Abstract
Adipose stem cells (ASCs) play an essential role in tumor microenvironments. These cells are altered by obesity (obASCs) and previous studies have shown that obASCs secrete higher levels of leptin. Increased leptin, which upregulates estrogen receptor alpha (ERα) and aromatase, enhances estrogen bioavailability and signaling in estrogen receptor positive (ER+) breast cancer (BC) tumor growth and metastasis. In this study, we evaluate the effect of obASCs on ER+BC outside of the ERα signaling axis using breast cancer models with constitutively active ERα resulting from clinically relevant mutations (Y537S and D538G). We found that while obASCs promote tumor growth and proliferation, it occurs mostly through abrogated estrogen signaling when BC has constitutive ER activity. However, obASCs have a similar promotion of metastasis irrespective of ER status, demonstrating that obASC promotion of metastasis may not be completely estrogen dependent. We found that obASCs upregulate two genes in both ER wild type (WT) and ER mutant (MUT) BC: SERPINE1 and ABCB1. This study demonstrates that obASCs promote metastasis in ER WT and MUT xenografts and an ER MUT patient derived xenograft (PDX) model. However, obASCs promote tumor growth only in ER WT xenografts.
Keywords: adipose stem cells, breast cancer, obesity, metastasis, estrogen receptor
1. Introduction
Breast cancer (BC) is the most common cancer and the second leading cause of cancer death in women [1]. An estimated 246,000 patients were diagnosed with new cases of BC in 2016 [1]. Among the many factors that influence BC, obesity has been shown to increase the rates of many types of cancer [2]. Studies have shown a positive correlation between adult body mass index (BMI) and the incidence of postmenopausal breast cancer, specifically ER+ breast cancer [2,3]. Obesity has also been found to increase both breast cancer recurrence and mortality [4]. Obesity is defined as having a BMI of ≥ 30 kg/m2 [5]. Rates of obesity have been increasing in the United States since the 1970s [6]. It is estimated that 21% of the world’s female population will be obese as of 2025, illustrating a critical need to interrogate the relationship between obesity and BC [7]. Obesity can promote breast cancer through many mechanisms including alterations to tumor biology to promote a more aggressive cancer phenotype or metabolic reprogramming. Many metabolic intermediates such as pyruvate kinase M2, sterol regulatory element-binding protein 1c, and peroxisome proliferator-activated receptors γ (PPARγ) are differentially regulated in obesity [8,9,10]. These factors have been implicated in reprogramming in the tumor microenvironment (TME) and have been shown to promote breast cancer proliferation and migration and even alter epigenetics leading to increased cancer incidence [11,12,13]. Data demonstrates targeting metabolic reprogramming with agents, such as statins or thiazolidinediones, can be as chemoprevention in rats [14,15]. It is clear that obesity alterations in the TME can alter cancer biology.
Adipose stem cells (ASCs) play a central role in the TME. They have been shown to support angiogenesis via recruitment of blood resident endothelial progenitors and promote inflammation [16]. ASCs effectively increase tumor growth, as well as the motility and invasive capacity of cancer cells via the stromal-derived factor 1/chemokine receptor type 4 (SDF-1/CXCR4) axis [17,18]. ASCs also induce an epithelial-to-mesenchymal (EMT) transition in cancer cells through platelet derived growth factor (PDGF) signaling [19], and a cancer stem-like phenotype in breast cancer through adipsin [20]. Additionally, cancer cells can induce a change in ASCs to cancer-associated fibroblasts (CAFs), which, in turn, increase secretion of factors that further enhance tumor proliferation, invasion, and metastasis [18,21]. Obesity increases the rate of CAF conversion, leading to enhanced proliferation and increased invasive capability of cancer cells [22].
It has previously been shown that ASCs from obese donors promote BC tumor growth and metastasis when compared to those cultured alone or with lean donor ASCs (BMI < 25) (lnASCs) [23]. Studies have demonstrated that obesity-altered ASCs (BMI > 30) (obASCs) increase the proliferation and tumor size of estrogen receptor positive (ER+) BC and increase lung and liver metastasis. Leptin, an adipokine abundantly secreted by obASCs relative to lnASCs, promotes ER+BC growth and metastasis by increasing expression of ERα and aromatase [24]. Previous reports show that knocking down leptin in obASCs reduces but does not entirely ameliorate the effect obASCs have on each of these processes. This suggests that obASCs may also act through non-estrogen pathways to promote ER+BC tumorigenesis and metastasis [23]. In this study, clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9 (CRISPR-Cas9) generation of ER+BC cell line MCF7 with a gnomically encoded Y537S mutation in the estrogen receptor alpha (ESR1) gene was used [25], and patient-derived xenograft models in which the patient developed the Y537S or D538G mutations were used. D538G and Y537S mutations in ERα result in constitutive ERα activity [26]. These are clinically relevant mutations that develop de novo in BC and result in BC that is resistant to endocrine therapies that target ERα [27]. By employing these genetically modified breast cancer cells (BCCs), the effect(s) obASCs have on ER+BC outside of the ERα-leptin signaling axis can be investigated.
