Abstract
Triple-negative breast cancer (TNBC) is a subtype of breast cancer. MicroRNA (miR)-214 is closely associated with controlling the development of tumor cells; therefore, in the present study, the target gene and effects of miR-214 on TNBC cells were explored. Luciferase activity was examined by luciferase reporter assay. The viability, invasion and migration of MDA-MB-231 TNBC cells were measured using Cell Counting kit-8, Transwell and wound-healing assays, respectively. The expression levels of various factors were determined using reverse transcription-quantitative polymerase chain reaction and western blotting. The results demonstrated that the expression levels of miR-214 were higher and the levels of α1-antitrypsin (α1-AT) were lower in TNBC tissues compared with in normal tissues. Subsequently, α1-AT was revealed to be a target of miR-214. Furthermore, inhibition of miR-214 decreased cell viability, invasion and migration, enhanced the expression of E-cadherin and tissue inhibitor of metalloproteinases-2, and reduced the expression of metastatic tumour antigen 1 and matrix metalloproteinase-2. Inhibition of miR-214 also significantly downregulated the phosphorylation of protein kinase B (Akt) and mammalian target of rapamycin (mTOR), and markedly downregulated that of phosphoinositide 3-kinase (PI3K); however, the expression levels of total PI3K, Akt and mTOR remained stable in all groups. Taken together, these findings indicated that α1-AT may be a target of miR-214. Downregulation of miR-214 markedly suppressed the viability, migration and invasion of MDA-MB-231 cells, and inhibited the PI3K/Akt/mTOR pathway. These findings suggested that miR-214 targeting α1-AT may be a potential mechanism underlying TNBC development.
Keywords: triple negative breast cancer, miR-214, α1-antitrypsin, PI3K/Akt/mTOR pathway
Introduction
Cancer is the second most common disease that threatens human health and mortality globally (1). Among women, breast cancer (BC) has the highest incidence of all malignant tumors (2). Triple-negative BC (TNBC) is a subtype of BC, which accounts for 10–20% of cases of BC globally. TNBC is characterized by negative expression of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2, and is considered an independent clinicopathological type of cancer that is characterized by strong invasiveness (3–5). Metastasis is a critical biological hallmark of malignant tumors and is the leading cause of mortality among patients with TNBC (6–8); however, the specific process and underlying mechanism remains unclear. Therefore, further study of the mechanism underlying TNBC metastasis and exploration of its effective prevention are of great significance in terms of improving the survival rate and quality of life for patients.
MicroRNAs (miRNAs/miRs) are non-coding single-stranded RNA molecules, which consist of ~22 nucleotides (9). The role of miRNAs is fulfilled by regulating the expression of target genes, which is regulated by complementary binding of the miRNA to the 3′-untranslated region (3′UTR) of the target gene mRNA (10,11). miRNAs may act as oncogenes or tumor suppressor genes in the development of cancer (12–15). Numerous studies have reported that the abnormal expression of miRNAs is closely associated with the development of various tumor types (13–18), and miRNAs are expected to be a novel molecular target for the diagnosis and treatment of cancer. The abnormal expression of miR-214 contributes to the formation of various human tumors, including ovarian cancer, colorectal cancer, gastric cancer and BC (19–22). Furthermore, miR-214 is closely associated with regulation of the development of tumor cells, including cell growth, apoptosis and metastasis (21,23–25); however, at present, the role and target of miR-214 in TNBC is not fully understood.
The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signal transduction pathway is one of the most important signaling pathways in cells. This pathway serves a vital role in regulating cell proliferation, apoptosis, differentiation and metabolism by affecting the activation state of several downstream effector molecules (26–28). In recent years, abnormal activation of the PI3K/Akt/mTOR pathway has been detected in numerous human malignant tumors, and activation of this pathway may be a critical factor leading to the proliferation and metastasis of tumor cells (29–31).
In the present study, the target gene of miR-214 in TNBC was analyzed using the microRNA.org website. In addition, the effects of miR-214 on the growth and metastasis of TNBC cells, and the associated pathways were analyzed.
Materials and methods
Tissue collection
Between June 2016 and November 2017, 37 TNBC, 37 non-TNBC BC and 37 normal tissues were collected from patients with TNBC (38–55 years), patients with n-TNBC (35–53 years) and normal control individuals (all female, 36–52 years) who were diagnosed and subjected to mastectomy at the Second Affiliated Hospital of Kunming Medical University (Kunming, China). All patients provided written informed consent and allowed their tissues to be used for research purposes. The present study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University.
Cell culture
The MDA-MB-231 human TNBC cell line was purchased from Cobioer Biosciences Co., Ltd. (Nanjing, China). The cells were cultured in RPMI-1640 medium (Huayueyang Biotechnology Co., Ltd., Beijing, China) supplemented with 10% fetal bovine serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China), and a mixture of penicillin (100 µg/ml) and streptomycin (100 U/ml; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in a 95% humidified incubator (Sanyo Electric Co., Ltd.; Panasonic Corporation, Kadoma, Japan) containing 5% CO2 at 37°C.
