Abstract
Microalgae are the primary producers of carbon in marine ecosystems, fixing carbon and subsequently generating various biomolecules such as carbohydrates, proteins and lipids. Most importantly, microalgae are the generators and main suppliers of ω3 polyunsaturated fatty acids (ω3PUFA) in the marine ecosystem, which have a fundamental importance for the functioning and quality of the whole marine food web. A meta-analysis of over 160 fatty acid profiles of 7 marine phytoplankton phyla reveals not only a phyla-specific, but also a highly class-specific PUFA production of marine phytoplankton. The highest EPA (Eicosapentaenoic acid; 20:5ω3) production per total fatty acids was found in 2 classes of Haptophyta and in Ochrophyta, while Dinophyta and the Haptophyte Emiliana huxleyi show the highest production of DHA (Docosahexaenoic acid; 22:6ω3). An important precursor for EPA, Stearidonic acid (SDA, 18:4ω3) is found in high proportions in Cryptophyta and the Chlorophta class Pyramimonadophyceae. Per unit of carbon, Chlorophyta and Cyanobacteria were the poorest producers of highly unsaturated fatty acids (HUFA). The remaining phyla had a similar HUFA contribution per unit of carbon but with different compositions. The nutritional and environmental effects on the phytoplankton PUFA production is summarized and shows a lowering of the PUFA content under stressful environmental conditions.
Keywords: marine phytoplankton, fatty acids, fatty acid synthesis, PUFA synthesis, EPA, DHA, SDA, environmental effects
1. Introduction
The basis of the marine pelagic ecosystem lies with the primary producers, the unicellular phytoplankton that fix inorganic carbon (CO2) with the aid of sunlight (photosynthesis). The carbon, fixed in the form of glucose within the phytoplankton, is directed into various types of molecular components mainly combined with phosphorus and/or nitrogen, making up the building blocks of the phytoplankton cell. These building blocks are protein, lipids and carbohydrates, and they are the nutritional foundation for the upper trophic levels in the marine environment, starting with the secondary producers, e.g., copepods and krill, controlling their growth, reproduction, fitness and survival. The ability of zooplankton to concentrate and store phytoplankton-based lipids [1,2,3] means that they are key trophic vectors, channeling these essential nutritional compounds towards fish, seabirds, marine mammals and eventually humans.
This review focuses on the chemical composition of marine phytoplankton as a nutritional source for marine zooplankton that could limit their reproduction and survival and therefore affect the efficiency of the entire marine food web. The starting point is the notion, taken from various studies, that different phytoplankton types offered as food for zooplankton differ greatly in their ability to support zooplankton growth and survival, ranging from being essentially non-nutritious to being excellent food [4,5,6,7]. This suggested some important differences in phytoplankton chemistry that mattered for the nutritional needs of their consumers.
Therefore, it was important to become familiar with the chemical composition of phytoplankton and establish which components determine the quality of the phytoplankton as food for their consumers. The nutritional components are usually the micro-molecules: the building blocks of the different macromolecules. Those building blocks could be, for example, the specific fatty acids, vitamins, trace metals and specific amino acids.
The essential nutrients are the ones that the organisms cannot synthesize themselves, which they have to obtain from their diet. Studies had shown that crustaceans do not or cannot easily biosynthesize the ω3 and ω6 polyunsaturated fatty acids (PUFAs), and that these fatty acids are found in crustaceans in proportion to their availability in their diet [1,3,8,9,10]. Therefore, the focus has been on the fatty acid composition of marine phytoplankton which has indeed shown the importance of PUFA on reproduction and the growth of secondary producers of the oceans [11,12,13,14,15].
Here, I begin by giving a general summary of the chemical composition of marine phytoplankton phyla, with a special emphasis on fatty acid synthesis and biochemistry. I will present the results of a meta-analysis of fatty acid profiles in various phytoplankton groups and summarize which environmental parameters affect the biochemical pathways of fatty acid synthesis.
2. The Gross Chemical Composition of Phytoplankton
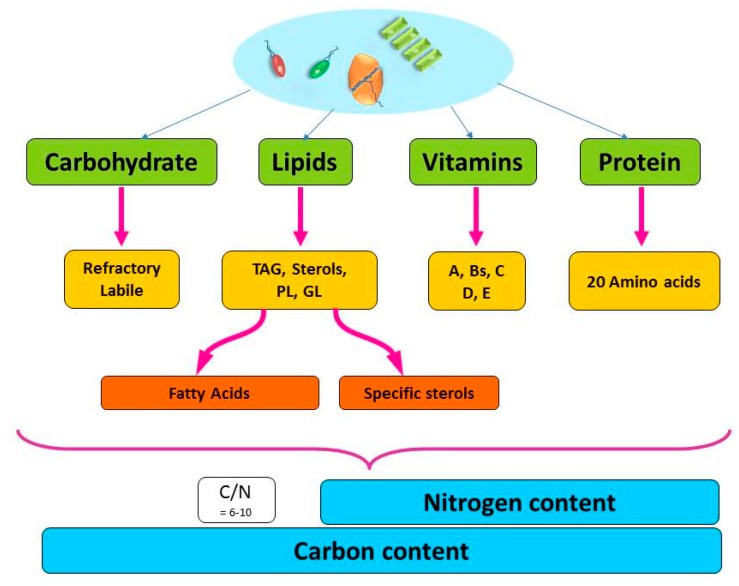
Carbon is the main element in most molecular structures in the phytoplankton cell and is often used as an indicator of the phytoplankton biomass. However, the quality of carbon can vary greatly based on the compound to which it is bound (Figure 1). In the phytoplankton cell, carbon is found in all macromolecules such as carbohydrates and lipids. Nitrogen is primarily bound in protein, and, as it is essential for phytoplankton growth, the nitrogen content has often been used to indicate the quality (i.e., the nutritional value) of the cell (most often as the C/N ratio). Nitrogen is also an essential part of vitamins, enzymes and some lipid complexes (Figure 1, Figure A1).
Figure 1.
Schematic diagram of the main macro-molecular structures of the phytoplankton cell and further fractionation of those macromolecules to the building blocks that make up the particulate carbon and nitrogen pool. TAG: Triacylglycerol, PL: Phospholipids, GL: Galactolipids.
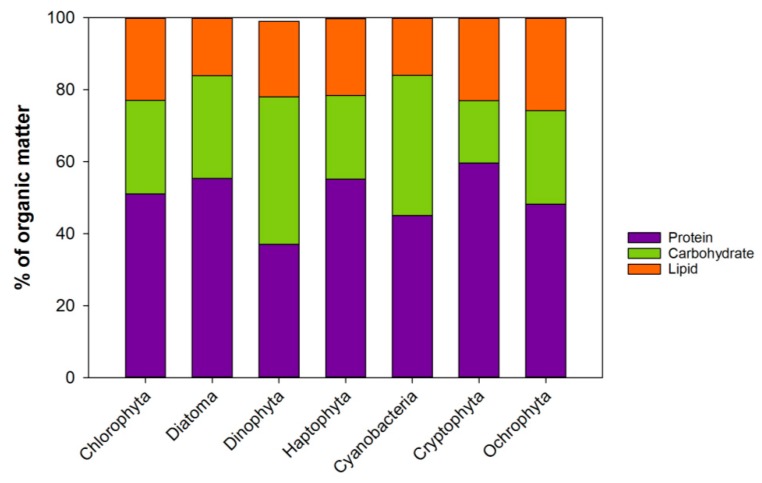
Protein is the main organic group measuring ca. 40–60% of the organic mass, with carbohydrates contributing approximately 17–40% and lipids about 16–26% (Figure 2, and references therein). This proportion is, however, dependent on the phytoplankton growth condition, as discussed in Section 3. In general, the average ratio of the protein, carbohydrates and lipids reported is remarkably similar between the different phytoplankton phyla, at approximately 5:3:2 (Figure 2). Proteins are engaged in almost all the tasks of the cellular activities. They are large complex molecules composed of one or more long chains of amino acids. They are important components of all membranes, involved in the transport of other molecules and ions across the membranes. Proteins are as diverse as the functions they serve. Most enzymes are proteins that organize, construct and receive signals, while the structural proteins maintain the shape of the cell. In animals, the structural proteins are the muscles and connective tissues. There are about 20 different types of amino acids that build up the protein structures, 10 of which are essential, (essential amino acids, EAA) i.e., cannot be synthesized de-novo by most organisms. The quality of marine proteins is measured by the presence of these EAAs, some of which have been found to be essential for the growth of some zooplankton species [16,17]. The synthesis of marine EAAs is found to be slower and more susceptible to nitrogen limitation compared to nonessential amino acids [18]. However, the protein quality (EAA) is found to be similar between many phytoplankton species [19], and the differences in protein quality appears to be secondary; that is, it is first evident when the other essential nutrients, such as essential fatty acids, are sufficient in the diet [19].
Figure 2.
The average biochemical composition of 7 phytoplankton phyla. Compilation of data from: [19,20,21,22]. Updated from [23].
Carbohydrates are either energy or structural compounds. Sugars are easily mobilized for energy (labile). Starch and glycogen are built up of longer branched polymers and are used for storage. Cellulose and chitin are structural and resistant to digestion (refractory). Marine phytoplankton carbohydrates are mainly glucose, galactose and mannose [19]. From a nutritional point of view, the refractory carbohydrates will not be of high nutritional value for zooplankton with a simple gut, while the labile carbohydrates would provide a more easily mobilized energy, without being of high nutritional value; they contain little in the way of nitrogen, phosphate or other trace elements.
Lipids comprise a wide range of compounds used in a wide variety of functions, such as energy storage, digestion, membrane structure, photosensitive pigments and more. The main lipid types in the phytoplankton cell are triacylglycerol (TAG), galactolipids (GL) and phospholipids (PL) (see Appendix). The building blocks of these lipid types are fatty acids which are hydrocarbon chains with a carboxyl end (-COOH) in the head of the molecule. The fatty acid chain can be saturated, that is, without a double bond (saturated fatty acid; SAFA), containing one double bond (monounsaturated; MUFA), or with 2 or more double bonds (polyunsaturated; PUFA, Appendix Figure A1).
