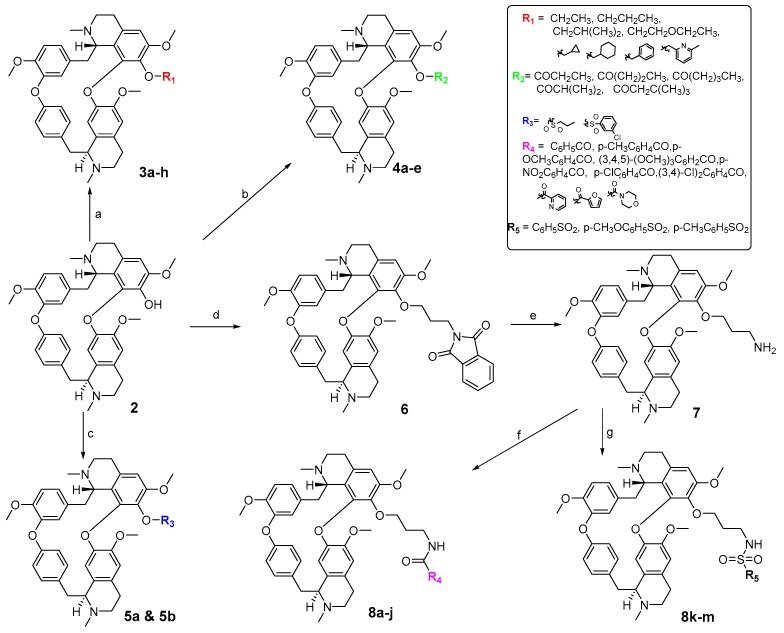
Scheme 1.
Reagents and conditions: (a) R1X, NaH, DMF, 0 °C–r.t., 29–30%; (b) R2COCl, TEA, CH2Cl2, 0 °C–r.t., 35–52%; (c) R3SO2Cl, TEA, CH2Cl2, 0 °C–r.t., 33–50%; (d) N-(3-bromopropyl)phthalimide, NaH, DMF, 0 °C–r.t., 50%; (e) H2NNH2·H2O, EtOH, overnight, 90%; (f) R4COCl, TEA, CH2Cl2, 0 °C–r.t., 22–71%; (g) R5SO2Cl, TEA, CH2Cl2, 0 °C–r.t., 21–52%.