Cell transfection
miR-214 mimics, miR-214 inhibitor and miRNA negative control (NC) were purchased from Vigene Biosciences (Rockville, MD, USA). The miRNA sequences were as follows: miR-214 mimics, 3′-UGACGGACAGACACGGACGACA-5′; miRNA inhibitor, 3′-ACUGCCUGUCUGUGCCUGCUGU-5′; and miRNA NC, 5′-UCUACUCUUUCUAGGAGGUUGUGA-3′. Cell transfection with miR-214 mimics (100 pmol), inhibitor (100 pmol) or miRNA NC (100 pmol) was performed when the confluence of MDA-MB-231 cells reached ~60%. Transfection was conducted using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 24 h. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis was performed to confirm successful transfection.
Luciferase reporter assay
Putative targets of miR-214 were predicted using miRanda version 3.3a (microRNA.org). Luciferase activity was determined using the Dual-Luciferase® Reporter Assay (Promega Corporation, Madison, WI, USA). Briefly, 293 cells (Cobioer Biosciences Co., Ltd.) were co-transfected with miR-214 mimics or miRNA NC and α1-AT-3′UTR or α1-AT-3′UTR mutant (mut) plasmids (400 ng) using Lipofectamine® 2000 at 37°C for 24 h; a QuikChange Site-Directed Mutagenesis Kit (Agilent Technologies, Inc., Santa Clara, CA, USA) was used to obtain α1-AT-3′UTR mutant. The cells were lysed using 1X passive lysis buffer at room temperature for 15 min and the suspension was transferred to the black enzyme plate. LARII (100 µl) and Stop&Glo® reagent (100 µl) were then added to the plates at room temperature, and luciferase activity normalized to Renilla luciferase was immediately measured using a GloMax® Discover Multimode Microplate Reader (GM3000; Promega Corporation).
RT-qPCR analysis
Total RNA was extracted from cells and tissues using RNA extraction kit (Takara Biotechnology Co., Ltd., Dalian, China). Subsequently, 1 µg RNA was used to synthesize cDNA using the SuperScript® VILO™ cDNA synthesis kit (Thermo Fisher Scientific, Inc.). The RT reaction conditions were as follows: 30°C for 10 min, 42°C for 30 min and 95°C for 5 min. cDNA was then amplified using Fast SYBR® Green Master Mix (Thermo Fisher Scientific, Inc.). The qPCR reaction conditions were as follows: 95°C for 10 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec, and a final step at 75°C for 1 min. Primer sequences are listed in Table I. U6 and β-actin were used as internal controls. mRNA expression levels were quantified using the 2−ΔΔCq method (32).
Table I.
Primer sequences.
| Primer name | Sequence (5′-3′) | Product size (bp) |
|---|---|---|
| miR-214-forward | ATAGAATTCTTTCTCCCTTTCCCCTTACTCTCC | 235 |
| miR-214-reverse | CCAGGATCCTTTCATAGGCACCACTCACTTTAC | |
| α1-AT-forward | TCAAGGACACCGAGGAAGAG | 190 |
| α1-AT-reverse | AGGTGCTGTAGTTTCCCCTC | |
| E-cadherin-forward | TTTGAAGATTGCACCGGTCG | 180 |
| E-cadherin-reverse | CAGCGTGACTTTGGTGGAAA | |
| TIMP2-forward | AGCACCACCCAGAAGAAGAG | 175 |
| TIMP2-reverse | TGATGCAGGCGAAGAACTTG | |
| MTA1-forward | CTACGACCCACAGCAGAAGA | 180 |
| MTA1-reverse | TGGTCGATCTGCTTGTCTGT | |
| MMP2-forward | TGGCTACACACCTGATCTGG | 184 |
| MMP2-reverse | GAGTCCGTCCTTACCGTCAA | |
| U6-forward | ACACCAAGCAGTCCGAAGAG | 220 |
| U6-reverse | ACAAAATTTCTCACGCCGGT | |
| β-actin-forward | GGGAAATCGTGCGTGACATT | 219 |
| β-actin-reverse | AGGTAGTTTCGTGGATGCCA |
α1-AT, α1-AT, α1-antitrypsin; miR-214, microRNA-214; MMP2, matrix metalloproteinase-2; MTA1, metastatic tumour antigen 1; TIMP2, tissue inhibitor of metalloproteinases-2.
