Abstract
Controlled ligand‐based redox‐activity and chemical non‐innocence are rapidly gaining importance for selective (catalytic) processes. This Concept aims to provide an overview of the progress regarding ligand‐to‐substrate single‐electron transfer as a relatively new mode of operation to exploit ligand‐centered reactivity and catalysis based thereon.
Keywords: non-innocent ligands, radical, redox-active ligands, single-electron transfer, substrate
Introduction
Storing and releasing redox‐equivalents (electrons) in non‐innocent or reactive ligand scaffolds is becoming a very important and effective strategy in both catalysis and renewable fuel research. Shuttling of electrons can be achieved using so‐called redox‐active ligands, that is, organic fragments that can reversibly shuttle between at least two well‐defined redox‐states whilst bound to a metal(loid) center, the oxidation state of which does not need to be significantly affected by this transformation (Figure 1). In cases where a more ambiguous overall electronic structure ensues from strong electronic coupling between a ligand and a metal center, the term redox‐noninnocence may be more appropriate.1 Well‐known examples of redox‐active ligands are dithiolenes,2 donor appended diarylamines,3 heterodienes such as 1,4‐diimine and related 2‐iminopyridines as well as bis(imino)pyridines,4 olefin‐appended diazadienes,5 salens and derivatives thereof,3j, 6 and dipyrrins as well as extended systems such as porphyrins.7 Newer additions have appeared as well, including verdazyl,8 and nindigo,9 formazanate,10 aminotropiminate,11 arylazopyridine,12 π‐coordinated arene and derivatives thereof,13 β‐diketiminate14 and various others.15 Particularly well‐studied are ligands bearing the redox‐active 1,2‐catechol or o‐phenylenediamine16, 17 moiety or the hybrid o‐aminophenol18, 19] fragment, which can bind to a metal center in three different oxidation states: the two‐electron reduced (dianionic), one‐electron reduced/oxidized (monoanionic ligand radical) and two‐electron oxidized form (neutral).
Figure 1.
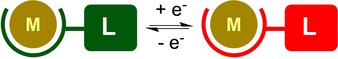
General depiction of reversible ligand centered redox process. Green=oxidized ligand; red=reduced ligand.
Modes of reactivity of redox‐active ligands
Beyond the intriguing spectroscopic and magnetochemical features of redox‐active ligands, which have been intense subject of research over the last decades recent developments have primarily focused on the possibilities for these platforms to accommodate and modulate chemical reactions at a bound transition metal. A major thrust for the use of redox‐active ligands has been as electron reservoir for storing and releasing redox equivalents that may be generated or required at a metal center during substrate activation.20 However, these ligand systems have been shown to also allow at least four other modes of operation, although each of these still need to be further explored to establish and exploit their full potential. Three of these modes are primarily related to the redox‐active nature of these ligand platforms: i) tuning of the Lewis acidity or basicity of a transition metal by changing the ligand redox‐state,21 ii) substrate activation by formation of a new ligand‐substrate bond via radical reactivity22 and iii) metal–ligand bifunctional substrate activation by a one (ligand) plus one (metal), overall two‐electron transfer23 (Figure 2). The fourth mode of operation (not displayed) relates to the potential chemical non‐innocent nature of these platforms in terms of reversible (de)protonation and proton‐shuttling abilities, which could allow for additional applications of redox‐active ligands for metal‐ligand bifunctional substrate activation.
Figure 2.
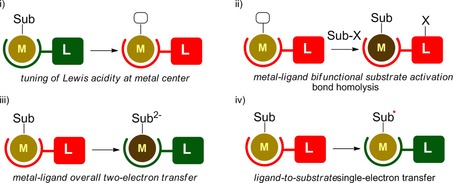
General depiction of three “established” modes (i–iii) of redox‐active ligand reactivity that go beyond two‐electron transfer to a metal center, as well as the new fourth mode (iv), highlighted in this Concept. Red=reduced ligand; green=oxidized ligand; light brown=metal in low oxidation state; dark brown=metal in high oxidation state.
Emerging strategies for radical catalysis
Several strategies for single‐electron transfer‐induced catalysis (e.g. photoredox catalysis, electron catalysis) have been developed, but although transition metals are sometimes involved as oxidant or reductant, many strategies result in the formation of (low concentrations of) free organic radicals. This may pose challenges with respect to, for example, chemoselectivity due to the highly reactive nature of many radical species.24, 25 Metal‐mediated atom transfer may afford more control, direct the reactivity of single‐electron activated substrates and thereby provide alternatives to free radical strategies in terms of for example, chemoselectivity and substrate scope.26 In extremis, radical‐type substrate species can be incorporated in the coordination sphere of a transition metal. One approach is metalloradical‐based catalysis using paramagnetic complexes based on a (transition) metal ion with only one unpaired electron (S= ), which allows for an electronic configuration wherein this unpaired electron can be transferred to an empty (acceptor) orbital at a bound substrate.27 This results in formal one‐electron redox‐events at both the metal (Mn+/(n+1)+) and substrate site. Only relatively few redox‐active metals (e.g. TiIII, FeII, CoII, RhII, CuII) are available to facilitate the desired one‐electron transfer using this strategy because the reduced and oxidized form of the redox‐couple should be reversibly accessible and properly stabilized by the same ligand set.28
An appealing alternative for metalloradical based catalysis would be to separate the locus of the redox‐chemistry from the metal‐centered substrate‐binding event, harnessing the ligand redox‐activity as one‐electron reservoir to facilitate single‐electron transfer with a substrate (Figure 2 iv). This relatively new mode of ligand reactivity in synthetic chemistry and catalysis is the focus of this Concept. This type of ligand‐centered redox‐chemistry also finds resonance in a number of biological metalloenzyme systems such as horseradish peroxidase,29 intradiol catechol dioxygenase,30 galactose oxidase31 and cytochrome P450 that operate through mechanisms in which a redox‐active ligand (e.g., a heme porphyrin, tyrosine‐based phenolate or a thiolate) shuttles a single electron to (or from) a substrate.32
The intrinsic differences between ligand‐centered single‐electron transfer and metalloradical‐based reactivity are outlined in Figure 3. In the ligand centered strategy, the need for a redox‐active metal is circumvented, allowing for more expanded coordination chemistry including noble, base and main‐group metals, thus offering a wider palette with respect to substrate binding and pre‐activation. Furthermore, there is more extensive opportunities to fine‐tune the redox‐properties of the ligand system by synthetic design (including strategies to extend to multinuclear architectures), and thus to potentially achieve control over the desirable redox‐processes.
Figure 3.
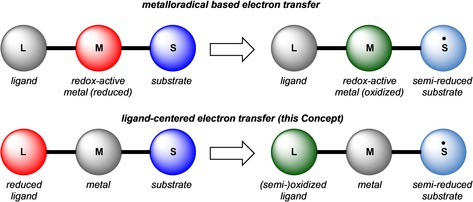
Metalloradical based vs. ligand‐centered single‐electron transfer reactivity. Red=reduced; green=oxidized (either metal or ligand).
Stoichiometric Ligand‐to‐Substrate Single‐Electron Transfer
The group of Soper reported that cis‐ReV(O)(Cl) precursor A bearing one amidophenolate ligand and a one‐electron oxidized ligand LISQ radical (i.e., a ligand mixed‐valent system) interacts with half an equivalent of triphenylmethyl radical (Ph3C.; Gomberg's reagent) to give triphenylmethanol (Ph3COH) and two new rhenium complexes, that is, a dichlorido‐species B and oxo‐bridged dimer C (Scheme 1, top).33 This outcome suggests that even on a coordinatively saturated metal center, the oxo ligands can be transformed into a reactive substrate. The atom‐transfer reaction was suggested to occur by mixing of a populated Re=O π‐bond with the ligand‐centered LISQ radical, which puts substantial spin density into the oxo oygen atom (de facto creating an oxyl fragment), enabling the low‐barrier radical coupling with the external organic radical, although computational studies were not performed to substantiate this proposal. Closed‐shell oxorhenium homologues were found to be inert to Ph3C., suggesting at least some role for the ligand radical to activate the oxo‐fragment during this transformation. The accompanying Re‐complexes were suggested to result from disproportionation of the Re‐species that are formed after O‐atom transfer. The origin of the H atom in the formed alcohol product is unknown.
Scheme 1.
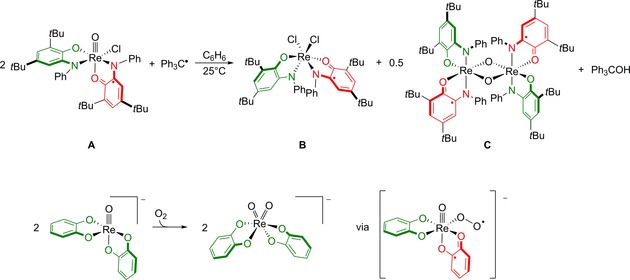
Top: Oxygen atom transfer reaction of A with Ph3C. to produce Ph3COH and two rhenium complexes B and C. Bottom: Homolysis of O2 on bis(catecholate) derivative of A to give ReV‐dioxo derivative.
Soper and co‐workers also demonstrated that the related ReV‐oxo complex bearing either two 2,4‐di‐tert‐6‐(phenylamido)phenolate ligands or two catecholate ligands allowed for the overall homolysis of O2 (Scheme 1, bottom).34 A combined kinetic and computational study led to the proposition that upon coordination of O2, single‐electron transfer from one of the redox‐active ligands generates a superoxide species rhenium(V)(O2 ・−), circumventing the rare formal ReVI oxidation state and creating a ligand mixed‐valent Re‐species. The accompanying spin density delocalization aids in lowering the activation barrier for the formally spin‐forbidden reaction between ReV and dioxygen by allowing spin‐crossover. Coupling with a second equivalent of the starting ReV‐oxo species leads to a μ‐peroxo dinuclear intermediate from which the final ReVII‐bis(oxo)‐product forms by O−O cleavage, with concomitant electron transfer back into the previously oxidized redox‐active ligand. Control experiments with redox‐inactive oxalate ligands gave no reaction, which is a strong indication of a crucial role for the ligand in this overall transformation. N−O cleavage in the stable nitroxyl radical 2,2,6,6‐tetramethylpiperidin‐1‐yl)oxyl (TEMPO⋅) was also possible using the same strategy.35
Although formally not a direct ligand‐to‐substrate single electron transfer, Heyduk and co‐workers demonstrated redox‐active ligand‐centered one‐electron oxidation to facilitate the addition of a chlorine atom (Cl.) on a d0 ZrIV metal center in complex D, ZrCl(THF)2(L), by reaction with 0.5 molar equivalents of the two‐electron oxidant PhICl2.36 This reaction yielded the paramagnetic S = complex E ZrCl2(THF)(L .). with the 1,2‐diaminobenzene‐derived tridentate trisanionic NNN ligand present as the one‐electron oxidized ligand‐centered radical L . (Scheme 2).
Scheme 2.
