Abstract
Ataxia-telangiectasia (A-T) is an autosomal recessive chromosome breakage disorder caused by mutations in the ATM serine/threonine kinase (ATM) gene. Typically, it presents in early childhood with progressive cerebellar dysfunction, accompanied by immunodeficiency and oculocutaneous telangiectasia. In the present study, the clinical and genetic findings of a Chinese family affected with A-T in two live siblings, the proband (II-2) and his elder brother (II-1), as well as a fetus (II-3) were reported. General health, clinical neurological, electrophysiological (motor and sensory nerve conduction) and magnetic resonance imaging evaluations revealed that patients II-1 and II-2 had similar symptoms of ataxia, dysarthria, conjunctival hyperemia and elevated serum α-fetoprotein, whereas patient II-1 had earlier A-T onset at 2 years old and more serious problems with movement and intelligence. Targeted sequencing followed by Sanger sequencing revealed that these two patients carried the compound heterozygotes of a novel nonsense mutation c.5170G>T (p.Glu1724Ter) and a known nonsense mutation c.748C>T (p.Arg250Ter) in the ATM gene. Each mutation was inherited from an asymptomatic parent, which therefore confirmed the diagnosis of A-T. Given this, proband's mother performed prenatal diagnosis in her third pregnancy. Unfortunately, the fetus had the same causal mutations as its siblings and the pregnancy was terminated. The findings of the present study expanded the mutation spectrum of the ATM gene and may help in understanding the genetic basis of A-T, in order to guide genetic counseling and prenatal diagnosis.
Keywords: ataxia-telangiectasia, mutation, ATM serine/threonine kinase
Introduction
Ataxia-telangiectasia (A-T; OMIM no. 208900) is a rare neurodegenerative disease inherited in an autosomal recessive manner with great phenotype heterogeneity (1,2). It is characterized by progressive cerebellar dysfunction, oculocutaneous telangiectasias, immunodeficiency and cancer predisposition (1). The estimated incidence in live births is 1 in 40,000 to 100,000 worldwide (2,3). The affected infant typically appears normal in the first 2–3 years, then staggering (ataxia) occurs. The majority of patients are wheelchair bound by 10 years old (4). The duration of disorder is associated with the severity of cerebellar atrophy, but not all the patients with severe cerebellar atrophy are unable to walk (5). The mildest atrophy has been observed in young patients with an average age of 5 years (5). The clinical manifestations of ataxia and oculocutaneous telangiectasia, combined with a series of laboratory tests, are helpful for the diagnosis of A-T (6,7). In most cases, A-T patients have elevated α-fetoprotein and carcinoembryonic antigen expression, as well as abnormal levels of serum-immunoglobulin. Although this disease cannot be cured at present, early diagnosis is important for symptomatic treatment, supportive care, genetic counseling and the avoidance of unnecessary and costly diagnostic tests.
It is well known that mutations in the ATM gene that result in complete inactivation or elimination of the ATM protein will lead to A-T (8,9). ATM protein has 3,050 amino acids and is a member of the phosphoinositide 3-kinase-related protein kinase super family. It serves important roles in regulating cell cycle, DNA alteration and restoration and cell death via phosphorylation of its substrates (10–12). As a redox thiol-sensitive protein kinase, ATM functions by activating multiple redox or phosphorylation sensitive mechanisms. During postnatal development, ATM is responsible for maintaining genomic, telomeric and chromosomal integrity under the conditions of genomic or redox stress (13–15). At present, >900 phosphorylation sites encompassing >700 proteins have been uncovered to be the targets of ATM, and the majority of these targets are associated with the DNA damage regulation (16).
The present study described a Chinese family which had two affected siblings with A-T and urgently required prenatal diagnosis of A-T on the third sibling. The clinical features of the two live patients were described and compared. Targeted sequencing was applied on the proband (the younger live sibling) for aiding A-T diagnosis, which revealed one novel, likely pathogenic, mutation c.5170G>T, as well as one known pathogenic mutation c.748C>T. Further validations were conducted on the remaining family members. The present study suggested that genetic testing is of great importance for aiding clinical and prenatal diagnoses.