2. Results
2.1. Obesity-Altered Adipose Stem Cells Promote Metastasis but Not Tumor Growth of Breast Cancer with Mutant ERα
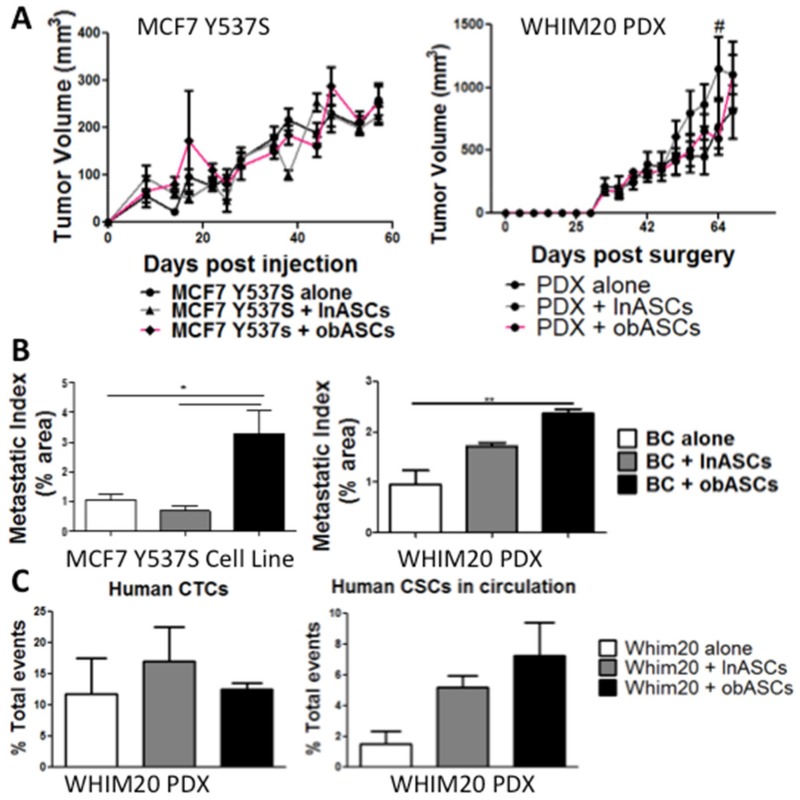
Previous studies have reported that obASCs promote growth and metastasis of MCF7 xenografts [24]. We demonstrate that obASCs enhance the growth of MCF7+estrogen pellet xenografts and have increased metastatic index (Supplementary Figure S1); however, MCF7-Y537S xenografts and WHIM20 patient-derived xenografts that have the Y537S mutation do not have enhanced growth in the presence of obASCs compared to lnASCs or control xenografts with no stem cells (Figure 1A). obASCs increase the metastatic index significantly in MCF7-Y537S xenografts with 3.30% ± 0.76 (Mean ± standard error of the mean (SEM)) area occupied by metastases in the obASC group compared to 0.69% ± 0.16 with lnASCs and 1.04% ± 0.21 with control (Figure 1B). Similarly, WHIM20 PDX demonstrated 2.36% ± 0.09 area occupied by metastases in the obASC group compared to 1.70% ± 0.08 with lnASCs and 0.95% ± 0.27 with control (Figure 1B). Flow cytometry was used to evaluate the presence of circulating tumor cells in the WHIM20 PDX model and found that mice with PDX tumors grown with obASCs had no significant difference in human (HLA1+) circulating tumor cells (CTCs); however CTCs from tumors grown with obASCs demonstrated a trend of enrichment for the breast cancer stem cell markers CD44+CD24− (Figure 1C). MCF7-Y537S and WHIM20 xenografts+estrogen pellets show a similar trend to xenografts without estrogen pellets where there is no effect of obASCs on tumor growth, but obASCs promote tumor metastasis and circulating tumor cells (CTCs) (Supplementary Figure S2).
Figure 1.
obASCs promote metastasis but not tumor growth of constitutively active ERα xenograft models—MCF7-Y537S and WHIM20 PDX (A) Tumor volume was tracked over time. (Day of injection = Day 0). There is no change in tumor volume when BC was implanted in the presence of lnASCs or obASCs compared to BC alone except lnASCs compared to control WHIM20 tumor volume at day 60 (# −ln vs. ctrl p < 0.05). Caliper measurements were taken every three to four days until the tumor volume reached 750–1000 mm3. Values reported are the mean (n = 5 mice/group). Data were analyzed using two-way analysis of variance (ANOVA) and a Bonferroni post-test. (B) Area of the lung occupied by metastasis (metastatic index) was evaluated at the endpoint. Groups, where BC was implanted with obASCs, had higher levels of metastasis compared to BC alone or grown with lnASCs. Data were analyzed using one-way ANOVA and Tukey post-test. Bars, ± SEM. * p < 0.05, ** p < 0.01. (C) Circulating tumor cells were analyzed in animals harboring patient-derived xenograft (WHIM20) at endpoint using flow cytometry. There was no change in human (HLA1+) cells across groups; however, analysis of circulating tumor cells enriched for the cancer stem cell marker CD44+CD24− was increased in PDX+obASCs compared to PDX alone. Data were analyzed using one-way ANOVA and Tukey post-test and no significant difference was found. Bars, ± SEM.
2.2. In Vitro obASCs Promote Proliferation and Migration of ER WT and ER MUT Cells
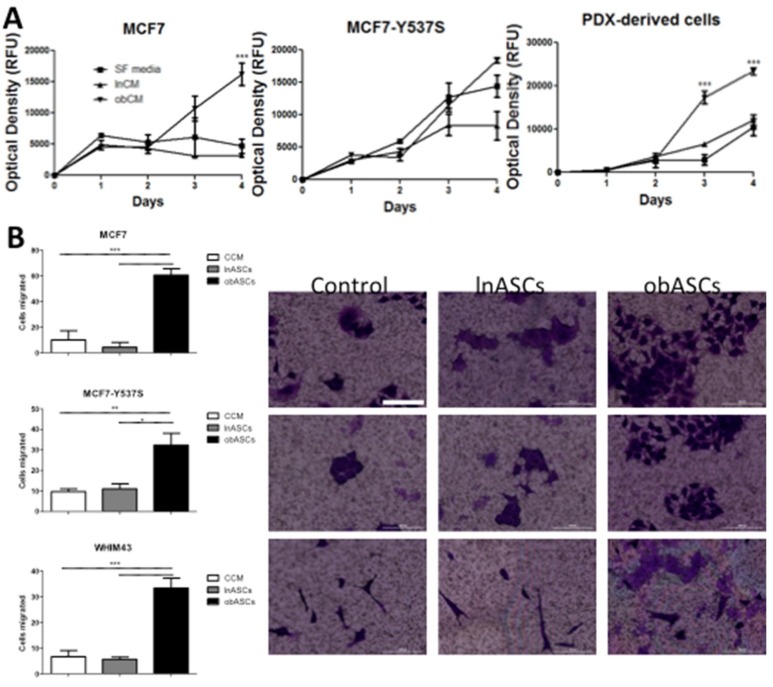
To evaluate in vitro the effects observed in vivo, conditioned media (CM) from ASCs was used and measured BCC proliferation over time. Secreted factors from obASCs promote proliferation of ER+BCCs in vitro. While obASC conditioned media (CM) had a greater effect on the proliferation of MCF7 than MCF7-Y537S, a trending increase in proliferation was observed when estrogen is constitutively active (Figure 2A). This demonstrates that obASCs exert some effect through the estrogen receptor on ER-dependent breast cancer cells. CM from obASCs did significantly increase the proliferation of PDX-derived cells with constitutive ER activity (Figure 2A). To study the metastatic phenotype in vitro, a migration assay was used and indicated that obASCs significantly promoted migration of ER+BCCs and a PDX-derived cell line irrespective of ER WT or MUT status (Figure 2B). Specifically, in ER WT BC (MCF7) an average of 60.7 cells ± 5.0 (Mean ± SEM) migrated to obASCs compared to 4.3 ± 3.8 cells to lnASCs, and 10.0 ± 7.2 cells migrated to CCM. ER MUT BC cell line (MCF7-Y537S) showed a similar trend with an average of 32.3 cells ± 5.8 (Mean ± SEM) migrated to obASCs compared to 11.0 ± 2.5 cells to lnASCs, and 9.7 ± 1.5 cells migrated to CCM, and ER MUT PDX derived cells (33.3 ± 3.8 to obASCs, 5.7 ± 0.9 to lnASCs, and 6.7 ± 2.4 to CCM) (Figure 2B). This further supports the hypothesis that obASCs are promoting metastasis in the TME outside of the estrogen-signaling axis.
