Abstract
Leucine zipper/EF-hand-containing transmembrane protein 1 (LETM1) has been identified as the gene responsible for Wolf-Hirschhorn syndrome (WHS), which is characterized by intellectual disability, epilepsy, growth delay and craniofacial dysgenesis. LETM1 is a mitochondrial inner membrane protein that encodes a homolog of the yeast protein Mdm38, which is involved in mitochondrial morphology. In the present review, the importance of LETM1 in WHS and its role within the mitochondrion was explored. LETM1 governs the mitochondrion ion channel and is involved in mitochondrial respiration. Recent studies have reported that LETM1 acts as a mitochondrial Ca2+/H+ antiporter. LETM1 has also been identified as a K+/H+ exchanger, and serves a role in Mg2+ homeostasis. The function of LETM1 in mitochondria regulation is regulated by its binding partners, carboxyl-terminal modulator protein and mitochondrial ribosomal protein L36. Therefore, we describe the remarkable role of LETM1 in mitochondrial network physiology and its function in mitochondrion-mediated cell death. In the context of these findings, we suggest that the participation of LETM1 in tumorigenesis through the alteration of cancer metabolism should be investigated. This review provides a comprehensive description of LETM1 function, which is required for mitochondrial homeostasis and cellular viability.
Keywords: leucine zipper/EF-hand-containing transmembrane protein 1, Wolf-Hirschhorn syndrome, mitochondria, tumorigenesis, mitophagy
1. Introduction
Wolf-Hirschhorn syndrome (WHS) patients display pleiotropic phenotypes including growth delay, intellectual disability, congenital hypotonia, distinct facial appearance, congenital heart defects, midline defects and seizures (1,2). The deletion of either of two critical regions (WHSCR and WHSCR-2) within chromosome band 4p16.3 has been proposed to be necessary for minimal clinical manifestation of WHS (3,4). A gene within WHSCR-2, leucine zipper/EF-hand-containing transmembrane protein 1 (LETM1), which is deleted in almost all patients with the full phenotype, and was therefore suggested as an excellent candidate gene for the seizure development (2,3,5). LETM1 was proposed to contribute to the neuromuscular features of WHS patients (6). Multiple genes are responsible for the characteristics of WHS disorders; thus, mouse models have been developed to complement ongoing patient-based studies (7). Phenotypes of a genetically modified mouse model for FGFR33, MAEA, Sax2/Nkx1-1 and CTBP1 showed skeletal malformations, hematopoietic dysgenesis, post-natal growth defects, and later growth defects, respectively (7). The most severe pathogenic phenotype of WHS is epilepsy, which has been shown in mouse lines carrying TACC3 and FAM53A mutantions (7,8). LETM1 is also accepted to be tightly linked to the epilepsy pathogenesis in WHS. In Drosophila melanogaster model, neuronal-specific knockdown of LETM1 resulted in the impairment of locomotor behavior in the fly and reduced synaptic neurotransmitter release. These results revealed the function of LETM1 in epilepsy: One of the severe pathogenic phenotypes of WHS (2,9). Decreased LETM1 levels in the neo-cortices of temporal lobe epilepsy patients and in the hippocampi and adjacent cortices of rats following the onset of seizures has suggested that reduction of LETM1 contributes to the seizure phenotype. Knockdown of LETM1 leads to reduced MT-CYB expression and induction of susceptibility to seizures, which suggested that dysfunctional LETM1 together with mitochondrial swelling and disturbed MT-CYB expression is important for the deteriorated behavioral phenotype of epilepsy (10). However, Nigericin, a K+/H+ ionophore, fails to prevent epileptic seizures, and does not reverse lentivirus-shLETM1-mediated facilitation of epilepsy, indicating that further studies are required to determine the detailed mechanism of LETM1 function and delineate the involvement of LETM1 in seizure pathogenesis (10). Therefore a detailed understanding of LETM1 function will provide great advantages for WHS patients.
