Abstract
Seven known sesquiterpene coumarins and a new sesquiterpene coumarin, anatolicin (8), were isolated from the dichloromethane extract of the roots of Heptaptera anatolica. Structures of these compounds were elucidated based on their spectral properties. While some of these sesquiterpene coumarins showed modest cytotoxic activity against COLO205, KM12, A498, UO31, and TC32 cancer cell lines, selective cytotoxicity of anatolicin (8) and 14′-acetoxybadrakemin (7) were observed at nanomolar level against the UO31 kidney cancer cell line.
Keywords: Heptaptera anatolica, Apiaceae, sesquiterpene coumarins, cytotoxic activity
1. Introduction
Cancer is the second leading cause of death worldwide according to WHO statistics [1]. Cancer is a highly complex disease that evolves through the acquisition of multiple biological capabilities by normal cells to become cancer cells [2]. In order to develop new cancer medicines, it is crucial to recognize the various pathways that provide immortality to cancer cells [3]. Natural products represent a major and highly diverse source for the development of novel cancer treatments [4]. As part of our continuing studies on potential anticancer phytochemicals from Apiaceae family, we report here the cytotoxic compounds of Heptaptera anatolica (Boiss.) Tutin.
Oleo-gum-resins obtained from the Ferula (Apiaceae) species have been used for the treatment of various tumors since ancient times; Dioscorides refers to the use of such drugs in the De Materia Medica [5], and Avicenna describes the application of Khiltit (the oleo-gum-resin of Ferula foetida) in the treatment of tumors, by direct application to the tumor after cutting it open, as described in the Al-Qanun fi al-Tibb (the Canon of Medicine) [6]. The sesquiterpene ethers found in the oleo-gum-resins of Ferula species are mainly responsible for their cytotoxic activities, in addition, they show many biological activities such as antiviral, antibacterial, antileishmanial, anti-inflammatory and P-glycoprotein inhibitory activities [7].
Heptaptera Marg. & Reuter is a small genus of the Apiaceae family represented by six species [8]. The majority of Heptaptera species are distributed throughout the countries of the Middle East (i.e., Turkey, Iran, Iraq, and Syria), except for Heptaptera angustifolia (Bertol.) Tutin and H. colladonioides Marg. & Reuter which are endemic to Italy and Greece, respectively. Characteristic secondary metabolites of Heptaptera (syn. Colladonia) sp. are sesquiterpene ethers of umbelliferone [9,10,11], in which the sesquiterpene moiety is either an acyclic sesquiterpene as in the case of umbelliprenin (1), or is a bicyclic drimane derivative, e.g., badrakemin (4). Similar sesquiterpene coumarins are also known from Ferula species, however, several drimane sesquiterpene ethers isolated from Heptaptera are biogenetically oxygenated at the C-14′ position that is a specific chemotaxonomic character of this genus and not been found in the sesquiterpene ethers isolated from Ferula species. In order to evaluate the cytotoxic activities of sesquiterpene coumarin ethers where C-14′ is oxygenated and related, compounds, we decided to investigate the dichloromethane root extract of H. anatolica.
2. Results
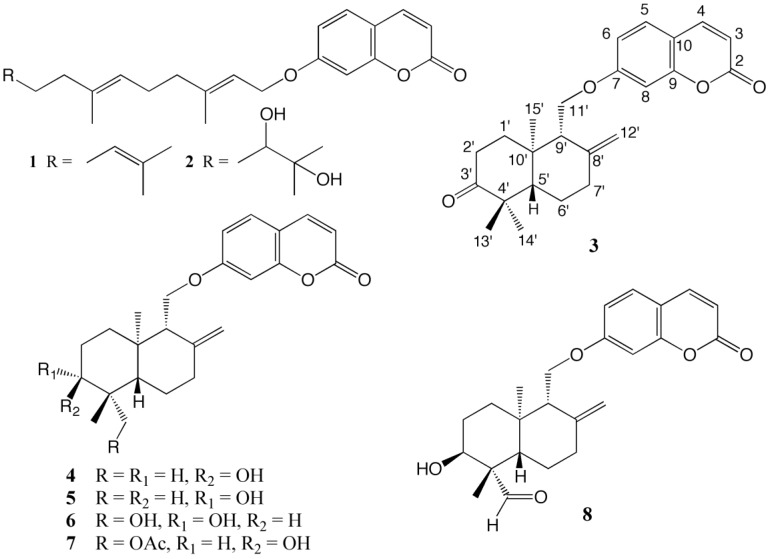
The known sesquiterpene coumarins were identified as umbelliprenin (1) [12], karatavicinol (2) [13], badrakemone (3) [9], badrakemin (4) [14], colladonin (5) [9], 14′-hydroxycolladonin (6) [9], and 14′-acetoxybadrakemin (7) [9] (Figure 1) by their spectral properties as well as direct comparison with the reference compounds where available. Due to their limited accessibility in the literature, high resolution 1H-NMR spectral data of known drimane sesquiterpene coumarins 3–8 are provided in Table 1 and Supplementary Materials.