Materials and methods
Patients
The present study was approved by the Ethics Committee of Wuhan Children's Hospital (Wuhan, China). Informed written consent was obtained from the parents of the studied family. The proband (II-2; age, 8) and his elder brother (II-1; age, 13) from a family with Han ethnicity in southern China were introduced to our clinic center due to signs of development retrogression. Based on clinical diagnostic criteria, they were initially diagnosed as A-T in our hospital. When the mother was pregnant again, she visited the clinic center for prenatal diagnosis.
Physical examination
Routine examination of general health as well as neurological evaluations were performed on two patients. Blood lymphocyte subsets (TBNK) was analyzed by flow cytometry (BD FACSCanto™ II system). α-fetoprotein was evaluated by electrochemiluminescence with the commercial kit (Roche Diagnostics GmbH, Mannheim, Germany). Serum IgG, IgA, IgM, C3, and C4 were determined by rate nephelometry. Sensory function was assessed with the measure of vibrotactile perception. Motor coordination was evaluated by finger-to-nose test and rapid alternating movement test. Reflex tests were conducted on knee, ankle and other joints. Muscular weakness was evaluated with common grading criteria (17).
Electrophysiological assessments
The motor and sensory nerve conduction assessments were performed by standard methods on the Natus Dantec™ Keypoint® G4 platform. The patients laid in a quiet, shielded room with room temperature of 20–22°C and limb temperature of 32–34°C. Surface electrodes were used for stimulation and recording. Motor conduction velocity (MCV), distal motor latency (DML) and compound muscle action potential (CMAP) were measured by stimulating the nerve segments of the ankle to the fibulae capitulum for the peroneal nerve, ankle to popliteal fossa for the tibial nerve, wrist to elbow for the median nerve, and wrist to elbow for the ulnar nerve, and recording from the extensor digitorum brevis, abductor hallucis, abductor pollicis brevis, and abductor digiti minim respectively. Sensory nerve conduction velocity (SCV), amplitude (Amp) and sensory nerve action potential (SNAP) were investigated through stimulating posterior leg (the place with 10 cm apart from the recording electrode) for the sural nerve, the median nerve and the ulnar nerve of the wrist, and then antidromic recording at lower part of ankle for the sural nerve, second digit for the median nerve and fifth digit for the ulnar nerve. Normal values of electromyography were defined as the normal values used in the Johns Hopkins Hospital in the United States adjusted for the age under the guidance from Cornblath (18), i.e. parameters of nerve conduction velocity are similar between adult and children older than 3 years old.
Standard intensity and duration of stimulation were applied firstly. For the motor nerve conduction stimulation, the intensity was 20–40 mA and the duration was 0.1 ms. If three consecutive stimulations leaded to stable waves with no more than 10% amplitude fluctuation, then the middle value of CMAP was recorded and used for calculating MCV. For the sensory nerve conduction stimulation, the intensity was 20–30 mA and the duration was 0.1 ms. The SNAP was generated by the equipment with the method of successive averages and recorded when there was no more than 10% amplitude fluctuation in the wave with stable shape. Then SNAP as well as the distance between stimulation and recording electrodes were used for calculating SCV.
Providing examinations failed with the aforementioned parameters, higher intensities and longer durations of stimulation were adopted, that is, intensities of 60–80 mA and a duration of 0.5 ms for the motor nerve conduction stimulation, and intensities of 20–40 mA and a duration of 0.5 ms for the sensory nerve conduction stimulation. If the SNAP wave fluctuated with >10% amplitude and unstable shape when the intensity increased to 40 mA and duration extended to 1.0 ms, then this examination was recorded as ‘-’.
