Abstract
Effective delivery of oxygen and essential nutrients to vital organs and tissues throughout the body requires adequate blood flow supplied through resistance vessels. The intimate relationship between intracellular calcium ([Ca2+]i) and regulation of membrane potential (Vm) is indispensable for maintaining blood flow regulation. In particular, Ca2+-activated K+ (KCa) channels were ascertained as transducers of elevated [Ca2+]i signals into hyperpolarization of Vm as a pathway for decreasing vascular resistance, thereby enhancing blood flow. Recent evidence also supports the reverse role for KCa channels, in which they facilitate Ca2+ influx into the cell interior through open non-selective cation (e.g., transient receptor potential; TRP) channels in accord with robust electrical (hyperpolarization) and concentration (~20,000-fold) transmembrane gradients for Ca2+. Such an arrangement supports a feed-forward activation of Vm hyperpolarization while potentially boosting production of nitric oxide. Furthermore, in vascular types expressing TRP channels but deficient in functional KCa channels (e.g., collecting lymphatic endothelium), there are profound alterations such as downstream depolarizing ionic fluxes and the absence of dynamic hyperpolarizing events. Altogether, this review is a refined set of evidence-based perspectives focused on the role of the endothelial KCa and TRP channels throughout multiple experimental animal models and vascular types. We discuss the diverse interactions among KCa and TRP channels to integrate Ca2+, oxidative, and electrical signaling in the context of cardiovascular physiology and pathology. Building from a foundation of cellular biophysical data throughout a wide and diverse compilation of significant discoveries, a translational narrative is provided for readers toward the treatment and prevention of chronic, age-related cardiovascular disease.
Keywords: Ca2+-activated K+ channels, transient receptor potential channels, intracellular Ca2+ homeostasis, endothelium-derived hyperpolarization, cardiovascular disease, aging
1. Introduction
Endothelial cells lining the lumen of resistance arteries command changes in vascular diameter as needed to meet the metabolic demand of vital organs and tissues throughout the body. In particular, the relationship of intracellular calcium ([Ca2+]i) to the hyperpolarization of membrane potential (Vm) is essential for a key signaling pathway underlying blood flow control, known as endothelium-derived hyperpolarization (EDH; i.e., activation of small- and intermediate-Ca2+-activated K+ (SKCa/IKCa) channels). In such a manner, endothelial cells coordinate with their surrounding smooth muscle cells via gap junctions for arterial relaxation and increased blood flow [1]. Physiological stimulation of EDH entails stimulation of Gq-protein-coupled receptors (GqPCRs) and then an increase in [Ca2+]i typically defined by initial Ca2+ release from the endoplasmic reticulum (ER) followed by a “plateau” phase of extracellular Ca2+ influx into the cellular interior through open transient receptor potential (TRP) channels [2,3]. Vascular TRP channels may be recruited by Orai for Ca2+ influx in response to cytosolic stromal interaction molecule (STIM) oligomers that form as a result of ER Ca2+ depletion [4,5,6,7]. The influx of Ca2+ contributes serves a dual role to (i) sustain activation of intracellular enzymes and plasma membrane ion channels for prolonged cellular function, and (ii) to refill ER Ca2+ (via smooth endoplasmic reticulum Ca2+ ATPase (SERCA) pump activity) to maintain consistent and repetitive physiological function. During elevation of overall [Ca2+]i, SKCa/IKCa channels are activated, and the cell interior local to the inner leaflet of the plasma membrane increases in negative charge (“hyperpolarization”) due to K+ efflux through open SKCa/IKCa channel pores [8,9]. Thus, the intimate relationship of endothelial [Ca2+]i and Vm is integral to blood flow regulation and, therefore, indispensable for cardiovascular function.
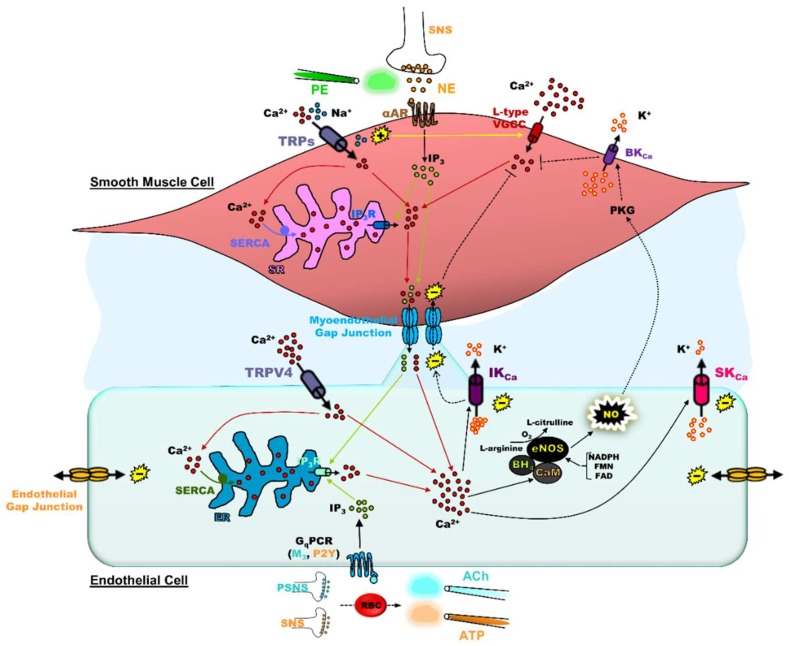
The aim of this review is to resolve the interaction of KCa and TRP channel function with [Ca2+]i and electrical signaling during physiology and pathology. The current working paradigm of the “microanatomy” of the [Ca2+]i-to-electrical signaling interface underlying EDH is located to endothelial projections that traverse through the internal elastic lamina to contact smooth muscle cells (Figure 1). Endothelial projections contain spatially proximal myoendothelial gap junctions, IKCa channels, TRP channels, and inositol 1,4,5-trisphosphate receptors (IP3Rs) of the endoplasmic reticulum [10,11,12]. When KCa channel function is present, robust Vm hyperpolarization occurs in response to sequential [Ca2+]i release and Ca2+ influx, notably during treatment with a GqPCR agonist (e.g., acetylcholine (ACh) or adenosine triphosphate (ATP)) or hydrogen peroxide (H2O2), which mimics conditions of oxidative stress during old age. When KCa channel function is absent, an otherwise rapid Vm hyperpolarization response converts into slow Vm depolarization due to accumulation of intracellular positive charges in the form of Na+ and Ca2+ having entered through activated non-selective cation channels. With perspectives for future directions, we examine recent evidence demonstrating the functional contrasts among “normal” or “healthy” vascular aging and the development of chronic disease (e.g., hypertension, diabetes, heart failure, and coronary artery disease).
Figure 1.
Anatomy of endothelium-derived hyperpolarization and myoendothelial coupling. Endothelium: Physiological stimulation of endothelial Gq-protein-coupled receptors (GqPCRs; muscarinic (M3), purinergic (P2Y)) occurs through neurotransmitter secretion (parasympathetic nervous system (PSNS), acetylcholine (ACh); sympathetic nervous system (SNS), adenosine triphosphate (ATP)) or ATP release from red blood cells (RBCs) as primary examples. ACh and ATP are pharmacologically applied using bulk flow or focal delivery via pipettes (iontophoresis and pressure ejection). Following endothelial GqPCR activation, inositol 1,4,5-trisphosphate (IP3) is produced, which in turn activates IP3 receptors (IP3Rs) to release Ca2+ from the endoplasmic reticulum (ER) into the cytosol. ER Ca2+ release is observed as the initial “peak” response in ∆[Ca2+]i. The ER Ca2+ stores are filled or refilled through uptake of Ca2+ from the cytosol into the ER through smooth endoplasmic reticulum Ca2+ ATPase (SERCA) pumps to sustain repetitive physiological signaling. The influx of Ca2+ occurs through TRP channels (e.g., vanilloid class, TRPV4) to help refill ER Ca2+ stores while integral to the secondary “plateau” phase of the ∆[Ca2+]i following GqPCR stimulation. The increase in [Ca2+]i leads to production of nitric oxide (NO) and/or activation of small- and intermediate-conductance Ca2+-activated K+ (SKCa and IKCa) channels. The production of NO is dependent on the conversion of l-arginine to l-citrulline in the presence of O2 via endothelial NO synthase (eNOS). Endothelial NOS contains an oxygenase domain to bind l-arginine, heme, Zn2+, and an essential cofactor tetrahydrobiopterin (BH4); a calmodulin (CaM) domain to bind Ca2+; and a reductase domain that binds to reducing agents nicotinamide adenine dinucleotide phosphate (NADPH), flavin mononucleotide (FMN), and flavin adenine dinucleotide (FAD). Activation of SKCa and IKCa channels generates hyperpolarization that is transmitted concomitantly along endothelial cells and to smooth muscle cells through myoendothelial gap junctions. Note that, regardless of endothelial Ca2+ mobilizing mechanism, endothelial [Ca2+]i increases can regulate vascular tone ranging from normalization of vasoconstriction for basal tone (steady-state blood flow) to net decreases in vascular resistance (increased blood flow). Smooth muscle: Endothelium-derived hyperpolarization deactivates L-type voltage-gated Ca2+ channels (VGCCs) to prevent Ca2+ entry into smooth muscle cells (see broken lines with flat ends). Additionally, endothelial production of NO diffuses to smooth muscle and increases the activity of K+ channels such as the large-conductance Ca2+-activated K+ (BKCa) channel via cGMP-dependent protein kinase (PKG) for hyperpolarization, another signaling input for deactivation of L-type VGCCs. Although not covered in detail in this review, TRP channels (TRPs; e.g., TRPM4) are also expressed in smooth muscle cells and play a general role for depolarization of Vm, thereby activating L-type VGCCs for myogenic constriction. Note that, regardless of smooth muscle Ca2+ mobilizing mechanism, smooth muscle [Ca2+]i increases can regulate vascular tone ranging from normalization of vasoconstriction for basal tone (steady-state blood flow) to net increases in vascular resistance (decreased blood flow). Myoendothelial feedback: Activation of α-adrenergic receptors (αARs) on smooth muscle by norepinephrine (NE) secreted by the SNS or the pharmacological α1R agonist phenylephrine (PE) results in IP3 production to elicit Ca2+ release through IP3Rs in the sarcoplasmic reticulum (SR) to evoke vascular contraction. When elevated in smooth muscle, IP3 and Ca2+ diffuse through myoendothelial gap junctions into the endothelium to activate SKCa/IKCa channels and/or NO production, providing negative feedback to smooth muscle contraction (see broken lines indicating signaling from endothelium back to smooth muscle). The (−) and (+) symbols indicate Vm hyperpolarization and depolarization respectively while the respective color of lines corresponds to Ca2+ (red), IP3 (lime green), or Na+ (light blue) signaling.
2. Significance of the Relationship of [Ca2+]i and Vm in the Vascular Wall of Blood Vessels
Whether influenced by hormones, transmitters, or the shear forces of blood flow itself, the interplay between smooth muscle and endothelium of the vascular wall regulates resistance to blood flow in the form of vascular dilation or constriction [1]. Myoendothelial gap junctions between the two cell layers allow for the transmission of IP3, Ca2+, cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), or the general distribution of electrogenic ions (e.g., Na+, K+, or Cl−) from endothelium to smooth muscle in response to stimulation of endothelial GqPCRs (e.g., muscarinic, purinergic) [13,14], or vice versa following smooth muscle α-adrenergic receptor stimulation [15]. Fundamentally, the central mediator underlying coordination of vascular reactivity is Ca2+, which activates endothelial nitric oxide synthase (eNOS) and the production of nitric oxide (NO) concomitant with stimulation of SKCa/IKCa channels for EDH. The oxidoreductase activity of eNOS is described by the conversion of l-arginine to NO and l-citrulline with additional requirements of the Ca2+ sensor calmodulin, the co-factor tetrahydrobiopterin (BH4; composes the oxygenase domain with heme), and nicotinamide adenine dinucleotide phosphate (NADPH; reductase domain) [16]; deficiency in l-arginine or BH4 results in eNOS “uncoupling”, whereby reactive oxygen species are produced [17]. Smooth muscle relaxation to eNOS-derived NO occurs via protein kinase G (PKG)-dependent phosphorylation of multiple ion pumps and channels (e.g., SERCAs or voltage-gated K+ channels) in a manner that establishes a hyperpolarized smooth muscle Vm, reduced smooth muscle [Ca2+]i and, thereby, reduced myosin light-chain phosphorylation [18,19]. Calmodulin also plays a primary role in SKCa/IKCa channel activation via the sensing of [Ca2+]i using C- and N-lobe EF hand domains to open channel pores and allow K+ efflux from the endothelial cell. However, in studies using Xenopus oocytes and the inside-out patch clamp configuration to examine intracellular regulation of SKCa channels, it was found that the C-lobe may play a dispensable role for modulating Ca2+ affinity, whereas the N-lobe in particular constitutively stabilizes KCa subunits for activation [20]. The resulting hyperpolarization of endothelial Vm transmits to the smooth muscle via myoendothelial gap junctions [21,22], whereby L-type voltage-gated Ca2+ channels are deactivated, and in like fashion with the NO/cGMP/PKG pathway, smooth muscle [Ca2+]i is ultimately reduced to promote vasodilation [23].