Western blot analysis
Total proteins were lysed from cells and tissues using radioimmunoprecipitation assay buffer (Beijing Solarbio Science & Technology Co., Ltd.), and protein concentrations were determined using bicinchoninic acid protein assay (Thermo Fisher Scientific, Inc.). Proteins (1 µg/µl; 10 µg/lane) were separated by 12% SDS-PAGE and were transferred onto polyvinylidene fluoride membranes (Hangzhou RENO Membrane Technology, Co., Ltd., Hangzhou, China, www.renomem.com). Subsequently, 5% non-fat milk was used to block the membranes at room temperature for 1.5 h. The membranes were then hybridized to anti-α1-AT (cat. no. ab179443, 1:1,000; Abcam, Cambridge, MA, USA), anti-E-cadherin (cat. no. ab15148, 1:800; Abcam), anti-tissue inhibitor of metalloproteinases-2 (TIMP2; cat. no. ab180630, 1:1,000; Abcam), anti-metastatic tumour antigen 1 (MTA1; cat. no. ab751, 1:1,000; Abcam), anti-matrix metalloproteinase-2 (MMP2; cat. no. ab37150, 1:1,200; Abcam), anti-phosphorylated (p)-PI3K (cat. no. ab182651, 1:1,000; Abcam), anti-PI3K (cat. no. MAB2686, 1:600; R&D Systems, Inc.), anti-p-Akt (cat. no. MAB887, 1:800; R&D Systems, Inc.), anti-Akt (cat. no. MAB2055, 1:800; R&D Systems, Inc.), anti-p-mTOR (cat. no. ab109268, 1:1,000; Abcam), anti-mTOR (cat. no. ab32028, 1:1,000; Abcam) and anti-β-actin (cat. no. ab8227, 1:600; Abcam) at 4°C for 24 h. Subsequently, the membranes were hybridized to the corresponding secondary antibodies (Abcam): Horseradish peroxidase (HRP)-conjugated donkey anti-mouse immunoglobulin (Ig)G H&L (cat. no. ab6820, 1:7,000), HRP-conjugated rabbit anti-mouse IgG H&L (cat. no. ab6728, 1:7,000) and HRP-conjugated goat anti-rabbit IgG H&L (cat. no. ab6721, 1:7,000) at 37°C for 60 min. The blots were detected by enhanced chemiluminescence detection reagent (Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China). VisionWorks® LS Image Acquisition and Analysis software version 7.0 (UVP, LLC, Phoenix, AZ, USA) was used to quantify band intensities.
Cell Counting kit-8 (CCK-8) assay
Cell viability was assessed by CCK-8 (Dalian Meilun Biotechnology Co., Ltd., Dalian, China). Briefly, cells were seeded in a 96-well plate (3×103 cells/well) at 37°C for 24 h. Subsequently, cells were exposed to PBS (control), miRNA NC or miR-214 inhibitor at 37°C for 6, 12 and 24 h. CCK-8 reagent (10 µl) was then added to the cells, which were incubated for a further 4 h at 37°C. The optical density value was then assessed at 450 nm using a microplate reader (SMR16.1; Uscn Life Sciences, Inc., Wuhan, China).
Wound-healing assay
Cells were seeded in a 6-well plate (6×104 cells/well) and were cultured for 24 h. The cells were then exposed to PBS (control), or were transfected with miRNA NC or miR-214 inhibitor. Subsequently, the cells were scratched using a 200-µl pipette tip (Thermo Fisher Scientific, Inc.). Following culturing at 37°C for 24 h, the cells were observed under a DSX100 optical microscope (Olympus Corporation, Tokyo, Japan).
Transwell assay
Matrigel (200 µg/ml, 100 µl; BD Biosciences, Franklin Lakes, NJ, USA) was added to the upper Transwell chamber at room temperature until it solidified, after which, cells were added to the upper chamber. The medium supplemented with 15% FBS was added to the lower chamber. The transfected cells were centrifuged at 5,000 × g for 20 min at 4°C, and the cell suspension (2×105 cells/ml) was cultured in the upper chamber at 37°C for 24 h. Crystal violet (0.1%; Shanghai Gefan Biotechnology Co., Ltd., Shanghai, China) was used to stain the cells for 20 min. Finally, cells were observed under a DSX100 optical microscope (Olympus Corporation).
Statistical analysis
All data are presented as the mean ± standard error of mean, as determined by Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Data were analyzed using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA). The differences among groups were determined by one-way analysis of variance, followed by Dunnett's test. Each experiment was performed in triplicate and repeated three times. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of miR-214 and α1-AT among patients with BC
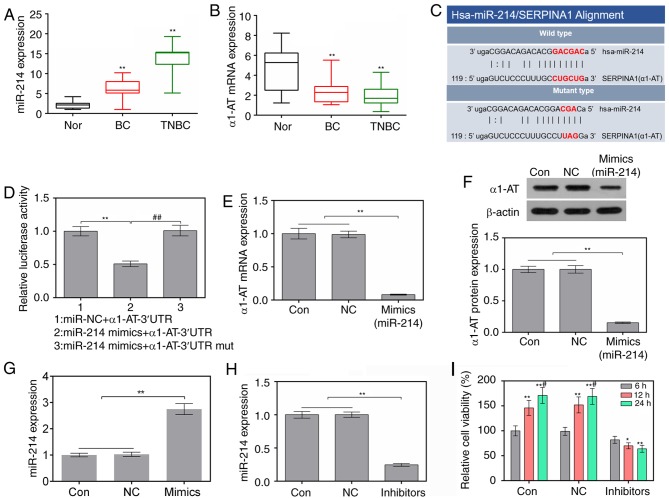
RT-qPCR was used to analyze the expression levels of miR-214 and α1-AT in normal, n-TNBC and TNBC tissues. In n-TNBC and TNBC tissues, the expression levels of miR-214 were higher and the mRNA expression levels of α1-AT were lower than in normal tissue (P<0.01; Fig. 1A and 1B). In addition, according to miRanda, α1-AT was predicted to possess an miR-214-binding site (Fig. 1C). Subsequently, it was revealed that the luciferase activity of α1-AT-3′UTR was reduced in cells transfected with miR-214 mimics; however, miR-214 mimics did not affect the luciferase activity of α1-AT-3′UTR mut (Fig. 1D). Furthermore, the expression levels of α1-AT were decreased following upregulation of miR-214 via transfection with miR-214 mimics (Fig. 1E-G). These results suggested that α1-AT may be a target of miR-214.