Figure 2.
In an estrogen-depleted environment, obASCs promote proliferation and migration of ER WT and ER MUT BCCs in vitro. (A) Conditioned media collected from ASCs after 24 h promotes proliferation of ER WT (MCF7) and ER MUT with constitutively active ERα (MCF7-Y537S and PDX-derived) cells. Data were analyzed using two-way ANOVA and Bonferroni post-test. *** p < 0.001 (B) obASCs promotes increased migration of MCF7, MCF7-Y537S and PDX derived cells (WHIM43) through a 0.4 um membrane. Scale bar represents 100 μm. Data were analyzed using one-way ANOVA and Tukey post-test. Bars, ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001. Values reported are the mean of three independent experiments each performed in triplicate.
2.3. Regulation of Breast Cancer Related Genes in ER WT and ER MUT Cells by obASCs
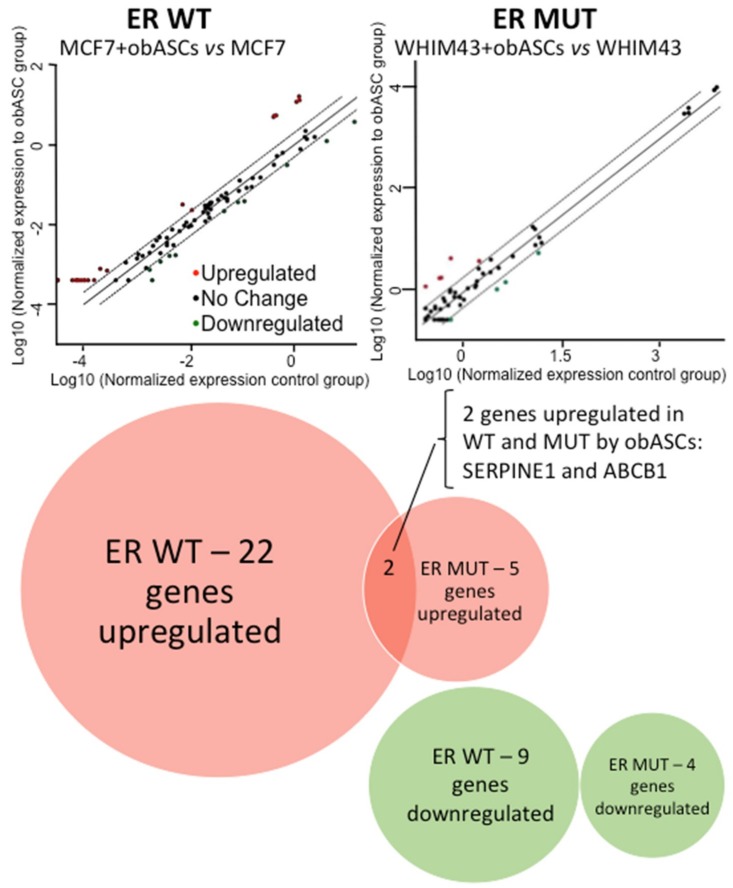
The Qiagen RT2 Profiler™ PCR Array Human Breast Cancer was used to determine genes and pathways altered in ER WT and MUT cells after 96-h Transwell co-culture with obASCs. A cutoff of 2-fold expression change was established, which resulted in the identification of 22 genes upregulated by obASCs in ER WT cells (MCF7), and 9 genes were downregulated by obASCs. In comparison, ER MUT (PDX-derived WHIM43 cells) had 5 genes upregulated by obASCs and 4 genes downregulated after co-culture with obASCs (Figure 3, Table 1). Of the 22 upregulated genes, obASCs upregulated estrogen-related genes that have been shown to promote tumor growth, such as ESR2; as well as cell-cycle-related genes associated with proliferation, such as CDKN1C and CDKN2A. ER WT and MUT cells upregulated 2 genes: SERPINE1 (2.51 fold in ER WT and 4.47 fold in ER MUT) and ABCB1 (6.48 fold in ER WT and 4.99 fold in ER MUT) (Figure 3, Table 1). In light of our results showing that obASCs promote metastasis but not tumor growth in ER MUT BC, it is likely that these overlapping gene changes are associated with the increased metastasis observed in ER WT and ER MUT BC when exposed to obASCs. These two genes could be a common mechanism outside of the estrogen axis through which obASCs promote metastasis of ER WT and ER MUT tumors.
Figure 3.
PCR array demonstrates that obASCs upregulate two common genes in ER WT and ER MUT cells. PCR array demonstrates that obASCs upregulate above a cutoff of 2x: 22 BC related genes in ER WT (MCF7) cells compared to 5 genes ER MUT (WHIM43) and downregulated 9 BC related genes in ER WT compared to 4 in ER MUT. Two genes were upregulated in both ER WT and ER MUT: SERPINE1 and ABCB1. PCR arrays were conducted (n = 1) for each condition.