Given the general similarity of the Protein/Carbohydrate/Lipid ratio (5/3/2) in the 7 phytoplankton phyla (Figure 2), these macromolecules cannot explain the variation observed in the consumer’s growth when fed different phytoplankton types. Therefore, it is necessary to look further into the more detailed structure of the classes, and as mentioned before, into the building blocks of lipids: the fatty acids.
2.1. From Glucose to Fatty Acids
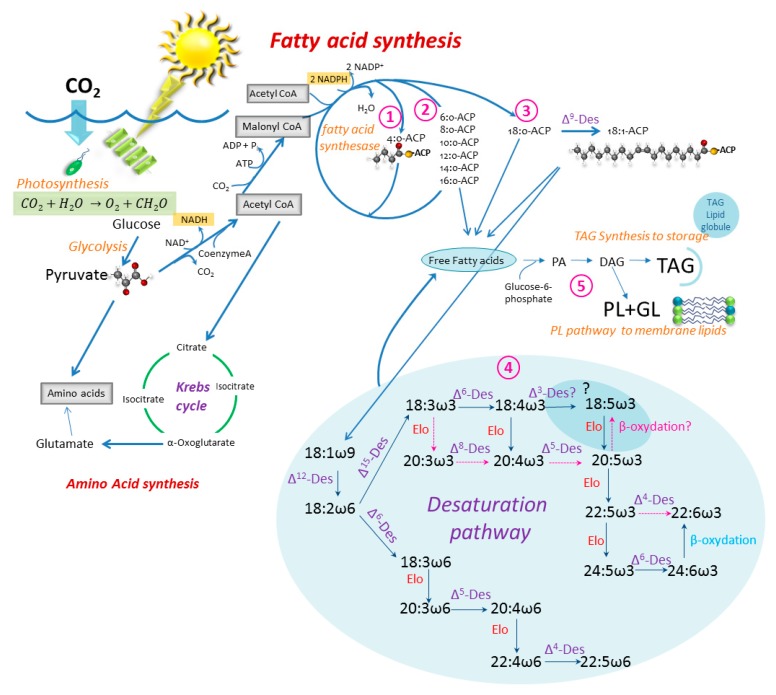
To get from glucose, produced by photosynthesis, to specific fatty acids and storage lipids, a series of complicated biochemical pathways are required. Knowing these pathways gives a better understanding of what controls and limits the fatty acid makeup of the phytoplankton cell (see references in the caption of Figure 3). A more detailed outline of the specific pathways and location of fatty acid synthesis in a eukaryotic organism can, for instance, be found in Zulu et al. [24] and Mühlroth et al. [25].
Figure 3.
Simplified sketch of the fatty acid synthesis in the phytoplankton cell; from photosynthesis (glucose) to triacylglycerol (TAG), phospholipid (PL) and galactolipid (GL). The path towards amino acid synthesis is shown. Des: Desaturase, Elo: elongase, ACP: Acyl carrier protein. Inspired by: [25,33,34,35,36,37,38]. The desaturation pathway from [39,40], with an alternative pathway to 18:5ω3 as suggested by [41], is shown by red arrows. Paths ①–⑤ are discussed in the text. Updated from [23].
Fatty acid synthesis in the algal cell occurs through an aerobic pathway [26] and takes place in the chloroplast and the endoplasmic reticulum. The glucose produced by photosynthesis is converted by glycolysis to pyruvate, which is the molecular basis for all metabolisms. Pyruvate is subjected to oxidative decarboxylation with the coenzyme A (CoASH) to form acetyl-CoA, which is taken into several directions; to the Krebs cycle in the cytosol or to form malonyl-CoA with biotin (acetyl-CoA carboxylase) driven by adenosine triphosphate (ATP). The acetyl-CoA may derive from either the chloroplast itself or from the cytosol [25]. This is the start of the fatty acid synthesis outlined in Figure 3. Fatty acid synthesis can be divided into several steps. Step 1: Fatty acid synthesis in the chloroplast where Malonyl-CoA and Acetyl-CoA contribute 2 carbons each to form the first fatty acid chain (4:0-ACP, Acyl Carrier Protein). Step 2: Associated fatty acid elongation where the 4:0-ACP is successively elongated with the aid of fatty acid synthesase 2 carbons at the time. The cycle ends up with 14–18 carbon length ACP-chains and either enters the fatty acid pool in the cytosol or is taken to step 3. The rest of the fatty acid production involves elongation and desaturation enzymes which occur in the endoplasmic reticulum (ER) in eukaryotic algae [25]. Step 3 involves the first unsaturation step, where 16 or 18:0-ACP with the help of Δ9 desaturase puts the first double bond on the 9th carbon from the ACP end of the chain. The first unsaturated ACP-chain enters the free fatty acid pool of the cell or is further desaturated. Step 4: The desaturation and elongation process takes place in the ER. It takes the 18:1ω9 (or 16:1ω7)-ACP by Δ12 desaturase to form 18:2ω6 further into either the ω6 pathway by Δ6 desaturase or into the ω3 pathway by Δ15 desaturase to form 18:3ω3. Different organisms utilize different desaturases to come to the different PUFAs, but Figure 3 shows two of several possible paths. Step 5: The final step in the lipid synthesis is the formation of storage lipids, usually triacylglycerols (TAG), phospholipids (PL) and galactolipids (GL), that occur in the smooth endoplasmic reticulum (ER) [25]. All require glucose-6-phosphate, to which a saturated fatty acyl-CoA is added with the help of acyl transferase. The second acyl-CoA is added, and phosphatidic acid (PA) is synthesized. Phosphatidic acid is the precursor to several lipid complexes, but diacylgcycerol (DAG), which is a precursor for TAG, PL and GL, is formed by hydrolysis of the phosphate group from phosphatidic acid.
Autotrophs (such as phytoplankton) are the only organisms in the marine environment that can produce linoleic (LA = 18:2ω6) and α-linolenic acid (ALA = 18:3ω3) de-novo from 18:0-ACP [18], but these are precursors for longer chain PUFAs (Figure 3). The reason for this is that higher organisms do not have the required Δ12- and Δ15- desaturase enzymes needed to synthesize LA and ALA from 18:0. A further elongation of LA and ALA to longer chain PUFAs is not easily completed by higher organisms such as the calanoid copepods (the main grazers of microplankton), but if done, they cannot biosynthesize these types of fatty acids with high enough efficiency to meet their growth requirements [27,28], except in some cases [28,29,30,31]. In mammals, such elongation is very slow and is limited by the availability of Delta-6-desaturase [32]. Therefore, phytoplankton are the major source of most PUFAs for most higher consumers [33], and LA, ALA and the longer chain length derivatives produced in the phytoplankton are, from a nutritional point of view, considered to be essential for most higher organisms.
2.2. Specific Lipid Content in the Phytoplankton
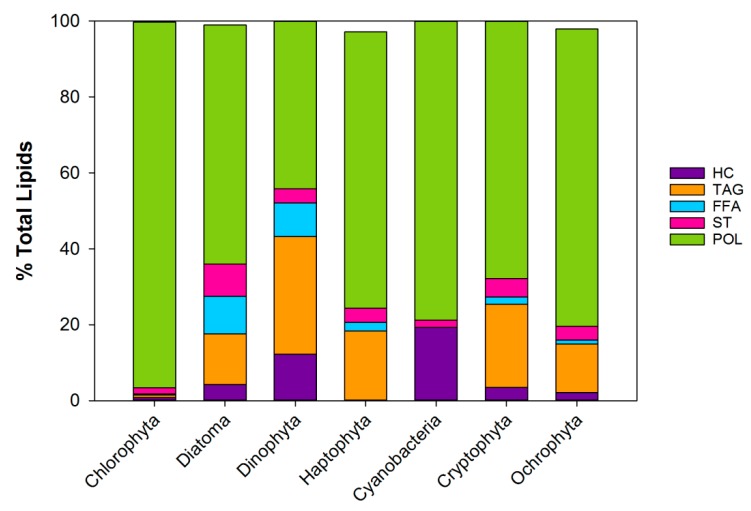
The different phytoplankton phyla contain different proportions of the lipid types hydrocarbon (HC), triacylglycerol (TAG), free fatty acids (FFA), sterols (ST), pigments, and polar lipids (POL), that include mainly phospholipids (PL) but also galactolipids (GL). Figure 4 depicts the average lipid class composition of 7 of the main phytoplankton phyla as % of the total lipids. The major lipid class in all the phyla are the polar lipids ranging from 40–95% of the total lipids (references in the figure’s caption).
Figure 4.
Lipid class composition of 7 phyla of phytoplankton as % of the total lipids excluding pigments. Based on a compilation of 9 articles [1,42,43,44,45,46,47,48,49]. HC: Hydrocarbon, TAG: Triacylglycerol, FFA: Free fatty acids, ST:sterol, POL:polar lipids (PL + GL). Updated from [23].
Triacylglycerol contributes up to 30%, free fatty acids up to 10% and sterol about 5% of the lipid pool (see Figure caption for references). Green algae (chlorophyta) have a very low neutral lipid content. Dinoflagellates (dinophyta) have the highest proportion of neutral lipids out of the 7 phyla. It should however be noted that the ratio between these lipid classes varies greatly with the growth condition of the cells (see Section 3).
2.2.1. Fatty Acid Profiles
The studies that report on marine and freshwater phytoplankton fatty acid profiles run in the hundreds, and are composed of thousands of fatty acid profiles (see [50,51]). The meta-analysis presented here is based on 38 publications that report fatty acid profiles of marine phytoplankton. These consisted of over 160 fatty acid profiles from 7 phytoplankton phyla (cited in the caption of Figure 5). Each species profile covered in this meta-analysis is listed in the Supplementary Material with additional phyla, class, and genus averages. Here, I identify specific aspects of the analyses, and draw up the apparent differences between classes or orders within a phylum (Figure 5). Figure A2 in the Appendix depicts the combined average fatty acid profile for the groups. While sch comparisons are a rather tedious read, it is important to understand the differences between phyla when using the fatty acids as biomarkers and tracers (e.g., [3,52,53,54]), when delving into the details of food quality [55,56], or when searching for a potential nutra- or pharmaceutical source for culturing.
