Abstract
Secretion from adipose tissue of adipokines or adipocytokines, comprising of bioactive peptides or proteins, immune molecules and inflammatory mediators, exert critical roles in inflammatory arthritis and obesity. This review considers the evidence generated over the last decade regarding the effects of several adipokines including leptin, adiponectin, visfatin, resistin, chemerin and apelin, in cartilage and bone homeostasis in the pathogenesis of rheumatoid arthritis and osteoarthritis, which has important implications for obesity.
Keywords: rheumatoid arthritis, osteoarthritis, adipokines, obesity
1. Introduction
Adipose tissue secretes various bioactive peptides or proteins, immune molecules and inflammatory mediators known as adipokines (only produced by the adipose tissue) or adipocytokines (mainly, but not solely, produced by adipocytes) (Figure 1). In this review, the term “adipokine” refers to these multifunctional molecules. Since the discovery in 1994 of the first adipokine, leptin, profiling studies have identified hundreds of adipokines in the human adipose proteome (adipokinome), all of which can potently modulate inflammation via autocrine/paracrine and endocrine pathways. Some of these multifunctional molecules are critical to the pathogenesis of rheumatoid arthritis (RA) and osteoarthritis (OA), modulating target tissues and cells in cartilage, synovium, bone, and various immune cells [1]. Thus, our review of data details adipose tissue paracrine signaling in RA and OA and discusses correlations identified between adipokines, obesity and the development of RA and OA. These are two of the most important and common arthritic diseases that lead to bone destruction and deformity; we therefore focused on the role of adipokines in these arthritic diseases. Our evidence is drawn from the period of January 2007 through October 2018, because the literature begins to extensively cover the role of adipose tissue and adipokines in obesity, RA and OA from 2007 and our literature search ended in October 2018.
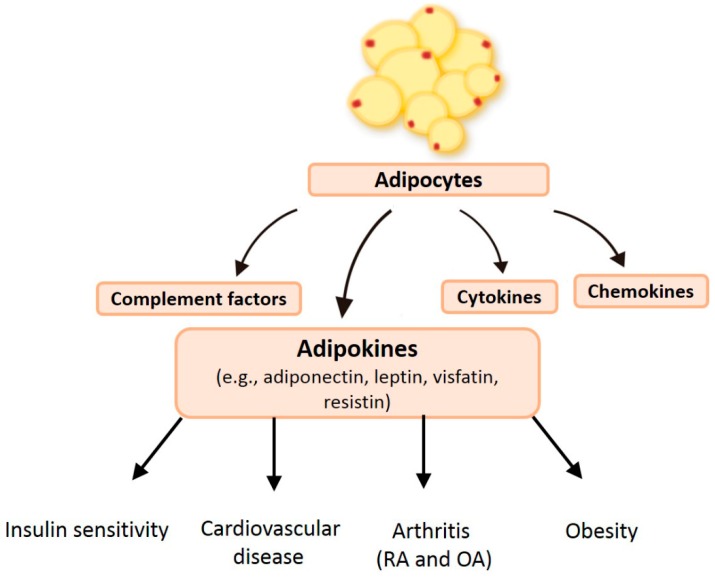
Figure 1.
The important role of adipokines. Adipokines are produced mainly by adipocytes and play critical roles in several major disorders including insulin sensitivity, cardiovascular disease, arthritic conditions (i.e., RA and OA), and obesity.
Rheumatoid arthritis, a chronic autoimmune disease marked by persistent synovial and systemic inflammation, damages joints, results in disability and increases cardiovascular burden. The pathogenesis of RA is uncertain, but the underlying pathology appears to commence outside the joints [2]. Obesity is accompanied by low-grade inflammation and is a recognized risk factor for several well-known health problems, including cardiovascular disorders, disorders of metabolic syndrome (MetS), various cancers, and some rheumatic diseases [3]. Evidence demonstrates that obesity independently increases the risk of RA developing in “at-risk”, autoantibody-positive people, and that higher birth weight is associated with the future onset of RA [4,5]. Conversely, other evidence suggests that obesity has no influence over the likelihood of developing RA. For instance, researchers have reported that in early RA, higher body mass index (BMI) not only does not influence the progression to clinical RA, but that it may be associated with less radiographic joint damage, with people who are obese developing fewer joint erosions and experiencing slower structural progression [6,7]. Interestingly, an association between lower BMI and progression of radiographic joint damage in early RA has been observed only in seropositive individuals [6,8,9].
In comparison to RA, a more definite link is established between higher BMI and the risk of developing hip and knee OA in men and women [10,11]. Evidence from the Framingham Heart Study reveals a 1.5- to 2-fold higher risk of developing knee OA among people who are obese compared with those who are leaner [1] and, in a US population-based study involving community-dwelling older adults (aged ≥70 years), a 5 kg/m2 increase in BMI increased the likelihood of developing knee OA by 32% [12]. Not only did a 200 pM increase in serum leptin increase the odds of knee OA by 11%, but also, approximately half of the BMI’s total effect on knee OA was attributed to leptin. In pooled relative risks (RRs) of a recent meta-analysis, overweight and obesity significantly increased the risk of knee OA by approximately 2.5 and 4.6 times, respectively, compared with normal weight [13]. Other risk factors that predispose to OA include joint trauma, and family history or medical disorders presenting with joint inflammation, such as hemochromatosis, septic arthritis, inflammatory arthritis, avascular necrosis, hemophilia, or gout [12].
As higher BMI fails to totally account for the development or progression of RA, researchers speculate that alterations in the production of inflammatory molecules from adipose tissue may help to activate the immune system and slow the process of damage in the joints [14]. Indeed, the release of adipokines from adipose tissue or joint compartments appears to have critical implications in inflammatory and immune responses of rheumatic diseases [15,16]. For instance, in a cohort of nonarthritic individuals with immunoglobulin M rheumatoid factor (IgM RF) and/or anti-citrullinated protein antibody (ACPA) positivity, serum vaspin levels at study entry related to the clinical manifestation of arthritis after a median 22 months of follow-up [17]. No such association was observed with other adipokines (adiponectin, resistin, leptin, chemerin, or omentin). Moreover, no associations were found between adiponectin, resistin or visfatin synovial expression and the development of arthritis [17]. Some researchers have proposed that lower levels of adiponectin in people with obesity are linked with high adiposity (a surrogate for high BMI) and less joint damage in RA [18].
2. The Involvement of Adiponectin in Arthritis
2.1. Adiponectin in RA
Adiponectin (also known as Acrp30, AdipoQ and GBP28) has attracted much attention for its potential therapeutic use in metabolic disorders, as this adipokine exerts pleiotropic metabolic effects on insulin sensitivity, inflammation and angiogenesis, primarily via the adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2), as well as the non-signaling binding protein T-cadherin, regulating glucose and lipid metabolism [19,20]. Evidence also indicates that adiponectin serves as a possible link between obesity and cancer [19]. Patients with RA have consistently higher serum [21,22,23] and synovial fluid [24] adiponectin levels than non-RA controls. The data are mixed as to the differential regulation of cytokines by adiponectin: On the one hand, adiponectin is capable of suppressing levels of proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) that are typically elevated in RA and adiponectin increases levels of the anti-inflammatory cytokine IL-10 in primary human macrophages activated with lipopolysaccharide (LPS) [25]. Conversely, increasing concentrations of adiponectin stimulate cultured synovial fibroblasts from RA and OA patients to produce IL-6 [26]. Adiponectin can also stimulate vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP) production in RA fibroblast-like synoviocytes (FLSs), leading to joint inflammation and destruction, respectively [27], and women with erosive OA of the hands have higher serum levels of adiponectin levels compared with those with nonerosive hand OA [28]. Moreover, adiponectin can mediate changes in effector cells in RA disease pathophysiology, inducing gene expression and protein synthesis in human RA synovial fibroblasts (RASFs), lymphocytes, endothelial cells and chondrocytes [29], enhancing prostaglandin E2 production in RASFs via AdipoR1 [30,31]. In untreated patients with early RA, serum adiponectin levels have been found to predict radiographic disease progression, independently of metabolic status and potentially confounding factors [32]. Other research has failed to find any association between high serum levels of adiponectin and either homeostasis model assessment for insulin resistance (HOMA-IR) index or common carotid artery intima-media thickness (IMT) measurements [23]. Other investigations into the mechanisms underlying adiponectin function have shown that adiponectin induces production of the proinflammatory cytokine, oncostatin M, in human osteoblasts [33].
