Abstract
Osteochondritis dissecans (OCD) lesions of the knee are a significant source of pain and disability. Although the pathologic process for this condition remains poorly understood, histologic studies suggest vascular insufficiency of the subchondral bone may be the underlying cause for focal necrosis and subsequent compromise of the overlying articular cartilage. These lesions most commonly affect the medial femoral condyle and can be found along the margins of the intercondylar notch. Because of significant bone involvement, osteochondral allograft (OCA) transplantation has emerged as a dominant treatment option for OCD lesions because it can accurately restore the entire osteochondral unit. Given the characteristic location and large, irregular shapes of these lesions, surgical management can be challenging. These lesions are often uncontained along the periphery of the condyle, which can compromise OCA graft fixation and healing. We describe our preferred technique for the treatment of large, uncontained OCD lesions of the medial femoral condyle using a unicompartmental OCA augmented with screw fixation.
Surgical management of osteochondritis dissecans (OCD) lesions can include palliative, reparative, and restorative techniques. Osteochondral allograft (OCA) transplantation represents a successful restorative option with well-established clinical outcomes and excellent graft survivorship.1, 2 It is important to note, however, the technical challenges associated with the surgical management of OCD lesions relative to other osteochondral defects. First, OCD lesions can be very large and irregularly shaped, thereby requiring multiple grafts in a “snowman” or “mastercard” configuration to restore the entire articular surface. Next, there is often significant bone involvement, which obviates standard technical approaches to minimize the depth of the recipient socket and the amount of allograft bone that is implanted to reduce immune reaction. Last, these lesions can be uncontained at the periphery of the femoral condyle, compromising the ability to achieve secure press-fit fixation of the OCA. In this article, we describe our preferred surgical approach for the treatment of large, uncontained OCD lesions of the MFC using a fresh OCA and the Arthrex BioUni Instrumentation System (Arthrex, Naples, FL) (Video 1). The surgical indications and contraindications for this procedure are summarized in Table 1, along with the potential pearls and pitfalls associated with this technique (Table 2).
Table 1.
Indications/Contraindications and Advantages/Disadvantages of BioUni Osteochondral Allograft Transplantation
| Indications |
| Symptomatic, full-thickness chondral or osteochondral lesions >2 cm2 |
| Femoral condylar lesions resulting from OCD, osteonecrosis, post-traumatic osteochondral defects, or localized osteoarthritis |
| Salvage procedure for previously failed cartilage restoration procedures |
| Young, high-demand patients who are not a candidate for arthroplasty |
| BMI <35 |
| Contraindications |
| Advanced degenerative changes affecting multiple compartments of the knee |
| Uncorrected lower extremity malalignment |
| Uncorrected ligamentous instability |
| Meniscal insufficiency |
| Inflammatory arthritis |
| Patient comorbidities (smoking, chronic steroid use, alcohol abuse, and/or drug abuse) |
| Advantages |
| Cartilage restoration can be performed in a single-stage procedure |
| Used to treat large, elongated defects with a single graft instead of using multiple dowels (e.g., “snowman technique”) |
| Restore defects with significant bone loss, potentially obviating the need for bone graft |
| No donor site morbidity |
| Disadvantages |
| Cost of fresh donor hemicondylar allograft |
| Limited availability of donor allografts can result in surgical delays |
BMI, body mass index; OCD, osteochondritis dissecans.
Table 2.
Technical Pearls/Pitfalls for BioUni Osteochondral Allograft Transplantation in the Setting of Uncontained OCD Lesions of the Medial Femoral Condyle
| Pearls | Pitfalls |
|---|---|
| In the setting of large bone defects, great care must be taken to envision the original geometry of the condyle along the intercondylar notch to select the most appropriate sizing template for BioUni instrumentation. | Ensure the sizing template is perpendicular to the condyle when inserting the guide pins for subsequent reaming. There is a potential tendency to deviate from this angle with missing bone along the intercondylar margin. Divergence of the template can lead to an inappropriate reaming angle and compromise the graft fit within the recipient defect site. |
| If the graft is proud or recessed within the donor trial, corresponding changes can be made with alternate drill stops when reaming the recipient site to ensure that the graft is placed flush to the surrounding articular surface. | |
| Pulse lavage the graft to remove antigenic marrow elements and soak the graft in BMAC while preparing the defect. This may aid in biologic healing and subsequent graft incorporation. | |
| Arthrex headless biocompression screws, countersunk to the graft surface, can be safely and effectively used to augment fixation for uncontained lesions. |
BMAC, bone marrow aspirate concentrate; OCD, osteochondritis dissecans.
