Abstract
For flawless translation of mRNA sequence into protein, tRNAs must undergo a series of essential maturation steps to be properly recognized and aminoacylated by aminoacyl-tRNA synthetase, and subsequently utilized by the ribosome. While all tRNAs carry a 3′-terminal CCA sequence that includes the site of aminoacylation, the additional 5′-G-1 position is a unique feature of most histidine tRNA species, serving as an identity element for the corresponding synthetase. In eukaryotes including yeast, both 3′-CCA and 5′-G-1 are added post-transcriptionally by tRNA nucleotidyltransferase and tRNAHis guanylyltransferase, respectively. Hence, it is possible that these two cytosolic enzymes compete for the same tRNA. Here, we investigate substrate preferences associated with CCA and G-1-addition to yeast cytosolic tRNAHis, which might result in a temporal order to these important processing events. We show that tRNA nucleotidyltransferase accepts tRNAHis transcripts independent of the presence of G-1; however, tRNAHis guanylyltransferase clearly prefers a substrate carrying a CCA terminus. Although many tRNA maturation steps can occur in a rather random order, our data demonstrate a likely pathway where CCA-addition precedes G-1 incorporation in S. cerevisiae. Evidently, the 3′-CCA triplet and a discriminator position A73 act as positive elements for G-1 incorporation, ensuring the fidelity of G-1 addition.
Keywords: CCA-addition, G-1 residue, tRNAHis guanylyltransferase, tRNAHis, tRNA maturation, tRNA nucleotidyltransferase
1. Introduction
Transfer RNAs (tRNAs) are essential key players in life. They function as adapter molecules, establishing an interface between the encoded genetic information in mRNA and the amino acid sequence at the protein level. To accomplish this critical biological function, tRNA transcripts undergo a large number of processing events in order to yield the mature tRNA species that are utilized by the translation machinery. Apart from the removal of 5′-leader, 3′-trailer and intron sequences (if present), the introduction of modifications at certain nucleotides and nucleotide editing are key maturation events [1]. Furthermore, all tRNAs carry the conserved CCA triplet at their 3′-end, generating the site of aminoacylation. While in some Bacteria and Archaea the CCA sequence is encoded in the tRNA genes, in all remaining organisms, especially in Eukarya, it is added post-transcriptionally by tRNA nucleotidyltransferase (CCA-adding enzyme) [2,3,4]. Nevertheless, CCA-adding enzymes occur in each domain of life, and according to their variable structural features and different mechanisms, they are divided into class I (Archaea) and class II (Bacteria and Eukarya) [5]. In class I CCA-adding enzymes, a highly conserved arginine residue in the active site and the sugar-phosphate-backbone of the tRNA acceptor stem collaborate in nucleotide selection and form hydrogen bonds with the incoming NTP [6], whereas in class II a pure amino acid based template exists. Here, the nucleotide binding pocket contains three highly conserved amino acids, forming Watson Crick-like hydrogen bonds to either CTP or ATP, without interacting with the tRNA [7]. Despite these differences, both types of CCA-adding enzymes incorporate the nucleotides at high fidelity to the tRNA 3′-end. Furthermore, they also play an important role in the quality control of tRNA transcripts [8,9,10,11].
Whereas the 3′-CCA-end is an indispensable feature for every mature tRNA, the addition of a single guanosine nucleotide at the tRNA 5′-end is a special maturation event occurring on tRNAHis. The presence of this single G at the -1 position is critical for recognition of tRNAHis by the histidyl tRNA synthetase (HisRS) in nearly all bacteria, as well as in a large number of eukaryotes, with only a few notable exceptions described so far in each domain [12,13]. While these bacterial and eukaryotic tRNAHis species share the same G-1 element, the mechanisms of incorporation vary between the two domains. In Bacteria, the genomically encoded G-1 is retained after 5′-end cleavage of the tRNAHis precursor transcript due to an alternative cleavage pattern in the 5′-leader sequence exhibited by Ribonuclease P (RNase P) [14]. In Eukarya, however, G-1 is not encoded and must be added post-transcriptionally by tRNAHis guanylyltransferase (Thg1) [15,16]. Thg1 adds a single G-1 residue across from an A73 discriminator nucleotide, forming an unconventional non-Watson Crick base pair. This unusual 3′-5′ nucleotidyltransferase protein is a member of a larger family of Watson Crick-dependent 3′-5′ polymerase enzymes that share structural similarity with canonical 5′-3′ DNA and RNA polymerases [16].
The fact that tRNAHis is subject to post-transcriptional addition of 5′- as well as 3′-terminal features by highly conserved polymerases that act on either end of a partially matured precursor raises important questions about coordination between these enzymatic activities. Indeed, prior to the activities of Thg1 and CCA-adding enzyme, the precursor tRNAHis species, like all other pre-tRNA, is transcribed in the nucleus with additional 5′-leader and 3′-trailer sequences that are removed by the nuclear enzymes RNase P and ribonuclease Z (RNase Z), respectively [1]. This 5′- and 3′-end processed tRNA is subsequently exported to the cytoplasm for additional maturation events, including the activities of Thg1 and CCA-adding enzyme, which are both localized to the cytoplasm in S. cerevisiae. Although both enzymes share the tRNA acceptor stem as a substrate, there is no evidence for interaction between Thg1 and the CCA-adding enzyme [17]. Therefore, the possibility of competition between these 5′- and 3′-end maturation activities for the same end-matured tRNAHis substrate is raised, and whether there is any apparent order associated with these enzymatic events has not been investigated.