Original investigations of the structural resolution of myoendothelial gap junctions [24,25] and functional determinations of myography and electrophysiology [26] altogether revealed regional contributions of EDH vs. NO to vasodilation along the vascular network. In particular, myoendothelial gap junctions are composed of connexins (Cxns) Cx37, Cx40, and Cx43 [11,27,28] as required for the spread of EDH from the endothelium to the smooth muscle, a mechanism that plays a prominent role in small arteries and arterioles [29]. Shimokawa et al. showed that the contribution of EDH to endothelium-dependent relaxations rises as vessel size (diameter) decreases in six- to eight-month-old male rats [26]. In particular, the range of the contribution of EDH was >2-fold when extending from aorta (~30%) to the proximal (~46%) and then to the distal (~72%) mesenteric arteries, whereas trends in NO-dependent vasodilation were the opposite (aorta: ~56%, proximal: ~17%, distal: ~20%). It is also worth noting that the contribution of prostacyclin (PGI2) was negligible regardless of blood vessel size. Thus, when examining Ca2+ and electrical signaling underlying EDH or NO, it is important to consider the anatomical position of the arterial segment throughout the conduit and resistance blood vessel network feeding into each organ in the body.
Altogether, regardless of source (intracellular release or plasma membrane entry), increased [Ca2+]i plays a dichotomous role in the smooth muscle vs. endothelial cell layers (See Figure 1 Legend; smooth muscle [Ca2+]i increase → depolarization → L-type Ca2+ channel activation → myosin light-chain phosphorylation → vasoconstriction vs. endothelial [Ca2+]i increase → SKCa/IKCa channel activation → hyperpolarization → myosin light-chain dephosphorylation → vasodilation) and maintains a narrow window of effective blood flow regulation [30,31] while preventing vascular rupture or ischemia. With some exception (e.g., direct PKG activation of myosin light-chain phosphatase and subsequent dephosphorylation of myosin light chain [32]), the cross-talk between [Ca2+]i and Vm is the “master regulator” for the coordination of blood flow throughout vascular resistance networks regardless of the mode of the upstream cellular signaling pathway. The most direct bridge between these two physiological variables is EDH with SKCa/IKCa channels as the transducers of increased [Ca2+]i to hyperpolarization of the Vm throughout the vascular wall.
Recent perspective points to an initial rapid role for EDH during vasodilation following the onset of physical activity and skeletal muscle contraction, whereas NO signaling underlies a secondary prolonged but slower vasorelaxation for sustained blood flow per lumenal sheer stress [1]. It is also worth noting that the spatial domain of signaling for NO is on the order of hundreds of microns vs. thousands of microns for EDH along the vascular wall encompassing from large extraparenchymal arteries to capillaries. Furthermore, a phenomenon of GqPCR-stimulated “slow” Ca2+ waves (~100 µm/s vs. cm/s for electrical conduction) among and along the endothelial cell layer may govern the spatial activation of both NO and EDH [33,34]. Although, as described, Ca2+ waves occur within an order of timing most consistent with the production and signaling of NO. It is possible that the amplitude, distance, and/or speed of a Ca2+ wave can be enhanced by direct opening of SKCa/IKCa channels to hyperpolarize Vm, thereby increasing the electrical gradient for Ca2+ to move into cells through open TRP channels, a phenomenon known as “hyperpolarization-induced Ca2+ entry” [3]. In its entirety for intra- and intercellular signaling, this hypothesis was proposed >5 years ago [34], and now has support with findings of enhancement in NO production [35] and the feed-forward activation of EDH [3,36]. Of course, there are limits to the effectiveness of SKCa/IKCa channel activation, vasodilation, and the delivery of blood flow, as we found that hyperpolarization of Vm >−60 mV results in current leak that reduces the spread of hyperpolarization among endothelial cells by more than half [37,38]. In addition to the diminishment of sufficient resting vascular tone [10], the consequence of the “over-activation” of SKCa/IKCa channels entails significant charge loss through electrically “leaky” membranes, thereby impairing effective cell-to-cell vasoreactivity coordination of blood flow along vascular resistance networks [1,21]. Accordingly, as the relationship between Vm and the increase in [Ca2+]i is only linear from ~−25 to −60 mV [3], and as only 10 to 15 mV of hyperpolarization from resting (~−30 to −40 mV) is needed for maximal vasodilation [39,40], “more” is certainly not “better” with respect to KCa channel function.
3. Significance of SKCa/IKCa and TRP Channels in the Blood Vasculature
Properties of transmembrane ion channel activity include the electrical and driving forces on the ionic species that are permeant through the channel pore, channel conductance for ease of current flow, and the number of channels (n) multiplied by their individual probability of opening (PO). The SKCa/IKCa channels are permeant to K+ ions which are favored for outward movement in accordance with the concentration gradient ([K+]o/[K+]i: ~0.030) despite an opposing electrical gradient (the intracellular side of the plasma membrane is negatively charged and attracts cations). The Vm must be as high as −90 mV at physiological temperature (37 °C) in order to bring transmembrane K+ flux to equilibrium (EK). For reference, vascular endothelial cells typically have a Vm of ~−30 to −40 mV during rest, and activation of SKCa/IKCa channels alone using NS309 can increase Vm to EK [37,41]. As a dominant form of K+ channel expressed in the endothelial membrane, the SKCa/IKCa channels are tetrameric (four subunits), voltage-independent (from Vm ~−80 to +10 mV [37]), and are constitutively bound to calmodulin binding sites for [Ca2+]i [42]. The physiological “agonist” is Ca2+, whereby the PO of the channels is determined by the [Ca2+]i needed to produce half-maximal activation for opening channel pores (K0.5) and cooperativity among the four subunits of the channel per number of Ca2+ ions (Hill coefficient; HC).
The SKCa channels were first identified for their role in the “after hyperpolarization” phase following the firing of individual action potentials in central neurons of the mammalian brain [43]. SKCa channels (KCa2.1, KCa2.2, and KCa2.3, or SK1, SK2, and SK3), have a relatively “small” conductance (~5 to 20 pS), a high affinity for Ca2+ (K0.5: ~400 to 700 nM), and a steep dependence on Ca2+, whereby at least four Ca2+ ions (HC = 3.9 to 4.8) are involved in the cooperativity of channels subunits for gating the pore ([43]; SK messenger RNA (mRNA) extracted from human (SK1) and rat (SK2 and SK3) brain and cloned in Xenopus oocytes). Later, the KCa2.3 (or SK3) channels in particular were demonstrated to play a role in endothelial cell regulation of vascular tone in mesenteric arteries and systemic blood pressure [44] using a SK3T/T mouse model, whereby the KCa2.3 gene promoter is governed by a tetracycline-sensitive transactivator protein [45]. Relative to wild type, the mesenteric arteries of SK3T/T mice contained elevated expression of KCa2.3 channels that were suppressed upon treatment with the tetracycline derivative doxycycline (DOX) to the extent that SKCa currents were ~25-fold lower in endothelial cells; thus, resting endothelial Vm was depolarized by ~14 mV. Also, vasoconstriction due to intravascular pressure (20 or 100 mmHg) or direct stimulation of smooth muscle α1-adrenergic receptors using phenylephrine was enhanced by >20% with blocking SKCa channels via apamin or removal of the endothelium in SK3T/T mice (no DOX) or by genetically suppressing SKCa channel expression in SK3T/T mice fed DOX. Furthermore, genetic deletion of SKCa channels increased diastolic (~94 to 118 mmHg) and systolic (~128 to 147 mmHg) blood pressure (mean arterial: ~105 to 128 mmHg) [44].
Relative to SKCa channels, IKCa (KCa 3.1, SK4, or IK1) channels have an “intermediate” conductance (=39 pS), reduced cooperativity of individual subunits per Ca2+ ion (>50%; HC = 1.7), and a similarly high Ca2+ affinity with a K0.5 of ~300 nM ([46]; IK1 mRNA extracted from human pancreas and cloned in Xenopus oocytes). The IKCa channels are generally absent in excitable cells such as neurons and cardiac myocytes [46,47,48] with an originally defined role characterized in immune cells [49]. However, similar to the role of SKCa channels for hyperpolarizing cellular Vm, the importance of the IKCa channel is now well established for endothelium-dependent vascular function [50]. A comprehensive demonstration of the cardiovascular role of endothelial KCa3.1 channels was achieved using a KCa3.1−/− (or IK−/−) mouse model with a genetic deletion of exon 4 coding the channel pore [51]. As a result, the overall endothelial KCa current densities were reduced by ~50%, while endothelial Vm hyperpolarization in response to ACh decreased by ~60%. Accordingly, measurements of maximal EDH-dependent vasodilation in response to ACh in isolated, pressurized carotid arteries ex vivo and cremaster arteries in vivo were less by ~30 to 50%. The physiological consequence entailed increased systolic and diastolic blood pressure by ~13 mmHg and ~12 mmHg, respectively (∆ mean arterial: ~14 mmHg) and mild left ventricular hypertrophy in KCa3.1−/− mice [51]. Thus, endothelial IKCa channels are fundamental for endothelial hyperpolarization underlying regulation of vascular tone, blood flow, and blood pressure, determinants that can altogether impact chronic cardiac remodeling as well.
Following thorough characterization of individual genetic models for SKCa and IKCa, further studies utilized SK3T/TIK−/− offspring generated by interbreeding SK3T/T and KCa3.1−/− mice, respectively [52]. This approach allowed for the delineation of the individual roles of SKCa and IKCa to EDH and regulation of blood pressure in a combinatorial manner. As expected, the combined genetic SKCa and IKCa deficiency eliminated endothelial K+ currents and substantially impaired (>50%) smooth muscle hyperpolarization of carotid arteries from IK1−/−/SK3T/T mice (+DOX). Consequently, IKCa deficiency (IK−/−/SK+/+ mice) reduced ACh-induced EDH-mediated vasodilation by ~75%, whereas deficit of both channels (IK−/−/SK3T/T + DOX mice) eliminated responses altogether (by ~99%). A reduction in ACh-induced vasodilation in either carotid arteries ex vivo or cremasteric arteries in vivo was not apparent in mice with a SKCa deficiency alone (IK+/+/SKT/T + DOX). The mean arterial pressure relative to wild-type mice (~100 mmHg) modestly increased with SKCa deficiency (to ~106 mmHg), IKCa deficiency (to ~108 mmHg), or combined SKCa/IKCa genetic deletion (to ~110 mmHg) [52]. An additional study of the double transgenic study concluded that SKCa channels, but not IKCa channels, played an integral role for vasodilation of cremasteric arterioles in response to tetanic muscle stimulation [53]. Also, it is also worth noting that the SKCa channel-driven vasodilation requires Cx40 endothelial and myoendothelial containing gap junctions [53], whereas IKCa-dependent responses do not require Cx40 during hyperemia of the mouse cremaster tissue [54]. These general findings of the endothelial role of SKCa vs. IKCa channels were further reinforced using an endothelium-specific SK3 knock-out mouse model generated from crossing a floxed SK3 mouse with another that expresses endothelial Cre recombinase driven by the endothelial receptor-specific tyrosine kinase (or Tie2) promoter [55].
The majority of the work delineating the individual contributions of SKCa and IKCa channels to vasodilation and tissue hyperemia was performed using mesenteric (gut) arteries as an accessible and abundant source of resistance arteries in the body [56,57,58]. However, to a lesser extent, studies also resolved clear contributions of respective KCa channel subtypes in the microcirculation of the eye [59], brain [60,61,62], heart [63,64], lung [65,66], skeletal muscle [8,67], and kidney [68,69,70]. While it is clear that both SKCa and IKCa channels are integral to overall cardiovascular health (systemic vascular resistance and blood pressure control), respective KCa contributions underlying hyperemia throughout organs may vary. A common feature at the biophysical level in isolated endothelium is that IKCa (vs. SKCa) channels play a predominant role in the regulation of Vm and membrane resistance during steady-state and pharmacological conditions in the presence of direct (e.g., NS309 and SKA-31) or indirect (e.g., ACh and ATP) KCa openers [8,71]. At the level of vasoreactivity and regulation of blood flow, evidence supports SKCa channels as the “affinity” component (sensitivity of vasodilation/hyperemia per unit stimulus), whereas IKCa channels define “efficacy” (amplitude of vasodilation/hyperemia per unit stimulus) [51,52,72].
