Abstract
TiO2 nanoparticles containing 0.0, 1.0, 5.0, and 10.0 wt.% Mo were prepared by a reverse micelle template assisted sol–gel method allowing the dispersion of Mo atoms in the TiO2 matrix. Their textural and surface properties were characterized by means of X-ray powder diffraction, micro-Raman spectroscopy, N2 adsorption/desorption isotherms at −196 °C, energy dispersive X-ray analysis coupled to field emission scanning electron microscopy, X-ray photoelectron spectroscopy, diffuse reflectance UV–Vis spectroscopy, and ζ-potential measurement. The photocatalytic degradation of Rhodamine B (under visible light and low irradiance) in water was used as a test reaction as well. The ensemble of the obtained experimental results was analyzed in order to discover the actual state of Mo in the final materials, showing the occurrence of both bulk doping and Mo surface species, with progressive segregation of MoOx species occurring only at a higher Mo content.
Keywords: TiO2 nanoparticles, Mo-doping, band-gap
1. Introduction
Titanium dioxide (TiO2), in virtue of its chemical stability and low toxicity, is currently used in a wide range of applications, from dye-sensitized solar cells, to sensor devices and paints [1,2,3]; most of all, TiO2 is one of the most investigated photocatalysts (along with ZnO), as it is able to decompose several organic/inorganic pollutants in both liquid and gas phases [4,5,6].
TiO2 may occur as anatase, rutile, or brookite phases; amorphous TiO2 may be obtained, as well, and by properly tailoring the synthesis conditions, pure or mixed phases are obtained. While in bulk TiO2, rutile is the stable phase above 600 °C, synthesis methods starting from solutions containing a Ti precursor generally lead to the formation of anatase. When TiO2 nanoparticles (NPs) are obtained, polymorphs that are usually metastable in the bulk may be stabilized instead. Among TiO2 polymorphs, anatase has the lowest surface energy, and NPs of 15–30 nm dimension are easily obtained [7,8,9], for instance, from alkoxide precursors. However, if a strong acid is added, brookite NPs are more likely to form [10,11].
As far as photocatalytic applications are concerned, anatase has a band-gap (Eg) of 3.2 eV, whereas that of rutile is 3.0 eV and that of brookite is in the range between 3.1 and 3.4 eV [12,13,14]: consequently, TiO2 is mainly active under UV light. Moreover, electron-hole pairs are characterized by a relatively fast recombination rate (from pico- to micro-seconds) [15] that decreases its photocatalytic efficiency. In addition, the TiO2 polar surface has a poor adsorption ability towards non-polar organic pollutants, further limiting its efficiency towards the removal of organic pollutants [16].
With the aim of improving TiO2 photocatalytic activity, doping with heteroatoms, including transition metals (Cr, Co, Fe, Ni, Mn, V, Cu, Ni, and Zn) and non-metals (vide infra), has been subject to many studies, since it should allow narrowing the band gap and improving its solar light absorption [17,18,19,20]. Concerning doping with transition metals, the absorption edge shifts towards longer wavelengths due to charge-transfer transitions between d electrons of the transition metals and the CB (conduction band) or the VB (valence band). Rare earths have good electron trapping properties, resulting in a large absorption edge shift towards longer wavelengths, whereas defects chemistry can also play an important role in the reactions kinetics and charge recombination.
Non-metals (C, B, I, F, S, and N) doping at the O sites of TiO2 NPS have also been largely exploited: N appears to be one of the most efficient (and investigated) dopants, the enhancement of charge separation being ascribed to the formation of paramagnetic species [O–Ti4+–N2−–Ti4+–VO], where VO is an oxygen vacancy. With non-metals doping, impurity states are located near the VB edge and their role as recombination centers might be minimized [17,21].
Other studies show, instead, that if TiO2 is modified by adding noble metals (such as Ag, Pt, Pd, Rh and Au), electron-hole recombination is hindered by the resulting Schottky barrier at the metal–TiO2 interface, where the former acts as a storing-transporting mediator of photogenerated electrons from the surface of TiO2 to an acceptor (in the reaction medium). Consequently, the photocatalytic activity increases as the charge carrier recombination rate decreases [22,23,24,25,26].
Concerning transition metals, Mo doping is very promising since it introduces an empty donor level below the CB (n-type doping), which only slightly perturbs the band [27], so avoiding strongly localized d states that can reduce the mobility of charge carriers [28].
High surface area NPs, with inter-and/or intra-particle porosity, can be obtained by using either hard or soft templates. Concerning soft-templates, mesoporous TiO2 particles are obtained by using either a triblock copolymer (e.g., Pluronic P123) or an ionic surfactant (e.g., cetyltrimethylammonium bromide), whereas when di-block copolymers are used (e.g., Brij-n), porosity forms among NPs [29].
Out of the different syntheses reported in the literature, the reverse micelles method proposed by Chandra et al. [30] allows obtaining high surface area Mo-doped TiO2, SiO2 and ZrO2 NPs where, simultaneously, Mo is fairly dispersed. With such a method, the self-assembly of surfactant molecules in the organic phase produces a “nanoreactor” where reactions in water phase take place; the size of the so-obtained reverse micelles, tunable through the polar head group and the alkyl tail length and structure, allows controlling both size and shape of the NPs [31]. The reverse micelle core provides a suitable environment for the controlled nucleation and growth of TiO2 NPs, simultaneously affording a good dispersion of MoOx species.
Here, we report the synthesis and the physico-chemical properties of a set of pure and Mo-doped TiO2 NPs, obtained by properly modifying the method reported by Chandra et al. and adapting it to TiO2 [30]. Here, different Mo contents were studied and also a different surfactant was employed, namely polyoxyethylene (20) oleyl ether, characterized by the presence of a double bond and a different molar mass, as both properties can affect the size of the reverse micelles and, consequently, that of the obtained NPs.
The samples properties were characterized by means of a set of techniques (X-ray powder diffraction, micro-Raman spectroscopy, N2 adsorption/desorption isotherms at −196 °C, energy dispersive X-ray analysis coupled to field emission scanning electron microscopy, X-ray photoelectron spectroscopy, diffuse reflectance UV–Vis spectroscopy, and ζ-potential measurement.), and the photocatalytic degradation of the model dye Rhodamine B under visible light was used as a test reaction to gain insights into the state of Mo in the materials.
2. Materials and Methods
All reagents were from Sigma-Aldrich (Milan, Italy), if not otherwise specified.
Pure and Mo-doped TiO2 NPs were prepared by sol–gel reverse micelle microemulsion method [14], where polyoxyethylene (20) oleyl ether (Brij O20) was the surfactant, cyclohexane the oil phase, titanium(IV) butoxide 97% (Ti-(BuO)4) and ammonium heptamolybdate tetrahydrate ((NH₄)₆Mo₇O₂₄*4H₂O, purum p.a. ≥ 99.9%) the Ti and Mo precursors, respectively.
