Abstract
In recent years, the demand for naturally derived products has hiked with enormous pressure to propose or develop state-of-the-art strategies to meet sustainable circular economy challenges. Microalgae possess the flexibility to produce a variety of high-value products of industrial interests. From pigments such as phycobilins or lutein to phycotoxins and several polyunsaturated fatty acids (PUFAs), microalgae have the potential to become the primary producers for the pharmaceutical, food, and agronomical industries. Also, microalgae require minimal resources to grow due to their autotrophic nature or by consuming waste matter, while allowing for the extraction of several valuable side products such as hydrogen gas and biodiesel in a single process, following a biorefinery agenda. From a Mexican microalgae biodiversity perspective, more than 70 different local species have been characterized and isolated, whereas, only a minimal amount has been explored to produce commercially valuable products, thus ignoring their potential as a locally available resource. In this paper, we discuss the microalgae diversity present in Mexico with their current applications and potential, while expanding on their future applications in bioengineering along with other industrial sectors. In conclusion, the use of available microalgae to produce biochemically revenuable products currently represents an untapped potential that could lead to the solution of several problems through green technologies. As such, if the social, industrial and research communities collaborate to strive towards a greener economy by preserving the existing biodiversity and optimizing the use of the currently available resources, the enrichment of our society and the solution to several environmental problems could be attained.
Keywords: microalgae, biodiversity, bioactive compounds, green extractions, pharmaceutical, secondary metabolites, biofuels
1. Introduction
Current needs demand high-level bio-compounds production coped with cutting-edge biotechnology. Several strategies to produce valuable compounds addressed by pharmaceutical and food industry rely on microorganism production. However, other bioactive products still rely on synthetic production processes. Plants, yeasts, bacteria, fungi, and microalgae are the most used organisms to produce such compounds naturally. Microalgae have a large number of species, and little is known about their potential uses in comparison to the diversity that is reported every day [1]. Microalgae are one of the most used bio-systems to produce different compounds in biotechnology (Figure 1). The utilization of microorganisms’ machinery helps to generate high-value bio-products [2]. As this is a bioprocess, it has several advantages over other techniques since it offers new environmental-friendly opportunities. The objective of this work is to compile a list of strains, show the relevance of new extraction techniques, and characterize current applications and potential future biotechnological microalgae opportunities.
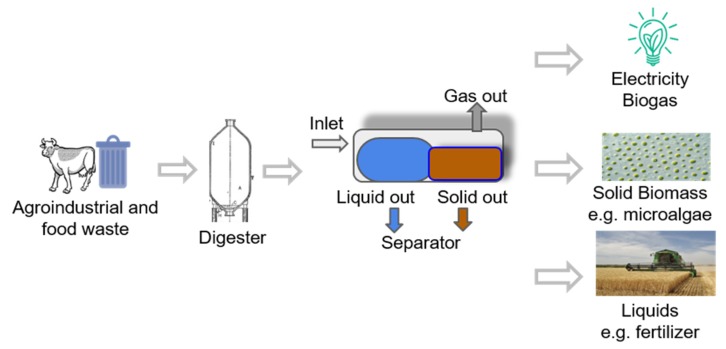
Figure 1.
Microalgae biotechnology valorization scheme to produce energy and bio-compounds from agro-industrial and food waste.
Microalgae or cyanobacteria are unicellular, cenobial, pluricellular, or colonial organisms adapted to live in water systems, soils, or as symbionts [1]. Depending on the species, they live in complex systems or as individual cells and interact with light through photosynthesis producing oxygen and consuming carbon dioxide [3]. Microalgae can produce biomass containing high-value bio-compounds and at the same time bio-fixate ions, both important factors to propel microalgae biotechnological applications in the new era of environment remediation [4,5,6]. Cultivation technologies to produce biomass include open ponds, photo-bioreactors, and fermentation reactors. A lack of attention to microalgae species is evident since only a few hundred have been investigated. It is believed that at least tens of thousands exist in the world [7]. Nonetheless, some countries had already looked for food production through microalgal cultivation. Mexican Aztecs used to cultivate and consume Spirulina from Texcoco salted lake [8]. Japan has been leading since the early 1950s [9] with the first industrial-scale production of Chlorella for human consumption to ingest as a nutritional supplement. Leading to use Chlorella as the main microalgae source of dietary supplement nowadays. Harvesting and drying of its biomass use expensive centrifuges and cells need to be broken therefor 5000 Mt of Chlorella biomass were sold for approximately $20,000/100 kg. Other successful microalgae with great industrial production are Spirulina. They have several advantages over Chlorella since they require a little inoculum, and is not easy to get contaminated, they grow in temperatures between 15 and 38° Celsius, high pH, and alkalinity. Spirulina market value, plant gate, is about $10,000 per ton and its main use is for food supplement [10]. Nowadays, Chlorella and Spirulina are the principal microalgae used for nutritional supplements, and their producers value them in the global market about $40–50 per kg [11]. The price and volume relations are even higher when pure fine-chemicals are obtained from algal cultivation. β-carotene is a pigment strongly used in the United States. In the market, its price is estimated from $300 to $3,000 USD/kg, the price depends entirely on the production, the fickle market, and the product purity [12]. The animal feeding price is about $10 USD/kg in the nutraceutical sector. On the other hand, for example, the same product for human consumption is sold at $120 US/kg [13].
Microalgal biomass is also used to get biofuels. In comparison with petroleum, biomass is more expensive. Assuming the lipid dry weight content within microalgae, 29.6% (lipid/biomass), the algal biomass must be produced at the cost of US $ 152.00 per ton to be competitive with petroleum [14]. Also, in this area, microalga biomass price along with its valued compound depend on where the product is located and the market status [15]. As discussed previously, it is evident that biotechnology focused on microalgae has a substantial potential application in the industry. This review presents several Mexican microalgae strains along with novel green extraction technologies applied to extract microalgae-based high-value compounds. Then, a comprehensive list of compounds is presented within five fields of applications, to be followed by potential applications and opportunities for improvement. Finally, concluding remarks and future perspectives are summarized.
2. Mexican Microalgae Biodiversity
Mexico is one of the countries with the highest biodiversity in the world thanks to its geography and size that covers several latitudes. The Mesoamerica region provides very different environmental conditions to support life [16]. Algal strains from all around the country were isolated and studied by research groups across México. To our knowledge, this is the first work to gather information from microalgae found in several locations, systems, and types of waterbodies in the country that also addresses its applications and prospect its potential applications in contrast to just freshwater biodiversity [17,18].
The Pacific Ocean is a vast water source with the unique condition of being warm. From this Mexican litoral, in Baja California, 21 species were isolated, but Aphanocapsa marina, Komvophoron sp., and Phormidium sp. were selected thanks to their capacity to produce fatty acids. The remaining strains were also characterized but were not selected for aquaculture farm food [19]. Raw microalgae biomass is used as a nutraceutical product in aquaculture activities directly in the country by increasing its productivity [20]. However, Rodríguez-Palacio et al. focused on a large microalgae diversity that causes algal blooms with toxic consequences for aquatic fauna in twelve locations of the country, where the harmful toxic microalgae affect fishes. In addition, they proposed the culture of those microalgae in order to evaluate changes in water pollution [21]. A list of microalgae found in Mexican water sources and isolated is presented in Table 1. Lately, the discovery of novel microorganisms is increasing, and it is expected to continue thanks to new research groups across the country. The panorama presented by the list of microalgae suggests potential applications since they were found in various environment growth conditions including volcano ponds, salted lakes, freshwaters, and seawaters. Even in extreme physiological conditions, microorganisms are capable of producing compounds with high value. To get an advantage, biotechnological processes like production, extraction, and purification require novel and environmentally friendly methods. The next section focuses on the description of green extraction methodologies.
Table 1.
Microalgae biodiversity in Mexico.
| State | Municipality/Location | Microalgae | References |
|---|---|---|---|
| Baja California | Ensenada | Aphanocapsa marina | [19] |
| Komvophoron sp. | |||
| Phormidium sp. | |||
| Tetraselmis suecica | |||
| Heterococcus sp. | |||
| Amphora sp. (7) | |||
| Cymbella sp. (2) | |||
| Navicula sp. (4) | |||
| Diploneis sp. | |||
| Grammatophora angulosa | |||
| Synedra sp. | |||
| Veracruz | Catemaco | Aphanothece comasii | [17] |
| Cyanotetras aerotopa | |||
| Cylindrospermopsis catemaco | |||
| Cylindrospermopsis taverae | |||
| Planktolyngbya regularis | |||
| San Luis Potosí | Cyanobacterium lineatum | ||
| Puebla | Alchichica | Cyclotella alchichicana | |
| Chroococcus deltoids | |||
| Baja California, Colima, Michoacan, Guerrero, Tamaulipas, Veracruz, Hidalgo, Mexico city |
Ensenada, Manzanillo, Lazaro Cardenas, Acapulco and Zihuatanejo, Laguna de Carpintero, Garrapatas and Barberena estuaries, Catemaco and Chalchoapan Lakes, Vicente Aguirre dam, Xochimilco Lake |
Alexandrium tamarense Amphidinium sp. Cochlodinium polykrikoides Heterocapsa pigmea Gyrodinium instriatum Gymnodinium catenatum Karlodinium veneficum Prorocentrum gracile Prorocentrum micans Prorocentrum triestimum Prorocentrum mexicanum Prorocentrum rathymum Protoceratium reticulatum Scrippsiella trochoidea Bacillaria paxilifera Cylindrotheca closterium Pseudonitszchia delicatisima Chattonella marina |
[21] |
| Mexico City | Mexico City | Spirulina maxima | [22] |
| Baja California Sur | La Paz | Rhabdonema sp. | [23] |
| Schizochytrium sp. | |||
| Nitzchia sp. | |||
| Navicula sp. | |||
| Grammatophora sp. | |||
| Mexico City | Mexico City | Spirulina platensis | [24] |
| Spirulina maxima | |||
| Queretaro | Not specified | Oscillatoria sp. | [25] |
| Guanajuato | Valle de Santiago | Actinastrum sp. | [26] |
| Baja California Sur | La Paz | Lyngbya sp. | [27] |
| Oscillatoria sp. | |||
| Microcoleus sp. | |||
| Anabaena sp. | |||
| Nuevo León | Apodaca | Scenedesmus sp. | [28] |
| Cadereyta | Chlorella sorokiniana | ||
| Campeche | El Carmen | Anabaena sp. | [29] |
| Oscillatoria sp. | |||
| Anabaena sp. | |||
| Cylindrospermopsis cuspis | |||
| Oaxaca | Zipolite | Dermocarpella sp. | [30] |
| Morelos | Tlaquiltenango | Nostoc sp. | [31] |
| Mexico City | Mexico City | Desmodesmus sp. | [32] |
| Coahuila | Cuatrociénegas | Scenedesmus sp. | [33] |
| Mexico City | Mexico City | Microcystis | [34] |
| Michoacan | Michoacan | Codium giraffa | [35] |
| Guerrero | Papanoa | Codium giraffa | [36] |
| Michoacán | Los Azufres | Trebouxiophyceae sp. | [37] |
3. State-Of-The-Art of Extraction Methods
Currently, most common extraction techniques consist of non-green processes. Millions of liters of organic solvents are used in the extraction process. Consequently, interest in green extraction techniques has recently increased as they are less expensive but cope with the global tendency of green legislation [38]. These processes are named, green extraction, since they do not negatively affect the environment and take advantage of the compound properties such as polarizability, charge, structure, as well as size [38]. Such processes include microwave extraction, supercritical fluid extraction, as well as ultrasound extraction. Using the appropriated method, bioactive compounds are extracted from microalgae, and valuable molecules remain functional after an efficient extraction. A list of compounds extracted from microalgae using ultrasound, microwave, and supercritical fluid extraction methods are presented in Table 2.