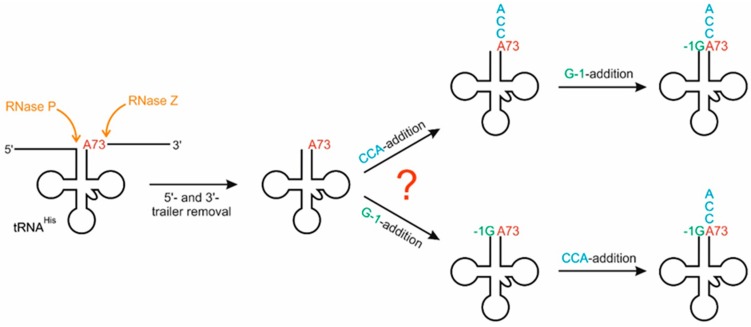
Here we sought to investigate the order of tRNAHis processing on the 5′- and 3′-ends of tRNAHis in S. cerevisiae using recombinant enzymes and assessing the in vitro substrate preferences for each enzyme for the matured vs. precursor form with respect to the other enzyme. By determining the biologically relevant and preferred substrate for each enzyme, we aimed to understand enzyme recognition and patterns of activity (Figure 1). Knowing that tRNAHis as well as CCA-adding enzyme and Thg1 are localized in the cytosol of S. cerevisiae, we identified a sequential order of tRNAHis processing. In this scenario, according to our results the enzymes do not compete for the substrate. Instead, the differing substrate preferences lead to a sequential order of nucleotide incorporation at 5′- and 3′-ends, resulting in a mature tRNAHis in the cytosol.
Figure 1.
Cytosolic tRNAHis processing in eukaryotes. After removal of 5′-leader and 3′-trailer sequences by the nucleus-localized RNase P and RNase Z (orange), CCA- (cyan) and G-1-addition (green) take place in the cytosol. However, it is not clear whether these events occur at random or follow a sequential order, due to different substrate preferences of the involved enzymes. Furthermore, these events can be affected by the nature of the discriminator base 73 that is located across the -1 position (as an example, A73 is indicated in red).
2. Results
2.1. Eukaryotic Cytosolic tRNAHis Processing—A Temporal Order of 5′- and 3′- Nucleotide Incorporation
2.1.1. Addition of the 3′-terminal CCA Sequence
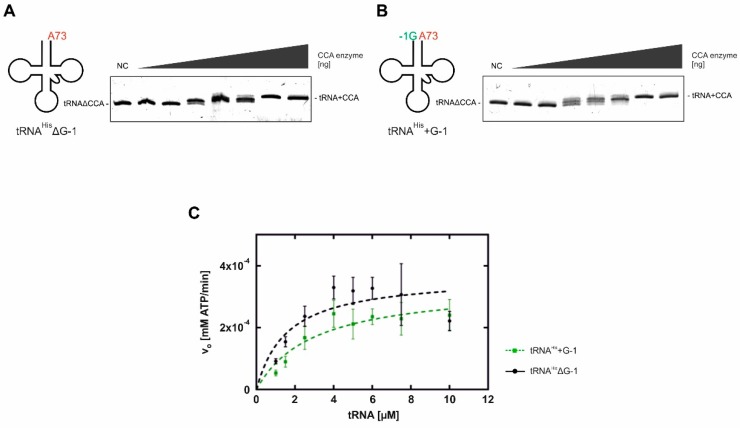
To figure out whether the maturation status of the 5′-end has an impact on the efficiency of CCA-addition at the 3′-end, in vitro transcribed cytosolic S. cerevisiae tRNAHisΔG-1 and tRNAHis+G-1 were incubated with increasing amounts of recombinant CCA-adding enzyme. Reaction products were separated on denaturing polyacrylamide gels and visualized by ethidium bromide staining. In the resulting band patterns of the reaction products, no remarkable differences in the efficiency of CCA-addition on these substrates were observed (Figure 2A,B). Therefore, the CCA-adding enzyme is able to act on both substrates and efficiently incorporates the complete CCA triplet at the 3′-end. To further quantify these results, steady-state Michaelis–Menten kinetics for the complete CCA-addition were performed (Figure 2C). As the limited solubility properties of RNA do not allow for using excessive saturating conditions in these analyses, the obtained parameters represent apparent values typical for CCA-addition kinetics [18,19,20,21]. For both transcripts, we obtained similar apparent KM values, indicating that the CCA-adding enzyme binds these two substrates with similar affinity. Furthermore, for both substrates tRNAHisΔG-1 and tRNAHis+G-1, comparable turnover numbers (apparent kcat) with values of approximately 25 min−1 were determined (Table 1). Hence, these data indicate that the 3′-terminal CCA-addition is not affected by the processing status at the 5′-end of tRNAHis.
Figure 2.
CCA-adding enzyme-catalyzed CCA incorporation on tRNAHis lacking G-1 (tRNAHisΔG-1; A) and tRNAHis+G-1 (B) from S. cerevisiae. tRNA variants were incubated with increasing amounts of CCA-adding enzyme (0.5, 1.5, 3.0, 4.5, 6.0, 60 und 300 ng). The reaction products were separated on a denaturing polyacrylamide gel and visualized by ethidium bromide staining. Both tRNA variants were processed at comparable efficiencies, resulting in a complete substrate turnover. NC, negative control without enzyme. (C) Kinetic analysis of CCA-addition for both tRNA variants. Increasing amounts of tRNAHisΔG-1 (black curve) and tRNAHis+G-1 (green curve) were incubated with CCA-adding enzyme, NTPs and α-32P-ATP under steady-state conditions. Michaelis–Menten data were calculated from triplicates using GraphPadPrism software (Table 1).
Table 1.