In severe, infliximab-refractory RA, a negative correlation has been observed between high-grade inflammation (C-reactive protein [CRP]) and low circulating plasma adiponectin levels [34]. That research documented independent, negative correlations between low adiponectin levels with atherogenic dyslipidemia and high plasma glucose levels, findings that are similar to those previously reported in individuals without RA disease, suggesting that low circulating adiponectin levels cluster with features of MetS that are implicated in RA atherogenesis [34]. If higher adiponectin levels are indeed protective against cardiovascular disease and obesity, it may not be wise to modulate those levels.
2.2. Adiponectin in OA
The somewhat puzzling findings as to the inflammatory activity associated with adiponectin is postulated to be because there are several isoforms that have differing, sometimes counteracting functions [35]. Their selective binding to AdipoR1 and AdipoR2 induces specific intracellular signaling cascades; the oligomerization and expression levels of these receptors determine adiponectin bioactivity [36]. For example, high-molecular-weight adiponectin induces IL-6 in human monocytes but has no effect upon LPS-induced IL-6 secretion, while low-molecular-weight adiponectin reduces LPS-induced IL-6 secretion and induces IL-10 in these cells [36]. Intriguingly, positive correlations have been observed between levels of synovial fluid from OA patients and levels of adiponectin and resistin, whereas conversely, the biological active free form of leptin (not the total leptin) appears to be negatively associated with IL-6 [37].
Much higher serum levels of adiponectin, leptin and resistin have been found in patients with severe knee OA compared with controls without radiographic knee OA; that same research also documented weak but positive associations between serum levels of adiponectin, leptin and resistin and synovial inflammation [38]. Another paper, involving female patients with knee OA, identified a significant correlation between synovial adiponectin levels and degradation markers of aggrecan, which suggests that adiponectin regulates the degeneration of cartilage matrix in OA [39]. In an investigation into the effects of adipokines upon the development of OA osteophytes, adiponectin and visfatin stimulated osteoblasts and chondrocytes, respectively, to increase their release of proinflammatory mediators [40]. Adiponectin has been found to enhance nitric oxide, IL-6, MMP-1 and MMP-3 production in OA cartilage and in primary chondrocytes via mitogen-activated protein kinase (MAPK) signaling [29,41]. Similarly, Junker and colleagues found that adiponectin induced p38 MAPK signaling in OA osteoblasts, whereas stimulation with adiponectin, resistin, or visfatin had no effect on Wnt signaling [40]. These findings indicate that adipokines do not directly influence osteophyte development, but that they do influence proinflammatory conditions in OA and that adiponectin possibly mediates cartilage destruction in OA. Other research has reported that monocyte adhesion to the human OA synovial fibroblast (OASF) monolayer is promoted by adiponectin-induced intercellular adhesion molecule 1 (ICAM-1) expression [42]. In contrast, some evidence suggests that serum adiponectin may be protective in OA; a significant, negative association between serum adiponectin and radiographic OA severity in patients with knee OA persisted after adjusting the analyses for age, sex, BMI and duration of disease [43].
3. Leptin Expression in RA and OA
Leptin, a 16 kDa non-glycosylated protein encoded by the obese (ob) gene, is mainly secreted by adipose tissue and regulates appetite and obesity by inducing anorexigenic factors and suppressing orexigenic neuropeptides [44]. The release of leptin into the circulation enables it to act peripherally and centrally [45]. After entering the brain via a saturable transport mechanism, leptin’s central location of action is the hypothalamus [45]. This adipokine also has direct effects on non-neural cells [46], as evidenced by its involvement in immunoregulatory functions, as it is capable of inducing TH1 immune reactions by increasing the TH1 phenotype of the effector CD4 T cell and suppressing the TH2 phenotype; leptin can also induce naïve CD4 T cells to proliferate and inhibit memory CD4 T cells from proliferating [47].
Increased serum leptin levels have been linked to erosion of cartilage and bone in OA [48], synovitis and cartilage defects, bone marrow lesions and osteophytes [49]. Notably, leptin expression correlates with DNA methylation in OA chondrocytes and leptin’s downregulation dramatically inhibits MMP-13 gene expression [50]. Single nucleotide polymorphism (SNP) analyses have suggested associations between the leptin gene and its receptor gene with OA in both normal weight and overweight Chinese populations [51,52]. Some research has found a significant correlation between leptin messenger RNA (mRNA) expression in advanced human OA cartilage and BMI, suggesting that leptin could serve as a metabolic link between obesity and OA [53]. That same research also found that leptin expression was significantly increased in synovial fluid, indicating that leptin was locally produced as opposed to diffusing from plasma to synovial fluid via the synovial membrane. The ability of leptin to stimulate IL-1β production and increase MMP-9 and MMP-13 protein expression in OA and normal chondrocytes supports the contention that leptin has proinflammatory and catabolic effects in the metabolism of cartilage [53]. Evidence implicates leptin in obesity and joint damage; a significant association has been found between baseline leptin levels and increased biomarkers of bone formation (osteocalcin and PINP) over a 2-year period; conversely, higher levels of soluble leptin receptor (sOB-Rb), which reduces leptin activity, were associated with lower osteocalcin levels at 2 years of follow-up [54]. Interestingly, serum leptin levels are significantly associated with increased knee OA cartilage volume, whereas serum adiponectin levels are significantly associated with lower levels of disease severity in radiographic OA [43].
Other research has found that adiposity in leptin-impaired mice does not lead to systemic inflammation and knee OA, which suggests that leptin directly influences knee OA pathogenesis rather than via any correlation with obesity and that the loss of leptin signaling pathways may help to prevent the development of OA [55]. Assessing leptin expression could potentially be used to measure RA and OA disease activity. Higher serum leptin levels are found in RA patients with high disease activity compared with those with low disease activity [22,56] and a small but significantly positive correlation exists between leptin levels and RA activity [57,58]; no such correlation exists between serum adiponectin levels and RA disease activity [58]. This lack of association between adiponectin levels and disease activity was seen in another study involving patients with knee OA, in whom synovial fluid leptin levels and plasma levels of adiponectin, soluble leptin receptor and free leptin were not significantly different across categories of OA severity, although the ratio of synovial fluid to plasma leptin level was significantly lower in advanced OA than in early disease [59]. In contrast, other research has revealed a close association between synovial fluid leptin levels and OA radiographic severity, which is highest in stage IV disease [60].
In vitro investigations suggest that leptin increases production of the proinflammatory cytokine IL-8 in RASFs and OASFs by binding to the leptin receptor (OBRI) and activating the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway, which in turn activates the insulin receptor substrate-1/phosphatidylinositol 3 kinase/Akt/nuclear factor-κB (IRS1/PI3K/Akt/NF-κB)-dependent pathway and leads to p300 recruitment [61]. Similarly, leptin induces IL-6 expression in OASFs by activating the OBRl receptor, which in turn activates the IRS-1, PI3K, Akt, and AP-1 signaling pathways and thus upregulates IL-6 expression [62]. Table 1 summarizes the involved receptors, downstream and targeted signaling molecules, and target genes involved in RA and OA. It shows that many leptin receptors exist in individual cells (osteoblasts, FLSs and chondrocytes), but not in the brain. Evidence of brain leptin receptor expression in RA/OA will require much more research. Leptin also increases vascular cell adhesion molecule 1 (VCAM-1) expression in human and murine chondrocytes [63]. Importantly, VCAM-1 functions as a cell adhesion molecule, mediating leukocyte recruitment and extravasation from circulating blood to inflamed joints [63]. Interestingly, leptin appears to induce MAPK signaling in both human chondrocytes [64] and RA FLSs [65]. The presence of leptin stimulates oncostatin M production in osteoblasts from healthy human donors [66].
Table 1.