Equipped with an enhanced electrical gradient via hyperpolarization [3] in accordance with a ~20,000-fold transmembrane concentration gradient [73], Ca2+ influx through open Ca2+-permeant TRP channels may serve at least two purposes: (i) to sustain the duration of elevated [Ca2+]i for prolonged control of blood flow, and (ii) to facilitate Ca2+ refilling of the endoplasmic reticulum to maintain continued, repetitive vascular function [8]. Note that endothelial TRP channels involved in EDH can be homo- or heteromeric among families and respective isoforms: canonical (TRPC; isoforms 1, 3, 4, 5, and 6), vanilloid (TRPV; isoforms 1, 3, and 4), ankyrin (TRPA; isoform 1), and polycystin (TRPP; isoform 2) [74,75]. Functional examples of heteromeric configurations of vascular TRPs include TRPC3-C4 [76], TRPV4-C1 [77,78,79], and TRPV4-C1-P2 [80]. Due to recent breakthroughs in pharmacological and genetic tools [81,82], the role of TRPV4-containing channels is the most widely studied thus far [3,83,84].
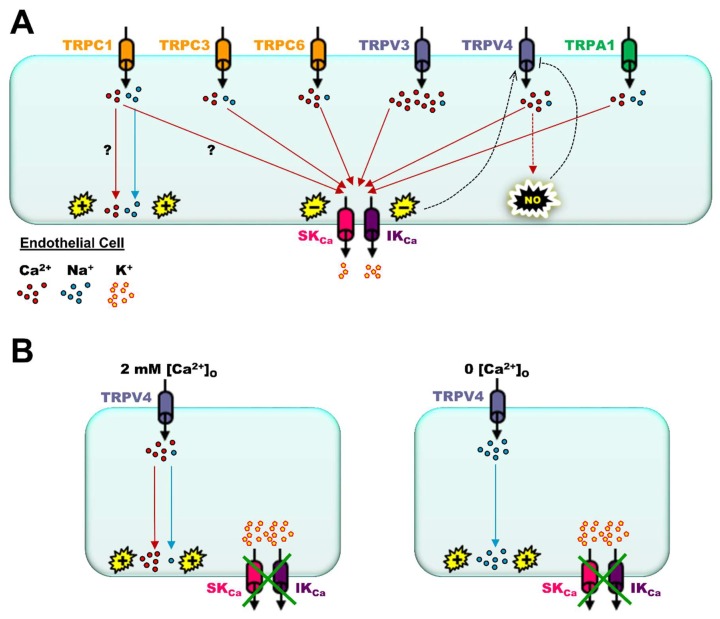
In general, most experimental evidence demonstrates that TRP channel function produces EDH via [Ca2+]i activation of SKCa and IKCa channels governing vascular dilation, thereby promoting increases in blood flow (Figure 2A). However, TRP channels can regulate EDH in a positive or negative manner based on relatively permeability of Ca2+ to Na+ ions of a particular TRP channel configuration. Whereas TRPC1-containing channels are relatively low conductance (~16 pS) and non-selective (PCa/PNa ~1:1; [85]), TRPC6 (~35 pS, PCa/PNa ~5:1; [86]) or TRPV4 (~90 pS, PCa/PNa ~6:1; [87]) channels are examples of high conductance, dominant for mediating Ca2+ (vs. Na+) influx. Consequently, TRPC1-containing channels in mouse resistance arteries mediate a net depolarizing influence on Vm, counteracting EDH, whereas TRPC6- [88] and TRPV4-containing channels [83] augment EDH in the presence of functional SKCa/IKCa channels. Depolarization of endothelial Vm is due to relatively higher Na+ permeability and influx, whereas net hyperpolarization is a result of prominent influx of Ca2+ because, unlike highly electrogenic Na+ ions, Ca2+ ions are primarily second messengers that rapidly bind with intracellular proteins such as calmodulin to activate SKCa/IKCa channels (as one example). Similar to endothelial TRPC1, smooth muscle TRP melastatin (TRPM, isoform 4; ~25pS, not Ca2+ permeant [89]) channels also permit Na+ influx to depolarize smooth muscle Vm, thereby activating L-type voltage Ca2+ channels for myogenic constriction [90]. Remarkably, blocking TRPM4 channels in rat mesenteric arteries using 9-phenanthrol also hyperpolarizes endothelial Vm via apparent enhancement of IKCa channel function [91]. Also, in mouse collecting lymphatic endothelium, where TRPV4 channels are present but functional SKCa/IKCa channels are absent (i.e., no EDH), depolarization of Vm in response to TRPV4 channel activation occurs almost exclusively due to Na+ influx [92] (Figure 2B). Subtle variations of positive or negative regulation of EDH will depend on oligomeric configurations of the TRP channel to govern permeability of one form of cationic species vs. another, and, in this regard, much remains to be resolved in physiological study models vs. heterologous cell culture systems.
Figure 2.
Functional contribution of major transient receptor potential (TRP) channel isoforms to activation of small- and intermediate-Ca2+-activated K+ (SKCa and IKCa) channels and endothelium-derived hyperpolarization. (A) Permeability of Ca2+ to Na+ ions (PCa/PNa) varies across the canonical (TRPC), vanilloid (TRPV), and ankyrin families of TRP channels. However, with the exception of TRPC1, endothelial TRP channels underlie significant influx of Ca2+ to activate SKCa and IKCa channels. As discussed in the text, note that these individual TRP isoforms can form tetrameric TRP channels via heteromeric combinations (e.g., TRPV4–TRPC1, TRPC3–TRPC4) in a physiological setting that may not be representative of determinations of homomeric channels expressed in heterologous culture systems. As it relates to the competition with Na+ influx, the “?” symbol indicates the unknown contribution of Ca2+ influx through TRPC1-containing channels for Vm depolarization vs. activation of SKCa and IKCa channels for Vm hyperpolarization as both are theoretically possible. Hyperpolarization via activation of SKCa/IKCa channels may stimulate Ca2+ influx through TRPV4-containing channels for further activation of SKCa and IKCa channels and production of NO (“positive” feedback; broken black arrow). Although, nitric oxide (NO) may inhibit TRPV4-containing channels (“negative” feedback) via S-nitrosylation (broken black line with flat end). (B) In the absence of functional SKCa and IKCa channels, Ca2+ and Na+ influx (PCa/PNa ~6:1; extracellular Ca2+ ([Ca2+]o) present) through TRPV4-containing channels leads to endothelial depolarization (left panel). In the absence of [Ca2+]o, Na+ influx through TRPV4 channels is robust, and depolarization occurs regardless of SKCa and IKCa channel presence and function (right panel). Green crosses over SKCa and IKCa channels denote their functional absence in the plasma membrane (e.g., collecting lymphatic endothelium). For all panels, (−) and (+) symbols indicate Vm hyperpolarization and depolarization respectively while the respective color of lines corresponds to Ca2+ (red) or Na+ (light blue) signaling. All lines with arrow ends indicate positive signaling of respective ions to ultimately effect a change in Vm (hyperpolarization or depolarization) or production of NO.
Since original myoendothelial investigations around the turn of the 21st century [24,25], the use of transgenic animal models and confocal microscopy greatly advanced cardiovascular studies of “microdomain” signaling among spatially localized KCa and TRP channels. The Taylor laboratory group in particular developed auto-detection and analysis algorithms to quantitate discrete Ca2+ events throughout cellular interior with primary data outputs of ∆[Ca2+]i event amplitude, frequency, duration, and spatial spread [93,94,95]. Throughout such studies, mesenteric arterial endothelium was examined using an en face study model, whereby experimentally feasible arteries (diameter: ~300 µm) for this protocol were cut open longitudinally and pinned down into silicone with the endothelium facing up toward the microscope objective. In comparison to wild type, the number of ACh-induced Ca2+ events during “plateau” responses (predominant TRP channel activity) are reduced by ≥60% in genetic elimination of IK1 (IK1−/−), whereas basal Ca2+ dynamics are similar [95]. In tandem with elimination of IK1, genetic SK3 suppression (IK1−/−/SK3T/T + DOX) or SK3 overexpression (IK1−/−/SK3T/T − DOX) has minimal effects on the occurrence of Ca2+ events under both basal and ACh-stimulated conditions [95]. In addition to removal of extracellular Ca2+, pharmacological corroboration of these findings was demonstrated with a block of SKCa and IKCa channels (apamin + charybdotoxin) or TRPV4 channel block with HC-067047, whereby ACh Ca2+ dynamics in wild-type arteries were reduced to the level of mice genetically deficient for both SK3 and IK1 (IK1−/−/SK3T/T + DOX) [95]. A follow-up study characterized endothelial dynamic Ca2+ signals during basal and conditions of Substance P-stimulated vasorelaxation in swine coronary endothelial cells. This investigation detected endothelial spatial and temporal events governing arterial tone that were not apparent using averaged, whole-field measurements of [Ca2+]i [96]. With the use of an endothelium-specific SK3 knock-out mouse model, it was found that the coupling of SK3 and TRPV4 channels in mesenteric arteries appears to elicit large-amplitude and slow-decay Ca2+ kinetics that may be consistent with relatively long-term physiological delivery of blood flow in response to metabolic demand of active tissues [55].
Another foundational study demonstrated how small (diameter: ~100 µm) mesenteric endothelial TRPV4 channel-mediated Ca2+ “sparklets” activate SKCa and IKCa channels using wild-type, endothelial genetic Ca2+ sensor (GCaMP2Cx40) and TRPV4−/− mice and customized Ca2+ event detection software; only a few open TRPV4 channels are required for maximal vasodilation [83]. Later, it was found that this coupling between endothelial KCa and TRP channels was due, at least in part, to the presence of protein kinase C (PKC) and PKC-anchoring protein AKAP150 or, otherwise, hypertension would ensue [12]. However, this scenario can change depending on vessel type as, in mouse pulmonary arteries, TRPV4 Ca2+ “sparklets” preferentially activate eNOS in a negative feedback manner, whereby NO-activated PKG inhibits cooperative opening of TRPV4 channels [66] (Figure 2A). Remarkably, a decrease in intralumenal pressure on its own (from 80 mmHg to ≤50 mmHg of rat cremasteric arterioles) can significantly increase TRPV4-mediated Ca2+ events that selectively activate IKCa vs. SKCa channels, leading to a loss of myogenic tone and blood flow autoregulation [10]. Also, using a novel gradient index (or GRIN) fluorescence microendoscopy method positioned inside of rat carotid arteries, ACh-induced widefield Ca2+ events (summation of IP3R release and TRP Ca2+ influx) decreased with increasing pressure (60, 110, and 160 mmHg), an effect that was diminished during late middle age (18 mo) vs. youth (3 mo) [97].
Although studied to a lesser extent vs. TRPV4 channels, the activation of SKCa and IKCa channels occurs downstream of other novel families and/or isoforms of TRP channels as well. In particular, TRPV3 channels contain ~2-fold the unitary conductance and Ca2+ permeability (~150 to 200 pS, PCa/PNa ~12:1; [98]) of TRPV4 channels [87] and, thus, may be more pertinent for activating eNOS and/or EDH. With use of total internal fluorescence (TIRF) microscopy and the oregano monoterpenoid carvacrol (TRPV3 activator; [99]), TRPV3-to-SKCa/IKCa channel coupling was characterized in rat cerebral parenchymal arterioles [100]. Notably, carvacrol-induced vasodilation was not sensitive to block of NO and cyclooxygenase signaling and was almost completely abolished upon conditions of either SKCa or IKCa block alone [100]. Another TRP channel of the ankyrin family (TRPA1; activated by mustard oil, PCa/PNa ~1:1; [101]) was found to contribute to endothelial SKCa and IKCa channel activation as well and in a manner insensitive to NO and cyclooxygenase signaling [102]. Despite very modest Ca2+-permeant properties vs. TRPV3- and TRPV4-containing channels, TRPA1 channel function may be involved in microdomain myoendothelial signaling for activation of KCa channels during physiology [103] and conditions of enhanced oxidative signaling [104]. Finally, in similar fashion to TRPV3, TRPV4, and TRPA1, the role of TRPC3 (~70 pS, PCa/PNa ~1.5:1; [105]) was thoroughly characterized for its coupling to SKCa/IKCa channels in mouse cerebral arteries [71] and rat mesenteric arteries [106] using a selective antagonist as the pyrazole compound Pyr3.