Table 2.
Compounds from microalgae extracted by novel green techniques.
| Compound(s) of Interest | Species | Extraction Technique | References |
|---|---|---|---|
| C-phycocyanin Pigments |
Spirulina maxima | Ultrasound | [39] |
| β-carotene | Chlorella sp. | Ultrasound | [40] |
| Polyphenols Flavonoids |
Spirulina platensis | Microwave and Ultrasound | [41] |
| Lipids | Scenedesmus sp. | Microwave | [42] |
| Lipids |
Scenedesmus obliquus &
Scenedesmus obtusiusculus |
Supercritical-CO2 | [43] |
| Oil | Spirulina platensis | Supercritical-CO2 | [44] |
| Docosahexaenoic acid | Schizochytrium limacinum | Supercritical-CO2 -vegetable oil | [45] |
| Lipids, Carotenoids | Chlorella vulgaris | Supercritical-CO2 | [46] |
| Lipids | Chlorella vulgaris | Ultrasound & Bligh and Dyer method | [47] |
| β-carotene | Spirulina platensis | Ultrasound | [48] |
| Vitamins Phycocyanin Fatty Acids |
Spirulina platensis | Microwave | [49] |
| Lipids | Chlorella sp. | Microwave and Ultrasound | [50] |
| Long-chain PUFAs | Schizochytrium sp. | Supercritical-CO2 | [51] |
| Carotenoids Fatty Acids |
Spirulina platensis | Microwave and Supercritical-CO2 -etOH |
[49] |
| C-phycocyanin | Spirulina platensis | Ultrasound | [52] |
| Neutral Lipids |
Chlorella vulgaris &
Nannochloropsis oculata |
Supercritical-CO2 | [53] |
| Chlorophyll | Chlorella vulgaris | Ultrasound | [54] |
| Lipids | Scenedesmus obliquus | Ultrasound + solvent | [55] |
3.1. Microwave-Based Extraction
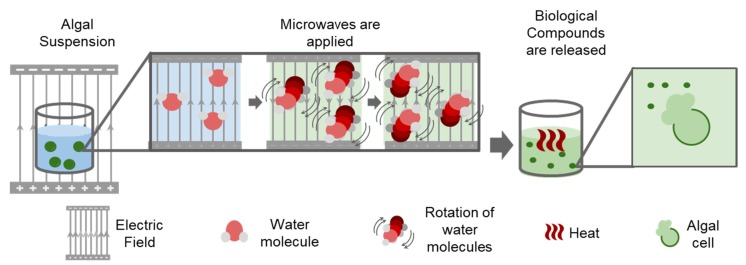
Microwave extraction involves heating of samples in a polar solution such as ionic liquids, ethanol, methanol, chloroform, acetone, and ultra-pure water by placing them in an alternating electric field. The solvent molecules align according to the applied electric field and quickly increase the temperature of the samples as a result of the inter- and intramolecular friction caused by this movement. The algal cell walls break with this sudden increase in temperature and release the compounds of interest (Figure 2). The resulting extract must undergo further purification as this process does not result in the isolated compound of interest [56]. This method differs from conventional heating extraction methods as it does not depend on the diffusion rate of the mixture mass. Therefore, microwaves heat the solvent which interacts with all the sample and prevents the formation of hot-spots and allowing for more homogeneous thermoregulation of the cell mixture. Further, it is quick and has been demonstrated to be at least ten times faster than conventional bath heating extraction methods, as mentioned by Balasubramanian et al. [56]. Additionally, the same study showed that the use of this method is especially beneficial when extracting lipids from microalgae, as it allows for a final product of better quality, with higher unsaturated lipids and antioxidant concentrations in comparison to the conventional Soxhlet extraction. In a second study involving pigment extraction from Dunaliella tertiolecta and Cylindrotheca closterium, it was demonstrated that microwave extraction techniques are more efficient than solvent-based techniques in overcoming mechanical resistance factors that limit solvent penetration into the cells [57].
Figure 2.
Scheme of microwave algal extraction.
The prospects for industrial implementation of Microwave extraction techniques have been previously discussed by Vinatoru et al. [58], where advantages such as shorter loading and downloading times as well as easier maintenance of the equipment are added to the lower energy consumption and faster extraction times. However, specific advantages depend on the type of material to be extracted (as previously discussed, microwave extraction methods are more efficient for certain mixtures due to their intrinsic properties, e.g., mechanical resistance). Nevertheless, pretreatment methods such as enzymatic digestion or milling could allow for a standardized development of this extraction method by improving its efficiency for a wide range of mixtures [58]. Also, the coupling of this method with other extraction techniques could also improve its efficiency. Still, the feasibility of scaling up microwave extraction techniques into an industrial level is still under research; because its high energy operating costs might present a major drawback of this process. For instance, the use of enzymes is expensive, especially for large scale applications when competing against other methods [59]. Moreover, this method cannot complete the separation process by itself and usually requires a subsequent centrifugation or filtration process [60].
3.2. Supercritical Fluid-Based Extraction
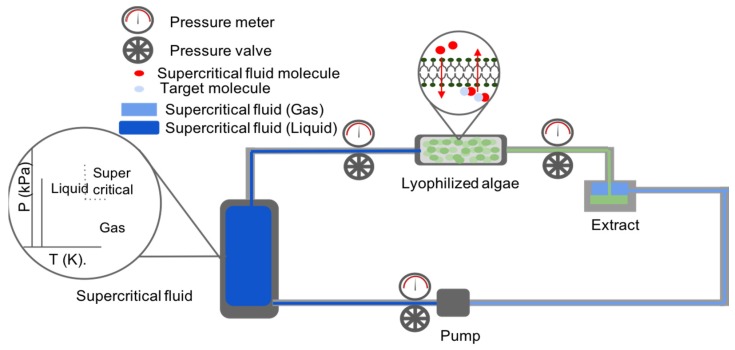
This extraction method depends on the usage of supercritical fluids (e.g., carbon dioxide, water, methane, ethane, methanol, ethanol, acetone, and nitrous oxide), induced either by temperature or pressure excitation, for the recovery of valuable products. Such fluids are capable of crossing the cellular membrane and wall of microalgae and solubilize internal metabolites to extract them from the cell (Figure 3). In most cases, supercritical fluids’ induction conditions range between for pressure and for temperature in order to allow for an energy efficient extraction (where, is the critical pressure constant and is the critical temperature constant for the supercritical fluid) [61]. The most common and preferred solvent used for this method is CO2 as it has an ambient critical temperature, is non-flammable, is chemically inert, non-toxic, and inexpensive [61]. As such, it is able to meet the ecological and chemical safety standards required for the extraction of high purity biological products (such as pigments for cosmetics and antiviral agent in medicine) as it leaves no harmful solvent residues and prevents thermal degradation of sensitive products. Moreover, with the addition of a polar entrainer (such as water) the solvent is able to dissolve polar compounds as well [61], thus allowing for a robust extraction procedure.
Figure 3.
Scheme of the supercritical fluid extraction process in a closed system.
The main advantage this method presents when compared to the conventional Soxhlet extraction method is the fact that it prevents the deposition of residual toxic matter in the extract, while also proving to be quite economical. The mentioned characteristics are because CO2 can reach a supercritical condition at a temperature of 31 °C (conventionally a temperature of 35 °C is preferred for extraction of biological materials). This critical temperature allows the liquid CO2 to be used without the consumption of excessive amounts of energy, and with a minimal reduction in temperature, most of the solvent will easily precipitate. Further, it is easily affordable, having a nominal price of 2.65 USD/kg in the year 2017 [62]. Also, as explained before, the non-extreme range of temperatures in which this method can operate prevents the degradation of valuable biological products, thus allowing for a high yield.
A recent study also analyzed the possibility of combining the use of supercritical fluid extraction with cold pressing to improve the extraction of fennel by supercritical fluid extraction alone [63]. The combination of these two extraction methods was originally developed by Johner et al. [64] for the extraction of pequi. This researchers demonstrated some promising results: a faster extraction rate with the consumption of less solvent. These results were validated with the fennel extraction, where the overall yield extraction was improved by 24.5%. Hatami et al. [63] inferred that this might be due to the increased exposure of oil to supercritical CO2 caused by the release of the substance from the compressed matter. These results also demonstrate that supercritical extraction can reduce operational time and costs while increasing yields if combined with other extraction procedures such as those including solvents like acetone or ethanol. All these factors proved that supercritical fluid extraction might be a promising alternative for a greener future. Nevertheless, supercritical fluid extraction machinery usually represents a high capital cost, regardless of the economic viability in the long run. For example, according to De Aguilar [62], Supercritical Fluid Extraction (SFE) units’ nominal prices ranged between 530,000 to 2,600,000 USD in 2017. Further, while temperature extraction conditions are amiable, pressure operating parameters still need to be decreased for general industrial implementation. While previous studies have been able to optimize industrial relevant conditions for supercritical extraction of lipids from Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina [65], optimal pressure conditions can range between 30 MPa and 50 MPa in some cases [59], thus representing an area of opportunity for further optimization of the standardized application. Polyunsaturated fatty acids like ω-3 and ω-6 types have been successfully extracted by SFE using CO2 and n-butane. Feller et al. [66] found a significant relationship between the content of carotenoids and the respective antioxidant activity. They used Phaeodactylum tricornutum, Nannochloropsis oculata, and Porphyridium cruentum strains and attributed the antioxidant activity of the marine microalgae to the carotenoid compounds [66]. In the case of subcritical n-butane, the procedure is the same as for the supercritical system. The difference relays in the control conditions using n-butane at 15 bar, 40 °C and solvent flow rate of 3 mL min−1 [66].
3.3. Ultrasound-Based Extraction
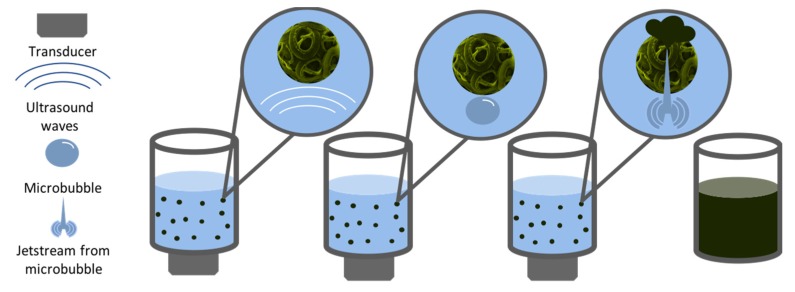
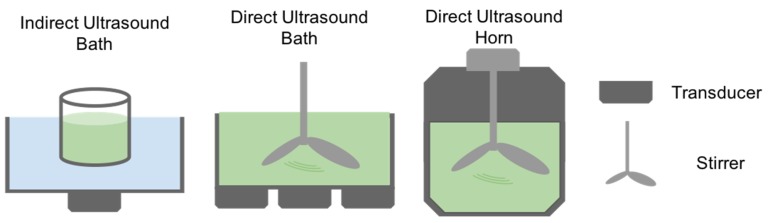
The technique of Ultrasound Extraction uses high-frequency sound waves to disrupt algal cell walls leading to the subsequent release of the compound of interest. As shown in Figure 4, the process depends on a physical phenomenon called cavitation where the disruption of the solvent caused by the sound waves, creates small bubbles. The bubbles generate strong jet streams as they implode by the effect of the acoustic cavitation force. When the bubble is close to a cell, allow for the puncturing of the cell wall and membrane [67]. The particular solvent used for this extraction method depends on the physical characteristics of the target compound, and subsequent fractionations are needed to isolate the compound of interest. Ultrasound extraction may occur directly as well as indirectly. Indirect ultrasound consists of placing a transducer touching the outer surface of a water bath. In the case of direct ultrasound extraction, the transducer can be close to the container where the bath occurs or in the form of an ultrasound horn in the sample (Figure 5). The advantages of this method include faster and substantial yields than other conventional techniques and moderate to low costs, in addition to minimal toxicity [68]. According to Kledjus et al. [69], it also allows for a more efficient extraction in freshwater algae species. On the other hand, the technique might negatively affect the quality of the oils as well as the integrity of polyunsaturated fatty acid rich oils. Furthermore, it cannot be scaled up [67].