Magnetic resonance imaging (MRI) material
MRI was performed with a GE Signa Excite 1.5T HD Echospeed platform according to the manufacture's manual. Analyzed sequences included T1WI FSE [fast spin echo; repetition time (TR) = 500–600 ms, echo time (TE) = 8–12 ms), T2WI FSE (TR = 3,000–4,000 ms, TE = 90–110 ms), T2 FLAIR (fluid-attenuated inversion recovery; TR = 8,000–9,000 ms, TE = 100–120 ms)] acquired in the axial, sagittal and coronal planes respectively. The parameters used in the DWI (diffusion weighted imaging) were as follows: TR = 5000 ms, TE = 82 ms, slice thickness = 6 mm, slice gap = 1 mm, field of view (FOV) = 24×24–36×36 cm, matrix = 256×256, and number of excitations = 2–4. The scanning results were confirmed by a board-certified neuroradiologist.
Genetic analysis
Targeted sequencing of genes associated with hereditary ataxias, including KCNA1, CACNA1A, CACNB4, SLC1A3, SACS, ABCB7, ATM, APTX and TTPA, was conducted on the proband (II-2), as described previously (19). Sanger sequencing of the identified pathogenic mutations was conducted on the parents, brother and fetus (II-3). Sanger sequencing for the fetus was performed at the Wuhan Children's Hospital. Remaining genetic testing and validation procedures were carried out in BGI Genomics (Shenzhen, China).
Variant interpretation adhered to the Standards and Guidelines for the interpretation of sequence variants: A Joint Consensus Recommendation of the American College of Medical Genetics And Genomics (ACMG) and the Association for Molecular Pathology (AMP), 2015 (20). According to dbSNP database (www.ncbi.nlm.nih.gov/SNP/), HapMap database (ftp.ncbi.nlm.nih.gov/hapmap/), HGMD (www.hgmd.cf.ac.uk/), 1000 genomes project database (www.1000genomes.org/), Exome Sequencing Project 6500 (evs.gs.washington.edu/EVS/), the Exome Aggregation Consortium (exac.broadinstitute.org/), local SNP databases of 100 normal Chinese (in-house) and available literature, the frequency and novelty of the variants were consequently determined (21). PolyPhen-2 (22) and SIFT programs (23) were used to evaluate the potential deleterious effect.
Results
The two live patients were from a Han family with unaffected parents. The 8-year-old proband (patient II-2) was born at full term without suffocation. At 1 year of age, he was observed to have normal development and intelligence, but weak limbs and poor memory. Typical symptoms of ataxia were noticed at 5 years old when he presented with slurred speech and evident regression of movement coordination, including unstable walking, trembling hands and clumsy action, as well as positive results in the finger-to-nose and rapid alternating movement tests. Conjunctival hyperemia was found in both eyes and hair was dry and dull. Proprioceptive sensibility was normal, but vibration sense was absent. The knee reflex was normal; however, the ankle reflex was not elicited. Muscle tensions of four limbs were normal, and muscle strength was graded as level V. Some abnormal results were found in the electromyography (EMG) examination (Tables I and II): i) The amplitude (AMP) of peroneal nerves and ulnar nerves was decreased on both sides; ii) the ulnar nerves were not elicited; and iii) the values of sensory conduction velocity (SCV) and AMP of median nerves on both sides were smaller than the normal limits. In addition, brain MRI examinations showed that the proband had enlarged cerebellar sulci (Fig. 1). According to descriptions from the parents, the proband was not susceptible to infectious diseases. However, significantly elevated serum α-fetoprotein (AFP; 170 IU/ml; normal range 0–3.07 IU/ml) (Table III) and slightly decreased CD4+/CD8+ T lymphocyte ratio (Table IV) was detected in the blood test, implying hepatic dysplasia and immunodeficiency in the patient. Other indicators in the blood test were normal or slightly decreased (Tables III and IV).
Table I.
