Abstract
The objectives of the present study were to determine the combined effects of chitosan and water activity (aW) on growth and mycotoxin production in situ on the two most important Fusarium species (F. proliferatum and F. verticillioides) present on maize, and on F. graminearum, the main pathogen causing Fusarium head blight on wheat. Results showed that low-molecular-weight chitosan with more than 70% deacetylation at the lowest dose used (0.5 mg/g) was able to reduce deoxynivalenol (DON) and fumonisin (FBs) production on irradiated maize and wheat grains. Growth rates of F. graminearum also decreased at the lowest chitosan dose used (0.5 mg/g), while F. verticillioides and F. proliferatum growth rates were reduced at 0.98 aW at the highest chitosan dose used (2 mg/g). Since mycotoxins are unavoidable contaminants in food and feed chains, their presence needs to be reduced in order to minimize their effects on human and animal health and to diminish the annual market loss through rejected maize and wheat; in this scenario, pre- and post-harvest use of chitosan could be an important alternative.
Keywords: chitosan, Fusarium, fumonisin, deoxynivalenol, wheat, maize
1. Introduction
Fusarium is one of the most economically important genera of phytopathogenic fungi. Several Fusarium species can infect small grain cereals (wheat, barley, and oat) and maize causing losses by seedling blight or reducing seed germination, or by causing seedling foot and stalk rots. However, the most important diseases in cereals, due to a severe reduction in yield and quality, are head blight of small cereals such as wheat, and ear rot of maize. Also, another risk is the presence of Fusarium toxins that contaminate cereals and are of concern because they could result in harmful contamination of foods and feedstuffs [1]. Maize can be infected by many toxigenic fungi [1,2,3], including Fusarium verticillioides and F. proliferatum [3,4,5,6,7]. These Fusarium species belong to the Fusarium fujikuroi species complex (FFSC) and are important pathogens of maize that can produce a high number of mycotoxins. Among them, fumonisins (FBs) are the most important in terms of occurrence and levels. Fumonisins are polyketides which are structurally similar to cellular sphingolipids, and they were shown to inhibit sphingolipid biosynthesis via the ceramide synthase pathway. Fumonisin toxicity is thought to result from the blockage of sphingolipid biosynthesis [8]. Dietary exposure to these mycotoxins was associated with leukoencephalomalacia in equine, hepatic, and renal toxicity in rodents, pulmonary edema in pigs, and esophageal cancer and neural tube defects in humans [9,10,11,12,13]. Recent studies suggest that exposure to fumonisins could also be related to stunting in children [14,15]. Due to the toxicological effect in humans and animals, fumonisin B1 (FB1) is classified as a “2B” carcinogen by the International Agency of Research on Cancer [16]. The Joint Food and Agriculture Organization and World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) determined a provisional maximum tolerable daily intake (PMTDI) of 2 µg/kg body weight per day for FB1, fumonisin B2 (FB2), and fumonisin B3 (FB3) alone or in combination [17]. Maximum fumonisin limits for human consumption in cereals and cereal-based foods were established by the European Union in 2007 (EC N°1126/2007), being 1000 ng/g for maize and sub-products used for human consumption.
One of the most important diseases of wheat and other cereals in many areas of the world is Fusarium head blight (FHB). This disease causes yield losses and often results in the accumulation of Fusarium mycotoxins in wheat grains [18]. In Argentina, the main pathogen associated with this disease is Fusarium graminearum sensu stricto [19,20,21]. Consequently, wheat is often contaminated with mycotoxins, with deoxinivalenol (DON) as the predominant one [19,22,23,24]. DON belongs to the type B trichothecenes. Historically, DON, also called vomitoxin, was notorious since it causes acute and chronic disease symptoms in humans and animals that consume contaminated grains [25]. Its toxic effects range from diarrhea to vomiting, gastro-intestinal inflammation, and necrosis of the intestinal tract, the bone marrow, and the lymphoid tissues [26]. Regarding DON legislation, the European Union sets limits of 1250 ng/g for unprocessed cereals, other than durum wheat, oats, and maize, and 1750 ng/g for unprocessed durum wheat and oats, while the United States of America (USA) and Canada legislation sets 2000 and 1000 ng/g, respectively, in wheat and wheat sub-products used for human consumption.
Considering the high impact of FBs and DON on human and animal health, and the economic losses related with the contamination of maize and wheat grains with mycotoxins, it is important to develop strategies in order to prevent their formation and/or to eliminate, inactivate, or reduce their presence in both cereals and food products. For this, several strategies such as a combination of agronomical practices, resistant cultivars, and fungicides are used [27]. However, chemical fungicides are reported to cause problems such as ambient pollution, chemical residues in food and feed, and the development of resistant pathogenic fungi [28]. Also, under certain conditions, they may act as a stress factor resulting in the induction of toxin biosynthesis; sub-lethal doses of some fungicides may lead to a stimulation of mycotoxin production by Fusarium species [29,30]. Due to these problems, there is an increased interest in the study and use of antifungal compounds obtained from natural sources to replace synthetic fungicides. Chitosan emerged as a promising eco-friendly alternative since it can be used to produce biodegradable fungicides. It induces several biological responses in plants; it enhances defense responses to abiotic and biotic stresses, stimulates plant growth, stimulates the effect of different enzyme activities to detoxify reactive oxygen species, interacts with chromatin, and directly affects gene expression [31]. Also, it is used to protect seeds [32]. Most importantly, it is considered as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA). Chitosan is a deacetylated form of chitin and has the ability to inhibit a wide variety of microorganisms such as fungi, bacteria, and viruses [33,34]. Chitosan is a lineal copolymer of β-(1,4) 2-acetamido-2deoxy-β-d-glucopyranose and 2-amino-2-deoxy-β-d-glucopyranose [35], and its biological properties are attributed to several traits, including deacetylation degree and molecular mass concentration [36]. Also, recently, the use of chitosan as a food preservative or adjuvant in agriculture increased to protect or stimulate the defense of different crops [33,34].
To date, only a few studies reported the effect of chitosan on both growth and mycotoxin production in different fungal species. For instance, on Aspergillus flavus and Aspergillus parasiticus [37,38,39] and Alternaria alternata f. sp. lycopersici [40]. It is surprising that these reports took no account of the interactions between the efficacy of chitosan and key environmental factors, such as water activity (aW) or temperature, since they are known for being main factors that influence fungal growth and mycotoxin production [41].
