Abstract
Among local faunas, the maximum body size and taxonomic affiliation of the top terrestrial vertebrate vary greatly. Does this variation reflect how food requirements differ between trophic levels (herbivores vs. carnivores) and with taxonomic affiliation (mammals and birds vs. reptiles)? We gathered data on the body size and food requirements of the top terrestrial herbivores and carnivores, over the past 65,000 years, from oceanic islands and continents. The body mass of the top species was found to increase with increasing land area, with a slope similar to that of the relation between body mass and home range area, suggesting that maximum body size is determined by the number of home ranges that can fit into a given land area. For a given land area, the body size of the top species decreased in the sequence: ectothermic herbivore > endothermic herbivore > ectothermic carnivore > endothermic carnivore. When we converted body mass to food requirements, the food consumption of a top herbivore was about 8 times that of a top carnivore, in accord with the factor expected from the trophic pyramid. Although top ectotherms were heavier than top endotherms at a given trophic level, lower metabolic rates per gram of body mass in ectotherms resulted in endotherms and ectotherms having the same food consumption. These patterns explain the size of the largest-ever extinct mammal, but the size of the largest dinosaurs exceeds that predicted from land areas and remains unexplained.
The size and taxonomic affiliation of the largest locally present species (“top species”) of terrestrial vertebrate vary greatly among faunas, raising many unsolved questions. Why are the top species on continents bigger than those on even the largest islands, bigger in turn than those on small islands? Why are the top mammals marsupials on Australia but placentals on the other continents? Why is the world's largest extant lizard (the Komodo dragon) native to a modest-sized Indonesian island, of all unlikely places? Why is the top herbivore larger than the top carnivore at most sites? Why were the largest dinosaurs bigger than any modern terrestrial species?
A useful starting point is the observation of Marquet and Taper (1), based on three data sets (Great Basin mountaintops, Sea of Cortez islands, and the continents), that the size of a landmass's top mammal increases with the landmass's area. To explain this pattern, they noted that populations numbering less than some minimum number of individuals are at high risk of extinction, but larger individuals require more food and hence larger home ranges, thus only large landmasses can support at least the necessary minimum number of individuals of larger-bodied species. If this reasoning were correct, one might expect body size of the top species also to depend on other correlates of food requirements and population densities, such as trophic level and metabolic rate. Hence we assembled a data set consisting of the top terrestrial herbivores and carnivores on 25 oceanic islands and the 5 continents to test 3 quantitative predictions.
- 1.
Within a trophic level, body mass of the top species will increase with land area, with a slope predictable from the slope of the relation between body mass and home range area.
- 2.
For a given land area, the top herbivore will be larger than the top carnivore by a factor predictable from the greater amounts of food available to herbivores than to carnivores.
- 3.
Within a trophic level and for a given area of landmass, top species that are ectotherms will be larger than ones that are endotherms, by a factor predictable from ectotherms' lower food requirements.
On reflection, one can think of other factors likely to perturb these predictions, such as environmental productivity, over-water dispersal, evolutionary times required for body size changes, and changing landmass area with geological time. Indeed, our database does suggest effects of these other factors. We propose our three predictions not because we expect them always to be correct, but because we expect them to describe broad patterns that must be understood in order to be able to detect and interpret deviations from those patterns.
Data
For continents and oceanic islands with a good fossil record for the last 65,000 years, Table 1 lists the identity and mean adult body mass of the top herbivore and top carnivore, most of them known only as Late Pleistocene or Holocene fossils. We chose a cutoff of 65,000 years ago because that is the approximate time of emergence of behaviorally modern humans (2), who may have been responsible for the subsequent extinctions of most of these top species.
Table 1.