Electromyography results of the common peroneal, tibial and sural nerves for the two patients.
| Left common peroneal nerve | Right common peroneal nerve | Left tibial nerve | Right tibial nerve | Left sural nerve | Right sural nerve | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | MCV (m/s) | AMP (mv) | MCV (m/s) | AMP (mv) | MCV (m/s) | AMP (mv) | MCV (m/s) | AMP (mv) | SCV (m/s) | AMP (mv) | SCV (m/s) | AMP (mv) |
| II:1 | 44.2 (n) | 1.2 (l) | 44.4 (n) | 1.9 (l) | 43.1 (n) | 8.4 (l) | 41.9 (nl) | 6.6 (l) | – | – | – | – |
| II:2 | 48.4 (n) | 1.2 (l) | 48.7 (n) | 0.8 (l) | 47.6 (n) | 21.5 (n) | 49.2 (nl) | 19.2 (n) | 67.6 (n) | 13.3 (n) | 54.6 (n) | 8.4 (n) |
Dashes indicate that stimulation did not lead to sensory nerve action potential. n, normal; l, low; nl, normal low limit.
Table II.
Electromyography results of the median and ulnar nerves for the two patients.
| Left median nerve | Right median nerve | Left ulnar nerve | Right ulnar nerve | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | MCV (m/s) | AMP (ms) | SCV (m/s) | AMP (µv) | MCV (m/s) | AMP (ms) | SCV (m/s) | AMP (µv) | MCV (m/s) | AMP (mv) | SCV (m/s) | MCV (m/s) | AMP (mv) | SCV (m/s) |
| II:1 | 58.5 (n) | 2.8 (n) | 47.9 (n) | 11 (l) | 59.5 (n) | 3.8 (n) | 43 (n) | 9.7 (l) | 56.8 (n) | 7.1 (n) | 35.5 (l) | 59.3 (nl) | 4.6 (l) | 40.7 (l) |
| II:2 | 50.4 (n) | 5.7 (n) | 42.7 (l) | 15 (l) | 52.6 (n) | 8.8 (n) | 30.3 (l) | 5.7 (l) | 48.5 (n) | 5.3 (l) | – | 45.6 (nl) | 4.8 (l) | – |
Dashes indicate that stimulation did not lead to sensory nerve action potential. n, normal; l, low; nl, normal low limit.
Figure 1.
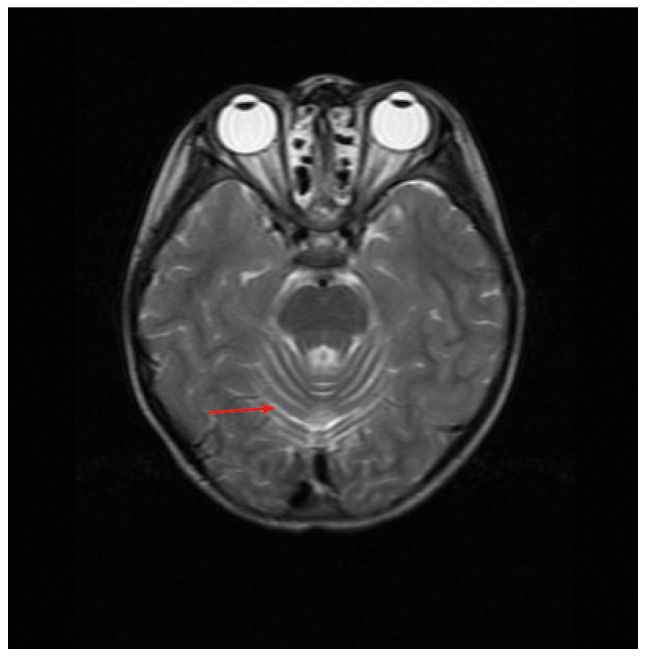
T2-weighted magnetic resonance imaging of the proband. Arrow indicates enlarged cerebellar sulci.
Table III.
Major clinical and laboratory features of two patients.
| Immunoglobulins | Complement | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age (years, months) | Age of ataxia onset (months) | Age of telangiectasia onset (years) | Cerebellar atrophy | α-fetoprotein (IU/ml)a | IgG (g/l) | IgA (g/l) | IgM (g/l) | C3c (g/l) | C4 (g/l) |
| II-1 | M | 13. 5 | 2 | Unknown | Yes | 234.8a | 9.68 | 1.74 | 2.39 | 0.76a | 0.15 |
| II-2 | M | 8. 1 | 1 | Unknown | Yes | 170a | 6.78 | – | – | – | – |
Normal blood levels are as follows: α-fetoprotein, 0–3.07 IU/ml; IgG, 7–16.5 g/l; IgA, 0.59–3.9 g/l; IgM, 0.56–3.45 g/l; C3c, 0.8–1.26 g/l; C4, 0.1–0.4 g/l.
abnormal result.