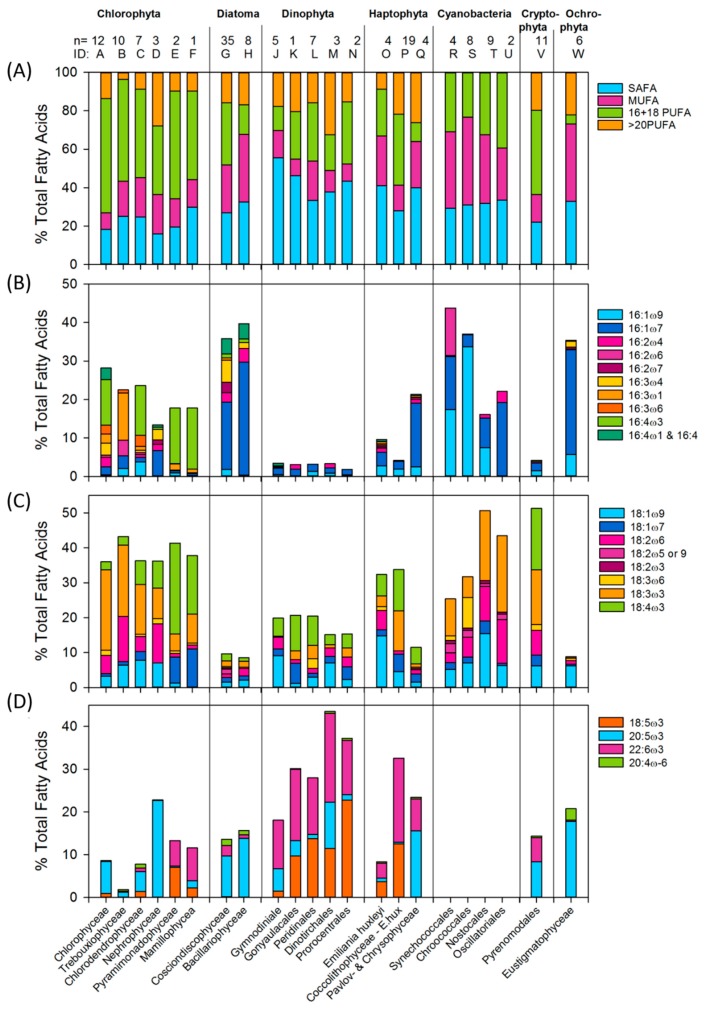
Figure 5.
Fatty acid profiles of 7 phytoplankton phyla and 22 classes/orders as % of the total fatty acids. n: number of profiles behind the analysis. The proportion of (A) Saturated fatty acids (SAFA), mono-unsaturated fatty acids (MUFA), 16-18 Poly unsaturated fatty acids (PUFA) and >20PUFA, (B) C16 fatty acids (C) C18 fatty acids excluding 18:5ω3 (D) Octadecapentaenoic acid (OPA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and arachidonic acid ARA. Upper case letters are the identification references to the classes listed on the x-axes where specific species are listed in the Supplementary Material. Compilation from: [20,43,44,45,46,55,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. Updated from [23].
By looking at the proportion of fatty acid unsaturation in the different groups, (Figure 5A) it is evident that the highest proportion of PUFA is in Chloro- and Cryptophyta, with about 60% of the total fatty acids. The lowest PUFA is found in Ochrophyta, Cyanobacteria (blue-green algae) and diatoms (22%, 26% and 28% respectively). The details of the differences is apparent in the other figure panels. Panel 5B shows the C16 fatty acids, and panel 5C lists the C18 fatty acids excluding 18:5ω3 (Octadecapentaenoic acid, OPA). Panel 5D contains the nutritionally most important PUFA, the highly unsaturated fatty acids (HUFA) 20:5ω3 (Eicosapentaenoic acid; EPA), 22:6ω3 (Docosahexaenoic acid; DHA) and 20:4ω6 (Arachidonic Acid; ARA), including the shorter 18:5ω3 fatty acid. I include OPA with EPA, DHA and ARA as it appears to be exclusive with EPA, i.e., it may appear in many instances that the phytoplankton synthesize either OPA or EPA, but rarely both.
For ease of reading, the classes and orders are identified by their ID letter, as shown in Figure 5 and in the Supplementary Tables.
Chlorophyta (green algae) contain 12–28% of their fatty acids as C16 fatty acids. They are split in 2 distinctive groups: B & D with 16:2 and 16:3 fatty acids, and the other classes (A, C, E & F) with 16:4 fatty acids. The C18 fatty acids make up 35–43% of the total fatty acids of green algae, and all groups have a high proportion of 18:3 ω3 fatty acids. The remaining C18 types split the classes into another combination of groups: E & F, having mainly 18:1ω7 and stearidonic acids 18:4ω3 (SDA); and the others (A, B, C & D), who have 18:1ω9 and 18:2ω6. A third combination between the green algal classes is apparent in the HUFA fraction, where A, B, C & D contain mainly EPA (10–25%) and E & F, mainly OPA and DHA (12%).
The C16 fatty acid group is a signature for silica rich Diatoms (>40%), especially the 16:1ω7, while having low levels of C18 fatty acids. The only difference between the two diatom classes shown here is in their 16:3 PUFA content, where 16:3ω3 and 16:3ω6 are present in class G but not in class H. Over 10% of the total diatom fatty acids are in the long chain EPA.
In contrast, Dinophyta (dinoflagellates) are high (20%) in C18 fatty acids and especially >20PUFA, where 22:6ω3 (DHA) and 18:5ω3 are the signature fatty acids for the phyla. One of the orders, J, has a low contribution of 18:5ω3 fatty acids but higher 20:5ω3.
The fatty acid profiles of the three orders of Haptophyta that are listed differ from each other in their C16, C18 and >20PUFA composition. Even within the class Coccolithophyceae, there are distinct differences, mainly because of a special composition of E. huxleyi diverging from the others within the class, which is therefore presented by itself. E. huxleyi (O) has a lower HUFA content, and a higher ω6 content compared to the rest of the class, with 18:3ω3 and a high proportion of DHA. The Pavlovo- and Chrysophyceae have similar profile and are combined as group Q. They differ from the other Haptophyta containing, high proportion of 20:5ω3 and 16:1ω7. The orders within Cyanobacteria have generally similar profiles, totally lacking the long chain PUFA. The 18:3ω3 fatty acid is conspicuous in all classes, and 16:1ω9 is about 20–30% of the total fatty acids in orders R and S, which also contain 18:3ω6, while the orders T&U have 16:1ω7 and lack 18:3ω6. The fatty acid composition and dynamics of marine Cryptophyta has recently been covered in more detail than here in [89]. Generally the Cryptophyta are low in C16 fatty acids but have equal mixtures of all 18:3ω3 and 18:4ω3 fatty acids, though they lack 18:5ω3 fatty acids. Both EPA and DHA are well represented within the profile. The Ochrophyta has a similar profile to diatoms with a high proportion of 16:1ω7 and EPA, but additionally has 16:1ω9 fatty acids and a total lack of DHA; however, it has Arachidonic acid (ARA, 20:4ω6), which is not present or reported in noticeable amounts in other phyla (See Figure 6).
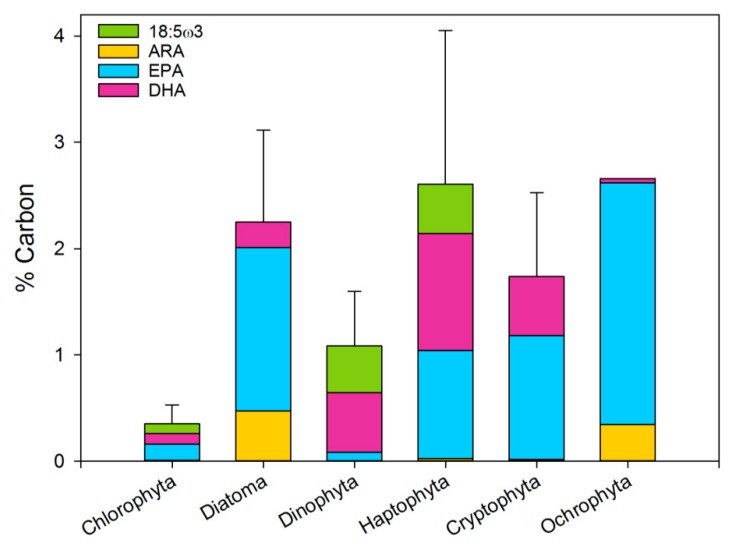
Figure 6.
The proportion of Arachidonic acid (ARA, 20:4ω6), EPA, DHA and 18:5ω3 of the PUFA pool. Error bars are the sum of all four fatty acid standard errors.
2.2.2. PUFA as a Fraction of Biomass
All the fatty acid profiles above are presented as % of the total fatty acid. However, what matters for a consumer is the actual amount of fatty acid in the food, or how much quality it receives per carbon (or dry weight) ingested. Very few of the studies with the fatty acid profiles give the specifics of the phytoplankton analyzed, such as the size, carbon or total fatty acids. If the size is given, it is usually possible to calculate the carbon content [90], while the total lipids or fatty acids per cell are seldom given. Table 1 summarizes several carbon-based specifics of the fatty acids available from the meta-analysis literature. The scarcity of data is reflected in the variation of the mean. The fatty acids in the different phytoplankton groups range between 5–14% of the carbon biomass, with the lowest percent occurring in dinoflagellates and Chlorophyta. Despite the relatively high PUFA content, the proportion of HUFA (including 18:5ω3) generates the difference between the phyla with Chlorophyta having the lowest proportion of HUFA. Other indicators of quality are the ω3/ω6 [82,87,91] and DHA/EPA ratios [55,92,93], both of which are high in diatoms.
Table 1.