In a previous study in vitro, we demonstrated the effect of chitosan on growth of F. verticillioides and F. proliferatum strains, as well as on FB production, under different aW at 25 °C, using a maize-based media. We observed that chitosan was able to significantly reduce growth rate and FB production, with maximum levels of reduction in both parameters obtained at the highest doses used [42]. Ramirez et al. [30] suggested that the effect of antifungal compounds on artificial substrate may not accurately represent the real situation on a natural substrate. The use of irradiated grains that retained viability may be a more appropriate system for screening antifungal and anti-mycotoxin compounds.
Under this scenario, chitosan could be proposed as a possible option to control fungal growth and mycotoxin accumulation in cereals, mainly due to its biological properties and its easy production by partial alkaline N-deacetylation of chitin [43].
The objectives of the present study were to determine the effect of chitosan under different aW at 25 °C on the (i) lag phase, (ii) growth rate, and (iii) mycotoxin (fumonisin and dexynivalenol) production by F. verticillioides, F. proliferatum, and F. graminearum on irradiated maize and wheat grains.
2. Results
2.1. Chitosan Characterization
The average chitosan molecular weight (Mw) in g/mol determined by the Mark–Houwink–Sakurada equation was 3.42 ± 0.08 × 103 g/mol. The deacetylation degree (DD), determined by an infrared spectroscopy analysis, was 77.6%.
2.2. Effect of Chitosan Concentration and aW on Growth Rates
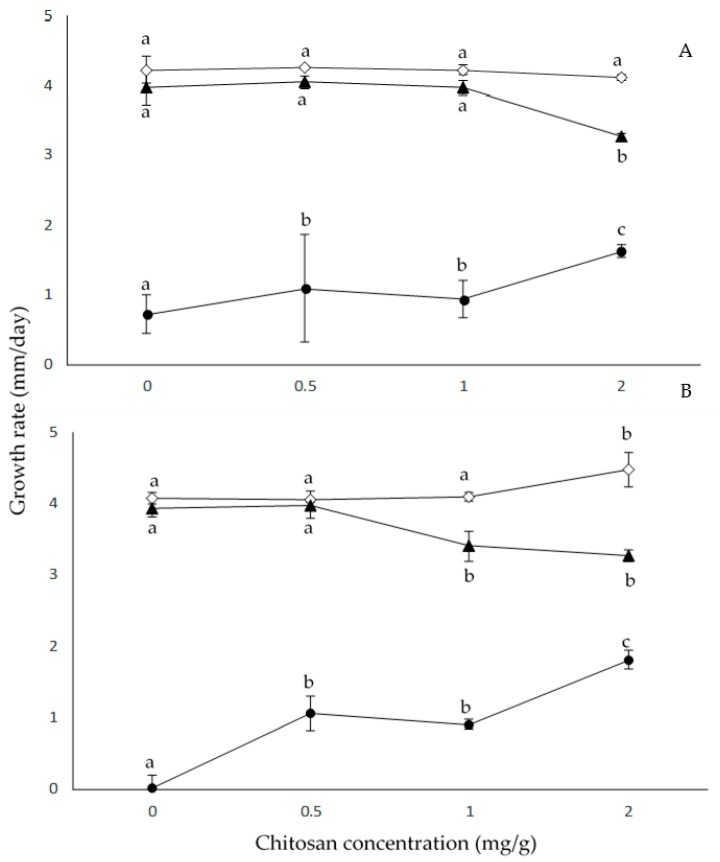
Figure 1 summarizes the effect of aW and chitosan doses on F. proliferatum RC2080 and F. verticillioides M7075 growth rates on irradiated maize grains. The results showed that increasing doses of chitosan at 0.99 aW in both Fusarium species did not affect their growth rates, while at 0.98 aW and 2 mg/mL chitosan, growth rates were significantly lower for both Fusarium species. Significant increases (p > 0.001) in growth rates were observed when F. proliferatum RC2080 and F. verticillioides M7075 were treated with chitosan at 0.95 aW; the increases were around 125% and 181% for F. proliferatum RC2080 and F. verticillioides M7075, respectively. No statistical differences were observed (p > 0.001) when F. proliferatum RC2080 was treated with chitosan doses of 0.5 and 1 mg/mL and 0.99 and 0.98 aW, regarding the control treatment. F. verticillioides M7075 treated with chitosan doses of 0.5 and 1 mg/mL and 0.99 aW showed no statistical differences (p > 0.001) compared to the control.
Figure 1.
Effect of chitosan and water activity (aW) (0.99 (◇), 0.98 (▲), 0.95 (⬤)) on growth rates of Fusarium proliferatum RC2080 (A) and F. verticillioides M7075 (B) strains on irradiated maize grains. Mean values based on biological triplicate data with letters in common for each aW are not significantly different according to the Tukey test (p > 0.001).
The statistical analysis using ANOVA of single factors (strain, chitosan dose, and aW) and of all interactions (two and three-way) on growth rate showed that the major effect was due to aW followed by the interaction chitosan × aW dose. The strain factor did not significantly affect the growth rate; therefore, it leads us to conclude that the strain behavior was the same in every condition tested (Table 1).
Table 1.
Analysis of variance on the effects of water activity (aW), chitosan dose (C), different strains (S), and their interactions on growth rates of Fusarium proliferatum and Fusarium verticilloides on irradiated maize grains.
| Source of Variation | df a | Growth Rates | |
|---|---|---|---|
| MS b | F c | ||
| S | 1 | 0.06 | 1.2 |
| C | 3 | 0.6 | 14 * |
| aW | 2 | 66.5 | 1364 * |
| S × C | 3 | 0.2 | 5.6 |
| S × aW | 2 | 0.07 | 1.4 |
| C × aW | 6 | 1.1 | 22 * |
| S × C × aW | 6 | 0.05 | 1.1 * |
* Significant at p < 0.001; a degrees of freedom; b mean square; c Snedecor-F.
Figure 2 summarizes the effect of aW and chitosan doses on F. graminearum strain growth rates on irradiated wheat grains. Under control conditions, maximum growth rates were observed at 0.995 and 0.98 aW depending on the F. graminearum strain tested. Growth rates of both F. graminearum strains significantly decreased when chitosan was used, as compared to the control conditions (Figure 2). The highest growth reduction with chitosan was observed at 0.98 aW for both strains. However, no significant differences were observed among chitosan concentrations at the same aW for both strains.
Figure 2.
Effect of chitosan and aW (0.995 (▲), 0.99 (⬜), 0.98 (⬤)) on growth rates of F. graminearum RCFG6001 (A) and F. graminearum RC22-2 (B) strains on irradiated wheat grains. Mean values based on biological triplicate data with letters in common for each aW are not significantly different according to the Tukey test (p > 0.001).