Table IV.
Blood lymphocyte subsets (TBNK) detected result of two patients.
| Patient | CD3+ TLs (%) | CD3+ TL count (per µl) | CD8+ TLs (%) | CD8+ TL count (per µl) | CD4+ TLs (%) | CD4+ TL count (per µl) | NK cells (%) | NK cell count (per µl) | CD19+ BLs (%) | CD19+ BL count (per µl) | CD4+/CD8+ TL ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| II-1 | 76.32a | 1,183 | 56.15a | 883 | 16.19 | 255a | 11.55 | 176a | 10.83a | 165a | 0.29a |
| II-2 | 63.72 | 577a | 32.66 | 297 | 24.2 | 220a | 24.44 | 220 | 8.94a | 81a | 0.74a |
Normal blood levels are as follows: CD3+ TLs, 38.56–70.06%; CD3+ TL count, 805–4,459/µl; CD8+ TLs, 13.24–38.53%; CD8+ TL count, 314–2,080/µl; CD4+ TLs, 14.21–36.99%; CD4+ TL count, 345–2,350/µl; NK cells, 7.92–33.99%; NK cell count, 210–1,514 µl; CD19+ BLs, 10.86–28.03%; CD19+ BL count, 240–1,317/µl; CD4+/CD8+ TL ratio, 0.96–2.05. TL, T lymphocyte; BL, B lymphocyte; NK, natural killer.
Abnormal result.
The proband's 13-year-old brother (patient II-1) had all typical symptoms of ataxia, as the proband did, as well as some additional clinical features. Patient II-1 presented earlier regression of movement coordination at 2 years old. The symptoms gradually progressed and as a result, he could not walk at 8 years old. Brain MRI showed cerebellar atrophy (data not shown), and intellectual retrogression was confirmed. Both eyes had conjunctival hyperemia and difficulties in seeing objects on the left, suggesting oculomotor apraxia. The head and neck had abnormally slow movement. Muscle strength of the upper and lower limbs were grade IV and III respectively. EMG results (Tables I and II) were similar in patients II-1 and II-2, but patient II-1's sural nerve, rather than ulnar nerve, was not elicited on both sides. Furthermore, AMPs of the tibial nerve were decreased on both sides. As shown in Tables II and III, serum AFP (234.8 IU/ml; normal range 0–3.07 IU/ml) was significantly increased and CD4+/CD8+ T lymphocyte ratio (0.29; normal range 0.96–2.05) was significantly decreased, implying severe immunodeficiency.
Targeted sequencing was performed on the proband. The generated data had a mean depth of 285.1-fold and a coverage of 99.71% across the targeted regions (Table V). In total, four non-synonymous and 10 synonymous variants were identified in nine genes associated with hereditary ataxias. Once filtered, two nonsense mutations c.748C>T (p.Arg250Ter) and c.5170G>T (p.Glu1724Ter) in the ATM gene were deemed to be pathogenic and likely pathogenic, respectively, according to the guidelines of ACMG/AMP (20). Both mutations were absent in 1000 Genomes Project database, Exome Sequencing Project 6500, The Exome Aggregation Consortium and local SNP database of normal Chinese. The mutation c.748C>T occurring in exon 7 converted arginine to stop codon at amino acid position 250, which has previously been reported as pathogenic (24–26). The c.5170G>T mutation located on exon 34 changed glutamic acid to stop codon at amino acid position 1,724. It is worth noting that no pathogenicity association between this mutation and A-T has been identified in the previous literature. Further Sanger sequencing was performed on the extensive family members to verify whether the two mutations c.748C>T and c.5170G>T segregated with the disease. It was found that all three siblings (patient II-1, II-2 and II-3) carried the same compound heterozygous mutations of c.748C>T and c.5170G>T, which were inherited from their father and mother, respectively (Fig. 2). These results confirmed A-T in all siblings and the parents decided to terminate the pregnancy (patient II-3).