Proper precursor amounts were used in order to obtain nominal contents of 0.0, 1.0, 5.0 and 10.0 wt.% Mo/(Mo+TiO2). A typical synthesis involves the following steps: the surfactant is dispersed in cyclohexane by stirring at 50 °C, while the salt precursor is dissolved in MilliQ water at the same temperature. Afterwards, the salt solution is added to the oil/surfactant mixture and stirred for 45 min, with formation of a water in oil (w/o) microemulsion of surfactant nanoreactors. Ti-(BuO)4 is then added dropwise to the emulsion. The mixture is stirred for 2 h at the constant temperature of 50 °C, and finally the emulsion is broken by addition of 2-propanol, followed by sonication. The solid phase is collected by centrifugation and dried at 100 °C for 24 h, followed by calcination in air at 500 °C for 2 h with a temperature ramp of 2.5 °C/min to remove the surfactant.
X-Ray Diffraction (XRD) patterns were obtained by means of X’Pert Phillips diffractometer operating (Phillips-PANalytical, Almelo, The Netherlands) at 40 kV and 40 mA equipped with Cu Kα radiation (step scan = 0.02 2θ, time per step = 2 s).
Raman spectra were acquired on a Renishaw InVia Reflex micro-Raman spectrometer (Renishaw plc, Wotton-under-Edge, UK) equipped with a cooled Charge-Coupled Device (CCD) camera. The Raman source was a diode laser (λex = 514.5 nm), and the inspection occurred over pelletized samples to ensure a “flat” surface, through a microscope objective (100X), in backscattering light collection. The following conditions were employed to collect each spectrum: 0.5 mW laser power, 10 s of exposure time and 1 accumulations.
N2 adsorption/desorption isotherms at −196 °C were obtained on a Micromeritics ASAP 2020Plus instrument (Micromeritics, Norcross, GA, USA) on powder samples previously outgassed at 120 °C for 1 h (temperature ramp = 5 °C/min), followed by an isothermal step of 2 h at 150 °C. The sample’s specific surface area (SSA) was calculated according to the Brunauer–Emmett–Teller (BET) method; pore total volume was measured at p/p0 = 0.99; pore size distribution was determined by applying the BJH (Brunauer–Joiner–Hallenda) method to the isotherm desorption branch.
Field Emission Scanning Electron Microscopy (FESEM) micrographs were taken on a ZEISS Supra 40 FESEM instrument (Carl-Zeiss AG, Oberkochen, Germany) equipped with an Energy Dispersive X-ray (EDX) probe used to determine the actual Mo/Ti atomic ratio, to be compared to nominal value, by a raster scan of ~0.05 mm2 of sample surface. For both FESEM and EDX analysis, a small amount of powder was pressed on the sample holder (a carbon conductive adhesive tape) without any conductive coating. FESEM micrographs were then analyzed by using the ImageJ software (version 1.50i), by arbitrarily selecting three parallel lines on the micrographs and by measuring particle edge-to-edge dimension, so obtaining the average particle size listed in Table 1.
Table 1.
Textural and surface properties of the studied samples as determined by N2 isotherms at −196 °C; FESEM; XRD, EDX and XPS (X-ray Photoelectron Spectroscopy) analyses.
| Sample | BET SSA (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Particle Size (±s.d. nm) | Average Crystallite Size (±s.d. nm) * | EDX Determined Mo/Ti (Nominal Mo/Ti) Atomic Ratios | XPS Determined Surface Mo/Ti Atomic Ratio [32] |
|---|---|---|---|---|---|---|
| TiO2 | 71 | 0.091 | 12 (3) | 10.3 (0.5) | - | - |
| Mo_1 | 76 | 0.112 | 21 (5) | 12.5 (0.9) | 0.0070 (0.0084) | 0.042 |
| Mo_5 | 74 | 0.141 | 22 (5) | 12.5 (0.4) | 0.05 (0.044) | 0.150 |
| Mo_10 | 96 | 0.137 | 18 (4) | 9.9 (0.5) | 0.090 (0.092) | 0.194 |
* Calculated anatase crystallite size as obtained according to the Williamson-Hall method.
X-ray Photoelectron Spectroscopy (XPS) analyses were performed with a Versa Probe II Scanning XPS Microprobe spectrometer (Physical Electronics GmbH, Ismaning, Germany). The measurements were done with a monochromatised Al Kα source (x-ray spot 100 μm), at a power of 24.8 W. Wide scans spectra were acquired in Fixed Analyzer Transmission (FAT) mode with a pass energy of 117.40. An electron gun was used for charge compensation (1.0V 20.0 μA). Data processing was performed by using the MultiPak software v. 9.8.0.19.
Diffuse reflectance (DR) UV-Visible (UV-Vis) spectra of the powders were recorded in the 200–800 nm range by using a UV–Vis Varian Cary 5000 spectrophotometer (Varian Instruments, Mulgrave, Australia) equipped with an integration sphere. The DR spectra is reported as Kubelka–Munk function (Equation (1)) where R is the absolute reflectance of the layer, s the scattering coefficient, and k the molar absorption coefficient under the assumption of “infinite” thick layer.
| (1) |
ζ-potential was measured on a Zetasizer Nano ZSP apparatus (Malvern Instruments Ltd., Malvern, Worcestershire, UK) on suspensions of 5 mg sample in 10 mL distilled water. Before the measurement, the suspension was sonicated for 5 min and pH was adjusted by using either HCl or NH4OH. The corresponding ζ-potential was obtained from the electrophoretic mobility, according to the Smoluchowski’s approximation.
To evaluate the photocatalytic response of the samples, Rhodamine B (RhB) was used as a model dye pollutant, the details of the photocatalytic evaluation and reactor being reported elsewhere [33]. In brief, a 9-Watt white fluorescent lamp (with maximum 3.00% UV portion) with very low irradiance (33 W/m2, to minimize dye sensitization effect) was used as visible light source (400–700 nm) to irradiate the dye solution containing 40 mg of synthesized sample and 50 mL of RhB dye at a concentration of 5 ppm.
3. Results and Discussion
Physico-Chemical Characterization of the Prepared Samples
The sol–gel chemistry of metal alkoxides is to some extent ruled by the rapid hydrolysis of the alkoxy groups, which explains why careful handling and storage of such reagents are required [34,35]. The vigorous reaction of Ti alkoxides with water leads to (undesired) formation of Ti oxo/hydroxo precipitates: the reverse micelles method is useful to control the rate of hydrolysis and condensation by the frequency of intermicellar exchanges and by the water content in the micelle core, in addition to the structure-directing role of the surfactant. The surfactant indeed provides a cage-like environment that limits the nucleation, growth, and agglomeration of the particles, which dimensions depend on the water to surfactant molar ratio and surfactant composition and morphology.
As reported by Wang et al. [36] and Chandra et al. [30] the size of reverse-micelle core depends on the synthesis parameters, predominantly the water to surfactant ratio and the nature of the solvent medium. Nevertheless, both size and shape of the NPs can be tuned by changing the polarity of hydrocarbon chain and the number polyoxyethylene groups. For instance, the non-ionic surfactant Brij-58 (linear formula C16H33(CH2CH2O)20OH; molar mass ~1124 g/mol) allows obtaining TiO2 NPs with an average size of about 23 nm [36]. In this study, instead, another surfactant was used, namely the Brij-98/O20 (linear formula C18H35(OCH2CH2)20OH; molar mass ~1150 g/mol): our purpose was to investigate the effect of another moiety on the size of the final NPs and on the overall Mo dispersion.