Kinetic parameters determined for the CCA-adding enzyme on tRNAHis variants carrying the discriminator A73.
| Substrate | KM [µM] | kcat [min−1] |
|---|---|---|
| tRNAHis∆G-1 | 1.6 ± 0.6 | 27.5 ± 3.3 |
| tRNAHis+G-1 | 2.9 ± 1.0 | 25.1 ± 3.3 |
Data are means ± SD; n = 3
2.1.2. Incorporation of the G-1 Nucleotide
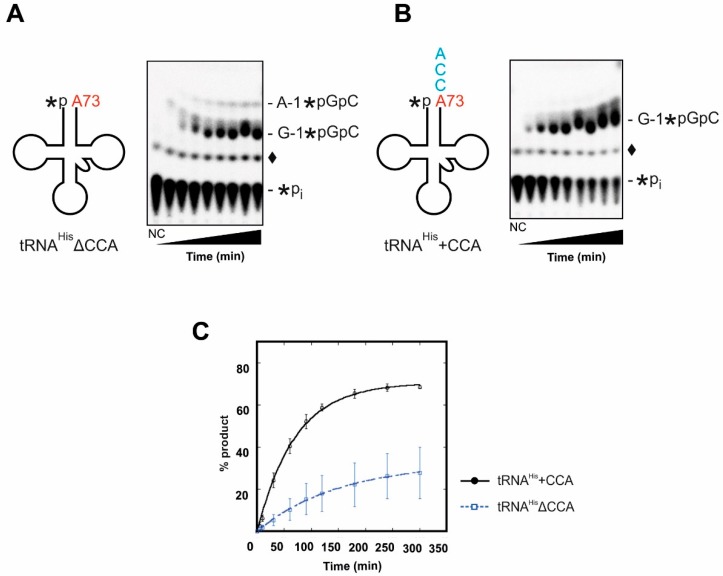
In a complementary series of experiments, we determined whether recombinant Thg1 prefers to act on tRNAHis before or after 3′-terminal CCA-addition. The unusual 3′-5′ nucleotide transfer mediated by Thg1 family proteins occurs in a 3-step reaction [22]. First, the 5′-monophosphate end of the substrate tRNA is adenylated via a 5′-5′-linkage with ATP as a co-substrate. Next, the 3′-hydroxyl of the incoming nucleotide (GTP) attacks the α-phosphate of the activated tRNA 5′-end, resulting in a phosphodiester bond. Lastly, pyrophosphate is released from the incorporated G-1 nucleotide. In order to allow this mechanism to proceed, the in vitro transcribed tRNA substrates with (tRNAHis+CCA) and without 3′-CCA-end (tRNAHisΔCCA) were monophosphorylated at the 5′-end using γ-32P-ATP. Thg1-catalyzed nucleotide incorporation on tRNAHisΔCCA and tRNAHis+CCA was monitored using a phosphatase protection assay which results in the protection of a labelled oligonucleotide (G-1*pGpC) from phosphatase activity if 5′-nucleotides have been incorporated (Figure 3A,B) [15]. As indicated by the intensity of the radioactive signals after resolution by TLC, Thg1 readily incorporates the G-1 nucleotide at the 5′-end of tRNAHis+CCA (Figure 3B). On the substrate lacking the CCA-end (tRNAHisΔCCA), however, the enzyme is less efficient. Furthermore, Thg1 forms an additional minor product on tRNAHisΔCCA, which resolves higher than the expected G-1 spot (Figure 3A). RNase T1 treatment and resolution by PEI cellulose TLC identified this spot as a side-reaction product of A-1 addition (A-1*pGpG) (Figure S2). It appears that when tRNAHis lacks the 3′-CCA-end, Thg1 exhibits a certain infidelity by incorporating some incorrect nucleotides at the -1 position.
Figure 3.
The CCA triplet affects the fidelity of G-1-addition by Thg1. 5′-labeled tRNAHis lacking the CCA-end (tRNAHisΔCCA); (A) or ending with CCA (tRNAHis+CCA); (B) were incubated with saturating amounts of Thg1 (15 µM) and assayed using a phosphatase protection assay, in which the 5′-32P-label on unreacted tRNA substrate is accessible to phosphatase, and visualized as inorganic phosphate (*Pi). On tRNAHisΔCCA, Thg1 exhibits a reduced fidelity and adds not only the correct G-1 (G-1*pGpC product) but to a certain amount also erroneously A-1 (A-1*pGpC product on panel A). However, when the tRNA substrate carries a 3′-terminal CCA sequence, Thg1 exclusively incorporates G-1, indicating that the CCA triplet contributes to the fidelity of the reaction. An additional non-enzymatic labeled species (♦) is visible in the no enzyme control (NC) and enzyme-containing reactions, as has been observed previously with these types of labeled tRNA assays [13,23]. (C) Single-turnover nucleotide incorporation was measured in triplicate and plotted as a function of time. 5′-labeled tRNAHisΔCCA (blue) and tRNAHis+CCA (black) were incubated with 15 µM Thg1 in the presence of 0.1 mM ATP and 1 mM GTP.
To address the differences in reaction efficiency on tRNAHisΔCCA compared to tRNAHis+CCA in more detail, G-1 addition was performed under single-turnover conditions ([E]>>[S]; E, enzyme; S, substrate) to obtain kobs values for each substrate. Although G-1 addition is clearly observed for both substrates, the measured kobs for nucleotide incorporation by Thg1 in the presence of tRNAHis+CCA is 2 times faster than that of tRNAHisΔCCA, supporting the qualitative observation in the TLC assays (Figure 3, Table 2). Furthermore, in addition to the effect on the rate of G-1 addition, the maximal amount of product formed in the absence of CCA was also significantly lower than in the presence of the intact 3′-end (Figure 3C). Finally, we obtained rates of nucleotide incorporation under varying enzyme concentrations, which enables the measurement of apparent dissociation constants (KD,app) for each tRNA substrate. A value of 5.8 ± 4.3 µM was obtained for tRNAHis+CCA (Table 1). This value aligned well with previously published data [15]. In comparison, a precise dissociation constant for tRNAHisΔCCA was not attainable due to the inability to saturate the observed rate even at the highest concentration of protein achievable in our assays, and therefore leading us to estimate that KD,app for tRNAHisΔCCA is ≥30 μM. Taken together, these results clearly indicate that tRNAHis+CCA is a better substrate for Thg1 than tRNAHisΔCCA in terms of nucleotide incorporation rates, fidelity, and binding.
Table 2.