Total average fatty acids (FA), polyunsaturated- and highly unsaturated fatty acids (PUFA and HUFA) as proportions of carbon content (% C ± SD), and ω3/ω6 and Eicosapentaenoic and docosahexaenoic acid ratio (EPA/DHA) (± standard deviation) of the 7 phytoplankton phyla.
| Phyla | Total FA | PUFA | HUFA | HUFA + 18:5 | ω3/ω6 | DHA/EPA |
|---|---|---|---|---|---|---|
| Chlorophyta | 8.6 ± 5.1 | 5.3 ± 3.6 | 0.3 ± 0.4 | 0.4 ± 0.5 | 8.1 ± 13.1 | 3.1 ± 4.8 |
| Diatoma | 13.6 ± 18.1 | 4.5 ± 6.0 | 2.3 ± 3.1 | 2.3 ± 3.1 | 15.2 ± 17.5 | 9.2 ± 9.0 |
| Dinophyta | 5.5 ± 2.2 | 1.6 ± 0.9 | 0.6 ± 0.5 | 1.1 ± 0.8 | 23.1 ± 15.8 | 0.3 ± 0.3 |
| Haptophyta | 14 ± 3.7 | 5.5 ± 2.2 | 2.5 ± 2.3 | 2.9 ± 2.0 | 5.0 ± 5.4 | 1.2 ± 3.2 |
| Cyanobacteria | ? | 0 | 0 | 0 | 2.5 ± 2.3 | - |
| Cryptophyta | 9.4 ± 7.7 | 5.9 ± 5.3 | 1.8 ± 1.2 | 1.8 ± 1.2 | 7.0 ± 7.3 | 1.8 ± 1.1 |
| Ochrophyta | 13.1 | 4.5 | 3.9 | 3.9 | 5.4 | 0 |
3. Environmental Effects on Lipid and Fatty Acid Composition
In the 1980s, Mayzaud et al. and Morris et al. [94,95] reported a strong seasonality in the carbon, nitrogen, protein, carbohydrate and lipid content of seston in nature. Additionally, both laboratory and field studies have shown that phytoplankton undergo compositional changes in their lipid classes and specific fatty acids as nutrient availability changes [55,87,94,96,97,98]; these compositional changes are also related to the age of the phytoplankton culture [57,60,99]. It was therefore inevitable that those changes would affect copepod egg production rates and growth, especially if those changes affect the essential nutritional components such as the ω3 fatty acids. A series of studies have focused on changes in the fatty acid content of phytoplankton, with a focus on change in physical condition, as well as nutritional and metal limitations.
3.1. Physical Environment
The temperature is one of the main environmental factors that can influence the biology of organisms. Each species (and even strain) has its own window of optimal growth and metabolic function, and a small change in temperature can change the dominance of phytoplankton species in an ecosystem. For lipid synthesis, temperature has been shown to affect the formation of the RuBisCO enzyme, which is a key factor in the carbon assimilation in algae and thus the ability to produce glucose – the precursor of fatty acid synthesis (Figure 3). Generally, studies show an increased lipid production, mainly TAG with increased temperatures. This is mainly shown as an increase in the SAFA and MUFA production. The saturation index of some GL has been shown to decrease with higher temperatures in a freshwater dinoflagellate. (Based on: [38,82,100,101,102,103]).
Light is needed for the generation of Nicotinamide adenine dinucleotide phosphate (NADPH) and acetyl-CoA carboxylase, both of which are essential for fatty acid synthesis. Elongation and desaturation of ARA to EPA and to DHA has been shown to be light-dependent in the haptophyte Pavlova lutheri (group Q), where HUFA production is active under low light conditions [104]. Other studies testing different light levels indicate that lipid production depends on the growth stage of the cultures and species, and that it appears to depend on the type of sugar used as an energy source to fuel the fatty acid production. (Based on: [19,38,100,105,106].
3.2. Nutrients
Many of the lipid measurements that represent nutrient limitations were conducted on cultures in a stationary growth phase, so the results are a combination of nutrient and light limitations.
Nitrogen is an essential part of amino acid synthesis, and when limited, the path is shifted towards non-nitrogenous compounds such as lipid or carbohydrate synthesis. When nutrient limitation becomes critical, it causes the size of thylakoids and other cell membranes to decrease, affecting the absolute amounts of PUFA, until the limitation becomes critical and affects the turnover of enzymes and the ability to repair or synthesize membranes; this results in the recycling PL and GL and the associated PUFA. The total sterol content of the cell (in diatoms and chlorophytes) has been shown to decrease with nitrogen stress. (Based on: [80,107,108,109]).
Studies on phosphate limitation show that SAFA and MUFA increase at the cost of PUFA. Total lipids increased in diatoms and prymnesiophytes, while total lipids decreased in chlorophytes—since chlorophytes store carbon as carbohydrates but not as lipids, and the P limitation affects their ability to synthesize phosphoglycerolipids. TAG and galactolipid content increased at the cost of phospholipids, indicating that the phosphate limitation pushes synthesis towards TAG and sugars. (Based on: [48,109,110,111]).
Silica limitation acts on diatoms, but mainly on their division rates. The lipid content, especially TAG, is found to increase with the Si limitation, and SAFA and MUFA are found in a higher proportion in the phytoplankton cell, compared to Si replete cultures. The lipid increase has been found to be equal to expectations in the 2 daughter cells as the frustule formation halts when Si is limited, while other processes continue causing an increase in the lipid storage. (Based on: [105,107,112,113]).
The reason why the PUFA and ω3 production is apparently affected by the N and P limitation is not clear, but it is most likely related to the need for NADP during desaturation and elongation.
3.3. Trace Metals
The trace metals essential for phytoplankton growth include manganese (Mn), Iron (Fe), cobalt (Co), copper (Cu), zinc (Zn) and nickel (Ni). Not many studies have been conducted to investigate the effects of trace metal limitation on fatty acid composition.
An increase in manganese (Mn+2) availability has been shown to cause an increase in PUFA in autotrophs [100]. Manganese is important in photosynthesis and has been shown to limit the chlorophyll content of cells [114].
Iron (Fe) is an important trace element used in the photosynthetic electron transport as Fe2S2, and it acts as an electron donor for the production of NATP. As mentioned above, lipid production is energy-dependent and requires, for example, 14 NADPH and 7 ATP for the production of one mole of palmitate (16:0). The limitation of iron results in a reduction of phytoplankton cell volumes by half and a significantly lower total lipid content in cells. The production of SDA is hindered in Fe-depleted cells compared to Fe-replete cells [87], which can be traced to the importance of Fe in the composition of the fatty acid desaturases (see above). Fe forms a reactive complex with oxygen (diiron) in the desaturation molecule, but oxygen reacts with carbon in the fatty acid chain and converts single bonds to double (From: [86,115,116]).
Generally, the different factors listed above limit the pathways shown in Figure 3 at different or various levels. Many are essential in the photosynthetic pathway where light activates the Mn+2 (and Mg+) dependent chlorophyll molecule (also containing nitrogen) and temperature affects the carbon fixation rates (RuBisCO), along with other rates. Iron is essential in the electron transport chain as an electron donor in the NADP formation. Nitrogen limits the amino acid production, as well as being an essential part of most enzymes, NADP and phospholipids. Phosphate is also essential in the energy transfer of ADP and ATP.
All these factors control phytoplankton growth and chemical composition in nature and can certainly be used to manipulate phytoplankton in cultures, for example by changing the light availability (density of cultures) and nutrients to attain the required and desired lipid and fatty acid composition.
It should be noted that most of the listed differences are most often relative (percentages) and do not reflect the absolute changes in the fatty acid composition. However, while the environmental and nutritional factors affect the relative fatty acid composition, the specificities of the fatty acid signature of the different phytoplankton phyla are relatively stable, and statistical analyses by Galloway and Winder [50] show that phylogenic fatty acid signatures are more robust than some fatty acid shifts that happen due to environmental factors. Therefore, using fatty acids as biomarkers is still a robust tool not greatly affected by environmental changes. However, the absolute amount of essential fatty acids is of crucial importance for the food web dynamics, and shifts in the absolute value of EFA will affect the quality of the phytoplankton as food.
4. Discussion
Lipids are immensely important for the functioning and well-being of marine ecosystems. Essential omega-3 lipids are produced by phytoplankton, and accumulated and transferred by zooplankton through the entire marine food web, part of which eventually ends up on our dinner tables. The quality, efficiency and productivity of the marine food web is highly dependent on the type of primary producer dominating at every moment, as is underscored by the great variation in the essential fatty acid content of the different phytoplankton phyla and classes.
Marine lipids are in huge demand [117,118] and have a high economic value [119]. The industrial uses of marine lipids are related to human consumption, fisheries, aquaculture, agriculture, health and cosmetics. Omega-3 PUFA are essential for the development and function of the brain, the nervous system and eyes, as well as serving as a preventative for heart disease and inflammation [120]. Therefore, EPA and DHA in particular are highly sought after by the nutra- and pharmaceutical industry.
Microalgae are an excellent source for acquiring ω3 and ω6 PUFA. The meta-analysis clearly reveals that some phytoplankton classes are more suitable sources for essential PUFA than others. For cultivation purposes, it is important to be aware that while the average lipid content of all phytoplankton phyla is similar (about 20% of their organic matter content), the lipid type differs greatly between phyla (Figure 4), as do the types and proportion of the ω3 and ω6 fatty acids. In the literature, fatty acid profiles are usually presented as a fraction of the total fatty acid pool. However, when looking at HUFA as a fraction of the biomass (Figure 6), it is clear that some phytoplankton types give more EPA and DHA per unit of carbon than others. The average PUFA content of the different phyla ranges from 0 (Cyanobacteria) to 2.5% (Ochrophyta) of the carbon biomass. Of these, the contribution of EPA and DHA to the PUFA mass varies both in proportion and in amount.
In the absence of EPA and DHA, SDA might be another PUFA of interest with human health benefits. SDA is synthesized from alpha-linolenic acid (18:3n3, ALA) with the aid of delta-6 desaturase, and as such is a precursor of EPA and DHA. Delta-6-desaturase is a limiting enzyme in humans [32] and is thought to decline in humans with age [120]. Delta-6-desaturase has several potential functions in the lipid desaturation pathway (Figure 3) that could compete for the generation of ω3 versus ω6 PUFA. In particular, the phytoplankton groups with a high contribution of SDA are Cryptophyta and the class Pyramionadophycea within the Chlorophyta.