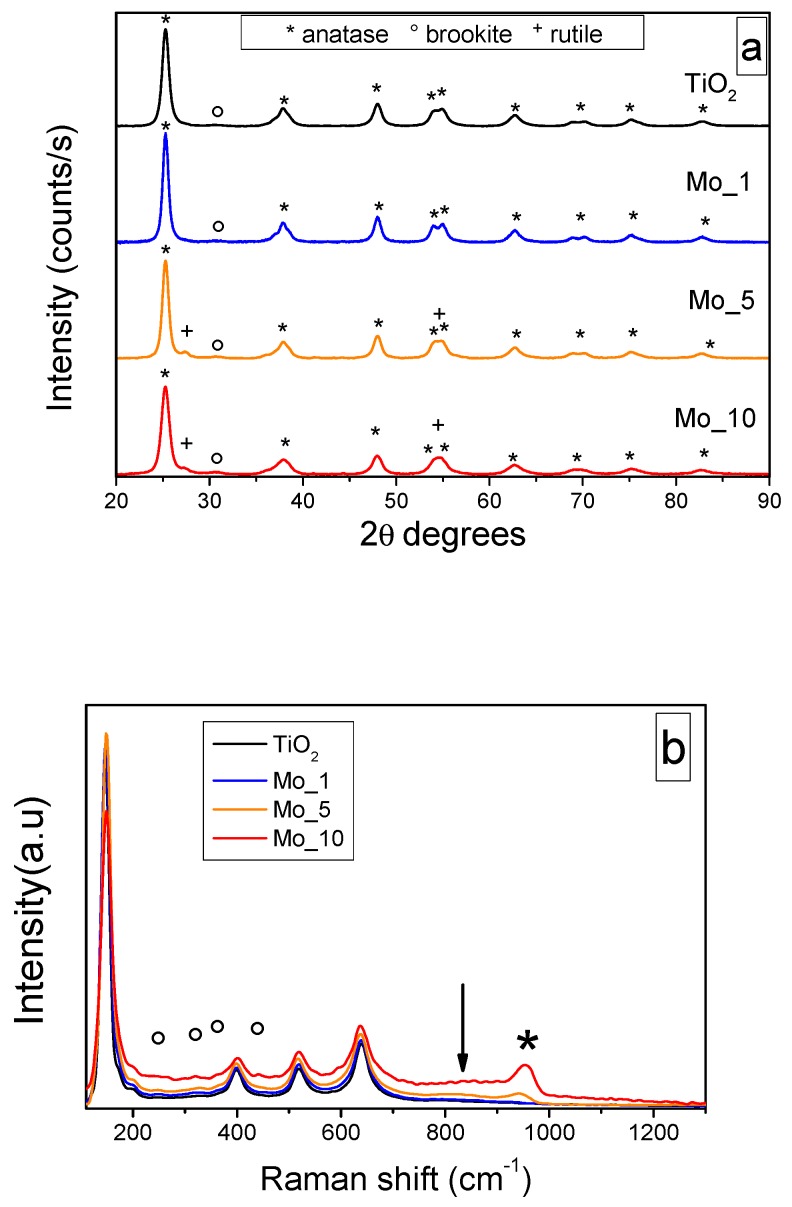
Figure 1a reports the powder XRD patterns of the synthesized samples in the 20–90 2θ range: in agreement with the calcination temperature, the TiO2 sample showed the main peaks of anatase (labelled by asterisks in the Figure, at the 2θ values of 25.2 (011), 37.8 (004), 47.9 (020), 53.8 (015), 54.9 (121), 62.6 (024), 68.7 (116), 70.1 (220), 74.9 (125), and 82.5 (224)), along with an additional broad and weak peak centered at ca. 30.7 2θ (circle) that can be ascribed to the (121) diffraction of brookite. The formation of such a phase, likely occurring in small amounts, can be assigned to the adopted synthesis. The XRD patterns of the Mo_1 sample did not differ much from those of TiO2, whereas with both Mo_5 and Mo_10 samples, two additional peaks (cross) at 27.2 and 54.4 2θ values are respectively assigned to the (110) and (211) diffraction peaks of rutile. Since the ionic radius of Mo6+ ion (0.059 nm) is very close to that of Ti4+ ion (0.0605 nm), Mo doping in TiO2 mostly occurs by substitution, with formation of impurities/defects. It is generally acknowledged that the structure of rutile is more tolerant to defects than that of anatase and so, based on the current XRD results, it can be inferred that the formation of rutile was favored at higher Mo content [37,38]. The Williamson–Hall method was used to calculate the crystallite sizes reported in Table 1: showing a slight increase with Mo_1 and Mo_5 samples. No signals ascribable to MoOx phases were detected, even at the highest Mo content: such a result indicates that Mo-containing phases, if present, are likely very well dispersed and cannot be detected by XRD.
Figure 1.
XRD patterns (a) and Raman spectra (b) of the studied powder samples (Phase symbols: * anatase; o brookite; + rutile).
The Raman spectra in Figure 1b basically confirm the XRD results: with all the samples, the Raman modes of anatase are observed at 147 (Eg), 199 (Eg), 399 (B1g), 519 (B1g) and 639 (Eg) cm−1. With the Mo_5 and Mo_10 samples, the band at 639 cm−1 is slightly red-shifted (637 cm−1) with respect to that of bare TiO2: the most intense mode of rutile (A1g) occurs indeed at 636 cm−1 and so the observed shift could be ascribed to the simultaneous presence of both rutile and anatase, in agreement with the corresponding XRD patterns. Additional Raman signals (circles in Figure 1b) were observed at 246, 327, 362, and 448 cm−1, their intensities increasing with the Mo content: they are assigned to the A1g (246 cm−1), B1g (327 and 448 cm−1), and B2g (362 cm−1) modes of brookite. The most intense band of brookite (B1g mode) was usually found at 152 cm−1 and, here, is likely superposed to that of anatase. Indeed, the maximum of the main peak was blue-shifted with the Mo_10 sample, indicating a strong interaction of the heteroatoms with the TiO2 matrix.
With the Mo_5 and Mo_10 samples, two additional Raman signals were observed: a band occurring at 944 cm−1 with the Mo_5 sample and at 956 cm−1 with the Mo_10 sample (asterisk), and a broad signal occurring in the 760–880 cm−1 range of the Mo_10 spectrum (arrow). The former signal was assigned to Mo=O groups stretching [39,40]: with systems where a comparable amount of MoO3 was supported on TiO2 by incipient wetness impregnation, a similar band was observed in the 934–954 cm−1 range, shifting to higher wavenumbers with the Mo content, and having a broad and asymmetric shape, as here. The band position suggested the presence of Mo7O246− or Mo8O264− species, where Mo is octahedrally coordinated. Tetrahedral hydrated MoO42− species (that should give a Raman band at 934 cm−1) were not observed here, even at the lowest Mo content, indicating that Mo- doping is mainly related to the TiO2 bulk, whereas at higher Mo contents, formation of polymolibdate species takes place at the surface of the NPs. The broad Raman signal in the 760–880 cm−1 range was ascribed to the Mo–O–Mo bond of the same Mo7O246− or Mo8O264− species, in agreement with the literature [41].