Kinetic parameters for Thg1 on variant tRNAHis substrates.
| tRNAHis+CCA | tRNAHis∆CCA | tRNAHis+CCA A73C | tRNAHis∆CCA A73C | |
|---|---|---|---|---|
|
kobs
(min−1) |
0.014 ± 0.001 | 0.0070 ± 0.0004 | 0.097 ± 0.008 | 0.013 ± 0.001 |
|
KD,app,tRNA
(µM) |
5.8 ± 4.3 | >30 * | 3.5 ± 1.7 | >30 * |
|
kmax/KD,app
(µM−1min−1) |
385 ± 45 | ND | 110 ± 7.7 | ND |
Data are means ± SD; n = 3; ND: not determined. * saturation was not reached even at the highest possible concentration of purified protein in the assays, leading to the lower estimate for KD,app of 30 µM
2.2. The 3′- A73CCA Sequence Serves as a Fidelity Determinant for Thg1
In contrast to almost all bacterial and archaeal tRNAHis species, which encode a cytosine residue at the discriminator position (C73), the eukaryotic cytosolic tRNAHis shows a conserved adenosine at position 73. Mutated tRNAHis variants that contain a C73 have been shown to affect the activity of Thg1, leading to the incorporation of multiple G residues onto the 5′-end of the tRNA, essentially “zipping” up the acceptor stem [24,25]. Since this 3′-5′ polymerase activity utilizes the 3′-CCA sequence as a template, the simultaneous presence of G-1 and CCA-end might lead to different consequences in the context of a C73 discriminator nucleotide instead of A73. We therefore tested whether the identity of the discriminator base has an impact on the correct incorporation of either the 3′-CCA sequence or the 5′-G-1 position. S. cerevisiae tRNAHis transcripts with and without G-1 were generated, carrying a C73 discriminator instead of the wild type A73 position (tRNAHisΔG-1 A73C and tRNAHis+G-1 A73C). Due to the additional base pair G-1/C73, tRNAHis+G-1 A73C carries an extended acceptor stem with a base-paired discriminator position (Figure S1B). However, this situation does not affect CCA-addition catalyzed by the CCA-adding enzyme and results in a similarly efficient CCA incorporation to tRNAHis+G-1 A73C compared to tRNAHisΔG-1 A73C (Figure S1A). Taken together, both tRNAHis substrates with a cytosine at the discriminator position are readily accepted as substrates for CCA-addition, showing comparable band patterns like the wild type tRNAHis containing an A73. Due to this identical substrate acceptance of the CCA-adding enzyme, no kinetic parameters were further determined.
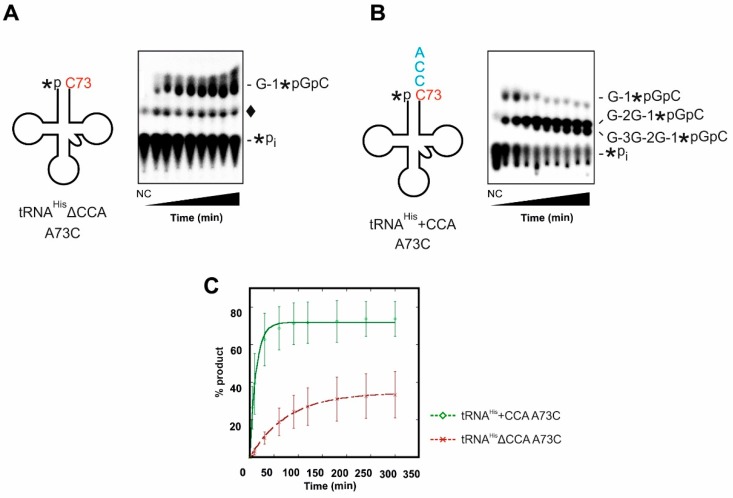
Next, G-1 incorporation on tRNAHis A73C variants with and without 3′-CCA-end was investigated. As shown for the tRNAHis with A73, the time course experiments indicate a significant preference of Thg1 for tRNAHis containing the 3′-CCA, both in terms of rate and maximal amount of product formed in the reactions (Figure 4). In the kinetic analysis, the rates of nucleotide incorporation (0.097 min−1 for tRNAHis+CCA A73C and 0.013 min−1 for tRNAHisΔCCA A73C) differ by nearly 10-fold (Figure 4C, Table 2). Finally, the substantial decrease in affinity for the tRNA in the absence of the 3′-CCA sequence was also observed here, as judged by the difference in KD,app measured by single-turnover assay (Table 2). Interestingly, in the presence of the 3′-CCA-end, Thg1 incorporated multiple (up to 3) G nucleotides at the tRNA 5′-end, as the 3′-CCA sequence on a tRNAHis with C73 obviously acts as a template for incorporation of multiple nucleotides as described by Jackman and Phizicky (compare Figure 4A vs. Figure 4B) [25].
Figure 4.
Thg1 activity on cytosolic tRNAHis carrying a cytosine residue at the discriminator position 73 (red). (A) On tRNAHisΔCCA A73C, the enzyme correctly adds a single G-1 residue (G-1*pGpC product), consistent with the absence of a 3′-end template sequence for further 3′-5′ polymerization. Other species observed in this assay include *Pi, which represents the remaining unreacted substrate tRNA, and ♦ which represents the non-enzymatic product that is visible in both no enzyme control (NC) and enzyme-containing reaction lanes. (B) If tRNAHis A73C additionally carries the 3′-CCA-end (cyan), Thg1 catalyzes multiple GTP incorporations in a 5′-template-dependent manner, resulting in two additional G-C base pairs with the 3′-CCA-end (G-2G-1*pGpC and G-3G-2G-1*pGpC products). In this case, the non-enzymatic side product is not visible because it is obscured by the strong signal from the multiple G-addition products. (C) Single-turnover nucleotide incorporation was measured in triplicate and plotted as a function of time. 5′-labeled tRNAHisΔCCA A73C (red graph) or tRNAHis+CCA A73C (green graph) were incubated with saturating amounts of enzyme (15 µM), 0.1 mM ATP and 1 mM GTP.