Figure 4.
Schematic representation of the steps involved in the ultrasound-assisted extraction method.
Figure 5.
Different methods involved in ultrasound assisted extraction.
4. Current Applications
Cyanobacterial have already shown potential applications in biotechnology, biomedicine, food, biofuel, fertilizers, pigments, waste treatment, among others. The production (general process scheme is presented in Figure 6) of various secondary metabolites includes toxins, antioxidants, vitamins, bio-adsorbents, enzymes, and pharmaceuticals. Considering cyanobacterial pluripotential biotechnological uses, an overview is presented. First, a list of microalgae strains found in Mexico and the bioactive compounds produced are presented in Table 3, followed by the description of the current applications of relevant works with the mentioned microalgae.
Figure 6.
Scheme of the biotechnological process to produce bio-compounds from microalgae biomass.
Table 3.
Summary of existing high-value products from Mexican microalgae species.
| Microalgae | Bioactive Compounds | Biological Activity | References |
|---|---|---|---|
| Oscillatoriaceae sp. | Malyngolide | Antibacterial | [70] |
| Lyngbyatoxins | PKC activator | ||
| Debromoaplysiatoxin | Inflammatory | ||
| Lyngbya majuscula | Curacin A | Microtubulin assembly inhibitors | [71] |
| Kalkitoxin | Sodium channel blocker | ||
| Cyclic polypeptide | Anti-HIV activity | ||
| Oscillatoria raoi | Acetylated sulfoglycolipids | Antiviral | [72] |
| Spirulina platensis | Spirulan | Antiviral | [73] |
| Nostocaceae sp. | Nostocyclamide | Antifungal | [74] |
| Chroococcaceae sp. | Kawaguchipeptin B | Antibacterial | [75] |
| Mycrosistis aeuregonosa | |||
| Scenedesmus sp. | Lutein | Anti-oxidant | [76,77,78,79,80] |
| Spirulina (Arthrospira) | γ-Linolenic acid (GLA) | The integrity of tissues, delay of aging | [81] |
| Spirulina (Arthrospira) | Phycocyanin | Antioxidant, anti-inflammatory | [15,82] |
| Tetracelmis suecica | α- tocopherol | Antioxidant | [15] |
| Chlorella sp. | Galactose, rhamnose, mannose, arabinose, N-acetyl glucosamide and N-acetyl galactosamine | Immune stimulatory activity | [83] |
| Spirulina platensis and Anabaena sp. | Proteins | [84,85,86] | |
| Anabaena sp. | Superoxide dismutase (SOD) |
Antioxidant, anti-inflammatory | [87,88,89,90] |
| Spirulina sp. | Vitamin C; vitamin K; vitamins , A and E; α-tocopherol |
Antioxidant; blood cell formation; blood clotting mechanism |
[15,91] |
| Chlorella sp. | Lutein, zeaxanthin, canthaxanthin |
Antioxidant | [15,92] |
| Lyngbya majuscula | Microlin- A | Immunosuppressive | [93] |
|
Chlorella sorokiniana and Scenedesmus spp. |
Mycosporine-like amino acids (MAA) | UV-screening agent; sunscreen | [94,95,96,97] |
| Chlorella sp. | α-carotene Astaxanthin | Lower risk of premature death | [98] |
| C. sorokiniana | β-carotene | Food colorant; antioxidant property; cancer preventive properties; prevent night blindness; prevent liver fibrosis |
[99,100] |
| Tretraselmis spp. | Zeaxanthin | Protect eye cells; antioxidant activity; neutralizing the free radicals |
[101,102] |
| Nitzschia spp. | Triglycerides and hydrocarbons | Biofuels | [95,103,104] |
| Tetraselmis spp. and T. suecica | Arachidonic acid (AA) Eicosapentaenoic acid (EPA) |
Nutritional supplements, aquaculture feeds | [105,106] |
| T. suecica | Sterols | Antidiabetic; anticancer; anti- inflammatory; anti-photoaging; anti-obesity; anti-inflammatory; antioxidant activities |
[107,108] |
| Chlorella spp. and C. sorokiniana | Vitamin B Vitamin C |
Decrease fatigue; reducing depression; protect against heart disease; protect the skin; anticancer activity Protect against cardiovascular disease; prenatal health problems; prevent from the eye disease; protect against skin wrinkling |
[85,99,109,110,111,112] |
| C. sorokiniana and T. suecica | Vitamin E | Protect against toxic pollutants; Premenstrual syndrome protects against eye disorders; anti- Alzheimer’s disease; anti- diabetic properties |
[85,98,111,113] |
4.1. Pigments - Phycobilins, Lutein, and Carotenoids
Phycobilins are produced only by algae such as Spirulina [114]. Phycobilins are photosynthetic pigments bonded to water-soluble proteins, building the so-called phycobiliproteins. Phycocyanin, phycoerythrin, and allophycocyanin are water-soluble and have a wide range of applications including food and cosmetic colors, as fluorescent tags for use in flow cytometry and immunology [52]. Other possible applications are as antioxidants in cosmetics, a component of functional foods, and photosensitizers in photodynamic cancer therapy [52,115]. Phycobilins are found in the stroma of chloroplasts of cyanobacteria, rhodophytes (red algae), glaucophytes, and some cryptomonads [116]. They forward the energy of the harvested light to chlorophylls for photosynthesis. Similar to carotenoids, those proteins serve as “secondary light-harvesting pigments” [117]. Besides these highly sophisticated applications as chemical tags, phycobilins are also used as food and cosmetic colorants due to their high yield [118].
Lutein is one of the most important carotenoids. Moreover, it is essential to the macula lutea in the retina and lens of eyes. Lutein industrial applications are as a colorant in food products [119]. Cancer, retinal degeneration, and cardiovascular diseases are some of the health applications [119]. As listed before the applications, the commercial potential of lutein from microalgae is high, but its large-scale production has not yet started to our knowledge [120]. Nonetheless, the basis for lutein production outdoors at a pilot scale for Scenedesmus has already been set up [121]. Carotenoids properties, which were discussed by Gille et al., [122], make them outstanding as functional foods. One of the most known is ß-carotene for its nature in sustaining growth and vision. Additionally, other carotenoids have been used as important food colorants [123]. That is the case of astaxanthin, another representative of the xanthophyll group of carotenoid pigments for its properties as a powerful antioxidant. It is important for humans to protect the skin from UV light as UV-induced photo-oxidation, antibody production, and anti-tumor therapy [124].
4.2. Nutraceutical Potentialities
Aztecs in Mexico used Spirulina sp. as food [125]. The application of these microalgae as food or dietary supplement has continued and have resulted in the research and finding of new species to be used in food applications. Besides direct consumption, derived products are used in food industry as colorants, antioxidants, and natural preservatives. The following paragraphs describe the most representative used species and respective applications. More recently, microalgae were incorporated into pasta, snack foods, candy bars or gums, and beverages as well [85,126]. Owing to their diverse chemical properties, they can act as a nutritional supplement or represent a source of natural food colorants [126]. The most relevant strains to commercial applications are Chlorella, Spirulina, Scenedesmus, and Nostoc. The polysaccharides from type β-1,3-glucan are known for its properties of immune-stimulation, reduction of free radicals and blood lipids, this substance is produced by Chlorella [127]. In addition, glucan has also benefited the immune system, reduce depression, protect the skin, and has anticancer properties [83,85,109,110,111,112]. Finally, phycobiliproteins and chlorophylls can be found as a food additive produced by P. cruentum [128].
As mentioned before, Spirulina has been used as a supplement in human nutrition. It is worth mentioning as it produces linolenic acid, which humans cannot synthesize [126], proteins, β-carotene, thiamine, riboflavin, vitamin B12. Spirulina also showed to be active against hyperlipidemia, hypertension, oxidative stress, arthritis, and serum glucose levels [129]. One attempt to introduce Spirulina into diet was by including it into cookies; in this study, the antioxidant properties were explored [130]. The most common microalgae strains used in the industry are Chlorella and Spirulina thanks to their high protein content and respective aminoacid profile, nutritive value, and standardized growth protocols with high biomass yield [131]. Although Scenedesmus was considered a pioneer as a food source, recently its application has been limited, and some extracts have been used in desserts, fruit puddings, and soups [83]. Besides other nutrients, Scenedesmus produces eicosapentaenoic acid (EPA), vitamins, and essential minerals [132].
4.3. Bioactive Compounds
Microalga have become a research target since they are a rich source of bioactive compounds [133,134,135]. The activities of the secondary metabolites isolated from microalga include antibacterial, antifungal, antialgal, antiprotozoal, and antiviral (Table 3). For example, the cyanobacterium Phormidium sp. has been reported to inhibit the growth of different Gram-positive and Gram-negative bacterial strains, yeasts, and fungi [136]. Lyngbya majuscula [70] that produces polyketides, lipopeptides, cyclic peptides, and many others compounds that have activities such as anti-HIV, anticancer, antifeedant, antifungal, anti-inflammatory, antimicrobial, antiviral, etc. [71]. Other biological activities of these compounds include protein kinase C activators and tumor promoters, inhibitors of microtubulin assembly, antimicrobial and antifungal, and sodium-channel blockers [71]. Antifungal compounds include fisherellin A, hapalindole, carazostatin, phytoalexin, tolytoxin, scytophycin, toyocamycin, tjipanazole, nostocyclamide and nostodione produced by cyanobacteria belonging to Nostocales and Oscillatoriales (Table 3) [75].
It is also important to mention the production of long-chain polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA) are important for human diet [15]. Besides, PUFAs have been used to prevent and treat chronic inflammations diseases (e.g., rheumatism, skin diseases, and inflammation of the mucosa of the gastrointestinal tract) [70,71]. In addition, studies show a positive effect on cardio-circulatory diseases, coronary heart diseases, atherosclerosis, hypertension, cholesterol, and cancer treatment [83,137]. Arachidonic acid (ARA), an essential component of membrane phospholipids with a function of vasodilator, shows anti-inflammatory effects [138]. Moreover, ARA is necessary for the repair and growth of skeletal muscle tissue and makes it a powerful dietary component in support of the anabolic muscle formulations [139]. The inclusion of Spirulina in malnourished children has shown improvement against anemia by increasing hemoglobin, protein, and vitamin levels [140]. Additionally, phycocyanin, γ-linolenic acid, vitamins, phenolic compounds, and minerals can help with malnourished children [141]. The consumption of S. platensis and S. maxima showed an increase of lactic acid bacteria increase in the gastrointestinal tract [83].
4.4. Bioremediation Potentialities
The ability to metabolize or bio-transform chemicals is one of the many properties of microalgae. Some of the remarkable studies are shown in the following paragraphs, including treatment against petroleum, herbicides, wastewater, etc. The potential of using the microalgae as a tool and profit from it is huge. Cyanobacteria have shown great potential against surfactants and herbicides as well [142,143,144]. Radwan et al. [145] showed the degradation of petroleum by using a functionalized mat with microalgae, and fluometuron and lindane degradation were investigated by the group of Mansy and El-Bestway [146], showing promising results when using a wide variety of microalgae.
