Abstract
The biologic and prognostic value of focal neuroendocrine differentiation (NED) in conventional prostate adenocarcinoma (PC) patients who undergo radical prostatectomy (RP) remains controversial. In this systematic review and meta-analysis, we assessed the association of focal NED in conventional PC with oncological outcomes after RP. A literature search using PubMed, Scopus, Web of Science, and Cochrane Library was conducted on December 2018 to find relevant studies according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. We used a fixed-effect model to analyze the impact of focal NED in RP specimen on progression-free survival defined by biochemical recurrence (BCR). A total of 16 studies with the outcomes of disease progression and survival were eligible. No patient in these studies received androgen deprivation therapy prior to RP. Eleven studies found no significant correlation between focal NED and outcomes of interest, while five studies reported a significant association of focal NED assessed by immunohistochemical chromogranin A or serotonin staining with BCR or survival. Focal NED was associated with higher BCR rates after RP with a pooled HR of 1.39 (95% CI 1.07‒1.81) in five studies. No heterogeneity was reported in this analysis (I2 = 21.7%, p = 0.276). In conclusion, focal NED in conventional PC is associated with worse prognosis after RP. Its presence should be reported in pathologic reports and its true clinical impact should be assessed in well-designed prospective controlled studies.
Keywords: neuroendocrine differentiation, chromogranin A, prostate cancer, radical prostatectomy, oncological outcome, survival
1. Introduction
Prostate cancer (PC) is the most common solid cancer and the second most common cause of cancer-related death in men [1]. Over 90% of newly diagnosed PCs in developed countries are clinically localized to the origin. The standard treatment for these tumors is either active surveillance or local therapy with radical prostatectomy (RP) or radiation therapy. While these therapies result in durable local and distant disease control [2,3]. A significant number of patients eventually experience biochemical recurrence (BCR) despite effective definitive local therapy with curative intent (up to 35% at 10 years following RP) [4,5,6,7].
Recently, increasing attention has been given to neuroendocrine differentiation (NED) of PC recognizing its potential diagnostic, prognostic, and therapeutic utility [8]. Neuroendocrine cells are androgen-independent because of their negative androgen receptor expression [8]. NED is an important factor influencing the development of PC toward an androgen-independent, lethal phenotype.
Focal NED in conventional prostatic adenocarcinoma (PC) is one of the pathologically defined neuroendocrine manifestations in prostate gland, and its diagnosis is based on the detection of neuroendocrine cells using immunohistochemical analysis of biomarkers such as chromogranin A (CgA) and serotonin. Poorly differentiated prostatic neuroendocrine carcinomas including small and large cell carcinoma have been shown to harbor an aggressive clinical behavior and poor prognosis [9]. However, the prognostic value of focal NED in conventional PC remains controversial and its diagnostic and, specifically, clinical impact is poorly investigated.
To elucidate the prognostic value of focal NED, we performed a systematic review and meta-analysis investigating the impact of focal NED in conventional PC after RP on oncological outcomes including disease progression (i.e., BCR, local recurrence, and distant metastasis) and survival outcomes.
2. Results
2.1. Results of Search
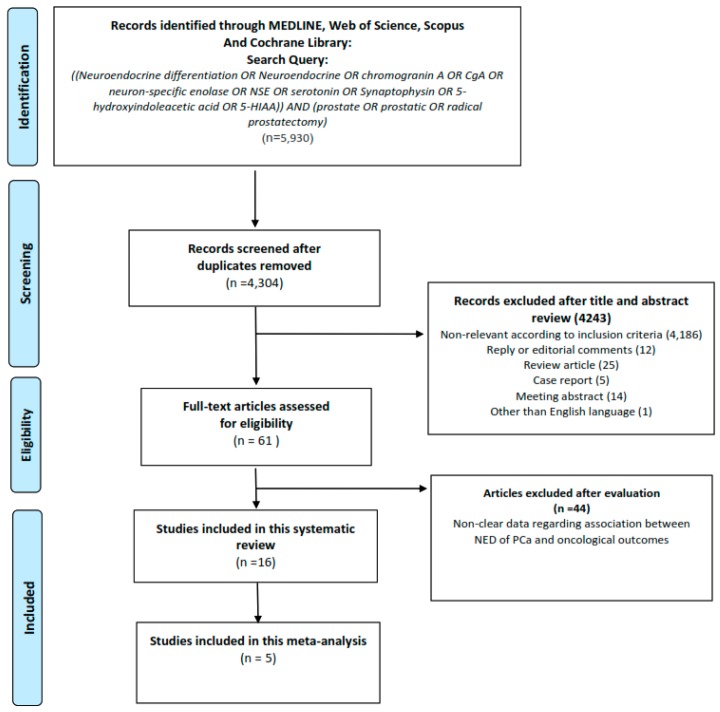
A total of 5930 studies were found for an initial assessment. Of these, 1626 duplicates were removed. After exclusion of non-relevant studies, review articles, meeting abstracts, case reports, replies, expert opinions, editorials or commentaries, and studies in languages other than English, 61 studies were reviewed. We finally identified 16 studies for systematic review and 5 studies for qualitative meta-analysis (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow chart for article selection process to analyze the impact of focal neuroendocrine differentiation in conventional prostate adenocarcinoma and oncological outcomes.