Surgical Technique
Patient Positioning and Bone Marrow Aspirate Concentrate Harvest
The patient is positioned supine on the operating room table, and general anesthesia is administered following application of a regional nerve block. Following sterile preparation, the anterior superior iliac spine is palpated and a trocar is percutaneously inserted into the iliac crest at a location 3 to 4 cm proximal to this landmark. A total of 60 mL of bone marrow is aspirated and processed into bone marrow aspirate concentrate (BMAC) using the Arthrex Angel Centrifuge System (Arthrex).
Diagnostic Arthroscopy
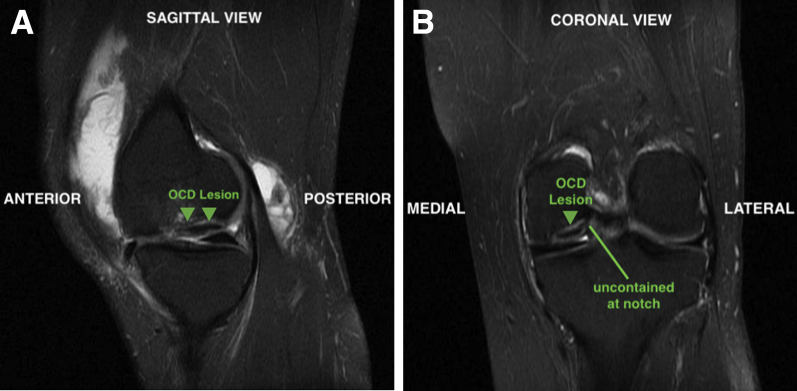
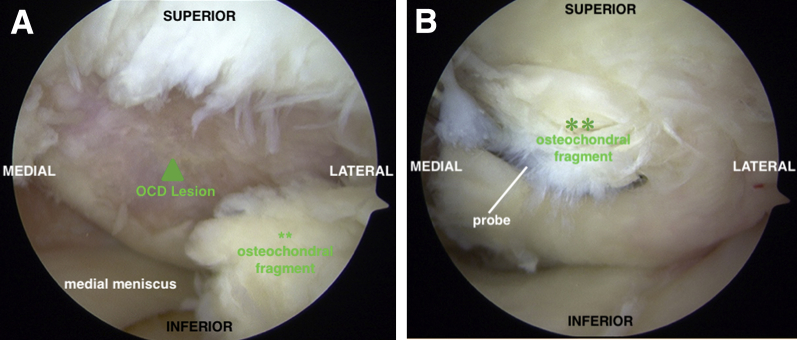
Preoperative magnetic resonance imaging revealed a large OCD lesion of the medial femoral condyle (MFC; Fig 1). A diagnostic arthroscopy using standard anterolateral and anteromedial portals is performed to confirm lesion characteristics and remove a loose body (Fig 2). Inspection of the medial compartment reveals a large, irregularly shaped 32 × 18-mm OCD lesion that is uncontained along the lateral margin of the MFC (Fig 3).
Fig 1.
Preoperative imaging demonstrates a large OCD lesion (arrows) of the left medial femoral condyle (arrows) in sagittal (A) and coronal (B) views. On the coronal view, the lesion is uncontained at the intercondylar notch. (OCD, osteochondritis dissecans.)
Fig 2.
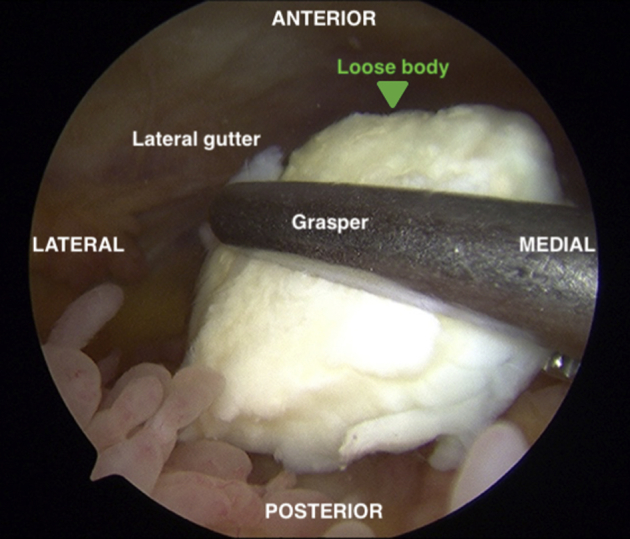
Diagnostic arthroscopy through an anterolateral viewing portal reveals a 10 × 10-mm osteochondral loose body in the lateral gutter of the left knee, which is removed through an accessory medial portal created under direct visualization.
