Abstract
To date, all altered patterns of seasonal interactions observed in insects, birds, amphibians, and plants associated with global warming during the latter half of the 20th century are explicable as variable expressions of plastic phenotypes. Over the last 30 years, the genetically controlled photoperiodic response of the pitcher-plant mosquito, Wyeomyia smithii, has shifted toward shorter, more southern daylengths as growing seasons have become longer. This shift is detectable over a time interval as short as 5 years. Faster evolutionary response has occurred in northern populations where selection is stronger and genetic variation is greater than in southern populations. W. smithii represents an example of actual genetic differentiation of a seasonality trait that is consistent with an adaptive evolutionary response to recent global warming.
The latter half of the 20th century has experienced a period of rapid global warming that is unprecedented over the last millennium (1). At least in eastern North America, the increase in mean surface temperature of the Earth has occurred more through the moderation of daily and annual minima than by raising extreme maxima (2–4). Hence, a major effect of global warming has been the earlier arrival of spring, longer growing seasons, and consequently, altered seasonal patterns and biotic interactions of insects, birds, amphibians, and plants (5–7). These altered seasonal interactions are explicable entirely as temperature-sensitive responses to the environment by individuals, i.e., as expressions of plastic phenotypes, rather than as actual genetic changes in populations. Cytogenetic changes consistent with recent global warming have been observed in Drosophila (8) but the “magnitude of genetic variation in natural populations for traits likely to be critical to survival and reproduction in future climates is largely unknown” (9). Herein we provide evidence for a microevolutionary (genetic) response to the longer growing seasons generated by global warming by documenting recent changes in the photoperiodic response of the pitcher-plant mosquito, Wyeomyia smithii, that are detectable over as short a time span as 5 years.
A wide variety of plants and animals use the length of day (photoperiod) as a pivotal environmental cue to program their seasonal patterns of dormancy, migration, development, and reproduction (10–12). Failure to accommodate to the novel seasonality is responsible for outbreeding depression in a managed population of the Hungarian Ibex (13) and is the major cause of the failure of insect populations introduced for biological control (14).
Among most temperate arthropods with an hibernal diapause (dormancy), long days sustain development and short days induce diapause. Hence, during the late summer, individuals perceive the shortening days and switch from active development and reproduction to diapause. It is important to note that it is the length of the growing season and the timing of the onset of winter that impose selection on the optimal time to switch from continuous development to diapause. In the northern hemisphere, winter arrives earlier in the north where daylengths are longer than in the south; consequently, to enter diapause on an earlier date in the north, insects must use a longer daylength to cue the switch from active development to diapause. This switching daylength, or critical photoperiod, increases regularly with latitude and altitude in a wide variety of arthropods (15–19). If populations have been adapting to longer growing seasons and later onsets of winter as a consequence of global warming, then they should show a more southern phenotype now than they did a few decades ago. Hence, we should see progressively shorter critical photoperiods now than in the recent past.
Materials and Methods
W. smithii.
As parts of different experiments run over the last 30 years, our lab has evaluated variation in photoperiodic response of multiple populations of the pitcher-plant mosquito, W. smithii, over a wide geographic range in eastern North America. W. smithii completes its preadult development entirely within the water-filled leaves of the purple pitcher plant, Sarracenia purpurea. Hence, W. smithii lives in a highly consistent microhabitat throughout its range. Both W. smithii and S. purpurea flourish in controlled-environment chambers where we are able to mimic field conditions common to all populations. The range of the mosquito tracks that of its host plant from the Gulf of Mexico to northern Canada (30–54°N). Throughout its range, W. smithii enter a larval diapause whose onset, maintenance, and termination are mediated by photoperiod (20). The critical photoperiod mediating the onset and maintenance of diapause is closely correlated with latitude and altitude, but not longitude, of origin with R2 repeatedly >90% (17, 21–23). Heritabilities of critical photoperiod within populations range from 15% to 70% (21) and hybrids between populations from distant latitudes and altitudes show intermediate phenotypes (20–22). Critical photoperiod in W. smithii is a genetically based, highly heritable, adaptive trait regulating the seasonal patterns of its life cycle.