Top herbivores and top carnivores for various land masses in the Late Pleistocene or Holocene
| Island | Area, km2 | Herbivore | Mass, kg | Ref. | Carnivore | Mass, kg | Ref. | Ratio†† |
|---|---|---|---|---|---|---|---|---|
| Plaza Sur | 0.12 | Conolophus subcristatus† (Galapagos land iguana) | 2.7 | 6 | Asio flammeus** (Short-eared owl) | |||
| Pinta | 59 | Geochelone nigra abingdoni (Galapagos tortoise) | 88 | § | Buteo galapagoensis** (Galapagos hawk) | |||
| Rodriguez | 108 | Pezophaps solitaria (Solitaire) | 23 | 7 | Mascarenotus murivorus (Owl) | 0.35 | ‖ | 17.7 |
| Aldabra | 129 | Geochelone gigantea (Aldabran tortoise) | 41 | 8 | Tyto alba (Barn owl) | 0.41 | ¶ | 5.0 |
| Barbuda | 172 | Oryzomyine rodent (Undescribed rice rat) | 0.79 | ¶ | Tyto neddi (Barn owl) | 1.0 | ¶ | 0.7 |
| Santa Rosa | 209 | Mammuthus exilis (Dwarf mammoth) | 1150 | ¶ | Haliaeetus leucocephalus** (Bald eagle) | |||
| New Providence | 228 | Geocapromys ingrahami (Bahaman hutia) | 0.71 | 9 | Titanohierax gloveralleni (Buteonine hawk) | 7.3 | ¶ | 0.2 |
| Tongatapu | 259 | Megapodius molistructor (Brush turkey) | 3.5 | ‖ | Accipiter cf. rufitorques (Hawk) | 0.28 | ¶ | 5.7 |
| Ibiza | 577 | Anser n. sp. (Goose) | 2.0 | ¶ | Haliaeetus albicilla (White-tailed eagle) | 4.8 | 10 | 0.6 |
| Mauritius | 1,874 | Raphus cucullatus (Dodo) | 19 | 7 | Circus alphonsi (Harrier) | 0.63 | ¶ | 10.3 |
| Mallorca | 3,667 | Myotragus balearicus (Cave goat) | 40 | 11 | Aquila chrysaetos (Golden eagle) | 4.2 | 10 | 5.2 |
| Crete | 8,259 | Elephas creutzburgi (Dwarf elephant) | 3200 | ¶ | Lutrogale cretensis (Cretan otter) | 11 | ‖ | 77.6 |
| Puerto Rico | 9,104 | Elasmodontomys obliquus (Rodent) | 50 | ‖ | Caracara sp. (Caracara) | 1.0 | 12 | 16.7 |
| Cyprus | 9,251 | Phanourios minutus (Dwarf hippopotomus) | 200 | 13 | Genetta cf. plesictoides (Genet) | 2 | 14 | 34.1 |
| Hawaii | 10,434 | “Large Hawaii goose” | 7.5 | ‖ | Haliaeetus albicilla‡‡ (White-tailed eagle) | 4.8 | 10 | 1.4 |
| Viti Levu | 10,531 | Megavitiornis altirostris (Pigeon) | 15 | ‖ | Volia athollandersoni (Crocodile) | 10 | ‖ | 8.7 |
| Jamaica | 10,991 | Heptaxodontidae: unnamed sp.A (Rodent) | 100 | ‖ | Accipitridae: gen/sp. ind. (Hawk/eagle) | 1.0 | ‖ | 28.4 |
| Flores | 14,154 | Feral species only | 15 | Varanus komodoensis (Komodo dragon) | 70 | 16 | ||
| New Caledonia | 16,648 | Sylviornis neocaledoniae (Brush turkey) | 40 | 17 | Mekosuchus inexpectatus (Crocodile) | 15 | ‖ | 11.8 |
| Sardinia | 24,090 | Megaloceros cazioti (Deer) | 70 | ¶ | Cynotherium sardous (Fox) | 15 | ‖ | 3.3 |
| Hispaniola | 76,192 | Megalocnus zile‡ (Ground sloth) | 150 | ‖ | Titanohierax sp. (Buteonine hawk) | 7.6 | ¶ | 9.6 |
| Cuba | 110,860 | Megalocnus rodens‡ (Ground sloth) | 150 | ‖ | Ornimegalonyx oteroi (Strigid owl) | 8.3 | ¶ | 9.0 |
| Sulawesi* | 189,216 | Bubalus depressicornis (Lowland anoa) | 225 | 14 | Crocodylus siamensis (Siamese crocodile) | 57 | ¶ | 14.5 |
| Macrogalidia musschenbroeki (Sulawesian palm civet) | 5.1 | 14 | ||||||
| New Zealand | 270,534 | Dinornis giganteus (Moa) | 117 | ‖ | Harpagornis moorei (New Zealand eagle) | 13 | 18 | 4.5 |
| Madagascar* | 587,040 | Aepyornis maximus (Elephant bird) | 440 | 19 | Crocodylus robustus (Crocodile) | 170 | ¶ | 6.51 |
| Cryptoprocta spelea (Fossa) | 17 | ‖ | ||||||
| New Guinea | 808,510 | Nototherium watutense (Diprotodontid marsupial) | 300 | 20 | Thylacinus cynocephalus (Tasmanian wolf) | 25 | ‖ | 5.23 |
| Australia* | 7,682,395 | Diprotodon opatum (Diprotodont marsupial) | 1150 | 21 | Megalania prisca (Varanid lizard) Thylacoleo carnifax (Marsupial lion) | 380 73 |
¶‖ | 3.5 |
| South America | 17,815,420 | Cuvieronius sp. (Gomphothere) | 4200 | ‖ | Smilodon fatalis (Sabretooth tiger) | 390 | 22 | 6.2 |
| North America | 24,680,331 | Mammuthus columbi (Columbian mammoth) | 6000 | ‖ | Panthera atrox (American lion) | 430 | 22 | 7.6 |
| Africa | 30,343,578 | Loxodonta africana (African elephant) | 3900 | 14 | Panthera leo (African lion) | 176 | 14 | 10.8 |
| Eurasia | 54,945,091 | Mammuthus primigenius (Woolly mammoth) | 5500 | ‖ | Panthera spelaea (Cave lion) | 380 | ¶ | 7.8 |
These three landmasses support a mammalian and a reptilian top carnivore of comparable food requirements, so we list both.
