Abstract
Objective
This study aimed to examine the relationship between serum alanine aminotransferase (ALT) and growth hormone (GH) in children and adolescents with short stature.
Methods
In this retrospective cohort study, 670 Chinese children and adolescents with short stature were included, and 253 of them received recombinant human GH (rhGH) therapy. Anthropometric and biochemical indicators were measured. GH peak levels were assessed after provocation tests with L-dopa and insulin. The subjects were divided into 3 groups according to the GH peak level. The association between the GH peak and ALT was analyzed. The change of ALT during rhGH therapy was assessed by a generalized additive mixed model.
Results
Serum ALT and incidence of ALT elevation were both decreased across the GH tertiles (P = 0.002, 0.012, respectively). A univariate analysis showed that the GH peak was negatively associated with ALT (β: -0.12; 95%CI: -0.22, -0.02; P = 0.023). Furthermore, multiple linear stepwise regression analysis demonstrated that the GH peak was independently related to ALT after adjusting for other confounding variables (β: -0.12; 95%CI: -0.24, -0.00; P = 0.042). Besides, mean values of the change in ALT from baseline displayed that, during the early stages of rhGH treatment, serum ALT level indicated a temporary upward trend, but it subsequently gradually decreased (β: -0.16; 95%CI: -0.23, -0.09; P < 0.001).
Conclusions
GH secretion level was strongly negatively correlated with ALT in short children and adolescents. And rhGH therapy could reduce ALT level over time.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a large spectrum of pathological changes that includes simple mild fatty liver to nonalcoholic steatohepatitis (NASH), fibrosis, and ultimately cirrhosis in the absence of excessive alcohol consumption, which is a common, serious disease that affects the health of adults, children, and adolescents [1, 2]. Moreover, many clinical and epidemiological studies have revealed that NAFLD is associated not only with liver-related morbidity and mortality but also with an increased risk of developing cardiometabolic diseases [3].
Various diagnostic tools, including ultrasound, computed tomography (CT), and magnetic resonance (MR), can be used to evaluate NAFLD but may not be suitable for large-scale epidemiologic studies due to time consumption and cost [4, 5]. As a common and useful indicator of hepatocyte injury, elevated serum alanine aminotransferase (ALT) level is a valuable way to screen for NAFLD that reflects liver histological progression [6–9]. Moreover, research has shown that serum ALT level is independently correlated with hepatic triglyceride content and might be more appropriate for use as a predictor of the degree of NAFLD than aspartate aminotransferase and gamma-glutamyl transferase [10].
The pathogenesis of NAFLD has not yet been clearly elaborated. Growth hormone (GH) is an anterior pituitary hormone that is a key regulator of fat metabolism, and the liver is a major target of GH. Experimental research has indicated that the liver GH receptor or dysfunction of its downstream signaling pathways could elevate intracellular lipid accumulation and promote the development of NAFLD [11–13]. Additionally, clinical data have shown that GH deficiency (GHD) might be closely associated with the occurrence and progression of NAFLD [14–17].
Short stature is a condition characterized by a height more than 2 standard deviations (SD) below the corresponding mean height for a given age, sex, and population group and can significantly affect the health-related quality of life of children, adolescents, and even adults [18]. One common etiology for short stature is involved in GHD [19]. Previous studies have demonstrated that GHD is induced by various causes, such as tumors, and plays an important role in the development of NAFLD in pediatric patients [14, 15]. However, there are few studies of the relationship between GH and liver enzyme in short children and adolescents, especially when caused by GHD.
The secretory pattern of GH is pulsatile, and a single fasting GH measurement might not accurately reflect GH level. A peak-stimulated GH after stimulation testing may be more suitable to assess the body's GH secretion status. In addition, recombinant human growth hormone (rhGH) is widely used for the treatment of short stature due to GH insufficiency and other growth disorders [20]. Consequently, the present study was carried out retrospectively with the purpose of analyzing the association between serum ALT and peak-stimulated GH levels, and observing the change of ALT during rhGH therapy in Chinese children and adolescents with short stature.