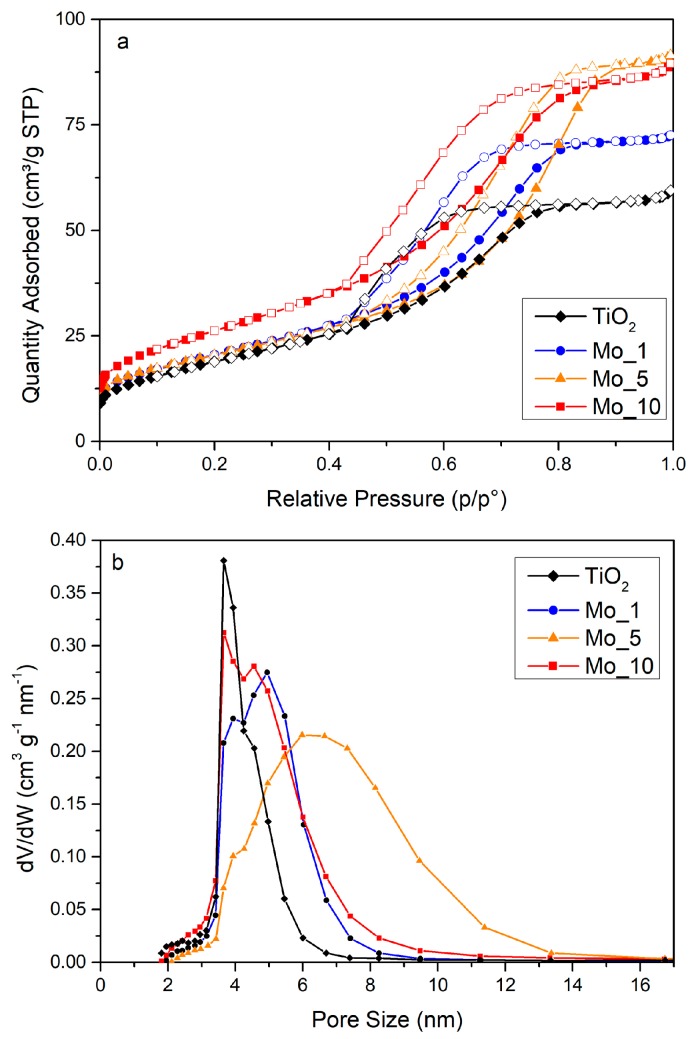
Figure 2 shows the N2 adsorption/desorption isotherms at −196 °C on both pure and Mo-doped samples, and the corresponding pore size distribution (PSD) as calculated by applying the BJH method to the isotherm desorption branch. All the samples show Type IV isotherms with Type H2 hysteresis loop, due to N2 condensation within inter-particles porosity. The BET SSA value (Table 1) was almost unaffected by the presence of Mo for heteroatom loadings up to 5 wt.%, while it increases with the Mo_10 sample.
Figure 2.
(a): N2 adsorption/desorption isotherms at −196 °C (full symbols: adsorption branch; hollow symbols: desorption branch); (b): pore size distributions (PSDs) of bare and Mo-doped TiO2.
The bare TiO2 showed a sharp Pore Size Distribution (PSD) in the 3–6 nm range (see Figure 2b), suggesting a homogeneous distribution of particles size. The same sample showed also the smallest particle size (see Table 1) and a quasi-round particle shape (see Figure 3a): from a merely geometrical point of view, the inter-particle porosity is expected to be rather homogeneous with smaller dimensions with respect to the particle size. Interestingly, the PSD became broader with Mo-doped samples. Actually, both the size and shape of the particles could affect the BET SSA and the PSDs, and, probably, less homogeneous (in both size and shape) particles were obtained in the presence of Mo. In particular, the Mo_5 sample showed the broadest PSD (in the 3–14 nm range): the sample showed indeed nearly the same BET SSA of Mo_1 (Table 1), but a larger pore volume. This could be due to the occurrence of NPs characterized by heterogeneous size and shape (see Figure 3b), although some aggregation/agglomeration phenomena may have occurred in Mo_5 as well.
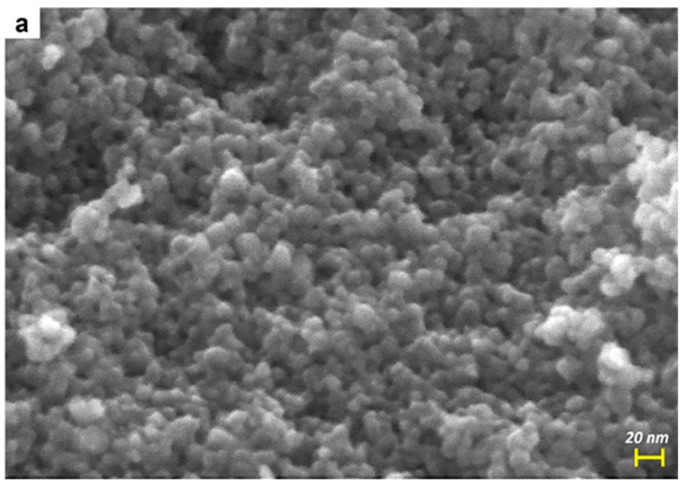
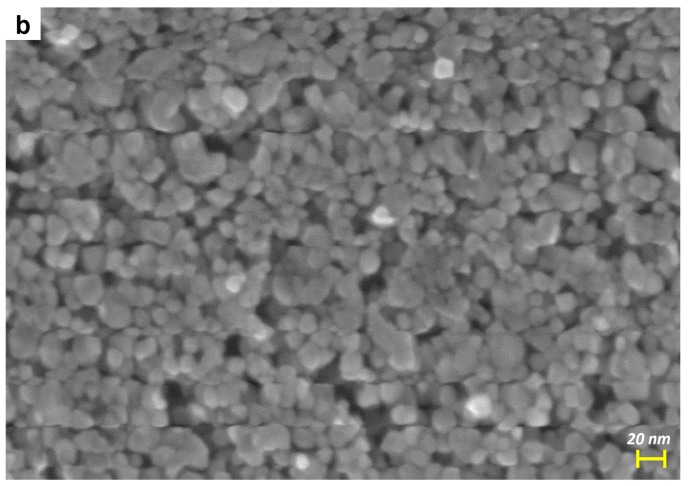
Figure 3.
FESEM micrographs of the bare TiO2 (a) and of the Mo_5 sample (b).
Figure 3a,b report the FESEM micrographs of two selected samples, namely TiO2 (a) and Mo_5 (b): NPs with quasi-round shape are observed, but the addition of Mo leads to a slight size increase due to induced structural changes (Table 1) and, indeed, the Mo_5 sample showed NPs with more heterogeneous size and shape, in agreement with the sample PSD (see Figure 2b).
The EDX analysis allowed us determining the Mo/Ti atomic ratios reported in Table 1, showing that in the explored composition range, the actual Mo content was very close to the nominal value.