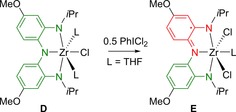
Ligand‐facilitated single‐electron oxidation on a ZrIV(NNN) complex.
Smith and co‐workers have reported the reactivity of coordinatively unsaturated CrIII(Cp) complexes, bearing a range of redox‐active diphenylamido NN ligands, towards organoazide substrates, with the outcome depending on both the substitution pattern of the azide reagent and the diamido ligand (Scheme 3).37 In selected cases, ligand‐to‐substrate single‐electron transfer was observed to furnish the one‐electron oxidized NN‐ligand radical concomitantly with conversion of the azide to the corresponding imido (F) or amido fragment (G) bound to Cr, in the latter case with adventitious H‐atom transfer. Using trisyl azide as substrate and 1,2‐bis(phenylamido)benzene as ligand, catalytic C−H insertion to provide a benzosultam product H was observed, although in modest turnover numbers (TON<5). The outcome of this particular reaction may be explained by ligand‐to‐substrate single‐electron transfer to generate an imidyl (or nitrene) radical species in the coordination sphere of chromium. Follow‐up intramolecular hydrogen atom abstraction and subsequent rebound (or alternatively direct C−H insertion) leads to product formation.
Scheme 3.
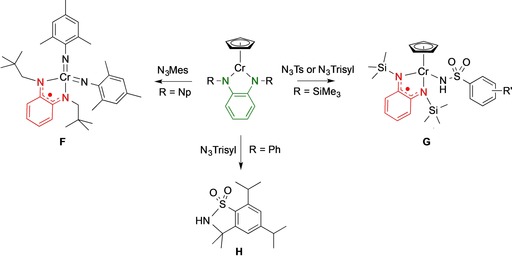
Ligand‐to‐substrate single‐electron transfer using o‐phenylenediamine‐derived chromium(III) complexes upon reaction with aryl and sulfonylazides, and catalytic formation of benzosultam product H.
Combining the well‐known aminophenol‐based redox‐chemistry with ever‐expanding chemistry related to pincer platforms, and building on expertise in the use of reactive pincer ligand systems,38 van der Vlugt et al. developed the NNHOH ligand L1H2 (L1H2=2,4‐di‐tert‐butyl‐6‐((2‐(pyridin‐2‐yl)propan‐2‐yl)amino)phenol). Exchanging the peripheral pyridine for a phosphine donor group provides the corresponding PNHOH framework L2H2. Both ligands readily coordinate to PdII as the ligand radical LISQ, giving access to crystalline paramagnetic square planar PdCl(LISQ) complexes with discrete well‐behaved ligand‐based electrochemical responses according to cyclic voltammetry, with the potentials for the respective redox‐couples subtly dependent on the peripheral donor groups whilst coordinated to PdII. They have also reported homodinuclear palladium complexes derived from these platforms and close analogs thereof and have studied the magnetochemistry and spectroelectrochemistry of their ligand‐radical binding pockets.39 Moreover, a route was devised to prepare heterodi‐ and trinuclear complexes with tunable d8–d10 metal interactions.40
In situ reduction of the PdCl(L2ISQ) complex in the presence of a stoichiometric amount of CoCp2 as reducing agent generated the fully reduced PNOAP platform I in an overall anionic Pd‐complex. Addition of TlPF6 as chloride removal agent enabled this system to facilitate the homolytic bond cleavage of disulfides through a ligand‐to‐substrate single‐electron‐transfer process (Scheme 4).41 Using a 1:1 ratio of Pd‐complex and disphenyl disulfide, the intermediacy of a free thiyl radical was evidenced by GC‐MS analysis, as PhSPh was detected from reaction of the thiyl radical with the benzene solvent after S−S cleavage. Use of di(tert‐butyl)disulfide allowed for spectroscopic observation of the corresponding disulfide adduct by NMR spectroscopy. The second part of the activated disulfide is transformed into a Pd‐bound thiolate, with reformation of the L2ISQ form in the coordination sphere of Pd. Follow‐up dimerization with reduced Pd(L2AP)‐complex resulted in the crystallographically characterized ligand mixed‐valent dinuclear species J (L2ISQ)Pd−SPh−Pd(L2AP). Clean formation of the dimeric species was obtained when using a 4:1 ratio of Pd‐complex to disulfide substrate.
Scheme 4.
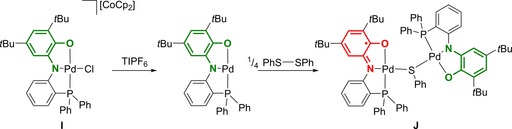
Ligand‐to‐substrate single‐electron transfer to homolytically cleave disulfides and generate mixed‐valent dinuclear Pd‐ligand species.
Catalytic Ligand‐to‐Substrate Single‐Electron Transfer
The group of Desage El‐Murr, partly in combination with the Fensterbank group, has demonstrated the use of square planar NiII complex K featuring two aminophenol‐based ligands or five coordinate CuII complex L bearing a redox‐active ONO pincer ligand, present in the one‐electron oxidized iminosemiquinone (LISQ) form, as an effective platform for the generation of free trifluoromethyl radicals (CF3 .), which formed upon single‐electron transfer from a metal‐bound iminosemiquinone ligand radical to a source of CF3 + (Scheme 5).42 It is postulated that no direct metal–CF3 bond occurs because the reactivity differs from that of isolated species with this bond present.43 Besides radical‐radical coupling with TEMPO⋅, catalytic trifluoromethylation of alkynes, heteroaromatics and vinyl silyl ethers was reported as well. The complexes formed after electron transfer were spectroscopically investigated but not isolated.
Scheme 5.
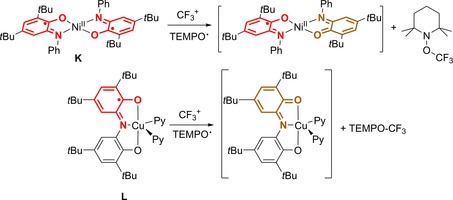
Selected examples of outer‐sphere ligand‐to‐substrate single‐electron transfer to CF3 +.
The concept of selective ligand‐to‐substrate single‐electron transfer can be exploited to generate nitrene radicals in the coordination sphere of, for example, a “redox‐inert”44 metal ion (PdII). In situ reduction of the Pd‐bound NNO‐ligand L1ISQ in complex M to the NNOAP form (N) using an equimolar amount of CoCp2, followed by activation of the organic azide substrate and irreversible expulsion of dinitrogen, initially generates the corresponding nitrene (Scheme 6). However, single‐electron transfer from the ligand to this hypovalent substrate featuring an empty p‐orbital can occur to form an open shell singlet “diradical” (nitrene radical‐ligand radical) O, as confirmed by DFT calculations. This effectively generates a nitrogen‐based substrate radical bound to PdII that is amenable to C−H insertion reactivity, as demonstrated by the formation of the N‐Boc‐pyrrolidine adduct P from the benchmark substrate 4‐phenylbutyl‐1‐azide by a two‐step H‐atom transfer and rebound, as supported experimentally by KIE data and DFT computations, and finally removal of the organic product by di‐tert‐butyldicarbonate (Boc‐anhydride).45 Although the turnover of this systems under optimized conditions is still modest (TON≈3), this proof‐of‐concept demonstrates the potential for ligand‐to‐substrate single‐electron transfer for radical‐type catalysis, even at redox‐inert metal centers.46
Scheme 6.
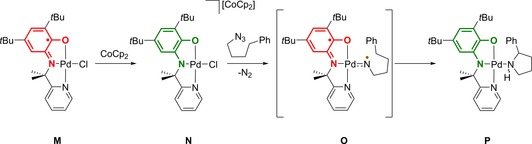
Generation of a nitrene radical in the coordination sphere of PdII by ligand‐to‐substrate single‐electron transfer and follow‐up C−H amination reactivity. Bottom: FeIII‐catalyzed C−H amination.
More recently, van der Vlugt et al. disclosed a well‐defined, crystallographically characterized, paramagnetic high‐spin (S=2) FeIII(Cl)2(L1ISQ) complex Q for the intramolecular C(sp3)−H amination of a broad range of organoazides, with TONs of up to 620 at 0.1 mol % catalyst loading (Scheme 7).47 This system is at least one order of magnitude more active than all other reported systems to date, all of which operate through metalloradical catalysis.48, 49, 50, 51 The FeIII‐complex could be quantitatively recycled and re‐used after catalysis. It was established that in situ reduction under the same conditions as for the Pd‐system led to Fe‐centered rather than ligand‐centered reduction, with subsequent disproportionation to afford a crystallographically characterized homoleptic FeII(L1ISQ)2 complex that was completely inactive for the C−H amination reaction. Furthermore, kinetics the FeIII‐based catalysis proved highly unusual, with zeroth order in the organoazide but first order in Boc‐anhydride. This hints to a pre‐activation step to generate a four‐coordinate derivative of the FeIII‐system Q′, potentially involving reversible reaction of the ligand radical with the Boc‐anhydride. The complexity of this system has precluded detailed computational or spectroscopic analyses to date.
Scheme 7.
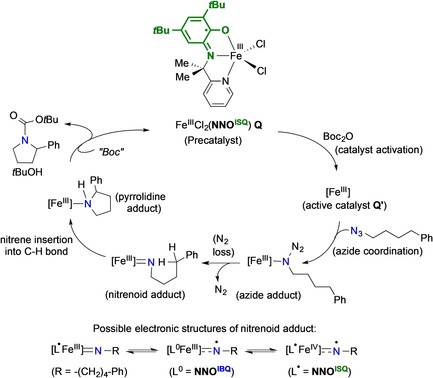
Postulated cycle for the C−H amination of a broad range of organoazides (4‐phenylbutyl azide chosen as an example) using crystallographically characterized FeIII‐precatalyst Q.
Following the same ligand‐to‐substrate single‐electron transfer strategy, Sugimoto, Itoh and co‐workers recently described C−H amination catalysis (maximum TON of 10) using the activated substrate tosyl azide and weak C−H bonds as in xanthene.52 The catalyst used consisted of a diamagnetic RhIII complex bearing a redox‐active ONNO‐type ligand, which switches from fully reduced trianionic to dianionic ligand radical R upon generation of the nitrene substrate radical bound to RhIII. Follow‐up work on this system established that the ligand may be considered chemically non‐innocent due to a fragmentation reaction occurring with the tosyl azide in the absence of xanthene substrate, forming R′, which is also active for the same C−H amination catalytic reaction (Scheme 8).53
Scheme 8.
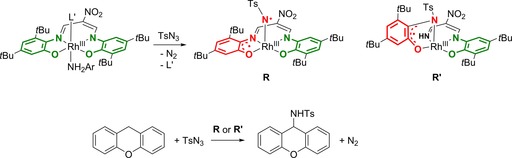
A RhIII(ONNO) catalyst for intermolecular C−H amination of xanthene using tosyl azide.