The statistical analysis using ANOVA of single factors (strains, aW, and chitosan dose) and their interactions on growth rates showed that aW and chitosan dose alone, as well as chitosan × aW interaction, were significant (p < 0.001), whereby chitosan was the factor that had the greatest effect on growth rates of both F. graminearum strains (Table 2).
Table 2.
Analysis of variance on the effects of aW, chitosan doses (C), and their interactions on growth rates of Fusarium graminearum RC22-2 and Fusarium graminearum RCFG6001 on irradiated wheat grains.
| Source of Variation | df a | Growth Rates | |
|---|---|---|---|
| MS b | F c | ||
| S | 1 | 56.4 | 1.8 |
| C | 3 | 8933.3 | 287.5 * |
| aW | 2 | 7043.5 | 226.6 * |
| S × C | 3 | 56.3 | 1.8 |
| S × aW | 2 | 95.9 | 3.1 |
| C × aW | 6 | 1095.3 | 35.2 * |
| S × C × aW | 6 | 88.4 | 2.8 |
* Significant at p < 0.001; a degrees of freedom; b mean square; c Snedecor-F.
2.3. Effect of Chitosan Concentration and aW on Mycotoxin Production
The F. verticillioides strain was able to produce higher amounts of fumonisins in almost all tested conditions in comparison with F. proliferatum. Maximum fumonisin production in the control condition for both strains was observed at 0.98 aW, and, at that aW, a significant reduction in FB levels was observed when chitosan treatments were used, reaching 88% and 95% reduction for F. proliferatum RC2080 and F. verticillioides M7075, respectively. For both strains, the lowest amounts of FBs were produced at 0.95 aW in the control condition. A high stimulation in fumonisin production by F. verticillioides M7075 was observed when the chitosan dose was 1 mg/g at 0.99 aW (Table 3); for the same strain, no significant differences were observed at 0.95 aW when chitosan was applied, while, for F. proliferatum, when chitosan dose was ≥1 mg/g at the same aW, FB production was significantly reduced. For both strains, in all conditions, FB1 was found in higher amounts than FB2 and FB3 (data not shown). The statistical analysis using ANOVA showed that single factors (strains, aW, and chitosan dose) and some interactions (S × aW and C × aW) were significant on fumonisin production. The major effect was given by aW, followed by strain factor (Table 4).
Table 3.
Combined effect of different concentrations of chitosan and aW on fumonisin (FB1 + FB2 + FB3 μg/g) accumulation by Fusarium verticillioides M7075 and Fusarium proliferatum RC2080 on irradiated maize at 25 °C.
| Strain | Chitosan Dose (mg/g) | aW | ||
|---|---|---|---|---|
| 0.99 | 0.98 | 0.95 | ||
| F. proliferatum RC2080 | 0 | 2017 ± 638 a | 5423 ± 2028 a | 819 ± 364 a |
| 0.5 | 2747 ± 651 a | 963 ± 118 b | 611 ± 489 a | |
| 1 | 1386 ± 333 b | 637 ± 98 c | 447 ± 284 b | |
| 2 | 2064 ± 537 a | 849 ± 403 b | 214 ± 219 c | |
| F. verticillioides M7075 | 0 | 4568 ± 645 a | 8582 ± 825 a | 211 ± 181 a |
| 0.5 | 1532 ± 1311 a | 2745 ± 525 b | 409 ± 81 a | |
| 1 | 9306 ± 1487 b | 1481 ± 843 b | 343 ± 44 a | |
| 2 | 3653 ± 931 a | 442 ± 57 d | 525 ± 71 a | |
Mean values of fumonisin concentration based on triplicate data with letters in common within a column and for each strain are not significantly different according to the Tukey HSD test (p > 0.001).
Table 4.
Analysis of variance on the effects of water activity (aW), chitosan dose (C), different strains (S), and their interactions on total fumonisin (FB1 + FB2 + FB3) production by Fusarium proliferatum and Fusarium verticillioides strains grown on irradiated maize grains, and on deoxynivalenol production by Fusarium graminearum strains grown on irradiated wheat grains.
| Source of Variation | Fumonisins | Deoxynivalenol | |||
|---|---|---|---|---|---|
| df a | MS b | F c | MS b | F c | |
| S | 1 | 42,955,342.1 | 27.7 * | 518.3 | 422.3 * |
| C | 3 | 18,021,595.5 | 11.6 * | 47.2 | 38.4 * |
| aW | 2 | 55,800,484.1 | 36.0 * | 43.2 | 35.2 * |
| S × C | 3 | 5,105,970.2 | 3.3 | 27.9 | 22.7 * |
| S × aW | 2 | 19,662,834.4 | 12.7 * | 7.8 | 6.4 |
| C × aW | 6 | 21,210,509.7 | 13.7 * | 12.7 | 10.4 * |
| S × C × aW | 6 | 6,420,659.2 | 4.1 | 5.9 | 4.8 * |
* Significant at p < 0.001; a degrees of freedom; b mean square; c Snedecor-F.
Regarding DON production, maximum concentrations were produced by the two F. graminearum strains at 0.995 aW in the control condition (without chitosan addition) on irradiated wheat grains. When chitosan treatments were applied to the irradiated wheat grains, DON levels were lower in comparison with the control condition, with the exception of F. graminearum RC22-2 at 0.99 aW, where a stimulation in DON production was observed. Overall, it was observed that the F. graminearum RC22-2 strain produced higher amounts of DON in comparison with the F. graminearum RCFG6001 strain (Table 5).
Table 5.
Combined effect of different concentration of chitosan and aW on deoxynivalenol (DON; ng/g) accumulation by Fusarium graminearum RCFG6001 and Fusarium graminearum RC22-2 on irradiated wheat at 25 °C.
| Strain | Chitosan Dose (mg/g) | aW | ||
|---|---|---|---|---|
| 0.995 | 0.99 | 0.98 | ||
| RCFG6001 | 0 | 21001 ± 1024 a | 119 ± 111 a | 177 ± 46 a |
| 0.5 | 93 ± 50 b | 148 ± 99 a | Nd b | |
| 1 | Nd c | 8.4 ± 6.8 b | Nd b | |
| 2 | Nd c | Nd c | Nd b | |
| RC22-2 | 0 | 32101 ± 1866 a | 1492 ± 489 a | 1918 ± 630 a |
| 0.5 | 16455 ± 1723 b | 2951 ± 392 b | 339 ± 68 b | |
| 1 | 5892 ± 210 c | 4094 ± 646 c | 2245 ± 564 a | |
| 2 | 8323 ± 934 c | 15466 ± 1135 d | 393 ± 201 b | |
Nd: not detected, lower than detection limit (<LOD). Mean values of DON concentration based on triplicate data with letters in common within a column for each strain are not significantly different according to the Tukey HSD test (p > 0.001).