This is the top herbivore on two islands, Plaza Sur and Baltra. We report it for the former because we found a mass estimate for individuals from that island but not Baltra.
Because the lifestyles of extinct giant ground sloths are unknown, we assume their food requirements to be equal to those of most placentals, rather than the low values of extant arboreal sloths.
Calculated, this study.
Personal communication: P. Christiansen (Cuvieronius sp., M. columbi, M. primigenius); R. Dewer (C. spelea); H. F. James (Hawaiian goose); B. Kear (T. carnifax, T. cynocephalus); R. D. E. MacPhee (E. obliquus, Heptaxodontidae: unnamed sp. A, M. rodens, M. zile); C. Mourer-Chauviré (M. murivorus); R. E. Molnar (V. athollandersoni); M. Palombo (C. sardous); D. W. Steadman (Accipitridae: gen/sp ind., M. molistructor); G. E. Willemsen (L. cretensis); P. Willis (M. inexpectatus); T. H. Worthy (M. altirostris, D. giganteus).
The top carnivore on this island is also present on larger islands within the archipelago, therefore we cannot use it for our mass/area calculations.
Fossils are known from three nearby Hawaiian islands, hence this species is presumed also to have occurred on Hawaii itself.
Ratio of estimated food consumption of top herbivore to that of top carnivore. If a landmass had two top carnivores (see ‡‡), the one with higher consumption was used to calculate the ratio.
We used mean adult mass of each species rather than mass of the largest known individual. In studies providing only a range of masses, we averaged the range. To generate a species mean, we averaged male and female body masses. When calculating the mean mass of extant reptiles, we included only mass estimates for individuals of breeding age and/or size. When no body mass values were available (e.g., for many extinct species), we estimated body mass from linear dimensions through comparisons with related extant species of known body mass, using regression equations (refs. 3 and 4; P. Christiansen, personal communication), or else assuming body mass to increase as the cube of linear dimensions.
In some cases, a top species occurred on multiple islands within an archipelago but was unlikely to disperse often among islands, hence each island must have had a nearly self-sustaining population. We report such a species only once, using the area of the largest island on which it was the top herbivore or carnivore. Because some avian carnivores (e.g., sea eagles Haliaeetus sp.) readily cross water gaps, we excluded them if they occurred on islands less than an arbitrarily defined 50 km from a larger landmass.
We included terrestrial and freshwater crocodiles known or suspected to prey on terrestrial vertebrates. We excluded salt-water and estuarine crocodilians, able to disperse among islands [e.g., Crocodylus porosus and Crocodylus aculatus (5)]. In gathering data on carnivores, we excluded omnivorous species (e.g., Brown bear Ursus arctos).
Because this article is concerned with resource consumption, we define the top herbivore or carnivore at each location as that species with the greatest food consumption, rather than greatest body mass. Given the 10-fold higher food consumption per gram of body mass for endotherms than for ectotherms,¶ an endotherm has the same food consumption as an ectotherm 10 times heavier. We used the taxon-specific equations of Nagy¶ (his equations 3, 5, 35, and 67) to calculate food consumption rates (grams of dry matter per day) from adult body masses.
Table 1 gives modern Holocene land areas. Because of Pleistocene lowered sea levels and resulting emergent continental shelves, most landmasses had Pleistocene areas somewhat greater than their modern areas. Only for the island of New Providence was the Pleistocene increase sufficiently large to influence our results, as we shall discuss. We excluded islands connected to nearby continents by Late Pleistocene land bridges, except that we included New Guinea, because it was a rainforest island connected to arid Australia. New Guinea's high percentage of endemic species suggests only limited faunal exchange with Australia.
Results and Discussion
The Main Patterns.
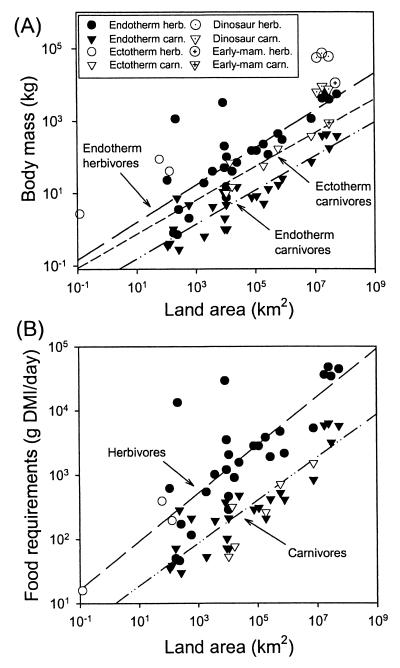
The body masses (in kg) of endothermic top herbivores (18 mammals, 9 birds), endothermic top carnivores (11 mammals, 14 birds), and ectothermic top carnivores (4 crocodilians, 2 lizards) all increased with increasing land area (in km2) according to the respective equations: Mass = 0.47 Area0.52 (r2 = 0.61, P < 0.0001); Mass = 0.05 Area0.47 (r2 = 0.81, P < 0.0001); and Mass = 0.25 Area0.47 (r2 = 0.78, P = 0.020) (Fig. 1A).