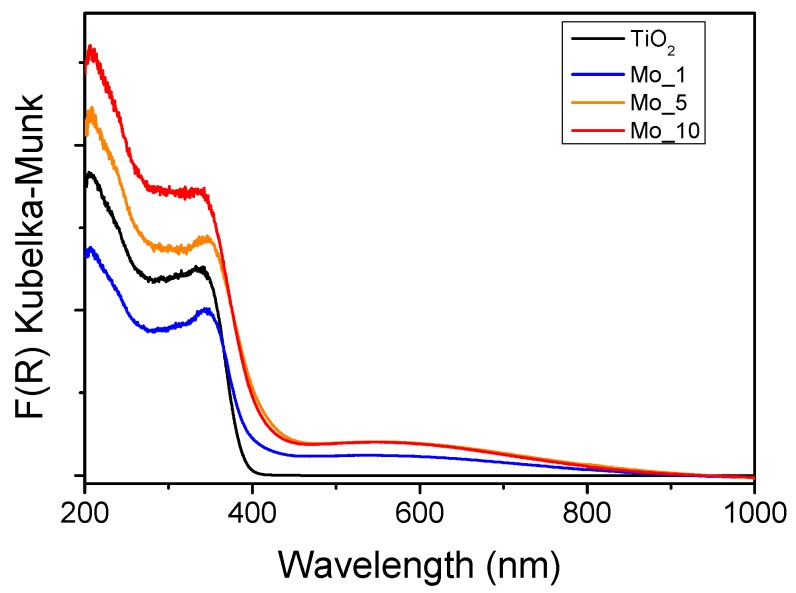
Figure 4 reports DR UV–Vis spectra of the samples. As expected, the TiO2 sample absorbed only below 400 nm, whereas introduction of Mo brought about two effects: a slight red-shift of the absorption edge and the appearance of a broad absorption centered at ca. 550 nm readily assigned to sub-band transitions related to mid-band gaps formed by Mo doping [42]. The red-shift of the absorption edge shows that Mo doping is modifying the band gap (Eg): the corresponding Eg values, as obtained by the Tauc’s plot method (not shown), are indeed 3.07 eV (TiO2), 2.86 ev (Mo_1), 2.58 eV (Mo_5) and 2.69 eV (Mo_10). Interestingly, the Mo_5 sample showed the lowest Eg value: at higher contents, Mo likely tends to form surface species, rather than being dispersed in the bulk, as also confirmed by Raman and XP spectroscopies (vide supra).
Figure 4.
DR–UV–Vis spectra of the samples TiO2 (black curve); Mo_1 (blue curve); Mo_5 (yellow curve) and Mo_10 (red curve).
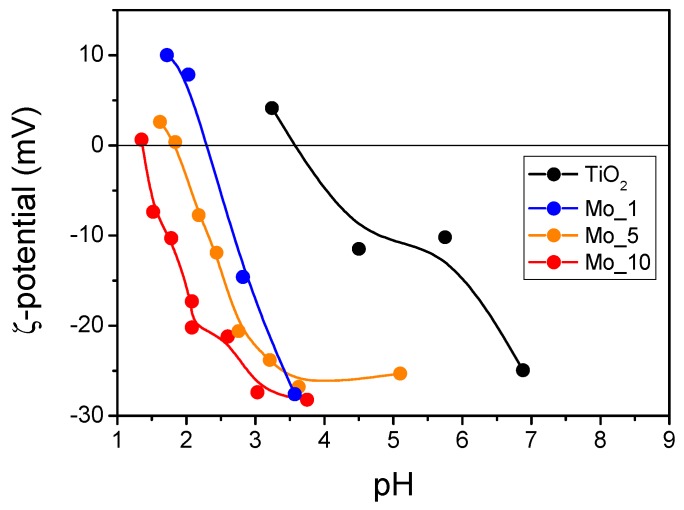
The ζ-potential measurements (Figure 5) show that the bare TiO2 has a Point of Zero Charge (PZC) of 3.6, that is, a value lower than usual for TiO2: for instance, Degussa P25 (with particle size between 20 and 40 nm) has a PZC around 6.2–6.9 [43,44]; for pure TiO2 NPs (with diameter in the range of 14–33 nm), a PZC of 6.8 [45] was reported; Allard et al. found a PZC of 6.1 with commercial anatase NPs (with a diameter of ca. 20 nm) [46]; Al-Hetlani et al. found a PZC of 5.98 for smaller anatase NPs (around 7.2 nm [47]); and Huijun et al. reported a PZC of 6.2 for anatase NPs (5–10 nm) [48]. The much lower value of PZC reported here may be ascribed, rather than to the size of TiO2 NPs, to the type of synthesis [49,50] that probably favors the formation of a very acidic surface, in agreement with previous work [43]. Addition of Mo leads to a further and progressive decrease of the PZC, even at the lowest Mo content (sample Mo_1). Such results confirm that this type of synthesis allows distribution of the heteroatoms not only in the bulk, but also at the NPs’ surface, with some superficial molybdenum ions lowering the PZC, as they are strong Lewis sites.
Figure 5.
ζ-potential measurement on the samples TiO2 (black); Mo_1 (blue); Mo_5 (yellow) and Mo_10 (red).
The ζ potential measurements allowed us to figure out that even at low Mo content, the surface of the NPs is affected by the presence of Mo: the ancillary XPS measurements (Supplementary Information) allowed us to measure the surface Mo/Ti atomic ratio in the studied samples: the corresponding values (reported in Table 1) show that the NPs surface is enriched in Mo atoms with respect to the bulk, in agreement with the type of synthesis adopted.
These obtained samples are negatively charged in a wide pH range (Figure 5), and so they should preferably interact with positively charged species, such as the Rhodamine B (RhB) dye [51].
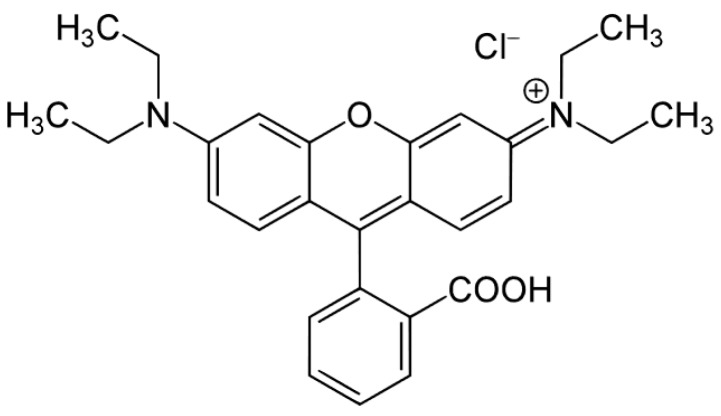
Indeed, RhB (Scheme 1) is a cationic Xanthene type dye, characterized by the presence of both diethylamine and carboxylic groups and is commonly used as a model dye pollutant [52,53]. For these reasons, the photocatalytic RhB degradation was studied in the presence of the Mo-doped samples and of the bare TiO2. Visible light irradiation with a low irradiance value of 33 W/m2 was used to avoid or at least minimize any dye-sensitization effect and to test the feasibility of sun-driven photocatalytic reaction [33].
Scheme 1.
Rhodamine B (RhB).