DON production by F. graminearum RCFG6001 strain was reduced as long as increasing chitosan doses were used, with reductions between 93% and 100% in all treatments and aW tested. DON production by F. graminearum RC22-2 was reduced at 0.995 and 0.98 aW when chitosan was applied at all used doses, with the exception of 0.98 aW when 1 mg/g was used and a slight stimulation in DON production was observed. Nevertheless, it was not significant (Table 5).
The analysis of the effect of individual factors (strains, chitosan dose, and aW) and most interactions (two- and three-way) on DON production showed that all the factors and interactions were statistically significant. Strain factor was the variable that most affected DON production, followed by chitosan dose and aW (Table 4).
3. Discussion
The present study highlights the potential of chitosan for controlling both growth and mycotoxin production (FBs and DON) by important Fusarium species, which are common cereal contaminants. However, it is important to consider that different responses to chitosan use were found among different fungal strains. Notably, at the lowest chitosan concentration used, we observed reductions in growth rates and an important effect on DON production by both F. graminearum strains, independently of the aW. The only exception was observed at 0.99 aW for strain RC22-2.
Regarding the Fusarium species that produce fumonisins (F. verticillioides and F. prolifeartum), we only observed a significant reduction in growth rates at 0.98 aw for both species at the highest chitosan dose used. Overall, important FB reductions were found at the lowest doses used (0.5 mg/g) at 0.98 aW, which is considered the most suitable aW for this mycotoxin production on maize [44]. Moreover, for the other aW, the most effective chitosan dose for fumonisin reduction was 1 mg/g with the exception of F. verticillioides at 0.99 aW.
The antifungal activity of chitosan over a broad range of phytopathgenic fungi was the subject of several review papers [35,45,46,47]. To the best of our knowledge, none of the previous studies reported on the interactions between the efficacy of chitosan or chitosan derivates and aW or temperature, which were demonstrated to be key parameters determining germination, growth, and mycotoxin production by different fungal species [20,48].
Also, there are no other studies in the literature evaluating both the chitosan and aW effect on Fusarium species growth and mycotoxin production on irradiated grains. There was only one study in vitro previously carried out in our laboratory by Ferrochio et al. [42], who demonstrated that different chitosan doses from 0.5 to 3.0 mg/L, combined with aW reduction, increased lag phases and decreased mycelial growth rates in F. verticillioides and F. proliferatum strains, when the study was performed in a wheat-based media at 25 °C. The lowest growth rate was obtained at the highest chitosan concentration tested (3 mg/mL) and at the lowest aW (0.93). However, these results are different from those obtained in the present study using the same F. verticillioides and F. proliferatum strains on irradiated maize grains. This could be explained by the fact that, although irradiated grains contained all the nutritional elements in order to allow the fungal development, they could represent a natural barrier in the penetration of Fusarium, thus restricting the fungal growth. However, good levels of fumonisin reduction were achieved in both studies at the lowest chitosan doses used (0.5 mg/g).
The antifungal activities of chitosan relied on several intrinsic and extrinsic factors, such as pH, fungal species, presence or absence of metal cations, pKa, Mw, DD, etc. [35]. Verlee et al. [45] criticized that most papers dealing with antifungal activity did not report the characterization (Mw and DD) of chitosan. For all these reasons, during the present work, some characteristics of the commercial chitosan used were determined. The results showed that chitosan presents a low molecular weight and 71% DD. For soluble chitosan, pH is a crucial factor related to solubility, and can further alter antifungal activity. The antifungal activity of chitosan is exhibited when the pH is below the representative pKa (~6.5), the value at which the soluble molecule could be dissociated as ions. In the present study, the pH of the chitosan solution was maintained below the pKa.
There were several attempts, in the last few years, to use chitosan for reducing FHB and DON contamination. Khan et al. [49] showed that both chitosan and Pseudomonas fluorescens (biological control agent) were very effective in reducing FHB disease caused by F. culmorum and DON contamination in wheat and barley grains, when they were applied at high disease pressure under glasshouse and field experiments. They concluded that, overall, chitosan was more effective than the potential biocontrol bacterium P. fluorescens strain MKB158. Also, Kheiri et al. [50], reported a good in vitro effect on growth of F. graminearum and FHB severity reduction in a glasshouse experiment using chitosan and chitosan nanoparticles, but they did not evaluate DON contamination.
In our group, we demonstrated the efficacy of Bacillus velezensis RC218 and Streptomyces albidoflavus RC87B for reducing FHB severity and DON accumulation by F. graminearum under glasshouse and field experiments [24]. Taking into account the good results obtained during the present work with chitosan and F. graminearum in situ, we are developing further experiments combining the biological control agent with chitosan in order to improve the achieved results.
In conclusion, the present study showed the combined effects of chitosan and aW on growth and mycotoxin production by the three most important Fusarium species present on maize and wheat. Also, it was demonstrated that low-Mw chitosan with more than 70% DD at 0.5 mg/mL was able to significantly reduce growth rate and mycotoxin production on irradiated grains. It is important to remark that the effect was strain-specific, and, for this reason, these types of antifungal tests need to be made with at least two strains. Since mycotoxins are unavoidable contaminants in food and feed chains, their presence needs to be reduced in order to reduce their effects on human and animal health and to decrease the annual market losses caused by rejected cereals. In this scenario, pre/post-harvest (for F. graminearum) and post-harvest (for F. verticillioides and F. proliferatum) use of chitosan could be an important alternative. The use of chitosan is proposed as a post-harvest alternative treatment in maize in Argentina since permanent storage capacity is not increasing at the same rate as cereal production. Thus, a substantial portion of the harvest (17 million tons) is stored in temporary hermetic storage systems called silo-bags that remain in the field for long periods (over five months). Normally, these bags are filled with maize at 14% to 16% moisture content (wet basis) (0.72–0.8 aw) [51]. Pacin et al. [52] evaluated fumonisin contamination in maize stored in silo-bags and observed that, in maize stored for around 200 days following good agricultural practices, fumonisin contamination increased significantly. Regarding wheat grains, we propose the use of chitosan as a pre- and post-harvest strategy. As the global wheat production increased steadily in recent years, a substantial portion of the harvest is stored under natural conditions with no controlled facilities in many developed countries. Yuan et al. [53] observed that DON levels increased up to 30% after three months of storage. Thus, post-harvest control strategies are necessary, and chitosan could also be used. However, it is necessary to continue with further basic studies that could contribute to explaining the effect of applying this polymer on cereals.