Figure 1.
Structures of the sesquiterpene coumarins isolated from the dichloromethane extract of the roots of Heptaptera anatolica.
Table 1.
1H-NMR data of compounds 3–7 (400 MHz, δ in ppm, m, J in Hz).
| Position | 3 (in CDCl3) | 4 (in CDCl3) | 5 (in CDCl3) | 6 (in CD3OD) | 7 (in CDCl3) |
|---|---|---|---|---|---|
| 3 | 6.25; d; 9.7; 1H | 6.23; d; 9.5; 1H | 6.24; d; 9.5; 1H | 6.24; d; 9.6; 1H | 6.23; d; 9.6; 1H |
| 4 | 7.63; d; 9.7; 1H | 7.62; d; 9.5; 1H | 7.62; d; 9.5; 1H | 7.88; d; 9.6; 1H | 7.62; d; 9.6; 1H |
| 5 | 7.36; d; 9.2; 1H | 7.34; d; 9.5; 1H | 7.35; d; 8.6; 1H | 7.52; d; 8.6; 1H | 7.34; d; 9.4; 1H |
| 6 | 6.82; dd; 9.2, 2.4; 1H * | 6.82; dd; 9.5, 2.4; 1H * | 6.82; dd; 8.6, 2.4; 1H * | 6.90; dd; 8.6, 2.4; 1H * | 6.82; dd; 9.4, 2.3; 1H * |
| 8 | 6.82; bd; 2.2 1H * | 6.82; bd; 2.1; 1H * | 6.81; bs, 1H * | 6.92; bd, 2.3; 1H * | 6.81; bd; 2.2 1H * |
| 1′α | 2.09; ddd; 3.6, 6.6, 13.2; 1H ** | 1.50; dt; 13.2, 3.3; 1H | 1.45; m; 1H ** | 1.73; m; 1H ** | 1.73; m; 1H ** |
| 1′β | 1.81; dt; 5.5, 13.2; 1H | 1.83; bdt; 3.2, 13.2; 1H | 1.76; m; 1H *** | 1.87; m; 1H *** | 1.84; dt; 2.8, 13.1; 1H |
| 2′α | 2.68; ddd; 6.9, 13.2, 15.1; 1H | 1.67; m; 1H ** | 1.45; m; 1H ** | 1.73; m; 1H ** | 1.73; m; 1H ** |
| 2′β | 2.41; ddd; 3.2, 5.3, 15.1; 1H | 1.67; m; 1H ** | 1.76; m; 1H *** | 1.87; m; 1H ** | 1.73; m; 1H ** |
| 3′ | - | 3.46; bt; 2.6; 1H | 3.30; dd; 4.4, 11.4; 1H | 3.43; bdd; 6.6, 10.0; 1H | 3.79; bt; 2.2; 1H |
| 5′ | 1.67; dd; 2.4, 12.1; 1H | 1.66; dd; 2.4, 12.9; 1H ** | 1.17; dd; 2.7, 12.5; 1H | 1.41; dd; 3.9, 12.6; 1H **** | 1.54; dd; 3.1, 12.3; 1H |