It can be argued that, globally speaking, the marine ecosystem is in a state of transition. The Arctic and subarctic North Atlantic are, in particular, transitioning to warmer waters and decreased salinity due to the melting of sea ice and the influx of glacial melt waters [121]. These systems are predominantly fuelled by the diatom vernal bloom that are highly characteristic for seasonal environments (e.g., polar and subpolar seas) and which make high quality EPA available for the marine food web. The warming and freshening of the subarctic waters is predicted to cause an increased stratification of the water masses, that will limit the nutrient input from deeper water masses causing nutrient limitation in the systems, and disrupting their highly productive seasonal cycle. Based on the studies listed above, both the increased temperatures and nutrient limitation decrease the quality of lipids in phytoplankton; while the total lipid content increases, the PUFA fraction goes down. At this stage, the question of what will happen with microplankton diversity and which organisms may take over from diatoms remains speculative, but it will most likely lessen the quality and efficiency of the Arctic food web. This is of great concern, and might even increase the need for additional production chains of PUFA in the near future, to fulfil the demand for this essential nutritional component for human consumption and health.
5. Methods
All metadata were from publications on marine phytoplankton, and freshwater species were not included in the analyses. The species were sorted according to phyla, class order and genus using the criteria from Algae Base [122]. The gross chemical composition of phytoplankton in the literature is usually presented as % dry weight or % organic weight. To facilitate comparisons, the data is presented here as the relative proportion between the 3 organic groups; protein, carbohydrates and lipids.
The presentation of fatty acid data is as a % of the total fatty acids. After an inspection of the profile similarities on the species and genus level, averages were taken on a class-level for most of the phyla. For Dinophycea, the orders were very different within the classes, and their profiles and are here presented on an order-level. There is some discrepancy between publications in the totality of the fatty acid profiles presented—e.g., some studies do not identify all fatty acids, and ω6 fatty acids may be underrepresented in some studies. It is not possible to evaluate if in those instances the specific fatty acids are not present in the sample or not analyzed/recognized. Therefore, the averages are based on >0.1% presence of the specific fatty acids, and zeros are not included in the averages. The original data is presented as Supplementary Material.
Acknowledgments
The author is grateful to Professors: Richard F. Lee, David D.M. Checkley, Andre W. Visser and the late Marie de Angelis for their useful comments on a previous version on this review.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/3/151/s1, File: Jonasdottir_Fatty Acid Profiles.
Appendix A
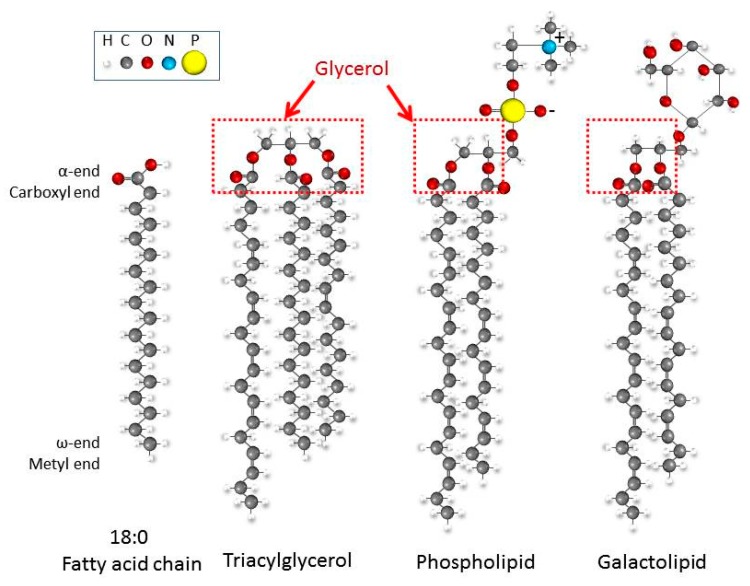
Figure A1.
Structures a fatty acid (18:0 SAFA) and the main phytoplankton lipid types Triacylglycerol and Phospho- and galactolipid molecules.
Lipids are a large group of molecules that include wax esters, sterols, fat-soluble vitamins (such as vitamins A, D, E, and K), diglycerides, triglycerides, phospholipids, galcatolipids and others. The molecules are composed of one or more fatty acids (Figure A1). Triacylglycerol is made from 3 fatty acids connected by an acyl bond. Most phospholipids contain 2 fatty acids bound in diglyceride, a phosphate group, and a hydrophilic head, while galacolipids have a glucose head.
Fatty acids are long carbon chains, and in phytoplankton they usually contain even numbers of carbon from C14-C24. One end (the head) of the fatty acid chain is called the α-end and contains carboxylic acid (-COOH), while the tail of the chain (also called ω-end) is a methyl (CH3). The chains may be saturated (SAFA), which is to say a straight chain without a double bond, indicated with a zero after a column, i.e., 16:0; monounsaturated (MUFA), containing one double bond (i.e., 16:1); or polyunsaturated (PUFA), containing 2 or more double bonds.
The location of the double bond on the fatty acid chain is indicated with “ω”. If the first double bond is on the 3rd carbon counted from the methyl or the ω-end, the fatty acid is a ω3 fatty acid, and similarly it’s a ω6 if the first bond is on the 6th carbon from the methyl end. The fatty acid 18:3ω3 is therefore 18 carbon long containing 3 double bonds where the first one is placed on the 3rd to the last carbon. In the literature, the use of highly saturated fatty acid HUFA covers >20 or 22 carbon chain lengths depending on the publication. Here I use HUFA for fatty acids at a ≥ 20 carbon length.
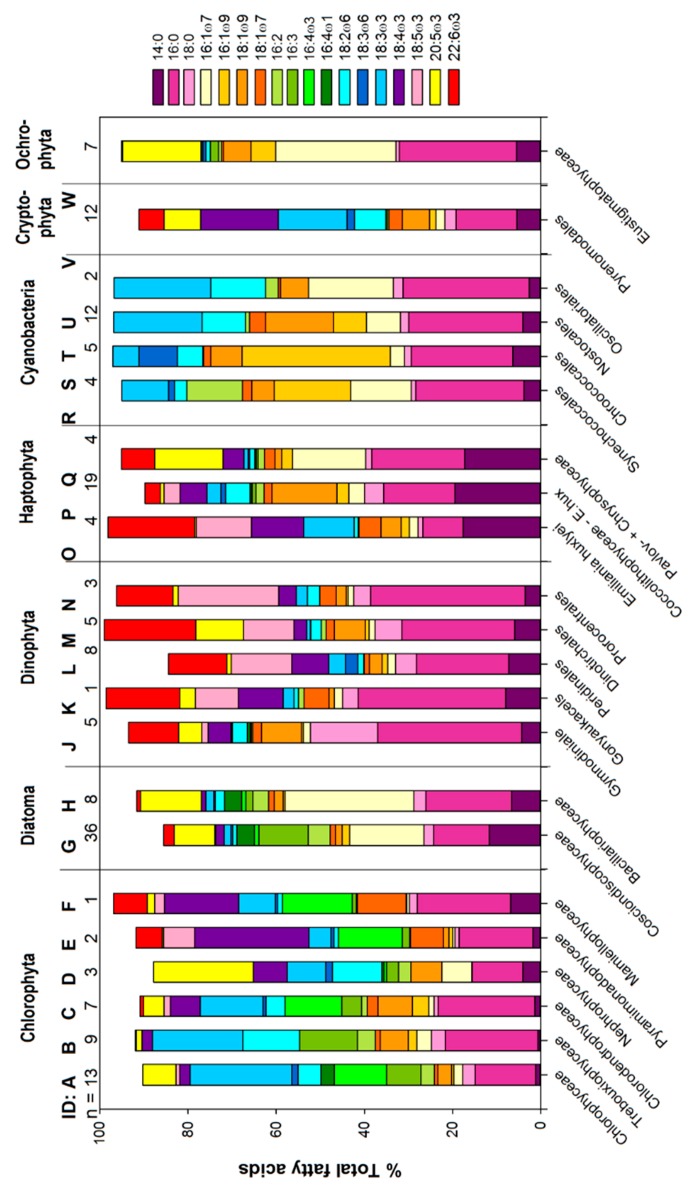
Figure A2.
Full fatty acid profiles of classes of the 7 phytoplankton phyla. The specifics of 16 and 18 PUFA are detailed in Figure 5 and full detailed profiles can be seen in the Supplementary Material.