Table 1.
PCR array fold changes of genes upregulated (red arrow) and downregulated (green arrow) by obASCs in ER WT (MCF7) and ER MUT (PDX-derived WHIM43) cells. This table demonstrates the specific genes and fold changes in gene expression after 96-h Transwell co-culture with pooled donors of obASCs. PCR arrays were conducted with an n = 1 for each condition.
| Gene Expression Changes after Transwell Co-Culture with obASCs | |||||
|---|---|---|---|---|---|
| ER WT | ER MUT | ||||
| Gene Name | Fold Change | Gene Name | Fold Change | ||
| ABCB1 | 6.48 | ↑ | ABCB1 | 4.99 | ↑ |
| ADAM23 | 3.93 | ↑ | CTNNB1 | 4.29 | ↑ |
| ATM | 0.3 | ↓ | CTSD | 0.41 | ↓ |
| CCNA1 | 0.2 | ↓ | MKI67 | 0.35 | ↓ |
| CCND2 | 12.51 | ↑ | MUC1 | 0.45 | ↓ |
| CDH13 | 5.41 | ↑ | NME1 | 0.34 | ↓ |
| CDKN1C | 0.41 | ↓ | PTEN | 2.36 | ↑ |
| CDKN2A | 12.51 | ↑ | SERPINE1 | 4.47 | ↑ |
| CSF1 | 3.72 | ↑ | VEGFA | 7.41 | ↑ |
| CST6 | 5.6 | ↑ | |||
| ESR2 | 4.98 | ↑ | |||
| GLI1 | 2.47 | ↑ | |||
| GSTP1 | 2.51 | ↑ | |||
| HIC1 | 3.31 | ↑ | |||
| IGF1 | 5.22 | ↑ | |||
| IGFBP3 | 4.18 | ↑ | |||
| IL6 | 4.42 | ↑ | |||
| KRT5 | 12.51 | ↑ | |||
| MMP2 | 12.51 | ↑ | |||
| PGR | 0.34 | ↓ | |||
| PLAU | 3.82 | ↑ | |||
| PTGS2 | 12.51 | ↑ | |||
| PYCARD | 0.42 | ↓ | |||
| RARB | 0.38 | ↓ | |||
| SERPINE1 | 2.51 | ↑ | |||
| SFRP1 | 12.51 | ↑ | |||
| SCL39A6 | 0.42 | ↓ | |||
| SNAI2 | 0.41 | ↓ | |||
| TFF3 | 0.29 | ↓ | |||
| TGFB1 | 2.01 | ↑ | |||
| TWIST1 | 12.51 | ↑ | |||
3. Discussion
Obesity is recognized as a leading preventable cause of cancer [28]. Obesity has also been shown to alter the way ASCs interact with cancer cells within the TME [29]. However, there is little research on obesity-specific cancer therapies that target the TME, despite the crucial role obesity-associated cytokines play in cancer development and progression. The current study aimed to reveal cross-talk between obASCs and ER+BCCs outside of estrogen-dependent signaling and to identify novel pathways through which obesity promotes BC metastasis.
Studies have demonstrated that ASCs promote breast cancer cell migration, stemness and promote angiogenesis [30,31,32]. ObASCs have been shown to promote tumor growth and metastasis of MCF7 through increased secretion of leptin, which upregulates aromatase and ERα [24]. In this study, we show that obASCs exert effects on BC through non-estrogen signaling pathways. We found that obASCs promote metastasis, but not tumor growth of ER+BC with mutations in ERα that result in constitutive ERα activity. We found that obASCs promote a metastatic phenotype in vitro, which correlates with increased circulating tumor cells and circulating cancer stem-like cells in PDX models and increased lung metastases in cell line and PDX models. To evaluate genes and pathways commonly activated in ER WT and ER MUT cells we used an array of 84 breast cancer- related genes. Some genes were differentially regulated in ER WT and ER MUT cells; however, only two genes were upregulated by obASCs in bot ER WT and ER MUT: ABCB1 and SERPINE1.
ABCB1, also known as multidrug resistance 1 or P-glycoprotein, is an efflux transporter with an ATP binding cassette [33,34]. Overexpression of ABCB1 in breast cancer is associated with poor response to first-line chemotherapies because ABCB1 can efflux many drugs used in the treatment of breast cancer such as taxanes, anthracyclines, and vinca alkaloids [35]. There are many different signal transduction pathways and transcription factors that can lead to ABCB1 transcription and ultimately chemoresistant tumors including: Ras [36,37,38], cyclic adenosine monophosphate (cAMP)/ Protein kinase A (PKA) pathway [39,40], protein kinase C [41,42], phosphatase and tensin homologue (PTEN) [43,44], phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway [45,46], and p53 [47,48]. These signaling pathways could be activated by any number of known secreted growth factors and cytokines from obASCs to upregulate ABCB1. ABCB1 is not known to play a role in the metastatic phenotype of breast cancer but is associated with a more aggressive cancer that is drug resistant leading to worse outcomes.
SERPINE1, also known as plasminogen activator inhibitor-1, which was also upregulated in both ER WT and ER MUT BC by obASCs is associated with tumor progression and invasion [49]. SERPINE1 is an inhibitor of urokinase plasminogen activator (uPA), which is itself an extracellular matrix-degrading protease associated with cancer invasion [50,51]. Based on its ability to suppress uPA, it was previously hypothesized that SERPINE1 would be tumor inhibitory; however, studies have now demonstrated that SERPINE1 plays a role in neoangiogenesis in the tumor microenvironment and thereby plays a role in tumor progression, invasion, and metastasis [52,53]. SERPINE1 in keratinocytes in a wound healing environment have been deemed the “molecular switch” from proliferation to migration by Simone et al. [54]. Because obASCs upregulated SERPINE1 in ER WT and MUT breast cancer and promoted metastasis, but not tumor growth in both xenograft models and a PDX model, we hypothesize that SERPINE1 could be a key mediator in obesity-altered ASCs promotion of metastasis. Future studies are needed to thoroughly investigate this hypothesis as well as evaluate the factors secreted by obASCs that upregulate SERPINE1 to develop therapeutic strategies to block this obesity-mediated promotion of metastatic disease.