2. Subjects and Methods
2.1. Subjects
This retrospective cohort study was performed by reviewing the medical records of patients with short stature from the Department of Endocrinology, Affiliated Hospital of Jining Medical University between March 2013 and July 2018. The inclusion criteria were (1) short stature, a condition characterized by a height more than 2SD below the corresponding mean height for a given age, sex, and population group; (2) normal weight and height at birth. The exclusion criteria were (1) subjects who had a history of liver disease, including viral hepatitis or genetic and autoimmune liver diseases; (2) subjects who suffered from chromosomal abnormalities, skeletal dysplasia, genetic metabolic diseases, thyroid dysfunction, and a history of use of medication that could affect GH secretion or function [21]. In all, six hundred and seventy children and adolescents with short stature and 10.2 ± 3.5 years of age were enrolled in this study. And among them, two hundred and fifty-three subjects received rhGH and were followed up.
The study was approved by the Human Ethics Committee of the Affiliated Hospital of Jining Medical University. All procedures were performed in accordance with ethical standards laid out in the Declaration of Helsinki. Written parental consent was obtained for all participants.
2.2. Anthropomorphic Measurements
The height of each participant was measured to the nearest 0.1 cm using the same height-measuring instrument (Nantong Best Industrial Co. Ltd., Jiangsu, China) and participants were measured without head coverings. Weight was measured using the same electronic scale (Xiangshan Weighing Apparatus Co. Ltd., Guangdong, China) in the fasting state and was accurate within ± 0.1 kg. Body mass index (BMI) was calculated with the following formula: weight (kg)/height (m2). Bone age (BA) was measured using an X-ray taken of the left hand, including the hand bone, wrist, and radial ulnar stem (3-4 cm). The image was scanned, and the BA was evaluated according to the Greulich-Pyle method by the same specially assigned investigator [22].
2.3. Laboratory Measurements
To assess GH secretion, provocation tests with L-dopa and insulin were performed. L-dopa (Levodopa Tablets®, He Feng, Guang Xi, China; dosing: body weight above 30 kg, 500 mg; below 30 kg, 250 mg) was administered orally, and insulin (Insulin Injection®, Wan Bang, Jiang Su, China, 0.1U/kg) was subcutaneously injected in the overnight fasting state. Blood samples were collected at 0, 30, 60, 90, and 120 min, respectively. GH concentration of each time point was measured using a chemiluminescence method (ACCESS2, Beckman Coulter; USA) with a sensitivity of 0.010 μg/L. Serum insulin-like growth factor-1 (IGF-1) was measured by the chemiluminescence immunometric method (DPC IMMULITE 1000 analyzer, SIEMENS, Germany). Lipid profiles, including total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol (HDL-c), and low density lipoprotein-cholesterol (LDL-c); fasting plasma glucose (FPG) and kidney function, including Cr and uric acid (UA) were all tested using a biochemical autoanalyzer (Cobas c 702, Roche; Shanghai, China). The serum ALT level was measured with an ALT kit (IFCC enzyme colorimetric method) (Roche Diagnostics, Mannheim Germany), and the mean and variabilities of with-run precision for ALT measurements were 47.5 U/L and 2.4%. The mean and variabilities of the intermediate precision were 39.9 U/L and 1.4%.
2.4. Statistical Analysis
Statistical analysis was performed using R statistical software (https://www.r-project.org) and EmpowerStats (http://www.empowerstats.com, X& Y solutions, Inc. Boston MA). Continuous variables were presented as the means ± SD, and categorical variables were presented as a percentage (%). Analysis of variance test was used for comparisons of continuous variables, and chi-square test for categorical variables. Univariate and multiple linear stepwise regression analyses were used to assess the association between ALT and GH peak levels. Generalized additive mixed model was used to analyze the change in ALT from baseline during rhGH therapy over time. P values (2-tailed) less than 0.05 were regarded as significant differences.