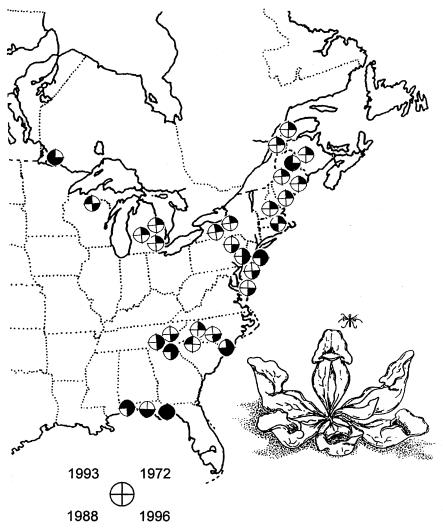
We collected W. smithii from Florida to Canada (Fig. 1) on four occasions: 1972 (17), 1988 (21), 1993 (22), and 1996 (23). For each year of collection, we determined photoperiodic response of all populations concurrently under rigorously controlled conditions in environmental chambers. Because we collected samples years to decades apart, we established genetic differences among years by comparing photoperiodic response between matched sets of populations by using the same method within each set. We used two methods: static daylengths in 1972 and 1996 and changing daylengths in 1988 and 1993. We therefore present the results of two separate comparisons, 1972 vs. 1996 and 1988 vs. 1993.
Figure 1.
Localities at 30–50°N latitude from which W. smithii were collected from S. purpurea (Inset) from the overwintering generation in 1972, 1988, 1993, and 1996. For each pie diagram, a blackened quadrant indicates a year that larvae were collected at that locality.
1972 vs. 1996.
Critical photoperiod determined with fixed daylengths is the 50% intercept on the photoperiodic response curve relating percentage development to hours of light per day, that is, the number of hours of light per day that initiates or maintains diapause in 50% of a sample population and averts or terminates diapause in the other 50%. In unchilled larvae, the critical photoperiod is the same for the initiation, maintenance, and termination of diapause (24). Separate samples of larvae are exposed to a range of fixed daylengths in half-hour increments at 25°C (1972) (17) or 23°C (1996) (23) until there is >90% development in the long-day controls. Percentage development is then plotted as a function of photoperiod and the critical photoperiod determined as the 50% intercept by simple interpolation. The 1972 critical photoperiods reflect the response of diapausing larvae collected directly from the field (fall 1971, 40–47°N) or the initiation of diapause in the F1 of larvae collected late winter, 1972 (30–36°N). The 1996 critical photoperiods reflect development of diapausing larvae in the F3 generation of larvae caught late winter 1996. The use of field-collected and F1 larvae directly for experiments may have introduced a bias in our results, but this bias is against showing a genetic change in photoperiodic response. Possible chilling of diapausing larvae in nature (25) before their collection in 1972 and the higher experimental temperatures (15, 16, 19) in 1972 than 1996 would, if anything, bias the results toward shorter, rather than the longer critical photoperiods we found in 1972.
1988 vs. 1993.
Critical photoperiod determined with changing daylengths is the mean daylength of pupation of a sample of diapausing larvae exposed to daylengths that start as short and increment by 3 min per day until all larvae pupate. This method is possible because diapausing W. smithii continue to respond to photoperiod and diapause is always terminated by long days (20, 24). Critical photoperiods were determined at temperatures that fluctuated in a smooth sine wave from 13° to 29°C with a mean of 21°C. Initially, the temperature cycle lagged the light cycle by 3 h and daylength was increased by advancing dawn 3 min per day. Experimental larvae were in the F2 (1988) and the F6 (1993) lab generation.
Results
1972 vs. 1996.
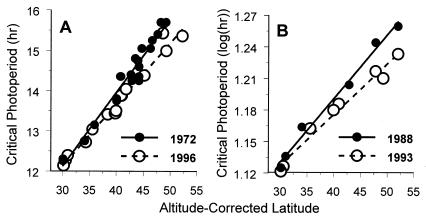
We estimated critical photoperiod in populations collected in 1972 and 1996 by using static daylengths. Critical photoperiod determined from the pooled 1972 and 1996 collections was positively correlated (R2 = 0.941) with latitude and altitude of origin (b ± SE: latitude = 0.173 ± 0.008, t = 22.62, P < 0.001; altitude = 0.00112 ± 0.00013, t = 8.53, P < 0.001). For each locality, we calculated the altitude-corrected latitude from the regression coefficients (b) of critical photoperiod regressed on latitude and altitude (20): ACL = latitude(°N) + altitude(m) × (bALTITUDE ÷ bLATITUDE). The critical photoperiods from 1972 showed a steeper regression on altitude-corrected latitude and were longer on average, than those from 1996 (Table 1; Fig. 2A). We were able to compare directly between years within localities for the seven specific populations [AL, FL, NC (3), NJ (2): 30–40°N] that were collected in both 1972 and 1996. All seven critical photoperiods were shorter from collections in 1996 than in 1972 [mean pair-wise difference in critical photoperiod (±SE) = 0.246 ± 0.073 h; t test for paired comparisons: t = 3.36, df = 6, P = 0.015]. Both analyses show significantly shorter critical photoperiods in the populations collected recently than in populations collected 24 years previously, and the differences in critical photoperiod between collection dates increase with latitude of origin. This shift toward shorter critical photoperiods over a 24-year period is in the direction predicted from a longer growing season, i.e., toward a more southern phenotype in more recent years.