Figure 1.
(A) Body masses of top endothermic and ectothermic carnivores and herbivores, as a function of Holocene area of landmass inhabited. Separate regression lines are fitted through the points for each set of species except for ectothermic herbivores, which were not fitted because we have only three data points. Slopes of the lines are 0.47–0.52 and do not differ significantly between the species sets (P > 0.60). Note that larger landmasses support larger top species, and that, for a given area of landmass, body masses decrease in the sequence: ectothermic herbivore (○) > endothermic herbivore (●) > ectothermic carnivore (▿) > endothermic carnivore (▾). The two deviant points (●) at 3,200 kg, 8,259 km2 and at 1,150 kg, 209 km2 are the Crete dwarf elephant and the Santa Rosa dwarf mammoth, respectively, discussed in the text. Dinosaurs and early mammals are coded separately and discussed in the text. (B) Daily food requirements [grams of dry matter intake (GMI) per day] of top species (coded by the same symbols as in A), as a function of Holocene area of landmass inhabited. Because of an ectotherm's lower metabolic rate per gram of body mass, its food requirements are lower than those of an endotherm of the same body mass. As a result, B shows that an ectothermic top carnivore (▿, reptile) has the same food requirements (P > 0.10) as a endothermic top carnivore (▾, mammal or bird) on a landmass of the same area (and similarly for herbivores, ● vs. ○), although A showed that the ectotherm had the larger body mass. The two lines have the same slope (P = 0.57).
The slopes of these three equations are statistically the same (P > 0.60) but their intercepts are different (P < 0.005), except that the difference between the intercepts of the first and third equations falls short of significance (P = 0.099). Our data set includes only three ectothermic top herbivores (two tortoises and one lizard), too few to calculate a regression equation, but on the average 16 times larger than predicted from the equation for endothermic top herbivores. Thus, for a given land area, the body size of top species decreased in the sequence: ectothermic herbivore > endothermic herbivore > ectothermic carnivore > endothermic carnivore.
When we made comparisons between trophic groups (Fig. 1A), for a given land area a top herbivore proved to be 14 times heavier than a top carnivore in the case of endotherms (ANCOVA, F1,49 = 49.3, P < 0.0001) and 33-fold heavier in the case of ectotherms. For example, as is well known, African elephants are much larger than African lions.
When we converted our body mass estimates to food consumed per day (Fig. 1B), the food consumption of a top herbivore was 7-fold greater than that of a top carnivore in the case of endotherms (ANCOVA F1,49 = 42.2, P < 0.0001) and 24-fold greater in ectotherms (comparing our three herbivores with the regression line for ectothermic carnivores). The greater food consumption of herbivores than of carnivores is as expected, because in trophic pyramids the energy available to herbivores exceeds that available to carnivores by a factor variously between 5 and 20 (23).
For a given land area, an ectothermic top carnivore was 5 times heavier than an endothermic top carnivore (ANCOVA, F1,28 = 12.9, P = 0.0012; see Fig. 1A), and an ectothermic top herbivore was 16 times heavier than an endothermic top herbivore. However, because ectotherms have lower mass-specific metabolic rates and hence food requirements than endotherms, the food requirements of ectothermic and endothermic top carnivores for a given land area proved to be the same (P = 0.17; see Fig. 1B). Similarly, for a given land area, the food consumption of our three ectothermic top herbivores was similar to that of endothermic top herbivores, falling within the 95% confidence intervals surrounding the endotherm regression line (Fig. 1B).
Significance of the Slopes and Intercepts.
The main patterns of Fig. 1 A and B agree qualitatively with predictions based on food requirements of individual animals and on minimum viable population sizes. A quantitative comparison is possible as follows.
Individual larger animals require more food than do smaller animals, hence they require a larger home range to supply that food. We compared our regression equations with one that relates mammalian home range area with body mass (24). To allow for comparison with our Fig. 1A, data from ref. 24 were replotted (D. Kelt, personal communication) with body mass as the dependent variable and home range area as the independent variable, omitting species with body masses <0.1 kg because at that body mass many biological characteristics appear to change sign (24). The resulting equation was Mass = 673 Area0.50 (r2 = 0.61, n = 232, P < 0.0001; D. Kelt, personal communication). Its slope (0.50) is almost identical to the slopes (0.47–0.52) of our equations of body mass of top species vs. land area.