3. Discussion
To participate in protein biosynthesis, tRNAs undergo many different maturation steps. Differences in subcellular localization of various components of the tRNA maturation machinery enforces a temporal order to many of these modification events, in which the tRNA substrate’s movement around the cell after transcription dictates the next steps in terms of its lifecycle [26]. In contrast, processing events that occur in the same compartment have been much less well described, and whether there are substrate specificity preferences that lead to sequential processing activities or whether these activities are more random in nature has not been investigated in many cases. The case of histidine tRNA provides a unique and perfect opportunity to investigate this question, since the addition of two indispensable features- the 3′-CCA-end and the 5′-G-1 position- are both added post-transcriptionally by conserved enzymes that are found in the cytosol of S. cerevisiae. Hence, it is conceivable that these two activities compete for the end-matured tRNAHis that is exported from the nucleus after initial processing events that remove leader and trailer sequences. Here, we used in vitro enzyme assays to investigate the 5′- and 3′-end maturation of eukaryotic cytosolic tRNAHis by the two enzymes responsible for these activities. Based on tRNAHis-dependent kinetic parameters as well as fidelity defects in the activity of Thg1, our results support a scenario in which the CCA-adding enzyme is likely to act prior to Thg1.
We note that the kinetic approaches applied to analysis of each of these enzymes were different, with CCA-adding activity measured under steady-state conditions, while Thg1 was measured using the single-turnover regime. This difference was largely driven by substantially different velocities known to be exhibited by these enzymes, which makes it challenging to perform the long-term courses needed to measure steady-state parameters of slowly-reacting Thg1, and would require highly specialized equipment to measure single-turnover parameters of the rapidly-reacting CCA-adding enzyme. Despite this disparity in approach, the kcat/KM value in steady-state and kmax/KD value in single-turnover are analogous reflections of the “specificity constant” for any particular substrate, and in each case support our conclusions that CCA-adding enzyme is likely to act prior to Thg1 (Table 1 and Table 2). Moreover, while these in vitro reaction rates may not precisely reflect the actual rates in vivo, especially given the use of otherwise unmodified tRNA transcripts for these assays, the trend of faster reaction with the CCA-adding enzyme also agrees well with our prediction that this enzyme is the first player in tRNAHis maturation in S. cerevisiae.
Taken together, the CCA incorporation assays and Michaelis–Menten kinetics indicate that the 3′-end of wild type tRNAHis from S. cerevisiae is not affected by the maturation status of the 5′-end (Figure 2, Table 1). The apparent KM values for CCA incorporation by yeast CCA-adding enzyme are in a low µM range, indicating similar affinities to both tRNA variants. Those evaluated apparent KM correspond to previously found KM values for tRNA structures or tRNA-like structures [27,28,29]. In addition, the kcat values do not show a significant difference in tRNA-turnover behavior. Contrary to previous observations that the CCA-adding enzyme prefers a single-stranded discriminator base or even more unpaired nucleotides at the 3′-end [30,31,32], the additional guanosine residue at the 5′-end does not appear to sterically hinder the binding of the 3′-OH group of the discriminator, which serves as the nucleophile for the subsequent CTP incorporation. Consequently, no inhibition of the incorporation of the essential CCA triplet is detectable even in the presence of G-1.
In contrast to the maturation status of the tRNAHis 5′-end, the presence of the 3′-terminal CCA sequence has a significant effect on G-1 addition as indicated by our time series experiments and the obtained kinetic parameters under single-turnover conditions, which all favor the +CCA substrate over the ΔCCA versions (Figure 3, Table 2). Moreover, the presence of the 3′-CCA exerts an interesting effect on the productive interaction with the tRNA substrate, evident from the significantly lower fractions of maximal product formation observed in time course assays with substrates that lack CCA (Figure 3C and Figure 4C). Although the precise molecular basis for this effect is not clear from existing structures, the absence of CCA appears to affect the ability of the tRNA to adopt a conformation that is accommodated correctly into the Thg1 active site for catalysis.
The preference of Thg1 for the CCA-containing tRNAHis extends beyond kinetics and appears to also be reflected in a modest loss of fidelity that could occur if the enzyme acted prior to the CCA-adding enzyme. Here, we clearly detected the incorporation of A-1 on tRNAHisΔCCA (Figure 3 and Figure S2). While this resulting product, tRNAHis+A-1, might be suitable for the CCA-adding enzyme, since there was little effect of the presence of a G-1 nucleotide on its activity, this could render the tRNAHis defective by affecting its fitness for aminoacylation by HisRS. However, in vitro kinetic analysis reveals that S. cerevisiae HisRS is only modestly impacted in its ability to aminoacylate tRNAHis transcripts containing A-1 instead of the natural G-1 nucleotide [33]. Moreover, several other studies have revealed that the identity of the nucleotide base at position N-1 is not as important as the presence of a single phosphate group on the incorporated N-1 residue [34], and that human tRNAHis even contains a relatively minor fraction of U-1-containing tRNAHis that is presumably generated by the Watson–Crick-dependent 3′-5′ polymerase activity of the human Thg1 homolog [35]. Nonetheless, it is possible that our in vitro assay conditions underestimate the extent of this fidelity issue since the experimental NTP concentrations employed for Thg1 assays do not match physiological conditions. In fact, the concentrations of ATP (0.1 mM; required for the tRNA 5′-adenylation step) and GTP (1 mM; to be incorporated at the -1 position) are ~30 times less and 2 times more, respectively, in our experiments than previously published physiological concentrations [36]. Taking this into consideration, one can postulate that Thg1 infidelity in terms of A-1 addition might be exacerbated in the presence of higher ATP (and lower GTP) concentrations in the cell, if the enzyme acts on tRNA prior to the action of the CCA-adding enzyme.