Table 1.
Analysis of covariance for critical photoperiod with altitude-corrected latitude (ACL) from pooled 2-year samples as a covariate and year sampled (Yr) as the treatment, using type III sum of squares in the glm procedure of SAS (26)
| Treatment | 1972 vs. 1996
|
1988 vs.
1993
|
||
|---|---|---|---|---|
| F1,34 | P | F1,10 | P | |
| ACL | 975.24 | <0.001 | 546.45 | <0.001 |
| Yr | 4.17 | 0.049 | 3.41 | 0.095 |
| ACL × Yr | 7.85 | 0.008 | 7.35 | 0.022 |
Figure 2.
Critical photoperiods of W. smithii collected during the overwintering generation from 1972 to 1996 determined from static (1972, 1996) or changing (1988, 1993) photoperiods. Analysis of covariance (Table 1) indicated significantly steeper slopes for the earlier year in each comparison, meaning that shifts toward shorter critical photoperiods (more southern phenotypes) increased with latitude.
1988 vs. 1993.
We estimated critical photoperiod in populations collected in 1988 and 1993 by using changing daylengths. This method reflects the mean response of many individuals rather than an interpolation between two smaller samples. Development under these conditions is log-normally distributed; the data were log10-transformed before averaging and critical photoperiods were calculated as mean log(hr). Critical photoperiod determined from the pooled 1988 and 1993 collections was positively correlated (R2 = 0.945) with latitude but not altitude (b ± SE: latitude = 0.00555 ± 0.00048, t = 11.56, P < 0.001; altitude = 0.0000243 ± 0.0000138, t = 1.75, P = 0.107). Critical photoperiods from 1988 showed a significantly steeper regression on altitude-corrected latitude, but were not longer, on average, than those from 1993 (Table 1; Fig. 2B). We were able to compare directly between years within localities for the three specific populations (FL, ME, ON: 30–49°N) that were collected in both 1988 and 1993. All three critical photoperiods from the 1993 collections were shorter than those from the 1988 collections, but, with only 2 df, the difference was not significant [X̄ ± SE = 0.019 ± 0.008 log(hr), t = 2.41, df = 2, P = 0.138]. The changes between the 1988 and 1993 collections were all in the same direction as the significant differences between the 1972 and 1996 collections; and, there was a significant, progressive shift at increasingly more northern latitudes toward shorter critical photoperiods at the later date in both the 1972 vs. 1996 and the 1988 vs. 1993 comparisons. Again, the shift in more northern populations toward shorter critical photoperiods over a 5-year period is in the direction predicted from a longer growing season, i.e., toward a more southern phenotype in more recent years.
Discussion
The shift toward shorter critical photoperiods has been more pronounced in the north than in the south (Fig. 2). From the regressions in Fig. 2A, at 50°N latitude the critical photoperiod declines from 15.79 to 15.19 h from 1972 to 1996, corresponding to 9 days later in the fall of 1996 than 1972. This value is strikingly similar to the advancement of other seasonal events in the north temperate region over the same time span: British birds began laying eggs an average of 8.8 days earlier in 1995 than in 1971 (27) and British frogs began spawning an average of 9–10 days earlier in 1994 than in 1978 (28). Land surface temperatures have generally increased faster in northeastern North America than in the southeast (2, 3, 29) and genetic variability underlying photoperiodic response in W. smithii increases with latitude (21). We therefore attribute the faster evolutionary response of the northern populations to a combination of their encountering stronger directional selection and their harboring a greater genetic capacity to evolve.
Because each matched set of experiments was run under a highly controlled, matched set of conditions, we conclude that differences in critical photoperiod among populations indicate genetic differences among them and that differences in critical photoperiod between collection dates represent genetic change toward shorter critical photoperiods at later dates. We also conclude that genetic change in critical photoperiod can take place over as short a time span as 5 years. This shift toward shorter critical photoperiods is consistent with an adaptive response to longer growing seasons and, therefore, with the indirect effects of global warming on seasonality. W. smithii represents an example of actual genetic differentiation of a seasonality trait that is consistent with an adaptive evolutionary (genetic) response to global warming. Our results suggest that other species may be in the process of analogous evolutionary responses to altered seasonality and that the composition of future biotic communities may depend on the relative abilities of their constituent species to adapt to altered seasonal interactions.