3. Results
3.1. Clinical and Biochemical Characteristics
As shown in Table 1, this study enrolled 670 participants with short stature. In accordance with the literature, the normal upper limit values for serum ALT were 25.8 U/L for males and 22.1 U/L for females [6, 7]. 9.0% of the subjects had ALT elevation in the present study. In addition, the GH peak value was defined as the highest level of GH at any time point in any excitation test. The participants were divided into three groups according to the GH peak levels (Table 2). The tertiles of GH peak were defined as follows: GH < 5 ng/mL; 5 ng/mL ≤ GH < 10 ng/mL; GH ≥ 10 ng/mL. The results displayed that the BMI, TG, LDL-c, ALT and incidence of ALT elevation decreased across the tertiles (all P < 0.05). However, there were no obvious differences in age, SBP, DBP, TC, HDL-c, FPG, Cr and IGF-1 in the GH tertiles (all P > 0.05).
Table 1.
Basic characteristics of the subjects.
| Variables | Total (n = 670) |
|---|---|
| Sex (Male, n, %) | 472 (70.5) |
| Age (years) | 10.2 ± 3.5 |
| Height (cm) | 125.6 ± 18.0 |
| HT SDS | -2.67 ± 0.57 |
| BMI (kg/m2) | 16.85 ± 2.81 |
| SBP (mmHg) | 104.4 ± 11.8 |
| DBP (mmHg) | 62.7 ± 8.9 |
| TC (mmol/L) | 3.83 ± 0.72 |
| TG (mmol/L) | 0.72 ± 0.33 |
| HDL-c (mmol/L) | 1.62 ± 6.18 |
| LDL-c (mmol/L) | 2.09 ± 1.14 |
| FPG (mmol/L) | 4.76 ± 0.69 |
| Cr (μmol/L) | 39.33 ± 14.74 |
| UA (μmol/L) | 262.64 ± 80.23 |
| GH peak (ng/mL) | 8.80 ± 6.17 |
| IGF-1(ng/ml) | 186.97 ± 147.99 |
| ALT (U/L) | 16.09 ± 8.20 |
| Increased ALT (n, %) | 60 (9.0) |
HT SDS: the standard deviation score of height; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglycerides; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; FPG: fasting plasma glucose; UA: uric acid; GH: growth hormone; IGF-1: insulin-like growth factor-1; ALT: alanine aminotransferase.
Table 2.
Comparison of anthropometric and biochemical characteristics.
| Variables | GH peak | P value | ||
|---|---|---|---|---|
| <5 (n = 182) | 5-10 (n = 271) | ≥10 (n = 217) | ||
| Age (years) | 10.3 ± 3.2 | 10.1 ± 3.4 | 10.3 ± 3.9 | 0.634 |
| BMI (kg/m2) | 17.97 ± 3.74 | 16.56 ± 2.15 | 16.28 ± 2.34 | <0.001 |
| SBP (mmHg) | 105.0 ± 12.1 | 103.6 ± 11.5 | 104.9 ± 12.0 | 0.370 |
| DBP (mmHg) | 63.3 ± 8.4 | 62.24 ± 8.3 | 62.7 ± 10.0 | 0.489 |
| TC (mmol/L) | 3.93 ± 0.76 | 3.82 ± 0.72 | 3.76 ± 0.69 | 0.080 |
| TG (mmol/L) | 0.80 ± 0.43 | 0.70 ± 0.29 | 0.67 ± 0.25 | <0.001 |
| HDL-c (mmol/L) | 1.43 ± 0.44 | 1.97 ± 9.71 | 1.35 ± 0.27 | 0.507 |
| LDL-c (mmol/L) | 2.28 ± 1.99 | 2.02 ± 0.59 | 2.02 ± 0.52 | 0.041 |
| FPG (mmol/L) | 4.71 ± 0.70 | 4.82 ± 0.76 | 4.73 ± 0.58 | 0.202 |
| Cr (μmol/L) | 38.66 ± 7.69 | 40.27 ± 20.37 | 38.73 ± 10.10 | 0.403 |
| UA (μmol/L) | 271.41 ± 71.64 | 247.70 ± 75.90 | 272.49 ± 89.61 | 0.003 |
| IGF-1 (ng/ml) | 182.56 ± 192.87 | 177.24 ± 108.56 | 202.73 ± 148.13 | 0.192 |
| ALT (U/L) | 17.85 ± 9.58 | 15.18 ± 7.83 | 15.76 ± 7.13 | 0.002 |
| Increased ALT (n, %) | 26 (14.29) | 20 (7.38) | 14 (6.45) | 0.012 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglycerides; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; FPG: fasting plasma glucose; UA: uric acid; GH: growth hormone; IGF-1: insulin-like growth factor-1; ALT: alanine aminotransferase.