Fig 3.
(A) Diagnostic arthroscopy of the left knee medial compartment through an anterolateral viewing portal facilitates complete evaluation of the OCD lesion characteristics. The lesion (arrow) measures approximately 32 × 18 mm, with significant bone involvement. (B) The overlying cartilage is degenerative. **Large unstable osteochondral fragment. (OCD, osteochondritis dissecans.)
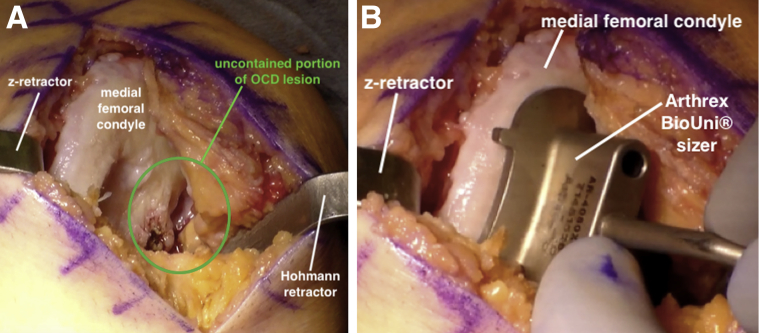
Surgical Approach and Graft Sizing
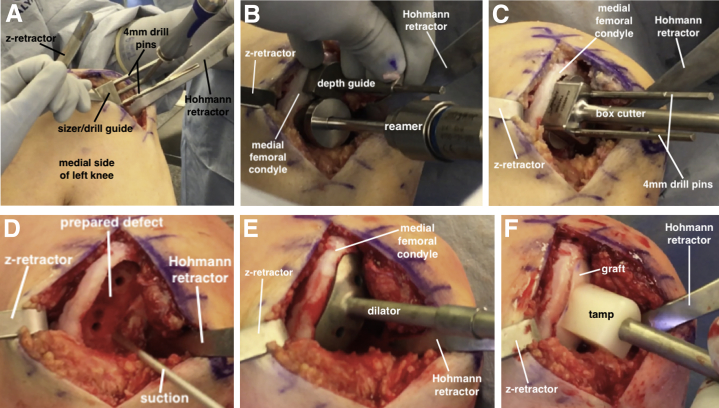
An 8-cm longitudinal incision is made over the anteromedial knee, incorporating the previously used anteromedial portal. A medial parapatellar arthrotomy is performed and the infrapatellar fat pad is partially excised. Using a large Hohmann and z-retractor, the MFC is exposed, revealing a large defect with significant bone loss along the intercondylar notch (Fig 4A). An Arthrex BioUni sizer that encompasses the entire lesion in the anteroposterior and mediolateral dimensions is selected (Fig 4B). It is critical to place the sizing template flush to the bone in a perpendicular fashion along the central axis of the MFC and envision where the normal surface contour along the intercondylar notch would be present. Appropriate determination of the sizing template is important because all subsequent instrumentation used for the defect and graft preparation is based on this template.
Fig 4.
(A) With the patient supine, the left medial femoral condyle is exposed using a medial parapatellar arthrotomy. The OCD lesion is uncontained along the intercondylar notch (circled). (B) An Arthrex BioUni sizer is selected that encompasses the entire lesion in the anteroposterior and mediolateral dimensions. (OCD, osteochondritis dissecans.)