The Desage El‐Murr group described the catalytic aziridination of cyclic and acyclic alkenes, non‐conjugated dienes and styrenes using tosyliminoiodinane as nitrene source and CuII(LISQ)2 as catalyst. This copper‐complex S has a doublet ground‐state due to antiferromagnetic coupling of one of the ligand‐centered radicals with the unpaired electron at Cu (but an S= 3/2 quartet is thermally accessible). A combined spectroscopic and computational study supported a mechanism involving initial formation of a nitrogen‐centered nitrene radical T after reaction with PhINTs and ligand‐to‐substrate single‐electron transfer. This five‐coordinate species has a quartet (S= 3/2) ground state. Reaction with a vinylic substrate (styrene in Scheme 9) generates U featuring a Cu‐bound amido‐fragment connected to a carbon‐centered radical. HYSCORE experiments at 5 K suggest a doublet ground state for this species because of a spin‐flip taking place at the iminosemiquinone ligand. Radical ring‐closure results in the organic aziridine product, most likely concomitant with activation of a new PhINTs substrate. Hence, two‐state reactivity involving spin‐state flipping at the ligand (rather than the metal, which is more commonly observed) is crucial for this catalytic reaction.54
Scheme 9.
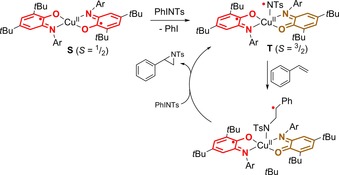
Copper(II) mediated aziridination of vinylic substrates by ligand‐centered two‐state reactivity.
Turning the Tables: Stoichiometric Single‐Electron Transfer to a Redox‐Active Ligand
There are at least two examples in which the (initial) electron transfer occurs from a substrate to a redox‐active ligand rather than, as in the above examples, ligand‐to‐substrate SET. Notably, such “oxidative” substrate activation is not reported to date for metalloradical‐based catalysis. The group of Bart has reported on the radical reductive elimination of 1,2‐diphenylethane by the release of a benzyl radical from UIV‐tetra(benzyl) complex V upon reaction with 3,5‐bis(tert‐butyl)iminobenzoquinone, followed by a second single‐electron‐transfer event and radical expulsion to yield amidophenolate‐bis(benzyl) UIV derivative W (Scheme 10).55
Scheme 10.
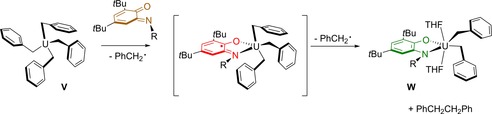
Stepwise single‐electron transfer from a UIV(tetrabenzyl) complex to an incoming iminobenzoquinone ligand to afford complex W.
The group of van der Vlugt reported the reaction of NaN3 with both cis‐ and trans‐RuIII(Cl)2(PPh3)(NNOISQ) X and Y, with NNOISQ being the ligand‐radical version of a redox‐active pincer ligand NNHOH ligand L1H2.56 The final product after reaction in ambient light is a trinuclear complex Z featuring bridging nitrido ligands that originate from the sodium azide as well as three fully reduced NNOAP fragments (Scheme 11). Isotope labeled 15N‐azide studies coupled to GC analysis of the formed N2 by‐product strongly suggests multiple events of substrate‐to‐ligand single‐electron transfer. There appears to be much potential to extrapolate this concept of activating substrates by a substrate‐to‐ligand single‐electron transfer to catalytic applications.
Scheme 11.
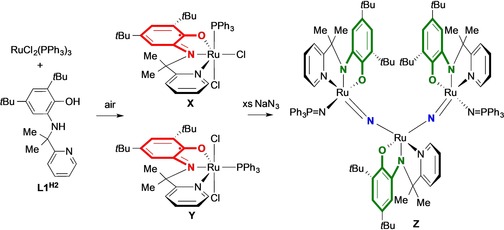
Formation of a trinuclear ruthenium complex with two bridging nitrides by single‐electron transfer from a nitride ligand to the redox‐active ligand.
Conclusions and Outlook
Single‐electron transfer to or from a redox‐active ligand to induce radical‐type reactivity on a metal‐bound substrate has rapidly emerged in the last ±5 years. Notable stoichiometric as well as catalytic applications in the area of C−H insertion and alkene functionalization exploit this new concept. Much more exciting reactivity based on selective single‐electron transfer between redox‐active ligands and metal‐bound substrates can be foreseen, with both early and late transition metals, lanthanides as well as possibly also main‐group elements. This may offer new ways to accomplish (catalytic) atom‐ and group‐transfer reactions as well as hydroadditions, to name a few important classes of transformations. In this context, it should be noted that also another type of “ligand reactivity”, that is, involving proton‐responsive ligands, has become prominent in the realm of both noble and base metal–ligand bifunctional substrate activation and catalysis in the last decade.57 However, strategies to effectively bridge redox non‐innocence and such chemical non‐innocence have been only scarcely identified to date.58, 59, 60, 61, 62 However, if this selective ligand‐centered hydrogen‐based chemistry could be exploited alongside the established electron‐based reactivity of such ligands, this might allow for interesting crossover studies between previously disparate ligand‐based reactivity patterns. Ultimately, this offers the foresight of selective and efficient proton‐coupled electron transfer63 in catalysis. It is therefore deemed only a matter of time before exciting extensions and novel additions to this field of redox‐active ligand‐based reactivity are developed.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Research in my own group on this topic was funded by the European Research Council (ERC) through a Starting Grant (Agreement 279097, EuReCat), by the Netherlands Council for Scientific Research NWO (ECHO Grant) and by the Research Priority Area Sustainable Chemistry of the University of Amsterdam.
J. I. van der Vlugt, Chem. Eur. J. 2019, 25, 2651.
References
- 1.
- 1a. Broere D. L. J., Plessius R., van der Vlugt J. I., Chem. Soc. Rev. 2015, 44, 6886; [DOI] [PubMed] [Google Scholar]
- 1b. Luca O. R., Crabtree R. H., Chem. Soc. Rev. 2013, 42, 1440–1459; [DOI] [PubMed] [Google Scholar]
- 1c. Praneeth V. K. K., Ringenberg M. R., Ward T. R., Angew. Chem. Int. Ed. 2012, 51, 10228–10234; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 10374–10380; [Google Scholar]
- 1d. Lyaskovskyy V., de Bruin B., ACS Catal. 2012, 2, 270–279; [Google Scholar]
- 1e. Chirik P. J., Inorg. Chem. 2011, 50, 8737–9740; [DOI] [PubMed] [Google Scholar]
- 1f. Chirik P. J., Wieghardt K., Science 2010, 327, 794–795; [DOI] [PubMed] [Google Scholar]
- 1g. Jørgensen C. K., Coord. Chem. Rev. 1966, 1, 164–178. [Google Scholar]
- 2.
- 2a. Eisenberg R., Gray H. B., Inorg. Chem. 2011, 50, 9741–9751; [DOI] [PubMed] [Google Scholar]
- 2b. Sproules S., Wieghardt K., Coord. Chem. Rev. 2011, 255, 837–860; [Google Scholar]
- 2c. Sproules S., Wieghardt K., Coord. Chem. Rev. 2010, 254, 1358–1382. [Google Scholar]
- 3.
- 3a. Adhikari D., Mossin S., Basuli F., Huffman J. C., Szilagyi R. K., Meyer K., Mindiola D. J., J. Am. Chem. Soc. 2008, 130, 3676–3682; [DOI] [PubMed] [Google Scholar]
- 3b. Hicks R. G., Angew. Chem. Int. Ed. 2008, 47, 7393–7395; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 7503–7505; [Google Scholar]
- 3c. Harkins S. B., Mankad N. P., Miller A. J. M., Szilagyi R. K., Peters J. C., J. Am. Chem. Soc. 2008, 130, 3478–3485; [DOI] [PubMed] [Google Scholar]
- 3d. Radosevich A. T., Melnick J. G., Stoian S. A., Bacciu D., Chen C. H., Foxman B. M., Ozerov O. V., Nocera D. G., Inorg. Chem. 2009, 48, 9214–9221; [DOI] [PubMed] [Google Scholar]
- 3e. Vreeken V., Siegler M. A., de Bruin B., Reek J. N. H., Lutz M., van der Vlugt J. I., Angew. Chem. Int. Ed. 2015, 54, 7055; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 7161; [Google Scholar]
- 3f. Wang D., Lindeman S. V., Fiedler A. T., Inorg. Chem. 2015, 54, 8744–8754; [DOI] [PubMed] [Google Scholar]
- 3g. Vreeken V., Broere D. L. J., Jans A. C. H., Lankelma M., Reek J. N. H., Siegler M. A., van der Vlugt J. I., Angew. Chem. Int. Ed. 2016, 55, 10042–11046; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 10196–10200; [Google Scholar]
- 3h. Vreeken V., Baij L., de Bruin B., Siegler M. A., van der Vlugt J. I., Dalton Trans. 2017, 46, 7145–7149; [DOI] [PubMed] [Google Scholar]
- 3i. Vreeken V., Siegler M. A., van der Vlugt J. I., Chem. Eur. J. 2017, 23, 5585–5594; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3j. Leconte N., Moutet J., Herasymchuk K., Clarke R. M., Philouze C., Luneau D., Storr T., Thomas F., Chem. Commun. 2017, 53, 2764–2767. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Gibson V. C., Redshaw C., Solan G. A., Chem. Rev. 2007, 107, 1745–1776; [DOI] [PubMed] [Google Scholar]
- 4b. Caulton K. G., Eur. J. Inorg. Chem. 2012, 435–443; [Google Scholar]
- 4c. Budzelaar P. H. M., Eur. J. Inorg. Chem. 2012, 530–534; [Google Scholar]
- 4d. Sieh D., Schlimm M., Andernach L., Angersbach F., Nückel S., Schöffel J., Šušnjar N., Burger P., Eur. J. Inorg. Chem. 2012, 444–462; [Google Scholar]
- 4e. Myers T. W., Berben L. A., Inorg. Chem. 2012, 51, 1480–1488; [DOI] [PubMed] [Google Scholar]
- 4f. Villa M., Miesel D., Hildebrandt A., Ragaini F., Schaarschmidt D., Jacobi von Wangelin A., ChemCatChem 2017, 9, 3203–3209; [Google Scholar]
- 4g. Chirik P. J., Angew. Chem. Int. Ed. 2017, 56, 5170–5181; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 5252–5265; [Google Scholar]; Heins S. P., Wolczanski P. T., Cundari T. R., MacMillan S. N., Chem. Sci. 2017, 8, 3410–3418; [DOI] [PMC free article] [PubMed] [Google Scholar]; Williams V. A., Hulley E. B., Wolczanski P. T., Lancaster K. L., Lobkovsky E. B., Chem. Sci. 2013, 4, 3636–3648; [Google Scholar]; Chirik P. J., Acc. Chem. Res. 2015, 48, 1687–1695; [DOI] [PubMed] [Google Scholar]; Bezdek M., Guo S., Chirik P. J., Inorg. Chem. 2016, 55, 3117–3127; [DOI] [PubMed] [Google Scholar]; Bowman A. C., Tondreau A. M., Lobkovsky E., Margulieux G. W., Chirik P. J., Inorg. Chem. 2018, 57, 9634–9643. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Büttner T., Geier J., Frison G., Harmer J., Calle C., Schweiger A., Schönberg H., Grützmacher H., Science 2005, 307, 235–238; [DOI] [PubMed] [Google Scholar]
- 5b. Maire P., Königsmann M., Sreekanth A., Harmer J., Schweiger A., Grützmacher H., J. Am. Chem. Soc. 2006, 128, 6578–6580; [DOI] [PubMed] [Google Scholar]
- 5c. Donati N., Stein D., Büttner T., Schönberg H., Harmer J., Anadaram S., Grützmacher H., Eur. J. Inorg. Chem. 2008, 4691–4703; [Google Scholar]
- 5d. Zweifel T., Naubron J.-V., Grützmacher J., Angew. Chem. Int. Ed. 2009, 48, 559–563; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 567–571; [Google Scholar]
- 5e. Rodríguez-Lugo R. E., Trincado M., Vogt M., Tewes F., Santiso-Quinones G., Grützmacher H., Nat. Chem. 2013, 5, 342–347; [DOI] [PubMed] [Google Scholar]
- 5f. Prokopchuk D. E., Lough A. J., Rodriguez-Lugo R. E., Morris R. H., Grützmacher H., Chem. Commun. 2016, 52, 6138–6141; [DOI] [PubMed] [Google Scholar]
- 5g. Rodríguez-Lugo R. E., de Bruin B., Trincado M., Grützmacher H., Chem. Eur. J. 2017, 23, 6795–6802; [DOI] [PubMed] [Google Scholar]
- 5h. Sinha V., Pribanic B., de Bruin B., Trincado M., Grützmacher H., Chem. Eur. J. 2018, 24, 5513–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.