3.2. Correlation Analysis between ALT and GH Peak
Table 3 shows the correlation between the ALT and GH peak and other variables according to univariate analysis. ALT was related positively to age, BMI, SBP, DBP, TG, UA, and IGF-1 (all P < 0.05) and negatively to GH peak level (P = 0.023). However, there was no significant association between ALT and TC, HDL-c, LDL-c, FPG, and Cr (all P > 0.05).
Table 3.
Correlation between the ALT and GH peak level by a univariate analysis.
| Variables | β (95%CI) | P value |
|---|---|---|
| Age | 0.36 (0.19, 0.54) | <0.001 |
| BMI | 0.59 (0.38, 0.81) | <0.001 |
| SBP | 0.12 (0.07, 0.17) | <0.001 |
| DBP | 0.12 (0.05, 0.19) | <0.001 |
| TC | 0.26 (-0.63, 1.15) | 0.573 |
| TG | 2.03 (0.09, 3.97) | 0.041 |
| HDL-c | -0.04 (-0.14, 0.07) | 0.466 |
| LDL-c | 0.07 (-0.49, 0.63) | 0.804 |
| FPG | 0.36 (0.51, 1.23) | 0.414 |
| Cr | 0.02 (-0.02, 0.06) | 0.416 |
| UA | 0.01 (0.00, 0.02) | 0.008 |
| GH peak | -0.12 (-0.22, -0.02) | 0.023 |
| IGF-1 | 0.00 (0.00, 0.01) | 0.024 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; FPG: fasting plasma glucose; UA: uric acid; GH: growth hormone; IGF-1: insulin-like growth factor-1; ALT: alanine aminotransferase.
Furthermore, the age, BMI, SBP, DBP, UA, TG, and IGF-1 were set as confounding variables based on the results of univariate correlation analysis. A multiple linear stepwise regression analysis was conducted in all subjects. The results showed that, after adjusting for the above confounding factors, the GH peak was independently related to the ALT (β: -0.12; 95%CI: -0.24, -0.00; P = 0.042).
3.3. Generalized Additive Mixed Model
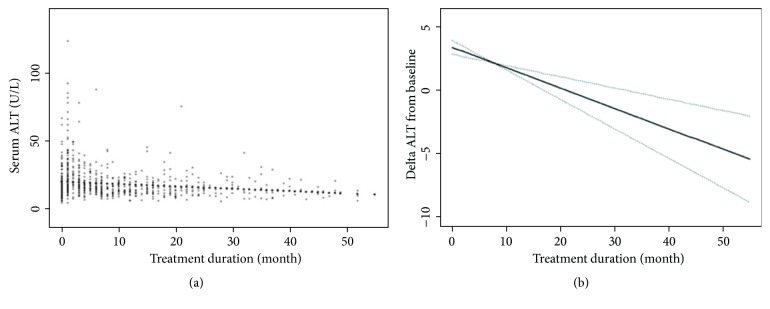
As shown in Figure 1, change of serum ALT during rhGH treatment over time was described by a generalized additive mixed model adjusted by age and gender. Figure 1(a) depicted the distribution of serum ALT at different follow-up times. Meanwhile, mean values of the change in ALT from baseline displayed that, during the early stages of rhGH treatment, serum ALT level indicated a temporary upward trend, but it gradually decreased subsequently (β: -0.16; 95%CI: -0.23, -0.09; P < 0.001) (Figure 1(b)).
Figure 1.
Change of serum ALT during rhGH treatment over time by a generalized additive mixed model adjusted for age and gender (n = 253). (a) Description of serum ALT. (b) Change in ALT from baseline. ALT: alanine aminotransferase; rhGH: recombinant human growth hormone.