| 6′α | 1.57; m; 1H | 1.42; dq; 4.4, 14.0; 1H | 1.62; m; 1H | 1.41; m; 1H **** | 1.42; dq; 4.3, 13.2; 1H |
| 6′β | 1.73; m; 1H | 1.96; ddt; 2.4, 3.7, 14.0; 1H | 1.76; m; 1H *** | 1.87; m; 1H *** | 1.93; ddt; 2.2, 2.8, 13.2 1H |
| 7′α | 2.53; ddd; 2.3, 4.0, 13.3; 1H | 2.45; ddd; 2.5, 4.2, 13.2; 1H | 2.46; ddd; 2.3, 4.0, 13.2; 1H | 2.46; ddd; 2.3, 3.9, 13.0; 1H | 2.46; ddd; 2.3, 4.1, 13.3; 1H |
| 7′β | 2.14; bdt; 4.8, 13.3; 1H ** | 2.13; bdt; 5.0, 13.2; 1H | 2.09; bdt; 5.1, 13.2; 1H | 2.07; bdt; 5.5, 13.2; 1H | 2.15; bdt; 5.0, 13.3; 1H |
| 9′ | 2.30; bt; 5.4; 1H | 2.33; bdd; 4.2, 7.3; 1H | 2.20; bdd; 4.3, 7.6; 1H | 2.22; bdd; 3.6, 6.9; 1H | 2.34; bdd; 4.5, 6.9; 1H |
| 11′a | 4.24; dd; 6.8, 9.8; 1H *** | 4.23; dd; 4.3, 10.0; 1H | 4.21; dd; 4.3, 9.7; 1H | 4.28; dd; 3.9, 10.3; 1H | 4.22; dd; 4.3, 9.9; 1H |
| 11′b | 4.17; dd; 5.2, 9.8; 1H *** | 4.16; dd; 7.6, 10.0; 1H | 4.17; dd; 7.6, 9.7; 1H | 4.22; dd; 7.7, 10.3; 1H | 4.17; dd; 7.4, 9.9; 1H ** |
| 12′a | 4.97; bs; 1H | 4.90; bd; 1.3; 1H | 4.91; bs; 1H | 4.89; bs; 1H | 4.90; bs; 1H |
| 12′b | 4.60; bs; 1H | 4.53; bd; 1.3; 1H | 4.53; bs; 1H | 4.56; bs; 1H | 4.54; bs; 1H |
| 13′ | 1.12; s; 3H | 0.98; s; 3H | 0.81; s; 3H | 1.23; s; 3H | 1.09; s; 3H |
| 14′a | 1.06; s; 3H | 0.86; s; 3H | 1.02; s; 3H | 4.13; d; 11.1; 1H | 4.18; d; 11.3; 1H ** |
| 14′b | 3.39; d; 11.1; 1H | 3.96; d; 11.3; 1H | |||
| 15′ | 1.04; s; 3H | 0.85; s; 3H | 0.84; s; 3H | 0.85; s; 3H | 0.84; s; 3H |
| OAc | - | - | - | - | 2.04; s; 3H |
*, **, ***, **** Partially overlapped signals.