Larger home ranges translate into lower population densities, hence lower population sizes (number of individuals constituting a population) for a given area. But some minimum population size is necessary for a population's long-term survival in evolutionary time (frequent estimates are a few thousand to at least 10,000 individuals; ref. 25). Hence, larger-bodied animals with larger home ranges require a larger landmass to achieve that required minimum population size. Because the trophic pyramid implies 5–20 times more food available to herbivores than to carnivores, a given area can support a population of a herbivore species whose individuals consume 5–20 times more than does a carnivore; we actually found a ratio of 8 for the median food consumption of top herbivores to top carnivores (Table 1). Because the high metabolic rates of endotherms result in their having food requirements equal to those of an ectotherm 10-fold heavier,¶ a given area can support a population of an ectotherm 10-fold heavier than an endotherm at the same trophic level; we actually found ratios of 5 for carnivores and 16 for herbivores.
The low metabolic rates of ectotherms probably explain why ectotherms are top carnivores on at least five islands whose herbivores are nevertheless endotherms (Viti Levu, Flores, New Caledonia, Sulawesi, and Madagascar). The most famous example is the world's largest extant lizard, the Komodo dragon of Flores (adult body mass averaging 70 kg). Flores formerly supported as its top herbivore a small elephant-like stegodont (not listed in Table 1 because of uncertainty whether it survived after 65,000 years ago). Fig. 1A shows Flores' area to be capable of supporting an endothermic top carnivore of only about 5 kg, no match for a stegodont. Instead, Flores supports the Komodo dragon, which today attacks feral horses and buffalo, and thus could have attacked a small stegodont. Despite its large size, the Komodo dragon has been able to maintain a viable population on Flores because of its low metabolic rate. Because more energy is available at lower trophic levels, Flores (and other islands with ectothermic top carnivores) can support large endothermic herbivores on which those carnivores prey. The same islands could not have supported endothermic top carnivores large enough to prey on those herbivores. For example, on Crete and Cyprus, the top carnivores are small endotherms, an 11-kg otter and a 2-kg genet, utterly incapable of preying on the top herbivores of these islands (a 3,200-kg elephant and a 200-kg hippopotamus, respectively).
Effect of Environmental Productivity.
Although the world's largest extant lizard is the Komodo dragon, Australia formerly supported a related larger lizard, the now-extinct 380-kg Megalania prisca. Why did Australia, uniquely among the continents, evolve a lizard as one of its top carnivores?
Part of the answer is that Australia is the smallest continent, thus from its area alone it would have had difficulty supporting an endothermic carnivore capable of preying on its top herbivore. The other part of the answer may be Australia's variable low rainfall and leached ancient soils, resulting in Australia having much lower net primary productivity than the other landmasses listed in Table 1. That is, in food availability and supportable population sizes, Australia is effectively even smaller than its actual area, so that “normal” large endotherms could not maintain viable populations, but species with lower metabolic rates could. In agreement with this interpretation, although Australia's other top carnivore (the “marsupial lion”) and its top herbivore (a diprotodont or “marsupial rhinoceros”) were mammals, they were not placentals but marsupials, whose metabolic rates and food requirements average 20% lower than those of placentals.¶ Australia's low productivity may explain why, even taking into account their low metabolic rates, the calculated food requirements of those two marsupials were still 50–60% below expectations for Australia's area (Fig. 1B). That is, not only were Australia's top mammals marsupials rather than placentals, but even those marsupials were smaller than would have been expected if Australia's productivity had equaled that of the other continents.
Interestingly, between about 115 and 54 million years ago, when it was still part of the supercontinent Gondwana, Australia supported putative placental mammals, which had become extinct by around 30 million years ago (26). The second-smallest continent, South America, also had marsupials (and birds) as its top carnivores, but placentals as its top herbivores, as long as it was isolated; only after South America became joined to North America around 2.8 million years ago were marsupial carnivores replaced by placental top carnivores (the sabretooth Smilodon, puma, and jaguar) whose ranges were much larger than the area of South America and extend to North America. In contrast, on the largest and most productive continents (Eurasia, North America, and Africa) all marsupials became replaced by placentals over the last 50 million years (27). Similarly, nontribosphenid mammals, whose representatives the monotremes have even lower metabolic rates than marsupials, were present on all continents until around 60 million years ago, but became extinct everywhere except Australia. Thus, considerations of the different metabolic rates of mammalian groups, and different areas and productivities of continents, help explain the differential survival of mammalian groups on the continents.
Effect of Dispersal.