Wastewater treatment by microalgae for the reduction of different contaminants is another bioremediation potential. The case to reduce calcium and chloride by the use of Oscillatoria sp. was studied by Uma and Subramanian [147]. Nitrogen and phosphorous reduction in wastewater by microalgae production is a strategy by combining it with bioremediation of amino acids, enzyme, or food industry effluents. Chlorella, Spirulina, and Scenedesmus are some of the species most used in these systems to reduce the eutrophication in water bodies [148]. The strategy to use the exopolysaccharides (EPS) produced in high amounts by cyanobacteria and microalgae as emulsifiers are driving the researcher’s attention. It can be applied to oil, metal, and dye recovery. Further, Matsunaga et al., [149] used Anabaena sp. to remove dyes from textile effluent, and Phormidium autumnale was used to degrade indigo dye in 20 days [150].
In addition, exopolysaccharides produced by some cyanobacteria have the capacity of capturing heavy metals suspended in water. The proper function of the EPS needs high purity, which is achieved by ionic resins treatment [151]. Moreover, novel studies suggest green extractions as alternative methods to get high purity EPS using membranes, ultrasound, and microwave. The bio-adsorption process occurs when negative charges present in EPS interact with heavy metal ions producing bonds. Chelation of positively charged ions on the microalgal polysaccharides layer is due to a high crosslink number that promotes fast kinetics. In recent literature, it has been demonstrated that the chelation of zinc, copper, cadmium, lead, arsenic, chromium, and mercury for the potential applications for heavy metals removal currently known as biosorption [152,153].
4.5. Bio-Fuels
The use of microalgae to generate biofuels has huge potential due to its oil content, biomass, and hydrocarbons production. The use of microalgae to produce energy is wide, and the biological conversion method of fermentation to generate hydrogen, ethanol, biodiesel, and biogas are the most important [154]. Hydrogen is the most efficient and cleanest energy carrier and Chlamydomonas, Arthrospira, and Chlorella microalgae species possess all the characteristics to photo produce hydrogen gas (Khetkorn et al.) [155]. The increment of photobiological production of hydrogen is related to the carbon content in biomass [156,157,158]. Other strains of interest are Anabaena able to produce hydrogen [159]. Additionally, S. platensis can produce hydrogen in dark conditions with photobiological hydrogen production [157].
4.5.1. Photosynthetically Production of Hydrogen
Cyanobacteria can produce hydrogen in two different ways. First, grown under nitrogen limiting conditions, as a byproduct of nitrogen fixation when the species nitrogenase-containing heterocysts. Second, a reversible activity of hydrogenases enzymes [160]. The two microalgae species studied for hydrogen production were Anabaena and Scenedesmus. When Anabaena was placed into a glass jar it produced hydrogen gas, and after a period of dark anaerobic “adaptation”, Scenedesmus sp. produces hydrogen at low rates, greatly stimulated by short periods of light [161,162].
4.5.2. Biodiesel/Bioethanol
The substitute for conventional diesel is biodiesel, the result of transesterification of lipids. The production of biodiesel is currently done by processing oily seeds from palm, castor bean, sunflower, corn, and cotton, among others [163,164,165]. As discussed before, microalgae are a huge producer of fatty acids that can be converted into biodiesel [59]. For instance, around 50% of Chlorella, Nannochloropsis, Dunaliella, Scenedesmus, and Scenedesmus composition are lipids. Microalgae biofuel has a high calorific value, low viscosity, and low-density properties turning it in a more suitable biofuel than lignocellulosic materials [166].
Among other benefits, the use of ethanol as combustible reduces levels of lead, sulfur, carbon monoxide, and particulates [167]. Normally, ethanol is produced from sugar from byproducts of sugar cane and corn through fermentation of biomass [168]. Microalgae have the fermentable potential substrate since they have high levels of carbon compounds, directly available or after a pre-treatment. Some fermentable microalgae, such as Chlorella sp., Oscillatoria sp., and S. platensis, have already been used to produce ethanol [169]. The ethanol should then be purified and used as efficient fuel and the CO2 can be recycled in the cultivation of more microalgae or use of residual biomass in the process of anaerobic digestion [170].
4.6. Antioxidants
Microalgae are rich in vitamins [85,112]. They can also accumulate vitamin E and fat-soluble phenols with antioxidant properties. Vitamin E has a wide range of applications. Some of its applications in medicine are to treat cancer, heart, eye, Alzheimer’s, Parkinson’s disease, and other medical conditions [171]. Harvested microalgae are used in the food industry as added preservatives, health-improving additives, and for photoprotection in skin creams [172].
Phycocyanin purified from cyanobacterium Synechococcus sp. R42DM showed antioxidant activity in vitro and in vivo. The cyanobacterium was isolated from a polluted industrial site in India [141]. The conditions showed stability in thermal and oxidative stress with Caenorhabditis elegans [141]. Geitlerinema sp. H8DM is another microalgae that produced a variation of phycocyanin [173].
4.7. Phycotoxins
One of the groups of microalgae responsible for producing phycotoxins are dinoflagellates that lead to harmful algal blooms and “red tides” [174]. Although, the same microalgae can produce a wide spectrum of secondary metabolites that may be applied to therapy as antitumor, antibiotic, antifungal, immunosuppressant, cytotoxic, and neurotoxic named as phycotoxins [175]. The other group is cyanobacteria. For example, Nostoc species are responsible for freshwater toxins with a potential pharmaceutical use such as borophycin used against human carcinoma, borophycin-8 as antibiotic, apratoxin A as a cytotoxin and anticancer, among another more than 30 metabolites [176,177]. Further, Anabaena produces bromoana-indolone, an antibiotic compound, along with balticidins and laxaphycins, antifungal metabolites against Candida albicans, Penicillium notatum, Saccharomyces cerevisiae, and Trichophyton mentagrophytes. The same species Anabaena produces sulfoglycolipids, antivirals that can inhibit the HIV-1 virus. Other antivirus families are lectins, e.g., cyanovirin-N which are produced by Nostoc sp. that prevents HI virus infections. It is effective against influenza A and B as well [178].
5. Opportunities for Improvement
Drug discovery through microalgae biotechnology is under-represented in the current pharmaceutical industry. Nevertheless, drug development by natural means like microalgae gives several advantages such as water solubility, membrane permeability, biodegradability, bioavailability, and biocompatibility [7]. Aquaculture feeds require a large volume of biomass for fish, mollusks, and crustaceans. However, accumulation of biomass can be difficult and expensive, but the inclusion of microalgal increases dietary value to feed with essential amino acids, fatty acids, high-quality protein, vitamins, micronutrients, and carotenoids [179]. Products from fisheries and aquaculture combined are supplying the world with 142 million tons of protein every year with a market value of 106 billion dollars calculated in 2008 [179].
Biofuels in the form of gas and liquid products are gaining impact by the world regulations of green economies. The use of microalgae to produce enzymes to be included in specific catalyze processes to generate combustibles. For instance, biodiesel is an opportunity with enzyme-chemistry [180]. More important is to address high-value products, as turbocine with more caloric power for future perspectives and include secondary products generated in the process to stack value and impact the current market. Carbon dioxide capture is another opportunity for microalgae. The utilization of microalgae cultures in industrial processes can capture harmful gas emissions [181]. The accumulation of carbon dioxide can be applied under specific conditions to match with proper algal strains in their mechanisms of photosynthesis, leading to a decrease in pollution and produce biomass [182].
The biopolymer field is a branch of chemistry and material science where the application of novel technologies are required to produce bioplastics. An urgent large-scale production of biodegradable materials for common use, like packaging and containers, is far from current technology. However, the application of biocompatible and biodegradable plastics with zero toxicity to mammal cells offers the initial impulse to study the production of chitosan, cellulose, polyhydroxyalkanoates, and other biopolymers by microalgae. A hot topic is the utilization of scaffolds to support cellular growth in prosthesis and patches to treat skin burns, and missing or destroyed bones [183]. Biosorption is a removal process of potentially toxic elements where adsorption, chelation, ion exchange, and surface precipitation may occur. Through microalgae, heavy metals can be removed from municipal and industrial wastewaters [153]. Its potential increases with the lack of fresh water and recently with the detection of emerging contaminants as pesticides and drug wastes from pharmacological industries [184].
6. Concluding Remarks and Future Perspectives
Discovery, isolation, and preservation of novel microalgae strains are evident as we showed in Table 1, where a large number of species were enumerated in recent years. Still, many more are waiting to be discovered in the vast fresh and marine water resources in Mexico. An opportunity to generate green solutions to local problems through science, innovation, technology development, and transfer becomes one of the most important objectives for the field of microalgae biotechnology applications. Investment in biodiversity has a relevant impact on preserving and studying the natural resources in the country. However, attractive applications for industry investment are imperative to have relevant participation in research. In addition, the involvement of industry, society, and the research community helps to protect Mexico’s biodiversity. A green economy means political strategies towards a low-carbon economy, resource efficiency, green investments, technological innovation and more recycling, green jobs, poverty eradication, and social inclusion. The whole idea points to a higher support for novel technologies that help in the mentioned point, especially towards the knowledge development of involving local ecosystems. Local and global problems list include waste management, water treatment and emerging pollutants with potential opportunities to provide innovative solutions. A close collaboration between society, industry, and research institutions will lead to the path for a sustainable development.
Acknowledgments
All authors are grateful to their representative institutes for providing literature facilities. The authors also appreciate the additional support from the Emerging Technologies Research Group of Tecnologico de Monterrey, Mexico. The first author (Juan Eduardo Sosa-Hernández) thankfully acknowledges a postdoctoral fellowship provided by Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico.
Author Contributions
Juan Eduardo Sosa-Hernández and Hafiz M. N. Iqbal conceptualized the overall layout and contents of the review. Kenya D. Romero-Castillo, Lizeth Parra-Arroyo, Mauricio A. Aguilar-Aguila-Isaías, and Isaac E. García-Reyes collected and analyzed the literature and compiled the initial draft. Lizeth Parra-Arroyo, Mauricio A. Aguilar-Aguila-Isaías, and Isaac E. García-Reyes designed the Figures. Juan Eduardo Sosa-Hernández and Kenya D. Romero-Castillo summarized the Tables. Ishtiaq Ahmed, and Roberto Parra-Saldivar pre-checked the collected literature and drafted the manuscript. Muhammad Bilal and Hafiz M. N. Iqbal made revisions and final editing of the final version. Hafiz M. N. Iqbal processed for publication. All the authors read and approved the final manuscript.