Figure 1.
Suppression of miR-214 attenuates the viability of MDA-MB-231 cells. (A and B) Expression levels of Hsa-miR-214 and α1-AT in normal tissues, TBNC tissues and BC tissues, as determined by RT-qPCR. **P<0.01 vs. Normal tissues (C) Binding sites of Hsa-miR-214 and α1-AT, as predicted by microRNA.org. (D) miR-214 mimics and α1-AT-3′UTR or α1-AT-3′UTR mut plasmids were co-transfected into 293 cells using Lipofectamine® 2000. Luciferase activity was measured by luciferase reporter assay. **P<0.01 vs. miR-NC + α1-AT-3′UTR group; ##P<0.01 vs. miR-214 mimics + α1-AT-3′UTR group. (E) mRNA expression levels of α1-AT in cells were assessed using RT-qPCR. **P<0.01 vs. control and NC cells. (F) Protein expression levels of α1-AT in cells were evaluated by western blotting. **P<0.01 vs. control and NC cells. (G) mRNA expression levels of miR-214 in MDA-MB-231 cells following transfection with miR-214 mimics were assessed using RT-qPCR. **P<0.01 vs. control and NC cells. (H) Expression levels of miR-214 in MDA-MB-231 cells following transfection with miR-214 inhibitors were assessed by RT-qPCR. **P<0.01 vs. control and NC cells. (I) Cell viability was determined using Cell Counting Kit-8. *P<0.05, **P<0.01 vs. 6 h; #P<0.05 vs. 12 h. 3′UTR, 3′-untranslated region; α1-AT, α1-AT, α1-antitrypsin; BC, breast cancer; miR-214, microRNA-214; mut, mutant; NC, negative control; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; TNBC, triple-negative BC.
Suppression of miR-214 attenuates the viability of MDA-MB-231 cells
In order to examine the effects of a miR-214 inhibitor on MDA-MB-231 cells, the expression levels of miR-214 and cell viability were detected by RT-qPCR and CCK-8 assay, respectively. The results of RT-qPCR revealed that the expression levels of miR-214 were significantly decreased in cells transfected with miR-214 inhibitor (Fig. 1H). In addition, the miR-214 inhibitor markedly suppressed cell viability at 12 and 24 h (Fig. 1I).
Suppression of miR-214 reduces the migratory and invasive abilities of MDA-MB-231 cells
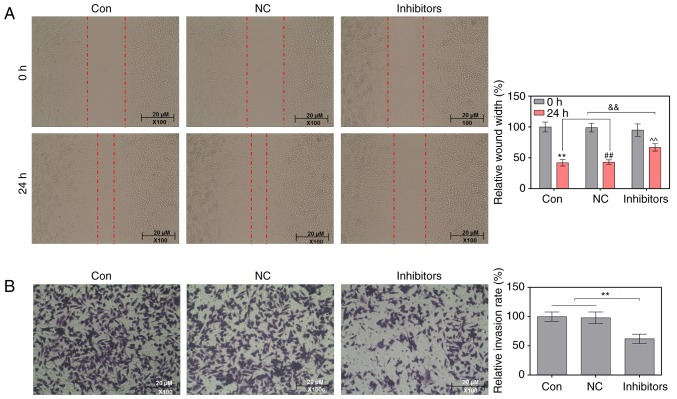
Wound-healing and Transwell assays were conducted to study the effects of a miR-214 inhibitor on the migratory and invasive abilities of MDA-MB-231 cells. As the wound-healing assay results indicated, in cells transfected with a miR-214 inhibitor, the relative wound width was markedly increased (Fig. 2A). The results of the Transwell assay indicated that the miR-214 inhibitor significantly decreased the relative invasion rate of cells (Fig. 2B).
Figure 2.
Suppression of microRNA-214 reduces the migratory and invasive abilities of MDA-MB-231 cells. (A) Relative wound width of cells was analyzed by a wound-healing assay. **P<0.01, ##P<0.01, ^^P<0.01 vs. 0 h; &&P<0.01 vs. control and NC cells. (B) Relative invasion rate of cells, as determined by Transwell assay. **P<0.01 vs. control and NC cells. NC, negative control.