Continuing dispersal from a mainland across a narrow water gap to a nearby island could lead to insular presences of mainland species much too large for the area of the island itself. This explanation may apply to two otherwise highly deviant data points. First, the modern top carnivore of the California Channel Islands, the Bald Eagle Haliaeetus leucocephalus (4.7 kg), is 7-fold larger than expected from Fig. 1A for the largest island's area but regularly flies and forages over water and belongs to the same population as that on the California mainland, only 30 km distant. Second, Santa Rosa Island off the California coast supported a species of dwarfed mammoth (Mammuthus exilis) only 20% the size of its mainland relative Mammuthus columbi, but still 50 times larger than expected from Fig. 1A even for Santa Rosa's Pleistocene area. However, Pleistocene Santa Rosa was separated by less than 8 km from the California coast. The high variance in adult body mass of M. exilis (ranging from 200 to 2,000 kg) and a few Santa Rosa records of 6,000-kg mainland-sized individuals suggest periodic dispersal of mainland individuals to Santa Rosa (28), as expected from the good swimming ability of elephants.
Effect of Evolutionary Time Required for Body Size Changes.
As illustrated by that example of the Santa Rosa mammoth, when a mainland species colonizes an island, it may arrive with the “wrong” size for a top herbivore or carnivore on an island of that area. It may then undergo an evolutionary decrease or increase in body size, resulting in the many insular dwarfs and giants discussed in biology textbooks. But evolutionary change takes time, so that at any instant there will inevitably be misfits between mass and island area.
Our database provides several examples of such misfits. The Wrangel Island mammoth declined by about 65% in body size within at most 5,000 years after the severing of the Late Pleistocene land bridge to Eurasia (29), but after those 5,000 years, it was still 40-fold too large for Wrangel's area. Crete was invaded over-water from the European mainland early in the Pleistocene by large elephants that eventually evolved into a 90-kg dwarf species (Elephas creticus) of a size more appropriate to Crete's area (Fig. 1A). Crete was then invaded in the Middle Pleistocene by mainland elephants that underwent a 50% evolutionary size reduction to become the endemic Elephas creutzburgi, but at the time of its Late Pleistocene extinction its 3,200-kg mass was still 60-fold too large for Crete's area (30). The pygmy hippopotamus of Cyprus, although dwarfed to 200 kg, was still 4-fold too large for Cyprus's area at the time of its Early Holocene extinction (13). Such episodes of invasion and dwarfing affected many other insular species of other islands of the Mediterranean and of Wallacea.
Conversely, some small-bodied mainland colonists of islands underwent evolutionary increase in size to become insular giants, such as the big flightless dodo of Mauritius (derived from a flying pigeon). The top herbivore of Hawaii was a 7.5-kg goose much larger than its likely ancestor but still much smaller than the 60-kg herbivore expected from Fig. 1A for Hawaii's area. In contrast to Mauritius, which is 10 million years old, Hawaii is one of the youngest islands included in Table 1, having formed no more than 375,000 years ago (31); perhaps a considerably longer time is required for a bird to evolve from 2 to 60 kg. Yet rapid changes in body size are known from the fossil record. For instance, the red deer of Jersey Island declined in mass by 80% in at most 6,000 years (32). By comparing sizes of other insular top species with sizes of ancestors and of top species expected for that area, it may eventually become possible to draw firmer conclusions about the time scale of evolutionary changes in body size.
Effect of Geological Time Required for Island Area Changes.
The 228-km2 Caribbean island of New Providence is expected from its area to support an endothermic top carnivore of only about 0.7 kg. Instead, its top carnivore was a giant hawk 10 times larger (7.3 kg) than expected, derived from an ancestor weighing only about 1 kg. Thus, the explanation for its excessive size cannot be evolutionary time delays in dwarfing—it evolved to be larger rather than smaller. Instead, the explanation is that the hawk's island became much smaller. At low-sea-level times of the Pleistocene, the shallow Great Bahama Bank emerged as a huge low-lying island of about 109,400 km2, of which only the highest hills remained above water as New Providence Island after the terminal Pleistocene rise in sea level (33). For an area of 109,400 km2, Fig. 1A predicts an endothermic top carnivore of 12 kg, close to the actual mass of the New Providence hawk. That is, the apparent discrepancy is an artifact of linking the New Providence hawk to an inappropriately small modern area, instead of to the large Pleistocene area in which it actually evolved. Similar artifacts are expected for other islands that suffered great area reductions at the end of the Pleistocene.
Earlier Species.
Our analysis has been restricted to species that lived within the past 65,000 years. Do similar mass/area relations describe earlier mammals and dinosaurs (Table 2)?
Table 2.
The largest dinosaurs and the largest terrestrial mammals of all time
| Species | Age (My) | Distribution | Area, ×106 km2 | Body mass,kg | Ref. |
|---|---|---|---|---|---|
| Dinosaurs | |||||
| Herbivores | |||||
| Sauroposeidon proteles | 110 | Central N. America | 12 | 55,000 | 39 |
| Argentinosaurus huincluensis | 100 | S. America | 18 | 73,000 | * |
| Paralititan stromeri | 95 | Africa | 30 | 59,000 | † |
| Carnivores | |||||
| Tyrannosaurus rex | 65 | Western N. America | 12 | 6,250 | 40 |
| Giganotosaurus carolinii | 100 | S. America | 18 | 9,000 | * |
| Caracharodontosaurus saharicus | 93 | Africa | 30 | 7,500 | * |
| Mammals | |||||
| Herbivore | |||||
| Indricotherium transouralicum | 30 | Asia | 50 | 11,000 | 41 |
| Carnivore | |||||
| Megistotherium osteothlastes | 20 | Africa | 30 | 880 | 34 |
Personal communication: P. Christiansen (A. huincluensis, G. carolinii); G. S. Paul (C. saharicus). My, million years.