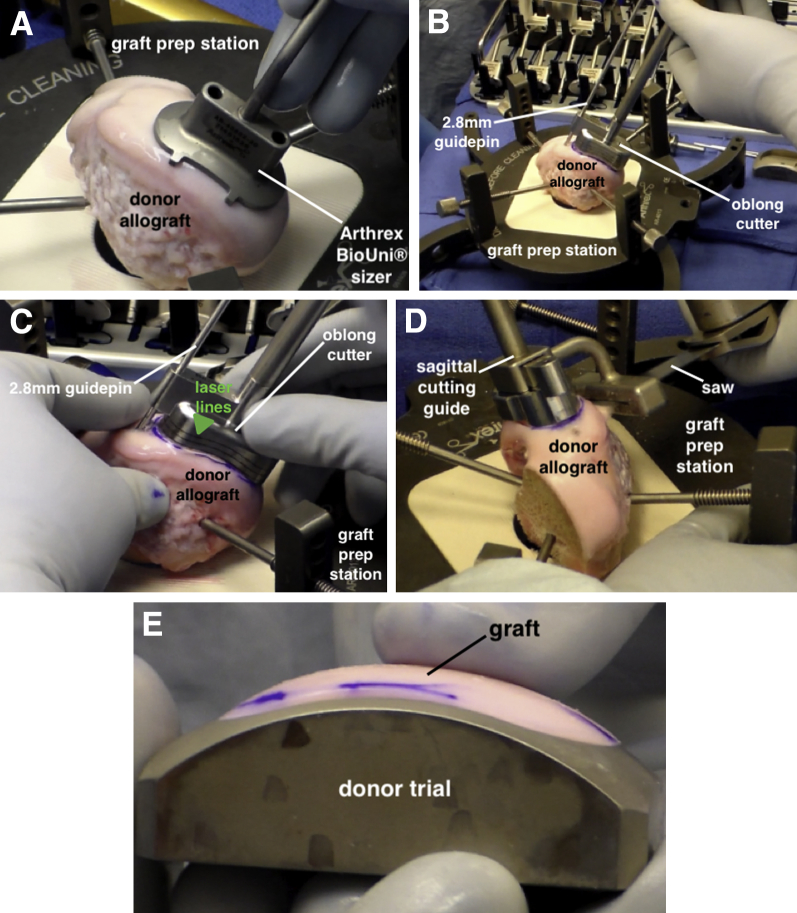
BioUni Donor Graft Preparation
A fresh MFC allograft is secured within the graft prep station, and the previously designated sizer is placed at an analogous position on the donor condyle (Fig 5A). The margins are outlined with a marker to ensure accurate alignment of the oblong cutter as it is secured to the graft. The cutter is held in place with a 2.8-mm guide pin (Fig 5B). An impactor handle is secured to the oblong cutter, and using a mallet, the cutter is advanced into the graft until the third laser line is flush to the surrounding cartilage (Fig 5C). It is important to periodically check that the cutter is uniformly advanced along the circumference of the graft, with subtle adjustments made as needed to avoid uneven insertion. Once fully impacted, the cutter is removed, and the corresponding sagittal cutting guide is placed within the graft (Fig 5D). A sagittal saw is used to cut the graft base and the allograft is removed. The graft is placed within the donor trial to evaluate the accuracy of the cuts. If the graft is proud or recessed within the trial, corresponding changes can be made when drilling the recipient site to ensure the graft is placed flush on final insertion. In this case, there were no areas of concern because the graft was flush along the entire circumference of the donor trial (Fig 5E). The graft is pulse lavaged to remove antigenic marrow elements and left to soak in the previously obtained BMAC while the recipient site is prepared.
Fig 5.
(A) The graft is secured within the graft prep station, and the selected sizer is placed at an analogous position on the donor condyle. (B) The oblong cutter is secured to the graft in the proper location with a 2.8-mm guide pin. (C) The oblong cutter is manually advanced into the graft. The cutter must be inserted into the graft evenly, with adjustments made as needed. The cutter is advanced until the third laser line (arrow) is flush with the cartilage surface. (D) The sagittal cutting guide is secured to the graft and a sagittal saw is used to cut the graft at its base. (E) The graft should be checked in the donor trial to assess the accuracy of the cuts. If the graft is proud or recessed, corresponding changes can be made when preparing the recipient site to ensure that the graft is placed flush within the defect.