Suppression of miR-214 regulates migration-associated factors in MDA-MB-231 cells
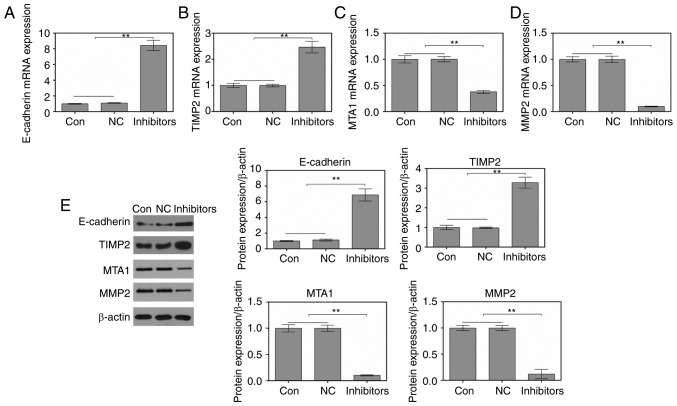
RT-qPCR and western blotting were performed to study the effects of miR-214 inhibition on the expression of migration-associated factors. As shown in Fig. 3A-D, the mRNA expression levels of E-cadherin and TIMP2 were increased, whereas the mRNA expression levels of MTA1 and MMP2 were decreased in cells transfected with a miR-214 inhibitor. In addition, alterations in the protein expression levels of E-cadherin, TIMP2, MTA1 and MMP2 were consistent with alterations in the mRNA expression levels (Fig. 3E).
Figure 3.
Suppression of microRNA-214 regulates migration/invasion-associated factors in MDA-MB-231 cells. Reverse transcription-quantitative polymerase chain reaction was conducted to detect the mRNA expression levels of (A) E-cadherin, (B) TIMP2, (C) MTA1 and (D) MMP2. (E) Western blotting was used to analyze the protein expression levels of E-cadherin, TIMP2, MTA1 and MMP2. **P<0.01 vs. control and NC cells. MMP2, matrix metalloproteinase-2; NC, negative control; MTA1, metastatic tumour antigen 1; TIMP2, tissue inhibitor of metalloproteinases-2.
Suppression of miR-214 inhibits the PI3K/Akt/mTOR signaling pathway in MDA-MB-231 cells
In order to investigate the effects of the miR-214 inhibitor on signaling in MDA-MB-231 cells, the PI3K/Akt/mTOR signaling pathway was examined by western blotting. The results revealed that transfection with the miR-214 inhibitor markedly downregulated phosphorylation of PI3K, Akt and mTOR. However, the expression levels of total PI3K, Akt and mTOR remained stable in the various groups (Fig. 4). The proportions of p-Akt/Akt and p-mTOR/mTOR were significantly reduced by the miR-214 inhibitor (Fig. 4).
Figure 4.
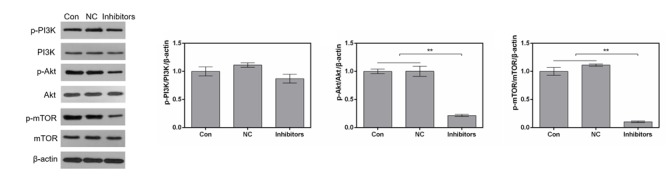
Suppression of miR-214 inhibits the PI3K/Akt/mTOR signaling pathway in MDA-MB-231 cells. The protein expression levels of p-PI3K, PI3K, p-Akt, Akt, p-mTOR and mTOR were determined by western blotting. The proportions of p-PI3K/PI3K/β-actin, p-Akt/Akt/β-actin and p-mTOR/mTOR/β-actin were semi-quantified. **P<0.01 vs. control and NC cells. Akt, protein kinase B; mTOR, mammalian target of rapamycin; NC, negative control; PI3K, phosphoinositide 3-kinase.
Discussion
Increasing evidence has demonstrated that miR-214 is aberrantly expressed and its function is altered in various types of tumor; in particular, low miR-214 expression has been detected in liver cancer and ovarian carcinoma (21,33), whereas high miR-214 expression has been observed in BC, gastric cancer and pancreatic cancer (20,24,34). Notably, Kalniete et al (20) detected high expression levels of miR-214 in patients with TNBC. Similar to this previous study, the present data demonstrated that miR-214 was highly expressed in TNBC tissues. α1-AT is a member of the serine protease inhibitor superfamily, which is dysregulated in lung cancer, prostate cancer and BC (35,36). Previous studies have reported that α1-AT may act as a tumor suppressor in BC cells (37,38). The present data revealed that the expression levels of α1-AT were decreased in TNBC tissues. According to microRNA.org, α1-AT was identified as a target gene of miR-214. In addition, when MDA-MB-231 cells were transfected with miR-214 mimics, the expression levels of α1-AT were downregulated. Therefore, these findings suggested that α1-AT may be a target gene of miR-214, and that miR-214 may exert its effect on TNBC through targeting α1-AT.
Cell proliferation, invasion and migration are known to be essential processes associated with tumor development. Wang et al (39) revealed that miR-214 increases the invasive capacity of BC cells. Zhang et al (40) also reported that miR-214 silencing decreases the proliferation of nasopharyngeal carcinoma cells. Furthermore, Xin et al (41) demonstrated that miR-214 facilitates the metastasis of gastric cancer cells. The present study explored the effects of miR-214 on the viability, invasion and migration of TNBC cells. The results demonstrated that inhibition of miR-214 significantly decreased the viability, migration and invasion of MDA-MB-231 cells.