Calculated, this study.
The largest known mammalian herbivore was the rhinocerotoid Indricotheriun transouralicun (estimated mass 11,000 kg) from Asia's Oligocene. At that time, Asia was separated from Europe and had an estimated area of 50,000,000 km2. With that estimated mass and area, Indricotheriun falls within the 95% confidence intervals surrounding our endothermic herbivore line (see Fig. 1A).
The largest known mammals usually considered to be carnivores were the 880-kg creodont Megistotherium osteothlastes from Africa's early Miocene (34), the 750-kg bear Agriotherium africanum from Africa's early Pliocene (35), and the mesonychid Andrewsarchus mongoliensis (600–900 kg; G. Paul, personal communication) from Asia's Oligocene. These species all fall above the 95% confidence interval for endothermic carnivores in Fig. 1A. However, the largest living terrestrial species of the order Carnivora today, whose diets are known with certainty, are bears (up to 800 kg), seven of whose extant eight species (all except the polar bear) are actually omnivores. Perhaps those earlier large presumed carnivores were actually omnivores as well (36).
The largest terrestrial vertebrates of all time were dinosaurs, of which Table 2 lists the largest known herbivores and carnivores for three continents. (Those listed top herbivores and top carnivores were not necessarily contemporaneous.) Their body masses all fall closer to the ectotherm lines than to the endotherm lines of Fig. 1A, suggesting that dinosaurs were “cold-blooded.” However, the top carnivorous dinosaurs were still 12 times heavier, and the herbivorous dinosaurs 1.5–3 times heavier, than predicted from the regression lines from Fig. 1A for extant ectotherms; therefore, the never-since-surpassed size of the largest dinosaurs remains unexplained. Perhaps the answer has to do with high net primary productivity during the dinosaur era: atmospheric CO2 levels then were up to 10 times the present levels (37) and elevated CO2 stimulates productivity of some plants (38).
Future Directions
Although the previous section suggested reasons why certain data points deviate from the main patterns of Fig. 1 A and B, there are other deviations that we cannot yet explain: the top herbivores of Barbuda, New Providence, and Ibiza are smaller than expected, and New Guinea's top herbivore and top carnivore are both of an appropriate mass but, being marsupials, have unexpectedly low food requirements.
We suggest three extensions of our study.
- 1.
Among the world's old rivers and lakes, does size of the largest fish increase with increasing volume of lake, length and outflow of river, and productivity? It is suggestive that three of the world's largest rivers—the Nile, Amazon, and Mississippi—contain some of the largest known fresh-water fish species.
- 2.
Among whales, the largest planktivore (Blue Whale Balaenoptera musculus) and carnivores (Sperm Whale Physeter catodon and Killer Whale Orcinus orca) all have worldwide distributions. Will it be possible to discern mass/area or mass/volume relations (importantly influenced by productivity) in the oceans, and to relate them to those on land?
- 3.
Our study applies to different landmasses around the world. Can similar mass/area relations be discerned for species isolated on separate islands within an archipelago?
Acknowledgments
For providing us with unpublished data, we thank Per Christiansen, Bob Dewer, Helen James, Ben Kear, Doug Kelt, Ross MacPhee, Cecile Mourer-Chauviré, Ralph Molnar, Patrick Nunn, Maria Rita Palombo, Greg Paul, Dave Steadman, Alan Tennyson, John Thorbjarnarson, Gerard Willemsen, Paul Willis, and Trevor Worthy. We thank Bob Hill for pointing out the relevance of Cretaceous atmospheric CO2 levels. Funding was provided by the Natural Sciences and Engineering Research Council (Canada).
Footnotes
Nagy, K. A. (2001) Nutr. Abstr. Rev., in press.