In addition to detectable amounts of A-1 addition, multiple nucleotide incorporation was observed on tRNAHis+CCA A73C, as has been observed previously (Figure 4) [25,37]. Incorporation of multiple G residues, up to G-3, on any tRNA would predictably be detrimental to the stability of the tRNA [38]. From an evolutionary standpoint, it appears to be very advantageous for eukaryotic organisms to encode tRNAHis A73 in order for Thg1 to incorporate a single G-1. Notably, this same observation raises interesting questions about the maturation of tRNAHis in some Bacteria and Archaea that encode homologs of both Thg1 and CCA-adding enzymes and naturally also encode tRNAHis with a C73 discriminator. This issue is further complicated by the fact that the 5′-G-1 and 3′-CCA are frequently genomically encoded in these species where the enzymes may also serve repair functions in vivo [39]. In contrast to Thg1, the CCA-adding enzyme does not show a favored discriminator base in the case of tested tRNAHis variants (Figure S1). An explanation for this equal acceptance by the CCA-adding enzyme might be related to the composition of the mitochondrial tRNAHis in yeast, which differs dramatically in the sequence within the acceptor stem. Like most bacterial tRNAHis sequences, the mt-tRNAHis from S. cerevisiae carries a genomically encoded G-1 across from C73, alleviating the need for Thg1 activity in mitochondria [40]. Due to this sequence difference between cytosolic and mitochondrial tRNAHis in yeast, the CCA adding enzyme has to accept both tRNA substrates and interact just as well with the cytosine at position 73, although it is not favored [27].
In summary, the CCA-adding enzyme has a higher turnover number and does not show preference for either tRNAHis substrate, while Thg1 relies on the presence of CCA for binding, rates of incorporation, and fidelity. Hence, these data indicate that although many tRNA maturation steps may occur in a rather random order, nucleotide incorporation at 5′- and 3′-ends of eukaryotic tRNAHis likely follows a temporal order with the CCA-adding enzyme incorporating 3′-CCA before Thg1 incorporates a single 5′-G-1.
4. Material and Methods
4.1. Preparation of Recombinat Enzymes
4.1.1. Yeast CCA-adding Enzyme
As the coding sequence for the CCA-adding enzyme from Saccharomyces cerevisiae contains no introns, genomic DNA was used for amplification using specific flanking primers. The PCR product was cloned into the Xho I and Nde I site of pET28a(+). The yeast CCA-adding enzyme was overexpressed with a C-terminal His-tag in E. coli BL21(DE3)cca−::cam as described [41] and stored at −80 °C in the presence of 40% glycerol until use. SDS-PAGE electrophoresis followed by staining with Coomassie Brilliant Blue R-250 (BioRad) revealed a >90% purity of the preparation. Protein concentration was measured according to Bradford [42].
4.1.2. Yeast tRNAHis Guanylyltransferase (Thg1)
Thg1 was overexpressed in E. coli BL21(DE3) pLysS cells and purified as described for N-terminally His6-tagged enzymes [16,23]. The protein was dialyzed to exchange into 50% glycerol and stored at −20 °C until use. Thg1 was assessed for >90% purity by visual inspection using standard SDS-PAGE. Concentration of Thg1 was determined by BioRad assay.
4.2. Preparation of tRNA Substrates
DNA templates for T7-based in vitro transcription were prepared by overlap extension PCR. Transcription of tRNAHis variants (tRNAHisΔG-1, tRNAHis+G-1, tRNAHisΔCCA, tRNAHis+CCA, tRNAHisΔG-1 A73C, tRNAHis+G-1 A73C, tRNAHisΔCCA A73C, and tRNAHis+CCA A73C) was carried out as described previously, using flanking ribozyme sequences to generate defined homogeneous 5′- and 3′-ends [43,44]. For internally labeled tRNAHis, transcription was performed in the presence of α-32P-ATP (Hartman Analytics). In vitro transcripts for Thg1-catalyzed G-1 addition were 5′-labeled using γ-32P-ATP (Perkin Elmer) and T4 polynucleotide kinase according to the manufacturer’s instructions (NEB).
4.3. In Vitro CCA-Addition
Standard CCA-addition and kinetic assays under steady-state conditions were performed as described [27,28]. Kinetic parameters of three independent experiments were analyzed using GraphPadPrism (curve fitting by nonlinear regression). Due to the solubility properties of RNA, no excessive saturation concentrations could be used, and the obtained values represent therefore apparent values, as frequently described for CCA-addition [18,19,20,21,45].
4.4. In Vitro G-1 Addition: Phosphate Protection Assay
Activity assays contained <200 fmol 5′-32P-tRNA (specific activity 6000 Ci/mmol) in 25 mM HEPES pH 7.5, 10 mM MgCl2, 3 mM DTT, 125 mM NaCl, 0.2 mg/ml bovine serum albumin (BSA), 0.1 mM ATP and 1 mM GTP. Enzyme was added to a desired final concentration. Aliquots were removed at time points and quenched with 1 mg/ml RNase A (Ambion) and 50 mM EDTA. Each sample was incubated at 50 °C for 10 min. RNase digested samples were treated with 0.05 U calf intestinal alkaline phosphatase (CIAP) (Invitrogen) and incubated at 37 °C for 1 hour. Reaction products were resolved using thin layer chromatography (TLC) in a 1-propanol:NH4OH:H2O (55:35:10) solvent system. TLC plates were visualized using a Typhoon (GE Healthcare) and quantified using ImageQuant software (GE Healthcare).
Single-turnover time courses were plotted and fit to a single exponential Equation (1) using Kaleidagraph (Synergy software). Pt is the fraction of product formed at each time point and ∆P is maximal amount of product formed for each time course.
| (1) |
kobs values obtained with varying concentrations of enzyme were plotted and fit to Equation (2) to yield pseudo first order maximal rate constants (kmax) and apparent dissociation constant (KD,app), which were used to derive the specificity constant kmax/KD,app. Reported errors are least fit squares of the standard deviation derived from the fit using Kaleidagraph [46].
| (2) |
Acknowledgments
We thank B. Klüver for expert technical assistance.