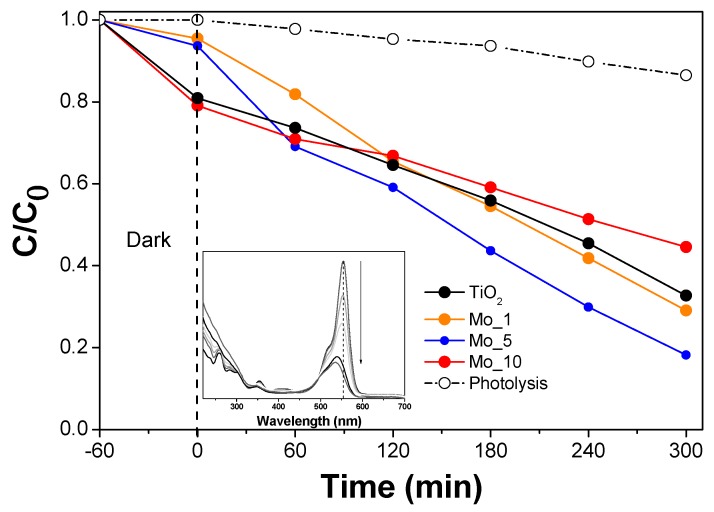
The obtained RhB degradation curves are shown in Figure 6, where, for comparison, the results concerning two blank experiments carried out without any catalyst (mere photolysis) and in the presence of a commercial TiO2 sample (Degussa P25) are reported, showing, respectively, that under Vis light, RhD photolysis occurs to a minor extent and that the commercial TiO2 was the least active of the tested samples. Concerning the photocatalytic activity of the bare TiO2 (Eg = 3.07 eV), XRD patterns shows that the sample also contained some brookite (see Figure 1): other mixed TiO2 phases containing brookite showed, indeed, promising photodegradation activity towards RhB under Vis light [54]. As compared to the bare TiO2, the Mo_10 sample showed similar behavior during the dark step, whereas under illumination, its RhB degradation efficiency was even lower than that of the bare TiO2 at a longer reaction time. Notwithstanding its smaller band gap (Eg = 2.69 eV) and lower PZC, which should favor, respectively, photocatalytic activity and the interaction with diethylamine groups (protonated in the adopted reaction conditions), the (high) Mo content of the Mo_10 sample likely had a detrimental effect on RhB degradation. At a higher content, Mo tends to form surface species, as shown by both Raman and XPS spectroscopies (the Mo_10 sample has indeed a slightly larger band gap (Eg = 2.69 eV) than Mo_5 (Eg = 2.58 eV)). Nonetheless, the formation of surface polymolibdates (as detected by Raman spectroscopy) could enhance surface electron/hole recombination, finally decreasing photocatalytic activity. Interestingly, an improved kinetic rate was achieved with the Mo_5 sample, characterized by the smallest Eg value (Eg = 2.58 eV). Such results suggest that Mo_5 has an optimum dopant concentration allowing a better exploitation of the light (due to the lowest band gap), notwithstanding the adsorption properties of the sample in dark conditions were worse than both TiO2 and Mo_10, likely due to the larger particles size of Mo_5 (22 nm, see Table 1). Similar considerations can be drawn for the adsorption behavior of the Mo_1 sample, where the lower Mo content was instead responsible of its lower activity with respect to Mo_5. Concerning the kind of dye–surface interaction, complex phenomena may occur, besides electrostatic attraction between the protonated diethylamine groups of the dye and the surface negative charge of the samples, as RhB also contains carboxylate groups and some positive surface charges may occur, especially when Mo ions are at the surface of the NPs. However, the interaction between protonated diethylamine groups of RhB and the negatively charged surface of the prepared samples could improve the degradation rate by cleavage of the Xantene group. This result could be further confirmed from the shift of the main RhB peak from 554 nm to 535 nm observed in the dye UV–Vis spectrum (inset to Figure 6): such shifts mainly occur when the dye undergoes photocatalytic degradation through a de-ethylation process/route and formation of triethylrhodamine, diethylrhodamine, and ethylrhodamine, with a different λmax, at 555, 539, 522 nm, repectively [52,53].
Figure 6.
Relative concentration profile of RhB degradation under visible light. Inset UV–Vis absorbance spectra carried out during the photodegradation of RhB in the presence of the Mo_5 sample.
4. Conclusions
Both pure and Mo-doped TiO2 NPs (with a size of ca. 10–20 nm) were obtained by a reverse micelle sol–gel synthesis method, allowing the dispersion of Mo both in the bulk and at the surface of the NPs, and substantially avoiding segregation of crystalline MoO3 event with 10 wt.% Mo.
The 5 wt.% Mo content was found to provide an optimal lowering of the band gap (from 3.07 V for the bare TiO2 to 2.58 eV), which resulted in the fastest kinetics during the photocatalytic degradation of the model dye Rhodamine B (used here as a characterization technique).
The synthesis also led to a very acidic (polar) surface, even in the absence of Mo: the resulting NPs were indeed negatively charged in a wide pH range. This surface negative charge, however, did not enhance the degradation of the dye studied here, especially at the highest Mo content, because the surface polymolibdate species likely acted as recombination centers of electron/hole pair. Despite all this, the high acidity of the NPs surface could be exploited in applications requiring a very polar surface, due to the possibility to polarize some organic pollutant, finally promoting its adsorption and consequent degradation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/12/6/937/s1, Figure S1: EDX spectra of the studied samples, Figure S2: XPS survey spectra of the studied samples [41].
Author Contributions
Both R.N. and S.E. are the primary authors, as they both realized the synthesis and most of the characterization of the materials; F.S.F. supervised the DR–UV–Vis measurements; M.A. and P.R. took part in the writing-review of the characterization part; T.A.G. and S.H. took part in the writing-review of the photocatalytic experiments; N.D. made the XPS measurements; B.B. was responsible of the writing of the paper.