- 6a. Clarke R. M., Herasymchuk K., Storr T., Coord. Chem. Rev. 2017, 352, 67–82; [Google Scholar]
- 6b. Clarke R. M., Storr T., Dalton Trans. 2014, 43, 9380–9391; [DOI] [PubMed] [Google Scholar]
- 6c. Clarke R. M., Hazin K., Thompson J. R., Savard D., Prosser K. E., Storr T., Inorg. Chem. 2016, 55, 762–774; [DOI] [PubMed] [Google Scholar]
- 6d. Clarke R. M., Storr T., J. Am. Chem. Soc. 2016, 138, 15299–15302; [DOI] [PubMed] [Google Scholar]
- 6e. Marín I. M., Cheisson T., Singh-Chauhan R., Herrero C., Cordier M., Clavaguéra C., Nocton G., Auffrant A., Chem. Eur. J. 2017, 23, 17940–17953; [DOI] [PubMed] [Google Scholar]
- 6f. de Bellefeuille D., Orio M., Barra A.-L., Aukauloo A., Journaux Y., Philouze C., Ottenwaelder X., Thomas F., Inorg. Chem. 2015, 54, 9013–9026; [DOI] [PubMed] [Google Scholar]
- 6g. Kochem A., Gellon G., Jarjayes O., Philouze C., du Moulinet d'Hardemare A., van Gastel M., Thomas F., Dalton Trans. 2015, 44, 12743–12756. [DOI] [PubMed] [Google Scholar]
- 7.Dipyrrins:
- 7a. Curcio M., Pankhurst J. R., Sproules S., Mignard D., Love J. B., Angew. Chem. Int. Ed. 2017, 56, 7939–7943; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 8047–8051; [Google Scholar]
- 7b. Pankhurst J. R., Bell N. L., Zegke M., Platts L. N., Alvarez Lamsfus C., Maron L., Natrajan L. S., Sproules S., Arnold P. L., Love J. B., Chem. Sci. 2017, 8, 108–116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7c. Gautam R., Astashkin A. V., Chang T. M., Shearer J., Tomat E., Inorg. Chem. 2017, 56, 6755–6762; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7d. Moutet J., Philouze C., du Moulinet d'Hardemare A., Leconte N., Thomas F., Inorg. Chem. 2017, 56, 6380–6392; [DOI] [PubMed] [Google Scholar]
- 7e. Tomat E., Comm. Inorg. Chem. 2016, 36, 327–342; [Google Scholar]
- 7f. Gautam R., Chang T. M., Astashkin A. V., Lincoln K. M., Tomat E., Chem. Commun. 2016, 52, 6585–6588; [DOI] [PubMed] [Google Scholar]
- 7g. Lecarme L., Chiang L., Moutet J., Leconte N., Philouze C., Jarjayes O., Storr T., Thomas F., Dalton Trans. 2016, 45, 16325–16334; [DOI] [PubMed] [Google Scholar]
- 7h. Gautam R., Loughrey J. J., Astashkin A. V., Shearer J., Tomat E., Angew. Chem. Int. Ed. 2015, 54, 14894–14897; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 15107–15110; [Google Scholar]
- 7i. Kochem A., Chiang L., Baptiste B., Philouze C., Leconte N., Jarjayes O., Storr T., Thomas F., Chem. Eur. J. 2012, 18, 14590–14593; [DOI] [PubMed] [Google Scholar]
- 7j. Schweyen P., Brandhorst K., Wicht R., Wolfram B., Bröring M., Angew. Chem. Int. Ed. 2015, 54, 8213–8216; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 8331–8334; Porphyrins: [Google Scholar]
- 7k. Stillman M. J., J. Porphyrins Phthalocyanines 2000, 4, 374–376; [Google Scholar]
- 7l. Scheidt W. R., J. Biol. Inorg. Chem. 2001, 6, 727–732; [DOI] [PubMed] [Google Scholar]
- 7m. Felton R. H., Davis D. G. in The Porphyrins, (Ed.: D. Dolphin), Academic Press, New York, 1978; [Google Scholar]
- 7n. Römelt C., Song J., Tarrago M., Rees J. A., van Gastel M., Weyhermüller T., DeBeer S., Bill E., Neese F., Ye S., Inorg. Chem. 2017, 56, 4745–4750. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Gilroy J. B., Koivisto B. D., McDonald R., Ferguson M. J., Hicks R. G., J. Mater. Chem. 2006, 16, 2618–2624; [Google Scholar]
- 8b. Gilroy J. B., McKinnon S. D. J., Koivisto B. D., Hicks R. G., Org. Lett. 2007, 9, 4837–4840; [DOI] [PubMed] [Google Scholar]
- 8c. McKinnon S. D. J., Patrick B. O., Lever A. B. P., Hicks R. G., Chem. Commun. 2010, 46, 773–775; [DOI] [PubMed] [Google Scholar]
- 8d. Sanz C. A., Ferguson M. J., McDonald R., Patrick B. O., Hicks R. G., Chem. Commun. 2014, 50, 11676–11678; [DOI] [PubMed] [Google Scholar]
- 8e. Johnston C. W., Schwantje T. R., Ferguson M. J., McDonald R., Hicks R. G., Chem. Commun. 2014, 50, 12542–12544; [DOI] [PubMed] [Google Scholar]
- 8f. Sanz C. A., McKay Z. R., MacLean S. W. C., Patrick B. O., Hicks R. G., Dalton Trans. 2017, 46, 12636–12644. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Oakley S. R., Nawn G., Waldie K. M., MacInnis T. D., Patrick B. O., Hicks R. G., Chem. Commun. 2010, 46, 6753–6755; [DOI] [PubMed] [Google Scholar]
- 9b. Nawn G., Waldie K. M., Oakley S. R., Peters B. D., Mandel D., Patrick B. O., McDonald R., Hicks R. G., Inorg. Chem. 2011, 50, 9826–9837; [DOI] [PubMed] [Google Scholar]
- 9c. Fortier S., Gonzalez del Moral O., Chen C.-H., Pink M., Le Roy J. J., Murugesu M., Mindiola D. J., Caulton K. G., Chem. Commun. 2012, 48, 11082–11084; [DOI] [PubMed] [Google Scholar]
- 9d. Nawn G., McDonald R., Hicks R. G., Inorg. Chem. 2013, 52, 10912–10919; [DOI] [PubMed] [Google Scholar]
- 9e. Mondal P., Ehret F., Bubrin M., Das A., Mobin S. M., Kaim W., Lahiri G. K., Inorg. Chem. 2013, 52, 8467–8475; [DOI] [PubMed] [Google Scholar]
- 9f. Boice G., Patrick B. O., McDonald R., Bohne C., Hicks R. G., J. Org. Chem. 2014, 79, 9196–9205; [DOI] [PubMed] [Google Scholar]
- 9g. Mondal P., Chatterjee M., Paretzki A., Beyer K., Kaim W., Lahiri G. K., Inorg. Chem. 2016, 55, 3105–3116. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Mondol R., Snoeken D. A., Chang M.-C., Otten E., Chem. Commun. 2017, 53, 513–516; [DOI] [PubMed] [Google Scholar]
- 10b. Travieso-Puente R., Broekman J. O. P., Chang M.-C., Demeshko S., Meyer F., Otten E., J. Am. Chem. Soc. 2016, 138, 5503–5506; [DOI] [PubMed] [Google Scholar]
- 10c. Chang M.-C., Roewen P., Travieso-Puente R., Lutz M., Otten E., Inorg. Chem. 2015, 54, 379–388; [DOI] [PubMed] [Google Scholar]
- 10d. Chang M.-C., Dann T., Day D. P., Lutz M., Wildgoose G. G., Otten E., Angew. Chem. Int. Ed. 2014, 53, 4118–4122; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 4202–4206; [Google Scholar]
- 10e. Maar R. R., Barbon S. M., Sharma N., Groom H., Luyt L. G., Gilroy J. B., Chem. Eur. J. 2015, 21, 15589–15599; [DOI] [PubMed] [Google Scholar]
- 10f. Barbon S. M., Staroverov V. N., Gilroy J. B., Angew. Chem. Int. Ed. 2017, 56, 8173–8177; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 8285–8289; [Google Scholar]
- 10g. Gilroy J. B., Ferguson M. J., McDonald R., Patrick B. O., Hicks R. G., Chem. Commun. 2007, 126–128; [DOI] [PubMed] [Google Scholar]
- 10h. Gilroy J. B., Otieno P. O., Ferguson M. J., McDonald R., Hicks R. G., Inorg. Chem. 2008, 47, 1279–1286; [DOI] [PubMed] [Google Scholar]
- 10i. Broere D. L. J., Mercado B. Q., Bill E., Lancaster K. M., Sproules S., Holland P. L., Inorg. Chem. 2018, 57, 9580–9591; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10j. Broere D. L. J., Mercado B. Q., Lukens J. T., Vilbert A. C., Banerjee G., Lant H. M. C., Lee S. H., Bill E., Sproules S., Lancaster K. M., Holland P. L., Chem. Eur. J. 2018, 24, 9417–9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Lichtenberg C., Krommenacher I., Chem. Commun. 2016, 52, 10044–10047; [DOI] [PubMed] [Google Scholar]
- 11b. Hanft A., Lichtenberg C., Dalton Trans. 2018, 47, 10578–10589; [DOI] [PubMed] [Google Scholar]
- 11c. Hanft A., Lichtenberg C., Eur. J. Inorg. Chem. 2018, 2018, 3361–3373. [Google Scholar]
- 12.