2.2. Characteristics of the Included Studies
The studies’ characteristics and patients’ clinical data are summarized in Table 1 and Table 2, respectively. The 16 studies comprised 2039 patients treated with RP. Included patients in these studies received no androgen deprivation therapy (ADT) prior to RP (as ADT can be a driver of neuroendocrine differentiation). The examined population was Northern American in seven studies, European in seven and Asian in two. All studies were designed retrospectively and were published between 1994 and 2017. All 16 included studies assessed CgA as a tissue marker for NED. Four [10,11,12,13] and three [10,14,15] studies used serotonin and neuron specific enolase (NSE) markers in addition to CgA to evaluate NED, respectively. BCR, the most frequently used oncologic outcome after RP, was reported in 13 studies [10,12,13,14,15,16,17,18,19,20,21,22,23]. Other reported oncologic endpoints included local recurrence, distant metastasis, and cancer-specific and overall survival. Follow-up ranged from 17.1 months to 17.3 years. Eleven studies found no significant association of focal NED with any oncologic outcome. Five studies comprising a total 1013 patients, in contrast, demonstrated a significant association of focal NED, as assessed using CgA or serotonin staining, with the prespecified oncologic endpoints of interest. [11,17,19,22,24]. The risk of bias in these16 studies is shown in Table 3.
Table 1.
Study characteristics of 16 studies assessing the role of neuroendocrine differentiation tissue markers in oncological outcomes after radical prostatectomy.
| Author | Year | Region | Design | Recruitment Period | No. pts | Markers | Oncological End Point |
|---|---|---|---|---|---|---|---|
| Cohen [15] | 1994 | USA | Retrospective | 1986–1989 | 38 | CgA, NSE | Disease progression (LR, BCR, DM) |
| Noordzij [25] | 1995 | Netherlands | Retrospective | 1977–1987 | 90 | CgA | Disease progression (LR, DM), CSS |
| Bubendorf [14] | 1996 | Switzerland | Retrospective | 1978–1993 | 137 | CgA, NSE | Disease progression (LR, BCR, DM) |
| Weinstein [22] | 1996 | USA | Retrospective | N/A | 104 | CgA | PFS (BCR) |
| Theodorescu [24] | 1997 | USA | Retrospective | 1970–1984 | 71 | CgA | DSS, Long-term Survival |
| Abrahamsson [10] | 1998 | USA | Retrospective | 1973–1989 | 87 | S, CgA, NSE | Disease progression (LR, BCR, DM) |
| Krupski [18] | 2000 | USA | Retrospective | 1970–1984 | 42 | CgA | DSS (LR, BCR, DM) |
| Ahlgren [16] | 2000 | Sweden | Retrospective | N/A | 53 | CgA | PFS (BCR) |
| Bostwick [11] | 2002 | USA | Retrospective | 1987-1992 | 196 | S, CgA | DM, CSD, All cause death |
| Revelos [20] | 2007 | Greece | Retrospective | N/A | 130 | CgA | PFS (BCR) |
| Gunia [17] | 2008 | Germany | Retrospective | 1996–2003 | 528 | CgA | BFS (BCR) |
| Veltri [21] | 2008 | USA | Retrospective | 1975–1991 | 105 | CgA | PFS (LR, BCR, DM) |
| Ishida [12] | 2008 | Japan | Retrospective | N/A | RP (50) Vs NADT +RP (46) | S, CgA | BCR |
| Ma [19] | 2010 | Japan | Retrospective | N/A | RP (114) of PCa cases (435) | CgA | BFS (BCR) |
| Heinrich [13] | 2011 | Germany | Retrospective | N/A | 175 | S, CgA | BCR |
| Genitsch [23] | 2017 | Switzerland | Retrospective | 1989–2006 | 119 | CgA | PFS (BCR), CSS, OS |
S: serotonin, CgA: chromogranin A, NSE: neuron-specific enolase, RP: radical prostatectomy, PCa: prostate cancer, N/A: not available, LR: local recurrence, BCR: biochemical recurrence, DM: distant metastasis, PFS: progression-free survival, CSD: cancer-specific death, BFS: biochemical free survival, CSS: cancer-specific survival, NADT: neo-adjuvant androgen deprivation therapy, OS: overall survival.