Acknowledgments
We thank M. C. Quebodeaux for determining the critical photoperiods from the 1996 collections and D. Udovic for reading earlier versions of this paper. Our research over the last 30 years has been supported by the National Science Foundation Programs in Population Biology and in Ecological and Evolutionary Physiology.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stott P A, Tett S F B, Jones G S, Allen M R, Mitchell J F B, Jenkins G J. Science. 2000;290:2133–2137. doi: 10.1126/science.290.5499.2133. [DOI] [PubMed] [Google Scholar]
- 2.Easterling D R, Meehl G A, Parmesan C, Changnon S A, Karl T R, Mearns L O. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 3.DeGaetano A T. J Climate. 1996;9:1646–1660. [Google Scholar]
- 4.Kerr R A. Science. 2001;288:589–590. doi: 10.1126/science.288.5466.589a. [DOI] [PubMed] [Google Scholar]
- 5.Hughes L. Trends Ecol Evol. 2000;15:56–61. doi: 10.1016/s0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- 6.Wuethrich B. Science. 2000;287:794–795. doi: 10.1126/science.287.5454.793. [DOI] [PubMed] [Google Scholar]
- 7.Thomas D W, Blondel J, Perret P, Lambrechts M M, Speakman J R. Science. 2001;291:2598–2600. doi: 10.1126/science.1057487. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Trelles F, Rodríguez M A. Evol Ecol. 1998;12:829–838. [Google Scholar]
- 9.Davis M B, Shaw R G. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- 10.Vaartaja O. Ecol Mongr. 1959;29:91–111. [Google Scholar]
- 11.Withrow R B, editor. Photoperiodism and Related Phenomena in Plants and Animals. Washington, DC: Am. Assoc. Advancement of Science; 1959. [Google Scholar]
- 12.Anonymous. Biological Clocks. Cold Spring Harbor, NY: Long Island Biological Assoc.; 1960. [Google Scholar]
- 13.Templeton A R. In: Conservation Biology: The Science of Scarcity and Diversity. Soulé E, editor. Sunderland, MA: Sinauer; 1986. pp. 105–116. [Google Scholar]
- 14.Stiling P. Am Entomol. 1993;39:31–37. [Google Scholar]
- 15.Tauber M J, Tauber C A, Masaki S. Seasonal Adaptations of Insects. New York: Oxford Univ. Press; 1986. [Google Scholar]
- 16.Danilevskii A S. Photoperiodism and Seasonal Development of Insects. Edinburgh: Oliver and Boyd; 1965. [Google Scholar]
- 17.Bradshaw W E. Nature (London) 1976;262:384–386. doi: 10.1038/262384b0. [DOI] [PubMed] [Google Scholar]
- 18.Taylor F, Spalding J. In: The Evolution of Insect Life Cycles. Taylor F, Karban R, editors. New York: Springer; 1986. pp. 66–85. [Google Scholar]
- 19.Danks H V. Insect Dormancy: An Ecological Perspective. Ottawa: Biological Survey of Canada; 1987. [Google Scholar]
- 20.Bradshaw W E, Lounibos L P. Evolution. 1977;31:546–567. doi: 10.1111/j.1558-5646.1977.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 21.Hard J J, Bradshaw W E, Holzapfel C M. Am Nat. 1993;142:457–473. doi: 10.1086/285549. [DOI] [PubMed] [Google Scholar]
- 22.Lair K P, Bradshaw W E, Holzapfel C M. Genetics. 1997;147:1873–1883. doi: 10.1093/genetics/147.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quebodeaux M C. M.S. thesis. Eugene: Univ. of Oregon; 1998. [Google Scholar]
- 24.Bradshaw W E, Lounibos L P. Can J Zool. 1972;50:713–719. [Google Scholar]
- 25.Bradshaw W E, Phillips D L. Oecologia (Berlin) 1980;44:311–316. doi: 10.1007/BF00545233. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous. SAS User's Guide: Statistics. Cary, NC: SAS Institute; 1985. , Version 5. [Google Scholar]
- 27.Crick H Q P, Dudley C, Glue D E, Thomspon D L. Nature (London) 1997;388:526. [Google Scholar]
- 28.Beebe T J C. Nature (London) 1995;374:219–220. [Google Scholar]
- 29.Hegerl G C, von Storch H, Hasselmann K, Santer B D, Cubasch U, Jones P D. J Climate. 1996;9:2281–2306. [Google Scholar]