E-cadherin is an intercellular adhesion molecule, and downregulation of E-cadherin decreases intercellular adhesion (42). The main function of MMPs is to degrade and reshape the dynamic balance of the extracellular matrix. A previous study reported that when tumor cell metastasis is inhibited, the levels of E-cadherin are upregulated and the levels of MMPs are downregulated (43). TIMPs are tissue inhibitors of the MMP family, and MTAs are also a class of critical tumor metastasis-associated genes. It has been reported that tumor necrosis factor α suppresses radiation-induced cell metastasis in neuroblastoma via increasing TIMP2 and E-cadherin levels, and decreasing MTA-2 and MMP levels (44). Therefore, this study examined the effects of miR-214 on the migration/invasion-associated factors using RT-qPCR and western blotting. It was revealed that miR-214 silencing decreased cell migration and invasion via upregulating E-cadherin and TIMP2, and downregulating MTA1 and MMP2.
Previous studies have demonstrated that miR-214 has a role in regulating the PI3K/Akt/mTOR pathway under various pathological conditions (25,45,46). Liu et al (25) reported that miR-214 controls the PI3K/Akt pathway via targeting phosphatase and tensin homolog in oophoroma. Zhao et al (46) also indicated that miR-214 expedited osteoclastogenesis via regulating PI3K/Akt signaling. Li et al (45) demonstrated that miR-214 mediates the PI3K/Akt/mTOR signaling pathway in rat muscle atrophy. Therefore, it was hypothesized that miR-214 may mediate the PI3K/Akt/mTOR pathway in TNBC. As expected, knockdown of miR-214 suppressed the PI3K/Akt/mTOR pathway in MDA-MB-231 cells.
In conclusion, this study demonstrated that miR-214 was highly expressed in TNBC tissues, and that α1-AT was a target gene for miR-214. Silencing of miR-214 markedly suppressed the proliferation, migration/invasion of MDA-MB-231 cells by upregulating E-cadherin and TIMP2, and downregulating MTA1 and MMP2. Furthermore, miR-214 silencing inhibited the PI3K/Akt/mTOR pathway. These findings indicated that miR-214 targeting α1-AT may be a potential mechanism underlying TNBC development.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JM made substantial contributions towards the design of the study. YT collected breast cancer and normal tissues. YiL performed gene expression experiments in tissues. YM performed target prediction for miR-214. YaL performed cell transfections. LD performed viability, migration and invasion assays. SL performed RT-qPCR assays. ZZ performed western blotting. YZ analyzed the data and drafted the manuscript. All authors read and approved of the final version of the manuscript.
Ethics approval and consent to participate
All patients provided written informed consent and allowed their tissues to be used for research purposes. The present study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University.
Patient consent for publication
All participants provided written informed consent prior to the present study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wu J, Goyal L, Nipp R, Wo J, Qadan M, Uppot RN. The Tipping Point: Key oncologic imaging findings resulting in critical changes in the management of malignant tumors of the gastrointestinal tract. Curr Probl Diagn Radiol. 2019;48:61–74. doi: 10.1067/j.cpradiol.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Dunne M, Dou YN, Drake DM, Spence T, Gontijo SML, Wells PG, Allen C. Hyperthermia-mediated drug delivery induces biological effects at the tumor and molecular levels that improve cisplatin efficacy in triple negative breast cancer. J Control Release. 2018;282:35–45. doi: 10.1016/j.jconrel.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Kong SY, Kwon Y, Kim MK, Sim SH, Joo J, Lee KS. Phase I/II clinical trial of everolimus combined with gemcitabine/cisplatin for metastatic triple-negative breast cancer. J Cancer. 2018;9:1145–1151. doi: 10.7150/jca.24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zenzola V, Cabezas-Quintario MA, Arguelles M, Perez-Fernandez E, Izarzugaza Y, Correa A, Garcia-Foncillas J. Prognostic value of Ki-67 according to age in patients with triple-negative breast cancer. Clin Transl Oncol. 2018;20:1448–1454. doi: 10.1007/s12094-018-1877-5. [DOI] [PubMed] [Google Scholar]
- 6.Farah M, Nagarajan P, Torres-Cabala CA, Curry JL, Amaria RN, Wargo J, Tawbi H, Ivan D, Prieto VG, Tetzlaff MT, Aung PP. Metastatic melanoma with balloon/histiocytoid cytomorphology after treatment with immunotherapy: A histologic mimic and diagnostic pitfall. J Cutan Pathol. 2018;45:545–549. doi: 10.1111/cup.13263. [DOI] [PubMed] [Google Scholar]