Adipokines implicated in rheumatoid arthritis and osteoarthritis.
| Stimulation | Target Factors | Effects in Tissue | Disease | Receptor | Known Pathways | References | |
|---|---|---|---|---|---|---|---|
| Adiponectin | |||||||
| IL-6 | ↑ | FLSs | OA & RA | AdipoR1 | AMPK/p38/IKKαβ and NF-κB | [26] | |
| VEGF, MMPs | ↑ | FLSs | RA | N/A | N/A | [27] | |
| IL-6, RANTES, MMP-3 | ↑ | FLSs, lymphocytes, endothelial cells, and chondrocytes | RA | N/A | PKA/NF-κB/p38MAPK/PKC | [29] | |
| PGE2 | ↑ | FLSs | RA | AdipoR1 | NF-κB | [30,31] | |
| OSM | ↑ | Osteoblasts | RA | N/A | PI3K/Akt and NF-κB | [33] | |
| IL-6, IL-8, and CCL2 | ↑ | Osteoblasts and chondrocytes | OA | N/A | p38/MAPK | [40] | |
| IL-6, MMP-1,-3 | ↑ | Chondrocytes | OA | N/A | p38/ERK1/2/JNK | [41] | |
| ICAM-1 | ↑ | FLSs | OA | AdipoR1 | LKB1, CaMKII, AMPK, and AP-1 | [42] | |
| VCAM-1 | ↑ | Chondrocytes | RA & OA | N/A | JAK2 and PI3K | [63] | |
| Leptin | |||||||
| MMP-13 | ↑ | Chondrocytes | OA | N/A | [50] | ||
| IL-1β, MMP-9 and MMP-13 | ↑ | Chondrocytes | OA | OBRb | [53] | ||
| IL-8 | ↑ | FLSs | RA & OA | OBRI | JAK2/STAT3 and IRS1/PI3K/Akt/NF-κB | [61] | |
| IL-6 | ↑ | FLSs | OA | OBRI | IRS-1/PI3K/Akt, and AP-1 | [62] | |
| VCAM-1 | ↑ | Chondrocytes | RA & OA | N/A | JAK2 and PI3K | [63] | |
| ADAMTS-4, -5 and -9 | ↑ | Chondrocytes | OA | N/A | MAPK and NF-κB | [64] | |
| IL-6 | ↑ | FLSs | RA | OBRb | JAK2/STAT3 | [65] | |
| OSM | ↑ | Osteoblasts | RA | OBRI | AKT/miR-93 | [66] | |
| Resistin | |||||||
| CXCL8, CCL2 and IL-6 | ↑ | FLSs | RA | N/A | CAP1 | [67] | |
| VEGF | ↑ | EPCs | RA | N/A | PKC-δ/AMPK/miR-206 | [68] | |
| Visfatin | |||||||
| IL-6 and IL-8, CCL2 and MMP-3 | ↑ | FLSs | RA | N/A | p38 pathway | [69] | |
| IGF-1 | ↓ | Chondrocytes | OA | IGF-1R | ERK/MAPK signaling pathway | [70] | |
| IL-6 and TNF-α | ↑ | FLSs | OA | N/A | ERK/p38/JNK and miR-199a-5p | [71] | |
| MMP-3, -12, and -13 | ↑ | Chondrocytes | OA | N/A | HIF-2a | [72] | |
| Other adipokines | |||||||
| MMP-1, -3 and -9, ADAMTS-4 and -5, IL-1β | Chondrocytes | OA | N/A | JNK, ERK and MAPK | [73] | ||
IL-6, interleukin 6; FLSs, fibroblast-like synoviocytes; OA, osteoarthritis; RA, rheumatoid arthritis; AdiopoR1, adiponectin receptor 1; AMPK, AMP-activated protein kinase; IKKα/β, IκB kinase alpha/beta; NF-κB, nuclear factor-kappa B; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase; RANTES, regulated upon activation, normal T cells expressed and secreted; PKA, protein kinase A; p38MAPK, P38 mitogen-activated protein kinase; PKC, protein kinase C; PGE2, prostaglandin E2; OSM, oncostatin M; PI3K, phosphatidylinositol 3 kinase; IL-8, interleukin-8; CCL2, chemokine (C-C motif) ligand 2; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; JNK, c-Jun N-terminal kinase; ICAM-1, intercellular adhesion molecule 1; LKB1, liver kinase B1; CaMKII, Ca2+/calmodulin-dependent protein kinase II; AP-1, activator protein 1; VCAM-1, vascular cell adhesion molecule 1; JAK2, Janus kinase 2; IL-1β, interleukin-1 beta; OBRb and OBRl, long isoform of leptin receptor; STAT3, signal transducer and activator of transcription 3; IRS1, insulin receptor substrate-1; ADAMTS-4, ADAM metallopeptidase with thrombospondin type 1 motif 4; ADAMTS-5, ADAM metallopeptidase with thrombospondin type 1 motif 5; ADAMTS-9, ADAM metallopeptidase with thrombospondin type 1 motif 9; miR, microRNA; CXCL8, C-X-C motif chemokine ligand 8; CAP1, adenylate cyclase-associated protein 1; EPCs, endothelial progenitor cells; IGF-1, insulin-like growth factor 1; IGF-1R, IGF-1 receptor; TNF-α, tumor necrosis factor alpha; HIF-2α, hypoxia-inducible factor 2 alpha.
4. Resistin in Arthritis
4.1. Resistin in RA
Adipocyte-derived expression and secretion of resistin, a small cysteine-rich adipokine, is linked to inflammation and insulin resistance [68,74]. Some researchers have documented positive correlations between serum resistin levels and inflammatory status (erythrocyte sedimentation rate [ESR], CRP) as well as clinical disease activity (28-joint count Disease Activity Score [DAS28]) in patients with RA [22,75]. Significantly higher resistin levels have been observed in synovial sublining layers from RA patients than from OA patients [75]. Šenolt and colleagues have documented resistin expression within several different cell types within the synovial tissue, including synovial fibroblasts, and in different inflammatory cell types found in RA synovium such as macrophages, B lymphocytes and plasma cells [75]. They proposed that resistin is a secreted signaling molecule that helps to activate these cell types in chronic inflammatory states such as RA. Subsequent investigations support this contention, showing positive associations between serum levels of resistin and leptin with CRP levels in RA, indicating that resistin and leptin act as proinflammatory cytokines in this disease [22]. Indeed, resistin has been found to specifically enhance the concentrations of chemokines CXCL8 and CCL2, as well as IL-6, in RA FLSs; transfecting the FLSs with adenylate cyclase-associated protein 1 (CAP1, a receptor for resistin) significantly reduced CXCL8 expression, which implicates the involvement of the resistin-CAP1 pathway in chemokine production in RA synovial tissue [67]. Plasma resistin levels also correlate with coronary artery calcification, a marker of coronary atherosclerosis [74].
Interestingly, despite finding evidence in support of resistin as a significant mediator of the inflammatory process in RA, Yoshino and colleagues (2011) found that serum resistin levels did not differ between RA patients and healthy controls [22], which is backed by other investigations [76,77]. In contrast, one small study found higher serum and synovial resistin levels in RA patients compared with OA patients, supporting a role for resistin in autoimmune inflammatory rheumatologic disease [78]. The study evidence also suggested that high synovial fluid resistin levels may be a poor prognostic factor for RA in terms of disease progression and radiologic joint damage.
Other researchers have described how resistin directly induces significant increases in VEGF expression in endothelial progenitor cells (EPCs) and promotes EPC homing into the synovium, inducing RA angiogenesis; inhibiting resistin reduces EPC homing into synovial fluid and angiogenesis in mice with collagen-induced arthritis [68]. Those researchers detail the involvement of the protein kinase C delta (PKC-δ) pathway in resistin-induced EPC migration and tube formation; this investigation was the first to show that resistin induces EPC migration and tube formation by downregulating microRNA 206 (miR-206) expression via the PKC-δ/AMPK (AMP-activated protein kinase) signaling pathway, which involves VEGF expression in primary EPCs. This clarification of the mechanisms underlying RA pathogenesis highlights resistin as a therapeutic target in RA and is confirmed by an investigation into resistin gene expression in pathogenetic leukocyte subsets from patients with active RA treated with TNF-α inhibitor therapy (adalimumab) for 3 months [79]. Among those who responded to adalimumab, resistin (RETN) gene expression was significantly downregulated in CD14+ and CD4+ monocytes, but was unchanged in CD8+ T cytotoxic lymphocytes and CD19+ B lymphocytes [79]. Conversely, RETN gene expression increased in a patient who failed to respond to adalimumab. Some research has explored whether selected gene polymorphisms in Chinese Han patients with RA and healthy controls are associated with RA susceptibility and clinicopathological characteristics [80]. The analysis examined four RETN single nucleotide polymorphisms (SNPs rs3745367, rs7408174, rs1862513, and rs3219175). Those carrying the C allele of the RETN SNP rs7408174 and those with the AG allele or who had at least one A allele of the SNP rs3219175 were more likely than wild-type carriers to develop RA. In addition, RA patients carrying the AG allele of the RETN SNP rs3219175 had higher serum CRP levels compared with controls, and had a high likelihood of being prescribed TNF inhibitors. Besides the risk for RA, genetic variation in RETN is linked to a higher likelihood of other diseases, such as MetS and colon cancer, while the RETN SNP rs186513 is implicated in a higher risk of type 2 diabetes [74]. Moreover, RETN SNPs correlated with lung cancer progression in patients with Chinese Han ethnicity [74].
4.2. Resistin in OA
Resistin may possibly serve as a drug target in OA: Positive correlations have been observed between resistin and inflammatory factors (IL-6, MMP-1 and MMP-3) in human OASFs [81]. Moreover, the researchers found a correlation between release of resistin from cultured OA cartilage and resistin levels in synovial fluid. Similarly, one study has reported that levels of circulating leptin resistin, IL-6 and IL-17, were positively correlated with clinical disease activity in Mexican patients with RA [82].