Author Contributions
This work is a sole compilation of S.H.J.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Lee R.F., Nevenzel J.C., Paffenhöfer G.-A. Importance of wax esters and other lipids in the marine food chain: Phytoplankton and copepods. Mar. Biol. 1971;9:99–108. doi: 10.1007/BF00348249. [DOI] [Google Scholar]
- 2.Graeve M., Kattner G., Hagen W. Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: Experimental evidence of trophic markers. J. Exp. Mar. Biol. Ecol. 1994;182:97–110. doi: 10.1016/0022-0981(94)90213-5. [DOI] [Google Scholar]
- 3.Sargent J.R., Falk-Petersen S. The lipid biochemistry of calanoid copepods. Hydrobiologia. 1988;167–168:101–114. doi: 10.1007/BF00026297. [DOI] [Google Scholar]
- 4.Marshall S., Orr A. On the biology of Calanus finmarchicus. VII. Factors affecting egg production. J. Mar. Biol. Assoc. UK. 1952;30:527–547. doi: 10.1017/S0025315400012959. [DOI] [Google Scholar]
- 5.Checkley D.M.D. The egg production of a marine planktonic copepod in relation to its food supply: Laboratory studies. Limnol. Oceanogr. 1980;25:430–446. doi: 10.4319/lo.1980.25.3.0430. [DOI] [Google Scholar]
- 6.Nassogne A. Influence of food organisms on the development and culture of pelagic copepods. Helgol. Wiss. Meeresunters. 1970;20:333–345. doi: 10.1007/BF01609911. [DOI] [Google Scholar]
- 7.Arnott G., Brand G., Kos L. Effects of food quality and quantity on the survival, development, and egg production of Gladioferens pectinatus (Brady) (Copepoda: Calanoida) Aust. J. Mar. Freshw. Res. 1986;37:467–473. doi: 10.1071/MF9860467. [DOI] [Google Scholar]
- 8.Castell J. Fatty acid metabolism in crustaceans. In: Pruder G.D., Langdon C.J., Conklin D.E., editors. Proceedings of the Second International Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition. Louisiana State University; Baton Rouge, LA, USA: 1982. pp. 124–145. [Google Scholar]
- 9.Bourdier G., Amblard C. Lipids in Acanthodiaptomus denticornis during starvation and fed on 3 different algae. J. Plankton Res. 1989;11:1201–1212. doi: 10.1093/plankt/11.6.1201. [DOI] [Google Scholar]
- 10.Fraser A.J., Sargent J.R., Gamble J.C., Seaton D.D. Formation and transfer of fatty acids in an enclosed marine food chain comprising phytoplankton, zooplankton and herring (Clupea harengus L.) larvae. Mar. Chem. 1989;27:1–18. doi: 10.1016/0304-4203(89)90024-8. [DOI] [Google Scholar]
- 11.Guisande C., Harris R. Effect of total organic content of eggs on hatching success and naupliar survival in the copepod Calanus helgolandicus. Limnol. Oceanogr. 1995;40:476–482. doi: 10.4319/lo.1995.40.3.0476. [DOI] [Google Scholar]
- 12.Koski M., Yebra L., Dutz J., Jónasdóttir S.H., Vidoudez C., Jakobsen H.H., Pohnert G., Nejstgaard J.C. The effect of egg versus seston quality on hatching success, naupliar metabolism and survival of Calanus finmarchicus in mesocosms dominated by Phaeocystis and diatoms. Mar. Biol. 2012;159:643–660. doi: 10.1007/s00227-011-1843-z. [DOI] [Google Scholar]
- 13.Jónasdóttir S.H., Visser A.W., Jespersen C. Assessing the role of food quality in the production and hatching of Temora longicornis eggs. Mar. Ecol. Prog. Ser. 2009;382:139–150. doi: 10.3354/meps07985. [DOI] [Google Scholar]
- 14.Kanazawa A., Teshima S., Tokiwa S. Nutritional requirements of prawn. 7. Effect of dieatry lipids on growth. Bull. Jpn. Soc. Sci. Fish. 1977;43:849–856. doi: 10.2331/suisan.43.849. [DOI] [Google Scholar]
- 15.Pond D.W., Harris R., Head R., Harbour D. Environmental and nutritional factors determining seasonal variability in the fecundity and egg viability of Calanus helgolandicus in coastal waters off Plymouth, UK. Mar. Ecol. Prog. Ser. 1996;143:45–63. doi: 10.3354/meps143045. [DOI] [Google Scholar]
- 16.Kleppel G., Burkart C. Egg production and the nutritional environment of Acartia tonsa: The role of food quality in copepod nutrition. ICES J. Mar. Sci. 1995;52:297–304. doi: 10.1016/1054-3139(95)80045-X. [DOI] [Google Scholar]
- 17.Guisande C., Riveiro I., Maneiro I. Comparisons among the amino acid composition of females, eggs and food to determine the relative importance of food quantity and food quality to copepod. Mar. Ecol. Prog. Ser. 2000;202:135–142. doi: 10.3354/meps202135. [DOI] [Google Scholar]
- 18.Grosse J., Brussaard C., Boschker H. Nutrient limitation driven dynamics of amino acids and fatty acids in coastal phytoplankton. Limnol. Oceanogr. 2018 doi: 10.1002/lno.11040. [DOI] [Google Scholar]
- 19.Brown M.R., Jeffrey S.W., Volkman J.K., Dunstan G. Nutritional properties of microaglae for mariculture. Aquaculture. 1997;151:315–331. doi: 10.1016/S0044-8486(96)01501-3. [DOI] [Google Scholar]
- 20.Ben-Amotz A., Fishler R., Schneller A. Chemical composition of dietary species of marine unicellular algae and rotifers with emphasis on fatty acids. Mar. Biol. 1987;95:31–36. doi: 10.1007/BF00447482. [DOI] [Google Scholar]
- 21.Parsons T.R., Stephens K., Strickland J.D.H. On the chemical composition of eleven species of marine phytoplankters. J. Fish. Board Can. 1961;18:1001–1016. doi: 10.1139/f61-063. [DOI] [Google Scholar]
- 22.Gatenby C.M., Orcutt D.M., Kreeger D.A., Parker B.C., Jones V.A., Neves R.J. Biochemical composition of three algal species proposed as food for captive freshwater mussels. J. Appl. Phycol. 2003;15:1–11. doi: 10.1023/A:1022929423011. [DOI] [Google Scholar]
- 23.Jónasdóttir S.H. Doctoral Thesis. National Institute of Aquatic Resources, Technical University of Denmark; Lyngby, Denmark: 2015. A Journey from Light to Darkness. Fatty Acids in the Marine Ecosystem: From Photosynthesis to Copepod Lipids and Sequestration; p. 118. [Google Scholar]
- 24.Zulu N.N., Zienkiewicz K., Vollheyde K., Feussner I. Current trends to comprehend lipid metabolism in diatoms. Prog. Lipid Res. 2018;70:1–16. doi: 10.1016/j.plipres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Mühlroth A., Li K., Røkke G., Winge P., Olsen Y., Hohmann-Marriott M., Vadstein O., Bones A. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar. Drugs. 2013;11:4662–4697. doi: 10.3390/md11114662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monroig Ó., Tocher D.R., Navarro J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs. 2013;11:3998–4018. doi: 10.3390/md11103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sargent J., Henderson R. Lipids. In: Corner E., O’Hara S., editors. The Biological Chemistry of Marine Copepods. Oxford Science Publications; New York, NY, USA: 1986. pp. 59–108. [Google Scholar]
- 28.Parrish C.C., French V.M., Whiticar M.J. Lipid class and fatty acid composition of copepods (Calanus finmarchicus, C. glacialis, Pseudocalanus sp., Tisbe furcata and Nitokra lacustris) fed various combinations of autotrophic and heterotrophic protists. J. Plankton Res. 2012;34:356–375. doi: 10.1093/plankt/fbs003. [DOI] [Google Scholar]
- 29.Caramujo M.-J., Boschker H.T.S., Admiraal W. Fatty acid profiles of algae mark the development and composition of harpacticoid copepods. Freshw. Biol. 2008;53:77–90. doi: 10.1111/j.1365-2427.2007.01868.x. [DOI] [Google Scholar]
- 30.De Troch M., Boeckx P., Cnudde C., Van Gansbeke D., Vanreusel A., Vincx M., Caramujo M.-J. Bioconversion of fatty acids at the basis of marine food webs: Insights from a compound-specific stable isotope analysis. Mar. Ecol. Prog. Ser. 2012;465:53–67. doi: 10.3354/meps09920. [DOI] [Google Scholar]
- 31.Arndt C., Sommer U. Effect of algal species and concentration on development and fatty acid composition of two harpacticoid copepods, Tisbe sp. and Tachidius discipes, and a discussion about their suitability for marine fish larvae. Aquac. Nutr. 2014;20:44–59. doi: 10.1111/anu.12051. [DOI] [Google Scholar]
- 32.Pelley J.W. 10-Fatty Acid and Triglyceride Metabolism. In: Pelley J.W., editor. Elsevier’s Integrated Review Biochemistry. 2nd ed. W.B. Saunders; Philadelphia, PA, USA: 2012. pp. 81–88. [Google Scholar]
- 33.Singh A., Ward O.P. Microbial production of docosahexaenoic acid. Adv. Appl. Microbiol. 1997;45:271–312. doi: 10.1016/s0065-2164(08)70266-1. [DOI] [PubMed] [Google Scholar]
- 34.Arts M., Evans M., Robarts R.D. Seasonal patterns of total and energy reserve lipids of dominant zooplanktonic crustaceans from a hyper-eutrophic lake. Oecologia. 1992;90:560–571. doi: 10.1007/BF01875451. [DOI] [PubMed] [Google Scholar]
- 35.Bell M., Tocher D. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: General pathways and new directions. In: Arts M.T., Brett M.T., Kainz M., editors. Lipids in Aquatic Ecosystems. Springer; New York, NY, USA: 2009. pp. 211–236. [Google Scholar]
- 36.Yu W.-L., Ansari W., Schoepp N.G., Hannon M.J., Mayfield S.P., Burkart M.D. Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb. Cell Factories. 2011;10:91. doi: 10.1186/1475-2859-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baba M., Shiraiwa Y. Biosynthesis of lipids and hydrocarbons in algae. In: Dubinsky S., editor. Photosynthesis. InTech; Vienna, Austria: 2013. pp. 331–356. [Google Scholar]
- 38.Heydarizadeh P., Poirier I., Loizeau D., Ulmann L., Mimouni V., Schoefs B., Bertrand M. Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar. Drugs. 2013;11:3425–3471. doi: 10.3390/md11093425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalsgaard J., St John M., Kattner G., Müller-Navarra D., Hagen W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003;46:225–340. doi: 10.1016/s0065-2881(03)46005-7. [DOI] [PubMed] [Google Scholar]