4. Materials and Methods
4.1. Human Subjects
All protocols were reviewed and approved by the Pennington Biomedical Research Center Institutional Review Board and all human participants provided written informed consent (PBRC #23040 approved in December, 2011) (LaCell, New Orleans, LA, USA). Human ASCs were isolated from 12 Caucasian females (2 groups, 6 donors per group) undergoing elective liposuction procedures, as previously described [24]. The mean BMI for each of the two donor groups was as follows: Obese (32.7 ± 3.7) and Lean (22.7 ± 1.9). The mean age of the subjects for each group of donors was as follows: Obese (42.5 ± 8.9) and Lean (38.8 ± 7.0). No statistical significance in age was observed between the donor groups.
4.2. Cell Culture
ASCs were isolated, cultured, and characterized as previously described [24]. MCF7 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). MCF7-Y537S cells were obtained from the Department of Surgery and Cancer at Imperial College London (London, England) and cultured as previously described [25]. Cells were cultured in complete culture media (CCM), which consisted of α-minimal essential media (αMEM; Gibco; Grand Island, NY, USA), 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, NJ, USA) 100 units per mL penicillin/100 ug/mL streptomycin (P/S; Gibco), and 2 mM L-Glutamine (Gibco). Cells were grown at 37 °C with 5% humidified CO2, CCM was changed every three to four days, and split 1:4 to 1:6 when they reached 90% confluency as previously described [24].
4.3. RT-qPCR
BCCs (5 × 104) were plated in the bottom of a 6-well plate (Nunc, ThermoFisher, Waltham, MA, USA) and six pooled donors of obASCs (BMI > 30) or lnASCs (BMI < 25) were seeded at a density of 5 × 104 cells in a 0.4 µm pore Transwell (Corning Inc., Corning, NY, USA). Cells were allowed to attach overnight. After 24 h, Transwell inserts containing ASCs were transferred to wells harboring BCCs for 96 h. RNA was isolated using Qiazol (Qiagen, Valencia, CA, USA) and an RNeasy Mini Kit (Qiagen). RNA was converted to cDNA using RT2 First Strand Kit (Qiagen). RT2 Profiler™ PCR Array Human Breast Cancer with RT2 SYBR Green qPCR Mastermix (Qiagen) was used to identify breast cancer genes/pathways upregulated by obASCs.
4.4. Conditioned Media Proliferation Assay
Lean and obese ASCs plated on a 150 mm2 dish were allowed to reach 70% confluence. Plates were washed with sterile phosphate buffered saline (PBS) and the medium was replaced with serum-free αMEM for 24 h. Media was collected and filtered through a cell strainer (0.2 μm nylon mesh; Fisher Scientific, Hampton, NH, USA) to remove cellular debris. BCCs were plated at 200 cells per well of a 96-well plate in triplicate in CCM and allowed to adhere overnight. Cells were then washed with PBS and 200 μL of lean or obese ASC CM, or serum-free αMEM was added. Proliferation assay was conducted with 10% Alamar blue reagent (Invitrogen, Carlsbad, CA, USA) per manufacturer’s instructions. Proliferation quantification was done by measuring relative fluorescent units (RFU) (excitation 530–560 nm; emission 590 nm).
4.5. Migration Assay
CCM or 0.5 × 106 ASCs in CCM were plated in the bottom of a 6 well plate and allowed to adhere overnight. 0.5 × 106 breast cancer cells were seeded in Transwells (0.4 μm pore; Corning Inc.) and allowed to adhere overnight. After 24 h Transwells were transferred to wells with CCM or ASCs in CCM and cultured for three days. Transwells were then fixed and stained with Crystal Violet (3% in methanol) for 30 min, washed with deionized water, and migrated cells were counted manually.
4.6. Orthotopic Xenografts
Four to six-week-old severe combined immunodeficiency (SCID)/beige (CB17.Cg-PrkdcscidLystbg-J/Crl) ovariectomized female mice were obtained from Charles River Laboratories (Wilmington, MA, USA). Mice were divided into six groups of five animals: MCF7-Y357S only, MCF7-Y357s plus obASCs (n = 6 donors), MCF7-Y357S plus lnASCs (n = 6 donors), MCF7-Y357S plus estradiol, MCF7-Y357S plus obASCs and estradiol (n = 6 donors), and MCF7-Y357S plus lnASCs and estradiol (n = 6 donors). Where indicated, estradiol pellets were implanted subcutaneously in the lateral area of the neck (0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL, USA) as previously described [24].
MCF7-Y357S cells (106) alone or MCF7-Y357S cells (106) in combination with ASCs (106) suspended in a total volume of 50 μL of sterile PBS were mixed with 100 μL of reduced growth factor Matrigel (BD Biosciences, Bedford, MA, USA). Cells were injected subcutaneously into the fifth mammary fat pad on both sides as previously described [24]. All procedures in animals were performed under anesthesia using a mixture of isoflurane and oxygen delivered by a nose cone. Tumor size was measured every three days using digital calipers and calculated as previously described [24]. At necropsy, animals were euthanized by cervical dislocation after exposure to CO2. Lungs were removed and fixed in 10% neutral buffered formalin and paraffin embedded for metastatic analysis. All procedures involving animals were conducted in compliance with State and Federal law, standards of the United States Department of Health and Human Services, and guidelines established by Tulane University Institutional Animal Care and Use Committee (IACUC). All protocols were approved by the Tulane IACUC (Protocol 4299R. Approved in December, 2013).