5. Visfatin in Arthritis
5.1. Visfatin in RA
Visfatin (otherwise known as pre-B-cell colony-enhancing factor [PBEF] or nicotinamide phosphoribosyltransferase [NAMPT]) is actively involved in the synthesis of cellular nicotinamide adenine dinucleotide (NAD+) and helps to regulate cellular growth, angiogenesis and apoptosis in mammalian cells [83]. Visfatin also triggers the release of cytokines, chemokines and proinflammatory enzymes that are characteristically present in RA joints [84] and is overexpressed in plasma and synovial fluid of several inflammatory diseases, including RA and OA [85,86], suggesting that visfatin promotes their development. This is supported by findings showing that visfatin/PBEF contributes to proinflammatory chemokine production in RASFs, matrix-degrading factors and pro-angiogenic molecules in RA synovial tissue [69]. Moreover, other researchers have demonstrated a positive correlation between circulating visfatin levels and RA disease activity as assessed by DAS28 and CRP levels [87]. Interestingly, although the study also reported significantly higher circulating adiponectin levels in RA patients than in controls, there was no apparent link between circulating adiponectin and disease activity.
Insulin-like growth factor-1 (IGF-1) is implicated in the synthesis and repair of cartilage matrix. Visfatin inhibits IGF-1 function in chondrocytes by prolonging the activation of the extracellular signal-regulated kinase (ERK)/MAPK signaling pathway, independently of IGF-1 receptor activation [70]. Visfatin also inhibits IGF-1-stimulated proteoglycan (PG) synthesis, basal and IGF-1-stimulated collagen type II expression and synthesis [70]. These findings help to clarify the local effects of visfatin on joint tissue and its effects upon inflammatory disease.
Substantial epidemiological data characterize cigarette smoking as an important risk factor for RA and attest to the negative impacts of smoking upon all stages of RA disease [88]. Cigarette smoking reduces the clinical response to antirheumatic therapy and to treatment with TNF inhibitors in particular, especially infliximab [88]. In preclinical and early-stage disease, evidence suggests that smoking may interact with HLA-DR shared epitope genes and encourage the development of anticitrulline antibody-positive RA [89]. A strong, positive association has been observed between smoking and radiographic progression in early RA [88]. In established RA disease, cigarette smoking has been associated with progressive joint damage, persistently active RA and the development of rheumatic nodules [89]. Smoking is also associated with high concentrations of inflammatory cytokines [88] and inversely associated with circulating levels of IGF-1 [89]. Intriguingly, researchers have demonstrated lower serum levels of leptin and adiponectin in smokers than in non-smokers, whereas smoking appears to have no such effect upon resistin and visfatin levels, which are similar between smokers and non-smokers [89].
Visfatin is known to increase cardiovascular risk. In untreated patients with early-stage RA, significant positive concentrations have been observed between visfatin and biochemical markers of severe metabolic disturbance (insulin and insulin resistance, total and LDL cholesterol and triglycerides), although other studies have failed to find an association between visfatin concentrations and coronary artery calcification scores in RA, between visfatin concentrations and carotid artery IMT, or any relationship between NAMPT polymorphisms, disease susceptibility and cardiovascular risk in RA [90]. In patients with established, treated RA, visfatin concentrations have been found to be independently associated with increased diastolic blood pressure and diabetes [90]. Moreover, visfatin concentrations were directly associated with levels of MMP-2 (a plaque stability mediator), even after adjusting for adiposity and Clinical Disease Activity Index (CDAI) scores. It is thought that elevated MMP-2 expression in RA might help to compensate for the visfatin-induced enhancement of cardiovascular risk [90]. Interestingly, several different types of cancers (i.e., colorectal, gastric, breast, prostatic, pancreas and esophageal) exhibit overexpression of visfatin [83].
5.2. Visfatin in OA
Investigations have confirmed an essential role for visfatin in the destruction of OA cartilage mediated by hypoxia-inducible factor 2-alpha (HIF-2α) [72]. Not only does HIF-2α directly target the Nampt gene in articular chondrocytes and OA cartilage, but also, visfatin upregulates mRNA levels and activities of MMP-3, MMP-12 and MMP-13 and downregulates aggrecan expression in chondrocytes, all of which are critical for OA pathogenesis. Inhibiting visfatin enzymatic activity blocks the destruction of OA cartilage [72]. In in vitro investigations, visfatin-induced promotion of IL-6 and TNF-α in human synovial fibroblasts occurs through the ERK, p38, and JNK signaling pathways [71].
6. Lipocalin-2 in RA and OA
Lipocalin-2 is upregulated in adipose tissue of obese animals and in vitro evidence suggests that lipocalin-2 homeostatically regulates inflammatory activity and inflammation-mediated adipocyte dysfunction in an autocrine or paracrine fashion [91]. This is supported by findings showing elevated levels of fecal lipocalin-2 in mice with collagen-induced arthritis and concomitant experimental colitis; induction of colitis delayed the onset of arthritis and reduced its severity as compared with the arthritis-only group [92]. Interestingly, the development of arthritis was not affected by colitis severity. Similarly, other researchers have reported that although higher serum lipocalin-2 levels can be used as an indicator of structural damage such as erosions in early-stage RA, they cannot be used to monitor disease activity [93]. A recent investigation into the expression and role of lipocalin-2 in OA osteoblasts and chondrocytes in osteochondral junctions has revealed its importance as a catabolic adipokine and its regulation in osteoblasts by inflammatory, catabolic, and anabolic factors [94]. That investigation also revealed that osteoblasts induced the paracrine expression of lipocalin-2. According to these findings, lipocalin-2 is apparently an active catabolic agent in OA joints and may serve as a link among obesity, aging and OA joint alterations.
7. Apelin in RA and OA
Early in vitro investigations into the role of apelin at any of the following concentrations of 0.5, 1, 10, or 100 nM in cartilage metabolism indicated that this cytokine stimulates chondrocyte proliferation and increases MMP-1, MMP-3 and MMP-9 transcript levels, as well as IL-1β protein expression [73]. These results were supported by in vivo findings: After rats were administered intra-articular injections of apelin (1 nM), MMP-1, MMP-3 and MMP-9 were upregulated, ADAMTS-4 and ADAMTS-5 mRNA levels were markedly increased, as were IL-1β levels, while levels of collagen II gene and protein expression were reduced. Moreover, proteoglycan was depleted in articular cartilage after apelin treatment. In patients with RA, research has reported finding a strong inverse association between apelin concentrations and those of MMP-9 [95]. Patients with early RA exhibit significantly lower serum apelin levels compared with apelin profiles of healthy controls [96]. The published evidence suggests that it could be worth investigating drugs that specifically target apelin and thus inhibit the development of arthritic diseases such as RA and OA.
8. Omentin, Vaspin and Nesfatin in RA, OA, and Other Arthritic Diseases
Similarly, another anti-inflammatory adipokine, omentin, is associated with lower levels of MMP-3 in RA [97], while nesfatin-1 has been found to be inversely associated with carotid IMT (80), suggesting that certain adipokines may protect against cardiovascular disease in RA. Patients with psoriatic arthritis exhibit higher serum levels of omentin and leptin, but lower levels of adiponectin and chemerin, compared with healthy controls [98]. Interestingly, whereas higher serum levels of omentin and leptin are positively correlated with numbers of osteoclast precursors in peripheral blood, lower serum adiponectin levels in psoriatic arthritis are negatively correlated with osteoclast precursors [98]. Those researchers also described finding a positive correlation between leptin and Psoriatic Arthritis Joint Activity Index scores. Serum levels of omentin are also significantly higher in patients with juvenile idiopathic arthritis (JIA) compared with healthy controls; moreover, omentin serum levels are higher in JIA with active joints compared with JIA without active joints, and a positive significant correlation has been observed between omentin serum levels and the presence of active joints in JIA [99]. That same investigation failed to find any such associations between these parameters of disease activity and serum levels of vaspin in JIA [99]. Omentin apparently has no effects upon central effector cells in RA pathophysiology, despite its presence in the synovium and synovial fluid from RA and OA patients [100]. Interestingly, not only do synovial fluid levels of omentin and vaspin appear to differ at the site of local inflammation in patients with RA and OA, but also, patients with RA have demonstrated lower levels of omentin and higher levels of vaspin in synovial fluid compared with patients with OA [101]. Those researchers found that synovial fluid levels of vaspin, but not of omentin, tended to correlated with DAS28 scores, but neither adipokine was correlated with serum CRP or synovial fluid leucocyte counts [101]. Although omentin seems to have anti-inflammatory and antiatherogenic properties in obesity and displays negative associations in inflammatory bowel disease and MetS, these effects may not occur in RA and OA [100]. It may be worth targeting nesfatin-1 in OA; elevated levels in serum and synovial fluid from patients with knee OA have been found to be significantly associated with disease severity as determined by Kellgren–Lawrence grading criteria [102]. Moreover, it is speculated that nesfatin-1 may contribute to pathophysiological changes in OA [103]. Nesfatin-1 has been found in articular cartilage in patients with knee OA, who exhibit significantly higher serum levels of nesfatin-1 compared with serum from healthy controls [103]. Furthermore, serum nesfatin-1 levels are significantly correlated with high-sensitivity CRP levels, while synovial nesfatin-1 is significantly correlated with IL-18 levels in patients with OA [103].