Table 2.
Patient characteristics in 16 studies assessing the prognostic role of neuroendocrine differentiation after radical prostatectomy.
| Author | Age, Year (mean/median) | Pre-Operative PSA, mg/dl (n) | Surgical GS (n) | Pathological Stage (n) | Follow-up Duration | Independent Correlation with Oncologic Outcomes |
|---|---|---|---|---|---|---|
| Cohen [15] | N/A | N/A | ≤6 (20), 7 (14), ≥8 (4) | II† (22), III (16) | Mean: 50.5 months (range, 2–77) | NS |
| Noordzij [25] | 62 (range, 47–74) | N/A | ≤6 (26)/7 (36)/≥8 (28) | T2 (22), T3 (66), T4 (2), N+ (7) | Mean: 86 months (range, 1–203) | NS |
| Bubendorf [14] | 65.3 (range, 45–82) | N/A | <7(68), ≥7 (69) | PT1 (4), PT2 (43), PT3 (90), N+(34) | Mean: 5.4 years (range, 1–15) |
NS |
| Weinstein [22] | N/A | N/A | ≤6 (59), >6 (45) | Organ confined (21%) SVI (0), LNI (0) |
Mean: 8 years (range,7–10) | S |
| Theodorescu [24] | 60.5 (range, 42–72) | N/A | ≤7 (48), ≥8 (23) | Capsular penetration: −(37), +(31), N/A(3) SVI: −(50), +(13), N/A(8) LNI: −(13), +(1), N/A(57) |
N/A | S |
| Abrahamsson [10] | 66 (range, 50–77) | N/A | Mean GS: 6–7 | A (1), B (27), C (50), D (9) | Mean: 4.2 years (range, 1.8–10.1) |
NS |
| Krupski [18] | 62 (range, 42–72) | N/A | ≤6 (22), 7 (3), ≥8 (17) | N/A | Median: 10 years (range, 3.5–20) | NS |
| Ahlgren [16] | N/A | <10 (24), 11–20 (22), >20 (7) | ≤6 (19), 7 (16), ≥8 (18) | T1b-T1c (22), T2-3 (31) | Mean: 39 ± 1 months | NS |
| Bostwick [11] | 65.7 (range, 47–79) | Median: 21.4 (range, 0.9–616) | N/A | N+ (196) | Mean: 6.8 years (range, 0.3–11) |
NS* |
| Revelos [20] | 66 (range, 47–76) | Median: 9.23 (2.5–45.0) | ≤6 (29), 7 (75), ≥8 (26) | ECE: +(70) −(60)/SVI: +(34) −(96), LNI: +(10) −(120) | Median: 28 months (1–97) | NS |
| Gunia [17] | 63.8 (range, 44–79) | ≤20 (472), >20 (56) | ≤6 (316), 7 (157), ≥8 (55) | T2 (367), T3 (149), T4 (12), N0 (412), N1 (38), Nx (78) | Median: 46.4 months (range, 10–116) | S |
| Veltri [21] | 59.62 | N/A | < 7 (64), ≥ 7 (41) | T2: (75), >T2 (30) SVI: +(1), −(104) LNI (0) |
Mean: 17.3 years (range:2–26) | NS |
| Ishida [12] | 69 (range, 54–78) | Mean 7.5 (range 0.0–50.3) | RP: ≤6 (25), 7 (21), ≥8 (4)/NADT +RP: ≤6 (13), 7 (8), ≥8 (12) | I (24), II (29), III (25), IV(12) | N/A | NS |
| Ma [19] | 70.28±7.43 | N/A | ≤6 (14), 7 (202), ≥8 (164), N/A (55) | T1a-bN0M0 (10), T1c-2N0M0 (191), T3-4N0M0 (83), T1-4N1M0-1 (25), T1-4N0-1M1 (126) | N/A | S |
| Heinrich [13] | 63.3 ± 5.9 years | N/A | ≤6 (86), 7 (63), ≥8 (24) | T2 (85), T3 (86), T4 (3) | Medium:17.1 months (range, 2–44) | NS |
| Genitsch [23] | 65 (range, 45–75) | N/A | ≤6 (12), 7 (63), ≥8 (44) | T2: 14, T3:105, N+ (119) | Median: 5.9 years (0.1–15.2) | NS |
RP: radical prostatectomy, N/A: not available, NED: neuroendocrine differentiation, NADT: neo-adjuvant androgen deprivation therapy, PSA: prostate-specific antigen, S: significant, NS: not-significant, ECE: extra capsular extension, SV: seminal vesicle invasion, LNI: lymph node invasion. *serotonin in benign epithelium was associated with cancer specific death but not distant metastasis or all cause survival. † Stage II: organ confined disease, III: extra capsular extension, seminal vesicle invasion, positive surgical margin.