4.7. Patient-Derived Xenografts
The PDX models used in this study WHIM20 (isolated from a patient who developed the Y537S mutation) and WHIM43 (D538G mutation) were obtained from Washington University in St. Louis (Horizons Discovery Group, Waterbeach UK). All animal procedures were reviewed and approved by Tulane University IACUC. SCID/beige (CB17.Cg-PrkdcscidLystbg-1/Crl) 4–6-week-old female mice were obtained from Charles River Laboratory. Intact tumor pieces were removed and sliced with a scalpel to 3mm x 3mm and coated with 100 uL phenol-free growth factor reduced Matrigel (BD Biosciences). Control groups had PDX tumor coated in Matrigel and implanted bilaterally in the mammary fat pads under isoflurane and oxygen. In ASC groups, 106 pooled donors (n = 6) of lnASCs or obASCs were resuspended in Matrigel and coated the tumor. Where indicated, estradiol pellets were implanted subcutaneously in the lateral area of the neck (0.72 mg, 60-day release; Innovative Research of America). Tumors were implanted into the fifth mammary fat pad bilaterally under isoflurane and oxygen anesthesia delivered by nose cone and animals were given 5mg/kg/day meloxicam for three days post-surgery. Tumors were measured by digital caliper every three to four days. At endpoint (tumors reach 750–1000 mm3) blood and lungs were collected for analysis. PDX derived cells were cloned out from PDX tumors.
4.8. Flow Cytometry
To identify circulating tumor cells whole blood was collected with 0.5 M EDTA (Gibco). Samples were incubated with 0.008% NH4CL (ThermoFisher) for red blood cell lysis and washed with PBS. Cells were then blocked with 1% bovine serum albumin (BSA) and 1% anti-CD16/anti-CD32 (EBioscience, ThermoFisher) in PBS and stained with antibodies against HLA1 (Invitrogen), CD24 (EBioscience), CD44 (EBioscience). Samples were analyzed with a Gallios Flow Cytometer (Beckman Coulter, Brea, CA, USA) with Kaluza software (Beckman Coulter). A minimum of 10,000 events were captured and analyzed.
4.9. Statistical Analysis
The analysis was performed using Prism (Graphpad Software, San Diego, CA, USA). All values are presented as means ± standard error. Statistical differences among two or more groups were determined by ANOVA, followed by post-hoc Tukey tests versus the respective control group. Statistical differences between two groups were performed by Student’s t-test. Statistical significance was set at p < 0.05.
5. Conclusions
ObASCs promote tumor growth and metastasis of ER WT BC, and promote metastasis of ER MUT BC. These data demonstrate that there are independent pathways promoted by obASCs that affect tumor growth and metastasis. The pathways that promote tumorigenesis are ER-dependent; however, in BC where these pathways are constitutively activated obASCs promote metastasis but have no effect on tumor growth. When 84 key breast cancer-related genes were evaluated in BCCs after Transwell co-culture with obASCs we found that obASCs upregulate more genes in ER WT than ER MUT cells. Interestingly, there were two genes upregulated in both cell types: ABCB1, a gene associated with multidrug resistance, and SERPINE1, a gene associated with an invasive metastatic phenotype. Future studies should aim to investigate the dependency of obASC promotion of metastasis on SERPINE1 expression as well as investigate the expression of SERPINE1 in human tumors from lean and obese women to see if these findings are translational.
Acknowledgments
The authors would like to thank Dina Gaupp and the Histology Core, as well as Alan Tucker and the Flow Cytometry core for their expertise.
Abbreviations
| ASCs | Adipose stem cells |
| obASCs | Obesity-altered ASCs |
| lnASCs | Lean ASCs |
| ER+ | Estrogen Receptor positive |
| BC | Breast cancer |
| WT | Wild Type |
| MUT | Mutant |
| BMI | Body mass index |
| EMT | Epithelial-to-mesenchymal transition |
| CAFs | Cancer-associated fibroblasts |
| BCCs | Breast cancer cells |
| CCM | Complete culture media |
| IACUC | Institutional animal care and use committee |
| PDX | Patient-derived xenografts |
| CTCs | Circulating tumor cells |
| CM | Conditioned media |
| TME | Tumor microenvironment |
| cAMP | Cyclic adenosine monophosphate |
| PKA | Protein Kinase A |
| PTEN | Phosphatase and tensin homolog |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase B |
| uPA | Urokinase plasminogen activator |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/6/1419/s1.
Author Contributions
Conceptualization, R.A.S., B.A.B., M.E.B.; methodology, R.A.S., A.B., P.G., R.M.W., M.A.A.H., B.A.O., B.N.S., J.D.L., M.D.M.; resources, M.R.B., G.W., M.E.B., B.M.C.B., B.A.B.; writing—original draft preparation, R.A.S., A.B., P.G., B.A.B.; writing—reviewing and editing, R.A.S., M.A.A.H., R.M.W., B.N.S., M.R.B., G.W., M.E.B., B.A.B.