9. Other Adipokines
Importantly, besides those adipokines covered in this review, we cannot discuss other more recently discovered adipokines including adipolin, acylation-stimulating protein, fasting-induced adipose factor, retinol-binding protein-4, and serum amyloid A3, because no available data provide evidence for their roles in RA and OA disease. Interestingly, related research has found that the novel adipokine fatty acid-binding protein 4 (FABP4), closely associated with obesity and metabolic diseases, is significantly higher in the serum and synovial fluid of patients with RA than in those of OA patients [104], while plasma and synovial fluid levels of FABP4 are significantly higher in OA patients than in those of healthy non-OA controls [105]. Furthermore, recent experimental research has indicated that in FABP4 knockout mice (KO) with obesity induced by a high-fat diet, cartilage degradation is significantly alleviated after 6 months of daily oral gavage with a selective FABP4 inhibitor has suggested that FABP4 may be a potential therapeutic target in OA [106]. Further explorations into the pathogenic aspects of novel adipokines involved in obesity may well uncover other such links into RA and OA disease activity; the space limitations of this review prevent us from researching this aspect.
10. Summary and Future Directions
Controversially, obesity does not necessarily influence the likelihood of developing RA; the evidential link is more definite for OA, where leptin appears to be a metabolic link between obesity and OA. Other adipokines, such as visfatin and resistin, also play important roles in arthritis pathogenesis (Table 1 and Figure 2). In agreement with previous summaries of evidence on the interaction of adipokines with RA and OA [107,108], the evidence discussed in this review suggests that it could be useful to develop therapeutic molecules that target individual adipokines. We have found no published evidence for any natural product that inhibits adipokines and potentially treats arthritis. To help overcome the lack of treatment opportunities, our laboratory is constructing a full-length adipokine containing luciferase for use as a screening model to identify and test natural products or pharmacochemical structures able to target specific adipokines involved in RA and OA disease. We are also working on the design of anti-adipokine antibodies that we will test for their ability to detect the early development of arthritic diseases, so that in future anti-arthritic therapy can be administered early to prevent disease progression.
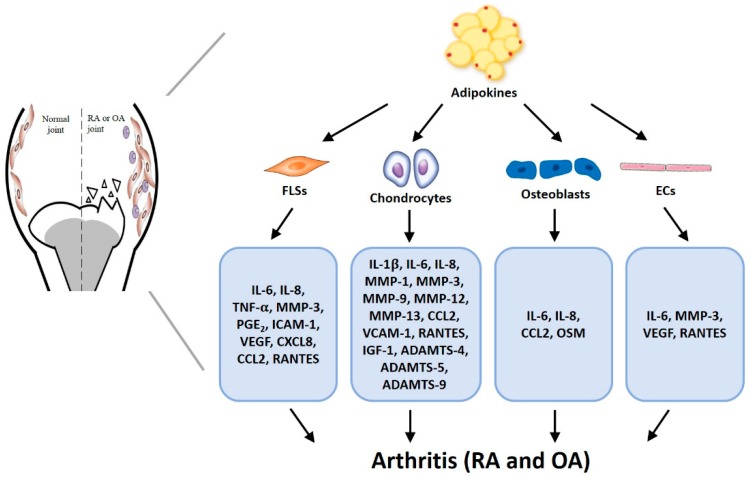
Figure 2.
Critical pathways involving adipokines in arthritic diseases. Adipose tissue paracrine signaling in RA and OA demonstrates systemic links between adipokines and arthritic disease.
Author Contributions
All authors except for I.J.M. and C.-C.H. conceptualized the topic for this review; S.-C.L. conceived ideas about the clinical application of anti-adipokine antibodies. S.-J.K. and C.-H.T. (Chun-Hao Tsai) were involved in drafting the article; C.-C.H. and C.-H.T. (Chih-Hsin Tang) critically revised the review for important intellectual content. All authors approved the final version to be published.
Funding
This work was supported by grants from the National Science Council of Taiwan (MOST107-2320-B-039-019-MY3; 105-2320-B-039-015-MY3; 107-2314-B-039-064-) and China Medical University Hospital, Taichung, Taiwan (DMR-108-070).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Azamar-Llamas D., Hernandez-Molina G., Ramos-Avalos B., Furuzawa-Carballeda J. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2017;2017:5468023. doi: 10.1155/2017/5468023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Q., Wang Y., Xu D., Nossent J., Pavlos N.J., Xu J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gremese E., Tolusso B., Gigante M.R., Ferraccioli G. Obesity as a risk and severity factor in rheumatic diseases (autoimmune chronic inflammatory diseases) Front. Immunol. 2014;5:576. doi: 10.3389/fimmu.2014.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Hair M.J., Landewe R.B., van de Sande M.G., van Schaardenburg D., van Baarsen L.G., Gerlag D.M., Tak P.P. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann. Rheum. Dis. 2013;72:1654–1658. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandl L.A., Costenbader K.H., Simard J.F., Karlson E.W. Is birthweight associated with risk of rheumatoid arthritis? Data from a large cohort study. Ann. Rheum. Dis. 2009;68:514–518. doi: 10.1136/ard.2007.080937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Helm-van Mil A.H., van der Kooij S.M., Allaart C.F., Toes R.E., Huizinga T.W. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann. Rheum. Dis. 2008;67:769–774. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 7.Baker J.F., George M., Baker D.G., Toedter G., Von Feldt J.M., Leonard M.B. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatology. 2011;50:2100–2107. doi: 10.1093/rheumatology/ker294. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto J., Garnero P., van der Heijde D., Miyasaka N., Yamamoto K., Kawai S., Takeuchi T., Yoshikawa H., Nishimoto N. A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod. Rheumatol. 2009;19:273–282. doi: 10.3109/s10165-009-0170-4. [DOI] [PubMed] [Google Scholar]
- 9.Westhoff G., Rau R., Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56:3575–3582. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 10.Lohmander L.S., Gerhardsson de Verdier M., Rollof J., Nilsson P.M., Engstrom G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: A population-based prospective cohort study. Ann. Rheum. Dis. 2009;68:490–496. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 11.Urban H., Little C.B. The role of fat and inflammation in the pathogenesis and management of osteoarthritis. Rheumatology. 2018;57(Suppl. S4):iv10–iv21. doi: 10.1093/rheumatology/kex399. [DOI] [PubMed] [Google Scholar]
- 12.Fowler-Brown A., Kim D.H., Shi L., Marcantonio E., Wee C.C., Shmerling R.H., Leveille S. The mediating effect of leptin on the relationship between body weight and knee osteoarthritis in older adults. Arthritis Rheumatol. 2015;67:169–175. doi: 10.1002/art.38913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng H., Chen C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open. 2015;5:e007568. doi: 10.1136/bmjopen-2014-007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senolt L. Adipokines: Role in local and systemic inflammation of rheumatic diseases. Expert Rev. Clin. Immunol. 2017;13:1–3. doi: 10.1080/1744666X.2017.1249850. [DOI] [PubMed] [Google Scholar]
- 15.Gomez R., Conde J., Scotece M., Gomez-Reino J.J., Lago F., Gualillo O. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 2011;7:528–536. doi: 10.1038/nrrheum.2011.107. [DOI] [PubMed] [Google Scholar]
- 16.Kontny E., Plebanczyk M., Lisowska B., Olszewska M., Maldyk P., Maslinski W. Comparison of rheumatoid articular adipose and synovial tissue reactivity to proinflammatory stimuli: Contribution to adipocytokine network. Ann. Rheum. Dis. 2012;71:262–267. doi: 10.1136/annrheumdis-2011-200123. [DOI] [PubMed] [Google Scholar]
- 17.Maijer K.I., Neumann E., Muller-Ladner U., Drop D.A., Ramwadhdoebe T.H., Choi I.Y., Gerlag D.M., de Hair M.J., Tak P.P. Serum Vaspin Levels Are Associated with the Development of Clinically Manifest Arthritis in Autoantibody-Positive Individuals. PLoS ONE. 2015;10:e0144932. doi: 10.1371/journal.pone.0144932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giles J.T., van der Heijde D.M., Bathon J.M. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 2011;70:1562–1568. doi: 10.1136/ard.2011.150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C.Y., Chang A.C., Chen H.T., Wang S.W., Lo Y.S., Tang C.H. Adiponectin promotes VEGF-C-dependent lymphangiogenesis by inhibiting miR-27b through a CaMKII/AMPK/p38 signaling pathway in human chondrosarcoma cells. Clin. Sci. 2016;130:1523–1533. doi: 10.1042/CS20160117. [DOI] [PubMed] [Google Scholar]