- 7.Hejduk B, Bobek-Billewicz B, Rutkowski T, Hebda A, Zawadzka A, Jurkowski MK. Application of Intravoxel Incoherent Motion (IVIM) model for differentiation between metastatic and non-metastatic head and neck lymph nodes. Pol J Radiol. 2017;82:506–510. doi: 10.12659/PJR.902275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Yang Y, Huo D, Wang Z, Zhai X, Chen J, Sun H, An W, Jie J, Yang P. LincRNA-RoR/miR-145 promote invasion and metastasis in Triple-negative breast cancer via targeting MUC1. Biochem Biophys Res Commun. 2018;500:614–620. doi: 10.1016/j.bbrc.2018.04.119. [DOI] [PubMed] [Google Scholar]
- 9.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 10.Abdellatif M. Differential expression of microRNAs in different disease states. Circ Res. 2012;110:638–650. doi: 10.1161/CIRCRESAHA.111.247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillai RS. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Jiang Y, Sang M, Xu C. Down-regulation of miR-373 increases the radiosensitivity of lung cancer cells by targeting TIMP2. Int J Biochem Cell Biol. 2018;99:203–210. doi: 10.1016/j.biocel.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhao Z, Li M, Liu M, Bahena A, Zhang Y, Zhang Y, Nambiar C, Liu G. Sulforaphane promotes apoptosis, and inhibits proliferation and self-renewal of nasopharyngeal cancer cells by targeting STAT signal through miRNA-124-3p. Biomed Pharmacother. 2018;103:473–481. doi: 10.1016/j.biopha.2018.03.121. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, He B, Xu T, Pan Y, Hu X, Chen X, Wang S. MiR-490-3p functions as a tumor suppressor by inhibiting oncogene VDAC1 expression in colorectal cancer. J Cancer. 2018;9:1218–1230. doi: 10.7150/jca.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polakovicova I, Jerez S, Wichmann IA, Sandoval-Borquez A, Carrasco-Veliz N, Corvalan AH. Role of microRNAs and exosomes in helicobacter pylori and epstein-barr virus associated gastric cancers. Front Microbiol. 2018;9:636. doi: 10.3389/fmicb.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan X, Zhang H, Zhang L, Zhou X, Wang T, Zhang J, Shu Y, Zhu W, Wen W, Liu P. Identification of four plasma microRNAs as potential biomarkers in the diagnosis of male lung squamous cell carcinoma patients in China Med 7. 2018:2370–2381. doi: 10.1002/cam4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Bao W, Liu Y, Wang S, Xu S, Li X, Li Y, Wu S. miR-98-5p contributes to cisplatin resistance in epithelial ovarian cancer by suppressing miR-152 biogenesis via targeting Dicer1. Cell Death Dis. 2018;9:447. doi: 10.1038/s41419-018-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T, Liu W, Meng W, Zhao H, Yang Q, Gu SJ, Xiao CC, Jia CC, Fu BS. Downregulation of miR-542-3p promotes cancer metastasis through activating TGF-β/Smad signaling in hepatocellular carcinoma. Onco Targets Ther. 2018;11:1929–1939. doi: 10.2147/OTT.S154416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu JL, He GY, Lan XL, Zeng ZC, Guan J, Ding Y, Qian XL, Liao WT, Ding YQ, Liang L. Inhibition of ATG12-mediated autophagy by miR-214 enhances radiosensitivity in colorectal cancer. Oncogenesis. 2018;7:16. doi: 10.1038/s41389-018-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalniete D, Nakazawa-Miklasevica M, Strumfa I, Abolins A, Irmejs A, Gardovskis J, Miklasevics E. High expression of miR-214 is associated with a worse disease-specific survival of the triple-negative breast cancer patients. Hered Cancer Clin Pract. 2015;13:7. doi: 10.1186/s13053-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Lin J, Zhai S, Sun C, Xu C, Zhou H, Liu H. MicroRNA-214 suppresses ovarian cancer by targeting β-Catenin. Cell Physiol Biochem. 2018;45:1654–1662. doi: 10.1159/000487733. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, Liu R, Zhang L, Ying G, Ba Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Wang YW, Zhu WJ, Li Y, Liu L, Yin G, Gao P. A four-microRNA signature predicts lymph node metastasis and prognosis in breast cancer. Hum Pathol. 2018;76:122–132. doi: 10.1016/j.humpath.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Li HL, Liang S, Cui JH, Han GY. Targeting of GSK-3β by miR-214 to facilitate gastric cancer cell proliferation and decrease of cell apoptosis. Eur Rev Med Pharmacol Sci. 2018;22:127–134. doi: 10.26355/eurrev_201801_14109. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Chen W, Zhang H, Liu T, Zhao L. miR-214 targets the PTEN-mediated PI3K/Akt signaling pathway and regulates cell proliferation and apoptosis in ovarian cancer. Oncol Lett. 2017;14:5711–5718. doi: 10.3892/ol.2017.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res Treat. 2018;169:397–406. doi: 10.1007/s10549-018-4697-y. [DOI] [PubMed] [Google Scholar]
- 27.Ramakrishnan V, Kumar S. PI3K/AKT/mTOR pathway in multiple myeloma: From basic biology to clinical promise. Leuk Lymphoma. 2018;1-11 doi: 10.1080/10428194.2017.1421760. [DOI] [PubMed] [Google Scholar]