Funding
This research received no external funding. The APC (ID: marinedrugs-17-00174) was funded by MDPI, St. Alban-Anlage 66, 4052 Basel, Switzerland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Centella M.H., Arévalo-Gallegos A., Parra-Saldivar R., Iqbal H.M. Marine-derived bioactive compounds for value-added applications in bio-and non-bio sectors. J. Clean. Prod. 2017;168:1559–1565. doi: 10.1016/j.jclepro.2017.05.086. [DOI] [Google Scholar]
- 2.Posten C. In: Microalgae Biotechnology. Chen S.F., editor. Springer International Publishing; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 3.Kasting J.F., Siefert J.L. Life and the evolution of Earth’s atmosphere. Science. 2002;296:1066–1068. doi: 10.1126/science.1071184. [DOI] [PubMed] [Google Scholar]
- 4.Arbib Z., Ruiz J., Álvarez-Díaz P., Garrido-Perez C., Perales J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014;49:465–474. doi: 10.1016/j.watres.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Lei A.P., Hu Z.L., Wong Y.S., Tam N.F.Y. Removal of fluoranthene and pyrene by different microalgal species. Bioresour. Technol. 2007;98:273–280. doi: 10.1016/j.biortech.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Mennaa F.Z., Arbib Z., Perales J.A. Urban wastewater treatment by seven species of microalgae and an algal bloom: Biomass production, N and P removal kinetics and harvestability. Water Res. 2015;83:42–51. doi: 10.1016/j.watres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Olaizola M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003;20:459–466. doi: 10.1016/S1389-0344(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 8.Farrar W.V. Tecuitlatl; a glimpse of Aztec food technology. Nature. 1966;211:341–342. doi: 10.1038/211341a0. [DOI] [Google Scholar]
- 9.Burlew J.S. Algal Culture. From Laboratory to Pilot Plant. Volume 600 Carnegie Institution of Washington Publication; Washington, DC, USA: 1953. [Google Scholar]
- 10.Belay A., Gershwin M.E. Spirulina in Human Nutrition and Health. CRC Press; Boca Raton, FL, USA: 2007. Spirulina (Arthrospira) pp. 11–35. [Google Scholar]
- 11.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Amotz A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Volume 273 Blackwell Publishing Ltd.; Hoboken, NJ, USA: 2004. Industrial production of microalgal cell-mass and secondary products-major industrial species. [Google Scholar]
- 13.Lamers P.P., Janssen M., De Vos R.C., Bino R.J., Wijffels R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008;26:631–638. doi: 10.1016/j.tibtech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Jorquera O., Kiperstok A., Sales E.A., Embirucu M., Ghirardi M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010;101:1406–1413. doi: 10.1016/j.biortech.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 16.Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 17.Oliva-Martínez M.G., Godínez-Ortega J.L., Zuñiga-Ramos C.A. Biodiversidad del fitoplancton de aguas continentales en México. Rev. Mex. Biodivers. 2014;85:54–61. doi: 10.7550/rmb.32706. [DOI] [Google Scholar]
- 18.Muciño-Márquez R.E., Figueroa-Torres M.G., Aguirre-León A. Cianofitas de los sistemas fluvio-lagunares Pom-Atasta y Palizada del Este, adyacentes a la Laguna de Términos, Campeche, México. Polibotánica. 2015;39:49–78. doi: 10.18387/polibotanica.39.3. [DOI] [Google Scholar]
- 19.Jiménez-Valera S., del Pilar Sánchez-Saavedra M. Growth and fatty acid profiles of microalgae species isolated from the Baja California Peninsula, México. Lat. Am. J. Aquat. Res. 2016;44:689–702. doi: 10.3856/vol44-issue4-fulltext-4. [DOI] [Google Scholar]
- 20.Dávila-Camacho C.A., Galaviz-Villa I., Lango-Reynoso F., Castañeda-Chávez M.D.R., Quiroga-Brahms C., Montoya-Mendoza J. Cultivation of native fish in Mexico: Cases of success. Rev. Aquac. 2018 doi: 10.1111/raq.12259. [DOI] [Google Scholar]
- 21.Rodríguez-Palacio M.C., Crisóstomo-Vázquez L., Álvarez-Hernández S., Lozano-Ramírez C. Strains of toxic and harmful microalgae, from waste water, marine, brackish and fresh water. Food Addit. Contam. Part A. 2012;29:304–313. doi: 10.1080/19440049.2011.596164. [DOI] [PubMed] [Google Scholar]
- 22.Ciferri O., Tiboni O. The biochemistry and industrial potential of Spirulina. Annu. Rev. Microbiol. 1985;39:503–526. doi: 10.1146/annurev.mi.39.100185.002443. [DOI] [PubMed] [Google Scholar]
- 23.Pacheco-Vega J.M., Cadena-Roa M.A., Ascencio F., Rangel-Dávalos C., Rojas-Contreras M. Assessment of endemic microalgae as potential food for Artemia franciscana culture. Lat. Am. J. Aquat. Res. 2015;43:23–32. doi: 10.3856/vol43-issue1-fulltext-3. [DOI] [Google Scholar]
- 24.Ramírez-Moreno L., Olvera-Ramírez R. Uso tradicional y actual de Spirulina sp. (Arthrospira sp.) Interciencia. 2006;31:657–663. [Google Scholar]
- 25.Cea-Barcia G., Buitrón G., Moreno G., Kumar G. A cost-effective strategy for the bio-prospecting of mixed microalgae with high carbohydrate content: Diversity fluctuations in different growth media. Bioresour. Technol. 2014;163:370–373. doi: 10.1016/j.biortech.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 26.Alcocer J., Hammer U.T. Saline lake ecosystems of Mexico. Aquat. Ecosyst. Health Manag. 1998;1:291–315. doi: 10.1016/S1463-4988(98)00011-6. [DOI] [Google Scholar]
- 27.Toledo G., Bashan Y., Soeldner A. Cyanobacteria and black mangroves in Northwestern Mexico: Colonization, and diurnal and seasonal nitrogen fixation on aerial roots. Can. J. Microbiol. 1995;41:999–1011. doi: 10.1139/m95-139. [DOI] [Google Scholar]
- 28.Reyna-Martinez R., Gomez-Flores R., López-Chuken U., Quintanilla-Licea R., Caballero-Hernandez D., Rodríguez-Padilla C., Beltrán-Rocha J.C., Tamez-Guerra P. Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State, México. PeerJ. 2018;6:e4358. doi: 10.7717/peerj.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poot-Delgado C.A., Okolodkov Y.B., Aké-Castillo J.A. Potentially harmful cyanobacteria in oyster banks of Términos lagoon, southeastern Gulf of Mexico. Acta Biológica Colomb. 2018;23:51–58. doi: 10.15446/abc.v23n1.65809. [DOI] [Google Scholar]
- 30.León-Tejera H., Montejano G. Dermocarpella (Cyanoprokaryota/Cyanophyceae/Cyanobacteria) from the pacific coast of Mexico. Cryptogam. Algol. 2000;21:259–272. doi: 10.1016/S0181-1568(00)00118-5. [DOI] [Google Scholar]
- 31.Valadez C.F., Carmona J., Cantoral U.E. Algas de ambientes lóticos en el estado de Morelos, México. An. Inst. Biol. Ser. Bot. 1996;67:227–282. [Google Scholar]
- 32.Komolafe O., Orta S.B.V., Monje-Ramirez I., Noguez I.Y., Harvey A.P., Ledesma M.T.O. Biodiesel production from indigenous microalgae grown in wastewater. Bioresour. Technol. 2014;154:297–304. doi: 10.1016/j.biortech.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Toledo-Cervantes A., Morales M., Novelo E., Revah S. Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresour. Technol. 2013;130:652–658. doi: 10.1016/j.biortech.2012.12.081. [DOI] [PubMed] [Google Scholar]
- 34.Arzate-Cárdenas M.A., Olvera-Ramírez R., Martínez-Jerónimo F. Microcystis toxigenic strains in urban lakes: A case of study in Mexico City. Ecotoxicology. 2010;19:1157–1165. doi: 10.1007/s10646-010-0499-7. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez-Hernandez S., De Lara-Isassi G., Arreguin-Espinoza R., Arreguin B., Hernandez-Santoyo A., Rodriguez-Romero A. Isolation and partial characterization of giraffine, a lectin from the Mexican endemic alga Codium giraffa Silva. Bot. Mar. 1999;42:573–580. doi: 10.1515/BOT.1999.064. [DOI] [Google Scholar]
- 36.Servín-Garcidueñas L.E., Martínez-Romero E. Complete mitochondrial and plastid genomes of the green microalga Trebouxiophyceae sp. strain MX-AZ01 isolated from a highly acidic geothermal lake. Eukaryot. Cell. 2012;11:1417–1418. doi: 10.1128/EC.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pena-Pereira F., Kloskowski A., Namieśnik J. Perspectives on the replacement of harmful organic solvents in analytical methodologies: A framework toward the implementation of a generation of eco-friendly alternatives. Green Chem. 2015;17:3687–3705. doi: 10.1039/C5GC00611B. [DOI] [Google Scholar]
- 38.Sosa-Hernández J., Escobedo-Avellaneda Z., Iqbal H., Welti-Chanes J. State-of-the-Art Extraction Methodologies for Bioactive Compounds from Algal Biome to Meet Bio-Economy Challenges and Opportunities. Molecules. 2018;23:2953. doi: 10.3390/molecules23112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi W.Y., Lee H.Y. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. Int. J. Mol. Sci. 2018;19:220. doi: 10.3390/ijms19010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh Y., Mazumder A., Giri A., Mishra H.N. Optimization of Ultrasound-Assisted Extraction of β-Carotene from Chlorella Biomass (MCC7) and its Use in Fortification of Apple Jam. J. Food Process Eng. 2017;40:e12321. doi: 10.1111/jfpe.12321. [DOI] [Google Scholar]
- 41.da Silva M.F., Casazza A.A., Ferrari P.F., Aliakbarian B., Converti A., Bezerra R.P., Porto A.L.F., Perego P. Recovery of phenolic compounds of food concern from Arthrospira platensis by green extraction techniques. Algal Res. 2017;25:391–401. doi: 10.1016/j.algal.2017.05.027. [DOI] [Google Scholar]
- 42.Guldhe A., Singh B., Rawat I., Bux F. Synthesis of biodiesel from Scenedesmus sp. by microwave and ultrasound assisted in situ transesterification using tungstated zirconia as a solid acid catalyst. Chem. Eng. Res. Des. 2014;92:1503–1511. doi: 10.1016/j.cherd.2014.05.012. [DOI] [Google Scholar]
- 43.Lorenzen J., Igl N., Tippelt M., Stege A., Qoura F., Sohling U., Brück T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst. Eng. 2017;40:911–918. doi: 10.1007/s00449-017-1755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crampon C., Nikitine C., Zaier M., Lépine O., Tanzi C.D., Vian M.A., Chemat F., Badens E. Oil extraction from enriched Spirulina platensis microalgae using supercritical carbon dioxide. J. Supercrit. Fluids. 2017;119:289–296. doi: 10.1016/j.supflu.2016.10.006. [DOI] [Google Scholar]
- 45.He B., Wang Y., Dou X., Chen Y.F. Supercritical CO2 extraction of docosahexaenoic acid from Schizochytrium limacinum using vegetable oils as entrainer. Algal Res. 2017;21:58–63. doi: 10.1016/j.algal.2016.10.007. [DOI] [Google Scholar]
- 46.Albarelli J.Q., Santos D.T., Ensinas A.V., Marechal F., Cocero M.J., Meireles M.A.A. Comparison of extraction techniques for product diversification in a supercritical water gasification-based sugarcane-wet microalgae biorefinery: Thermoeconomic and environmental analysis. J. Clean. Prod. 2018;201:697–705. doi: 10.1016/j.jclepro.2018.08.137. [DOI] [Google Scholar]