- 20.Ye R., Scherer P.E. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2013;2:133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otero M., Lago R., Gomez R., Lago F., Dieguez C., Gomez-Reino J.J., Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino T., Kusunoki N., Tanaka N., Kaneko K., Kusunoki Y., Endo H., Hasunuma T., Kawai S. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern. Med. 2011;50:269–275. doi: 10.2169/internalmedicine.50.4306. [DOI] [PubMed] [Google Scholar]
- 23.Ozgen M., Koca S.S., Dagli N., Balin M., Ustundag B., Isik A. Serum adiponectin and vaspin levels in rheumatoid arthritis. Arch. Med. Res. 2010;41:457–463. doi: 10.1016/j.arcmed.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Senolt L., Pavelka K., Housa D., Haluzik M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35:247–252. doi: 10.1016/j.cyto.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Folco E.J., Rocha V.Z., Lopez-Ilasaca M., Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 2009;284:25569–25575. doi: 10.1074/jbc.M109.019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C.H., Chiu Y.C., Tan T.W., Yang R.S., Fu W.M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J. Immunol. 2007;179:5483–5492. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- 27.Choi H.M., Lee Y.A., Lee S.H., Hong S.J., Hahm D.H., Choi S.Y., Yang H.I., Yoo M.C., Kim K.S. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res. Ther. 2009;11:R161. doi: 10.1186/ar2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filkova M., Liskova M., Hulejova H., Haluzik M., Gatterova J., Pavelkova A., Pavelka K., Gay S., Muller-Ladner U., Senolt L. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Ann. Rheum. Dis. 2009;68:295–296. doi: 10.1136/ard.2008.095737. [DOI] [PubMed] [Google Scholar]
- 29.Frommer K.W., Zimmermann B., Meier F.M., Schroder D., Heil M., Schaffler A., Buchler C., Steinmeyer J., Brentano F., Gay S., et al. Adiponectin-mediated changes in effector cells involved in the pathophysiology of rheumatoid arthritis. Arthritis Rheum. 2010;62:2886–2899. doi: 10.1002/art.27616. [DOI] [PubMed] [Google Scholar]
- 30.Kusunoki N., Kitahara K., Kojima F., Tanaka N., Kaneko K., Endo H., Suguro T., Kawai S. Adiponectin stimulates prostaglandin E(2) production in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010;62:1641–1649. doi: 10.1002/art.27450. [DOI] [PubMed] [Google Scholar]
- 31.Zuo W., Wu Z.H., Wu N., Duan Y.H., Wu J.T., Wang H., Qiu G.X. Adiponectin receptor 1 mediates the difference in adiponectin-induced prostaglandin E2 production in rheumatoid arthritis and osteoarthritis synovial fibroblasts. Chin. Med. J. 2011;124:3919–3924. [PubMed] [Google Scholar]
- 32.Meyer M., Sellam J., Fellahi S., Kotti S., Bastard J.P., Meyer O., Liote F., Simon T., Capeau J., Berenbaum F. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: Results from the ESPOIR cohort. Arthritis Res. Ther. 2013;15:R210. doi: 10.1186/ar4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su C.M., Lee W.L., Hsu C.J., Lu T.T., Wang L.H., Xu G.H., Tang C.H. Adiponectin Induces Oncostatin M Expression in Osteoblasts through the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2016;17:29. doi: 10.3390/ijms17010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Gay M.A., Llorca J., Garcia-Unzueta M.T., Gonzalez-Juanatey C., De Matias J.M., Martin J., Redelinghuys M., Woodiwiss A.J., Norton G.R., Dessein P.H. High-grade inflammation, circulating adiponectin concentrations and cardiovascular risk factors in severe rheumatoid arthritis. Clin. Exp. Rheumatol. 2008;26:596–603. [PubMed] [Google Scholar]
- 35.Krysiak R., Handzlik-Orlik G., Okopien B. The role of adipokines in connective tissue diseases. Eur. J. Nutr. 2012;51:513–528. doi: 10.1007/s00394-012-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann E., Frommer K.W., Vasile M., Muller-Ladner U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 2011;63:1159–1169. doi: 10.1002/art.30291. [DOI] [PubMed] [Google Scholar]
- 37.Gross J.B., Guillaume C., Gegout-Pottie P., Mainard D., Presle N. Synovial fluid levels of adipokines in osteoarthritis: Association with local factors of inflammation and cartilage maintenance. Bio-med. Mater. Eng. 2014;24(Suppl. S1):17–25. doi: 10.3233/BME-140970. [DOI] [PubMed] [Google Scholar]
- 38.De Boer T.N., van Spil W.E., Huisman A.M., Polak A.A., Bijlsma J.W., Lafeber F.P., Mastbergen S.C. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr. Cartil. 2012;20:846–853. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Hao D., Li M., Wu Z., Duan Y., Li D., Qiu G. Synovial fluid level of adiponectin correlated with levels of aggrecan degradation markers in osteoarthritis. Rheumatol. Int. 2011;31:1433–1437. doi: 10.1007/s00296-010-1516-0. [DOI] [PubMed] [Google Scholar]
- 40.Junker S., Frommer K.W., Krumbholz G., Tsiklauri L., Gerstberger R., Rehart S., Steinmeyer J., Rickert M., Wenisch S., Schett G., et al. Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts. Matrix Biol. 2017;62:75–91. doi: 10.1016/j.matbio.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Koskinen A., Juslin S., Nieminen R., Moilanen T., Vuolteenaho K., Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res. Ther. 2011;13:R184. doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H.T., Tsou H.K., Chen J.C., Shih J.M., Chen Y.J., Tang C.H. Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts. PLoS ONE. 2014;9:e92741. doi: 10.1371/journal.pone.0092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng S., Xu J., Xu S., Zhang M., Huang S., He F., Yang X., Xiao H., Zhang H., Ding C. Association between circulating adipokines, radiographic changes, and knee cartilage volume in patients with knee osteoarthritis. Scand. J. Rheumatol. 2016;45:224–229. doi: 10.3109/03009742.2015.1083053. [DOI] [PubMed] [Google Scholar]
- 44.Francisco V., Perez T., Pino J., Lopez V., Franco E., Alonso A., Gonzalez-Gay M.A., Mera A., Lago F., Gomez R., et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J. Orthop. Res. 2018;36:594–604. doi: 10.1002/jor.23788. [DOI] [PubMed] [Google Scholar]
- 45.Tang C.H., Lu D.Y., Yang R.S., Tsai H.Y., Kao M.C., Fu W.M., Chen Y.F. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappaB, and p300 pathway in microglia. J. Immunol. 2007;179:1292–1302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- 46.Huang C.Y., Yu H.S., Lai T.Y., Yeh Y.L., Su C.C., Hsu H.H., Tsai F.J., Tsai C.H., Wu H.C., Tang C.H. Leptin increases motility and integrin up-regulation in human prostate cancer cells. J. Cell. Physiol. 2011;226:1274–1282. doi: 10.1002/jcp.22455. [DOI] [PubMed] [Google Scholar]
- 47.Hasenkrug K.J. The leptin connection: Regulatory T cells and autoimmunity. Immunity. 2007;26:143–145. doi: 10.1016/j.immuni.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Scotece M., Conde J., Lopez V., Lago F., Pino J., Gomez-Reino J.J., Gualillo O. Adiponectin and leptin: New targets in inflammation. Basic Clin. Pharmacol. Toxicol. 2014;114:97–102. doi: 10.1111/bcpt.12109. [DOI] [PubMed] [Google Scholar]
- 49.Karvonen-Gutierrez C.A., Harlow S.D., Jacobson J., Mancuso P., Jiang Y. The relationship between longitudinal serum leptin measures and measures of magnetic resonance imaging-assessed knee joint damage in a population of mid-life women. Ann. Rheum. Dis. 2014;73:883–889. doi: 10.1136/annrheumdis-2012-202685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iliopoulos D., Malizos K.N., Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: Possible molecular target for osteoarthritis therapeutic intervention. Ann. Rheum. Dis. 2007;66:1616–1621. doi: 10.1136/ard.2007.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin J., Shi D., Dai J., Zhu L., Tsezou A., Jiang Q. Association of the leptin gene with knee osteoarthritis susceptibility in a Han Chinese population: A case-control study. J. Hum. Genet. 2010;55:704–706. doi: 10.1038/jhg.2010.86. [DOI] [PubMed] [Google Scholar]