4. Materials and Methods
4.1. Chitosan Solution
Low-viscosity chitosan was used (Fluka 50494; LVC; viscosity: ≤200 mPa∙s). A stock solution was prepared by dissolving 10 g/L of chitosan in 1% acetic acid (AcH), and the solution was stirred for 24 hours at 28 °C. The pH of the chitosan solution was adjusted to 5.6 using NaOH in order to ensure that all the chitosan amino groups were positively charged [35,54]. Then, the solution obtained was autoclaved at 121 °C for 15 min, and maintained at 4 °C until use.
4.2. Chitosan Characterization
The viscosity–average molecular weight (Mw) of chitosan was determined using the intrinsic viscometric method using the Mark–Houwink–Sakurada equation [55]. The percentage of the chitosan amino groups (degree of deacetylation, DD) was determined using an infrared spectroscopy (Bruker Tensor 27) analysis, applying the following equation:
| DD (%) = 97.67 − 26.486 × (A1655/A3450), | (1) |
where A1655 is the absorbance at 1655 cm−1 of the amide I band and A3450 is the absorbance at 3450 cm−1 of the hydroxyl band [37].
4.3. Fungal Strains
One F. verticillioides (M7075) strain and one F. proliferatum (RC2080) strain were used. Both strains were isolated from maize in Argentina [56,57] and were characterized using a polyphasic approach: morphological, biological, and genetic (amplified fragment length polymorphisms; AFLP). Also, fumonisin production capability was demonstrated. Moreover, two strains identified as F. graminearum sensu stricto, RC22-2 and RCFG6001, respectively, isolated from wheat spikes with Fusarium head blight (FBH) symptoms, were included in the present study. These strains were identified morphologically, by AFLP, and by partial sequencing of elongation factor gene (EF-1α); DON production capability was also demonstrated in previous studies [21,58]. The strains were deposited at the Department of Microbiology and Immunology, Universidad Nacional de Rio Cuarto culture collection (RC). Cultures were maintained in 15% glycerol at −80 °C (Table S1 in the Supplementary Materials).
4.4. Grains
Maize and wheat grains were gamma-irradiated (10–12 kGy) using a cobalt radiation source and were stored aseptically at 4 °C. Grains contained no fungal infection or contamination, but retained germinative capacity. The initial values of aW of the grains were 0.75 and 0.65 for maize and wheat, respectively. Irradiated maize and wheat grains were weighed and placed into sterile flasks and rehydrated to the required aW (0.99, 0.98, and 0.95 for maize, and 0.995, 0.99, and 0.98 for wheat) via addition of sterile distilled water using a moisture absorption curve. An appropriate aliquot of water was replaced by chitosan stock solution to give to the irradiated grains a final concentration (0.5, 1, and 2 mg/g). Flasks were subsequently refrigerated at 4 °C for 48 h with periodic shaking to allow absorption and equilibration. At the end of this period, the aW was checked with an Aqualab Series 3 (Decagon Devices, Inc, WA, USA). The rehydrated maize and wheat were placed in sterile 9-cm Petri dishes to form thin layers of grains (~20 g).
In order to discard fungal inhibition due to acetic acid in the chitosan solution, irradiated grains without chitosan (control AcH) were prepared for each aW level used. Those AcH control plates were prepared by adding the same volume of 1% AcH (pH adjusted to 5.6) used to prepare 2 mg/g of chitosan-amended wheat and maize grains.
4.5. Inoculation, Incubation, and Growth Assessment
All plates were inoculated with a 4-mm-diameter agar disc that was taken from the margin of a seven-day-old colony of each strain grown on synthetic nutrient agar at 25 °C [59]. The discs were transferred face-down onto the center of each plate. Inoculated Petri plates containing grains of the same aW were enclosed in plastic containers together with two beakers of NaCl–water solutions of the same aW as the treatments to maintain constant equilibrium relative humidity (ERH) inside the boxes. Containers were incubated at 25 °C for 28 and 21 days for irradiated maize grains and irradiated wheat grains, respectively.
Experiments were carried out in triplicate for each treatment. Assessment of growth was made daily during the incubation period, and two diameters of the growing colonies were measured at right angles to each other until the colony reached the edge of the plate. The radii of the colonies were plotted against time, and linear regression was applied in order to obtain the growth rate (mm/day) as the slope of the line. After the incubation period, three complete Petri plate cultures per treatment were sampled, dried at 50 °C for 24 h, and stored at −20 °C until toxin analysis (FB extraction from irradiated maize grains, and DON extraction from irradiated wheat grains).
4.6. Mycotoxin Determination
4.6.1. Fumonisin Determination in Maize
Each sample was finely ground and mixed well. A sub-sample (15 g) was extracted with 40 mL of a mixture of acetonitrile–water (1:1 v/v) for 30 min using an orbital shaker (150 rpm) and then filtering the extracts through a paper filter (Nº 4, Whatman International Ltd, Maidstone, Kent, United Kingdom). An aliquot of extract (1000 μL) was taken and stored in an Eppendorf tube at −20 °C until HPLC analysis.
An aliquot (50 μL) of this solution was derivatized with 200 μL of an o-phthaldialdehyde (OPA) solution obtained by adding 5 mL of 0.1 M sodium tetraborate and 50 µL of 2-mercaptoethanol to 1 mL of methanol containing 40 mg of OPA [60]. The fumonisin OPA derivates (50 μL solution) were analyzed using a reversed-phase HPLC/fluorescence detection system. The HPLC system consisted of a Hewlett-Packard 1100 pump (Hewlett-Packard, Palo Alto, CA, USA) connected to a Hewlett-Packard 1046A programmable fluorescence detector and a data module Hewlett-Packard Kayak XA (HP ChemStation Rev. A.06.01). Chromatographic separations were performed on a stainless-steel, C18 reversed-phase column (150 × 4.6 mm inner diameter (i.d.), 5 μm particle size; Luna-Phenomenex, Torrance, CA, USA) connected to Security Guard cartridge (4 × 3 mm i.d., 5 μm particle size; Phenomenex, Torrance, CA, USA) filled with the same phase. Methanol–0.1 M sodium dihydrogen phosphate (75:25, v/v) solution adjusted to pH 3.35 with orthophosphoric acid was used as the mobile phase, at a flow rate of 1.5 mL/min. Fluorescence of the fumonisin OPA derivatives was recorded at excitation and emission wavelengths of 335 and 440 nm, respectively. Fumonisins were measured as peak heights and compared with reference standard solutions (Sigma Chemical Co, St. Louis, MO, USA). A mixed acetonitrile–water (1:1, v/v) stock solution of FB1, FB2, and FB3 containing 50 μg/mL of each toxin was prepared. Four mixed working calibrant solutions (0.25, 0.5, 1.0, and 2.0 μg/mL) were prepared by diluting an aliquot of the stock solution with the appropriate volume of acetonitrile–water (1:1, v/v). The retention times of FB1, FB3, and FB2 were 7.5, 16.7, and 18.5 min, respectively. Appropriate dilutions of standards and/or sample extracts were made with acetonitrile–water (1:1). The detection limit (LOD) of the analytical method for the three fumonisins was 1 µg/g based on the signal-to-noise ratio 3:1.