When interpreting studies on TRP channel expression and function, it is important to bear in mind that TRP channels are tetrameric in their physiological form and may contain only one isoform (homotetrameric) or two to four different isoforms (heterotetrameric). Parameters of conductance and Ca2+ permeability are primarily determined in heterologous cell culture systems that only express homotetrameric TRP channels to determine parameters of individual TRP isoforms. Furthermore, genetic tools (e.g., TRP knock-out animals) do not necessarily manifest results consistent with pharmacological agents (e.g., TRP blockers) [3,107], as the former method prevents genetic expression of the TRP isoform and perhaps decreases the total number of functional TRP channels overall, whereas the latter strategy modulates a post-translational TRP channel product. Again, for pharmacological interventions, heterotetrameric TRP channel configurations were considered and, thus, potential insensitivity to agonists or antagonists were demonstrated to be effective for homotetrameric channels formed in a heterologous system.
4. Impact of Adrenergic Tone on SKCa/IKCa and TRP Channels & Significance of In Vivo vs. Ex Vivo Observations
A central (and extremely complex) research topic entails how the stimulation of smooth muscle α-adrenergic receptors (α1ARs) influences the interaction between endothelial KCa and TRP channels during “myoendothelial feedback” [24,30,31]. In isolated mouse mesenteric arteries, activation of smooth muscle α1ARs elicits vasoconstriction for ~2 min followed by a slow dilation induced by increases in endothelial TRPV4-mediated Ca2+ influx and activation of KCa channels, a mechanism that may cease prolonged vascular resistance and hypertension as observed in TRPV4−/− mice [108]. Whether the ionic species directly passing from smooth muscle to endothelial cells through gap junctions is in the form of IP3 (activator of IP3Rs and Ca2+ release from endothelial ER) [109], Ca2+ [14], or perhaps both [110], in the context of animal species (e.g., rat vs. mouse), blood vessel type (e.g., mesenteric vs. skeletal muscle) and/or experimental conditions (e.g., ex vivo vs. in vivo) remains a topic of active investigation. In addition, there is a complex interplay among the relative contributions of endothelial NO, SKCa channels, and IKCa channels as it pertains to the coordination of local and conducted vasodilation signaling. As a key example, a recent study demonstrated that isolated rat mesenteric arteries in the presence of norepinephrine mediate myoendothelial feedback primarily through activation of IKCa channels, whereas, with ongoing physiological sheer stress to blood flow, feedback is in the form of SKCa channel activation and NO production in the intact mesenteric network [111].
Despite efforts for experimental controls and proposals for simplified working models across vascular types and methods of pre-constricted tone, studies of myoendothelial feedback remain empirically convoluted by nature. To begin with, it is inherently difficult to delineate Ca2+ signaling events among respective smooth muscle and endothelial cell layers with common molecular targets (e.g., TRP channels and ER IP3Rs) of interest. Next, there are factors of interventional drug delivery (ablumenal vs. lumenal), permeability (cell-permeant vs. extracellular), and target selectivity to contend with. In addition, whether via means of a chemical fluorescent dye or genetic sensor, compaction or dilution of Ca2+ sensor molecules can alter with cellular mechanical movement regardless of distinct stimuli or pharmacological interventions. Also, as in the case of in vivo experiments, halogenated (e.g., isoflurane) or injectable (e.g., pentobarbital) anesthetics can influence vascular function on their own toward the alteration of endothelial function [112,113]. Furthermore, even agents that are commonly used for cell/tissue-specific genetic manipulations (e.g., tamoxifen (estrogen receptor partial agonist/antagonist) and DOX (tetracycline antibiotic)) and, interestingly, the choice of vehicle solvent (e.g., sunflower vs. peanut oil) [114] may introduce experimental artefacts. Thus, scientific corroboration among complementary methods such as a vascular co-culture method [115], pressure myography [116], microendoscopy imaging [117], and intravital microscopy [118] helps to clarify myoendothelial signaling events during health and disease.
5. Role of Familial Mutations in KCa and TRP Channels in the Emergence of Cardiovascular Disease
The cardiovascular consequences of deliberate experimental mutations of major components of EDH (KCa and TRP channels, and gap junction Cxns) were discussed, but genetic mutations are also present during inheritable diseases. A single autosomal dominant mutation in KCNN3 (guanine (mutant) substitution for cytosine (wild type) at nucleotide 1348 in coding DNA (or c.1348 C>G) and the KCa2.3 channel subunit (leucine (mutant) substitution for valine (wild type) at amino acid 450 (or V450L)) underlies development of idiopathic non-cirrhotic portal hypertension [119]. Also, a variety of single-nucleotide polymorphisms for KCNN3 (chromosome 1q21 locus) are involved in lone atrial fibrillation [120], presumably due to dysregulation in the repolarization of action potentials in cardiac atria. As cardiac myocytes are also rich in gap junctions for coordinating electrical activity, it is not surprising that germline heterozygous missense mutations in GJA5 (Cx40; e.g., phenylalanine for isoleucine, I75F [121]) also underlie lone atrial fibrillation. Autosomal dominant KCNN4 (KCa3.1; “Gardos” channel) mutations on chromosome 19 lead to decreases in erythrocyte production ([122]; Diamond–Blackfan anemia) and health ([123]; xerocytosis, dehydration due to excess K+ and H2O loss). TRP “channelopathy” mutations are linked to an assortment of disorders throughout the entire body, including cardiovascular maladies of ischemic heart disease, hypertension, and atherosclerosis (see review [124]). However, there are a few adequately described cases of familial mutations accompanying cardiovascular pathology, including TRPC3 (Williams–Beuren syndrome, deletion of 26 to 28 genes in 7q11.23; blood vessel stenosis [125]) and TRPM4 (progressive familial heart block type I, missense mutations in 19q13.32, and gain of function; atrioventricular block [126]).
Although not discussed here, inheritable mutations in KCa channels, TRP channels, and Cxns also involve prominent neurological (e.g., Parkinson’s) and developmental (e.g., skeletal dysplasia) disorders. Further investigation is needed to delineate de novo germline mutations from post-zygotic mutations that arise and provide susceptibility to development of cardiovascular pathology (e.g., arrhythmias or hypertension) with advancing age and select conditions of environmental exposure. In addition, albeit indirectly, the functional efficiency of EDH may also be affected by germline and somatic mutations of the upstream (e.g., CHRM3; muscarinic type 3 GqPCR, M3R [127]) and/or downstream (e.g., MYLK; myosin light-chain kinase, MLCK [128]) signaling components of vascular function. As of the present, human genetic mutations in key components involved in EDH appear extremely rare while not selective toward cardiovascular impairments alone. As a clearer foundation for understanding mammalian cardiovascular function and pathology, focus on the impact of aging and associated pathology (primarily using rodent models) is discussed next.
6. Endothelial SKCa and IKCa during Aging and Chronic Pathology
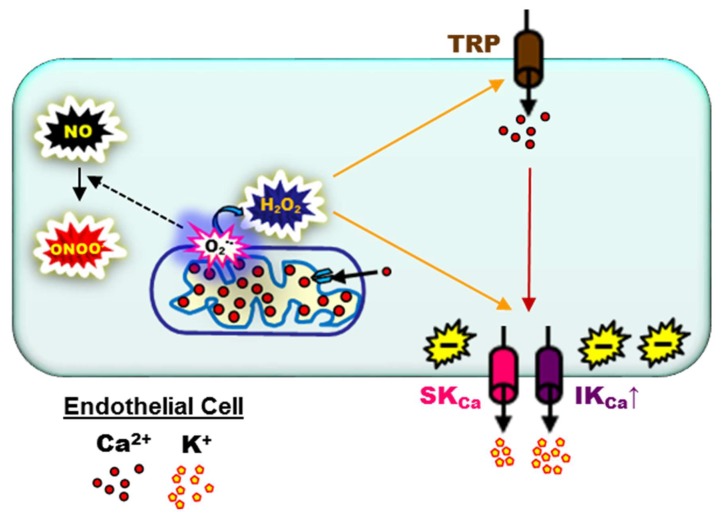
The leading cause of death in the United States is cardiovascular disease [129] with aging [130,131] and age-related vascular endothelial dysfunction [132,133] as key risk factors. A central mechanism of endothelial function for vasodilation and coordination of blood flow along and among vascular networks is EDH [8,134]. The physiological consequences of enhancement or diminishment of EDH during development of disease relative to young, healthy cardiovascular conditions remain altogether unclear. It is worth noting that IKCa function was found to increase in coronary arteries [135] and saphenous arteries [136] of obese rats, and mesenteric arteries of hypertensive [137] and diabetic [138] rats. At least in part, EDH compensates for reduced NO bioavailability during diabetic conditions in coronary arteries of dogs [139] and subcutaneous arteries of humans [140]. In aortae of apolipoprotein E knock-out mice, basal KCa channel function appears to be reduced with enhancement of peak hyperpolarization to indirect (ACh) and direct (SKA-31 or NS1619) stimulation of the channels [141]. Remarkably, EDH may also emerge as a prominent contributor to the dilation of cremaster arterioles in developing mice of 15 weeks of age fed a high-fat diet continuously, beginning from at least four weeks prior to conception vs. control or temporally split control/high-fat diet treatments [142]. With respect to aging, increased enhanced IKCa function was observed in the aortae of 75- to 100-week-old mice [143] and skeletal muscle feed arteries of 24- to 26-month-old mice [38,144]. Remarkably, large-conductance Ca2+-activated K+ (BKCa) channels also emerge in vascular endothelium as a result of conditions associated with chronic hypoxia (48 h), forming microdomain arrangements among Caveolin-1, TRPV4 channels, and BKCa channels (rat gracilis arteries) [145]. It is worth noting that, either on its own or in combination with deficient gap junction transmission, KCa over-activation may significantly diminish conducted hyperpolarization and/or vasodilation during old age (≥24-month-old mouse skeletal muscle arteries [38], and ≥64-year-old human coronary arteries; [63]), hypertension (Cx40-deficient mice [146]), hyperhomocysteinemia (cystathionine β-synthase mouse gluteus maximus arterioles [147]), and chronic hyperglycemia (diabetic mouse mesenteric arteries [148]). Currently, the upregulation of EDH during aging and development of chronic pathology is best explained by enhanced oxidative signaling (Figure 3). In brief, superoxide (O2•−) production emerges from a variety of intracellular sources (NADPH oxidases, mitochondria, uncoupled NO synthase, and xanthine oxidase) which inactivates NO to peroxynitrite (ONOO−) [149,150] and, via superoxide dismutase, O2•− is rapidly converted to H2O2 with spontaneous breakdown products such as hydroxyl radicals (OH•) [151] which increase EDH [8,38,152]. Thus, with this mechanistic basis in mind, SKCa/IKCa channel function may “compensate” for sustaining local vasodilation [153] at the expense of a restricted spatial domain of the spread of coordinated blood flow due to “leaky” endothelial plasma membranes [8].
Figure 3.
Working model for the upregulation of endothelium-derived hyperpolarization during aging and development of chronic pathology per enhanced oxidative signaling. Cardiovascular aging and the development of chronic disease is associated with a progressive increase in endothelial oxidative signaling. Mitochondrial respiration is a primary source of superoxide (O2•−) which inactivates NO to peroxynitrite (ONOO−) and, via superoxide dismutase, O2•− is rapidly converted to H2O2. H2O2/OH• activates SKCa and IKCa (primarily IKCa) channels directly and/or indirectly (Ca2+ influx through TRP channels). Thus, SKCa/IKCa channel function may “compensate” for decreased NO bioavailability to sustain local vasodilation. The (−) symbol indicates Vm hyperpolarization while the respective color of lines corresponds to O2•− (black), H2O2 (orange), or Ca2+ (red) signaling.
On the flip side, there is support that the contribution of EDH may also decrease during conditions of cardiovascular disease. In rats, respective contributions of NO and EDH to the dilation of mesenteric arteries are reduced during diabetes [154] or of kidney arterioles at 18 months of age relative to 3 months [155]. It was also determined that physiological contribution of SKCa/IKCa channel function of saphenous arteries was less in 34- and 64-week-old (vs. 12-week-old) mice [156]. Finally, recent reports also conclude that EDH-dependent dilation of mesenteric arteries decreases in genetically hypertensive rats [157] or in 32-week-old rats fed a high-fat and/or high-fructose diet beginning at four weeks of age [158]. Discrepancies in conclusions for remodeled EDH function can be attributed to several differences among studies (e.g., diet application protocol or vascular study model). However, with consideration for “healthy” aging alone, perhaps impaired vascular IKCa channel function is not a consistent observation for rodents >20 months of age.