- 12a. Doslik N., Sixt T., Kaim W., Angew. Chem. Int. Ed. 1998, 37, 2403–2404; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 1998, 110, 2521–2522; [Google Scholar]
- 12b. Dogan A., Sarkar B., Klein A., Lissner F., Schleid T., Fiedler J., Záliš S., Jain V. K., Kaim W., Inorg. Chem. 2004, 43, 5973–5980; [DOI] [PubMed] [Google Scholar]
- 12c. Patra S., Sarkar B., Maji S., Fiedler J., Urbanos F. A., Jimenez-Aparicio R., Kaim W., Lahiri G. K., Chem. Eur. J. 2006, 12, 489–498; [DOI] [PubMed] [Google Scholar]
- 12d. Paul N. D., Samanta S., Goswami S., Inorg. Chem. 2010, 49, 2649–2655; [DOI] [PubMed] [Google Scholar]
- 12e. Joy S., Krämer T., Paul N. D., Banerjee P., McGrady J. E., Goswami S., Inorg. Chem. 2011, 50, 9993–10004; [DOI] [PubMed] [Google Scholar]
- 12f. Jana R., Lissner F., Schwederski B., Fiedler J., Kaim W., Organometallics 2013, 32, 5879–5886; [Google Scholar]
- 12g. Ghosh P., Samanta S., Roy S. K., Demeshko S., Meyer F., Goswami S., Inorg. Chem. 2014, 53, 4678–4686; [DOI] [PubMed] [Google Scholar]
- 12h. Sengupta D., Ghosh P., Chatterjee T., Datta H., Paul N. D., Goswami S., Inorg. Chem. 2014, 53, 12002–12013; [DOI] [PubMed] [Google Scholar]
- 12i. Sengupta D., Chowdhury N. S., Samanta S., Ghosh P., Seth S. K., Demeshko S., Meyer F., Goswami S., Inorg. Chem. 2015, 54, 11465–11476; [DOI] [PubMed] [Google Scholar]
- 12j. Sinha S., Das S., Sikari R., Parua S., Brandaõ P., Demeshko S., Meyer F., Paul N. D., Inorg. Chem. 2017, 56, 14084–14100; [DOI] [PubMed] [Google Scholar]
- 12k. Roy S., Pramanik S., Patra S. C., Adhikari B., Mondal A., Ganguly S., Pramanik K., Inorg. Chem. 2017, 56, 12764–12774; [DOI] [PubMed] [Google Scholar]
- 12l. Sengupta A., Rajput A., Varman S. K., Mukherjee R., Dalton Trans. 2017, 46, 11291–11305. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a.Review on π-coordinated ligand systems: Verhoeven D. G. A., Moret M.-E., Dalton Trans. 2016, 45, 15762; [DOI] [PubMed] [Google Scholar]
- 13b. Horak K. T., Velian A., Day M. W., Agapie T., Chem.Commun. 2014, 50, 4427–4429; [DOI] [PubMed] [Google Scholar]
- 13c. Henthorn J. T., Lin S., Agapie T., J. Am. Chem. Soc. 2015, 137, 1458–1464; [DOI] [PubMed] [Google Scholar]
- 13d. Henthorn J. T., Agapie T., Inorg. Chem. 2016, 55, 5337–5342; [DOI] [PubMed] [Google Scholar]
- 13e. Horak K. T., Agapie T., J. Am. Chem. Soc. 2016, 138, 3443–3452. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Ghosh P., Naastepad R., Riemersma C. F., Lutz M., Moret M.-E., Klein Gebbink R. J. M., Chem. Eur. J. 2017, 23, 10732–10737; for related work on diketiminate (nacnac) redox-noninnocence, see: [DOI] [PubMed] [Google Scholar]
- 14b. Khusniyarov M. M., Bill E., Weyhermüller T., Bothe E., Wieghardt K., Angew. Chem. Int. Ed. 2011, 50, 1652–1655; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 1690–1693; [Google Scholar]
- 14c. Marshak M. P., Chambers M. B., Nocera D. G., Inorg. Chem. 2012, 51, 11190–11197; [DOI] [PubMed] [Google Scholar]
- 14d. Morris V. A., Wolczanski P. T., Sutter J., Meyer K., Lobkovsky E. B., Cundari T. R., Inorg. Chem. 2014, 53, 4459–4474; [DOI] [PubMed] [Google Scholar]
- 14e. Morris W. D., Wolczanski P. T., Sutter J., Meyer K., Cundari T. R., Lobkovsky E. B., Inorg. Chem. 2014, 53, 7467–7484; [DOI] [PubMed] [Google Scholar]
- 14f. Takaichi J., Morimoto Y., Ohkubo K., Shimokawa C., Hojo T., Mori S., Asahara H., Sugimoto H., Fujieda N., Nishiwaki N., Fukuzumi S., Itoh S., Inorg. Chem. 2014, 53, 6159–6169; [DOI] [PubMed] [Google Scholar]
- 14g. Takaichi J., Ohkubo K., Sugimoto H., Nakano M., Usa D., Maekawa H., Fujieda N., Nishiwaki N., Seki S., Fukuzumi S., Itoh S., Dalton Trans. 2013, 42, 2438–2444. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Bezpalko M. W., Foxman B. M., Thomas C. M., Inorg. Chem. 2013, 52, 12329–12331; [DOI] [PubMed] [Google Scholar]
- 15b. Harris C. F., Bayless M. B., van Leest N. P., Bruch Q. J., Livesay B. N., Bacsa J., Hardcastle K. I., Shores M. P., de Bruin B., Soper J. D., Inorg. Chem. 2017, 56, 12421–12435; [DOI] [PubMed] [Google Scholar]
- 15c. Mahoney J. K., Martin D., Moore C. E., Rheingold A. L., Bertrand G., J. Am. Chem. Soc. 2013, 135, 18766–18769; [DOI] [PubMed] [Google Scholar]
- 15d. Munz D., Chu J., Melaimi M., Bertrand G., Angew. Chem. Int. Ed. 2016, 55, 12886–12890; [DOI] [PubMed] [Google Scholar]
- 15e. Eberle B., Kaifer E., Himmel H.-J., Angew. Chem. Int. Ed. 2017, 56, 3360–3363; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 3408–3412; [Google Scholar]
- 15f. Taylor J. W., McSkimming A., Guzman C. F., Harman W. H., J. Am. Chem. Soc. 2017, 139, 11032–11035; [DOI] [PubMed] [Google Scholar]
- 15g. Rosen E. L., C. D. Varnado, Jr. , Tennyson A. G., Khramov D. M., Kamplain J. W., Sung D. H., Creswell P. T., Lynch V. M., Bielawski C. W., Organometallics 2009, 28, 6695–6706; [Google Scholar]
- 15h. Weinberg D. R., Hazari N., Labinger J. A., Bercaw J. E., Organometallics 2010, 29, 89–100; [Google Scholar]
- 15i. Romain C., Choua S., Colllin J.-P., Heinrich M., Bailly C., Karmazin-Brelot L., Bellemin-Laponnaz S., Dagorne S., Inorg. Chem. 2014, 53, 7371–7376; [DOI] [PubMed] [Google Scholar]
- 15j. Hettmanczyk L., Suntrup L., Klenk S., Hoyer C., Sarkar B., Chem. Eur. J. 2017, 23, 576–585; [DOI] [PubMed] [Google Scholar]
- 15k. Schrempp D. F., Schneider E., Kaifer E., Wadepohl H., Himmel H.-J., Chem. Eur. J. 2017, 23, 11636–11648; [DOI] [PubMed] [Google Scholar]
- 15l. Ruamps M., Bastin S., Rechignat L., Saquet A., Valyaev D. A., Mouesca J.-M., Lugan N., Maurel V., Cesar V., Chem. Commun. 2018, 54, 7653–7656; [DOI] [PubMed] [Google Scholar]
- 15m. Jongbloed L. S., Vogt N., Sandleben A., de Bruin B., Klein A., van der Vlugt J. I., Eur. J. Inorg. Chem. 2018, 2408–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a. Pierpont C. G., Coord. Chem. Rev. 2001, 219–221, 415–433; [Google Scholar]
- 16b. Pierpont C. G., Coord. Chem. Rev. 2001, 216–217, 99–125; [Google Scholar]
- 16c. Pierpont C. G., Inorg. Chem. 2011, 50, 9766–9772; [DOI] [PubMed] [Google Scholar]
- 16d. Mederos A., Domínguez S., Hernández-Molina R., Sanchiz J., Brito F., Coord. Chem. Rev. 1999, 193–195, 857–911; [Google Scholar]
- 16e. Mederos A., Domínguez S., Hernández-Molina R., Sanchiz J., Brito F., Coord. Chem. Rev. 1999, 193–195, 913–939; [Google Scholar]
- 16f. Ciccione J., Leconte N., Luneau D., Philouze C., Thomas F., Inorg. Chem. 2016, 55, 649–665; [DOI] [PubMed] [Google Scholar]
- 16g. van der Meer M., Manck S., Sobottka S., Plebst S., Sarkar B., Organometallics 2015, 34, 5393–5400; [Google Scholar]
- 16h. Martyanov K., Kuropatov V., Inorganics 2018, 6, 48. [Google Scholar]
- 17.