3. Discussion
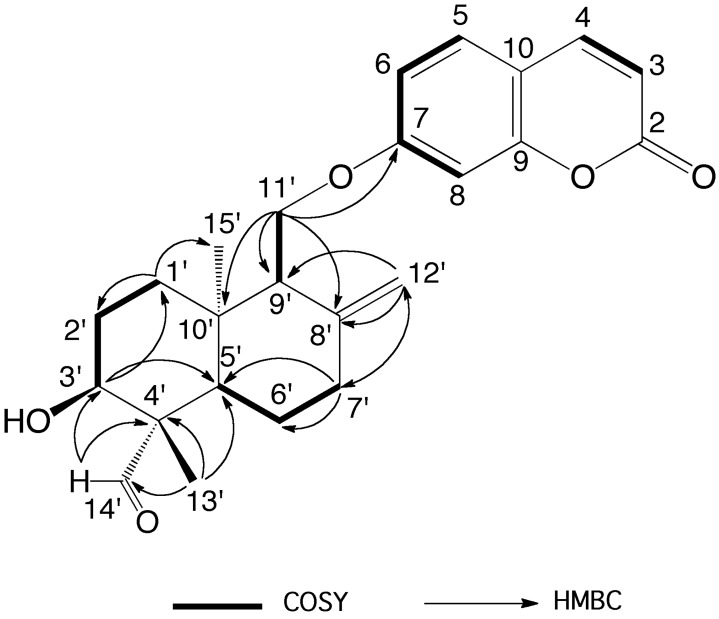
The molecular formula of anatolicin was determined as C24H28O5 by the 13C-NMR data (Table 1) and the observed [M + H] ion at m/z 397.2010 (calcd. for C24H29O5, m/z 397.2010) indicating 11 degrees of unsaturation. The 1H-NMR and 2D NMR spectroscopic data of anatolicin (8) (Table 1, supplementary materials) were similar to that of badrakemin (4) (Table 2) with the exception of a missing methyl signal of the drimane sesquiterpene moiety, the presence of a tertiary aldehyde group signal at δ 9.76 ppm strongly suggesting that it was biogenetically oxidized to an aldehyde group in 8. The strong anisotropic deshielding effect of the aldehyde carbonyl group on the chemical shifts of H-3′ and H-5′ protons (i.e., 0.70 and 0.34 ppm, respectively) in anatolicin (8) in comparison with those observed in badrakemin (4) suggests that one of the methyl groups located on the C-4′ position (i.e., C-13′ or C-14′) of the drimane skeleton must be biogenetically oxidized to an aldehyde group. The 2D HMBC spectra data (Table 1 and Figure 2) also confirm this observation.
Table 2.
1H-NMR (600 MHz), 13C-NMR (150 MHz), and HMBC data of anatolicin (8) (in CDCl3).
| Position | δH (in ppm, m, J in Hz) | δC (in ppm) | HMBC (H -> C) Correlations |
|---|---|---|---|
| 2 | - | 161.41 | |
| 3 | 6.25; d; 9.7; 1H | 113.22 | C-2; C-10 |
| 4 | 7.63; d; 9.7; 1H | 143.32 | C-2; C-5; C-9; C-10 |
| 5 | 7.35; d; 8.3; 1H | 128.90 | C-4; C-7; C-9 |
| 6 | 6.82; dd; 8.3, 2.2; 1H * | 113.19 | C-7; C-8; C-10 |
| 7 | - | 162.21 | |
| 8 | 6.81; bs, 1H * | 101.46 | C-7; C-9; C-10 |
| 9 | - | 156.00 | |
| 10 | - | 112.64 | |
| 1′α | 1.57; dt; 13.2, 3.3; 1H | 31.85 | C-2′; C-15′ |
| 1′β | 1.83; ddd; 3.3, 13.2, 13.2; 1H | ||
| 2′α | 1.73; m; 1H | 26.65 | C-1′ |
| 2′β | 1.92; m; 1H | ||
| 3′ | 4.16; bt; 3.1; 1H ** | 69.10 | C-1′; C-5′ |
| 4′ | - | 52.42 | |
| 5′ | 2.00; dd; 2.8, 13.3; 1H | 48.60 | C-4′; C-6′; C-13′; C-14′; C-15′ |
| 6′α | 1.76; m; 1H | 23.12 | C-5′; C-7′ |
| 6′β | 1.96; m; 1H | ||
| 7′α | 2.53; ddd; 2.6, 4.5, 13.2; 1H | 37.78 | C-5′; C-6′; C-8′; C-9′; C-12′ |
| 7′β | 2.15; ddd; 4.5, 13.2, 13.2; 1H | ||
| 8′ | - | 145.69 | |
| 9′ | 2.36; bdd; 3.5, 7; 1H | 53.58 | C-8′; C-10′; C-11′; C-12′ |
| 10′ | 39.30 | ||
| 11′a | 4.23; dd; 4.0, 9.8; 1H | 65.67 | C-7; C-8′; C-9′; C-10′ |
| 11′b | 4.17; dd; 7.8, 9.8; 1H ** | ||
| 12′a | 4.96; bs; 1H | 108.68 | C-7′; C-8′; C-9′ |
| 12′b | 4.58; bs; 1H | ||
| 13′ | 1.15; s; 3H | 20.13 | C-3′; C-4′; C-5′; C-14′ |
| 14′ | 9.76; s; 1H | 204.49 | C-3′; C-4′ |
| 15′ | 0.74; s; 3H | 14.34 | C-1′; C-5′; C-9′; C-10′ |
*, ** Partially overlapped signals.