Table V.
Bioinformatics quality control matrices of the proband's targeted next generation sequencing data.
| Measure | Result |
|---|---|
| Number of genes | 9 |
| Length of target region (bp) | 65,439 |
| Coverage of target region (%) | 99.71 |
| Average depth of target region (-fold) | 285.1 |
| Proportion of target region with sequencing depth of >30-fold (%) | 97.50 |
Figure 2.
Segregation analysis of the two mutations in ATM. (A) Pedigree. Sanger sequencing verified the heterozygous ATM mutations. Arrow indicates the proband. (B) c.5170G>T (p.Glu1724Ter) in all the siblings and their mother, and (C) c.748C>T (p.Arg250Ter) in all the siblings and their father.
Discussion
Ataxia-telangiectasia, characterized by progressive difficulty with coordinating movement, is a rare inherited disorder that affects the nervous and immune system, as well as other processes (1). A series of clinical criteria for A-T diagnosis have been identified (7), but there are still limitations to prenatal diagnosis, and cases with variable phenotypes or late onset. By conducting targeted sequencing and Sanger sequencing on an A-T family with variable clinical signs, novel nonsense ‘likely pathogenic’ and known nonsense ‘pathogenic’ mutations were found in the ATM gene, which confirmed the A-T in two patients and then aided the prenatal diagnosis of A-T in the third child of this family. Therefore, genetic testing is of crucial importance for confirming A-T, particularly in the initial phases of the disease.
The clinical features of patients II-1 and II-2 were similar with those of the A-T patients in previous reports, such as a combination of progressive cerebellar ataxia, dysarthria, conjunctival hyperemia and elevated serum AFP levels (27–29). However, it was found that the two patients distinguished themselves with onset, severity and development of the disease. For example, symptoms of A-T had presented since 2 years old in patient II-1, but at 5 years old in patient II-2; at 8 years old, patient II-1 was not able to walk, whereas patient II-2 could walk slowly; further, patient II-1 had obvious intellectual retrogression, while patient II-2 had normal intelligence. According to the clinical examinations, it was speculated that the more severe symptoms in patient II-1 might be explained by the following findings: i) Patient II-1's sural nerve, rather than ulnar nerve, was not elicited in the EMG, therefore disrupting his walking ability; ii) while patient II-2 had wide cerebellar sulci, the initial stage of cerebellar atrophy, patient II-1 had cerebellar atrophy, thus causing more critical consequences; and iii) the indexes of serum AFP and CD4+/CD8+ T lymphocyte ratio in patient II-1 were more severely shifted away from the normal ranges, indicating a more serious immunodeficiency. Taken together, these findings demonstrated that the phenotypes of A-T were quite heterogeneous, especially in the initial phases of the disease, which presents difficulties in making accurate clinical diagnoses.
The majority of ATM mutations causing A-T are nonsense and frameshift mutations, resulting in truncation of ATM protein (30–33). Consistent with these previously findings, the present study detected two nonsense mutations. The mutation c.748C>T has been reported to be pathogenic in A-T patients (24–26). To the best of knowledge, this is the first paper to identify c.5170G>T to be associated with A-T pathogenicity. This mutation produces a truncated premature protein at amino acid position 1724, which is conserved in multiple species, including rhesus, mouse, dog, elephant, wild yak, and bonobo.
In conclusion, one novel mutation and one known disease-inducing mutation of A-T was identified. The present study not only expanded the mutation spectrum of ATM-associated A-T, but also contributed valuable guidance on the genetic diagnosis and the prenatal screening of A-T.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current study are available in the CNGB Nucleotide Sequence Archive (CNSA, http://db.cngb.org./cnsa) with accession number CNP0000265.
