffold, and the scaffold is cut using tenotomy scissors. It is helpful to have an assistant hold the corners of the graft and Tegaderm stable with small forceps while cutting. (MACI, matrix-induced autologous chondrocyte implantation.)
The bone graft is removed from the BMAC solution and impacted into the defect with a small bone tamp to a level even with the subchondral bone (Fig 6A). The base of the defect is filled with Tisseel fibrin glue, and the scaffold is placed cell side down in the proper orientation (Fig 6B and C). Light digital pressure is held against the graft for 3 minutes while the fibrin glue solidifies (Fig 6D). Additional fibrin glue is placed along the periphery of the lesion for additional fixation. The knee is fully cycled a minimum of 10 to 15 times to ensure graft stability, and the wound is closed in a layered fashion.
Fig 6.
(A) With the patient supine and left knee slightly flexed, the defect is filled with BMAC-soaked bone graft to a level flush with the subchondral bone (arrow). (B) The base of the defect is filled with Tisseel fibrin glue. (C) The scaffold is placed flush into the lesion with the cell side down. It is helpful to insert the scaffold with it still attached to the foil template (arrow), to allow for stable insertion in the proper orientation. (D) After removing the template and holding light digital pressure on the graft for 3 minutes, the fibrin glue has solidified and the graft (arrow) is now secured. (BMAC, bone marrow aspirate concentrate.)
Postoperative Rehabilitation
Following treatment of patellofemoral cartilage lesions, the patient is permitted to bear full weight on the operative extremity in a hinged knee brace with crutches. The brace is kept locked during ambulation until quadriceps function is satisfactory. Typically, the brace is discontinued at approximately 4 weeks, and patients are allowed to ambulate without crutches at 6 to 8 weeks postoperatively if they demonstrate sufficient proximal hip and quadriceps strength with a normal gait pattern. A continuous passive motion machine is used for a minimum of 6 hours daily for 6 weeks. Patients are encouraged to return to most activities of daily living at 3 months and are permitted to participate in noncontact inline athletic activities (e.g., swimming, rollerblading, hiking, elliptical) at approximately 4 to 6 months. Full return to unrestricted athletic activities is usually permitted after 12 months of supervised rehabilitation.
Discussion
There are several advantages to the MACI procedure relative to prior ACI techniques (Table 2). The collagen membrane stabilizes the chondrocytes in situ and obviates the need for a periosteal membrane, which has innate vasculogenic and hypertrophic potential, to secure the cultured chondrocytes. Next, the scaffold can be easily secured within the base of the defect using fibrin glue, which precludes the use of sutures, thereby facilitating a faster, more minimally invasive technique through a mini-arthrotomy or arthroscopic approach.6 Finally, the compliant properties of the MACI scaffold make it particularly suitable for chondral defects in the patellofemoral joint, where the geometry of the anatomic surfaces are multiplanar and variable among patients. The primary limitation of MACI compared with prior ACI techniques is the expense of the MACI scaffold. Compared with non-ACI techniques for treating osteochondral defects, such as osteochondral allograft transplantation, disadvantages of MACI include that it is a 2-stage procedure and may necessitate a longer rehabilitation for graft maturation.
Table 2.
Advantages/Disadvantages for MACI Transplantation Augmented With Autologous Bone Graft
| Advantages | Disadvantages |
|---|---|
| Accurate restoration of the osteochondral unit given the compliant properties of the cancellous bone graft and MACI scaffold | 2-stage procedure |
| Minimally invasive technique performed through a mini-arthrotomy or arthroscopic procedure | Cost of MACI scaffold |
| Ability to treat lesions that were once difficult to access, including tibial defects | Given the slower graft maturation process observed with MACI, it is important to counsel athletes that return to sports is unlikely to occur before 12 months. |
MACI, matrix-induced autologous chondrocyte implantation.
The ACI “sandwich” technique is a successful procedure that requires impaction bone grafting of the lesion and 2 collagen membranes to separately secure the bone graft and cultured chondrocytes.7 The first type I/III collagen bilayer membrane is positioned over the bone graft and secured to the defect with fibrin glue and sutures. A second membrane is then sutured to the articular surface overlying the first collagen membrane, and the chondrocytes are injected between the 2 membranes. A study by Vijayan et al.8 reported on 14 patients treated with a sandwich technique using MACI scaffolds and found significant improvements at a mean follow-up of 5.2 years, with only 1 instance of graft failure (7.1%).