Recent support of SKCa/IKCa and TRP channel function as a “double-edged sword” that promotes the gain or loss of blood flow control is intriguing and important for a modern understanding of acute and chronic cardiovascular disease. The extent of SKCa/IKCa channel activation coincident with Vm ≥−60 mV may be excessive [3,38] as it pertains to the loss of myogenic tone and significant impairment of blood flow control [10]. Remarkably, pharmacological block or genetic deletion of IKCa channels may actually serve as protection as demonstrated in KCa3.1−/− mice that avoid an otherwise fatal pulmonary circulatory collapse in response to the activation of Ca2+-permeant TRPV4 channels with GSK1016790A (high dose: 10 nM [159]; see Reference [160] for a thorough review on the interaction of pulmonary KCa and TRPV4 channels). Furthermore, direct genetic or pharmacological inhibition of TRPV4 prevents inflammatory cytokine signaling and endothelial dysfunction in septic mice (via cecal ligation and puncture or injection of tumor necrosis factor α or lipopolysaccharide) [161] and cardiac left ventricular cell/tissue damage in aged mice (24 to 27 months) exposed to hypoosmotic conditions (250 mOsm vs. physiological, ~300 mOsm) representative of ischemia–reperfusion injury [162]. Theoretically, enhanced smooth muscle α-adrenergic receptor activation via perivascular sympathetic nerves during aging and chronic vascular disorders (e.g., hypertension) may further overstimulate endothelial IKCa channels in response to high IP3 and Ca2+ concentrations transmitted from smooth muscle through myoendothelial gap junctions [1,21]. Thus, in conditions of excessive stimulation of GqPCRs, such as adrenergic receptors or TRPV4 channels that ultimately cause supraphysiological increases in vascular wall [Ca2+]i, a deleted or diminished role for endothelial IKCa channels may actually alleviate the consequences of pathological burden of [Ca2+]i associated with the development of acute and chronic disease.
7. What May Be Next for Investigative Studies of Endothelial Function: Novel Physiological and Pharmacological Molecular Signaling Pathways
The number of “endothelium” publications recognized by the National Library of Medicine significantly increased from ~5000 in the early 1990s [163] to the present with >150,000. Under this heading, studies of EDH as they relate to the interaction of KCa and TRP channels are a rapidly growing research landscape for resolving cardiovascular physiology (rest and exercise) and the development of chronic disease. In general, characterizations of the essential components of EDH (GqPCRs, IP3Rs, KCa, and TRP channels) were thoroughly investigated across animal species and blood vessel types as discussed in this review. Finally, as a key intracellular organelle for the integration of Ca2+ and oxidative signaling in the vasculature, a physiological role for endothelial mitochondria is being investigated during ATP-dependent control of Ca2+ homeostasis [164], enhanced EDH during vascular aging [144], and metabolic stress during conditions of hyperglycemia [165] as a few recent examples. Also, additional clarification regarding endothelial mitochondrial KATP channels [166] and BKCa channels [167], and delineation of their actions of depolarization of the inner mitochondrial membrane vs. the well-characterized plasma membrane K+ channels (hyperpolarization of the plasma membrane) is required.
In addition to NO, there are other gases now recognized to play a role during blood flow control including hydrogen sulfide (H2S) and carbon monoxide (CO). As a reducing agent (vs. oxidizing H2O2), H2S is generated from substrates homocysteine (via cystathionine β-synthase), cysteine (3-mercaptopyruvate sulfurtransferase), and thiosulfate (cystathionine γ-lyase), [168]. Overall evidence supports H2S as a vasodilator that increases TRPV4 channel-dependent Ca2+ events and activation of endothelial BKCa channels of rat mesenteric arteries [169]. H2S generated by cystathionine γ-lyase in particular may activate endothelial SKCa and IKCa channels for vasodilation of mouse mesenteric arteries [170]. CO is produced from heme via the actions of heme oxygenase [171]. Although there is some evidence to suggest that CO is integral to endothelial function [167] and vasodilation [172], further investigation is needed.
There are several other endogenous and therapeutic molecules (lipids and polyphenols) that operate via endothelium-dependent vasodilation. In particular, legalization and social acceptance for the recreational and medicinal use of cannabis among American populations increased since 2007 [173]. Thus, the investigation of cannabinoids is perhaps currently among the highest of priorities in biomedical research. Although reports are presently scarce, there is evidence to support that the endocannabinoid 2-arachidonoylglycerol induces vasorelaxation of rat mesenteric arteries as dependent on KCa and TRPV channels [174]. In human mesenteric arteries, this vasodilation appears to be dependent on cyclooxygenase metabolism and prostanoid signaling [175]. Recent evidence also suggests that endothelial cannabinoid receptors are not existent, and vasodilatory effects are directly mediated via BKCa channels [176]. As representative of studies testing cannabis Δ9-tetrahydrocannabinol (THC; CB1 receptor agonist) on vasoreactivity, there is evidence demonstrating the opposite as well, whereby the application of THC reduces EDH-dependent vasorelaxation in mouse arteries [177]. Regardless, a consensus is presently lacking across the mechanisms of action of various cannabinoids, respective pharmacological kinetics, presence/classification of vascular cannabinoid receptors, and contribution of KCa channels vs. NO vs. cyclooxygenases among animal species and blood vessel types. Other studies support the role of endothelial KCa channel function during arterial treatment with omega-3 polyunsaturated acids [178], fruit-derived polyphenols [179], Rhododendron flavonoids [180], or sex hormones (testosterone [181] and estrogen [182]). All of such studies and therapeutic strategies that are either ancillary to, or perhaps circumvent, classical GqPCR pathways (e.g., purinergic and muscarinic) are in need of further investigation.
8. Summary and Conclusions
Cardiovascular disease is the number one killer of Americans [129] with age-related vascular endothelial dysfunction at the center [132,133]. Coordinated blood flow and the perfusion of organs depend on the endothelial lining of major arteries and respective microcirculatory networks (arterioles and capillaries) that travel ≥100 km throughout the body [183,184]. The signaling pathway integral to the promotion of blood flow is endothelium-derived hyperpolarization (EDH). EDH sequentially entails Gq-protein-coupled receptor stimulation in intracellular Ca2+ release from the endoplasmic reticulum and Ca2+ influx through transient receptor potential (TRP) channels to activate Ca2+-activated K+ (KCa) channels and produce hyperpolarization [8]. The structural and functional arrangement among TRP and KCa channels in the blood vasculature is diverse across animal species, vessel type, and modes of local vs. conducted signaling to tune blood flow. Under- or over-activation of EDH is associated with aging and/or the development of chronic cardiovascular disease.
The future of cardiovascular physiology will likely entail progressive recruitment of state-of-the-art methods in the form of genetics, pharmacology, and engineering (e.g., stem-cell therapy [185], nanoparticle delivery of antioxidant molecules [186], and “blood–brain barrier on a chip” [187]) to examine classical components of genetics, structure, and function using cell/tissue/animal models of aging and disease. However, a present priority includes comprehensively defining a role of endothelial mitochondria as a nexus for vascular Ca2+ and oxidative signaling and modulatory input of novel molecules in the form of gases, lipids, hormones, and phytochemicals. Regardless of physiological examination and therapeutic strategies, we anticipate that fully understanding mechanisms underlying blood flow regulation as it relates to the aging cardiovascular system will ultimately lead to the prevention of chronic disease.
Acknowledgments
The authors would like to thank John N. Buchholz for kindly going through the manuscript and providing an overall assessment of its quality.
Author Contributions
E.J.B. and M.A.H. interpreted results of the studies cited; E.J.B. and M.A.H. prepared figures; E.J.B. and M.A.H. drafted manuscript; E.J.B. edited and revised manuscript; E.J.B. and M.A.H. approved final version of the manuscript.
Funding
The authors’ contributions to this review were supported by National Institutes of Health Grants R00AG047198 and R56AG062169 to Erik J. Behringer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare no conflicts of interest
References
- 1.Socha M.J., Segal S.S. Microvascular mechanisms limiting skeletal muscle blood flow with advancing age. J. Appl. Physiol. (1985) 2018;125:1851–1859. doi: 10.1152/japplphysiol.00113.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himmel H.M., Whorton A.R., Strauss H.C. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- 3.Behringer E.J., Segal S.S. Membrane potential governs calcium influx into microvascular endothelium: Integral role for muscarinic receptor activation. J. Physiol. 2015;593:4531–4548. doi: 10.1113/JP271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giuro C.M.L., Shrestha N., Malli R., Groschner K., van Breemen C., Fameli N. Na+/Ca2+ exchangers and Orai channels jointly refill endoplasmic reticulum (ER) Ca2+ via ER nanojunctions in vascular endothelial cells. Pflug. Arch. 2017;469:1287–1299. doi: 10.1007/s00424-017-1989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila-Medina J., Mayoral-Gonzalez I., Dominguez-Rodriguez A., Gallardo-Castillo I., Ribas J., Ordonez A., Rosado J.A., Smani T. The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells. Front. Physiol. 2018;9:257. doi: 10.3389/fphys.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J. Physiol. 2012;590:4201–4208. doi: 10.1113/jphysiol.2012.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Gueguinou M., Trebak M. Store-Independent Orai Channels Regulated by STIM. In: Kozak J.A., Putney J.W. Jr., editors. Calcium Entry Channels in Non-Excitable Cells. CRC Press; Boca Raton, FL, USA: 2018. pp. 197–214. [PubMed] [Google Scholar]
- 8.Behringer E.J. Calcium and electrical signaling in arterial endothelial tubes: New insights into cellular Physiol.ogy and cardiovascular function. Microcirculation. 2017;24 doi: 10.1111/micc.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledoux J., Taylor M.S., Bonev A.D., Hannah R.M., Solodushko V., Shui B., Tallini Y., Kotlikoff M.I., Nelson M.T. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagher P., Beleznai T., Kansui Y., Mitchell R., Garland C.J., Dora K.A. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc. Natl. Acad. Sci. USA. 2012;109:18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandow S.L., Neylon C.B., Chen M.X., Garland C.J. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: Possible relationship to vasodilator function? J. Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonkusare S.K., Dalsgaard T., Bonev A.D., Hill-Eubanks D.C., Kotlikoff M.I., Scott J.D., Santana L.F., Nelson M.T. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci. Signal. 2014;7:ra66. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson G.G., Segal S.S. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ. Res. 2000;87:474–479. doi: 10.1161/01.RES.87.6.474. [DOI] [PubMed] [Google Scholar]
- 14.Garland C.J., Bagher P., Powell C., Ye X., Lemmey H.A.L., Borysova L., Dora K.A. Voltage-dependent Ca2+ entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci. Signal. 2017;10:eaal3806. doi: 10.1126/scisignal.aal3806. [DOI] [PubMed] [Google Scholar]