- 52.Ma X.J., Guo H.H., Hao S.W., Sun S.X., Yang X.C., Yu B., Jin Q.H. Association of single nucleotide polymorphisms (SNPs) in leptin receptor gene with knee osteoarthritis in the Ningxia Hui population. Yi Chuan = Hered. 2013;35:359–364. doi: 10.3724/SP.J.1005.2013.00359. [DOI] [PubMed] [Google Scholar]
- 53.Simopoulou T., Malizos K.N., Iliopoulos D., Stefanou N., Papatheodorou L., Ioannou M., Tsezou A. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthr. Cartil. 2007;15:872–883. doi: 10.1016/j.joca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Berry P.A., Jones S.W., Cicuttini F.M., Wluka A.E., Maciewicz R.A. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011;63:700–707. doi: 10.1002/art.30182. [DOI] [PubMed] [Google Scholar]
- 55.Griffin T.M., Huebner J.L., Kraus V.B., Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935–2944. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S.W., Park M.C., Park Y.B., Lee S.K. Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol. Int. 2007;27:537–540. doi: 10.1007/s00296-006-0253-x. [DOI] [PubMed] [Google Scholar]
- 57.Lee Y.H., Bae S.C. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: A meta-analysis. Z. fur Rheumatol. 2016;75:1021–1027. doi: 10.1007/s00393-016-0050-1. [DOI] [PubMed] [Google Scholar]
- 58.Cao H., Lin J., Chen W., Xu G., Sun C. Baseline adiponectin and leptin levels in predicting an increased risk of disease activity in rheumatoid arthritis: A meta-analysis and systematic review. Autoimmunity. 2016;49:547–553. doi: 10.1080/08916934.2016.1230847. [DOI] [PubMed] [Google Scholar]
- 59.Staikos C., Ververidis A., Drosos G., Manolopoulos V.G., Verettas D.A., Tavridou A. The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology. 2013;52:1077–1083. doi: 10.1093/rheumatology/kes422. [DOI] [PubMed] [Google Scholar]
- 60.Ku J.H., Lee C.K., Joo B.S., An B.M., Choi S.H., Wang T.H., Cho H.L. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin. Rheumatol. 2009;28:1431–1435. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 61.Tong K.M., Shieh D.C., Chen C.P., Tzeng C.Y., Wang S.P., Huang K.C., Chiu Y.C., Fong Y.C., Tang C.H. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-kappaB/p300 binding in human synovial fibroblasts. Cell. Signal. 2008;20:1478–1488. doi: 10.1016/j.cellsig.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Yang W.H., Liu S.C., Tsai C.H., Fong Y.C., Wang S.J., Chang Y.S., Tang C.H. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PLoS ONE. 2013;8:e75551. doi: 10.1371/journal.pone.0075551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conde J., Scotece M., Lopez V., Gomez R., Lago F., Pino J., Gomez-Reino J.J., Gualillo O. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE. 2012;7:e52533. doi: 10.1371/journal.pone.0052533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaykasli K.O., Hatipoglu O.F., Yaykasli E., Yildirim K., Kaya E., Ozsahin M., Uslu M., Gunduz E. Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-kB signaling pathways in human chondrocytes. Cell Biol. Int. 2015;39:104–112. doi: 10.1002/cbin.10336. [DOI] [PubMed] [Google Scholar]
- 65.Muraoka S., Kusunoki N., Takahashi H., Tsuchiya K., Kawai S. Leptin stimulates interleukin-6 production via janus kinase 2/signal transducer and activator of transcription 3 in rheumatoid synovial fibroblasts. Clin. Exp. Rheumatol. 2013;31:589–595. [PubMed] [Google Scholar]
- 66.Yang W.H., Tsai C.H., Fong Y.C., Huang Y.L., Wang S.J., Chang Y.S., Tang C.H. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int. J. Mol. Sci. 2014;15:15778–15790. doi: 10.3390/ijms150915778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato H., Muraoka S., Kusunoki N., Masuoka S., Yamada S., Ogasawara H., Imai T., Akasaka Y., Tochigi N., Takahashi H., et al. Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res. Ther. 2017;19:263. doi: 10.1186/s13075-017-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su C.M., Hsu C.J., Tsai C.H., Huang C.Y., Wang S.W., Tang C.H. Resistin Promotes Angiogenesis in Endothelial Progenitor Cells Through Inhibition of MicroRNA206: Potential Implications for Rheumatoid Arthritis. Stem Cells. 2015;33:2243–2255. doi: 10.1002/stem.2024. [DOI] [PubMed] [Google Scholar]
- 69.Meier F.M., Frommer K.W., Peters M.A., Brentano F., Lefevre S., Schroder D., Kyburz D., Steinmeyer J., Rehart S., Gay S., et al. Visfatin/pre-B-cell colony-enhancing factor (PBEF), a proinflammatory and cell motility-changing factor in rheumatoid arthritis. J. Biol. Chem. 2012;287:28378–28385. doi: 10.1074/jbc.M111.312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yammani R.R., Loeser R.F. Extracellular nicotinamide phosphoribosyltransferase (NAMPT/visfatin) inhibits insulin-like growth factor-1 signaling and proteoglycan synthesis in human articular chondrocytes. Arthritis Res. Ther. 2012;14:R23. doi: 10.1186/ar3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu M.H., Tsai C.H., Huang Y.L., Fong Y.C., Tang C.H. Visfatin Promotes IL-6 and TNF-alpha Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int. J. Mol. Sci. 2018;19:190. doi: 10.3390/ijms19010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang S., Ryu J.H., Oh H., Jeon J., Kwak J.S., Kim J.H., Kim H.A., Chun C.H., Chun J.S. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2alpha, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 2015;74:595–602. doi: 10.1136/annrheumdis-2013-204355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu P.F., Chen W.P., Tang J.L., Bao J.P., Wu L.D. Apelin plays a catabolic role on articular cartilage: In vivo and in vitro studies. Int. J. Mol. Med. 2010;26:357–363. [PubMed] [Google Scholar]
- 74.Yang W.H., Wang S.J., Chang Y.S., Su C.M., Yang S.F., Tang C.H. Association of Resistin Gene Polymorphisms with Oral Squamous Cell Carcinoma Progression and Development. BioMed Res. Int. 2018;2018:9531315. doi: 10.1155/2018/9531315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senolt L., Housa D., Vernerova Z., Jirasek T., Svobodova R., Veigl D., Anderlova K., Muller-Ladner U., Pavelka K., Haluzik M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann. Rheum. Dis. 2007;66:458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forsblad d’Elia H., Pullerits R., Carlsten H., Bokarewa M. Resistin in serum is associated with higher levels of IL-1Ra in post-menopausal women with rheumatoid arthritis. Rheumatology. 2008;47:1082–1087. doi: 10.1093/rheumatology/ken187. [DOI] [PubMed] [Google Scholar]
- 77.Alkady E.A., Ahmed H.M., Tag L., Abdou M.A. Serum and synovial adiponectin, resistin, and visfatin levels in rheumatoid arthritis patients. Relation to disease activity. Z. fur Rheumatol. 2011;70:602–608. doi: 10.1007/s00393-011-0834-2. [DOI] [PubMed] [Google Scholar]
- 78.Fadda S.M., Gamal S.M., Elsaid N.Y., Mohy A.M. Resistin in inflammatory and degenerative rheumatologic diseases. Relationship between resistin and rheumatoid arthritis disease progression. Z. fur Rheumatol. 2013;72:594–600. doi: 10.1007/s00393-013-1146-5. [DOI] [PubMed] [Google Scholar]
- 79.Nagaev I., Andersen M., Olesen M.K., Nagaeva O., Wikberg J., Mincheva-Nilsson L., Andersen G.N. Resistin Gene Expression is Downregulated in CD4(+) T Helper Lymphocytes and CD14(+) Monocytes in Rheumatoid Arthritis Responding to TNF-alpha Inhibition. Scand. J. Immunol. 2016;84:229–236. doi: 10.1111/sji.12464. [DOI] [PubMed] [Google Scholar]