Few studies have evaluated outcomes of segmental bone defects with MACI scaffolds. In our description, we used 1 MACI membrane given the small osseous defect (6 × 8 mm) within the chondral surface lesion. The fibrin glue is used to provide a watertight seal to prevent displacement of the bone graft and contamination of the graft site with marrow-derived cells. Although we have noted successful early results with this technique for very small segmental defects, we recommend using a formal sandwich technique with a second MACI scaffold for larger bone defects (>20% of the total surface area) to ensure satisfactory containment of the underlying bone graft (Table 3). Further follow-up is under way to determine clinical results following this procedure and establish formal treatment guidelines.
Table 3.
Technical Pearls/Pitfalls for MACI Transplantation Augmented With Autologous Bone Graft
| Pearls | Pitfalls |
|---|---|
| It is important to thoroughly debride the cavitary bone defect, including the sclerotic margins. A small k-wire can be used to enhance the blood supply if necessary, and BMAC may be useful to aid in bone graft incorporation and successful healing. | The MACI graft should be positioned flat within the defect. It is important to cut the margins of the graft so that they do not overlap the surrounding articular cartilage surface, because this could predispose the graft to delamination and early failure. |
| Ensure that enough bone graft is obtained to adequately fill the defect flush to the surrounding subchondral bone. | Pay close attention to the orientation of the MACI graft as it is prepared and positioned within the defect. It is extremely important to ensure that the cellular side of the scaffold is facing down toward the subchondral bone. |
| Use a sufficient amount of fibrin glue to ensure that the area overlying the bone graft has a watertight seal. | |
| In most cases, fibrin glue provides adequate graft stability, but if there is concern about graft fixation, No. 6-0 resorbable suture can be used to provide additional fixation. |
BMAC, bone marrow aspirate concentrate; MACI, matrix-induced autologous chondrocyte implantation.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: K.J.J. reports nonfinancial support from Aesculap and Arthrex, grants from the Musculoskeletal Transplant Foundation, personal fees from Joint Restoration Foundation Ortho and Vericel, and is a board member for the American Orthopaedic Society for Sports Medicine. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Demonstration of a technique for the treatment of osteochondral lesions of the femoral trochlea using a single MACI scaffold and autologous bone grafting for a segmental osseous defect. The procedure is performed with the patient supine. (MACI, matrix-induced autologous chondrocyte implantation.)
References
- 1.McCormick F., Harris J.D., Abrams G.D. Trends in the surgical treatment of articular cartilage lesions in the United States: An analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30:222–226. doi: 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Krill M., Early N., Everhart J.S., Flanigan D.C. Autologous chondrocyte implantation (ACI) for knee cartilage defects: A review of indications, technique, and outcomes. JBJS Rev. 2018;6:e5. doi: 10.2106/JBJS.RVW.17.00078. [DOI] [PubMed] [Google Scholar]
- 3.Brittberg M., Recker D., Ilgenfritz J., Saris D.B.F., SUMMIT Extension Study Group Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46:1343–1351. doi: 10.1177/0363546518756976. [DOI] [PubMed] [Google Scholar]
- 4.Kreuz P.C., Kalkreuth R.H., Niemeyer P., Uhl M., Erggelet C. Long-term clinical and MRI results of matrix-associated autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. February 1, 2018 doi: 10.1177/1947603518756463. [E-pub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marlovits S., Aldrian S., Wondrasch B. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40:2273–2280. doi: 10.1177/0363546512457008. [DOI] [PubMed] [Google Scholar]
- 6.Edwards P.K., Ebert J.R., Janes G.C., Wood D., Fallon M., Ackland T. Arthroscopic versus open matrix-induced autologous chondrocyte implantation: Results and implications for rehabilitation. J Sports Rehabil. 2014;23:203–215. doi: 10.1123/jsr.2013-0042. [DOI] [PubMed] [Google Scholar]
- 7.Minas T., Ogura T., Headrick J., Bryant T. Autologous chondrocyte implantation “sandwich” technique compared with autologous bone grafting for deep osteochondral lesions in the knee. Am J Sports Med. 2018;46:322–332. doi: 10.1177/0363546517738000. [DOI] [PubMed] [Google Scholar]
- 8.Vijayan S., Bartlett W., Bentley G. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: A two- to eight-year follow-up study. J Bone Joint Surg Br. 2012;94:488–492. doi: 10.1302/0301-620X.94B4.27117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of a technique for the treatment of osteochondral lesions of the femoral trochlea using a single MACI scaffold and autologous bone grafting for a segmental osseous defect. The procedure is performed with the patient supine. (MACI, matrix-induced autologous chondrocyte implantation.)