- 15.Billaud M., Lohman A.W., Johnstone S.R., Biwer L.A., Mutchler S., Isakson B.E. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharm. Rev. 2014;66:513–569. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia V., Sessa W.C. Endothelial NOS: Perspective and recent developments. Br. J. Pharm. 2019;176:189–196. doi: 10.1111/bph.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feil R., Lohmann S.M., de Jonge H., Walter U., Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: Insights from genetically modified mice. Circ. Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 19.Irvine J.C., Favaloro J.L., Kemp-Harper B.K. NO- activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Halling D.B., Hall A.W., Aldrich R.W. EF hands at the N-lobe of calmodulin are required for both SK channel gating and stable SK-calmodulin interaction. J. Gen. Physiol. 2009;134:281–293. doi: 10.1085/jgp.200910295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behringer E.J., Segal S.S. Spreading the signal for vasodilatation: Implications for skeletal muscle blood flow control and the effects of ageing. J. Physiol. 2012;590:6277–6284. doi: 10.1113/jphysiol.2012.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emerson G.G., Neild T.O., Segal S.S. Conduction of hyperpolarization along hamster feed arteries: Augmentation by acetylcholine. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H102–H109. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- 23.Nelson M.T., Quayle J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Pt 1Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 24.Dora K.A., Doyle M.P., Duling B.R. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc. Natl. Acad. Sci. USA. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandow S.L., Hill C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000;86:341–346. doi: 10.1161/01.RES.86.3.341. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa H., Yasutake H., Fujii K., Owada M.K., Nakaike R., Fukumoto Y., Takayanagi T., Nagao T., Egashira K., Fujishima M., et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric Circulation. J. Cardiovasc. Pharm. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Haas T.L., Duling B.R. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc. Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- 28.Hakim C.H., Jackson W.F., Segal S.S. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandow S.L., Tare M., Coleman H.A., Hill C.E., Parkington H.C. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ. Res. 2002;90:1108–1113. doi: 10.1161/01.RES.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 30.Nagaraja S., Kapela A., Tran C.H., Welsh D.G., Tsoukias N.M. Role of microprojections in myoendothelial feedback—A theoretical study. J. Physiol. 2013;591:2795–2812. doi: 10.1113/jphysiol.2012.248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran C.H., Taylor M.S., Plane F., Nagaraja S., Tsoukias N.M., Solodushko V., Vigmond E.J., Furstenhaupt T., Brigdan M., Welsh D.G. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am. J. Physiol.-Cell Physiol. 2012;302:C1226–C1242. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole W.C., Welsh D.G. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch. Biochem. Biophys. 2011;510:160–173. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Uhrenholt T.R., Domeier T.L., Segal S.S. Propagation of calcium waves along endothelium of hamster feed arteries. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1634–H1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 34.Socha M.J., Behringer E.J., Segal S.S. Calcium and electrical signalling along endothelium of the resistance vasculature. Basic Clin. Pharm. Toxicol. 2012;110:80–86. doi: 10.1111/j.1742-7843.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr P.M., Wei R., Tam R., Sandow S.L., Murphy T.V., Ondrusova K., Lunn S.E., Tran C.H., Welsh D.G., Plane F. Activation of endothelial IKCa channels underlies NO-dependent myoendothelial feedback. Vasc. Pharm. 2015;74:130–138. doi: 10.1016/j.vph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Stankevicius E., Dalsgaard T., Kroigaard C., Beck L., Boedtkjer E., Misfeldt M.W., Nielsen G., Schjorring O., Hughes A., Simonsen U. Opening of small and intermediate calcium-activated potassium channels induces relaxation mainly mediated by nitric-oxide release in large arteries and endothelium-derived hyperpolarizing factor in small arteries from rat. J. Pharm. Exp. 2011;339:842–850. doi: 10.1124/jpet.111.179242. [DOI] [PubMed] [Google Scholar]
- 37.Behringer E.J., Segal S.S. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca2+-activated K+ channels. Circ. Res. 2012;110:1311–1321. doi: 10.1161/CIRCRESAHA.111.262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behringer E.J., Shaw R.L., Westcott E.B., Socha M.J., Segal S.S. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arter. Thromb. Vasc. Biol. 2013;33:1892–1901. doi: 10.1161/ATVBAHA.113.301514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emerson G.G., Segal S.S. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ. Res. 2000;86:94–100. doi: 10.1161/01.RES.86.1.94. [DOI] [PubMed] [Google Scholar]
- 40.Wolfle S.E., Chaston D.J., Goto K., Sandow S.L., Edwards F.R., Hill C.E. Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation. Pt 10J. Physiol. 2011;589:2607–2623. doi: 10.1113/jphysiol.2010.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakim M.A., Buchholz J.N., Behringer E.J. Electrical dynamics of isolated cerebral and skeletal muscle endothelial tubes: Differential roles of G-protein-coupled receptors and K+ channels. Pharm. Res. Perspect. 2018;6:e00391. doi: 10.1002/prp2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stocker M. Ca2+-activated K+ channels: Molecular determinants and function of the SK family. Nat. Rev. Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 43.Kohler M., Hirschberg B., Bond C.T., Kinzie J.M., Marrion N.V., Maylie J., Adelman J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 44.Taylor M.S., Bonev A.D., Gross T.P., Eckman D.M., Brayden J.E., Bond C.T., Adelman J.P., Nelson M.T. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ. Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 45.Bond C.T., Sprengel R., Bissonnette J.M., Kaufmann W.A., Pribnow D., Neelands T., Storck T., Baetscher M., Jerecic J., Maylie J., et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–1946. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- 46.Ishii T.M., Silvia C., Hirschberg B., Bond C.T., Adelman J.P., Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei A.D., Gutman G.A., Aldrich R., Chandy K.G., Grissmer S., Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharm. Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 48.Kohler R., Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflug Arch. Eur. J. Phys. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- 49.Logsdon N.J., Kang J., Togo J.A., Christian E.P., Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- 50.Grgic I., Kaistha B.P., Hoyer J., Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery. Br. J. Pharm. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Si H., Heyken W.T., Wolfle S.E., Tysiac M., Schubert R., Grgic I., Vilianovich L., Giebing G., Maier T., Gross V., et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ. Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 52.Brahler S., Kaistha A., Schmidt V.J., Wolfle S.E., Busch C., Kaistha B.P., Kacik M., Hasenau A.L., Grgic I., Si H., et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 53.Milkau M., Kohler R., de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 2010;24:3572–3579. doi: 10.1096/fj.10-158956. [DOI] [PubMed] [Google Scholar]
- 54.Radtke J., Schmidt K., Wulff H., Kohler R., de Wit C. Activation of KCa3.1 by SKA-31 induces arteriolar dilatation and lowers blood pressure in normo- and hypertensive connexin40-deficient mice. Br. J. Pharm. 2013;170:293–303. doi: 10.1111/bph.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yap F.C., Weber D.S., Taylor M.S., Townsley M.I., Comer B.S., Maylie J., Adelman J.P., Lin M.T. Endothelial SK3 channel-associated Ca2+ microdomains modulate blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H1151–H1163. doi: 10.1152/ajpheart.00787.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doughty J.M., Plane F., Langton P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Pt 2Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- 57.Sandow S.L., Haddock R.E., Hill C.E., Chadha P.S., Kerr P.M., Welsh D.G., Plane F. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clin. Exp. Pharm. Physiol. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 58.Garland C.J., Dora K.A. EDH: Endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. (Oxf.) 2017;219:152–161. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 59.Otani S., Nagaoka T., Omae T., Tanano I., Kamiya T., Ono S., Hein T.W., Kuo L., Yoshida A. Histamine-Induced Dilation of Isolated Porcine Retinal Arterioles: Role of Endothelium-Derived Hyperpolarizing Factor. Investig. Ophthalmol. Vis. Sci. 2016;57:4791–4798. doi: 10.1167/iovs.15-19038. [DOI] [PubMed] [Google Scholar]
- 60.Hannah R.M., Dunn K.M., Bonev A.D., Nelson M.T. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J. Cereb. Blood Flow Metab. 2011;31:1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marrelli S.P., Eckmann M.S., Hunte M.S. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 62.McNeish A.J., Sandow S.L., Neylon C.B., Chen M.X., Dora K.A., Garland C.J. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 63.Feher A., Broskova Z., Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H1595–H1601. doi: 10.1152/ajpheart.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y., Terata K., Chai Q., Li H., Kleinman L.H., Gutterman D.D. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ. Res. 2002;91:1070–1076. doi: 10.1161/01.RES.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]
- 65.Lin M.T., Jian M.Y., Taylor M.S., Cioffi D.L., Yap F.C., Liedtke W., Townsley M.I. Functional coupling of TRPV4, IK, and SK channels contributes to Ca2+-dependent endothelial injury in rodent lung. Pulm. Circ. 2015;5:279–290. doi: 10.1086/680166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marziano C., Hong K., Cope E.L., Kotlikoff M.I., Isakson B.E., Sonkusare S.K. Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries. J. Am. Heart Assoc. 2017;6:e007157. doi: 10.1161/JAHA.117.007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinkler S.Y., Segal S.S. Rapid versus slow ascending vasodilatation: Intercellular conduction versus flow-mediated signalling with tetanic versus rhythmic muscle contractions. J. Physiol. 2017;595:7149–7165. doi: 10.1113/JP275186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bussemaker E., Popp R., Binder J., Busse R., Fleming I. Characterization of the endothelium-derived hyperpolarizing factor (EDHF) response in the human interlobar artery. Kidney Int. 2003;63:1749–1755. doi: 10.1046/j.1523-1755.2003.00910.x. [DOI] [PubMed] [Google Scholar]
- 69.Salomonsson M., Brasen J.C., Sorensen C.M. Role of renal vascular potassium channels in Physiology and pathophysiology. Acta Physiol. (Oxf.) 2017;221:14–31. doi: 10.1111/apha.12882. [DOI] [PubMed] [Google Scholar]
- 70.Waeckel L., Bertin F., Clavreul N., Damery T., Kohler R., Paysant J., Sansilvestri-Morel P., Simonet S., Vayssettes-Courchay C., Wulff H., et al. Preserved regulation of renal perfusion pressure by small and intermediate conductance KCa channels in hypertensive mice with or without renal failure. Pflug. Arch. 2015;467:817–831. doi: 10.1007/s00424-014-1542-y. [DOI] [PubMed] [Google Scholar]
- 71.Kochukov M.Y., Balasubramanian A., Abramowitz J., Birnbaumer L., Marrelli S.P. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J. Am. Heart Assoc. 2014;3:e000913. doi: 10.1161/JAHA.114.000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaston D.J., Baillie B.K., Grayson T.H., Courjaret R.J., Heisler J.M., Lau K.A., Machaca K., Nicholson B.J., Ashton A., Matthaei K.I., et al. Polymorphism in endothelial connexin40 enhances sensitivity to intraluminal pressure and increases arterial stiffness. Arter. Thromb. Vasc. Biol. 2013;33:962–970. doi: 10.1161/ATVBAHA.112.300957. [DOI] [PubMed] [Google Scholar]
- 73.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 74.Dietrich A., Gudermann T. TRP channels in the cardiopulmonary vasculature. Adv. Exp. Med. Biol. 2011;704:781–810. doi: 10.1007/978-94-007-0265-3_41. [DOI] [PubMed] [Google Scholar]