Table 3.
Risk of bias assessment for individual studies using the “Risk Of Bias In Non-randomised Studies - of Interventions” tool (ROBINS-I).
| Author | Confounding | Participant Selection | Classification of Interventions | Deviations from Intended Intervention | Missing Data | Measurement of Outcomes | Selection of the Reported Result |
Overall |
|---|---|---|---|---|---|---|---|---|
| Abrahamsson [10] | Serious | Serious | Low | Serious | Low | Moderate | Low | Serious |
| Ahlgren [16] | Serious | Serious | Low | Moderate | Low | Low | Low | Serious |
| Bostwick [11] | Serious | Serious | Low | Serious | Low | Moderate | Moderate | Serious |
| Bubendorf [14] | Serious | Serious | Low | Moderate | Low | Low | Moderate | Serious |
| Cohen [15] | Serious | Serious | Low | Serious | Low | Moderate | Low | Serious |
| Gunia [17] | Serious | Serious | Low | Serious | Low | Moderate | Low | Serious |
| Heinrich [13] | Serious | Serious | Low | Serious | Low | Low | Moderate | Serious |
| Ishida [12] | Serious | Serious | Low | Serious | Low | Moderate | Moderate | Serious |
| Krupski [18] | Serious | Serious | Low | Serious | Low | Moderate | Moderate | Moderate |
| Ma [19] | Serious | Serious | Low | Low | Low | Moderate | Low | Serious |
| Noordzij [25] | Serious | Serious | Low | Moderate | Low | Low | Moderate | Serious |
| Revelos [20] | Moderate | Serious | Low | Serious | Low | Low | Moderate | Serious |
| Theodorescu [24] | Serious | Moderate | Low | Serious | Low | Moderate | Low | Serious |
| Veltri [21] | Serious | Serious | Low | Serious | Low | Low | Moderate | Serious |
| Weinstein [22] | Moderate | Moderate | Low | Moderate | Low | Moderate | Low | Moderate |
| Genitsch [23] | Serious | Serious | Low | Serious | Low | Moderate | Moderate | Serious |
2.3. Meta-Analysis
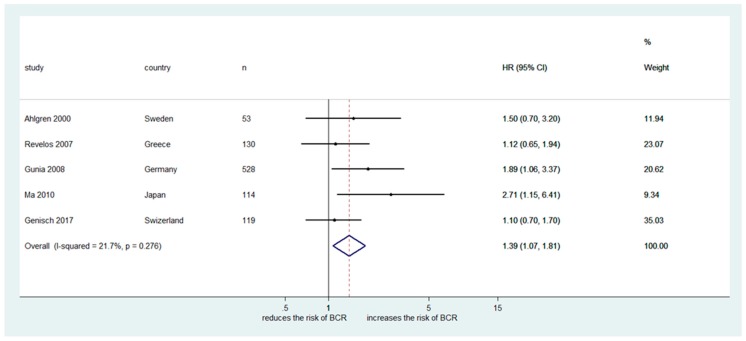
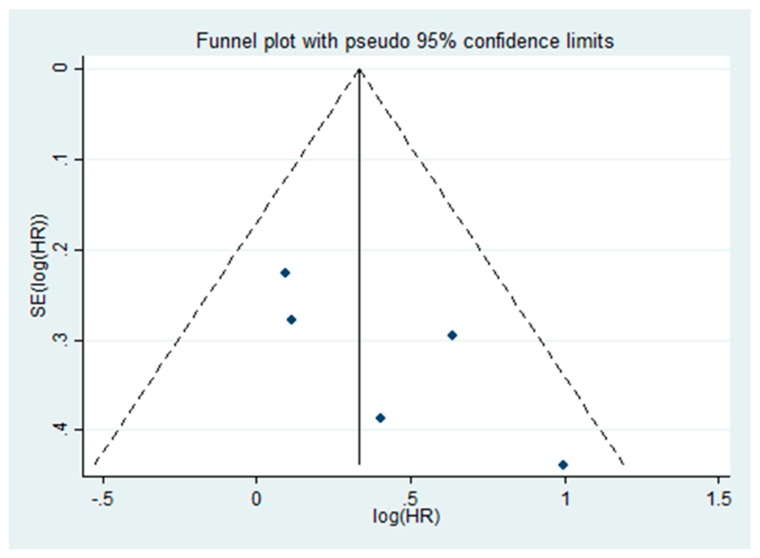
The impact of focal NED in RP specimen on BCR using HR was investigated in five studies including a total of 944 patients [16,17,19,20,23]. All five studies used immunohistochemical CgA expression as NED marker. The Cochrane Q test (chi-square 3.24, p = 0.276) and the I2 test (I2 = 21.7%) revealed no heterogeneity. Therefore, we used a fixed-effect model. The forest plot (Figure 2) shows that focal NED was significantly associated with BCR after RP (pooled HR: 1.39, 95% CI 1.07‒1.81). Funnel plot analysis did not identify any publication bias (Figure 3).