Recipient Femoral Condyle Defect Preparation and Graft Insertion
The sizer/drill guide is placed flush to the MFC surface in a perpendicular fashion along the central axis of the condyle. There will be a tendency to allow the template to deviate from the perpendicular angle given the significant bone defect along the lateral margin of the condyle. One must avoid any angular divergence, because the two 4-mm drill pins are inserted to secure the guide (Fig 6A). Next, the scoring device is inserted over the drill pins and impacted 2- to 3-mm deep to the surface. The appropriately sized drill depth stops (proud, recessed, or flush) are selected based on the fit of the graft within the donor trial. The reamer is advanced over the superior and inferior guide pins until the depth stop is reached, thereby creating 2 large sockets (Fig 6B). The box cutter is placed over the guide pins and inserted flush to the base of the defect (Fig 6C). The remaining bone is removed with a curette, and the base of the defect (Fig 6D) is drilled with a k-wire. A dilator is impacted flush to the surface of the defect to ensure that the graft will be easily inserted (Fig 6E). The graft is removed from the BMAC and inserted with manual pressure. A tamp and mallet is then used to gently seat the graft flush to the surrounding articular surface (Fig 6F).
Fig 6.
(A) With the patient supine, the sizer/drill guide is oriented over the OCD lesion on the left medial femoral condyle, and two 4-mm drill pins are inserted through the sizer/drill guide. (B) The sizer/drill guide is removed, and the scoring device is inserted over the drill pins and impacted 2- to 3-mm deep to the cartilage surface. The appropriately sized drill depth guides are selected based on the fit of the graft within the donor trial (i.e., proud, recessed, flush). The reamer is advanced over each guide pin until a hard stop is reached, thereby creating 2 large sockets. (C) The box cutter is advanced over the guide pins and inserted flush to the base of the defect. (D) The remaining bone is removed with a curette to finalize defect preparation. (E) A dilator is trialed to ensure it sits flush within the defect. (F) The graft is manually inserted into the defect and then gently impacted with a tamp until it is flush to the surrounding articular surface. (OCD, osteochondritis dissecans.)
Graft Fixation
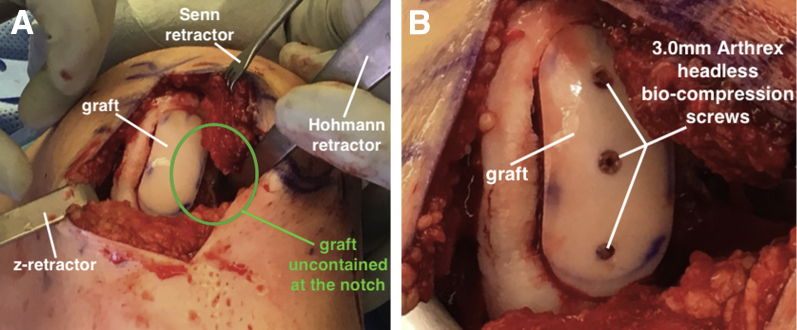
In this case, we noted that approximately 40% to 50% of the graft circumference was not contained at the intercondylar notch because of pre-existing bone loss (Fig 7A). Given concerns for graft fixation and stability with an accelerated rehabilitation protocol, 3 Arthrex 3.0-mm headless biocompression screws are used to augment fixation. The screws are inserted along the central axis of the graft and countersunk to the articular surface (Fig 7B). The tourniquet is deflated, and hemostasis is achieved. The wound is copiously irrigated and closed in a layered fashion.
Fig 7.
(A) The graft, now secured in the left medial femoral condyle, is uncontained at the intercondylar notch (circled). (B) Fixation is augmented with three 3.0-mm Arthrex headless biocompression screws.
Postoperative Rehabilitation
The patient is placed in a hinged knee brace and allowed to begin toe-touch weightbearing with crutches immediately after surgery. A continuous passive motion machine is used for a minimum of 4 hours per day for the first 4 weeks. Full weightbearing is typically allowed at 4 weeks postoperatively if the patient demonstrates satisfactory quadriceps strength and no joint effusion. Progressive exercises are used to strengthen the proximal lower extremity with a focus on the hips and core. The return to sport phase may begin at 16 to 18 weeks postoperatively, with an expected return to unrestricted activity at 8 to 10 months postoperatively.