- 28.Simioni C, Martelli AM, Zauli G, Vitale M, McCubrey JA, Capitani S, Neri LM. Targeting the phosphatidylinositol 3-kinase/Akt/mechanistic target of rapamycin signaling pathway in B-lineage acute lymphoblastic leukemia: An update. J Cell Physiol. 2018;233:6440–6454. doi: 10.1002/jcp.26539. [DOI] [PubMed] [Google Scholar]
- 29.Du L, Li X, Zhen L, Chen W, Mu L, Zhang Y, Song A. Everolimus inhibits breast cancer cell growth through PI3K/AKT/mTOR signaling pathway. Mol Med Rep. 2018;17:7163–7169. doi: 10.3892/mmr.2018.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh YH, Hsiao HF, Yeh YC, Chen TW, Li TK. Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J Exp Clin Cancer Res. 2018;37:70. doi: 10.1186/s13046-018-0730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Xu HL, Wang YC, Lu ZY, Yu XF, Sui DY. 20(S)-Protopanaxadiol-induced apoptosis in MCF-7 breast cancer cell line through the inhibition of PI3K/AKT/mTOR signaling pathway. Int J Mol Sci. 2018;19(pii):E1053. doi: 10.3390/ijms19041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Fan Y, Qian X, Zhang C. U/G SNP rs111904020 in 3′UTR of STAT3 regulated by miR-214 promotes hepatocellular carcinoma development in Chinese population. Tumour Biol. 2016;37:14629–14635. doi: 10.1007/s13277-016-5352-z. [DOI] [PubMed] [Google Scholar]
- 34.Kuninty PR, Bojmar L, Tjomsland V, Larsson M, Storm G, Ostman A, Sandstrom P, Prakash J. MicroRNA-199a and −214 as potential therapeutic targets in pancreatic stellate cells in pancreatic tumor. Oncotarget. 2016;7:16396–16408. doi: 10.18632/oncotarget.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Akawi ZJ, Al-Hindawi FK, Bashir NA. Alpha-1 antitrypsin (alpha1-AT) plasma levels in lung, prostate and breast cancer patients. Neuro Endocrinol Lett. 2008;29:482–484. [PubMed] [Google Scholar]
- 36.Hamrita B, Chahed K, Trimeche M, Guillier CL, Hammann P, Chaieb A, Korbi S, Chouchane L. Proteomics-based identification of alpha1-antitrypsin and haptoglobin precursors as novel serum markers in infiltrating ductal breast carcinomas. Clin Chim Acta. 2009;404:111–118. doi: 10.1016/j.cca.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 37.García-Orad A, Arizti P, Durán L, Urcelay B, De Pancorbo MM. Alpha-1-antitrypsin phenotypes among breast cancer patients in the basque population. Hum Hered. 1994;44:203–208. doi: 10.1159/000154218. [DOI] [PubMed] [Google Scholar]
- 38.Lópezárias E, Aguilarlemarroy A, Felipe JL, Morganvillela G, Mariscalramírez I, Martínezvelázquez M, Alvarez AH, Gutiérrezortega A, Hernándezgutiérrez R. Alpha 1-antitrypsin: A novel tumor-associated antigen identified in patients with early-stage breast cancer. Electrophoresis. 2012;33:2130–2137. doi: 10.1002/elps.201100491. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Lv P, Liu X, Zhu M, Qiu X. microRNA-214 enhances the invasion ability of breast cancer cells by targeting p53. Int J Mol Med. 2015;35:1395–1402. doi: 10.3892/ijmm.2015.2123. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZC, Li YY, Wang HY, Fu S, Wang XP, Zeng MS, Zeng YX, Shao JY. Knockdown of miR-214 promotes apoptosis and inhibits cell proliferation in nasopharyngeal carcinoma. PLoS One. 2014;9:e86149. doi: 10.1371/journal.pone.0086149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin R, Bai F, Feng Y, Jiu M, Liu X, Bai F, Nie Y, Fan D. MicroRNA-214 promotes peritoneal metastasis through regulating PTEN negatively in gastric cancer. Clin Res Hepatol Gastroenterol. 2016;40:748–754. doi: 10.1016/j.clinre.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Pieters T, van Roy F. Role of cell-cell adhesion complexes in embryonic stem cell biology. J Cell Sci. 2014;127:2603–2613. doi: 10.1242/jcs.146720. [DOI] [PubMed] [Google Scholar]
- 43.Che F, Xie X, Wang L, Su Q, Jia F, Ye Y, Zang L, Wang J, Li H, Quan Y, et al. B7-H6 expression is induced by lipopolysaccharide and facilitates cancer invasion and metastasis in human gliomas. Int Immunopharmacol. 2018;59:318–327. doi: 10.1016/j.intimp.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Aravindan S, Natarajan M, Herman TS, Aravindan N. Radiation-induced TNFalpha cross signaling-dependent nuclear import of NFκB favors metastasis in neuroblastoma. Clin Exp Metastasis. 2013;30:807–817. doi: 10.1007/s10585-013-9580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Li QS, Li WB, Wei J, Chang WK, Chen Z, Qiao HY, Jia YW, Tian JH, Liang BS. miRNA targeted signaling pathway in the early stage of denervated fast and slow muscle atrophy. Neural Regen Res. 2016;11:1293–1303. doi: 10.4103/1673-5374.189195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D, Peng J, Wang A, Li Q, Song J, et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12:343–353. doi: 10.1080/15476286.2015.1017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.