- 80.Wang L., Tang C.H., Lu T., Sun Y., Xu G., Huang C.C., Yang S.F., Su C.M. Resistin polymorphisms are associated with rheumatoid arthritis susceptibility in Chinese Han subjects. Medicine. 2018;97:e0177. doi: 10.1097/MD.0000000000010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koskinen A., Vuolteenaho K., Moilanen T., Moilanen E. Resistin as a factor in osteoarthritis: Synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP-3. Scand. J. Rheumatol. 2014;43:249–253. doi: 10.3109/03009742.2013.853096. [DOI] [PubMed] [Google Scholar]
- 82.Bustos Rivera-Bahena C., Xibille-Friedmann D.X., Gonzalez-Christen J., Carrillo-Vazquez S.M., Montiel-Hernandez J.L. Peripheral blood leptin and resistin levels as clinical activity biomarkers in Mexican Rheumatoid Arthritis patients. Reumatol. Clin. 2016;12:323–326. doi: 10.1016/j.reuma.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 83.Mohammadi M., Mianabadi F., Mehrad-Majd H. Circulating visfatin levels and cancers risk: A systematic review and meta-analysis. J. Cell. Physiol. 2019;234:5011–5022. doi: 10.1002/jcp.27302. [DOI] [PubMed] [Google Scholar]
- 84.Brentano F., Schorr O., Ospelt C., Stanczyk J., Gay R.E., Gay S., Kyburz D. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007;56:2829–2839. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- 85.Jacques C., Holzenberger M., Mladenovic Z., Salvat C., Pecchi E., Berenbaum F., Gosset M. Proinflammatory actions of visfatin/nicotinamide phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and Nampt enzymatic activity. J. Biol. Chem. 2012;287:15100–15108. doi: 10.1074/jbc.M112.350215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laiguillon M.C., Houard X., Bougault C., Gosset M., Nourissat G., Sautet A., Jacques C., Berenbaum F., Sellam J. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res. Ther. 2014;16:R38. doi: 10.1186/ar4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee Y.H., Bae S.C. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int. J. Rheum. Dis. 2018;21:664–672. doi: 10.1111/1756-185X.13038. [DOI] [PubMed] [Google Scholar]
- 88.Chang K., Yang S.M., Kim S.H., Han K.H., Park S.J., Shin J.I. Smoking and rheumatoid arthritis. Int. J. Mol. Sci. 2014;15:22279–22295. doi: 10.3390/ijms151222279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erlandsson M.C., Doria Medina R., Toyra Silfversward S., Bokarewa M.I. Smoking Functions as a Negative Regulator of IGF1 and Impairs Adipokine Network in Patients with Rheumatoid Arthritis. Mediat. Inflamm. 2016;2016:3082820. doi: 10.1155/2016/3082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson C., Tsang L., Solomon A., Woodiwiss A.J., Gunter S., Mer M., Hsu H.C., Gomes M., Norton G.R., Millen A.M.E., et al. Nesfatin-1 and visfatin expression is associated with reduced atherosclerotic disease risk in patients with rheumatoid arthritis. Peptides. 2018;102:31–37. doi: 10.1016/j.peptides.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J., Wu Y., Zhang Y., Leroith D., Bernlohr D.A., Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 2008;22:1416–1426. doi: 10.1210/me.2007-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hablot J., Peyrin-Biroulet L., Kokten T., El Omar R., Netter P., Bastien C., Jouzeau J.Y., Sokol H., Moulin D. Experimental colitis delays and reduces the severity of collagen-induced arthritis in mice. PLoS ONE. 2017;12:e0184624. doi: 10.1371/journal.pone.0184624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gulkesen A., Akgol G., Poyraz A.K., Aydin S., Denk A., Yildirim T., Kaya A. Lipocalin 2 as a clinical significance in rheumatoid arthritis. Cent. -Eur. J. Immunol. 2017;42:269–273. doi: 10.5114/ceji.2017.70969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villalvilla A., Garcia-Martin A., Largo R., Gualillo O., Herrero-Beaumont G., Gomez R. The adipokine lipocalin-2 in the context of the osteoarthritic osteochondral junction. Sci. Rep. 2016;6:29243. doi: 10.1038/srep29243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gunter S., Solomon A., Tsang L., Woodiwiss A.J., Robinson C., Millen A.M., Norton G.R., Dessein P.H. Apelin concentrations are associated with altered atherosclerotic plaque stability mediator levels and atherosclerosis in rheumatoid arthritis. Atherosclerosis. 2017;256:75–81. doi: 10.1016/j.atherosclerosis.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 96.Di Franco M., Spinelli F.R., Metere A., Gerardi M.C., Conti V., Boccalini F., Iannuccelli C., Ciciarello F., Agati L., Valesini G. Serum levels of asymmetric dimethylarginine and apelin as potential markers of vascular endothelial dysfunction in early rheumatoid arthritis. Mediat. Inflamm. 2012;2012:347268. doi: 10.1155/2012/347268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robinson C., Tsang L., Solomon A., Woodiwiss A.J., Gunter S., Millen A.M., Norton G.R., Fernandez-Lopez M.J., Hollan I., Dessein P.H. Omentin concentrations are independently associated with those of matrix metalloproteinase-3 in patients with mild but not severe rheumatoid arthritis. Rheumatol. Int. 2017;37:3–11. doi: 10.1007/s00296-016-3541-0. [DOI] [PubMed] [Google Scholar]
- 98.Xue Y., Jiang L., Cheng Q., Chen H., Yu Y., Lin Y., Yang X., Kong N., Zhu X., Xu X., et al. Adipokines in psoriatic arthritis patients: The correlations with osteoclast precursors and bone erosions. PLoS ONE. 2012;7:e46740. doi: 10.1371/journal.pone.0046740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cantarini L., Simonini G., Fioravanti A., Generoso M., Bacarelli M.R., Dini E., Galeazzi M., Cimaz R. Circulating levels of the adipokines vaspin and omentin in patients with juvenile idiopathic arthritis, and relation to disease activity. Clin. Exp. Rheumatol. 2011;29:1044–1048. [PubMed] [Google Scholar]
- 100.Frommer K.W., Vasile M., Muller-Ladner U., Neumann E. The Adipokine Omentin in Late-stage Rheumatoid Arthritis and Endstage Osteoarthritis. J. Rheumatol. 2017;44:539–541. doi: 10.3899/jrheum.161267. [DOI] [PubMed] [Google Scholar]
- 101.Senolt L., Polanska M., Filkova M., Cerezo L.A., Pavelka K., Gay S., Haluzik M., Vencovsky J. Vaspin and omentin: New adipokines differentially regulated at the site of inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1410–1411. doi: 10.1136/ard.2009.119735. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y., Shui X., Lian X., Wang G. Serum and synovial fluid nesfatin-1 concentration is associated with radiographic severity of knee osteoarthritis. Med Sci. Monit. 2015;21:1078–1082. doi: 10.12659/MSM.892875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang L., Bao J., Zhou X., Xiong Y., Wu L. Increased serum levels and chondrocyte expression of nesfatin-1 in patients with osteoarthritis and its relation with BMI, hsCRP, and IL-18. Mediat. Inflamm. 2013;2013:631251. doi: 10.1155/2013/631251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andres Cerezo L., Kuklova M., Hulejova H., Vernerova Z., Pesakova V., Pecha O., Veigl D., Haluzik M., Pavelka K., Vencovsky J., et al. The level of fatty acid-binding protein 4, a novel adipokine, is increased in rheumatoid arthritis and correlates with serum cholesterol levels. Cytokine. 2013;64:441–447. doi: 10.1016/j.cyto.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Zhang C., Li T., Chiu K.Y., Wen C., Xu A., Yan C.H. FABP4 as a biomarker for knee osteoarthritis. Biomark. Med. 2018;12:107–118. doi: 10.2217/bmm-2017-0207. [DOI] [PubMed] [Google Scholar]
- 106.Zhang C., Chiu K.Y., Chan B.P.M., Li T., Wen C., Xu A., Yan C.H. Knocking out or pharmaceutical inhibition of fatty acid binding protein 4 (FABP4) alleviates osteoarthritis induced by high-fat diet in mice. Osteoarthr. Cartil. 2018;26:824–833. doi: 10.1016/j.joca.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Conde J., Scotece M., Lopez V., Gomez R., Lago F., Pino J., Gomez-Reino J.J., Gualillo O. Adipokines: Novel players in rheumatic diseases. Discov. Med. 2013;15:73–83. [PubMed] [Google Scholar]
- 108.Poonpet T., Honsawek S. Adipokines: Biomarkers for osteoarthritis? World J. Orthop. 2014;5:319–327. doi: 10.5312/wjo.v5.i3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]