Figure 2.
Forest plot presenting significant association of focal neuroendocrine differentiation in conventional prostate adenocarcinoma and biochemical recurrence after radical prostatectomy.
Figure 3.
Funnel plot demonstrates no publication bias in five assessed studies in this meta-analysis.
3. Discussion
This systematic review and meta-analysis aimed to elucidate the prognostic value of focal NED in conventional PC on disease progression and survival after RP. We found that PC patients with focal NED in their RP specimen have an increased probability of BCR compared to patients without focal NED. However, this conclusion is based on mostly small retrospective studies making strong recommendations impossible. Moreover, the impact of focal NED in RP specimen on other endpoints such as cancer-specific and overall survival was only assessed in a limited number of studies, further weakening the possibility of making solid recommendations.
Neuroendocrine cells do not express prostate specific antigen (PSA) and androgen receptors [8,9]. Rapid disease progression in patients with a low serum PSA in pure neuroendocrine PC such as small cell carcinoma reflects the aggressive behavior of these tumors and the difference from standard follow-up strategies used for PC [26]. There is no clear understanding regarding the exact function of neuroendocrine cells in the prostate, but these cells are known to contribute to the inhibition of cellular apoptosis in PC through modulation of factors such as survivin, thereby enhancing the likelihood of BCR after RP [27,28,29]. Moreover, neuroendocrine cells may regulate the growth process of epithelial cells in prostate tissue by secretion of neuropeptides (e.g., bombesin, calcitonin, and serotonin), growth factors (e.g., vascular endothelial growth factor), and factors degrading the extracellular matrix (e.g., urokinase plasminogen activation system) [30,31,32,33].
Pathologic characteristics such as Gleason score and TNM classification of malignant tumors are well established prognostic factors after RP providing important information regarding the likelihood of BCR and survival [2]. NED formation in conventional PC treated with ADT is associated with rapidly progressive hormone resistant disease [34]. However, it still remains controversial whether patients with focal NED in conventional PC without history of ADT have worse prognosis when compared to those without focal NED. In our study, the number of patients included in studies reporting significant impact of NED on defined oncologic outcomes are comparable to those studies that showed no significant difference. One could conclude that there was no sample size effect on the difference of the results between studies. Currently, there is no recommendation for routine immunohistochemical staining of prostatic adenocarcinoma for neuroendocrine markers [35].
Neuroendocrine PC cells may not produce and/or leak PSA in the same amount as conventional PC. Therefore, monitoring with serum PSA evaluation is not ideal to identify progressive disease in patients harboring focal NED PC [36]. Due to the significant effect of focal NED on disease-specific and overall survival outcomes in some of the included studies in our review, serum neuroendocrine markers such as CgA and NSE might be considered as tumor markers for monitoring of PC patients who harbor focal NED on their RP [37]. This assumption needs, however, to be validated in well-designed studies.
Although the current study represents the first systematic review and meta-analysis demonstrating the prognostic impact of focal NED in conventional PC on significant oncologic outcomes including BCR after RP, it has some limitations. The retrospective nature of the studies, generally small cohorts, variability in CgA immunohistochemistry and scoring, variation in patients’ characteristics, and endpoint heterogeneity across studies limited the quality of the data and precluded further strong recommendations. Further prospective well designed studies considering other NED tissue markers such as NSE and synaptophysin might help clarify the prognostic value of NED in conventional PC.
4. Materials and Methods
4.1. Searching Strategy
Two independent reviewers conducted a full electronic literature search using PubMed, Scopus, Web of Science, and Cochrane Library on December 2018 to find relevant studies for this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. The search terms used were (“neuroendocrine differentiation” OR “neuroendocrine” OR “chromogranin A” OR “CgA” OR “neuron-specific enolase” OR “NSE” OR “serotonin” OR “Synaptophysin” OR “5-hydroxyindoleacetic acid OR 5-HIAA”) AND (“prostate” OR “prostatic” OR “prostate cancer” OR “radical prostatectomy”). Disease progression (including BCR, local recurrence, and distant metastasis) and survival data were our primary outcomes of interest.