Funding
Research reported in this publication was supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center and OD11104 from the Office of the Director at the National Institutes for Health (BAB). These studies were also supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001418 (RAS). This publication was made possible by funding from the NIMHD-RCMI grant number 5G12MD007595 from the National Institute on Minority Health and Health Disparities and the NIGMS-BUILD grant number 8UL1GM118967 (MRB and GW). This publication was also made possible by the Louisiana Cancer Research Consortium and was facilitated in part by the Cell, Molecular, and Bioinformatics Core facility at Xavier University (MRB and GW). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIMHD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H., Stein K.D., Alteri R., Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.Enger S.M., Ross R.K., Paganini-Hill A., Carpenter C.L., Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: Results from two case-control studies. Cancer Epidemiol. Biomark. Prev. 2000;9:681–687. [PubMed] [Google Scholar]
- 4.Loi S., Milne R.L., Friedlander M.L., McCredie M.R., Giles G.G., Hopper J.L., Phillips K.A. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol. Biomark. Prev. 2005;14:1686–1691. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 5.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am. J. Clin. Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell N.S., Catenacci V.A., Wyatt H.R., Hill J.O. Obesity: Overview of an epidemic. Psychiatr. Clin. N. Am. 2011;34:717–732. doi: 10.1016/j.psc.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaboration N.C.D. Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahid H., Subbaramaiah K., Iyengar N.M., Zhou X.K., Chen I.C., Bhardwaj P., Gucalp A., Morrow M., Hudis C.A., Dannenberg A.J., et al. Leptin regulation of the p53-hif1alpha/pkm2-aromatase axis in breast adipose stromal cells: A novel mechanism for the obesity-breast cancer link. Int. J. Obes. 2018;42:711–720. doi: 10.1038/ijo.2017.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettinelli P., Videla L.A. Up-regulation of ppar-gamma mrna expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to srebp-1c induction. J. Clin. Endocrinol. Metab. 2011;96:1424–1430. doi: 10.1210/jc.2010-2129. [DOI] [PubMed] [Google Scholar]
- 10.Oberkofler H., Fukushima N., Esterbauer H., Krempler F., Patsch W. Sterol regulatory element binding proteins: Relationship of adipose tissue gene expression with obesity in humans. Biochim. Biophys. Acta. 2002;1575:75–81. doi: 10.1016/S0167-4781(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 11.Bao J., Zhu L., Zhu Q., Su J., Liu M., Huang W. Srebp-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol. Lett. 2016;12:2409–2416. doi: 10.3892/ol.2016.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi M., Fujii N., Narita T., Higami Y. Srebp-1c-dependent metabolic remodeling of white adipose tissue by caloric restriction. Int. J. Mol. Sci. 2018;19:3335. doi: 10.3390/ijms19113335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L., Shi Y., Liu S., Cao Y., Wang X., Tao Y. Pkm2: The thread linking energy metabolism reprogramming with epigenetics in cancer. Int. J. Mol. Sci. 2014;15:11435–11445. doi: 10.3390/ijms150711435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bojkova B., Garajova M., Kajo K., Pec M., Kubatka P., Kassayova M., Kiskova T., Orendas P., Ahlersova E., Ahlers I. Pioglitazone in chemically induced mammary carcinogenesis in rats. Eur. J. Cancer Prev. 2010;19:379–384. doi: 10.1097/CEJ.0b013e32833ca233. [DOI] [PubMed] [Google Scholar]
- 15.Kubatka P., Stollarova N., Skarda J., Zihlavnikova K., Kajo K., Kapinova A., Adamicova K., Pec M., Dobrota D., Bojkova B., et al. Preventive effects of fluvastatin in rat mammary carcinogenesis. Eur. J. Cancer Prev. 2013;22:352–357. doi: 10.1097/CEJ.0b013e32835b385d. [DOI] [PubMed] [Google Scholar]
- 16.Eterno V., Zambelli A., Pavesi L., Villani L., Zanini V., Petrolo G., Manera S., Tuscano A., Amato A. Adipose-derived mesenchymal stem cells (ascs) may favour breast cancer recurrence via hgf/c-met signaling. Oncotarget. 2014;5:613–633. doi: 10.18632/oncotarget.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muehlberg F.L., Song Y.H., Krohn A., Pinilla S.P., Droll L.H., Leng X., Seidensticker M., Ricke J., Altman A.M., Devarajan E., et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 18.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated sdf-1/cxcl12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Devarajan E., Song Y.H., Krishnappa S., Alt E. Epithelial-mesenchymal transition in breast cancer lines is mediated through pdgf-d released by tissue-resident stem cells. Int. J. Cancer. 2012;131:1023–1031. doi: 10.1002/ijc.26493. [DOI] [PubMed] [Google Scholar]
- 20.Goto H., Shimono Y., Funakoshi Y., Imamura Y., Toyoda M., Kiyota N., Kono S., Takao S., Mukohara T., Minami H. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene. 2019;38:767–779. doi: 10.1038/s41388-018-0477-8. [DOI] [PubMed] [Google Scholar]
- 21.Yamamura Y., Asai N., Enomoto A., Kato T., Mii S., Kondo Y., Ushida K., Niimi K., Tsunoda N., Nagino M., et al. Akt-girdin signaling in cancer-associated fibroblasts contributes to tumor progression. Cancer Res. 2015;75:813–823. doi: 10.1158/0008-5472.CAN-14-1317. [DOI] [PubMed] [Google Scholar]
- 22.Strong A.L., Pei D.T., Hurst C.G., Gimble J.M., Burow M.E., Bunnell B.A. Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int. 2017;2017:9216502. doi: 10.1155/2017/9216502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strong A.L., Ohlstein J.F., Biagas B.A., Rhodes L.V., Pei D.T., Tucker H.A., Llamas C., Bowles A.C., Dutreil M.F., Zhang S., et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strong A.L., Strong T.A., Rhodes L.V., Semon J.A., Zhang X., Shi Z., Zhang S., Gimble J.M., Burow M.E., Bunnell B.A. Obesity associated alteration in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013;5:R102. doi: 10.1186/bcr3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrod A., Fulton J., Nguyen V.T.M., Periyasamy M., Ramos-Garcia L., Lai C.F., Metodieva G., de Giorgio A., Williams R.L., Santos D.B., et al. Genomic modelling of the esr1 y537s mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene. 2017;36:2286–2296. doi: 10.1038/onc.2016.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toy W., Shen Y., Won H., Green B., Sakr R.A., Will M., Li Z., Gala K., Fanning S., King T.A., et al. Esr1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanning S.W., Mayne C.G., Dharmarajan V., Carlson K.E., Martin T.A., Novick S.J., Toy W., Green B., Panchamukhi S., Katzenellenbogen B.S., et al. Estrogen receptor alpha somatic mutations y537s and d538g confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. eLife. 2016;5:e12792. doi: 10.7554/eLife.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haakinson D.J., Leeds S.G., Dueck A.C., Gray R.J., Wasif N., Stucky C.C., Northfelt D.W., Apsey H.A., Pockaj B. The impact of obesity on breast cancer: A retrospective review. Ann. Surg. Oncol. 2012;19:3012–3018. doi: 10.1245/s10434-012-2320-8. [DOI] [PubMed] [Google Scholar]