Authors' contributions
AZ and XT conceived the study. JC and BM analyzed the patients and collected clinical data. JC, RS, WZ, BM, QS and RZ performed data analyses and prepared the manuscript. ZL, BZ, XC, CZ, ML, PH and JW conducted the genetic experiments. All authors reviewed and approved the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Children's Hospital of Wuhan (Wuhan, China). The parents of the patients provided written informed consent.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gilad S, Chessa L, Khosravi R, Russell P, Galanty Y, Piane M, Gatti RA, Jorgensen TJ, Shiloh Y, Bar-Shira A. Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am J Hum Genet. 1998;62:551–561. doi: 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoenaker MH, Suarez F, Szczepanski T, Mahlaoui N, Loeffen JL. Treatment of acute leukemia in children with ataxia telangiectasia (A-T) Eur J Med Genet. 2016;59:641–646. doi: 10.1016/j.ejmg.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Ataxia telangiectasia, corp-author. https://www.cancer.gov/about-cancer/causes-prevention/genetics/ataxia-fact-sheet. [Dec 18;2018 ];Fact sheet.
- 4.Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst) 2004;3:1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Tavani F, Zimmerman RA, Berry GT, Sullivan K, Gatti R, Bingham P. Ataxia-telangiectasia: The pattern of cerebellar atrophy on MRI. Neuroradiology. 2003;45:315–319. doi: 10.1007/s00234-003-0945-9. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann TA, McIntire KR. Serum-alpha-fetoprotein levels in patients with ataxia-telangiectasia. Lancet. 1972;2:1112–1115. doi: 10.1016/S0140-6736(72)92717-1. [DOI] [PubMed] [Google Scholar]
- 7.Jason JM, Gelfand EW. Diagnostic considerations in ataxia-telangiectasia. Arch Dis Child. 1979;54:682–686. doi: 10.1136/adc.54.9.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatti RA, Berkel I, Boder E, Braedt G, Charmley P, Concannon P, Ersoy F, Foroud T, Jaspers NG, Lange K, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22-23. Nature. 1988;336:577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- 9.Chun HH, Sun X, Nahas SA, Teraoka S, Lai CH, Concannon P, Gatti RA. Improved diagnostic testing for ataxia-telangiectasia by immunoblotting of nuclear lysates for ATM protein expression. Mol Genet Metab. 2003;80:437–443. doi: 10.1016/j.ymgme.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Gao P, Lv X, Zhang L, Zhang J. The role of the ataxia telangiectasia mutated gene in lung cancer: Recent advances in research. Ther Adv Respir Dis. 2017;11:375–380. doi: 10.1177/1753465817725716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodarzi AA, Jonnalagadda JC, Douglas P, Young D, Ye R, Moorhead GB, Lees-Miller SP, Khanna KK. Autophosphorylation of ataxia-telangiectasia mutated is regulated by protein phosphatase 2A. EMBO J. 2014;23:4451–4461. doi: 10.1038/sj.emboj.7600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlov SV, Graham ME, Jakob B, Tobias F, Kijas AW, Tanuji M, Chen P, Robinson PJ, Taucher-Scholz G, Suzuki K, et al. Autophosphorylation and ATM activation: Additional sites add to the complexity. J Biol Chem. 2011;286:9107–9119. doi: 10.1074/jbc.M110.204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlow C, Dennery PA, Shigenaga MK, Smith MA, Morrow JD, Roberts LJ, II, Wynshaw-Boris A, Levine RL. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci USA. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan M, Qiang W, Liu N, Shen J, Lynn WS, Wong PK. The ataxia-telangiectasia gene product may modulate DNA turnover and control cell fate by regulating cellular redox in lymphocytes. FASEB J. 2001;15:1132–1138. doi: 10.1096/fj.00-0601com. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Zhu C, Liu N, Jiang Y, Scofield VL, Riggs PK, Qiang W, Lynn WS, Wong PK. ATM controls c-Myc and DNA synthesis during postnatal thymocyte development through regulation of redox state. Free Radic Biol Med. 2006;41:640–648. doi: 10.1016/j.freeradbiomed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 17.Medical Research Council, corp-author. Memorandum No. Vol. 45. Medical Research Council; London: 1976. Aids to examination of the peripheral nervous system. [Google Scholar]