4.2. Inclusion Criteria
The population, intervention, comparator, outcome, and study design (PICOS) approach was used to define the eligibility criteria: full text studies which assessed the association between focal NED in conventional prostate adenocarcinoma (population) in RP (intervention) specimen without history of neo-adjuvant therapy and post-operative prognosis included disease progression or survival were considered eligible. We excluded studies in languages other than English, review articles, meeting abstracts, case reports, replies, expert opinions, editorials, or commentaries. To perform meta-analysis, we included studies comparing positive RP specimen tissue staining for NED using predefined markers with patients without tissue staining for NED (comparator) to determine independent predictors of the mentioned oncological outcomes (outcome) after RP using multivariate Cox regression or logistic regression analysis (study design).
4.3. Data Extraction
The full text of relevant studies were evaluated by two independent authors. In case of more than one study of the same cohort, we included only the largest or most recent study. Data were extracted on first author, year of publication, country of study, study design, recruitment period, total number of patients, NED tissue markers, oncological end outcomes, demographic and clinicopathological characteristics, and follow-up duration. Independent correlation of concomitant focal NED in prostate adenocarcinoma with oncologic outcomes were retrieved.
4.4. Statistical Analyses and Bias Risk Assessment
We extracted reported HRs and 95% CIs to calculate cumulative effect size of studies which presented the association between focal NED of RP specimen as a prognostic factor and progression-free survival defined by BCR rate. Studies presenting HR using multivariate Cox proportional hazard regression model were included in meta-analysis. STATA/MPTM, version 14.2 (Stata-Corp, College Station, TX, USA) was used to perform meta-analysis. Heterogeneity between the studies included in the meta-analysis was assessed using Cochrane Q test and I2 statistics. An I2 > 50% and p-value < 0.05 in the Cochrane Q test implied that heterogeneity existed. With no heterogeneity among selected studies, we considered fixed effect models to calculate pooled HRs. Visual inspection of a funnel plot was carried out to identify publication bias in our meta-analysis. We used the ROBINS-I (“Risk Of Bias In Non-randomised Studies - of Interventions”) to assess the risk of bias in 16 included studies [38].
5. Conclusions
In this systematic review and meta-analysis, we detected a significant association of focal NED in conventional PC with oncologic outcomes including BCR after RP. Nevertheless, well-designed prospective studies overcoming inherent limitations of the current data are needed to confirm these findings. We suggest the assessment of focal NED in RP specimen, to prospectively assess the prognostic value in clinical decision making (i.e., ADT) and patient counselling.
Abbreviations
| NED | neuroendocrine differentiation |
| PC | prostate adenocarcinoma |
| RP | radical prostatectomy |
| BCR | biochemical recurrence |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| CgA | chromogranin A |
| NSE | neuron specific enolase |
| PSA | prostate specific antigen |
| ADT | androgen deprivation therapy |
Author Contributions
Conceptualization, S.F.S., M.K.P., and P.I.K.; methodology, S.F.S., M.K.P., and D.E.; software, M.K.P. and S.K.; formal analysis, M.K.P., T.I., and G.H.; investigation, M.K.P., F.J., D.E., and M.A.; writing—original draft preparation, M.K.P.; writing—review and editing, S.F.S.; visualization, S.F.S., L.M.R., and G.H.; supervision, S.F.S.; project administration, S.F.S. and M.K.P.
Funding
This work had no specific funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M., Fossati N., Gross T., Henry A.M., Joniau S., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Singh H., Canto E.I., Shariat S.F., Kadmon D., Miles B.J., Wheeler T.M., Slawin K.M. Improved detection of clinically significant, curable prostate cancer with systematic 12-core biopsy. J. Urol. 2004;171:1089–1092. doi: 10.1097/01.ju.0000112763.74119.d4. [DOI] [PubMed] [Google Scholar]