- 47.Araujo G.S., Matos L.J., Fernandes J.O., Cartaxo S.J., Gonçalves L.R., Fernandes F.A., Farias W.R. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013;20:95–98. doi: 10.1016/j.ultsonch.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 48.Ma Q.Y., Fang M., Zheng J.H., Ren D.F., Lu J. Optimised extraction of β-carotene from Spirulina platensis and hypoglycaemic effect in streptozotocin-induced diabetic mice. J. Sci. Food Agric. 2016;96:1783–1789. doi: 10.1002/jsfa.7286. [DOI] [PubMed] [Google Scholar]
- 49.Esquivel-Hernández D.A., Ibarra-Garza I.P., Rodríguez-Rodríguez J., Cuéllar-Bermúdez S.P., Rostro-Alanis M.D.J., Alemán-Nava G.S., García-Pérez J.S., Parra-Saldívar R. Green extraction technologies for high-value metabolites from algae: A review. Biofuels Bioprod. Biorefining. 2017;11:215–231. doi: 10.1002/bbb.1735. [DOI] [Google Scholar]
- 50.Ma Y.A., Cheng Y.M., Huang J.W., Jen J.F., Huang Y.S., Yu C.C. Effects of ultrasonic and microwave pretreatments on lipid extraction of microalgae. Bioprocess Biosyst. Eng. 2014;37:1543–1549. doi: 10.1007/s00449-014-1126-4. [DOI] [PubMed] [Google Scholar]
- 51.Zinnai A., Sanmartin C., Taglieri I., Andrich G., Venturi F. Supercritical fluid extraction from microalgae with high content of LC-PUFAs. A case of study: Sc-CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids. 2016;116:126–131. doi: 10.1016/j.supflu.2016.05.011. [DOI] [Google Scholar]
- 52.Tavanandi H.A., Mittal R., Chandrasekhar J., Raghavarao K.S.M.S. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018;31:239–251. doi: 10.1016/j.algal.2018.02.008. [DOI] [Google Scholar]
- 53.Obeid S., Beaufils N., Camy S., Takache H., Ismail A., Pontalier P.Y. Supercritical carbon dioxide extraction and fractionation of lipids from freeze-dried microalgae Nannochloropsis oculata and Chlorella vulgaris. Algal Res. 2018;34:49–56. doi: 10.1016/j.algal.2018.07.003. [DOI] [Google Scholar]
- 54.Kong W., Liu N., Zhang J., Yang Q., Hua S., Song H., Xia C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014;51:2006–2013. doi: 10.1007/s13197-012-0706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ido A.L., de Luna M.D.G., Capareda S.C., Maglinao A.L., Jr., Nam H. Application of central composite design in the optimization of lipid yield from Scenedesmus obliquus microalgae by ultrasonic-assisted solvent extraction. Energy. 2018;157:949–956. doi: 10.1016/j.energy.2018.04.171. [DOI] [Google Scholar]
- 56.Balasubramanian S., Allen J.D., Kanitkar A., Boldor D. Oil extraction from Scenedesmus obliquus using a continuous microwave system–design, optimization, and quality characterization. Bioresour. Technol. 2011;102:3396–3403. doi: 10.1016/j.biortech.2010.09.119. [DOI] [PubMed] [Google Scholar]
- 57.Pasquet V., Chérouvrier J.R., Farhat F., Thiéry V., Piot J.M., Bérard J.B., Kaas R., Serive B., Patrice T., Cadoret J.P., et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011;46:59–67. doi: 10.1016/j.procbio.2010.07.009. [DOI] [Google Scholar]
- 58.Vinatoru M., Mason T.J., Calinescu I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017;97:159–178. doi: 10.1016/j.trac.2017.09.002. [DOI] [Google Scholar]
- 59.Harris J., Viner K., Champagne P., Jessop P.G. Advances in microalgal lipid extraction for biofuel production: A review. Biofuels Bioprod. Biorefining. 2018;12:1118–1135. doi: 10.1002/bbb.1923. [DOI] [Google Scholar]
- 60.Kumar S.J., Kumar G.V., Dash A., Scholz P., Banerjee R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017;21:138–147. doi: 10.1016/j.algal.2016.11.014. [DOI] [Google Scholar]
- 61.Mendes R.L., Nobre B.P., Cardoso M.T., Pereira A.P., Palavra A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta. 2003;356:328–334. doi: 10.1016/S0020-1693(03)00363-3. [DOI] [Google Scholar]
- 62.de Aguiar A.C., Osorio-Tobón J.F., Silva L.P.S., Barbero G.F., Martínez J. Economic analysis of oleoresin production from malagueta peppers (Capsicum frutescens) by supercritical fluid extraction. J. Supercrit. Fluids. 2018;133:86–93. doi: 10.1016/j.supflu.2017.09.031. [DOI] [Google Scholar]
- 63.Hatami T., Johner J.C.F., Meireles M.A.A. Extraction and fractionation of fennel using supercritical fluid extraction assisted by cold pressing. Ind. Crop. Prod. 2018;123:661–666. doi: 10.1016/j.indcrop.2018.07.041. [DOI] [Google Scholar]
- 64.Johner J.C., Hatami T., Meireles M.A.A. Developing a supercritical fluid extraction method assisted by cold pressing for extraction of pequi (Caryocar brasiliense) J. Supercrit. Fluids. 2018;137:34–39. doi: 10.1016/j.supflu.2018.03.005. [DOI] [Google Scholar]
- 65.Solana M., Rizza C.S., Bertucco A. Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: Comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J. Supercrit. Fluids. 2014;92:311–318. doi: 10.1016/j.supflu.2014.06.013. [DOI] [Google Scholar]
- 66.Feller R., Matos Â.P., Mazzutti S., Moecke E.H., Tres M.V., Derner R.B., Oliveira J.V., Junior A.F. Polyunsaturated ω-3 and ω-6 fatty acids, total carotenoids and antioxidant activity of three marine microalgae extracts obtained by supercritical CO 2 and subcritical n-butane. J. Supercrit. Fluids. 2018;133:437–443. doi: 10.1016/j.supflu.2017.11.015. [DOI] [Google Scholar]
- 67.Wei F., Gao G.Z., Wang X.F., Dong X.Y., Li P.P., Hua W., Wang X., Wu X.M., Chen H. Quantitative determination of oil content in small quantity of oilseed rape by ultrasound-assisted extraction combined with gas chromatography. Ultrason. Sonochem. 2008;15:938–942. doi: 10.1016/j.ultsonch.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Mercer P., Armenta R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011;113:539–547. doi: 10.1002/ejlt.201000455. [DOI] [Google Scholar]
- 69.Klejdus B., Lojková L., Plaza M., Šnóblová M., Štěrbová D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A. 2010;1217:7956–7965. doi: 10.1016/j.chroma.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Burja A.M., Banaigs B., Abou-Mansour E., Burgess J.G., Wright P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron. 2001;57:9347–9377. doi: 10.1016/S0040-4020(01)00931-0. [DOI] [Google Scholar]
- 71.Shimizu Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003;6:236–243. doi: 10.1016/S1369-5274(03)00064-X. [DOI] [PubMed] [Google Scholar]
- 72.Reshef V., Mizrachi E., Maretzki T., Silberstein C., Loya S., Hizi A., Carmeli S. New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 1997;60:1251–1260. doi: 10.1021/np970327m. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi K., Hayashi T., Kojima I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir. 1996;12:1463–1471. doi: 10.1089/aid.1996.12.1463. [DOI] [PubMed] [Google Scholar]
- 74.Moore R.E., Patterson G.M., Carmichael W.W. New pharmaceuticals from cultured blue-green algae. Biomed. Importance Mar. Org. 1988;13:143–150. [Google Scholar]
- 75.Dahms H.-U., Xu Y., Pfeiffer C. Antifouling potential of cyanobacteria: A mini-review. Biofouling. 2006;22:317–327. doi: 10.1080/08927010600967261. [DOI] [PubMed] [Google Scholar]
- 76.Piccaglia R., Marotti M., Grandi S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crop. Prod. 1998;8:45–51. doi: 10.1016/S0926-6690(97)10005-X. [DOI] [Google Scholar]
- 77.Blanco A.M., Moreno J., Del Campo J.A., Rivas J., Guerrero M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007;73:1259–1266. doi: 10.1007/s00253-006-0598-9. [DOI] [PubMed] [Google Scholar]
- 78.Choudhari S.M., Ananthanarayan L., Singhal R.S. Use of metabolic stimulators and inhibitors for enhanced production of β-carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Bioresour. Technol. 2008;99:3166–3173. doi: 10.1016/j.biortech.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 79.Sánchez J.F., Fernández-Sevilla J.M., Acién F.G., Cerón M.C., Pérez-Parra J., Molina-Grima E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008;79:719–729. doi: 10.1007/s00253-008-1494-2. [DOI] [PubMed] [Google Scholar]
- 80.Fernández-Sevilla J.M., Fernández F.A., Grima E.M. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010;86:27–40. doi: 10.1007/s00253-009-2420-y. [DOI] [PubMed] [Google Scholar]
- 81.Cohen Z. Production of polyunsaturated fatty acids (EPA, ARA, and GLA) by the microalgae Porphyridium and Spirulina. Ind. Appl. Single Cell Oils. 1992:243–273. [Google Scholar]
- 82.Mao T.K., Water J.V.D., Gershwin M.E. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J. Med. Food. 2005;8:27–30. doi: 10.1089/jmf.2005.8.27. [DOI] [PubMed] [Google Scholar]
- 83.Barrow C., Shahidi F., editors. Marine Nutraceuticals and Functional Foods. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 84.Becker E.W. Microalgae: Biotechnology and Microbiology. Volume 10 Cambridge University Press; Cambridge, UK: 1994. [Google Scholar]
- 85.Becker W. Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Volume 312 Blackwell Publishing Ltd.; Hoboken, NJ, USA: 2004. Microalgae in Human and Animal Nutrition. [Google Scholar]
- 86.Rasmussen R.S., Morrissey M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007;52:237–292. doi: 10.1016/S1043-4526(06)52005-4. [DOI] [PubMed] [Google Scholar]
- 87.García-González A., Ochoa J.L. Anti-inflammatory activity of Debaryomyces hansenii Cu, Zn-SOD. Arch. Med. Res. 1999;30:69–73. doi: 10.1016/S0188-0128(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 88.Guzmán-Murillo M.A., López-Bolaños C.C., Ledesma-Verdejo T., Roldan-Libenson G., Cadena-Roa M.A., Ascencio F. Effects of fertilizer-based culture media on the production of exocellular polysaccharides and cellular superoxide dismutase by Phaeodactylum tricornutum (Bohlin) J. Appl. Phycol. 2007;19:33–41. doi: 10.1007/s10811-006-9108-9. [DOI] [Google Scholar]
- 89.Priya B., Premanandh J., Dhanalakshmi R.T., Seethalakshmi T., Uma L., Prabaharan D., Subramanian G. Comparative analysis of cyanobacterial superoxide dismutases to discriminate canonical forms. BMC Genom. 2007;8:435. doi: 10.1186/1471-2164-8-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thepenier C., Chaumont D., Gudin C. Mass culture of Porphyridium cruentum: A multiproduct stategy for the biomass valorisation. In: Stadler T., editor. Algal Biotechnology. Elsevier Applied Science Publishers; London, UK: 1988. pp. 413–420. [Google Scholar]
- 91.Antia N.J., Desai I.D., Romilly M.J. The tocopherol, vitamin K, and related isoprenoid quinone composition of a unicellular red alga (Porphyridium cruentum) J. Phycol. 1970;6:305–312. [Google Scholar]