Figure 2.
Key COSY and HMBC correlations of anatolicin (8).
Clear interactions observed between the aldehyde proton and C-13′ and H-6α proton (Figure 3) in the 2D NOESY spectrum of anatolicin indicate that the C-14′ axillary methyl group on the C-4′ of the drimane skeleton was biogenetically oxidized to an aldehyde carbonyl, thus, anatolicin is an oxidized C-14′ derivative of badrakemin (4). The C-14′ oxidation pattern observed in 6, 7, and 8 is in agreement with the biogenetic oxidation pattern of the sesquiterpene coumarin ethers isolated from Heptaptera species. Although C-14′ hydroxymethylene derivatives of drimane sesquiterpene coumarin ethers have been reported previously [9,10,11], anatolicin (8) is the first drimane sesquiterpene coumarin ether with an aldehydic functional group.
Figure 3.
NOE interactions observed in the 2D NOESY spectrum of anatolicin (8) [15].
The sesquiterpene coumarin ethers isolated from Heptaptera anatolica were tested against COLO205, KM12, A498, UO31, and TC32 cancer cell lines, and the cytotoxic activities observed with these compounds are shown in Table 3.
Table 3.
Cytotoxic activities of sesquiterpene coumarin ethers isolated from Heptaptera anatolica.
| Compounds | Cytotoxic Activity (IC50 values in μM) | |||||
|---|---|---|---|---|---|---|
| COLO205 | KM12 | A498 | UO31 | A673 | TC32 | |
| 1 | >50 | >50 | >50 | 1.8 | >50 | >50 |
| 2 | >50 | >50 | >50 | 7.6 | >50 | >50 |
| 3 | >50 | >50 | >50 | 11 | >50 | >50 |
| 4 | >50 | 9.1 | 20 | 0.38 | >50 | >50 |
| 5 | 19 | 2.5 | 21 | 0.75 | >50 | 45 |
| 6 | >50 | >50 | >50 | >50 | >50 | >50 |
| 7 | >50 | >50 | >50 | 0.017 | >50 | >50 |
| 8 | >50 | >50 | >50 | 0.024 | >50 | >50 |
While most of the sesquiterpene coumarin ethers were active against the UO31 kidney cancer cell line, 14′-acetoxybadrakemin (7) and anatolicin (8) were the most active compounds with 17 and 24 nM IC50 values, respectively. Colladonin (5) was also active against the UO31 cell line with a 0.75 μM IC50 value and, furthermore, showed similar activity against the KM12 colon cancer cell line, was moderately active against the COLO205 and A498 cell lines, and weakly active against the TC32 cell line. In addition, badrakemin (4) also showed strong activity against the UO31 cell line and moderate activity against the KM12 and A498 cell lines.