- 40.Harwood J.L., Guschina I.A. The versatility of algae and their lipid metabolism. Biochimie. 2009;91:679–684. doi: 10.1016/j.biochi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Sayanova O., Haslam R.P., Calerón M.V., López N.R., Worthy C., Rooks P., Allen M., Napier J.A. Identification and functional characterisation of genes encoding the omega-3 polyunsaturated fatty acid biosynthetic pathway from the coccolithophore Emiliania huxleyi. Phytochemistry. 2011;72:594–600. doi: 10.1016/j.phytochem.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Henderson R., Leftley J.W., Sargent J.R. Lipid composition and biosynthesis in the marine dinoflagellate Crypthecodinium cohnii. Phytochemistry. 1988;27:1679–1683. doi: 10.1016/0031-9422(88)80425-4. [DOI] [Google Scholar]
- 43.Volkman J.K., Jeffrey S.W., Nichols P.D., Rogers G.I., Garland C.D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989;128:219–240. doi: 10.1016/0022-0981(89)90029-4. [DOI] [Google Scholar]
- 44.Al-Hasan R.H., Ali A.M., Radwan S.S. Lipids, and their constituent fatty acids, of Phaeocystis sp. from the Arabian Gulf. Mar. Biol. 1990;105:9–14. doi: 10.1007/BF01344265. [DOI] [Google Scholar]
- 45.Dunstan G., Volkman J.K., Jeffrey S.W., Barrett S. Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinohyceae. 2. Lipid classes and fatty acids. J. Exp. Mar. Biol. Ecol. 1992;161:115–134. doi: 10.1016/0022-0981(92)90193-E. [DOI] [Google Scholar]
- 46.Dunstan G., Volkman J.K., Barrett S. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae) Phytochemistry. 1993;35:155–161. doi: 10.1016/S0031-9422(00)90525-9. [DOI] [Google Scholar]
- 47.Parrish C.C., Bodennec G., Gentien P. Time courses of intracellular and extracellular lipid classes in batch cultures of the toxic dinoflagellate, Gymnodinium cf. nagasakiense. Mar. Chem. 1994;48:71–78. doi: 10.1016/0304-4203(94)90063-9. [DOI] [Google Scholar]
- 48.Lombardi A.T., Wangersky P.J. Particulate lipid class composition of three marine phytoplankters Chaetoceros gracilis, Isochrysis galbana (Tahiti) and Dunaliella tertiolecta grown in batch culture. Hydrobiologia. 1995;306:1–6. doi: 10.1007/BF00007853. [DOI] [Google Scholar]
- 49.Nordbäck J., Lundberg E., Christie W. Separation of lipid classes from marine particulate material by HPLC on a polyvinyl alcohol-bonded stationary phase using dual-channel evaporative light-scattering detection. Mar. Chem. 1998;60:165–175. doi: 10.1016/S0304-4203(97)00109-6. [DOI] [Google Scholar]
- 50.Galloway A.W.E., Winder M. Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS ONE. 2015;10:e0130053. doi: 10.1371/journal.pone.0130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cañavate J.P. Advancing assessment of marine phytoplankton community structure and nutritional value from fatty acid profiles of cultured microalgae. Rev. Aquac. 2018 doi: 10.1111/raq.12244. [DOI] [Google Scholar]
- 52.Scott C.C.L., Kwasniewski S., Falk-Petersen S., Sargent J.R.J. Species differences, origins and functions of fatty alcohols and fatty acids in the wax esters and phospholipids of Calanus hyperboreus, C. glacialis and C. finmarchicus from Arctic waters. Mar. Ecol. Prog. Ser. 2002;235:127–134. doi: 10.3354/meps235127. [DOI] [Google Scholar]
- 53.Falk-Petersen S., Hagen W., Kattner G., Clarke A., Sargent J. Lipids, trophic relationships, and biodiversity in Arctic and Antarctic krill. Can. J. Fish. Aquat. Sci. 2000;57:178–191. doi: 10.1139/f00-194. [DOI] [Google Scholar]
- 54.Stübing D., Hagen W. Fatty acid biomarker ratios-suitable trophic indicators in Antarctic euphausiids? Polar Biol. 2003;26:774–782. doi: 10.1007/s00300-003-0550-8. [DOI] [Google Scholar]
- 55.Jónasdóttir S.H. Effects of food quality on the reproductive success of Acartia tonsa and Acartia hudsonica: Laboratory observations. Mar. Biol. 1994;121:67–81. doi: 10.1007/BF00349475. [DOI] [Google Scholar]
- 56.Müller-Navarra D.C., Brett M.T., Liston A.M., Goldman C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature. 2000;403:74–77. doi: 10.1038/47469. [DOI] [PubMed] [Google Scholar]
- 57.Ackman R.G., Jangaard P., Hoyle R.R.J., Brockerhoff H. Origin of marine fatty acids. I. Analyses of the fatty acids produced by the diatom Skeletonema costatum. J. Fish. Board Can. 1964;21:747–756. doi: 10.1139/f64-067. [DOI] [Google Scholar]
- 58.Ackman R.G., Tocher C.S., McLachlan J. Marine phytoplankter fatty acids. J. Fish. Board Can. 1968;25:1603–1620. doi: 10.1139/f68-145. [DOI] [Google Scholar]
- 59.Chuecas L., Riley J.P. Component fatty acids of the total lipids of some marine phytoplankton. J. Mar. Biol. Assoc. UK. 1969;49:97–116. doi: 10.1017/S0025315400046439. [DOI] [Google Scholar]
- 60.Pugh P.R. Changes in the fatty acid composition of Coscinodiscus eccentricus with culture-age and salinity. Mar. Biol. 1971;124:118–124. [Google Scholar]
- 61.Whyte J.N. Fatty acid profiles from direct methanolysis of lipids in tissue of cultured species. Aquaculture. 1988;75:193–203. doi: 10.1016/0044-8486(88)90032-4. [DOI] [Google Scholar]
- 62.Claustre H.A., Poulet S.A., Williams R., Marty J.C., Coombs S., Ben Mlih F., Jezequel-Martin V. A biochemical investigation of a Phaeocystis sp. bloom in the Irish Sea. J. Mar. Biol. Assoc. UK. 1990;70:197–207. doi: 10.1017/S0025315400034317. [DOI] [Google Scholar]
- 63.Mourente G., Lubian L.M., Odriozola J.M. Total fatty acid composition as a taxonomic index of some marine microalgae used as food in marine aquaculture. Hydrobiologia. 1990;203:147–154. doi: 10.1007/BF00005683. [DOI] [Google Scholar]
- 64.Ahlgren G., Lundstedt L., Brett M., Forsberg C. Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankter. J. Plankton Res. 1990;12:809–818. doi: 10.1093/plankt/12.4.809. [DOI] [Google Scholar]
- 65.Ahlgren G., Gustafsson I.B., Bobeg M. Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 1992;28:37–50. doi: 10.1111/j.0022-3646.1992.00037.x. [DOI] [Google Scholar]
- 66.Nichols P.D., Skerratt J.H., Davidson A., Burton H., Mcmeekin T.A. Lipids of cultured Phaeocystis pouchetii: Signatures for food-web, biogeochemical and environmental studies in Antarctica and the Southern ocean. Phytochemistry. 1991;30:3209–3214. doi: 10.1016/0031-9422(91)83177-M. [DOI] [Google Scholar]
- 67.Virtue P., Nichols P.D.D., Nicol S., McMinn A., Sikes E.L.L. The lipid composition of Euphausia superba Dana in relation to the nutritional value of Phaeocystis pouchetii (Hariot) Lagerheim. Antarct. Sci. 1993;5:169–177. doi: 10.1017/S0954102093000239. [DOI] [Google Scholar]
- 68.Viso A.C.A.C., Marty J.C.J.C. Fatty acids from 28 marine microalgae. Phytochemistry. 1993;34:1521–1533. doi: 10.1016/S0031-9422(00)90839-2. [DOI] [Google Scholar]
- 69.Ederington M.C., McManus G.B., Harvey H.R. Trophic transfer of fatty acids, sterols, and a triterpenoid alcohol between bacteria, a ciliate, and the copepod Acartia tonsa. Limnol. Oceanogr. 1995;40:860–867. doi: 10.4319/lo.1995.40.5.0860. [DOI] [Google Scholar]
- 70.Zhukova N., Aizdaicher N. Fatty acid composition of 15 species of marine microalgae. Phytochemistry. 1995;39:351–356. doi: 10.1016/0031-9422(94)00913-E. [DOI] [Google Scholar]
- 71.Berge J., Gouygou J., Dubacq J., Durand P. Reassessment of lipid composition of the diatom, Skeletonema costatum. Phytochemistry. 1995;39:1017–1021. doi: 10.1016/0031-9422(94)00156-N. [DOI] [Google Scholar]
- 72.Bell M., Pond D.W. Lipid composition during growth of motile and coccolith forms of Emiliania huxleyi. Phytochemistry. 1996;41:465–471. doi: 10.1016/0031-9422(95)00663-X. [DOI] [Google Scholar]
- 73.Klein Breteler W., Schogt N., Baas M., Schouten S., Kraay G.W. Trophic upgrading of food quality by protozoans enhancing copepod growth: Role of essential lipids. Mar. Biol. 1999;135:191–198. doi: 10.1007/s002270050616. [DOI] [Google Scholar]
- 74.Riebesell U., Revill A.T., Holdsworth D.G., Volkman J.K. The effects of varying CO2 concentration on lipid composition and carbon isotope fractionation in Emiliania huxleyi. Geochim. Cosmochim. Acta. 2000;64:4179–4192. doi: 10.1016/S0016-7037(00)00474-9. [DOI] [Google Scholar]
- 75.Cotonnec G., Brunet C., Sautour B., Thoumelin G. Nutritive value and selection of food particles by copepods during a spring bloom of Phaeocystis sp. in the English Channel, as determined by pigment and fatty acid analyses. J. Plankton Res. 2001;23:693–703. doi: 10.1093/plankt/23.7.693. [DOI] [Google Scholar]
- 76.Dembitsky V.M., Shkrob I., Go J.V. Dicarboxylic and fatty acid compositions of cyanobacteria of the genus Aphanizomenon. Biochem. Mosc. 2001;66:72–76. doi: 10.1023/A:1002837830653. [DOI] [PubMed] [Google Scholar]
- 77.Tang K.W., Jakobsen H.H., Visser A.W. Phaeocystis globosa (Prymnesiophyceae) and the planktonic food web: Feeding, growth, and trophic interactions among grazers. Limnol. Oceanogr. 2001;46:1860–1870. doi: 10.4319/lo.2001.46.8.1860. [DOI] [Google Scholar]
- 78.Gugger M., Lyra C., Suominen I., Tsitko I., Humbert J.-F., Salkinoja-Salonen M.S., Sivonen K. Cellular fatty acids as chemotaxonomic markers of the genera Anabaena, Aphanizomenon, Microcystis, Nostoc and Planktothrix (cyanobacteria) Int. J. Syst. Evol. Microbiol. 2002;52:1007–1015. doi: 10.1099/00207713-52-3-1007. [DOI] [PubMed] [Google Scholar]
- 79.Broglio E., Jónasdóttir S.H., Calbet A., Jakobsen H.H., Saiz E. Effect of heterotrophic versus autotrophic food on feeding and reproduction of the calanoid copepod Acartia tonsa: Relationship with prey fatty acid composition. Aquat. Microb. Ecol. 2003;31:267–278. doi: 10.3354/ame031267. [DOI] [Google Scholar]