4.6.2. Deoxynivalenol Determination in Wheat
The DON analysis was done using a modified version of that originally reported by Cooney et al. [61]. Each sample was finely ground and mixed well. A sub-sample (15 g) was extracted by mixing with acetonitrile–methanol (14:1; 40 mL), shaken for 2 h, and then filtered through filter paper (Whatman N° 1, Whatman International Ltd, Maidstone, Kent, United Kingdom). A syringe was plugged with glass wool and dry-packed with alumina/carbon (20:1; 300 mg) to form a mini-cleanup column. A 2-mL aliquot of extract was applied to the column and allowed to drain under gravity, and the eluent was collected. The column was washed with 500 µL of a mixture of acetonitrile–methanol–water (80:5:15 v/v), and the combined eluant was evaporated to dryness using N2 at 50 °C. The cleaned-up residue was dissolved in 500 µL of methanol–water (5:95 v/v) and stored until HPLC analysis. The HPLC system consisted of a Hewlett-Packard model 1100 pump (Palo Alto, CA) connected to a Hewlett-Packard 1100 Series variable wavelength detector and a data module Hewlett-Packard Kayak XA (HP ChemStation Rev. A.06.01). Chromatographic separations were performed on a Luna™ C18 reversed-phase column (100 × 4.6 mm, 5 μm particle size) connected to a guard column, SecurityGuard™ (4 × 3.0 mm), filled with the same phase. The mobile phase consisted of methanol–water (12:88, v/v), at a flow rate of 1.5 mL∙min−1. The detector was set at 220 nm with an attenuation of 0.01 AUFS (absorbance units full scale). The injection volume was 50 μL. Quantification was relative to external standards of DON (Sigma-Aldrich Co. St Louis, MO) of 1 to 4 μg/mL in methanol–water (5:95). The quantification (LOD) limit was 5 ng/g.
4.7. Statistical Treatment of Results
The growth rates, lag phases, and mycotoxin concentrations were evaluated by analysis of variance (ANOVA) to determine the effect of chitosan doses, aW, Fusarium species, and two- and three-way interactions. When the analysis was statistically significant, the post hoc Tukey´s multiple comparison procedure was used for separation of the means. Statistical significance was judged at the level p ≤ 0.001. All the analyses were done using SigmaStat for Windows Version 2.03 (SPSS Inc.)
Acknowledgments
E.C. and M.J.N. are fellows of CONICET, and V.G.L.Z., M.L.R., and S.N.C. are members of the Research Career of CONICET.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/8/1/29/s1, Table S1: Characteristics of the Fusarium strains used.
Author Contributions
M.L.R. and S.N.C. designed the entire experiment. V.G.L.Z. was responsible for carrying out the entire experimental work. M.J.N. and V.G.L.Z. performed mycotoxin analysis. E.C. and V.G.L.Z. were responsible for data analysis. E.C. and M.L.R. wrote the paper, and M.J.N., V.G.L.Z., and S.N.C. revised the manuscript.
Funding
This research was supported by grants from SECyT-UNRC (Secretaria de Ciencia y Técnica, Universidad Nacional de Río Cuarto) and ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica) PICT-2015-1253.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Marin S., Sanchis V., Ramos A.J., Vinas I., Magan N. Environmental factors, in vitro interactions, and niche overlap between Fusarium moniliforme, F. proliferatum, and F. graminearum, Aspergillus and Penicillium species from maize grain. Mycol. Res. 1998;102:831–837. doi: 10.1017/S0953756297005777. [DOI] [Google Scholar]
- 2.Kriek N.P.J., Marases W.F.O., Thiel P.G. Hepato-and Cardiotoxicity of Fusarium verticillioides (F. moniliforme) isolates from southern african maize. Food Cosmet. Toxicol. 1981;19:447–456. doi: 10.1016/0015-6264(81)90449-1. [DOI] [PubMed] [Google Scholar]
- 3.Cardwell K.F., Kling J.G., Maziya-Dixon B., Bosque-Pérez N.A. Interactions between Fusarium verticillioides, Aspergillus flavus, and insect infestation in four maize genotypes in Lowland Africa. Phytopathology. 2000;90:276–284. doi: 10.1094/PHYTO.2000.90.3.276. [DOI] [PubMed] [Google Scholar]
- 4.Marasas W.F.O., Kriek N.P.J., Wiggins V.M., Steyn P.S., Towers D.K., Hastie T.J. Incidence, geographic distribution, and toxigenicity of Fusarium species in South African corn. Phytopathology. 1979;69:1181–1185. doi: 10.1094/Phyto-69-1181. [DOI] [Google Scholar]
- 5.González H.H.L., Resnik S.L., Boca R.T., Marasas W.F.O. Mycoflora of Argentinian corn harvested in the main production area in 1990. Mycopathologia. 1995;130:29–36. doi: 10.1007/BF01104346. [DOI] [PubMed] [Google Scholar]
- 6.Chulze S.N., Ramirez M.L., Farnochi M.C., Pascale M., Visconti A., March G. Fusarium and fumonisin occurrence in Argentinian corn at different ear maturity stages. J. Agric. Food Chem. 1996;44:2797–2801. doi: 10.1021/jf950381d. [DOI] [Google Scholar]
- 7.Picco M., Nesci A., Barros G., Cavaglieri L., Etcheverry M. Aflatoxin B1 and fumosin B1 in mixed cultures of Aspergillus flavus and Fusarium proliferatum on maize. Nat. Toxins. 1999;7:331–336. doi: 10.1002/1522-7189(199911/12)7:6<331::AID-NT89>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Munkvold G.P. Fusarium species and their associated mycotoxins. In: Moretti A., Susca A., editors. Mycotoxigenic Fungi: Methods and Protocols, Methods in Molecular Biology. Volume 1542. Humana Press; New York, NY, USA: 2017. pp. 51–106. [DOI] [PubMed] [Google Scholar]
- 9.Marasas W.F.O. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001;109:5. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marasas W.F.O., Riley R.T., Hendricks K.A., Stevens V.L., Sadler T.W., Gelineau-van Waes J., Missmer S.A., Cabrera J., Torres O., Gelderblom W.C.A., et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 11.Missmer S.A., Suarez L., Felkner M., Wang E., Merrill A.H., Rothman K.J., Hendricks K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas–Mexico border. Environ. Health Perspect. 2006;114:237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun G., Wang S., Hu X., Su J., Huang T., Yu J., Tang L., Gao W., Wang J.-S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007;24:181–185. doi: 10.1080/02652030601013471. [DOI] [PubMed] [Google Scholar]