4. Materials and Methods
4.1. General Experimental Procedures
Melting points were determined on a Reichert microscope equipped with a Kofler heating stage and were uncorrected (Wien, Austria). Optical rotations were measured using a Perkin-Elmer Model 241 polarimeter in a 100 × 2 mm cell (units 10−1 deg cm2 g−1) in dichloromethane. IR spectra (neat) were recorded on a Perkin-Elmer FT-IR Spectrometer, SPECTRUM 2000 (Waltham, MA, USA). NMR spectra of anatolicin (8) were acquired on a Bruker Avance III spectrometer (Billerica, MA, USA) operating at 600 MHz for 1H and 150 MHz for 13C and equipped with a 3 mm cryogenically cooled probe. 1H-NMR spectrum of compound 1 was acquired on a Bruker Avance III spectrometer (Rheinstetten, Germany) operating at 500 MHz and equipped with a 5 mm indirect observation probe and 1H-NMR spectra of compounds 2–7 were acquired on a Varian (Agilent) Mercury Spectrometer (Palo Alto, CA, USA) operating at 400 MHz and equipped with a 5 mm probe. 1H and 13C spectra were referenced to the residual deuterated solvent peaks. HRESIMS data were acquired on an Agilent 6530 Accurate Mass Q-TOF instrument (Santa Clara, CA, USA) and on a Triple TOF 5600 mass spectrometer (AB SCIEX, Framingham, MA, USA). Initial purification of the dichloromethane extract was carried out on a Sephadex LH-20 (GE Healthcare, Chicago, IL, USA) column. Further purification of column fractions was performed using silica gel F254 PLC plates (1 mm thickness) (Merck KGaA, Darmstadt, Germany).
4.2. Plant Material
The roots of Heptaptera anatolica were collected from the Izmir province in Western Anatolia in June 2013 and identified by Prof. A. Duran. A voucher specimen (A. Duran 9703) was deposited in the Herbarium of Selcuk University, Faculty of Sciences, Department of Biology.
4.3. Extraction and Isolation
Air-dried and coarsely powdered roots (100 g) of H. anatolica were extracted with dichloromethane at room temperature and concentrated, in vacuo, to dryness (5.15 g). The dichloromethane extract (4 g) was separated using a Sephadex LH-20 column (4 × 64 cm) packed in hexane/dichloromethane/methanol (14:9:1) followed by prep. TLC (1 mm thickness, silica gel F254 developed with hexane/ethyl acetate at 9:1, 7:3, or 1:1) for final purification of compounds. The known compounds isolated from the dichloromethane extract were umbelliprenin (1, 30 mg), karatavicinol (2, 18 mg), badrakemone (3, 14 mg), badrakemin (4, 309 mg), colladonin (5, 326 mg), 14′-hydroxycolladonin (6, 13 mg), and 14′-acetoxybadrakemin (7, 345 mg).Anatolicin (8, 38 mg). White needles from hexane/dichloromethane/methanol (14:9:1), m.p. 139–141 °C; −21.8 (c 2.25, CH2Cl2); IR (NaCl) νmax: 3488, 3112, 2932, 2900, 1733, 1709, 1612, 1556, 1509, 1398, 1352, 1282, 1230, 1015, 892, 835 cm−1; UV (MeOH) λmax (log ε): 322 (4.11), 297 (sh) (3.86), 253 (sh) (3.25), 219 (sh) (4.02); 1H and 13C-NMR (see Table); HRESIMS m/z 397.2010 [M + H]+ (calcd. for C24H28O5 [M]+; 396.1937; err. 0.05 ppm).
4.4. Cytotoxic Activity Assay
The primary assay used for this study was a two-day, two-cell-line XTT bioassay [16], an in vitro antitumor colorimetric assay developed by the MTP Assay Development and Screening Section. Renal cancer cell lines used were UO31 and A498. Cells were harvested and plated (45 μL) at a seeding density of 1.5 × 104 cells per well for both the UO31 and A498 cell line into a 384-well “assay plate” and then incubated for 24 h. DMSO solutions of the test materials (8 μL) were diluted 1:25 with medium (192 μL) and then subjected to five 2:1 serial dilutions (100 μL each) on a 96-well plate. Duplicate 40 μL aliquots of each sample concentration were then transferred to a 384-well “dilution plate”, which could accommodate the duplicate samples from two 96-well plates. A 5 μL aliquot of each solution on the dilution plate was transferred to the cell cultures on the 384-well assay plate to give a final volume of 50 μL and a DMSO concentration of 0.4%. Cells were incubated for 48 h at 37 °C in the presence of the test samples and then treated with the tetrazolium salt XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide). Viable cells reduced the XTT to a colored formazan product, and after an additional 4 h incubation period, the amount of formazan produced was quantified by absorption at 450 nm using the absorption at 650 nm as a reference. Sanguinarine was used on each plate as a positive control. Similar methodology and seeding densities were used for COLO205, KM12 (colon), A673, and TC32 (Ewing sarcoma) cell lines.