- 4.Freedland S.J., Humphreys E.B., Mangold L.A., Eisenberger M., Dorey F.J., Walsh P.C., Partin A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Trinh Q.D., Bjartell A., Freedland S.J., Hollenbeck B.K., Hu J.C., Shariat S.F., Sun M., Vickers A.J. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur. Urol. 2013;64:786–798. doi: 10.1016/j.eururo.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lughezzani G., Briganti A., Karakiewicz P.I., Kattan M.W., Montorsi F., Shariat S.F., Vickers A.J. Predictive and prognostic models in radical prostatectomy candidates: A critical analysis of the literature. Eur. Urol. 2010;58:687–700. doi: 10.1016/j.eururo.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun F.K., Briganti A., Shariat S.F., Graefen M., Montorsi F., Erbersdobler A., Steuber T., Salonia A., Currlin E., Scattoni V., et al. Significant upgrading affects a third of men diagnosed with prostate cancer: Predictive nomogram and internal validation. BJU Int. 2006;98:329–334. doi: 10.1111/j.1464-410X.2006.06262.x. [DOI] [PubMed] [Google Scholar]
- 8.Surcel C.I., van Oort I.M., Sooriakumaran P., Briganti A., De Visschere P.J., Fütterer J.J., Ghadjar P., Isbarn H., Ost P., van den Bergh R.C., et al. Prognostic effect of neuroendocrine differentiation in prostate cancer: A critical review. Urol. Oncol. 2015;33:265.e1–265.e7. doi: 10.1016/j.urolonc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Mazzucchelli R., Morichetti D., Lopez-Beltran A., Cheng L., Scarpelli M., Kirkali Z., Montironi R. Neuroendocrine tumours of the urinary system and male genital organs: Clinical significance. BJU Int. 2009;103:1464–1470. doi: 10.1111/j.1464-410X.2009.08451.x. [DOI] [PubMed] [Google Scholar]
- 10.Abrahamsson P.A., Cockett A.T., di Sant’Agnese P.A. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. Prostate Suppl. 1998;8:37–42. doi: 10.1002/(SICI)1097-0045(1998)8+<37::AID-PROS7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick D.G., Qian J., Pacelli A., Zincke H., Blute M., Bergstralh E.J., Slezak J.M., Cheng L. Neuroendocrine expression in node positive prostate cancer: Correlation with systemic progression and patient survival. J. Urol. 2002;168:1204–1211. doi: 10.1016/S0022-5347(05)64626-5. [DOI] [PubMed] [Google Scholar]
- 12.Ishida E., Nakamura M., Shimada K., Tasaki M., Konishi N. Immunohistochemical analysis of neuroendocrine differentiation in prostate cancer. Pathobiology. 2009;76:30–38. doi: 10.1159/000178153. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich E., Trojan L., Friedrich D., Voss M., Weiss C., Michel M.S., Grobholz R. Neuroendocrine tumor cells in prostate cancer: Evaluation of the neurosecretory products serotonin, bombesin, and gastrin—Impact on angiogenesis and clinical follow-up. Prostate. 2011;71:1752–1758. doi: 10.1002/pros.21392. [DOI] [PubMed] [Google Scholar]
- 14.Bubendorf L., Sauter G., Moch H., Schmid H.P., Gasser T.C., Jordan P., Mihatsch M.J. Ki67 labelling index: An independent predictor of progression in prostate cancer treated by radical prostatectomy. J. Pathol. 1996;178:437–441. doi: 10.1002/(SICI)1096-9896(199604)178:4<437::AID-PATH484>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Cohen M.K., Arber D.A., Coffield K.S., Keegan G.T., McClintock J., Speights V.O., Jr. Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumor progression. Cancer. 1994;74:1899–1903. doi: 10.1002/1097-0142(19941001)74:7<1899::AID-CNCR2820740712>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Ahlegren G., Pedersen K., Lundberg S., Aus G., Hugosson J., Abrahamsson P. Neuroendocrine differentiation is not prognostic of failure after radical prostatectomy but correlates with tumor volume. Urology. 2000;56:1011–1015. doi: 10.1016/S0090-4295(00)00838-4. [DOI] [PubMed] [Google Scholar]
- 17.Gunia S., Albrecht K., Koch S., Herrmann T., Ecke T., Loy V., Linke J., Siegsmund M., May M. Ki67 staining index and neuroendocrine differentiation aggravate adverse prognostic parameters in prostate cancer and are characterized by negligible inter-observer variability. World J. Urol. 2008;26:243–250. doi: 10.1007/s00345-008-0257-0. [DOI] [PubMed] [Google Scholar]
- 18.Krupski T., Petroni G.R., Frierson H.F., Jr., Theodorescu J.U. Microvessel density, p53, retinoblastoma, and chromogranin A immunohistochemistry as predictors of disease-specific survival following radical prostatectomy for carcinoma of the prostate. Urology. 2000;55:743–749. doi: 10.1016/S0090-4295(99)00598-1. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z., Tsuchiya N., Yuasa T., Huang M., Obara T., Narita S., Horikawa Y., Tsuruta H., Saito M., Satoh S., et al. Clinical significance of polymorphism and expression of chromogranin a and endothelin-1 in prostate cancer. J. Urol. 2010;184:1182–1188. doi: 10.1016/j.juro.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 20.Revelos K., Petraki C., Scorilas A., Stefanakis S., Malovrouvas D., Alevizopoulos N., Kanellis G., Halapas A., Koutsilieris M. Correlation of androgen receptor status, neuroendocrine differentiation and angiogenesis with time-to-biochemical failure after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2007;27:3651–3660. [PubMed] [Google Scholar]