Discussion
OCD is an idiopathic condition attributed to potential biologic and/or mechanical factors that result in focal osseous necrosis, collapse, and destabilization of the overlying articular cartilage.3 These debilitating lesions are most commonly observed in the knee, with a specific predisposition for the MFC.4 Fresh OCA transplantation has become a reliable treatment option for OCD lesions with favorable graft survivorship (93% at 10 years) and significant improvements in patient reported outcomes1, 2; however, surgical management can be challenging because defect fragmentation creates a heterogeneous articular surface with large amounts of bone loss. The use of a unicompartmental OCA provides an alternative to traditional techniques, especially for large, irregularly shaped defects. A recent study by Waterman et al.5 demonstrated higher graft failure rates (33.3%) when the “snowman” technique was used to treat elongated cartilage lesions relative to patients that underwent isolated, single graft transplants. With the BioUni instrumentation, a single elongated OCA graft can be used to treat these larger lesions, thereby addressing potential concerns with graft survivorship when multiple overlapping dowels are used to restore elongated defects. The BioUni graft also provides approximately 10 mm of bone restoration at its maximum depth, thereby facilitating treatment of OCD lesions with significant amounts of pathologic bone loss. Given the larger amount of bone associated with these grafts, we recommend the use of BMAC to aid in biologic healing and graft incorporation.6 Last, careful intraoperative evaluation of graft stability is of utmost importance in the setting of uncontained defects, and strong consideration for screw fixation should be made on an individualized basis.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: K.J.J. reports nonfinancial support from Aesculap and Arthrex, grants from the Musculoskeletal Transplant Foundation, personal fees from Vericel, and is a board or committee member for the American Orthopaedic Society for Sports Medicine, outside the submitted work. R.J.W. reports an unpaid consultancy for Aperion; intellectual property royalties and paid consultancy from Arthrex; research support, paid consultancy, and nonfinancial support from Histogenics, paid consultancy for JRF Ortho, royalties and financial and material support from Springer Publishing; and stock or stock options from Cymedica, Gramercy Extremity Orthopedics, Pristine Surgical, and RecoverX. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Demonstration of a unicompartmental fresh osteochondral allograft transplantation for the management of an elongated, uncontained OCD lesion of the left medial femoral condyle using Arthrex BioUni instrumentation and Arthrex headless biocompression screws for graft fixation. The patient is in the supine position for this procedure. (OCD, osteochondritis dissecans.)
References
- 1.Cotter E.J., Frank R.M., Wang K.C. Clinical outcomes of osteochondral allograft transplantation for secondary treatment of osteochondritis dissecans of the knee in skeletally mature patients. Arthroscopy. 2018;34:1105–1112. doi: 10.1016/j.arthro.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 2.Sadr K.N., Pulido P.A., McCauley J.C., Bugbee W.D. Osteochondral allograft transplantation in patients with osteochondritis dissecans of the knee. Am J Sports Med. 2016;44:2870–2875. doi: 10.1177/0363546516657526. [DOI] [PubMed] [Google Scholar]
- 3.Andriolo L., Crawfrod D.C., Reale D. Osteochondritis dissecans of the knee: Etiology and pathogenetic mechanisms. A systematic review. Cartilage. July 1, 2018 doi: 10.1177/1947603518786557. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hevesi M., Sanders T.L., Pareek A. Osteochondritis dissecans in the knee of skeletally immature patients: Rates of persistent pain, osteoarthritis, and arthroplasty at mean 14-years’ follow-up. Cartilage. July 1, 2018 doi: 10.1177/1947603518786545. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter E.J., Hannon C.P., Lansdown D.A., Waterman B.R., Frank R.M., Cole B.J. Clinical outcomes of multiple osteochondral allograft transplantation of the knee: An analysis of snowman technique and multifocal lesions. Am J Sports Med. 2018;46:2884–2893. doi: 10.1177/0363546518793405. [DOI] [PubMed] [Google Scholar]
- 6.Oladeji L.O., Stannard J.P., Cook C.R. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am J Sports Med. 2017;45:2797–2803. doi: 10.1177/0363546517715725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of a unicompartmental fresh osteochondral allograft transplantation for the management of an elongated, uncontained OCD lesion of the left medial femoral condyle using Arthrex BioUni instrumentation and Arthrex headless biocompression screws for graft fixation. The patient is in the supine position for this procedure. (OCD, osteochondritis dissecans.)