4. Discussion
This retrospective study investigated the association between the ALT and GH in short children and adolescents. The results showed that the GH peak level was independently and negatively related to serum ALT. Moreover, mean values of the change in ALT from baseline displayed that although serum ALT level indicated a temporary upward trend during the early stages of rhGH treatment, it gradually decreased over time.
There are various methods for screening and diagnosis of NAFLD. Among them, imaging examinations, including ultrasound, CT, and MR, play important roles. Recently, Esterson et al. [5] reviewed and discussed the sensitivity, specificity, advantages and limitations of the above examinations, which may not be suitable for large-scale epidemiological studies due to their characteristics, including required time and cost. Fortunately, serum ALT is cheaper and more accessible, and in numerous national guidelines, the use of ALT is recommended to screen for NAFLD in children [23, 24]. According to the literature for the definition of ALT elevation [6, 7, 25, 26], the proportion of ALT elevation was relatively high in this study. However, it should be stressed that the data from liver biopsy specimens in children with NAFLD showed that significant histological abnormalities can occur with normal or mildly elevated ALT levels [7]. Thus, the appropriate ALT cutoff values must be further explored and defined.
NAFLD is a multifactorial disease involved in various pathophysiological factors. Numerous studies have shown that dysfunction of the hypothalamic-pituitary axes might be an important etiology for NALFD [27]. GH is mainly synthesized and secreted in the anterior pituitary gland, which can regulate not only the body's growth but also its metabolism and composition [28, 29]. Many studies have shown that low levels of GH are causally related to hepatic lipid accumulation and promote the occurrence and progression of NAFLD. However, these studies mainly focused on the effects of GH in the adult liver [30]. Notably, some studies have demonstrated the roles of GH in pediatric patients. Gilliland et al. [15] reported a pediatric case of NASH secondary to panhypopituitarism from craniopharyngioma and found that GH replacement therapy led to rapid and complete resolution of hepatic steatosis. Similarly, Fujio et al. [14] showed that GH therapy could reduce transaminase levels in a pediatric patient with NAFLD associated with hypopituitarism. Therefore, the data suggest that NAFLD should be identified and screened in pediatric patients with GHD.
In our study, we found that serum ALT and incidence of ALT elevation significantly increased in short children and adolescents with the low GH state, and univariate analysis showed a negative correlation between ALT and GH levels. It is well known that obesity is a major risk factor for NAFLD [31]. BMI is useful indicator for evaluating obesity and nutritional status [32], and there is a strong relationship between BMI and prospectively recorded diagnoses of NAFLD [33]. We found that there was a close relationship between ALT and BMI. However, GH peak level was still independently related to ALT after adjusting for BMI. In addition, GH stimulates IGF-1 generation mainly in the liver [34], and as an important member of the GH/IGF-1 axis, there was a negative correlation between IGF-1 and NAFLD [35]. Low serum IGF-1 level was associated with increased histologic severity [36]. Inconsistent with previous studies, univariate analysis showed that IGF-1 was positively related to ALT, and GH fit well in our regression model after adjusting for IGF-1. The above data suggest that GH level might be more correlated with ALT than other variables. Moreover, some studies showed that the decrease in ALT might be a valid monitoring biomarker of histologic improvement of NAFLD [37, 38]. In this study, the results displayed that the ALT level was gradually decreased during rhGH therapy, which suggest that GH probably has potential values in prevention and treatment of NAFLD in short children and adolescents with the low GH state.
There are several limitations to our current study. Serum ALT was used as an independent variable to screen the suspected NAFLD, but imaging examinations were still needed to diagnose NAFLD. In fact, abdominal ultrasound was used to diagnose NAFLD in our study, but the results showed that NAFLD was not discovered. As ultrasound is not ideal for detecting the early stages of NAFLD [5], a more accurate method should be used to assess histologic severity in the liver in the future.
In conclusion, GH secretion level was strongly correlated with ALT in short children and adolescents, and rhGH therapy could reduce ALT level over time. This suggests that it will be important to screen for NAFLD and use rhGH treatment in short stature, especially when caused by GHD.