Abbreviations
| aaRS | aminoacyl-tRNA synthetase |
| DTT | dithiothreitol |
| EDTA | 2,2′,2″,2‴-(Ethane-1,2-diyldinitrilo)tetraacetic acid |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HisRS | histidyl-tRNA synthetase |
| PEI | Polyethylenimine |
| Thg1 | tRNAHis guanylyltransferase |
| TLC | thin layer chromatography |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/6/1384/s1.
Author Contributions
M.-T.P., T.M.R., H.B., J.E.J. and M.M. designed and evaluated the experiments and wrote the manuscript. M.-T.P. and T.M.R. performed the experiments. M.-T.P. and T.M.R. contributed equally to this work and represent joint first authors.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (MO 634/8-2 to MM) and the National Institutes of Health (R01 GM087543 to JEJ). We acknowledge support from the Deutsche Forschungsgemeinschaft (DFG) and Leipzig University within the program of Open Access Publishing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Phizicky E.M., Hopper A.K. tRNA biology charges to the front. Genes Dev. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopper A.K. Transfer RNA Post-Transcriptional Processing, Turnover, and Subcellular Dynamics in the Yeast Saccharomyces cerevisiae. Genetics. 2013;194:43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann R.K., Gößringer M., Späth B., Fischer S., Marchfelder A. Chapter 8 The Making of tRNAs and More—RNase P and tRNase Z. In: Condon C., editor. Molecular Biology of RNA Processing and Decay in Prokaryotes. Volume 85. Elsevier; Amsterdam, The Netherlands: 2009. pp. 319–368. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L., Deutscher M.P. tRNA nucleotidyltransferase is not essential for Escherichia coli viability. EMBO J. 1987;6:2473–2477. doi: 10.1002/j.1460-2075.1987.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betat H., Rammelt C., Mörl M. tRNA nucleotidyltransferases: Ancient catalysts with an unusual mechanism of polymerization. Cell. Mol. Life Sci. 2010;67:1447–1463. doi: 10.1007/s00018-010-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong Y., Steitz T.A. Mechanism of transfer RNA maturation by CCA-adding enzyme without using an oligonucleotide template. Nature. 2004;430:640–645. doi: 10.1038/nature02711. [DOI] [PubMed] [Google Scholar]
- 7.Li F., Xiong Y., Wang J., Cho H.D., Tomita K., Weiner A.M., Steitz T.A. Crystal structures of the Bacillus stearothermophilus CCA-adding enzyme and its complexes with ATP or CTP. Cell. 2002;111:815–824. doi: 10.1016/S0092-8674(02)01115-7. [DOI] [PubMed] [Google Scholar]
- 8.Wellner K., Betat H., Mörl M. A tRNA’s fate is decided at its 3′ end: Collaborative actions of CCA-adding enzyme and RNases involved in tRNA processing and degradation. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:433–441. doi: 10.1016/j.bbagrm.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Betat H., Mörl M. The CCA-adding enzyme: A central scrutinizer in tRNA quality control. BioEssays. 2015;37:975–982. doi: 10.1002/bies.201500043. [DOI] [PubMed] [Google Scholar]
- 10.Wilusz J.E., Whipple J.M., Phizicky E.M., Sharp P.A. tRNAs marked with CCACCA are targeted for degradation. Science. 2011;334:817–821. doi: 10.1126/science.1213671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou Y.-M. CCA Addition to tRNA: Implications for tRNA Quality Control. IUBMB Life. 2010;62:251–260. doi: 10.1002/iub.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C., Sobral B.W., Williams K.P. Loss of a universal tRNA feature. J. Bacteriol. 2007;189:1954–1962. doi: 10.1128/JB.01203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao B.S., Mohammad F., Gray M.W., Jackman J.E. Absence of a universal element for tRNAHis identity in Acanthamoeba castellanii. Nucleic Acids Res. 2013;41:1885–1894. doi: 10.1093/nar/gks1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orellana O., Cooley L., Söll D. The additional guanylate at the 5′ terminus of Escherichia coli tRNAHis is the result of unusual processing by RNase P. Mol. Cell. Biol. 1986;6:525–529. doi: 10.1128/MCB.6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackman J.E., Phizicky E.M. tRNAHis guanylyltransferase adds G-1 to the 5′ end of tRNAHis by recognition of the anticodon, one of several features unexpectedly shared with tRNA synthetases. RNA. 2006;12:1007–1014. doi: 10.1261/rna.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu W., Jackman J.E., Lohan A.J., Gray M.W., Phizicky E.M. tRNAHis maturation: An essential yeast protein catalyzes addition of a guanine nucleotide to the 5′ end of tRNAHis. Genes Dev. 2003;17:2889–2901. doi: 10.1101/gad.1148603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghaemmaghami S., Huh W.-K., Bower K., Howson R.W., Belle A., Dephoure N., O’Shea E.K., Weissman J.S. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 18.Tomita K., Ishitani R., Fukai S., Nureki O. Complete crystallographic analysis of the dynamics of CCA sequence addition. Nature. 2006;443:956–960. doi: 10.1038/nature05204. [DOI] [PubMed] [Google Scholar]
- 19.Ernst F.G.M., Erber L., Sammler J., Jühling F., Betat H., Mörl M. Cold adaptation of tRNA nucleotidyltransferases: A tradeoff in activity, stability and fidelity. RNA Biol. 2018;15:144–155. doi: 10.1080/15476286.2017.1391445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H.D., Verlinde C.L., Weiner A.M. Archaeal CCA-adding enzymes: Central role of a highly conserved beta-turn motif in RNA polymerization without translocation. J. Biol. Chem. 2005;280:9555–9566. doi: 10.1074/jbc.M412594200. [DOI] [PubMed] [Google Scholar]