Funding
This research was funded by the CARIPLO foundation, grant number 2015–0186 DeN–Innovative technologies for the abatement of N-containing pollutants in water.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hoffmann M.R., Martin S.T., Choi W., Bahnemann D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995;95:69–96. doi: 10.1021/cr00033a004. [DOI] [Google Scholar]
- 2.Linsebigler A.L., Lu G., Yates J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995;95:735–758. doi: 10.1021/cr00035a013. [DOI] [Google Scholar]
- 3.Chen X., Mao S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007;107:2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 4.Martin S.T., Lee A.T., Hoffmann M.R. Chemical mechanism of inorganic oxidants in the TiO2/UV process: Increased rates of degradation of chlorinated hydrocarbons. Environ. Sci. Technol. 1995;29:2567–2573. doi: 10.1021/es00010a017. [DOI] [PubMed] [Google Scholar]
- 5.Choi W., Hoffmann M.R. Novel Photocatalytic Mechanisms for CHCl3, CHBr3, and CCl3CO2− Degradation and the Fate of Photogenerated Trihalomethyl Radicals on TiO2. Environ. Sci. Technol. 1997;31:89–95. doi: 10.1021/es960157k. [DOI] [Google Scholar]
- 6.Prairie M.R., Evans L.R., Stange B.M., Martinez S.L. An investigation of titanium dioxide photocatalysis for the treatment of water contaminated with metals and organic chemicals. Environ. Sci. Technol. 1993;27:1776–1782. doi: 10.1021/es00046a003. [DOI] [Google Scholar]
- 7.Zhang H., Banfield J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B. 2000;104:3481–3487. doi: 10.1021/jp000499j. [DOI] [Google Scholar]
- 8.Zhang Z.B., Wang C.C., Zakaria R., Ying J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. Biol. 1998;102:10871–10878. doi: 10.1021/jp982948+. [DOI] [Google Scholar]
- 9.Zhang H., Banfield J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998;8:2073–2076. doi: 10.1039/a802619j. [DOI] [Google Scholar]
- 10.Oskam G., Nellore A., Penn R.L., Searson P.C. The growth kinetics of TiO2 nanoparticles from titanium (IV) alkoxide at high water/titanium ratio. J. Phys. Chem. B. 2003;107:1734–1738. doi: 10.1021/jp021237f. [DOI] [Google Scholar]
- 11.Wu M., Lin G., Chen D., Wang G., He D., Feng S., Xu R. Sol-hydrothermal synthesis and hydrothermally structural evolution of nanocrystal titanium dioxide. Chem. Mater. 2002;14:1974–1980. doi: 10.1021/cm0102739. [DOI] [Google Scholar]
- 12.Kavan L., Grätzel M., Gilbert S.E., Klemenz C., Scheel H.J. Electrochemical and Photoelectrochemical Investigation of Single-Crystal Anatase. J. Am. Chem. Soc. 1996;118:6716–6723. doi: 10.1021/ja954172l. [DOI] [Google Scholar]
- 13.Reyes-Coronado D., Rodríguez-Gattorno G., Espinosa-Pesqueira M.E., Cab C., de Coss R., Oskam G. Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology. 2008;19:145605. doi: 10.1088/0957-4484/19/14/145605. [DOI] [PubMed] [Google Scholar]
- 14.Asahi R., Taga Y., Mannstadt W. Electronic and optical properties of anatase. Phys. Rev. B Condens. Matter Mater. Phys. 2000;61:7459–7465. doi: 10.1103/PhysRevB.61.7459. [DOI] [Google Scholar]
- 15.Ozawa K., Emori M., Yamamoto S., Yukawa R., Yamamoto S., Hobara R., Fujikawa K., Sakama H., Matsuda I. Electron–Hole Recombination Time at TiO2 Single-Crystal Surfaces: Influence of Surface Band Bending. J. Phys. Chem. Lett. 2014;5:1953–1957. doi: 10.1021/jz500770c. [DOI] [PubMed] [Google Scholar]
- 16.Szczepanik B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017;141:227–239. doi: 10.1016/j.clay.2017.02.029. [DOI] [Google Scholar]
- 17.Moma J., Baloyi J. Photocatalysts-Applications and Attributes. IntechOpen; London, UK: 2018. Modified Titanium Dioxide for Photocatalytic Applications. [Google Scholar]
- 18.Clarizia L., Vitiello G., Pallotti D.K., Silvestri B., Nadagouda M., Lettieri S., Luciani G., Andreozzi R., Maddalena P., Marotta R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrogen Energy. 2017;42:28349–28362. doi: 10.1016/j.ijhydene.2017.09.093. [DOI] [Google Scholar]
- 19.Freyria F.S., Compagnoni M., Ditaranto N., Rossetti I., Piumetti M., Ramis G., Bonelli B. Pure and Fe-doped mesoporous titania catalyse the oxidation of acid orange 7 by H2O2 under different illumination conditions: Fe doping improves photocatalytic activity under simulated solar light. Catalysts. 2017;7:213. doi: 10.3390/catal7070213. [DOI] [Google Scholar]
- 20.Piumetti M., Freyria F.S., Armandi M., Geobaldo F., Garrone E., Bonelli B. Fe- and V-doped mesoporous titania prepared by direct synthesis: Characterization and role in the oxidation of AO7 by H2O2 in the dark. Catal. Today. 2014;227:71–79. doi: 10.1016/j.cattod.2013.11.013. [DOI] [Google Scholar]
- 21.Zeng L., Lu Z., Li M., Yang J., Song W., Zeng D., Xie C. A modular calcination method to prepare modified N-doped TiO2 nanoparticle with high photocatalytic activity. Appl. Catal. B Environ. 2016;183:308–316. doi: 10.1016/j.apcatb.2015.10.048. [DOI] [Google Scholar]
- 22.Hossain M.A., Elias M., Sarker D.R., Diba Z.R., Mithun J.M., Azad M.A.K., Siddiquey I.A., Rahman M.M., Uddin J., Uddin M.N. Synthesis of Fe- or Ag-doped TiO2–MWCNT nanocomposite thin films and their visible-light-induced catalysis of dye degradation and antibacterial activity. Res. Chem. Intermed. 2018;44:2667–2683. doi: 10.1007/s11164-018-3253-z. [DOI] [Google Scholar]
- 23.Guayaquil-Sosa J.F., Serrano-Rosales B., Valadés-Pelayo P.J., de Lasa H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B Environ. 2017;211:337–348. doi: 10.1016/j.apcatb.2017.04.029. [DOI] [Google Scholar]
- 24.Lavorato C., Argurio P., Mastropietro T.F., Pirri G., Poerio T., Molinari R. Pd/TiO2 doped faujasite photocatalysts for acetophenone transfer hydrogenation in a photocatalytic membrane reactor. J. Catal. 2017;353:152–161. doi: 10.1016/j.jcat.2017.07.015. [DOI] [Google Scholar]
- 25.Jin C., Dai Y., Wei W., Ma X., Li M., Huang B. Effects of single metal atom (Pt, Pd, Rh and Ru) adsorption on the photocatalytic properties of anatase TiO2. Appl. Surf. Sci. 2017;426:639–646. doi: 10.1016/j.apsusc.2017.07.065. [DOI] [Google Scholar]
- 26.Zou Z., Zhou Z., Wang H., Yang Z. Effect of Au clustering on ferromagnetism in Au doped TiO2 films: theory and experiments investigation. J. Phys. Chem. Solids. 2017;100:71–77. doi: 10.1016/j.jpcs.2016.09.011. [DOI] [Google Scholar]
- 27.Gai Y., Li J., Li S., Xia J., Wei S. Design of Narrow-Gap TiO2: A Passivated Codoping Approach for Enhanced Photoelectrochemical Activity. Phys. Rev. Lett. 2009;36402:23–26. doi: 10.1103/physrevlett.102.036402. [DOI] [PubMed] [Google Scholar]
- 28.Umebayashi T., Yamaki T., Itoh H., Asai K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J. Phys. Chem. Solids. 2002;63:1909–1920. doi: 10.1016/S0022-3697(02)00177-4. [DOI] [Google Scholar]