- 21.Veltri R.W., Isharwal S., Miller M.C., Epstein J.I., Mangold L.A., Humphreys E., Partin A.W. Long-term assessment of prostate cancer progression free survival: Evaluation of pathological parameters, nuclear shape and molecular biomarkers of pathogenesis. Prostate. 2008;68:1806–1815. doi: 10.1002/pros.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstein M.H., Partin A.W., Veltri R.W., Epstein J.I. Neuroendocrine differentiation in prostate cancer: Enhanced prediction of progression after radical prostatectomy. Hum. Pathol. 1996;27:683–687. doi: 10.1016/S0046-8177(96)90398-6. [DOI] [PubMed] [Google Scholar]
- 23.Genitsch V., Zlobec I., Seiler R., Thalmann G.N., Fleischmann A. Neuroendocrine Differentiation in Metastatic Conventional Prostate Cancer Is Significantly Increased in Lymph Node Metastases Compared to the Primary Tumors. Int. J. Mol. Sci. 2017;18:1640. doi: 10.3390/ijms18081640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodorescu D., Broder S.R., Boyd J.C., Mills S.E., Frierson H.F., Jr. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997;80:2109–2119. doi: 10.1002/(SICI)1097-0142(19971201)80:11<2109::AID-CNCR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Noordzij M.A., van der Kwast T.H., van Steenbrugge G.J., Hop W.J., Schroder F.H. The prognostic influence of neuroendocrine cells in prostate cancer: Results of a long-term follow-up study with patients treated by radical prostatectomy. Int. J. Cancer. 1995;62:252–258. doi: 10.1002/ijc.2910620304. [DOI] [PubMed] [Google Scholar]
- 26.Parimi V., Goyal R., Poropatich K., Yang X.J. Neuroendocrine differentiation of prostate cancer: A review. Am. J. Clin. Exp. Urol. 2014;2:273–285. [PMC free article] [PubMed] [Google Scholar]
- 27.Xing N., Qian J., Bostwick D., Bergstralh E., Young C.Y. Neuroendocrine cells in human prostate over-express the anti-apoptosis protein survivin. Prostate. 2001;48:7–15. doi: 10.1002/pros.1076. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu R., Lucca I., Vartolomei M.D., Mbeutcha A., Klatte T., Seitz C., Karakiewicz P.I., Fajkovic H., Sun M., Lotan Y., et al. Role of survivin expression in predicting biochemical recurrence after radical prostatectomy: A multi-institutional study. BJU Int. 2017;119:234–238. doi: 10.1111/bju.13472. [DOI] [PubMed] [Google Scholar]
- 29.Shariat S.F., Lotan Y., Saboorian H., Khoddami S.M., Roehrborn C.G., Slawin K.M., Ashfaq R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100:751–757. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 30.Puca L., Vlachostergios P.J., Beltran H. Neuroendocrine Differentiation in Prostate Cancer: Emerging Biology, Models, and Therapies. Cold Spring Harb. Perspect. Med. 2018;29:a030593. doi: 10.1101/cshperspect.a030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terry S., Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol. 2014;25:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shariat S.F., Anwuri V.A., Lamb D.J., Shah N.V., Wheeler T.M., Slawin K.M. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J. Clin. Oncol. 2004;22:1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 33.Shariat S.F., Roehrborn C.G., McConnell J.D., Park S., Alam N., Wheeler T.M., Slawin K.M. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J. Clin. Oncol. 2007;25:349–355. doi: 10.1200/JCO.2006.05.6853. [DOI] [PubMed] [Google Scholar]
- 34.Wang H.T., Yao Y.H., Li B.G., Tang Y., Chang J.W., Zhang J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: Factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J. Clin. Oncol. 2014;32:3383–3390. doi: 10.1200/JCO.2013.54.3553. [DOI] [PubMed] [Google Scholar]
- 35.Epstein J.I., Amin M.B., Beltran H., Lotan T.L., Mosquera J.M., Reuter V.E., Robinson B.D., Troncoso P., Rubin M.A. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 2014;38:756–767. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shariat S.F., Semjonow A., Lilja H., Savage C., Vickers A.J., Bjartell A. Tumor markers in prostate cancer I: Blood-based markers. Acta Oncol. 2011;50:61–75. doi: 10.3109/0284186X.2010.542174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niedworok C., Tschirdewahn S., Reis H., Lehmann N., Szücs M., Nyirády P., Romics I., Rübben H., Szarvas T. Serum Chromogranin A as a Complementary Marker for the Prediction of Prostate Cancer-Specific Survival. Pathol. Oncol. Res. 2017;23:643–650. doi: 10.1007/s12253-016-0171-5. [DOI] [PubMed] [Google Scholar]
- 38.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]