5. Conclusions
Investigation of the dichloromethane extract of the roots of Heptaptera anatolica yielded seven known and one new sesquiterpene coumarin. Although cytotoxic activity of sequiterpene coumarins were described in the literature earlier [7], high potency and selectivity of C-3′-β-hydroxy and C-14′-acetoxy or C-14′-keto derivatives (i.e., 14′-acetoxybadrakemin (7) and anatolicin (8)) toward UO31 kidney cancer cell line had not been reported previously.
Acknowledgments
We thank A. Duran for the collection and identification of plant material.
Supplementary Materials
The Supplementary Materials are available online. the 1D and 2D NMR data, HRESIMS data of anatolicin (8), as well as the 1H-NMR spectra of compounds 1–7.
Author Contributions
F.T. suggested the idea of the investigations; F.T. and M.M. designed the experiments obtained; F.T. and M.M. purified and characterized all compounds for biological assays; M.M. wrote the paper; F.T., M.M. and J.A.B. contributed to the discussion of results; T.T.R and J.A.B. measured the cytotoxic activity and interpreted the results. All authors read and approved the final manuscript.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (1ZIA BC01146907) and with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
Conflicts of Interest
The authors declare no conflict of interest. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does it mention trade names, commercial products, or organizations implying endorsement by the U.S. Government.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H., Stein K.D., Alteri R., Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 5.Gunther R.T. The Greek Herbal of Dioscorides. Hafner Publishing Co.; New York, NY, USA: 1959. pp. 328–331. [Google Scholar]
- 6.Eisenman S.W., Zaurov D.E., Struwe L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan. Springer; New York, NY, USA: Heidelberg/Berlin, Germany: Dordrecht, The Netherlands: London, UK: 2013. p. 10. [Google Scholar]
- 7.Nazari Z.E., Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 8.Herrnstadt I., Heyn C.C. Studies in Heptaptera (Umbelliferae) II: Taxonomic revision. Notes R. Bot. Gard. Edinb. 1971;31:91–107. [Google Scholar]
- 9.Appendino G., Özen H.C., Tagliapietra S., Cisero M. Coumarins from Heptaptera anisoptera. Phytochemistry. 1992;31:3211–3213. doi: 10.1016/0031-9422(92)83477-G. [DOI] [Google Scholar]
- 10.Appendino G., Özen H.C., Nano G.M., Cisero M. Sesquiterpene coumarin ethers from the genus Heptaptera. Phytochemistry. 1992;31:4223–4226. doi: 10.1016/0031-9422(92)80447-M. [DOI] [Google Scholar]
- 11.Appendino G., Özen H.C., Jakupovic J. A Sesquiterpene coumarin ether and a coniferyl ester from Heptaptera anisoptera. Fitoterapia. 1993;64:505–506. [Google Scholar]
- 12.Roselli S., Maggio A., Bellone G., Formisano C., Basile A., Cicala C., Alfieri A., Mascolo N., Bruno M. Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magydaris tomentosa. Planta Med. 2006;72:116–120. doi: 10.1055/s-2006-951772. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A.A. Sesquiterpene coumarins and sesquiterpenes from Ferula sinaica. Phytochemistry. 1999;50:109–112. doi: 10.1016/S0031-9422(98)00489-0. [DOI] [Google Scholar]
- 14.Eshbakova K.A., Saidkhodzhaev A.I., Vdovin A.D., Abdullaev N.D. Terpenoid coumarins from Ferula feruloides. Chem. Nat. Comp. 2009;45:708–709. doi: 10.1007/s10600-009-9426-7. [DOI] [Google Scholar]
- 15.Wavefunction, Inc.; Irvine, CA, USA: 2014. The tube model of anatolicin (8) was generated using the Spartan’14 Molecular Modeling Software Package ver. 1.2.0. [Google Scholar]
- 16.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J.B., Vistica D., Warren J.T., Bokesch H.R., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.