- 17a. Blackmore K. J., Ziller J. W., Heyduk A. F., Inorg. Chem. 2005, 44, 5559–5561; [DOI] [PubMed] [Google Scholar]
- 17b. Haneline M. R., Heyduk A. F., J. Am. Chem. Soc. 2006, 128, 8410–8411; [DOI] [PubMed] [Google Scholar]
- 17c. Blackmore K. J., Lal N., Ziller J. W., Heyduk A. F., J. Am. Chem. Soc. 2008, 130, 2728–2729; [DOI] [PubMed] [Google Scholar]
- 17d. Zarkesh R. A., Ziller J. W., Heyduk A. F., Angew. Chem. Int. Ed. 2008, 47, 4715–4718; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 4793–4796; [Google Scholar]
- 17e. Ketterer N. A., Fan H., Blackmore K. J., Yang X., Ziller J. W., Baik M., Heyduk A. F., J. Am. Chem. Soc. 2008, 130, 4364–4374; [DOI] [PubMed] [Google Scholar]
- 17f. Nguyen A. I., Blackmore K. J., Carter S. M., Zarkesh R. A., Heyduk A. F., J. Am. Chem. Soc. 2009, 131, 3307–3316; [DOI] [PubMed] [Google Scholar]
- 17g. Zarkesh R. A., Heyduk A. F., Organometallics 2011, 30, 4890–4898; [Google Scholar]
- 17h. Heyduk A. F., Zarkesh R. A., Nguyen A. I., Inorg. Chem. 2011, 50, 9849–9863; [DOI] [PubMed] [Google Scholar]
- 17i. Munhá R. F., Zarkesh R. A., Heyduk A. F., Inorg. Chem. 2013, 52, 11244–11255; [DOI] [PubMed] [Google Scholar]
- 17j. Hananouchi S., Krull B. T., Ziller J. W., Furche F., Heyduk A. F., Dalton Trans. 2014, 43, 17991–18000; [DOI] [PubMed] [Google Scholar]
- 17k. Ghosh S., Baik M.-H., Chem. Eur. J. 2015, 21, 1780–1789. [DOI] [PubMed] [Google Scholar]
- 18.See Ref. [7a]and:
- 18a. Sarkar B., Schweinfurth D., Deibel N., Weisser F., Coord. Chem. Rev. 2015, 293, 250–262; [Google Scholar]
- 18b. Skara G., Pinter B., Geerlings P., De Proft F., Chem. Sci. 2015, 6, 4109–4117; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18c. Poddel′sky A. I., Cherkasov V. K., Abakumov G. A., Coord. Chem. Rev. 2009, 253, 291–324; [Google Scholar]
- 18d. Pierpont C. G., Lange C. W., Prog. Inorg. Chem. 1994, 41, 331–442; [Google Scholar]
- 18e. Pierpont C. G., Buchanan R. M., Coord. Chem. Rev. 1981, 38, 45–87. [Google Scholar]
- 19.
- 19a. Ali A., Dhar D., Barman S. K., Lloret F., Mukherjee R., Inorg. Chem. 2016, 55, 5759–5771; [DOI] [PubMed] [Google Scholar]
- 19b. Ali A., Barman S. K., Mukherjee R., Inorg. Chem. 2015, 54, 5182–5194; [DOI] [PubMed] [Google Scholar]
- 19c. Rajput A., Sharma A. K., Barman S. K., Koley D., Steinert M., Mukherjee R., Inorg. Chem. 2014, 53, 36–48; [DOI] [PubMed] [Google Scholar]
- 19d. Ali A., Barman S. K., Mukherjee R., Inorg. Chem. 2014, 53, 4062–4067;24679042 [Google Scholar]
- 19e. Heinze K., Huttner G., Zsolnai L., Jacobi A., Schober P., Chem. Eur. J. 1997, 3, 732–743; [Google Scholar]
- 19f. Ghumaan S., Mukherjee S., Kar S., Roy D., Mobin S. M., Sunoj R. B., Lahiri G. K., Eur. J. Inorg. Chem. 2006, 4426–4441; [Google Scholar]
- 19g. Das A., Kundu T., Mobin S. M., Priego J. L., Jiménez-Aparicio R., Lahiri G. K., Dalton Trans. 2013, 42, 13733–13746; [DOI] [PubMed] [Google Scholar]
- 19h. Ansari M. A., Mandal A., Beyer K., Paretzki A., Schwederski B., Kaim W., Lahiri G. K., Dalton Trans. 2017, 46, 15589–15598; [DOI] [PubMed] [Google Scholar]
- 19i. Poddel'sky A., Piskunov A. V., Druzhkov N. O., Fukin G. K., Cherkasov V. K., Abakumov G. A., Z. Anorg. Allg. Chem. 2009, 635, 2563–2571; [Google Scholar]
- 19j. Deibel N., Sommer M. G., Hohloch S., Schwann J., Schweinfurth D., Ehret F., Sarkar B., Organometallics 2014, 33, 4756–4765; [Google Scholar]
- 19k. Schweinfurth D., Rechkemmer Y., Hohloch S., Deibel N., Peremykin I., Fiedler J., Marx R., Neugebauer P., van Slageren J., Sarkar B., Chem. Eur. J. 2014, 20, 3475–3486. [DOI] [PubMed] [Google Scholar]
- 20.This has predominantly been exploited using either i) direct two-electron transfer to or from a single-ligand platform to facilitate chemical transformations at the neighboring, coordinatively unsaturated early or late transition metal or ii) overall two-electron transfer using two ligand fragments that each act as a single-electron reservoir. See ref. [7] for recent overviews of this chemistry.
- 21.
- 21a. Ringenberg M. R., Kokatam S. L., Heiden Z. M., Rauchfuss T. B., J. Am. Chem. Soc. 2008, 130, 788–789; [DOI] [PubMed] [Google Scholar]
- 21b. Boyer J. L., Cundari T. R., DeYonker N. J., Rauchfuss T. B., Wilson S. R., Inorg. Chem. 2009, 48, 638–645; [DOI] [PubMed] [Google Scholar]
- 21c. Bubrin M., Schweinfurth D., Ehret F., Záliš S., Kvapilová H., Fiedler J., Zeng Q., Hartl F., Kaim W., Organometallics 2014, 33, 4973–4985; [Google Scholar]
- 21d. Lindner R., van den Bosch B., Lutz M., Reek J. N. H., van der Vlugt J. I., Organometallics 2011, 30, 499–510; [Google Scholar]
- 21e. Hübner R., Weber S., Strobel S., Sarkar B., Záliš S., Kaim W., Organometallics 2011, 30, 1414–1418; [Google Scholar]
- 21f. Paretzki A., Bubrin M., Fiedler J., Záliš S., Kaim W., Chem. Eur. J. 2014, 20, 5414–5422. [DOI] [PubMed] [Google Scholar]
- 22.
- 22a. Ouch K., Mashuta M. S., Grapperhaus C. A., Inorg. Chem. 2011, 50, 9904–9914; [DOI] [PubMed] [Google Scholar]
- 22b. Grapperhaus C. A., Ouch K., Mashuta M. S., J. Am. Chem. Soc. 2009, 131, 64–65; [DOI] [PubMed] [Google Scholar]
- 22c. Königsmann M., Donati N., Stein D., Schönberg H., Harmer J., Sreekanth A., Grützmacher H., Angew. Chem. Int. Ed. 2007, 46, 3567–3570; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 3637–3640; [Google Scholar]
- 22d. Chaudhuri P., Wieghardt K., Weyhermüller T., Paine T. K., Mukherjee S., Mukherjee C., Biol. Chem. 2005, 386, 1023–1033; [DOI] [PubMed] [Google Scholar]
- 22e. Wang K., Stiefel E. I., Science 2001, 291, 106–109; [DOI] [PubMed] [Google Scholar]
- 22f. Chaudhuri P., Hess M., Flörke U., Wieghardt K., Angew. Chem. Int. Ed. 1998, 37, 2217–2220; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 1998, 110, 2340–2343. [Google Scholar]
- 23.
- 23a. Hoyt J. M., Sylvester K. T., Semproni S. P., Chirik P. J., J. Am. Chem. Soc. 2013, 135, 4862–4877; [DOI] [PubMed] [Google Scholar]
- 23b. Bittner M. M., Lindeman S. V., Popescu C. V., Fiedler A. T., Inorg. Chem. 2014, 53, 4047–4061; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23c. Pappas I., Treacy S., Chirik P. J., ACS Catal. 2016, 6, 4105–4109. [Google Scholar]
- 24.
- 24a. Studer A., Curran D. P., Angew. Chem. Int. Ed. 2016, 55, 58–102; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 58–106; [Google Scholar]
- 24b. Frontana-Uribe B. A., Little R. D., Ibanez J. G., Palma A., Vasquez-Medrano R., Green Chem. 2010, 12, 2099–2119; [Google Scholar]
- 24c. Schäfer H. J., C. R. Chim. 2011, 14, 745–765; [Google Scholar]
- 24d. Sambiagio C., Sterckx H., Maes B. U. W., ACS Cent. Sci. 2017, 3, 686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.
- 25a. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]
- 25b. Dadashi-Silab S., Doran S., Yagci Y., Chem. Rev. 2016, 116, 10212–10275; [DOI] [PubMed] [Google Scholar]
- 25c. Prier C. K., Rankic D. A., MacMillan D. W. C., Chem. Rev. 2013, 113, 5322–5363; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25d. Narayanam J. N. R., Stephenson C. J., Chem. Soc. Rev. 2011, 40, 102–113; [DOI] [PubMed] [Google Scholar]
- 25e. Matsui J. K., Lang S. B., Heitz D. R., Molander G. A., ACS Catal. 2017, 7, 2563–2575; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25f. Tellis J. C., Kelly C. B., Primer D. N., Jouffroy M., Patel N. R., Molander G. A., Acc. Chem. Res. 2016, 49, 1429–1439; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25g. Fabry D. C., Rueping M., Acc. Chem. Res. 2016, 49, 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selected recent examples:
- 26a. Wiebe A., Gieshoff T., Möhle S., Rodrigo E., Zirbes M., Waldvogel S. R., Angew. Chem. Int. Ed. 2018, 57, 5594–5619; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 5694–5721; [Google Scholar]
- 26b. Möhle S., Zirbes M., Rodrigo E., Gieshoff T., Wiebe A., Waldvogel S. R., Angew. Chem. Int. Ed. 2018, 57, 6018–6041; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 6124–6149; [Google Scholar]
- 26c. Lo J. C., Kim D., Pan C.-M., Edwards J. T., Yabe Y., Gui J., Qin T., Gutieŕrez S., Giacoboni J., Smith M. W., Holland P. L., Baran P. S., J. Am. Chem. Soc. 2017, 139, 2484–2503; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26d. Badalyan A., Stahl S. S., Nature 2016, 535, 406–410; [DOI] [PubMed] [Google Scholar]
- 26e. Boyer C., Corrigan N. A., Jung K., Nguyen D., Nguyen T.-K., Adnan N. N. M., Oliver S., Shanmugam S., Yeow J., Chem. Rev. 2016, 116, 1803–1949; [DOI] [PubMed] [Google Scholar]
- 26f. Zhang Y.-Q., Jakoby V., Stainer K., Schmer A., Klare S., Bauer M., Grimme S., Cuerva J. M., Gansäuer A., Angew. Chem. Int. Ed. 2016, 55, 1523–1526; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 1546–1550; [Google Scholar]
- 26g. Roizen J. L., Harvey M. E., Du Bois J., Acc. Chem. Res. 2012, 45, 911–922; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26h. Matyjaszewski K., Macromolecules 2012, 45, 4015–4039; [Google Scholar]
- 26i. Satoh K., Kamigaito M., Chem. Rev. 2009, 109, 5120–5156. [DOI] [PubMed] [Google Scholar]
- 27.Recent reviews on metalloradical catalysis:
- 27a. Kuijpers P. F., van der Vlugt J. I., Schneider S., de Bruin B., Chem. Eur. J. 2017, 23, 13819–13829; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27b. Clark A. J., Eur. J. Org. Chem. 2016, 2231–2243; [Google Scholar]
- 27c. Xue Z., He D., Xie X., Polym. Chem. 2015, 6, 1660–1687; [Google Scholar]
- 27d. Olivos Suarez A. I., Lyaskovskyy V., Reek J. N. H., van der Vlugt J. I., de Bruin B., Angew. Chem. Int. Ed. 2013, 52, 12510–12529; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 12740–12760; [Google Scholar]
- 27e. Jahn U., Top. Curr. Chem. 2011, 320, 191–322. [DOI] [PubMed] [Google Scholar]
- 28.