- 75.Yue Z., Xie J., Yu A.S., Stock J., Du J., Yue L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H157–H182. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M.X., Romanin C., Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 77.Ma X., Cao J., Luo J., Nilius B., Huang Y., Ambudkar I.S., Yao X. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arter. Thromb. Vasc. Biol. 2010;30:2249–2255. doi: 10.1161/ATVBAHA.110.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang P., Mao A.Q., Sun C.Y., Zhang X.D., Pan Q.X., Yang D.T., Jin J., Tang C.L., Yang Z.Y., Yao X.Q., et al. Translocation of PKG1alpha acts on TRPV4-C1 heteromeric channels to inhibit endothelial Ca2+ entry. Acta Pharm. Sin. 2016;37:1199–1207. doi: 10.1038/aps.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greenberg H.Z.E., Carlton-Carew S.R.E., Khan D.M., Zargaran A.K., Jahan K.S., Vanessa Ho W.S., Albert A.P. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vasc. Pharm. 2017;96–98:53–62. doi: 10.1016/j.vph.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du J., Ma X., Shen B., Huang Y., Birnbaumer L., Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 2014;28:4677–4685. doi: 10.1096/fj.14-251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thorneloe K.S., Sulpizio A.C., Lin Z., Figueroa D.J., Clouse A.K., McCafferty G.P., Chendrimada T.P., Lashinger E.S., Gordon E., Evans L., et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J. Pharm. Exp. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 82.Cheung M., Bao W., Behm D.J., Brooks C.A., Bury M.J., Dowdell S.E., Eidam H.S., Fox R.M., Goodman K.B., Holt D.A., et al. Discovery of GSK2193874: An Orally Active, Potent, and Selective Blocker of Transient Receptor Potential Vanilloid 4. ACS Med. Chem. Lett. 2017;8:549–554. doi: 10.1021/acsmedchemlett.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sonkusare S.K., Bonev A.D., Ledoux J., Liedtke W., Kotlikoff M.I., Heppner T.J., Hill-Eubanks D.C., Nelson M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bubolz A.H., Mendoza S.A., Zheng X., Zinkevich N.S., Li R., Gutterman D.D., Zhang D.X. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: Role of Ca2+ entry and mitochondrial ROS signaling. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H634–H642. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zitt C., Zobel A., Obukhov A.G., Harteneck C., Kalkbrenner F., Luckhoff A., Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/S0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- 86.Hofmann T., Obukhov A.G., Schaefer M., Harteneck C., Gudermann T., Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 87.Strotmann R., Schultz G., Plant T.D. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J. Biol. Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt K., Dubrovska G., Nielsen G., Fesus G., Uhrenholt T.R., Hansen P.B., Gudermann T., Dietrich A., Gollasch M., de Wit C., et al. Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br. J. Pharm. 2010;161:1722–1733. doi: 10.1111/j.1476-5381.2010.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Launay P., Fleig A., Perraud A.L., Scharenberg A.M., Penner R., Kinet J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/S0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 90.Earley S., Waldron B.J., Brayden J.E. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ. Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 91.Garland C.J., Smirnov S.V., Bagher P., Lim C.S., Huang C.Y., Mitchell R., Stanley C., Pinkney A., Dora K.A. TRPM4 inhibitor 9-phenanthrol activates endothelial cell intermediate conductance calcium-activated potassium channels in rat isolated mesenteric artery. Br. J. Pharm. 2015;172:1114–1123. doi: 10.1111/bph.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Behringer E.J., Scallan J.P., Jafarnejad M., Castorena-Gonzalez J.A., Zawieja S.D., Moore J.E., Jr., Davis M.J., Segal S.S. Calcium and electrical dynamics in lymphatic endothelium. J. Physiol. 2017;595:7347–7368. doi: 10.1113/JP274842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Francis M., Qian X., Charbel C., Ledoux J., Parker J.C., Taylor M.S. Automated region of interest analysis of dynamic Ca2+ signals in image sequences. Am. J. Physiol. Cell Physiol. 2012;303:C236–C243. doi: 10.1152/ajpcell.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Francis M., Waldrup J., Qian X., Taylor M.S. Automated analysis of dynamic Ca2+ signals in image sequences. J. Vis. Exp. 2014 doi: 10.3791/51560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian X., Francis M., Kohler R., Solodushko V., Lin M., Taylor M.S. Positive feedback regulation of agonist-stimulated endothelial Ca2+ dynamics by KCa3.1 channels in mouse mesenteric arteries. Arter. Thromb. Vasc. Biol. 2014;34:127–135. doi: 10.1161/ATVBAHA.113.302506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Francis M., Waldrup J.R., Qian X., Solodushko V., Meriwether J., Taylor M.S. Functional Tuning of Intrinsic Endothelial Ca2+ Dynamics in Swine Coronary Arteries. Circ. Res. 2016;118:1078–1090. doi: 10.1161/CIRCRESAHA.115.308141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson C., Saunter C.D., Girkin J.M., McCarron J.G. Advancing Age Decreases Pressure-Sensitive Modulation of Calcium Signaling in the Endothelium of Intact and Pressurized Arteries. J. Vasc. Res. 2016;53:358–369. doi: 10.1159/000454811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung M.K., Lee H., Mizuno A., Suzuki M., Caterina M.J. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Earley S., Gonzales A.L., Garcia Z.I. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol. Pharm. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pires P.W., Sullivan M.N., Pritchard H.A., Robinson J.J., Earley S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H2031–H2041. doi: 10.1152/ajpheart.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 102.Earley S., Gonzales A.L., Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qian X., Francis M., Solodushko V., Earley S., Taylor M.S. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation. 2013;20:138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sullivan M.N., Gonzales A.L., Pires P.W., Bruhl A., Leo M.D., Li W., Oulidi A., Boop F.A., Feng Y., Jaggar J.H., et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal. 2015;8:ra2. doi: 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kiselyov K., Xu X., Mozhayeva G., Kuo T., Pessah I., Mignery G., Zhu X., Birnbaumer L., Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 106.Senadheera S., Kim Y., Grayson T.H., Toemoe S., Kochukov M.Y., Abramowitz J., Housley G.D., Bertrand R.L., Chadha P.S., Bertrand P.P., et al. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc. Res. 2012;95:439–447. doi: 10.1093/cvr/cvs208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jackson W.F. Endothelial cell ion channel expression and function in arterioles and resistance arteries. In: Levitan I., Dopico A.M., editors. Vascular Ion Channels in Physiology and Disease. Springer; Cham, Switzerland: 2016. pp. 3–36. [Google Scholar]
- 108.Hong K., Cope E.L., DeLalio L.J., Marziano C., Isakson B.E., Sonkusare S.K. TRPV4 (Transient Receptor Potential Vanilloid 4) Channel-Dependent Negative Feedback Mechanism Regulates Gq Protein-Coupled Receptor-Induced Vasoconstriction. Arter. Thromb. Vasc. Biol. 2018;38:542–554. doi: 10.1161/ATVBAHA.117.310038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nausch L.W., Bonev A.D., Heppner T.J., Tallini Y., Kotlikoff M.I., Nelson M.T. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H594–H602. doi: 10.1152/ajpheart.00773.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isakson B.E., Ramos S.I., Duling B.R. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ. Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 111.Wei R., Lunn S.E., Tam R., Gust S.L., Classen B., Kerr P.M., Plane F. Vasoconstrictor stimulus determines the functional contribution of myoendothelial feedback to mesenteric arterial tone. J. Physiol. 2018;596:1181–1197. doi: 10.1113/JP274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aguirre J.A., Lucchinetti E., Clanachan A.S., Plane F., Zaugg M. Unraveling Interactions between Anesthetics and the Endothelium: Update and Novel Insights. Anesth. Analg. 2016;122:330–348. doi: 10.1213/ANE.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 113.Tran C.H., Gordon G.R. Astrocyte and microvascular imaging in awake animals using two-photon microscopy. Microcirculation. 2015;22:219–227. doi: 10.1111/micc.12188. [DOI] [PubMed] [Google Scholar]
- 114.Suarez-Martinez A.D., Peirce S.M., Isakson B.E., Nice M., Wang J., Lounsbury K.M., Scallan J.P., Murfee W.L. Induction of microvascular network growth in the mouse mesentery. Microcirculation. 2018;25:e12502. doi: 10.1111/micc.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biwer L.A., Lechauve C., Vanhoose S., Weiss M.J., Isakson B.E. A Cell Culture Model of Resistance Arteries. J. Vis. Exp. 2017 doi: 10.3791/55992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pires P.W., Dabertrand F., Earley S. Isolation and Cannulation of Cerebral Parenchymal Arterioles. J. Vis. Exp. 2016 doi: 10.3791/53835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilson C., Saunter C.D., Girkin J.M., McCarron J.G. Pressure-dependent regulation of Ca2+ signalling in the vascular endothelium. J. Physiol. 2015;593:5231–5253. doi: 10.1113/JP271157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bagher P., Segal S.S. The mouse cremaster muscle preparation for intravital imaging of the microCirc.ulation. J. Vis. Exp. 2011 doi: 10.3791/2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koot B.G., Alders M., Verheij J., Beuers U., Cobben J.M. A de novo mutation in KCNN3 associated with autosomal dominant idiopathic non-cirrhotic portal hypertension. J. Hepatol. 2016;64:974–977. doi: 10.1016/j.jhep.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 120.Ellinor P.T., Lunetta K.L., Glazer N.L., Pfeufer A., Alonso A., Chung M.K., Sinner M.F., de Bakker P.I., Mueller M., Lubitz S.A., et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat. Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun Y., Yang Y.Q., Gong X.Q., Wang X.H., Li R.G., Tan H.W., Liu X., Fang W.Y., Bai D. Novel germline GJA5/connexin40 mutations associated with lone atrial fibrillation impair gap junctional intercellular communication. Hum. Mutat. 2013;34:603–609. doi: 10.1002/humu.22278. [DOI] [PubMed] [Google Scholar]
- 122.Ghanshani S., Coleman M., Gustavsson P., Wu A.C., Gargus J.J., Gutman G.A., Dahl N., Mohrenweiser H., Chandy K.G. Human calcium-activated potassium channel gene KCNN4 maps to chromosome 19q13.2 in the region deleted in diamond-blackfan anemia. Genomics. 1998;51:160–161. doi: 10.1006/geno.1998.5333. [DOI] [PubMed] [Google Scholar]
- 123.Rapetti-Mauss R., Lacoste C., Picard V., Guitton C., Lombard E., Loosveld M., Nivaggioni V., Dasilva N., Salgado D., Desvignes J.P., et al. A mutation in the Gardos channel is associated with hereditary xerocytosis. Blood. 2015;126:1273–1280. doi: 10.1182/blood-2015-04-642496. [DOI] [PubMed] [Google Scholar]
- 124.Nilius B., Szallasi A. Transient receptor potential channels as drug targets: From the Science of basic research to the art of medicine. Pharm. Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- 125.Letavernier E., Rodenas A., Guerrot D., Haymann J.P. Williams-BEur.en syndrome hypercalcemia: Is TRPC3 a novel mediator in calcium homeostasis? Pediatrics. 2012;129:e1626–e1630. doi: 10.1542/peds.2011-2507. [DOI] [PubMed] [Google Scholar]
- 126.Daumy X., Amarouch M.Y., Lindenbaum P., Bonnaud S., Charpentier E., Bianchi B., Nafzger S., Baron E., Fouchard S., Thollet A., et al. Targeted resequencing identifies TRPM4 as a major gene predisposing to progressive familial heart block type I. Int. J. Cardiol. 2016;207:349–358. doi: 10.1016/j.ijcard.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 127.Deng A.Y., deBlois D., Laporte S.A., Gelinas D., Tardif J.C., Thorin E., Shi Y., Raignault A., Menard A. Novel Pathogenesis of Hypertension and Diastolic Dysfunction Caused by M3R (Muscarinic Cholinergic 3 Receptor) Signaling. Hypertension. 2018;72:755–764. doi: 10.1161/HYPERTENSIONAHA.118.11385. [DOI] [PubMed] [Google Scholar]
- 128.Wang L., Guo D.C., Cao J., Gong L., Kamm K.E., Regalado E., Li L., Shete S., He W.Q., Zhu M.S., et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am. J. Hum. Genet. 2010;87:701–707. doi: 10.1016/j.ajhg.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 130.Abete P., Della-Morte D., Gargiulo G., Basile C., Langellotto A., Galizia G., Testa G., Canonico V., Bonaduce D., Cacciatore F. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res. Rev. 2014;18:41–52. doi: 10.1016/j.arr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 131.Berridge M.J. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150434. doi: 10.1098/rstb.2015.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bugiardini R., Manfrini O., Pizzi C., Fontana F., Morgagni G. Endothelial function predicts future development of coronary artery disease: A study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 133.Schachinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 134.Segal S.S. Integration and Modulation of Intercellular Signaling Underlying Blood Flow Control. J. Vasc. Res. 2015;52:136–157. doi: 10.1159/000439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Climent B., Moreno L., Martinez P., Contreras C., Sanchez A., Perez-Vizcaino F., Garcia-Sacristan A., Rivera L., Prieto D. Upregulation of SK3 and IK1 channels contributes to the enhanced endothelial calcium signaling and the preserved coronary relaxation in obese Zucker rats. PLoS ONE. 2014;9:e109432. doi: 10.1371/journal.pone.0109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chadha P.S., Haddock R.E., Howitt L., Morris M.J., Murphy T.V., Grayson T.H., Sandow S.L. Obesity up-regulates intermediate conductance calcium-activated potassium channels and myoendothelial gap junctions to maintain endothelial vasodilator function. J. Pharm. Exp. 2010;335:284–293. doi: 10.1124/jpet.110.167593. [DOI] [PubMed] [Google Scholar]
- 137.Giachini F.R., Carneiro F.S., Lima V.V., Carneiro Z.N., Dorrance A., Webb R.C., Tostes R.C. Upregulation of intermediate calcium-activated potassium channels counterbalance the impaired endothelium-dependent vasodilation in stroke-prone spontaneously hypertensive rats. Transl. Res. 2009;154:183–193. doi: 10.1016/j.trsl.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schach C., Resch M., Schmid P.M., Riegger G.A., Endemann D.H. Type 2 diabetes: Increased expression and contribution of IKCa channels to vasodilation in small mesenteric arteries of ZDF rats. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1093–H1102. doi: 10.1152/ajpheart.00240.2013. [DOI] [PubMed] [Google Scholar]