Acknowledgments
This research was supported by grants from the Natural Science Foundation of Shandong Province (no. ZR2014HP058), the Health and Family Planning Commission of Shandong Province (no. 2014-032), the Jining Science and Technology Bureau (no. 2017SMNS007), and the PhD Research Foundation of the Affiliated Hospital of Jining Medical University (no. 2017-BS-12).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Baolan Ji and Mei Zhang are co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Bo Ban conceived and designed the study. Baolan Ji and Mei Zhang participated in the design and drafted the manuscript. Qianqian Zhao and Yuntian Chu performed the statistical analysis. Yanying Li and Hui Pan revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Kleiner D. E., Makhlouf H. R. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children. Clinics in Liver Disease. 2016;20(2):293–312. doi: 10.1016/j.cld.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temple J. L., Cordero P., Li J., Nguyen V., Oben J. A. A guide to non-alcoholic fatty liver disease in childhood and adolescence. International Journal of Molecular Sciences. 2016;17(6, article no. 947) doi: 10.3390/ijms17060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstee Q. M., Targher G., Day C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature Reviews Gastroenterology & Hepatology. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 4.Di Martino M., Koryukova K., Bezzi M., Catalano C. Imaging Features of Non-Alcoholic Fatty Liver Disease in Children and Adolescents. Children. 2017;4(8):p. 73. doi: 10.3390/children4080073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esterson Y. B., Grimaldi G. M. Radiologic imaging in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clinics in Liver Disease. 2018;22(1):93–108. doi: 10.1016/j.cld.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Fraser A., Longnecker M. P., Lawlor D. A. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133(6):1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molleston J. P., Schwimmer J. B., Yates K. P., et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. Journal of Pediatrics. 2014;164(4, article e703):707–713. doi: 10.1016/j.jpeds.2013.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballestri S., Nascimbeni F., Romagnoli D., Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features: insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatology Research. 2016;46(11):1074–1087. doi: 10.1111/hepr.12656. [DOI] [PubMed] [Google Scholar]
- 9.Seko Y., Sumida Y., Tanaka S., et al. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatology Research. 2015;45(10):E53–E61. doi: 10.1111/hepr.12456. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z., Han C.-K., Pan L.-L., et al. Serum alanine aminotransferase independently correlates with intrahepatic triglyceride contents in obese subjects. Digestive Diseases and Sciences. 2014;59(10):2470–2476. doi: 10.1007/s10620-014-3214-3. [DOI] [PubMed] [Google Scholar]
- 11.Barclay J. L., Nelson C. N., Ishikawa M., et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152(1):181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]
- 12.Cordoba-Chacon J., Majumdar N., List E. O., et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015;64(9):3093–3103. doi: 10.2337/db15-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y., Fang X., Tajima A., et al. Evolution of hepatic steatosis to fibrosis and adenoma formation in liver-specific growth hormone receptor knockout mice. Frontiers in Endocrinology. 2014;5, article 218 doi: 10.3389/fendo.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujio A., Kawagishi N., Echizenya T., et al. Long-term survival with growth hormone replacement after liver transplantation of pediatric nonalcoholic steatohepatitis complicating acquired hypopituitarism. The Tohoku Journal of Experimental Medicine. 2015;235(1):61–67. doi: 10.1620/tjem.235.61. [DOI] [PubMed] [Google Scholar]
- 15.Gilliland T., Dufour S., Shulman G. I., Petersen K. F., Emre S. H. Resolution of non-alcoholic steatohepatitis after growth hormone replacement in a pediatric liver transplant patient with panhypopituitarism. Pediatric Transplantation. 2016;20(8):1157–1163. doi: 10.1111/petr.12819. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto R., Fukuoka H., Iguchi G., et al. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Hormone & IGF Research. 2014;24(5):174–179. doi: 10.1016/j.ghir.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y., Iida K., Takahashi K., et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132(3):938–943. doi: 10.1053/j.gastro.