- 92.Shi X.M., Chen F. Algae and Their Biotechnological Potential. Springer; Dordrecht, The Netherlands: 2001. High yield production of lutein by heterotrophic Chlorella protothecoides in fed-batch systems; pp. 107–119. [Google Scholar]
- 93.Arya V., Gupta V.K. A review on marine immunomodulators. Int. J. Pharm. Life Sci. 2011;2:751–758. [Google Scholar]
- 94.Xiong F., Kopecky J., Nedbal L. The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta) Aquat. Bot. 1999;63:37–49. doi: 10.1016/S0304-3770(98)00106-5. [DOI] [Google Scholar]
- 95.Chu W.L. Biotechnological applications of microalgae. IeJSME. 2012;6:S24–S37. [Google Scholar]
- 96.Duval B., Shetty K., Thomas W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 1999;11:559. doi: 10.1023/A:1008178208949. [DOI] [Google Scholar]
- 97.Karsten U., Lembcke S., Schumann R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta. 2007;225:991–1000. doi: 10.1007/s00425-006-0406-x. [DOI] [PubMed] [Google Scholar]
- 98.Matsukawa R., Hotta M., Masuda Y., Chihara M., Karube I. Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J. Appl. Phycol. 2000;12:263–267. doi: 10.1023/A:1008141414115. [DOI] [Google Scholar]
- 99.Barbosa M.J., Zijffers J.W., Nisworo A., Vaes W., van Schoonhoven J., Wijffels R.H. Optimization of biomass, vitamins, and carotenoid yield on light energy in a flat-panel reactor using the A-stat technique. Biotechnol. Bioeng. 2005;89:233–242. doi: 10.1002/bit.20346. [DOI] [PubMed] [Google Scholar]
- 100.Egeland E.S., Guillard R.R., Liaaen-Jensen S. Additional carotenoid prototype representatives and a general chemosystematic evaluation of carotenoids in Prasinophyceae (Chlorophyta) Phytochemistry. 1997;44:1087–1097. doi: 10.1016/S0031-9422(96)00650-4. [DOI] [Google Scholar]
- 101.Cha K.H., Koo S.Y., Lee D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008;56:10521–10526. doi: 10.1021/jf802111x. [DOI] [PubMed] [Google Scholar]
- 102.Leya T., Rahn A., Lütz C., Remias D. Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol. Ecol. 2009;67:432–443. doi: 10.1111/j.1574-6941.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 103.Ramaraj S., Hemaiswarya S., Raja R., Ganesan V., Anbazhagan C., Carvalho I.S., Juntawong N. Environmental Sustainability. Springer; New Delhi, India: 2015. Microalgae as an attractive source for biofuel production; pp. 129–157. [Google Scholar]
- 104.Mata T.M., Martins A.A., Caetano N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010;14:217–232. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- 105.Reitan K.I., Rainuzzo J.R., Olsen Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae 1. J. Phycol. 1994;30:972–979. doi: 10.1111/j.0022-3646.1994.00972.x. [DOI] [Google Scholar]
- 106.Cheng-Wu Z., Cohen Z., Khozin-Goldberg I., Richmond A. Characterization of growth and arachidonic acid production of Parietochloris incisa comb. nov (Trebouxiophyceae, Chlorophyta) J. Appl. Phycol. 2002;14:453–460. doi: 10.1023/A:1022375110556. [DOI] [Google Scholar]
- 107.Ponomarenko L.P., Stonik I.V., Aizdaicher N.A., Orlova T.Y., Popovskaya G.I., Pomazkina G.V., Stonik V.A. Sterols of marine microalgae Pyramimonas cf. cordata (Prasinophyta), Attheya ussurensis sp. nov.(Bacillariophyta) and a spring diatom bloom from Lake Baikal. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004;138:65–70. doi: 10.1016/j.cbpc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 108.Cardozo K.H., Guaratini T., Barros M.P., Falcão V.R., Tonon A.P., Lopes N.P., Campos S., Torres M.A., Souza A.O., Colepicolo P., et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Vilchez C., Garbayo I., Lobato M.V., Vega J. Microalgae-mediated chemicals production and wastes removal. Enzym. Microb. Technol. 1997;20:562–572. doi: 10.1016/S0141-0229(96)00208-6. [DOI] [Google Scholar]
- 110.Uhlik D.J., Gowans C.S. Synthesis of nicotinic acid in Chlamydomonas eugametos. Int. J. Biochem. 1974;5:79–84. doi: 10.1016/0020-711X(74)90047-0. [DOI] [Google Scholar]
- 111.Borowitzka M.A. Progress in Industrial Microbiology. Volume 35. Elsevier; Amsterdam, The Netherlands: 1999. Commercial production of microalgae: Ponds, tanks, and fermenters; pp. 313–321. [Google Scholar]
- 112.Running J.A., Severson D.K., Schneider K.J. Extracellular production of L-ascorbic acid by Chlorella protothecoides, Prototheca species, and mutants of P. moriformis during aerobic culturing at low pH. J. Ind. Microbiol. Biotechnol. 2002;29:93–98. doi: 10.1038/sj.jim.7000275. [DOI] [PubMed] [Google Scholar]
- 113.Carballo-Cárdenas E.C., Tuan P.M., Janssen M., Wijffels R.H. Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol. Eng. 2003;20:139–147. doi: 10.1016/S1389-0344(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 114.Chandra R., Parra R., MN Iqbal H. Phycobiliproteins: A Novel Green Tool from Marine Origin Blue-Green Algae and Red Algae. Protein Pept. Lett. 2017;24:118–125. doi: 10.2174/0929866523666160802160222. [DOI] [PubMed] [Google Scholar]
- 115.Du S.W., Zhang L.K., Han K., Chen S., Hu Z., Chen W., Hu K., Yin L., Wu B., Guan Y.Q. Combined Phycocyanin and Hematoporphyrin Monomethyl Ether for Breast Cancer Treatment via Photosensitizers Modified Fe3O4 Nanoparticles Inhibiting the Proliferation and Migration of MCF-7 Cells. Biomacromolecules. 2017;19:31–41. doi: 10.1021/acs.biomac.7b01197. [DOI] [PubMed] [Google Scholar]
- 116.Koller M., Muhr A., Braunegg G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014;6:52–63. doi: 10.1016/j.algal.2014.09.002. [DOI] [Google Scholar]
- 117.Parmar A., Singh N.K., Kaushal A., Sonawala S., Madamwar D. Purification, characterization and comparison of phycoerythrins from three different marine cyanobacterial cultures. Bioresour. Technol. 2011;102:1795–1802. doi: 10.1016/j.biortech.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 118.Yu P., Wu Y., Wang G., Jia T., Zhang Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. Nutr. 2017;57:3840–3849. doi: 10.1080/10408398.2016.1167668. [DOI] [PubMed] [Google Scholar]
- 119.Luengo E., Martínez J.M., Bordetas A., Álvarez I., Raso J. Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015;29:15–22. doi: 10.1016/j.ifset.2015.02.012. [DOI] [Google Scholar]
- 120.Lin J.H., Lee D.J., Chang J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015;184:421–428. doi: 10.1016/j.biortech.2014.09.099. [DOI] [PubMed] [Google Scholar]
- 121.Del Campo J.A., García-González M., Guerrero M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007;74:1163–1174. doi: 10.1007/s00253-007-0844-9. [DOI] [PubMed] [Google Scholar]
- 122.Gille A., Neumann U., Louis S., Bischoff S.C., Briviba K. Microalgae as a potential source of carotenoids: Comparative results of an in vitro digestion method and a feeding experiment with C57BL/6J mice. J. Funct. Foods. 2018;49:285–294. doi: 10.1016/j.jff.2018.08.039. [DOI] [Google Scholar]
- 123.Rodriguez-Amaya D.B. Update on natural food pigments-A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2018 doi: 10.1016/j.foodres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 124.Chue K.T., Ten L.N., Oh Y.K., Woo S.G., Lee M., Yoo S.A. Carotinoid compositions of five microalga species. Chem. Nat. Compd. 2012;48:141–142. doi: 10.1007/s10600-012-0183-7. [DOI] [Google Scholar]
- 125.García J.L., de Vicente M., Galán B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017;10:1017–1024. doi: 10.1111/1751-7915.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soni R.A., Sudhakar K., Rana R.S. Spirulina–From growth to nutritional product: A review. Trends Food Sci. Technol. 2017;69:157–171. doi: 10.1016/j.tifs.2017.09.010. [DOI] [Google Scholar]
- 127.Matos J., Cardoso C., Bandarra N.M., Afonso C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017;8:2672–2685. doi: 10.1039/C7FO00409E. [DOI] [PubMed] [Google Scholar]
- 128.Fuentes M.R., Fernández G.A., Pérez J.S., Guerrero J.G. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000;70:345–353. doi: 10.1016/S0308-8146(00)00101-1. [DOI] [Google Scholar]
- 129.Ovando C.A., Carvalho J.C.D., Vinícius de Melo Pereira G., Jacques P., Soccol V.T., Soccol C.R. Functional properties and health benefits of bioactive peptides derived from Spirulina: A review. Food Rev. Int. 2018;34:34–51. doi: 10.1080/87559129.2016.1210632. [DOI] [Google Scholar]
- 130.Batista A.P., Niccolai A., Fradinho P., Fragoso S., Bursic I., Rodolfi L., Biondi N., Tredici M.R., Sousa I., Raymundo A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017;26:161–171. doi: 10.1016/j.algal.2017.07.017. [DOI] [Google Scholar]
- 131.Ventura S.P.M., Nobre B.P., Ertekin F., Hayes M., Garciá-Vaquero M., Vieira F., Koc M., Gouveia L., Aires-Barros M.R., Palavra A.M.F. Microalgae-Based Biofuels and Bioproducts. Elsevier; Woodhead Publishing: 2017. Extraction of value-added compounds from microalgae; pp. 461–483. [Google Scholar]
- 132.Markovits A., Conejeros R., López L., Lutz M. Evaluation of marine microalga Nannochloropsis sp. as a potential dietary supplement. Chemical, nutritional and short term toxicological evaluation in rats. Nutr. Res. 1992;12:1273–1284. [Google Scholar]
- 133.Rania M.A., Hala M.T. Antibacterial and antifungal activity of Cyanobacteria and green Microalgae evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. Glob. J. Biotechnol. Biochem. 2008;3:22–31. [Google Scholar]
- 134.Ruiz-Ruiz F., Mancera-Andrade E.I., Iqbal H.M. Marine-derived bioactive peptides for biomedical sectors: A review. Protein Pept. Lett. 2017;24:109–117. doi: 10.2174/0929866523666160802155347. [DOI] [PubMed] [Google Scholar]
- 135.de Vera C., Díaz Crespín G., Hernández Daranas A., Montalvão Looga S., Lillsunde K.E., Tammela P., Perälä M., Hongisto V., Virtanen J., Rischer H., et al. Marine Microalgae: Promising Source for New Bioactive Compounds. Mar. Drugs. 2018;16:317. doi: 10.3390/md16090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bloor S., England R.R. Elucidation and optimization of the medium constituents controlling antibiotic production by the cyanobacterium Nostoc muscorum. Enzym. Microb. Technol. 1991;13:76–81. doi: 10.1016/0141-0229(91)90192-D. [DOI] [PubMed] [Google Scholar]