- 18.Cornblath D. Electrophysiology in Guillain-Barre syndrome. Ann Neurol. 1990;27(Suppl):S17–S20. doi: 10.1002/ana.410270706. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Liao Y, Xu M, Ji Z, Xu Y, Zhou L, Wei X, Hu P, Han P, Yang F, et al. A novel mutation R190H in the AT-hook 1 domain of MeCP2 identified in an atypical Rett syndrome. Oncotarget. 2017;8:82156–82164. doi: 10.18632/oncotarget.18955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, Ju X, Yi X, Zhu Q, Qu N, Liu T, Chen Y, Jiang H, Yang G, Zhen R, et al. Identification of sequence variants in genetic disease-causing genes using targeted next-generation sequencing. PLoS One. 2013;6:e29500. doi: 10.1371/journal.pone.0029500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 24.Moulton J. Functional characterization and targeted correction of ATM mutations identified in Japanese patients with ataxia-telangiectasia. Hum Mutat. 2015;33:198–208. doi: 10.1002/humu.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teraoka SN, Telatar M, Becker-Catania S, Liang T, Onengüt S, Tolun A, Chessa L, Sanal O, Bernatowska E, Gatti RA, Concannon P. Splicing defects in the ataxia-telangiectasia gene, ATM: Underlying mutations and consequences. Am J Hum Genet. 1999;64:1617–1631. doi: 10.1086/302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzin CH, Gatti RA, Nguyen VQ, Wen CY, Mitui M, Sanal O, Chen JS, Nozari G, Mengos A, Li X, Fujimura F, Sommer SS. Comprehensive scanning of the ATM gene with DOVAM-S. Hum Mutat. 2010;21:123–131. doi: 10.1002/humu.10158. [DOI] [PubMed] [Google Scholar]
- 27.Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Ann Rev Immunol. 2003;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AM, Byrd PJ, McConville CM, Thacker S. Genetic and cellular features of ataxia telangiectasia. Int J Radiat Biol. 1994;65:65–70. doi: 10.1080/09553009414550091. [DOI] [PubMed] [Google Scholar]
- 29.Kraus M, Lev A, Simon AJ, Levran I, Nissenkorn A, Levi YB, Berkun Y, Efrati O, Amariglio N, Rechavi G, Somech R. Disturbed B and T cell homeostasis and neogenesis in patients with ataxia telangiectasia. J Clin Immunol. 2014;34:561–572. doi: 10.1007/s10875-014-0044-1. [DOI] [PubMed] [Google Scholar]
- 30.Wright J, Teraoka S, Onengut S, Tolun A, Gatti RA, Ochs HD, Concannon P. A high frequency of distinct ATM gene mutations in ataxia-telangiectasia. Am J Hum Genet. 1996;59:839–846. [PMC free article] [PubMed] [Google Scholar]
- 31.Concannon P, Gatti RA. Diversity of ATM gene mutations detected in patients with ataxia-telangiectasia. Hum Mutat. 1997;10:100–107. doi: 10.1002/(SICI)1098-1004(1997)10:2<100::AID-HUMU2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Li A, Swift M. Mutations at the ataxia-telangiectasia locus and clinical phenotypes of A-T patients. Am J Med Genet. 2010;92:170–177. doi: 10.1002/(SICI)1096-8628(20000529)92:3<170::AID-AJMG3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Jacquemin V, Rieunier G, Jacob S, Bellanger D, d'Enghien CD, Laugé A, Stoppa-Lyonnet D, Stern MH. Underexpression and abnormal localization of ATM products in ataxia telangiectasia patients bearing ATM missense mutations. Eur J Hum Genet. 2012;20:305–312. doi: 10.1038/ejhg.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available in the CNGB Nucleotide Sequence Archive (CNSA, http://db.cngb.org./cnsa) with accession number CNP0000265.