- 80.Ahlgren G., Hyenstrand P. Nitrogen limitation effects of different nitrogen sources on nutritional quality of two freshwater organisms, Scenedesmus quadricauda (Chlorophyceae) and Synechococcus sp. (Cyanophyceae) J. Phycol. 2003;39:906–917. doi: 10.1046/j.1529-8817.2003.02026.x. [DOI] [Google Scholar]
- 81.Řezanka T., Dor I., Prell A., Dembitsky V.M., Řezanka T., Dor I., Prell A., Dembitsky V.M. Fatty acid composition of six freshwater wild cyanobacterial species. Folia Microbiol. 2003;48:71–75. doi: 10.1007/BF02931279. [DOI] [PubMed] [Google Scholar]
- 82.Shin K., Jang M.C., Jang P.K., Ju S.J., Lee T.K., Chang M. Influence of food quality on egg production and viability of the marine planktonic copepod Acartia omorii. Prog. Oceanogr. 2003;57:265–277. doi: 10.1016/S0079-6611(03)00101-0. [DOI] [Google Scholar]
- 83.Arendt K.E., Jónasdóttir S.H., Hansen P.J., Gärtner S. Effects of dietary fatty acids on the reproductive success of the calanoid copepod Temora longicornis. Mar. Biol. 2005;146:513–530. doi: 10.1007/s00227-004-1457-9. [DOI] [Google Scholar]
- 84.Thor P., Koski M., Tang K.W., Jónasdóttir S.H. Supplemental effects of diet mixing on absorption of ingested organic carbon in the marine copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 2007;331:131–138. doi: 10.3354/meps331131. [DOI] [Google Scholar]
- 85.Dutz J., Koski M., Jónasdóttir S.H. Copepod reproduction is unaffected by diatom aldehydes or lipid composition. Limnol. Oceanogr. 2008;53:225–235. doi: 10.4319/lo.2008.53.1.0225. [DOI] [Google Scholar]
- 86.Chen M., Liu H., Chen B. Effects of dietary essential fatty acids on reproduction rates of a subtropical calanoid copepod, Acartia erythraea. Mar. Ecol. Prog. Ser. 2012;455:95–110. doi: 10.3354/meps09685. [DOI] [Google Scholar]
- 87.Jónasdóttir S.H., Kiørboe T. Copepod recruitment and food composition: Do diatoms affect hatching success? Mar. Biol. 1996;125:743–750. doi: 10.1007/BF00349257. [DOI] [Google Scholar]
- 88.Mansour M.P., Volkman J.K., Jackson A.E., Blackburn S.I. The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 1999;35:710–720. doi: 10.1046/j.1529-8817.1999.3540710.x. [DOI] [Google Scholar]
- 89.Peltomaa E., Johnson M.D., Taipale S.J. Marine cryptophytes are great sources of EPA and DHA. Mar. Drugs. 2017;16:3. doi: 10.3390/md16010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menden-Deuer S., Lessard E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000;45:569–579. doi: 10.4319/lo.2000.45.3.0569. [DOI] [Google Scholar]
- 91.Jónasdóttir S.H., Fields D., Pantoja S. Copepod egg production in Long Island Sound, USA, as a function of the chemical composition of seston. Mar. Ecol. Prog. Ser. 1995;119:87–98. doi: 10.3354/meps119087. [DOI] [Google Scholar]
- 92.Md Amin R., Koski M., Båmstedt U., Vidoudez C. Strain-related physiological and behavioral effects of Skeletonema marinoi on three common planktonic copepods. Mar. Biol. 2011;158:1965–1980. doi: 10.1007/s00227-011-1706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters J., Dutz J., Hagen W. Role of essential fatty acids on the reproductive success of the copepod Temora longicornis in the North Sea. Mar. Ecol. Prog. Ser. 2007;341:153–163. doi: 10.3354/meps341153. [DOI] [Google Scholar]
- 94.Mayzaud P., Chanut J.P., Ackman R.G. Seasonal changes of the biochemical composition of marine particulate matter with special reference to fatty acids and sterols. Mar. Ecol. Prog. Ser. 1989;56:189–204. doi: 10.3354/meps056189. [DOI] [Google Scholar]
- 95.Morris R.J., McCartney M.J., Robinson G.A. Studies of a spring phytoplankton bloom in an enclosed experimental ecosystem. I. Biochemical changes in relation to the nutrient chemistry of water. J. Exp. Mar. Biol. Ecol. 1983;70:249–262. doi: 10.1016/0022-0981(83)90092-8. [DOI] [Google Scholar]
- 96.Kattner G., Gercken G., Eberlein K. Development of lipids during a spring plankton bloom in the northern North Sea. I. Particulate fatty acids. Mar. Chem. 1983;14:149–162. doi: 10.1016/0304-4203(83)90038-5. [DOI] [Google Scholar]
- 97.Parrish C.C. Time series of particulate and dissolved lipid classes during spring phytoplankton blooms in Bedford Basin, a marine inlet. Mar. Ecol. Prog. Ser. 1987;35:129–139. doi: 10.3354/meps035129. [DOI] [Google Scholar]
- 98.Parrish C.C., Wangersky P. Particulate and dissolved lipid classes in cultures of Phaeodactylum tricornutum grown in cage culture turbidostats with a range of nitrogen supply rates. Mar. Ecol. Prog. Ser. 1987;35:119–128. doi: 10.3354/meps035119. [DOI] [Google Scholar]
- 99.Webb K., Chu F.-L. Phytoplankton as a food source for bivalve larvae. In: Pruder G., Langdon C., Conklin D., editors. Proceedings of the Second International Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition. Louisiana State University; Baton Rouge, LA, USA: 1982. pp. 272–291. [Google Scholar]
- 100.Cohen Z. Products from microalgae. In: Richmond A., editor. Handbook for Micralgal Mass Culture. CRC Press Inc.; Boca Raton, FL, USA: 1986. pp. 421–454. [Google Scholar]
- 101.Thompson P.A., Guo M., Harrison P.J. Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplantkton. J. Phycol. 1992;28:481–488. doi: 10.1111/j.0022-3646.1992.00481.x. [DOI] [Google Scholar]
- 102.Rousch J.M.M., Bingham S.E.E., Sommerfeld M.R.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003;295:145–156. doi: 10.1016/S0022-0981(03)00293-4. [DOI] [Google Scholar]
- 103.Flaim G., Obertegger U., Guella G. Changes in galactolipid composition of the cold freshwater dinoflagellate Borghiella dodgei in response to temperature. Hydrobiologia. 2012;698:285–293. doi: 10.1007/s10750-012-1070-8. [DOI] [Google Scholar]
- 104.Guihéneuf F., Mimouni V., Tremblin G., Ulmann L. Light intensity regulates LC-PUFA incorporation into lipids of Pavlova lutheri and the final desaturase and elongase activities involved in their biosynthesis. J. Agric. Food Chem. 2015;63:1261–1267. doi: 10.1021/jf504863u. [DOI] [PubMed] [Google Scholar]
- 105.Thompson P.A., Harrison P.J., Whyte J.N.C. Influence of irradiance on the fatty acid composition of phytoplankton. J. Phycol. 1990;26:278–288. doi: 10.1111/j.0022-3646.1990.00278.x. [DOI] [Google Scholar]
- 106.Wainman B.C., Smith R.E., Rai H., Furgal J.A. Irradiance and lipid production in natural algal populations. In: Arts M.T., Wainmann B.C., editors. Lipids in Freshwater Ecosystems. Springer; New York, NY, USA: 1999. pp. 45–70. [Google Scholar]
- 107.Shifrin N.S., Chisholm S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-drak cycles. J. Phycol. 1981;17:374–384. doi: 10.1111/j.0022-3646.1981.00374.x. [DOI] [Google Scholar]
- 108.Groeger A., Schram M., Marzolf G. Influence of food quality on growth and reproduction in Daphnia. Freshw. Biol. 1991;26:11–19. doi: 10.1111/j.1365-2427.1991.tb00504.x. [DOI] [Google Scholar]
- 109.Klein Breteler W., Schogt N., Rampen S. Effect of diatom nutrient limitation on copepod development: The role of essential lipids. Mar. Ecol. Prog. Ser. 2005;291:125–133. doi: 10.3354/meps291125. [DOI] [Google Scholar]
- 110.Reitan K.I., Rainuzzo J.R., Olsen Y. Effect of Nutrient Limitation on Fatty-Acid and Lipid-Content of Marine Microalgae. J. Phycol. 1994;30:972–979. doi: 10.1111/j.0022-3646.1994.00972.x. [DOI] [Google Scholar]
- 111.Dörmann P. Encyclopedia of Life Sciences: Els. John Wiley & Sons Ltd.; Chichester, UK: 2001. [(accessed on 31 August 2015)]. Galactolipids in plant membranes. Available online: http://www.els.net. [Google Scholar]
- 112.Taguchi S., Hirata J.A., Laws E.A. Silicate Deficiency and Lipid Synthesis of Marine Diatoms. J. Phycol. 1987;23:260–267. doi: 10.1111/j.1529-8817.1987.tb04133.x. [DOI] [Google Scholar]
- 113.Roessler P.G. Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica. J. Phycol. 1988;24:394–400. doi: 10.1111/j.1529-8817.1988.tb00189.x. [DOI] [Google Scholar]
- 114.Gavalas N., Clark H. On the Role of Manganese in Photosynthesis Kinetics of Photoinhibition in Manganese-deficent and 3-(4-Chlorophenyl)-1, 1-dimethylurea-inhibited Euglena gracilis. Plant Physiol. 1971;47:139–143. doi: 10.1104/pp.47.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shanklin J., Cahoon E.B. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- 116.Los D.A., Murata N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta. 1998;1394:3–15. doi: 10.1016/S0005-2760(98)00091-5. [DOI] [PubMed] [Google Scholar]
- 117.Sprague M., Dick J.R., Tocher D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016;6:21892. doi: 10.1038/srep21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pike I.H., Jackson A. Fish oil: Production and use now and in the future. Lipid Technol. 2010;22:59–61. doi: 10.1002/lite.201000003. [DOI] [Google Scholar]
- 119.Greenberg P. The Omega Principle: Seafood and the Quest for a Long Life and a Healthier Planet. Penguin Books; London, UK: 2018. [Google Scholar]
- 120.Calder P.C. Mechanisms of Action of (n-3) Fatty Acids, 2. J. Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 121.IPCC Climate Change 2014 Synthesis Report Summary Chapter for Policymakers. IPCC; Geneva, Switzerland: 2014. p. 32. [Google Scholar]
- 122.Guiry M.D., Guiry G.M. AlgaeBase. World-Wide Electronic Publication. National University of Ireland Galway; Galway, Ireland: 2018. [(accessed on 20 December 2018)]. Available online: http://www.algaebase.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.