- 28a. Wang Y., Wen X., Cui X., Zhang X. P., J. Am. Chem. Soc. 2018, 140, 4792–4796; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28b. Gu Z.-Y., Liu Y., Wang F., Bao X., Wang S.-Y., Ji S.-J., ACS Catal. 2017, 7, 3893–3899; [Google Scholar]
- 28c. Liu J., Hu L., Wang L., Chen H., Deng L., J. Am. Chem. Soc. 2017, 139, 3876–3888; [DOI] [PubMed] [Google Scholar]
- 28d. Jiang H., Lang K., Lu H., Wojtas L., Zhang X. P., J. Am. Chem. Soc. 2017, 139, 9164–9167; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28e. Xu X., Wang Y., Cui X., Wojtas L., Zhang X. P., Chem. Sci. 2017, 8, 4347–4351; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28f. Wang Y., Wen X., Cui X., Wojtas L., Zhang X. P., J. Am. Chem. Soc. 2017, 139, 1049–1052; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28g. Jiang H., Lang K., Lu H., Wojtas L., Zhang X. P., Angew. Chem. Int. Ed. 2016, 55, 11604–11608; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 11776–11780; [Google Scholar]
- 28h. te Grotenhuis C., van den Heuvel N., van der Vlugt J. I., de Bruin B., Angew. Chem. Int. Ed. 2018, 57, 140; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 146; [Google Scholar]
- 28i. te Grotenhuis C., Das B. G., Kuijpers P. F., Hageman W., Trouwborst M., de Bruin B., Chem. Sci. 2017, 8, 8221–8230; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28j. Das B. G., Chirila A., Tromp M., Reek J. N. H., de Bruin B., J. Am. Chem. Soc. 2016, 138, 8968–8975; [DOI] [PubMed] [Google Scholar]
- 28k. Paul N. D., Mandal S., Otte M., Cui X., Zhang X. P., de Bruin B., J. Am. Chem. Soc. 2014, 136, 1090–1096; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28l. Gansäuer A., Hildebrandt S., Michelmann A., Dahmen T., von Laufenberg D., Kube C., Fianu G. D., R. A. Flowers II , Angew. Chem. Int. Ed. 2015, 54, 7003–7006; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 7109–7112; [Google Scholar]
- 28m. Gansäuer A., Hildebrandt S., Vogelsang E., R. A. Flowers II , Dalton Trans. 2016, 45, 448–452; [DOI] [PubMed] [Google Scholar]
- 28n. Hildebrandt S., Gansäuer A., Angew. Chem. Int. Ed. 2016, 55, 9719–9722; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 9871–9874; [Google Scholar]
- 28o. Hennessy E. T., Betley T. A., Science 2013, 340, 591–595; [DOI] [PubMed] [Google Scholar]
- 28p. de Bruin B., Dzik W. I., Li S., Wayland B. B., Chem. Eur. J. 2009, 15, 4312–4320; [DOI] [PubMed] [Google Scholar]
- 28q. Takaoka A., Moret E.-M., Peters J. C., J. Am. Chem. Soc. 2012, 134, 6695–6706. [DOI] [PubMed] [Google Scholar]
- 29. Veitch N. C., Phytochemistry 2004, 65, 249–259. [DOI] [PubMed] [Google Scholar]
- 30.
- 30a. Costas M., Mehn M. P., Jensen M. P., L. Que, Jr. , Chem. Rev. 2004, 104, 939–986; [DOI] [PubMed] [Google Scholar]
- 30b. Lipscomb J. D., Curr. Opin. Struct. Biol. 2008, 18, 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.
- 31a. Whittaker J. W., Arch. Biochem. Biophys. 2005, 433, 227–239; [DOI] [PubMed] [Google Scholar]
- 31b. Whittaker J. W., Chem. Rev. 2003, 103, 2347–2363. [DOI] [PubMed] [Google Scholar]
- 32. Rittle J., Green M. T., Science 2010, 330, 933–937. [DOI] [PubMed] [Google Scholar]
- 33. Lippert C. A., Arnstein S. A., Sherrill C. D., Soper J. D., J. Am. Chem. Soc. 2010, 132, 3879–3892. [DOI] [PubMed] [Google Scholar]
- 34. Lippert C. A., Hardcastle K. I., Soper J. D., Inorg. Chem. 2011, 50, 9864–9878. [DOI] [PubMed] [Google Scholar]
- 35. Lippert C. A., Soper J. D., Inorg. Chem. 2010, 49, 3682–3684. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen A. I., Zarkesh R. A., Lacy D. C., Thorson M. K., Heyduk A. F., Chem. Sci. 2011, 2, 166–169. [Google Scholar]
- 37. Zhou W., Patrick B. O., Smith K. M., Chem. Commun. 2014, 50, 9958–9960. [DOI] [PubMed] [Google Scholar]
- 38.
- 38a. van der Vlugt J. I., Pidko E. A., Vogt D., Lutz M., Spek A. L., Meetsma A., Inorg. Chem. 2008, 47, 4442–4444; [DOI] [PubMed] [Google Scholar]
- 38b. van der Vlugt J. I., Pidko E. A., Vogt D., Lutz M., Spek A. L., Inorg. Chem. 2009, 48, 7513–7515; [DOI] [PubMed] [Google Scholar]
- 38c. van der Vlugt J. I., Lutz M., Pidko E. A., Vogt D., Spek A. L., Dalton Trans. 2009, 1016–1023; [DOI] [PubMed] [Google Scholar]
- 38d. van der Vlugt J. I., Siegler M. A., Janssen M., Vogt D., Spek A. L., Organometallics 2009, 28, 7025–7032; [Google Scholar]
- 38e. van der Vlugt J. I., Pidko E. A., Bauer R. C., Gloaguen Y., Rong M. K., Lutz M., Chem. Eur. J. 2011, 17, 3850–3854; [DOI] [PubMed] [Google Scholar]
- 38f. Bauer R. C., Gloaguen Y., Lutz M., Reek J. N. H., de Bruin B., van der Vlugt J. I., Dalton Trans. 2011, 40, 8822–8829; [DOI] [PubMed] [Google Scholar]
- 38g. de Boer S. Y., Gloaguen Y., Reek J. N. H., Lutz M., van der Vlugt J. I., Dalton Trans. 2012, 41, 11276–11283; [DOI] [PubMed] [Google Scholar]
- 38h. de Boer S. Y., Gloaguen Y., Lutz M., van der Vlugt J. I., Inorg. Chim. Acta 2012, 380, 336–342; [Google Scholar]
- 38i. Jongbloed L. S., de Bruin B., Reek J. N. H., Lutz M., van der Vlugt J. I., Chem. Eur. J. 2015, 21, 7297–7305; [DOI] [PubMed] [Google Scholar]
- 38j. Oldenhof S., Terrade F. G., Lutz M., van der Vlugt J. I., Reek J. N. H., Organometallics 2015, 34, 3209–3215; [Google Scholar]
- 38k. Oldenhof S., Lutz M., van der Vlugt J. I., Reek J. N. H., Chem. Commun. 2015, 51, 15200–15203; [DOI] [PubMed] [Google Scholar]
- 38l. Tang Z., Otten E., Reek J. N. H., van der Vlugt J. I., de Bruin B., Chem. Eur. J. 2015, 21, 12683–12693; [DOI] [PubMed] [Google Scholar]
- 38m. Jongbloed L. S., García-Loṕez D., van Heck R., Siegler M. A., Carbó J. J., van der Vlugt J. I., Inorg. Chem. 2016, 55, 8041–8047; [DOI] [PubMed] [Google Scholar]
- 38n. Oldenhof S., van der Vlugt J. I., Reek J. N. H., Catal. Sci. Technol. 2016, 6, 404–408; [Google Scholar]
- 38o. Jongbloed L. S., de Bruin B., Reek J. N. H., Lutz M., van der Vlugt J. I., Catal. Sci. Technol. 2016, 6, 1320–1327; [Google Scholar]
- 38p. Devillard M., Alvarez Lamsfus C., Vreeken V., Maron L., van der Vlugt J. I., Dalton Trans. 2016, 45, 10989–10998; [DOI] [PubMed] [Google Scholar]
- 38q. de Boer S. Y., Korstanje T. J., La Rooij S. R., Kox R., Reek J. N. H., Siegler M. A., van der Vlugt J. I., Organometallics 2017, 36, 1541–1549; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38r. Devillard M., de Bruin B., Siegler M. A., van der Vlugt J. I., Chem. Eur. J. 2017, 23, 13628–13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.
- 39a. Broere D. L. J., Plessius R., Tory J., Demeshko S., de Bruin B., Siegler M. A., Hartl F., van der Vlugt J. I., Chem. Eur. J. 2016, 22, 13965–13975; [DOI] [PubMed] [Google Scholar]
- 39b. Broere D. L. J., Demeshko S., de Bruin B., Pidko E. A., Reek J. N. H., Siegler M. A., Lutz M., van der Vlugt J. I., Chem. Eur. J. 2015, 21, 5879–5886. [DOI] [PubMed] [Google Scholar]
- 40. Broere D. L. J., Modder D. K., Blokker E., Siegler M. A., van der Vlugt J. I., Angew. Chem. Int. Ed. 2016, 55, 2406–2410; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 2452–2456. [Google Scholar]
- 41. Broere D. L. J., Metz L. L., de Bruin B., Reek J. N. H., Siegler M. A., van der Vlugt J. I., Angew. Chem. Int. Ed. 2015, 54, 1516–1520; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 1536–1540. [Google Scholar]
- 42.
- 42a. Jacquet J., Blanchard S., Derat E., Desage-El Murr M., Fensterbank L., Chem. Sci. 2016, 7, 2030–2036; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42b. Jacquet J., Cheaib K., Ren Y., Vezin H., Orio M., Blanchard S., Fensterbank L., Desage-El Murr M., Chem. Eur. J. 2017, 23, 15030–15034. [DOI] [PubMed] [Google Scholar]
- 43.
- 43a. Jacquet J., Chaumont P., Gontard G., Orio M., Vezin H., Blanchard S., Desage-El Murr M., Fensterbank L., Angew. Chem. Int. Ed. 2016, 55, 10712–10716; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 10870–10874; [Google Scholar]
- 43b. Jacquet J., Salanouve E., Orio M., Vezin H., Blanchard S., Derat E., Desage-El Murr M., Fensterbank L., Chem. Commun. 2014, 50, 10394. [DOI] [PubMed] [Google Scholar]
- 44.Although the redox-chemistry of Pd ions spans the oxidation states 0–IV, palladium is not prone to undergo or support single-electron-transfer steps, hence the term “redox-inert” in this context.