- 21.Betat H., Rammelt C., Martin G., Mörl M. Exchange of regions between bacterial poly(A) polymerase and the CCA-adding enzyme generates altered specificities. Mol. Cell. 2004;15:389–398. doi: 10.1016/j.molcel.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Jahn D., Pande S. Histidine tRNA guanylyltransferase from Saccharomyces cerevisiae. II. Catalytic mechanism. J. Biol. Chem. 1991;266:22832–22836. [PubMed] [Google Scholar]
- 23.Abad M.G., Rao B.S., Jackman J.E. Template-dependent 3′-5′ nucleotide addition is a shared feature of tRNAHis guanylyltransferase enzymes from multiple domains of life. Proc. Natl. Acad. Sci. USA. 2010;107:674–679. doi: 10.1073/pnas.0910961107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackman J.E., Gott J.M., Gray M.W. Doing it in reverse: 3′-to-5′ polymerization by the Thg1 superfamily. RNA. 2012;18:886–899. doi: 10.1261/rna.032300.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackman J.E., Phizicky E.M. tRNAHis guanylyltransferase catalyzes a 3′-5′ polymerization reaction that is distinct from G-1 addition. Proc. Natl. Acad. Sci. USA. 2006;103:8640–8645. doi: 10.1073/pnas.0603068103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee K., Nostramo R.T., Wan Y., Hopper A.K. tRNA dynamics between the nucleus, cytoplasm and mitochondrial surface: Location, location, location. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:373–386. doi: 10.1016/j.bbagrm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wende S., Bonin S., Götze O., Betat H., Mörl M. The identity of the discriminator base has an impact on CCA addition. Nucleic Acids Res. 2015;43:5617–5629. doi: 10.1093/nar/gkv471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf J., Obermaier-Kusser B., Jacobs M., Milles C., Mörl M., von Pein H.D., Grau A.J., Bauer M.F. A new mitochondrial point mutation in the transfer RNA(Lys) gene associated with progressive external ophthalmoplegia with impaired respiratory regulation. J. Neurol. Sci. 2012;316:108–111. doi: 10.1016/j.jns.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Williams K.R., Schofield P. Kinetic mechanism of tRNA nucleotidyltransferase from Escherichia coli. J. Biol. Chem. 1977;252:5589–5597. [PubMed] [Google Scholar]
- 30.Giegé R., Jühling F., Pütz J., Stadler P., Sauter C., Florentz C. Structure of transfer RNAs: Similarity and variability. Wiley Interdiscip. Rev. RNA. 2012;3:37–61. doi: 10.1002/wrna.103. [DOI] [PubMed] [Google Scholar]
- 31.Lizano E., Scheibe M., Rammelt C., Betat H., Mörl M. A comparative analysis of CCA-adding enzymes from human and E. coli: Differences in CCA addition and tRNA 3′-end repair. Biochimie. 2008;90:762–772. doi: 10.1016/j.biochi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Li Z., Sun Y., Thurlow D.L. RNA minihelices as model substrates for ATP/CTP:tRNA nucleotidyltransferase. Biochem. J. 1997;327:847–851. doi: 10.1042/bj3270847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nameki N., Asahara H., Shimizu M., Okada N., Himeno H. Identity elements of Saccharomyces cerevisiae tRNA(His) Nucleic Acids Res. 1995;23:389–394. doi: 10.1093/nar/23.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen A.E., Brooks B.S., Guth E., Francklyn C.S., Musier-Forsyth K. Evolutionary conservation of a functionally important backbone phosphate group critical for aminoacylation of histidine tRNAs. RNA. 2006;12:1315–1322. doi: 10.1261/rna.78606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigematsu M., Kirino Y. 5′-Terminal nucleotide variations in human cytoplasmic tRNAHisGUG and its 5′-halves. RNA. 2017;23:161–168. doi: 10.1261/rna.058024.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traut T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 37.Preston M.A., Phizicky E.M. The requirement for the highly conserved G-1 residue of Saccharomyces cerevisiae tRNAHis can be circumvented by overexpression of tRNAHis and its synthetase. RNA. 2010;16:1068–1077. doi: 10.1261/rna.2087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohmichi T., Nakano S.-I., Miyoshi D., Sugimoto N. Long RNA Dangling End Has Large Energetic Contribution to Duplex Stability. J. Am. Chem. Soc. 2002;124:10367–10372. doi: 10.1021/ja0255406. [DOI] [PubMed] [Google Scholar]
- 39.Betat H., Long Y., Jackman J.E., Mörl M. From end to end: TRNA editing at 5′- and 3′-terminal positions. Int. J. Mol. Sci. 2014;15:23975–23998. doi: 10.3390/ijms151223975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sibler A.P., Martin R.P., Dirheimer G. The nucleotide sequence of yeast mitochondrial histidine-tRNA. FEBS Lett. 1979;107:182–186. doi: 10.1016/0014-5793(79)80491-3. [DOI] [PubMed] [Google Scholar]
- 41.De Wijn R., Hennig O., Ernst F.G.M., Lorber B., Betat H., Mörl M., Sauter C. Combining crystallogenesis methods to produce diffraction-quality crystals of a psychrophilic tRNA-maturation enzyme. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018;74:747–753. doi: 10.1107/S2053230X18014590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 43.Mörl M., Lizano E., Willkomm D.K., Hartmann R.K. Production of RNAs with Homogeneous 5′- and 3′-Ends. In: Hartmann R.K., Bindereif A., Schön A., Westhof E., editors. Handbook of RNA Biochemistry. Wiley-VCH; Weinheim, Germany: 2005. pp. 22–35. [Google Scholar]
- 44.Schürer H., Lang K., Schuster J., Mörl M. A universal method to produce in vitro transcripts with homogeneous 3′ ends. Nucleic Acids Res. 2002;30:e56. doi: 10.1093/nar/gnf055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomita K., Fukai S., Ishitani R., Ueda T., Takeuchi N., Vassylyev D.G., Nureki O. Structural basis for template-independent RNA polymerization. Nature. 2004;430:700–704. doi: 10.1038/nature02712. [DOI] [PubMed] [Google Scholar]
- 46.Smith B.A., Jackman J.E. Kinetic analysis of 3′-5′ nucleotide addition catalyzed by eukaryotic tRNA(His) guanylyltransferase. Biochemistry. 2012;51:453–465. doi: 10.1021/bi201397f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.