References
- 1.Marquet P A, Taper M L. Evol Ecol. 1998;12:127–139. [Google Scholar]
- 2.Klein R J. The Human Condition. Chicago: University of Chicago Press; 1999. [Google Scholar]
- 3.Leslie A J. Dissertation. Philadelphia: Drexel University; 1997. [Google Scholar]
- 4.Webb G J W, Messel H. Aust J Zool. 1978;26:1–27. [Google Scholar]
- 5.Groombridge B. In: Wildlife Management: Crocodiles and Alligators. Webb G J W, Manolis S C, Whitehead P J, editors. Chipping Norton, U.K.: Surrey Beatty; 1987. pp. 9–21. [Google Scholar]
- 6.Snell H L, Christian K A. Herpetologica. 1985;41:437–442. [Google Scholar]
- 7.Livezey B C. J Zool. 1993;230:247–292. [Google Scholar]
- 8.Coe M J, Bourn D, Swingland I R. Philos Trans R Soc London B. 1979;286:163–176. [Google Scholar]
- 9.Clough G C. J Mammal. 1972;53:807–823. [Google Scholar]
- 10.Dunning J B., Jr . CRC Handbook of Avian Body Masses. Boca Raton, FL: CRC; 1993. [Google Scholar]
- 11.Alcover J A, Pérez-Obiol R, Yll E I, Bover P. Biol J Linn Soc. 1999;66:57–74. [Google Scholar]
- 12.Morrison J L. In: The Birds of North America. Poole A, Gill F, editors. Philadelphia: Acad. Nat. Sci.; 1996. , No. 249. [Google Scholar]
- 13.Simmons A H. Faunal Extinction in an Island Society: Pygmy Hippopotamus Hunters of Cyprus. New York: Kluwer; 1999. [Google Scholar]
- 14.Nowak R M. Walker's Mammals of the World. Baltimore: Johns Hopkins Univ. Press; 1999. [Google Scholar]
- 15.van den Bergh G D. Scr Geol. 1999;117:1–419. [Google Scholar]
- 16.Ciofi C. Sci Am. 1999;280:85–91. [Google Scholar]
- 17.Steadman D W. Zool Verh. 1999;327:7–21. [Google Scholar]
- 18.Brathwaite D H. Notornis. 1992;39:239–247. [Google Scholar]
- 19.Amadon D. Condor. 1947;49:159–164. [Google Scholar]
- 20.Flannery T F, Roberts R G. In: Extinctions in Near Time. MacPhee R D E, editor. New York: Kluwer; 1999. pp. 239–253. [Google Scholar]
- 21.Murray P. In: Vertebrate Palaeontology of Australasia. Vickers-Rich P, Monaghan J M, Baird R F, Rich T H, editors. Melbourne: Pioneer Design Studio; 1991. pp. 1071–1163. [Google Scholar]
- 22.Anyonge W. J Zool. 1993;231:339–350. [Google Scholar]
- 23.Ricklefs R E. The Economy of Nature. New York: Freeman; 1996. [Google Scholar]
- 24.Kelt D A, Van Vuren D H. Am Nat. 2001;157:637–645. doi: 10.1086/320621. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C D. Conserv Biol. 1990;4:324–327. [Google Scholar]
- 26.Rich T H, Vickers-Rich P, Constantine A, Flannery T F, Kool L, van Klaveren N. Science. 1997;278:1438–1442. doi: 10.1126/science.278.5342.1438. [DOI] [PubMed] [Google Scholar]
- 27.Szalay F S. Evolutionary History of the Marsupials and an Analysis of Osteological Characters. New York: Cambridge Univ. Press; 1994. [Google Scholar]
- 28.Roth V L. In: The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives. Shoshani J, Tassy P, editors. Oxford: Oxford Univ. Press; 1996. pp. 249–253. [Google Scholar]
- 29.Lister A M. Nature (London) 1993;362:288–289. doi: 10.1038/362288a0. [DOI] [PubMed] [Google Scholar]
- 30.Caloi L, Kotsakis T, Palombo M R, Petronio C. In: The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives. Shoshani J, Tassy P, editors. Oxford: Oxford Univ. Press; 1996. pp. 234–239. [Google Scholar]
- 31.Nunn P D. Oceanic Islands. Oxford: Blackwell; 1994. [Google Scholar]
- 32.Lister A M. Nature (London) 1989;342:539–542. doi: 10.1038/342539a0. [DOI] [PubMed] [Google Scholar]
- 33.Morgan G S. In: Biogeography of the West Indies: Past, Present, and Future. Woods C A, editor. Gainesville, FL: Sandhill Crane; 1989. pp. 685–740. [Google Scholar]
- 34.Savage R J G. Bull Br Mus (Nat Hist), Geol. 1973;22:485–511. [Google Scholar]
- 35.Hendey Q B. Langebaanweg. A Record of Past Life. Cape Town, South Africa: South African Museum; 1982. [Google Scholar]
- 36.Farlow J O. Am J Sci. 1993;293:167–199. [Google Scholar]
- 37.Ekart D D, Cerling T E, Montanez I P, Tabor N J. Am J Sci. 1999;299:805–827. [Google Scholar]
- 38.Saxe H, Ellsworth D S, Heath J. New Phytol. 1998;139:395–436. [Google Scholar]
- 39.Wedel M J, Cifelli R L, Sanders R K. Acta Palaeontol Pol. 2000;45:343–388. [Google Scholar]
- 40.Christiansen P. J Vert Paleontol. 1999;19:666–680. [Google Scholar]
- 41.Fortelius M, Kappelman J. Zool J Linn Soc. 1993;107:85–101. [Google Scholar]