- 13.Wild C.P., Gong Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimanya M.E., De Meulenaer B., Roberfroid D., Lachat C., Kolsteren P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- 15.Shirima C.P., Kimanya M.E., Routledge M.N., Srey C., Kinabo J.L., Humpf H.-U., Wild C.P., Gong Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during Early Childhood in Tanzania. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1408097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. World Health Organization and International Agency for Research on Cancer . Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Volume 82. IARC (International Agency for Research on Cancer); Geneva, Switzerland: 1980. pp. 301–366. [Google Scholar]
- 17.World Health Organization (WHO) Safety Evaluation of Certain Mycotoxins in Food. Food & Agriculture Org.; Rome, Italy: 2001. [Google Scholar]
- 18.Bottalico A., Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. In: Logrieco A., Bailey J.A., Corazza L., Cooke B.M., editors. Mycotoxins in Plant Disease. Springer; Dordrecht, The Netherlands: 2002. pp. 611–624. [Google Scholar]
- 19.Lori G.A., Sisterna M.N., Haidukowski M., Rizzo I. Fusarium graminearum and deoxynivalenol contamination in the durum wheat area of Argentina. Microbiol. Res. 2003;158:29–35. doi: 10.1078/0944-5013-00173. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez M.L., Chulze S., Magan N. Temperature and water activity effects on growth and temporal deoxynivalenol production by two Argentinean strains of Fusarium graminearum on irradiated wheat grain. Int. J. Food Microbiol. 2006;106:291–296. doi: 10.1016/j.ijfoodmicro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez M.L., Reynoso M.M., Farnochi M.C., Torres A.M., Leslie J.F., Chulze S.N. Population genetic structure of Gibberella zeae isolated from wheat in Argentina. Food Addit. Contam. 2007;24:1115–1120. doi: 10.1080/02652030701546487. [DOI] [PubMed] [Google Scholar]
- 22.Dalcero A., Torres A., Etcheverry M., Chulze S., Varsavsky E. Occurrence of deoxynivalenol and fusarium graminearum in Argentinian wheat. Food Addit. Contam. 1997;14:11–14. doi: 10.1080/02652039709374492. [DOI] [PubMed] [Google Scholar]
- 23.González H.H.L., Pacin A., Resnik S.L., Martinez E.J. Deoxynivalenol and contaminant mycoflora in freshly harvested Argentinian wheat in 1993. Mycopathologia. 1996;135:129–134. doi: 10.1007/BF00436463. [DOI] [PubMed] [Google Scholar]
- 24.Palazzini J.M., Alberione E., Torres A., Donat C., Köhl J., Chulze S. Biological control of Fusarium graminearum sensu stricto, causal agent of Fusarium head blight of wheat, using formulated antagonists under field conditions in Argentina. Biol. Control. 2016;94:56–61. doi: 10.1016/j.biocontrol.2015.12.009. [DOI] [Google Scholar]
- 25.Audenaert K., Vanheule A., Höfte M., Haesaert G., Audenaert K., Vanheule A., Höfte M., Haesaert G. Deoxynivalenol: A major player in the multifaceted response of Fusarium to its environment. Toxins. 2013;6:1–19. doi: 10.3390/toxins6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pestka J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- 27.Sevastos A., Markoglou A., Labrou N.E., Flouri F., Malandrakis A. Molecular characterization, fitness and mycotoxin production of Fusarium graminearum laboratory strains resistant to benzimidazoles. Pest. Biochem. Physiol. 2016;128:1–9. doi: 10.1016/j.pestbp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Sun B., Zhang L., Yang L., Zhang F., Norse D., Zhu Z. Agricultural non-point source pollution in China: Causes and mitigation measures. Ambio. 2012;41:370–379. doi: 10.1007/s13280-012-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mateo E.M., Gómez J.V., Gimeno-Adelantado J.V., Romera D., Mateo-Castro R., Jiménez M. Assessment of azole fungicides as a tool to control growth of Aspergillus flavus and aflatoxin B1 and B2 production in maize. Food Addit. Contam. Part A. 2017;34:1039–1051. doi: 10.1080/19440049.2017.1310400. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez M.L., Chulze S., Magan N. Impact of environmental factors and fungicides on growth and deoxinivalenol production by Fusarium graminearum isolates from Argentinian wheat. Crop Protec. 2004;23:117–125. doi: 10.1016/j.cropro.2003.07.005. [DOI] [Google Scholar]
- 31.Malerca M., Cerana R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016;17:996. doi: 10.3390/ijms17070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alburquenque C., Bucarey S.A., Neira-Carrillo A., Urza B., Hermosilla G., Tapia C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010;48:1018–1023. doi: 10.3109/13693786.2010.486412. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H., Li R., Liu W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. J. Mol. Sci. 2011;12:917–934. doi: 10.3390/ijms12020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayakumar R., Prabaharan M., Sudheesh Kumar P.T., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Ziani K., Fernández-Pan I., Royo M., Maté J.I. Antifungal activity of films and solutions based on chitosan against typical seed fungi. Food Hydrocoll. 2009;23:2309–2314. doi: 10.1016/j.foodhyd.2009.06.005. [DOI] [Google Scholar]
- 37.Cota-Arriola O., Cortez-Rocha M.O., Rosas-Burgos E.C., Burgos-Hernández A., López-Franco Y.L., Plascencia-Jatomea M. Antifungal effect of chitosan on the growth of Aspergillus parasiticus and production of aflatoxin B1. Polym. Int. 2011;60:937–944. doi: 10.1002/pi.3054. [DOI] [Google Scholar]
- 38.Cuero R.G., Osuji G., Washington A. N-carboxymethyl chitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991;13:441–444. doi: 10.1007/BF01030998. [DOI] [Google Scholar]