- 137.Galano J.M., Roy J., Durand T., Lee J.C.Y., Le Guennec J.Y., Oger C., Demion M. Biological activities of non-enzymatic oxygenated metabolites of polyunsaturated fatty acids (NEO-PUFAs) derived from EPA and DHA: New anti-arrhythmic compounds? Mol. Asp. Med. 2018;64:161–168. doi: 10.1016/j.mam.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 138.Abdulrazaq M., Innes J.K., Calder P.C. Effect of ω-3 polyunsaturated fatty acids on arthritic pain: A systematic review. Nutrition. 2017;39:57–66. doi: 10.1016/j.nut.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Markworth J.F., Mitchell C.J., D’Souza R.F., Aasen K.M., Durainayagam B.R., Mitchell S.M., Chan A.H., Sinclair A.J., Garg M., Cameron-Smith D. Arachidonic acid supplementation modulates blood and skeletal muscle lipid profile with no effect on basal inflammation in resistance exercise trained men. Prostaglandins Leukot. Essent. Fat. Acids. 2018;128:74–86. doi: 10.1016/j.plefa.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 140.Habib M.A.B. Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. Food and Agriculture Organization of the United Nations; Quebec, QC, Canada: 2008. [Google Scholar]
- 141.Sonani R.R., Patel S., Bhastana B., Jakharia K., Chaubey M.G., Singh N.K., Madamwar D. Purification and antioxidant activity of phycocyanin from Synechococcus sp. R42DM isolated from industrially polluted site. Bioresour. Technol. 2017;245:325–331. doi: 10.1016/j.biortech.2017.08.129. [DOI] [PubMed] [Google Scholar]
- 142.Yan G.A., Jiang J.W., Wu G., Yan X. Disappearance of linear alkylbenzene sulfonate from different cultures with Anabaena sp. HB 1017. Bull. Environ. Contam. Toxicol. 1998;60:329–334. doi: 10.1007/s001289900630. [DOI] [PubMed] [Google Scholar]
- 143.Radwan S.S., Al-Hasan R.H. The Ecology of Cyanobacteria. Springer; The Netherlands: 2000. Oil pollution and cyanobacteria; pp. 307–319. [Google Scholar]
- 144.Raghukumar C., Vipparty V., David J., Chandramohan D. Degradation of crude oil by marine cyanobacteria. Appl. Microbiol. Biotechnol. 2001;57:433–436. doi: 10.1007/s002530100784. [DOI] [PubMed] [Google Scholar]
- 145.Radwan S.S., Al-Hasan R.H., Salamah S., Al-Dabbous S. Bioremediation of oily sea water by bacteria immobilized in biofilms coating macroalgae. Int. Biodeterior. Biodegrad. 2002;50:55–59. doi: 10.1016/S0964-8305(02)00067-7. [DOI] [Google Scholar]
- 146.Mansy A.E.R., El-Bestawy E. Toxicity and biodegradation of fluometuron by selected cyanobacterial species. World J. Microbiol. Biotechnol. 2002;18:125–131. doi: 10.1023/A:1014490811121. [DOI] [Google Scholar]
- 147.Uma L., Subramanian G. Effective use of cyanobacteria in effluent treatment; Proceedings of the National Symposium Cyanobacerial Nitrogen Fixation; IARI, New Delhi, India. 1990. pp. 437–444. [Google Scholar]
- 148.Scherer M.D., de Oliveira A.C., Magalhães Filho F.J.C., Ugaya C.M.L., Mariano A.B., Vargas J.V.C. Environmental study of producing microalgal biomass and bioremediation of cattle manure effluents by microalgae cultivation. Clean Technol. Environ. Policy. 2017;19:1745–1759. doi: 10.1007/s10098-017-1361-x. [DOI] [Google Scholar]
- 149.Matsunaga T., Sudo H., Takemasa H., Wachi Y., Nakamura N. Sulfated extracellular polysaccharide production by the halophilic cyanobacterium Aphanocapsa halophytia immobilized on light-diffusing optical fibers. Appl. Microbiol. Biotechnol. 1996;45:24–27. doi: 10.1007/s002530050643. [DOI] [Google Scholar]
- 150.Dellamatrice P.M., Silva-Stenico M.E., de Moraes L.A.B., Fiore M.F., Monteiro R.T.R. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017;48:25–31. doi: 10.1016/j.bjm.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pierre G., Zhao J.M., Orvain F., Dupuy C., Klein G.L., Graber M., Maugard T. Seasonal dynamics of extracellular polymeric substances (EPS) in surface sediments of a diatom-dominated intertidal mudflat (Marennes–Oléron, France) J. Sea Res. 2014;92:26–35. doi: 10.1016/j.seares.2013.07.018. [DOI] [Google Scholar]
- 152.Bhunia B., Uday U.S.P., Oinam G., Mondal A., Bandyopadhyay T.K., Tiwari O.N. Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr. Polym. 2018;179:228–243. doi: 10.1016/j.carbpol.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 153.Bilal M., Rasheed T., Sosa-Hernández J., Raza A., Nabeel F., Iqbal H. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs. 2018;16:65. doi: 10.3390/md16020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shuba E.S., Kifle D. Microalgae to biofuels:‘Promising’alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018;81:743–755. doi: 10.1016/j.rser.2017.08.042. [DOI] [Google Scholar]
- 155.Khetkorn W., Rastogi R.P., Incharoensakdi A., Lindblad P., Madamwar D., Pandey A., Larroche C. Microalgal hydrogen production–A review. Bioresour. Technol. 2017;243:1194–1206. doi: 10.1016/j.biortech.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 156.Batyrova K., Hallenbeck P.C. Hydrogen production by a Chlamydomonas reinhardtii strain with inducible expression of photosystem II. Int. J. Mol. Sci. 2017;18:647. doi: 10.3390/ijms18030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ainas M., Hasnaoui S., Bouarab R., Abdi N., Drouiche N., Mameri N. Hydrogen production with the cyanobacterium Spirulina platensis. Int. J. Hydrog. Energy. 2017;42:4902–4907. doi: 10.1016/j.ijhydene.2016.12.056. [DOI] [Google Scholar]
- 158.Sengmee D., Cheirsilp B., Suksaroge T.T., Prasertsan P. Biophotolysis-based hydrogen and lipid production by oleaginous microalgae using crude glycerol as exogenous carbon source. Int. J. Hydrog. Energy. 2017;42:1970–1976. doi: 10.1016/j.ijhydene.2016.10.089. [DOI] [Google Scholar]
- 159.Vargas S.R., dos Santos P.V., Zaiat M., do Carmo Calijuri M. Optimization of biomass and hydrogen production by Anabaena sp. (UTEX 1448) in nitrogen-deprived cultures. Biomass Bioenergy. 2018;111:70–76. doi: 10.1016/j.biombioe.2018.01.022. [DOI] [Google Scholar]
- 160.Nagarajan D., Lee D.J., Kondo A., Chang J.S. Recent insights into biohydrogen production by microalgae–From biophotolysis to dark fermentation. Bioresour. Technol. 2017;227:373–387. doi: 10.1016/j.biortech.2016.12.104. [DOI] [PubMed] [Google Scholar]
- 161.Wünschiers R., Lindblad P. Hydrogen in education—A biological approach. Int. J. Hydrog. Energy. 2002;27:1131–1140. doi: 10.1016/S0360-3199(02)00098-8. [DOI] [Google Scholar]
- 162.Benemann J.R. Hydrogen production by microalgae. J. Appl. Phycol. 2000;12:291–300. doi: 10.1023/A:1008175112704. [DOI] [Google Scholar]
- 163.Milano J., Ong H.C., Masjuki H.H., Chong W.T., Lam M.K., Loh P.K., Vellayan V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016;58:180–197. doi: 10.1016/j.rser.2015.12.150. [DOI] [Google Scholar]
- 164.Park J.Y., Park M.S., Lee Y.C., Yang J.W. Advances in direct transesterification of algal oils from wet biomass. Bioresour. Technol. 2015;184:267–275. doi: 10.1016/j.biortech.2014.10.089. [DOI] [PubMed] [Google Scholar]
- 165.Lee S.Y., Cho J.M., Chang Y.K., Oh Y.K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017;244:1317–1328. doi: 10.1016/j.biortech.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 166.Miao X., Wu Q., Yang C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis. 2004;71:855–863. doi: 10.1016/j.jaap.2003.11.004. [DOI] [Google Scholar]
- 167.Willke T.H., Vorlop K.D. Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl. Microbiol. Biotechnol. 2004;66:131–142. doi: 10.1007/s00253-004-1733-0. [DOI] [PubMed] [Google Scholar]
- 168.Gaurav N., Sivasankari S., Kiran G.S., Ninawe A., Selvin J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017;73:205–214. doi: 10.1016/j.rser.2017.01.070. [DOI] [Google Scholar]
- 169.Rizza L.S., Smachetti M.E.S., Do Nascimento M., Salerno G.L., Curatti L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res. 2017;22:140–147. doi: 10.1016/j.algal.2016.12.021. [DOI] [Google Scholar]
- 170.Harun R., Danquah M.K., Forde G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010;85:199–203. doi: 10.1002/jctb.2287. [DOI] [Google Scholar]
- 171.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 172.Alberts D.S., Goldman R., Xu M.J., Dorr R.T., Quinn J., Welch K., Guillen-Rodriguez J., Aickin M., Peng Y.M., Loescher L., et al. Disposition and metabolism of topically administered α-tocopherol acetate: A common ingredient of commercially available sunscreens and cosmetics. Nutr. Cancer. 1996;26:193–201. doi: 10.1080/01635589609514475. [DOI] [PubMed] [Google Scholar]
- 173.Patel H.M., Rastogi R.P., Trivedi U., Madamwar D. Structural characterization and antioxidant potential of phycocyanin from the cyanobacterium Geitlerinema sp. H8DM. Algal Res. 2018;32:372–383. doi: 10.1016/j.algal.2018.04.024. [DOI] [Google Scholar]
- 174.Camacho F.G., Rodríguez J.G., Mirón A.S., García M.C., Belarbi E.H., Chisti Y., Grima E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007;25:176–194. doi: 10.1016/j.biotechadv.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 175.Sathasivam R., Radhakrishnan R., Hashem A., Abd_Allah E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2017 doi: 10.1016/j.sjbs.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vijayakumar S., Menakha M. Pharmaceutical applications of cyanobacteria—A review. J. Acute Med. 2015;5:15–23. doi: 10.1016/j.jacme.2015.02.004. [DOI] [Google Scholar]
- 177.Nowruzi B., Haghighat S., Fahimi H., Mohammadi E. Nostoc cyanobacteria species: A new and rich source of novel bioactive compounds with pharmaceutical potential. J. Pharm. Health Serv. Res. 2018;9:5–12. doi: 10.1111/jphs.12202. [DOI] [Google Scholar]
- 178.Niedermeyer T.H.J. Anti-infective natural products from Cyanobacteria. Planta Med. 2015;81:1309–1325. doi: 10.1055/s-0035-1546055. [DOI] [PubMed] [Google Scholar]
- 179.Yaakob Z., Ali E., Zainal A., Mohamad M., Takriff M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res.-Thessalon. 2014;21:6. doi: 10.1186/2241-5793-21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sandoval G., Casas-Godoy L., Bonet-Ragel K., Rodrigues J., Ferreira-Dias S., Valero F. Enzyme-Catalyzed Production of Biodiesel as Alternative to Chemical-Catalyzed Processes: Advantages and Constraints. Curr. Biochem. Eng. 2017;4:109–141. doi: 10.2174/2212711904666170615123640. [DOI] [Google Scholar]
- 181.Hosseini N.S., Shang H., Scott J.A. Biosequestration of industrial off-gas CO2 for enhanced lipid productivity in open microalgae cultivation systems. Renew. Sustain. Energy Rev. 2018;92:458–469. doi: 10.1016/j.rser.2018.04.086. [DOI] [Google Scholar]
- 182.Packer M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy. 2009;37:3428–3437. doi: 10.1016/j.enpol.2008.12.025. [DOI] [Google Scholar]
- 183.Kavitha G., Rengasamy R., Inbakandan D. Polyhydroxybutyrate production from marine source and its application. Int. J. Biol. Macromol. 2018;111:102–108. doi: 10.1016/j.ijbiomac.2017.12.155. [DOI] [PubMed] [Google Scholar]
- 184.Archer E., Petrie B., Kasprzyk-Hordern B., Wolfaardt G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere. 2017;174:437–446. doi: 10.1016/j.chemosphere.2017.01.101. [DOI] [PubMed] [Google Scholar]