- 29.Bonelli B., Esposito S., Freyria F.S. Titanium Dioxide. Intech; London, UK: 2017. Mesoporous Titania: Synthesis, properties and comparison with non-porous titania. [Google Scholar]
- 30.Chandra P., Doke D.S., Umbarkar S.B., Biradar A.V. One-pot synthesis of ultrasmall MoO3 nanoparticles supported on SiO2, TiO2, and ZrO2 nanospheres: An efficient epoxidation catalyst. J. Mater. Chem. A. 2014;2:19060–19066. doi: 10.1039/C4TA03754E. [DOI] [Google Scholar]
- 31.Ghosh S. Comparative studies on brij reverse micelles prepared in benzene/surfactant/ethylammonium nitrate systems: Effect of head group size and polarity of the hydrocarbon chain. J. Colloid Interface Sci. 2011;360:672–680. doi: 10.1016/j.jcis.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Gadhi T.A., Hernández S., Castellino M., Chiodoni A., Husak T., Barrera G., Allia P., Russo N., Tagliaferro A. Single BiFeO3 and mixed BiFeO3/Fe2O3/Bi2Fe4O9 ferromagnetic photocatalysts for solar light driven water oxidation and dye pollutants degradation. J. Ind. Eng. Chem. 2018;63:437–448. doi: 10.1016/j.jiec.2018.03.004. [DOI] [Google Scholar]
- 33.Esposito S., Turco M., Bagnasco G., Cammarano C., Pernice P. New insight into the preparation of copper/zirconia catalysts by sol–gel method. Appl. Catal. A Gen. 2011;403:128–135. doi: 10.1016/j.apcata.2011.06.024. [DOI] [Google Scholar]
- 34.Esposito S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials. 2019;12:668. doi: 10.3390/ma12040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Li X., Zhang S., Lu R. Facile synthesis of ultrasmall monodisperse “raisin–bun”-type MoO3/SiO2 nanocomposites with enhanced catalytic properties. Nanoscale. 2013;5:4823. doi: 10.1039/c3nr01097j. [DOI] [PubMed] [Google Scholar]
- 36.Wang X.H., Li J.G., Kamiyama H., Ishigaki T. Fe-doped TiO2 nanopowders by oxidative pyrolysis of organometallic precursors in induction thermal plasma: Synthesis and structural characterization. Thin Solid Films. 2006;506–507:278–282. doi: 10.1016/j.tsf.2005.08.069. [DOI] [Google Scholar]
- 37.Batzill M., Morales E.H., Diebold U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006;96:1–4. doi: 10.1103/PhysRevLett.96.026103. [DOI] [PubMed] [Google Scholar]
- 38.Ciambelli P., Sannino D., Palma V., Vaiano V., Bickley R.I. Reaction mechanism of cyclohexane selective photo-oxidation to benzene on molybdena/titania catalysts. Appl. Catal. A Gen. 2008;349:140–147. doi: 10.1016/j.apcata.2008.07.019. [DOI] [Google Scholar]
- 39.Del Arco M., Carrazhn S.R.G., Rives V., Garcla-Ramos J.V. A Laser Raman Spectroscopy Study of Surface Species Existing in MoO3/A12O3 Catalysts. Spectrosc. Lett. 1992;25:73–82. doi: 10.1080/00387019208020758. [DOI] [Google Scholar]
- 40.Stampfl S.R., Chen Y., Dumesic J.A., Niu C., Hill C.G. Interactions of molybdenum oxide with various oxide supports: Calcination of mechanical mixtures. J. Catal. 1987;105:445–454. doi: 10.1016/0021-9517(87)90072-8. [DOI] [Google Scholar]
- 41.Nasi R., Gadhi T.A., Freyria F.S., Ditaranto N., Esposito S., Hernandez S., Armandi M., Bonelli B. Surface chemical characterization of Mo doped TiO2 nanoparticles for photocatalytic dye degradation; Proceedings of the Incontro Spettroscopia Analitica ISA 2018; Cagliari, Italy. 5–8 June 2018; pp. 5–6. [Google Scholar]
- 42.Gomathi Devi L., Narasimha Murthy B. Characterization of Mo Doped TiO2 and its Enhanced Photo Catalytic Activity Under Visible Light. Catal. Lett. 2008;125:320–330. doi: 10.1007/s10562-008-9568-4. [DOI] [Google Scholar]
- 43.Suttiponparnit K., Jiang J., Sahu M., Suvachittanont S., Charinpanitkul T., Biswas P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2010;6:27. doi: 10.1007/s11671-010-9772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmberg J.P., Ahlberg E., Bergenholtz J., Hassellöv M., Abbas Z. Surface charge and interfacial potential of titanium dioxide nanoparticles: Experimental and theoretical investigations. J. Colloid Interface Sci. 2013;407:168–176. doi: 10.1016/j.jcis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Pirinejad L., Maleki A., Shahmoradi B., Daraei H., Yang J.-K., Lee S.-M. Synthesis and application of Fe-N-Cr-TiO2 nanocatalyst for photocatalytic degradation of Acid Black 1 under LED light irradiation. J. Mol. Liq. 2019;279:232–240. doi: 10.1016/j.molliq.2019.01.135. [DOI] [Google Scholar]
- 46.Allard M.M., Merlos S.N., Springer B.N., Cooper J., Zhang G., Boskovic D.S., Kwon S.R., Nick K.E., Perry C.C. Role of TiO2 Anatase Surface Morphology on Organophosphorus Interfacial Chemistry. J. Phys. Chem. C. 2018;122:29237–29248. doi: 10.1021/acs.jpcc.8b08641. [DOI] [Google Scholar]
- 47.Al-Hetlani E., Amin M.O., Madkour M. Detachable photocatalysts of anatase TiO2 nanoparticles: Annulling surface charge for immediate photocatalyst separation. Appl. Surf. Sci. 2017;411:355–362. doi: 10.1016/j.apsusc.2017.03.151. [DOI] [Google Scholar]
- 48.He H., Cheng Y., Yang C., Zeng G., Zhu C., Yan Z. Influences of anion concentration and valence on dispersion and aggregation of titanium dioxide nanoparticles in aqueous solutions. J. Environ. Sci. 2017;54:135–141. doi: 10.1016/j.jes.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Azeez F., Al-Hetlani E., Arafa M., Abdelmonem Y., Nazeer A.A., Amin M.O., Madkour M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018;8:7104. doi: 10.1038/s41598-018-25673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao D.L., Wu G.S., Liao B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009;348:270–275. doi: 10.1016/j.colsurfa.2009.07.036. [DOI] [Google Scholar]
- 51.Lops C., Ancona A., Di K., Dumontel B., Garino N. Applied Catalysis B: Environmental Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019;243:629–640. doi: 10.1016/j.apcatb.2018.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rochkind M., Pasternak S., Paz Y., Rochkind M., Pasternak S., Paz Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules. 2014;20:88–110. doi: 10.3390/molecules20010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadhi T.A., Hernández-Gordillo A., Bizarro M., Jagdale P., Tagliaferro A., Rodil S.E. Efficient α/β-Bi2O3 composite for the sequential photodegradation of two-dyes mixture. Ceram. Int. 2016;42:13065–13073. doi: 10.1016/j.ceramint.2016.05.087. [DOI] [Google Scholar]
- 54.Boppella R., Basak P., Manorama S.V. Viable method for the synthesis of biphasic TiO2 nanocrystals with tunable phase composition and enabled visible-light photocatalytic performance. ACS Appl. Mater. Interfaces. 2012;4:1239–1246. doi: 10.1021/am201354r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.