- 39.Fonseca Moreira da Silva J., Prado G., Gazzinelli Cruz Madeira J.E., Silva Oliveira M., Gomes Faraco A.A., Martins Malta C., Nicoli J.R., Sanzio Pimenta R. Utilização de filme de quitosana para o controle de aflatoxinas em amendoim. Bragantia. 2015;74:467–475. doi: 10.1590/1678-4499.0120. [DOI] [Google Scholar]
- 40.Reddy M.V.B., Arul J., Ait-Barka E., Angers P., Richard C., Castaigne F. EVect of Chitosan on Growth and Toxin Production by Alternaria alternata f. sp. lycopersici. Biocontrol Sci. Technol. 1998;8:33–43. doi: 10.1080/09583159830414. [DOI] [Google Scholar]
- 41.Mateo E.M., Valle-algarra F.M., Mateo R., Jiménez M., Magan N. Effect of fenpropimorph, prochloraz and tebuconazole on growth and production of T-2 and HT-2 toxins by Fusarium langsethiae in oat-based medium. Int. J. Food Microbiol. 2011;151:289–298. doi: 10.1016/j.ijfoodmicro.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Ferrochio L.V., Cendoya E., Zachetti V.G.L., Farnochi M.C., Massad W., Ramirez M.L. Combined effect of chitosan and water activity on growth and fumonisin production by Fusarium verticillioides and Fusarium proliferatum on maize-based media. Int. J. Food Microbiol. 2014;185:51–56. doi: 10.1016/j.ijfoodmicro.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Chung Y.C., Wang H.L., Chen Y.M., Li S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003;88:179–184. doi: 10.1016/S0960-8524(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 44.Marín S., Sanchis V., Teixido A., Saenz R., Ramos A.J., Vinas I., Magan N. Water and temperature relations and microconidial germination of Fusarium moniliforme and Fusarium proliferatum from maize. Can. J. Microbiol. 1996;42:1045–1050. doi: 10.1139/m96-134. [DOI] [PubMed] [Google Scholar]
- 45.Verlee A., Mincke S., Stevens C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017;164:268–283. doi: 10.1016/j.carbpol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Hosseinnejad M., Jafari S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016;85:467–475. doi: 10.1016/j.ijbiomac.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Sahariah P., Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules. 2017;18:3846–3868. doi: 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- 48.Sanchis V., Magan N. Environmental conditions affecting mycotoxins. In: Magan N., Olsen M., editors. Mycotoxins in Food: Detection and Control. Woodhead Publishing Ltd.; Oxford, UK: 2004. pp. 174–189. [Google Scholar]
- 49.Khan M.R., Doohan F.M. Comparison of the efficacy of chitosan with that of a fluorescent pseudomonad for the control of Fusarium head blight disease of cereals and associated mycotoxin contamination of grain. Biol. Control. 2009;48:48–54. doi: 10.1016/j.biocontrol.2008.08.014. [DOI] [Google Scholar]
- 50.Kheiri A., Moosawi Jorf S.A., Mallihipour A., Saremi H., Nikkhah M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016;93:1261–1272. doi: 10.1016/j.ijbiomac.2016.09.072. [DOI] [PubMed] [Google Scholar]
- 51.Chulze S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. Part A. 2010;27:651–657. doi: 10.1080/19440040903573032. [DOI] [PubMed] [Google Scholar]
- 52.Pacin A.M., Ciancio Bovier E., González H.H.L., Whitechurch E.M., Martínez E.J., Resnik S.L. Fungal and fumonisins contamination in Argentine maize (Zea mays L.) silo bags. J. Agric. Food Chem. 2009;57:2778–2781. doi: 10.1021/jf803609c. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Q.-S., Yang P., Wu A.-B., Zuo D.-Y., He W.-J., Guo M.-W., Huang T., Li H.-P., Liao Y.-C. Variation in the microbiome, trichothecenes, and aflatoxins in stored wheat grains in Wuhan, China. Toxins. 2018;10:171. doi: 10.3390/toxins10050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H., Du Y., Wang X., Sun L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004;95:147–155. doi: 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Knaul J.Z., Kasaii M.R., Bui V.T., Creber K.A. Characterization of deacetylated chitosan and chitosan molecular weight review. Can. J. Chem. 1998;76:1699–1706. doi: 10.1139/cjc-76-11-1699. [DOI] [Google Scholar]
- 56.Etcheverry M., Torres A., Ramirez M.L., Chulze S., Magan N. In vitro control of growth and fumonisin production by Fusarium verticillioides and F. proliferatum using antioxidants under different water availability and temperature regimes. J. Appl. Microbiol. 2002;92:624–632. doi: 10.1046/j.1365-2672.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 57.Reynoso M.M., Torres A.M., Chulze S.N. Fusaproliferin, beauvericin and fumonisin production by different mating populations among the Gibberella fujikuroi complex isolated from maize. Mycol. Res. 2004;108:154–160. doi: 10.1017/S095375620300892X. [DOI] [PubMed] [Google Scholar]
- 58.Palacios S.A., Giaj Merlera G., Erazo J., Reynoso M.M., Farnochi M.C., Torres A.M. Trichothecene genotype and genetic variability of Fusarium graminearum and F. cerealis isolated from durum wheat in Argentina. Eur. J. Plant Pathol. 2017;149:969–981. doi: 10.1007/s10658-017-1247-0. [DOI] [Google Scholar]
- 59.Gerlach W., Nirenberg H. The genus Fusarium—A pictorial atlas. Mitteilungen aus der Biologischen Bundesanstalt fur Land- und Forstwirtschaft Berlin-Dahlem. 1982;209:1–406. doi: 10.2307/3792677. [DOI] [Google Scholar]
- 60.Shephard G.S., Sydenham E.W., Thiel P.G., Gelderblom W.C.A. Quantitative determination of fumonisins B1 and B2 by High-Performance Liquid Chromatography with fluorescence detection. J. Liq. Chromatogr. 1990;13:2077–2087. doi: 10.1080/01483919008049014. [DOI] [Google Scholar]
- 61.Cooney J.M., Lauren D.R., Di Menna M.E. Impact of competitive fungi on trichothecene production by Fusarium graminearum. J. Agric. Food Chem. 2001;49:522–526. doi: 10.1021/jf0006372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.