- 29.Strong A.L., Burow M.E., Gimble J.M., Bunnell B.A. Concise review: The obesity cancer paradigm: Exploration of the interactions and crosstalk with adipose stem cells. Stem Cells. 2015;33:318–326. doi: 10.1002/stem.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Liu J., Jiang Q., Deng J., Xu F., Chen X., Cheng F., Zhang Y., Yao Y., Xia Z., et al. Human adipose-derived mesenchymal stem cell-secreted cxcl1 and cxcl8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells. 2017;35:2060–2070. doi: 10.1002/stem.2643. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y., Zhang X., Zhao H., Wang J., Zhang Q. Cxcl5 secreted from adipose tissue-derived stem cells promotes cancer cell proliferation. Oncol. Lett. 2018;15:1403–1410. doi: 10.3892/ol.2017.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koellensperger E., Bonnert L.C., Zoernig I., Marme F., Sandmann S., Germann G., Gramley F., Leimer U. The impact of human adipose tissue-derived stem cells on breast cancer cells: Implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res. Ther. 2017;8:121. doi: 10.1186/s13287-017-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: Complete cdna sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- 34.Higgins C.F. Abc transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 35.Sekine I., Shimizu C., Nishio K., Saijo N., Tamura T. A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with breast cancer. Int. J. Clin. Oncol. 2009;14:112–119. doi: 10.1007/s10147-008-0813-z. [DOI] [PubMed] [Google Scholar]
- 36.Nakagami H., Soukupova H., Schikora A., Zarsky V., Hirt H. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in arabidopsis. J. Biol. Chem. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 37.Wei N., Sun H., Wang F., Liu G. H1, a novel derivative of tetrandrine reverse p-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of p-glycoprotein. Cancer Chemother. Pharmacol. 2011;67:1017–1025. doi: 10.1007/s00280-010-1397-7. [DOI] [PubMed] [Google Scholar]
- 38.Bark H., Choi C.H. Psc833, cyclosporine analogue, downregulates mdr1 expression by activating jnk/c-jun/ap-1 and suppressing nf-kappab. Cancer Chemother. Pharmacol. 2010;65:1131–1136. doi: 10.1007/s00280-009-1121-7. [DOI] [PubMed] [Google Scholar]
- 39.Rohlff C., Glazer R.I. Regulation of multidrug resistance through the camp and egf signalling pathways. Cell Signal. 1995;7:431–443. doi: 10.1016/0898-6568(95)00018-K. [DOI] [PubMed] [Google Scholar]
- 40.Ziemann C., Riecke A., Rudell G., Oetjen E., Steinfelder H.J., Lass C., Kahl G.F., Hirsch-Ernst K.I. The role of prostaglandin e receptor-dependent signaling via camp in mdr1b gene activation in primary rat hepatocyte cultures. J. Pharmacol. Exp. Ther. 2006;317:378–386. doi: 10.1124/jpet.105.094193. [DOI] [PubMed] [Google Scholar]
- 41.Fine R.L., Patel J., Chabner B.A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc. Natl. Acad. Sci. USA. 1988;85:582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blobe G.C., Sachs C.W., Khan W.A., Fabbro D., Stabel S., Wetsel W.C., Obeid L.M., Fine R.L., Hannun Y.A. Selective regulation of expression of protein kinase c (pkc) isoenzymes in multidrug-resistant mcf-7 cells. Functional significance of enhanced expression of pkc alpha. J. Biol. Chem. 1993;268:658–664. [PubMed] [Google Scholar]
- 43.Keniry M., Parsons R. The role of pten signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 44.Maehama T., Dixon J.E. The tumor suppressor, pten/mmac1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 45.Pacold M.E., Suire S., Perisic O., Lara-Gonzalez S., Davis C.T., Walker E.H., Hawkins P.T., Stephens L., Eccleston J.F., Williams R.L. Crystal structure and functional analysis of ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/S0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 46.Kuo M.T., Liu Z., Wei Y., Lin-Lee Y.C., Tatebe S., Mills G.B., Unate H. Induction of human mdr1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate nf-kappab signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 47.Oka M., Kounoura K., Narasaki F., Sakamoto A., Fukuda M., Matsuo I., Ikeda K., Tsurutani J., Ikuno N., Omagari K., et al. P-glycoprotein is positively correlated with p53 protein accumulation in human colorectal cancers. Jpn. J. Cancer Res. 1997;88:738–742. doi: 10.1111/j.1349-7006.1997.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuhashi N., Saio M., Matsuo A., Sugiyama Y., Saji S. The evaluation of gastric cancer sensitivity to 5-fu/cddp in terms of induction of apoptosis: Time- and p53 expression-dependency of anti-cancer drugs. Oncol. Rep. 2005;14:609–615. doi: 10.3892/or.14.3.609. [DOI] [PubMed] [Google Scholar]
- 49.Dhanda J., Triantafyllou A., Liloglou T., Kalirai H., Lloyd B., Hanlon R., Shaw R.J., Sibson D.R., Risk J.M. Serpine1 and sma expression at the invasive front predict extracapsular spread and survival in oral squamous cell carcinoma. Br. J. Cancer. 2014;111:2114–2121. doi: 10.1038/bjc.2014.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deryugina E.I., Quigley J.P. Cell surface remodeling by plasmin: A new function for an old enzyme. J. Biomed. Biotechnol. 2012;2012:564259. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beaufort N., Plaza K., Utzschneider D., Schwarz A., Burkhart J.M., Creutzburg S., Debela M., Schmitt M., Ries C., Magdolen V. Interdependence of kallikrein-related peptidases in proteolytic networks. Biol. Chem. 2010;391:581–587. doi: 10.1515/bc.2010.055. [DOI] [PubMed] [Google Scholar]
- 52.Masuda T., Hattori N., Senoo T., Akita S., Ishikawa N., Fujitaka K., Haruta Y., Murai H., Kohno N. Sk-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol. Cancer Ther. 2013;12:2378–2388. doi: 10.1158/1535-7163.MCT-13-0041. [DOI] [PubMed] [Google Scholar]
- 53.Bajou K., Noel A., Gerard R.D., Masson V., Brunner N., Holst-Hansen C., Skobe M., Fusenig N.E., Carmeliet P., Collen D., et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 54.Simone T.M., Higgins C.E., Czekay R.P., Law B.K., Higgins S.P., Archambeault J., Kutz S.M., Higgins P.J. Serpine1: A molecular switch in the proliferation-migration dichotomy in wound-“activated” keratinocytes. Adv. Wound Care (New Rochelle) 2014;3:281–290. doi: 10.1089/wound.2013.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.