- 45. Broere D. L. J., de Bruin B., Reek J. N. H., Lutz M., Dechert S., van der Vlugt J. I., J. Am. Chem. Soc. 2014, 136, 11574–11577. [DOI] [PubMed] [Google Scholar]
- 46. Broere D. L. J., van Leest N. P., de Bruin B., Siegler M. A., van der Vlugt J. I., Inorg. Chem. 2016, 55, 8603–8611. [DOI] [PubMed] [Google Scholar]
- 47. Bagh B., Broere D. L. J., Sinha V., Kuijpers P. F., van Leest N. P., de Bruin B., Demeshko S., Siegler M. A., van der Vlugt J. I., J. Am. Chem. Soc. 2017, 139, 5117–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.See ref. [28];
- 48a. Hennessy E. T., Liu R. Y., Iovan D. A., Duncan R. A., Betley T. A., Chem. Sci. 2014, 5, 1526–1532; [Google Scholar]
- 48b. Iovan D. A., Betley T. A., J. Am. Chem. Soc. 2016, 138, 1983–1993; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48c. Iovan D. A., Wilding M. J. T., Baek Y., Hennessy E. T., Betley T. A., Angew. Chem. Int. Ed. 2017, 56, 15599–15602; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 15805–15808; [Google Scholar]
- 48d. Wilding M. J. T., Iovan D. A., Wrobel A. T., Lukens J. T., MacMillan S. N., Lancaster K. M., Betley T. A., J. Am. Chem. Soc. 2017, 139, 14757–14766; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48e. Wilding M. J. T., Iovan D. A., Betley T. A., J. Am. Chem. Soc. 2017, 139, 12043–12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuijpers P. F., Tiekink M. J., Breukelaar W. B., Broere D. L. J., van Leest N. P., van der Vlugt J. I., Reek J. N. H., de Bruin B., Chem. Eur. J. 2017, 23, 7945–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.
- 50a. Thacker N. C., Lin Z., Zhang T., Gilhula J. C., Abney C. W., Lin W., J. Am. Chem. Soc. 2016, 138, 3501–3509; [DOI] [PubMed] [Google Scholar]
- 50b. Lin Z., Thacker N. C., Sawano T., Drake T., Ji P., Lan G., Cao L., Liu S., Wang C., Lin W., Chem. Sci. 2018, 9, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Very recently, Figueroa, Cohen and co-workers reported a highly active Mn-MOF system for the C−H amination using PhINTs as sn N-transfer agent: Wang L., Agnew D. W., Yu X., Figueroa J. S., Cohen S. M., Angew. Chem. Int. Ed. 2018, 57, 511–518; [Google Scholar]; Angew. Chem. 2018, 130, 520–524. [Google Scholar]
- 52. Fujita D., Sugimoto H., Shiota Y., Morimoto Y., Yoshizawa K., Itoh S., Chem. Commun. 2017, 53, 4849–4852. [DOI] [PubMed] [Google Scholar]
- 53. Fujita D., Sugimoto H., Morimoto Y., Itoh S., Inorg. Chem. 2018, 57, 9738–9747. [DOI] [PubMed] [Google Scholar]
- 54. Ren Y., Jacquet J., Vezin H., Fensterbank L., Orio M., Blanchard S., Desage El-Murr M., Chem. Eur. J. 2018, 24, 5086–5090. [DOI] [PubMed] [Google Scholar]
- 55. Matson E. M., Franke S. M., Anderson N. H., Cook T. D., Fanwick P. E., Bart S. C., Organometallics 2014, 33, 1964–1971. [Google Scholar]
- 56. Bagh B., Broere D. L. J., Siegler M. A., van der Vlugt J. I., Angew. Chem. Int. Ed. 2016, 55, 8381–8385; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 8521–8525. [Google Scholar]
- 57.
- 57a. Khusnutdinova J., Milstein D., Angew. Chem. Int. Ed. 2015, 54, 12236–12273; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 12406–12445; [Google Scholar]
- 57b. Li H., Zheng B., Huang K.-W., Coord. Chem. Rev. 2015, 293–294, 116–138; [Google Scholar]
- 57c. Morris R. H., Acc. Chem. Res. 2015, 48, 1494–1502; [DOI] [PubMed] [Google Scholar]
- 57d. Kuwata S., Ikariya T., Chem. Commun. 2014, 50, 14290–14300; [DOI] [PubMed] [Google Scholar]
- 57e. Zhao B., Han Z., Ding K., Angew. Chem. Int. Ed. 2013, 52, 4744–4788; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 4844–4889; [Google Scholar]
- 57f. Askevold B., Schneider S., Roesky H. W., ChemCatChem 2012, 4, 307–320; [Google Scholar]
- 57g. van der Vlugt J. I., Eur. J. Inorg. Chem. 2012, 363–375; [Google Scholar]
- 57h. Kuwata S., Ikariya T., Dalton Trans. 2010, 39, 2984–2992; [DOI] [PubMed] [Google Scholar]
- 57i. van der Vlugt J. I., Reek J. N. H., Angew. Chem. Int. Ed. 2009, 48, 8832–8846; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 8990–9004. [Google Scholar]
- 58.
- 58a. Myers T. W., Berben L. A., J. Am. Chem. Soc. 2013, 135, 9988–9990; [DOI] [PubMed] [Google Scholar]
- 58b. Myers T. W., Berben L. A., Chem. Sci. 2014, 5, 2771–2777; [Google Scholar]
- 58c. Thompson E. J., Berben L. A., Angew. Chem. Int. Ed. 2015, 54, 11642–11646; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 11808–11812; [Google Scholar]
- 58d. Sherbow T. J., Carr C. R., Saisu T., Fettinger J. C., Berben L. A., Organometallics 2016, 35, 9–14; [Google Scholar]
- 58e. Sherbow T. J., Fettinger J. C., Berben L. A., Inorg. Chem. 2017, 56, 8651–8660. [DOI] [PubMed] [Google Scholar]
- 59.
- 59a. Wright D. D., Brown S. N., Inorg. Chem. 2013, 52, 7831–7833; [DOI] [PubMed] [Google Scholar]
- 59b. Hoffman J. M., Oliver A. G., Brown S. N., J. Am. Chem. Soc. 2017, 139, 4521–4531. [DOI] [PubMed] [Google Scholar]
- 60.
- 60a. Wong J. L., Higgins R. F., Bhowmick I., Cao D. X., Szigethy G., Ziller J. W., Shores M. P., Heyduk A. F., Chem. Sci. 2016, 7, 1594–1599; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60b. Wong J. L., Sánchez R. H., Logan J. G., Zarkesh R. A., Ziller J. W., Heyduk A. F., Chem. Sci. 2013, 4, 1906–1910; [Google Scholar]
- 60c. Lu F., Zarkesh R. A., Heyduk A. F., Eur. J. Inorg. Chem. 2012, 467–470. [Google Scholar]
- 61.
- 61a. Haddad A. Z., Cronin S. P., Mashuta M. S., Buchanan R. M., Grapperhaus C. A., Inorg. Chem. 2017, 56, 11254–11265; [DOI] [PubMed] [Google Scholar]
- 61b. Haddad A. Z., Garabato B. D., Kozlowski P. M., Buchanan R. M., Grapperhaus C. A., J. Am. Chem. Soc. 2016, 138, 7844–7847; [DOI] [PubMed] [Google Scholar]
- 61c. Haddad A. Z., Kumar D., Sampson K. O., Matzner A. M., Mashuta M. S., Grapperhaus C. A., J. Am. Chem. Soc. 2015, 137, 9238–9241. [DOI] [PubMed] [Google Scholar]
- 62.
- 62a. Bruch Q. J., Lindley B. M., Askevold B., Schneider S., Miller A. J. M., Inorg. Chem. 2018, 57, 1964–1975; [DOI] [PubMed] [Google Scholar]
- 62b. Lindley B. M., Bruch Q. J., White P. S., Hasanayn F., Miller A. J. M., J. Am. Chem. Soc. 2017, 139, 5305–5308; [DOI] [PubMed] [Google Scholar]
- 62c. Schneck F., Finger M., Tromp M., Schneider S., Chem. Eur. J. 2017, 23, 33–37; [DOI] [PubMed] [Google Scholar]
- 62d. Mondal M. K., Tiwarib A., Mukherjee C., Chem. Commun. 2016, 52, 11995–11998; [DOI] [PubMed] [Google Scholar]
- 62e. Nadif S. S., O'Reilly M. E., Ghiviriga I., Abboud K. A., Veige A. S., Angew. Chem. Int. Ed. 2015, 54, 15138–15142; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 15353–15357; [Google Scholar]
- 62f. Semproni S. P., Atienza C. C. H. A., Chirik P. J., Chem. Sci. 2014, 5, 1956–1960; [Google Scholar]
- 62g. Gloaguen Y., Rebreyend C., Lutz M., Kumar P., Huber M., van der Vlugt J. I., Schneider S., de Bruin B., Angew. Chem. Int. Ed. 2014, 53, 6814–6818; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 6932–6936; [Google Scholar]
- 62h. Khaskin E., Diskin-Posner Y., Weiner L., Leitus G., Milstein D., Chem. Commun. 2013, 49, 2771–2773; [DOI] [PubMed] [Google Scholar]
- 62i. Ringenberg M. R., Rauchfuss T. B., Eur. J. Inorg. Chem. 2012, 490–495; [Google Scholar]
- 62j. Lin S., Day M. W., Agapie T., J. Am. Chem. Soc. 2011, 133, 3828–3831; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62k. Mondol R., Otten E., Inorg. Chem. 2018, 57, 9720–9727; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62l. Conner K. M., Arostegui A. C., Swanson D. D., Brown S. N., Inorg. Chem. 2018, 57, 9696–9707; [DOI] [PubMed] [Google Scholar]; Margulieux G., Bezdek M. J., Turner Z. R., Chirik P. J., J. Am. Chem. Soc. 2017, 139, 6110–6113. [DOI] [PubMed] [Google Scholar]
- 63.
- 63a. Himmel H.-J., Synlett 2018, 29, 1957–1977; [Google Scholar]
- 63b. Hoffmann N., Eur. J. Org. Chem. 2017, 1982–1992; [Google Scholar]
- 63c. Gentry E. C., Knowles R. R., Acc. Chem. Res. 2016, 49, 1546–1556; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63d. Elgrishi N., McCarthy B. D., Rountree E. S., Dempsey J. L., ACS Catal. 2016, 6, 3644–3659; [Google Scholar]
- 63e. Weinberg D. R., Gagliardi C. J., Hull J. F., Murphy C. F., Kent C. A., Westlake B. C., Paul A., Ess D. H., Granville MacCafferty D., Meyer T. J., Chem. Rev. 2012, 112, 4016–4093; [DOI] [PubMed] [Google Scholar]
- 63f. Warren J. J., Tronic T. A., Mayer J. M., Chem. Rev. 2010, 110, 6961–7001. [DOI] [PMC free article] [PubMed] [Google Scholar]