- 139.Yada T., Shimokawa H., Tachibana H. Endothelium-dependent hyperpolarization-mediated vasodilatation compensates nitric oxide-mediated endothelial dysfunction during ischemia in diabetes-induced canine coronary collateral microcirculation in vivo. Microcirculation. 2018;25:e12456. doi: 10.1111/micc.12456. [DOI] [PubMed] [Google Scholar]
- 140.Mokhtar S.S., Vanhoutte P.M., Leung S.W., Yusof M.I., Wan Sulaiman W.A., Mat Saad A.Z., Suppian R., Rasool A.H. Endothelium dependent hyperpolarization-type relaxation compensates for attenuated nitric oxide-mediated responses in subcutaneous arteries of diabetic patients. Nitric Oxide. 2016;53:35–44. doi: 10.1016/j.niox.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 141.Bondarenko A.I., Panasiuk O., Okhai I., Montecucco F., Brandt K.J., Mach F. Ca2+-dependent potassium channels and cannabinoid signaling in the endothelium of apolipoprotein E knockout mice before plaque formation. J. Mol. Cell. Cardiol. 2018;115:54–63. doi: 10.1016/j.yjmcc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 142.Stead R., Musa M.G., Bryant C.L., Lanham S.A., Johnston D.A., Reynolds R., Torrens C., Fraser P.A., Clough G.F. Developmental conditioning of endothelium-derived hyperpolarizing factor-mediated vasorelaxation. J. Hypertens. 2016;34:452–463. doi: 10.1097/HJH.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi S., Kim J.A., Li H.Y., Shin K.O., Oh G.T., Lee Y.M., Oh S., Pewzner-Jung Y., Futerman A.H., Suh S.H. KCa3.1 upregulation preserves endothelium-dependent vasorelaxation during aging and oxidative stress. Aging Cell. 2016;15:801–810. doi: 10.1111/acel.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Behringer E.J., Segal S.S. Impact of Aging on Calcium Signaling and Membrane Potential in Endothelium of Resistance Arteries: A Role for Mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:1627–1637. doi: 10.1093/gerona/glx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naik J.S., Walker B.R. Endothelial-dependent dilation following chronic hypoxia involves TRPV4-mediated activation of endothelial BK channels. Pflug. Arch. 2018;470:633–648. doi: 10.1007/s00424-018-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jobs A., Schmidt K., Schmidt V.J., Lubkemeier I., van Veen T.A., Kurtz A., Willecke K., de Wit C. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension. 2012;60:1422–1429. doi: 10.1161/HYPERTENSIONAHA.112.201194. [DOI] [PubMed] [Google Scholar]
- 147.Givvimani S., Narayanan N., Armaghan F., Pushpakumar S., Tyagi S.C. Attenuation of conducted vasodilation in skeletal muscle arterioles during hyperhomocysteinemia. Pharmacology. 2013;91:287–296. doi: 10.1159/000350394. [DOI] [PubMed] [Google Scholar]
- 148.Lemmey H.A.L., Ye X., Ding H.C., Triggle C.R., Garland C.J., Dora K.A. Hyperglycaemia disrupts conducted vasodilation in the resistance vasculature of db/db mice. Vasc. Pharm. 2018;103–105:29–35. doi: 10.1016/j.vph.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bachschmid M.M., Schildknecht S., Matsui R., Zee R., Haeussler D., Cohen R.A., Pimental D., Loo B. Vascular aging: Chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann. Med. 2013;45:17–36. doi: 10.3109/07853890.2011.645498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muller-Delp J.M., Gurovich A.N., Christou D.D., Leeuwenburgh C. Redox balance in the aging microCirc.ulation: New friends, new foes, and new clinical directions. Microcirculation. 2012;19:19–28. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chidgey J., Fraser P.A., Aaronson P.I. Reactive oxygen species facilitate the EDH response in arterioles by potentiating intracellular endothelial Ca2+ release. Free Radic. Biol. Med. 2016;97:274–284. doi: 10.1016/j.freeradbiomed.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Feletou M. Endothelium-Dependent Hyperpolarization and Endothelial Dysfunction. J. Cardiovasc. Pharm. 2016;67:373–387. doi: 10.1097/FJC.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 154.Leo C.H., Hart J.L., Woodman O.L. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br. J. Pharm. 2011;162:365–377. doi: 10.1111/j.1476-5381.2010.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Long D.A., Newaz M.A., Prabhakar S.S., Price K.L., Truong L.D., Feng L., Mu W., Oyekan A.O., Johnson R.J. Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in aging. Kidney Int. 2005;68:2154–2163. doi: 10.1111/j.1523-1755.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 156.Chennupati R., Lamers W.H., Koehler S.E., De Mey J.G. Endothelium-dependent hyperpolarization-related relaxations diminish with age in murine saphenous arteries of both sexes. Br. J. Pharm. 2013;169:1486–1499. doi: 10.1111/bph.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seki T., Goto K., Kiyohara K., Kansui Y., Murakami N., Haga Y., Ohtsubo T., Matsumura K., Kitazono T. Downregulation of Endothelial Transient Receptor Potential Vanilloid Type 4 Channel and Small-Conductance of Ca2+-Activated K+ Channels Underpins Impaired Endothelium-Dependent Hyperpolarization in Hypertension. Hypertension. 2017;69:143–153. doi: 10.1161/HYPERTENSIONAHA.116.07110. [DOI] [PubMed] [Google Scholar]
- 158.Gradel A.K.J., Salomonsson M., Sorensen C.M., Holstein-Rathlou N.H., Jensen L.J. Long-term diet-induced hypertension in rats is associated with reduced expression and function of small artery SKCa, IKCa, and Kir2.1 channels. Clin. Sci. (Lond.) 2018;132:461–474. doi: 10.1042/CS20171408. [DOI] [PubMed] [Google Scholar]
- 159.Wandall-Frostholm C., Dalsgaard T., Bajoriunas V., Olivan-Viguera A., Sadda V., Beck L., Mogensen S., Stankevicius E., Simonsen U., Kohler R. Genetic deficit of KCa 3.1 channels protects against pulmonary Circ.ulatory collapse induced by TRPV4 channel activation. Br. J. Pharmacol. 2015;172:4493–4505. doi: 10.1111/bph.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Simonsen U., Wandall-Frostholm C., Olivan-Viguera A., Kohler R. Emerging roles of calcium-activated K channels and TRPV4 channels in lung oedema and pulmonary Circ.ulatory collapse. Acta Physiol. (Oxf.) 2017;219:176–187. doi: 10.1111/apha.12768. [DOI] [PubMed] [Google Scholar]
- 161.Dalsgaard T., Sonkusare S.K., Teuscher C., Poynter M.E., Nelson M.T. Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice. Sci. Rep. 2016;6:33841. doi: 10.1038/srep33841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jones J.L., Peana D., Veteto A.B., Lambert M.D., Nourian Z., Karasseva N.G., Hill M.A., Lindman B.R., Baines C.P., Krenz M., et al. TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc. Res. 2019;115:46–56. doi: 10.1093/cvr/cvy156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nachman R.L., Jaffe E.A. Endothelial cell culture: Beginnings of modern vascular biology. J. Clin. Investig. 2004;114:1037–1040. doi: 10.1172/JCI23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wilson C., Lee M.D., Heathcote H.R., Zhang X., Buckley C., Girkin J.M., Saunter C.D., McCarron J.G. Mitochondrial ATP production provides long-range control of endothelial inositol trisphosphate-evoked calcium signaling. J. Biol. Chem. 2019;294:737–758. doi: 10.1074/jbc.RA118.005913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Durand M.J., Ait-Aissa K., Levchenko V., Staruschenko A., Gutterman D.D., Beyer A.M. Visualization and Quantification of Mitochondrial Structure in the Endothelium of Intact Arteries. Cardiovasc. Res. 2018 doi: 10.1093/cvr/cvy294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Busija D.W., Rutkai I., Dutta S., Katakam P.V. Role of Mitochondria in Cerebral Vascular Function: Energy Production, Cellular Protection, and Regulation of Vascular Tone. Compr. Physiol. 2016;6:1529–1548. doi: 10.1002/cphy.c150051. [DOI] [PubMed] [Google Scholar]
- 167.Kaczara P., Motterlini R., Rosen G.M., Augustynek B., Bednarczyk P., Szewczyk A., Foresti R., Chlopicki S. Carbon monoxide released by CORM-401 uncouples mitochondrial respiration and inhibits glycolysis in endothelial cells: A role for mitoBKCa channels. Biochim. Biophys. Acta. 2015;1847:1297–1309. doi: 10.1016/j.bbabio.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 168.Kanagy N.L., Kevil C.G. The pleiotropic effects of hydrogen sulfide. Am. J. Physiol. Heart Circ. Physiol. 2018;314:H1–H2. doi: 10.1152/ajpheart.00613.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Naik J.S., Osmond J.M., Walker B.R., Kanagy N.L. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1437–H1444. doi: 10.1152/ajpheart.00465.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tang G., Yang G., Jiang B., Ju Y., Wu L., Wang R. H2S is an endothelium-derived hyperpolarizing factor. Antioxid. Redox Signal. 2013;19:1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 171.Durante W. Targeting heme oxygenase-1 in vascular disease. Curr. Drug Targets. 2010;11:1504–1516. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McRae K.E., Pudwell J., Peterson N., Smith G.N. Inhaled carbon monoxide increases vasodilation in the microvascular circulation. Microvasc. Res. 2019;123:92–98. doi: 10.1016/j.mvr.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 173.Rezkalla S., Kloner R.A. Cardiovascular effects of marijuana. Trends Cardiovasc. Med. 2018 doi: 10.1016/j.tcm.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 174.Ho W.S., Zheng X., Zhang D.X. Role of endothelial TRPV4 channels in vascular actions of the endocannabinoid, 2-arachidonoylglycerol. Br. J. Pharm. 2015;172:5251–5264. doi: 10.1111/bph.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stanley C.P., O’Sullivan S.E. Cyclooxygenase metabolism mediates vasorelaxation to 2-arachidonoylglycerol (2-AG) in human mesenteric arteries. Pharm. Res. 2014;81:74–82. doi: 10.1016/j.phrs.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bondarenko A.I., Panasiuk O., Drachuk K., Montecucco F., Brandt K.J., Mach F. The quest for endothelial atypical cannabinoid receptor: BKCa channels act as cellular sensors for cannabinoids in in vitro and in situ endothelial cells. Vasc. Pharm. 2018;102:44–55. doi: 10.1016/j.vph.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brandes R.P., Schmitz-Winnenthal F.H., Feletou M., Godecke A., Huang P.L., Vanhoutte P.M., Fleming I., Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Limbu R., Cottrell G.S., McNeish A.J. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS ONE. 2018;13:e0192484. doi: 10.1371/journal.pone.0192484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Idris Khodja N., Chataigneau T., Auger C., Schini-Kerth V.B. Grape-derived polyphenols improve aging-related endothelial dysfunction in rat mesenteric artery: Role of oxidative stress and the angiotensin system. PLoS ONE. 2012;7:e32039. doi: 10.1371/journal.pone.0032039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Han J., Xu H.H., Chen X.L., Hu H.R., Hu K.M., Chen Z.W., He G.W. Total Flavone of Rhododendron Improves Cerebral Ischemia Injury by Activating Vascular TRPV4 to Induce Endothelium-Derived Hyperpolarizing Factor-Mediated Responses. Evid.-Based Complement. Altern. Med. 2018;2018:8919867. doi: 10.1155/2018/8919867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ruamyod K., Watanapa W.B., Shayakul C. Testosterone rapidly increases Ca2+-activated K+ currents causing hyperpolarization in human coronary artery endothelial cells. J. Steroid Biochem. Mol. Biol. 2017;168:118–126. doi: 10.1016/j.jsbmb.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 182.Mazzuca M.Q., Mata K.M., Li W., Rangan S.S., Khalil R.A. Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]i in mesenteric microvessels of female rat. J. Pharm. Exp. 2015;352:291–304. doi: 10.1124/jpet.114.219865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aird W.C. Spatial and temporal dynamics of the endothelium. J. Thromb. Haemost. 2005;3:1392–1406. doi: 10.1111/j.1538-7836.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- 184.Fishman A.P. Endothelium: A distributed organ of diverse capabilities. Ann. N. Y. Acad. Sci. 1982;401:1–8. doi: 10.1111/j.1749-6632.1982.tb25702.x. [DOI] [PubMed] [Google Scholar]
- 185.Hielscher D., Kaebisch C., Braun B.J.V., Gray K., Tobiasch E. Stem Cell Sources and Graft Material for Vascular Tissue Engineering. Stem Cell Rev. 2018;14:642–667. doi: 10.1007/s12015-018-9825-x. [DOI] [PubMed] [Google Scholar]
- 186.Mauricio M.D., Guerra-Ojeda S., Marchio P., Valles S.L., Aldasoro M., Escribano-Lopez I., Herance J.R., Rocha M., Vila J.M., Victor V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxid. Med. Cell. Longev. 2018;2018:6231482. doi: 10.1155/2018/6231482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Phan D.T., Bender R.H.F., Andrejecsk J.W., Sobrino A., Hachey S.J., George S.C., Hughes C.C. Blood-brain barrier-on-a-chip: MicroPhysiol.ogical systems that capture the complexity of the blood-central nervous system interface. Exp. Biol. Med. (Maywood) 2017;242:1669–1678. doi: 10.1177/1535370217694100. [DOI] [PMC free article] [PubMed] [Google Scholar]