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Quitmann J. H., Bullinger M., Sommer R., Rohenkohl A. C., Silva N. M. B. D. Associations between psychological problems and quality of life in pediatric short stature from patients' and parents' perspectives. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0153953.e0153953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogol A. D., Hayden G. F. Etiologies and early diagnosis of short stature and growth failure in children and adolescents. Journal of Pediatrics. 2014;164(5, article e16):S1–S14. doi: 10.1016/j.jpeds.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Franklin S. L., Geffner M. E. Growth hormone: the expansion of available products and indications. Pediatric Clinics of North America. 2011;58(5):1142–1165. doi: 10.1016/j.pcl.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Cohen P., Rogol A. D., Deal C. L., et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. The Journal of Clinical Endocrinology & Metabolism. 2008;93(11):4210–4217. doi: 10.1210/jc.2008-0509. [DOI] [PubMed] [Google Scholar]
- 22.Satoh M. Bone age: Assessment methods and clinical applications. Clinical Pediatric Endocrinology. 2015;24(4):143–152. doi: 10.1297/cpe.24.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.August G. P., Caprio S., Fennoy I., et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. The Journal of Clinical Endocrinology & Metabolism. 2008;93(12):4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlow S. E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 25.Malespin M., Sleesman B., Lau A., Wong S. S., Cotler S. J. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese American children. Journal of Clinical Gastroenterology. 2014 doi: 10.1097/MCG.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 26.Song P., Yu J., Wang M., Chang X., Wang J., An L. Prevalence and correlates of suspected nonalcoholic fatty liver disease in chinese children. International Journal of Environmental Research and Public Health. 2017;14(5) doi: 10.3390/ijerph14050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann A., Muller H. L. Novel perspectives on hypothalamic-pituitary dysfunction as a risk factor for non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42(2):132–144. doi: 10.23736/S0391-1977.16.02500-1. [DOI] [PubMed] [Google Scholar]
- 28.Audí L., Fernández-Cancio M., Camats N., Carrascosa A. Growth hormone deficiency: an update. Minerva Endocrinologica. 2013;38(1):1–16. [PubMed] [Google Scholar]
- 29.Vijayakumar A., Yakar S., LeRoith D. The intricate role of growth hormone in metabolism. Frontiers in Endocrinology. 2011;2, article 32 doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi Y. The role of growth hormone and insulin-like growth factor-I in the liver. International Journal of Molecular Sciences. 2017;18(7) doi: 10.3390/ijms18071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo Baidal J. A., Lavine J. E. The intersection of nonalcoholic fatty liver disease and obesity. Science Translational Medicine. 2016;8(323):323rv1–323rv1. doi: 10.1126/scitranslmed.aad8390. [DOI] [PubMed] [Google Scholar]
- 32.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents and adults. The Lancet. 2017;390(10113):2627–2642. doi: 10.1530/ey.15.13.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loomis A. K., Kabadi S., Preiss D., et al. Body mass index and risk of nonalcoholic fatty liver disease: two electronic health record prospective studies. The Journal of Clinical Endocrinology & Metabolism. 2016;101(3):945–952. doi: 10.1210/jc.2015-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkes C. P., Grimberg A. Insulin-like growth factor-I is a marker for the nutritional state. Pediatr Endocrinol Rev. 2015;13(2):499–511. [PMC free article] [PubMed] [Google Scholar]
- 35.Liang S., Cheng X., Hu Y., Song R., Li G. Insulin-like growth factor 1 and metabolic parameters are associated with nonalcoholic fatty liver disease in obese children and adolescents. Acta Paediatrica. 2017;106(2):298–303. doi: 10.1111/apa.13685. [DOI] [PubMed] [Google Scholar]
- 36.Dichtel L. E., Corey K. E., Misdraji J., et al. The association between IGF-1 levels and the histologic severity of nonalcoholic fatty liver disease. Clinical and Translational Gastroenterology. 2017;8(1, article e217) doi: 10.1038/ctg.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arsik I., Frediani J., Frezza D., et al. Alanine aminotransferase as a monitoring biomarker in children with nonalcoholic fatty liver disease: a secondary analysis using TONIC trial data. Children. 2018;5(6) doi: 10.3390/children5060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vuppalanchi R., Jain A. K., Deppe R., et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2014;12(12):2121–2130. doi: 10.1016/j.cgh.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.