2. LETM1 functions in mitochondria
LETM1 structure and localization
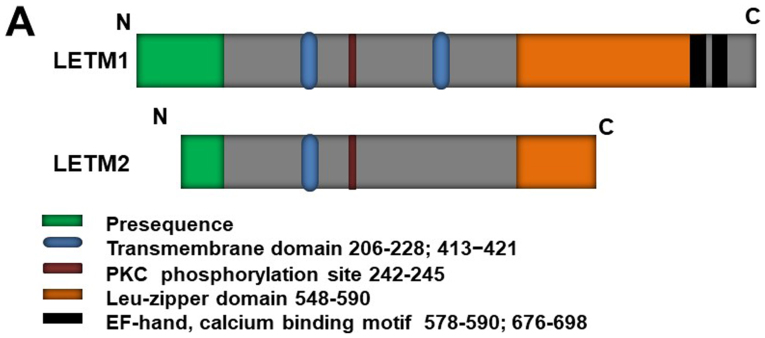
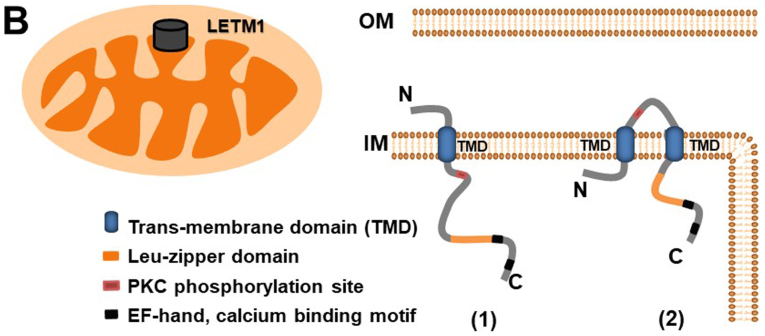
LETM1 is a protein with a molecular mass of 70 kDa; it has also been identified as a 83-kDa endogenous protein in HeLa cells, suggesting that it is synthesized as a cytosolic precursor with a presequence (11). LETM1, like its yeast counterpart Mdm38 (12), is a mitochondrial inner membrane protein (1,9,13); it comprises a C-terminal domain bearing two EF-hand domains (578–590 and 676–698) and an N-terminal domain bearing a protein kinase C phosphorylation site (Fig. 1A) (6,14). LETM1 is an integral mitochondrial inner membrane protein facing toward the matrix (Fig. 1B, right). LETM1 is co-localized with HSP60, a mitochondria matrix protein (11). Partial digestion of LETM1 under hypotonic conditions has suggested that LETM1 contains a small and/or protease-resistant region exposed to the inner membrane space (11). The N-terminus of LETM1 is located in the inter-membrane space, connected to the inner membrane by a transmembrane domain containing three conserved proline residues (206–228), and the C-terminus extends to the mitochondrial matrix (6,15) [Fig. 1B, left (1)]. However, a recent study (16) revealed that LETM1 contains another transmembrane domain, from residues 413 to 421. This discovery has led to controversy regarding LETM1 N-terminal localization, which was strongly suggested to face the matrix [Fig. 1B, left (2)] (15).
Figure 1.
LETM1 structure and localization. (A) Schematic representation of LETM1 and LETM2. Specific domains and sites are symbolized by respective colors as indicated. (B) Sub-mitochondrial localization of LETM1. OM, Outer Membrane; IM, inner membrane; LETM1, leucine zipper/EF-hand-containing transmembrane protein 1.
LETM2, a homolog of LETM1, has already been identified (11,17). The LETM2 gene is located at human chromosome 8 and was also found in rat through a homolog search using human LETM1 cDNA (17). LETM2 is a mitochondrial inner membrane protein that shares some features with LETM1 (e.g., they are both mitochondrial inner membrane proteins with a leucine-zipper motif); however, it has a function distinct from that of LETM1, as demonstrated by its failure to suppress mitochondrial swelling caused by LETM1 knockdown (11). LETM1 has been detected in nearly all rat tissues, whereas LETM2 is expressed specifically in the testis, with a molecular size of 45 kDa, during the developmental stages from spermatocyte to spermatozoon. These expression environments indicate its probable function in inner and crista membrane reorganization in the mitochondria during spermatogenesis (11).
LETM1 regulates mitochondrial ion-channels
As indicated in Fig. 2, LETM1 was recently reported to function as a mitochondrial ion channel regulator. The presence of two putative Ca2+ binding sites on LETM1 suggestes that impaired mitochondrial Ca2+ homeostasis due to lack of LETM1 could explain the seizures observed in some WHS patients (1). An RNAi screen was conducted to identify the genes that control mitochondrial Ca2+ transport in Drosophila S2 cells which stably express mitochondria-targeted ratiometric pericam. CG4589, the Drosophila homolog of the human gene LETM1, was identified as a gene strongly affecting (Ca2+)mito and (H+)mito responses (18). LETM1 catalyzes the 1:1 electrogenic exchange of Ca2+ for H+ which is driven by the negative potential of mitochondria and by protons leaving the mitochondrial matrix (18,19). The mitochondrial calcium unipoter (mCU) drives rapid and massive Ca2+entry, but only at the high cytosolic Ca2+ concentrations (>10 µM) that are reached in microdomains near Ca2+ release channels on the endoplasmic reticulum (ER) (20). The new Ca2+/H+ antiporter operates at low cytosolic Ca2+ concentrations (<100 nM) and is limited by the pH gradient generated by the mitochondrial electron transport chain (21). LETM1 shares a role with mCU in catalyzing Ca2+ uptake into mitochondria; this role can be inhibited by ruthenium red. Thus, unlike mCU, which is critical only for Ca2+ uptake, LETM1 catalyzes Ca2+ uptake into mitochondria in exchange for H+, implying that proton efflux from mitochondria can drive LETM1-dependent Ca2+ entry into mitochondria. mCU conveys rapid Ca2+ transient from the cytosol to the matrix but exposes mitochondria to Ca2+ overload and alterations in ER Ca2+ handling. LETM1 is bidirectional and can extrude Ca2+ along with the Na+/Ca2+ exchanger during large Ca2+ loads, allowing mitochondria to bear small cytosolic Ca2+ elevations without risking Ca2+ overload (21). Knockdown and overexpression of LETM1 in cells has been shown to alter [Ca2+]mito and [pH]mito responses in a pattern consistent with Ca2+/H+ exchange (18). Reconstitution of the purified protein in liposomes confirmed that LETM1 mediates Ca2+/H+ exchange. This transport is electrogenic and therefore blocked by ruthenium red. Functional data in LETM1-depleted HeLa cells have indicated that the new antiporter can mediate both Ca2+ uptake and Ca2+ extrusion from mitochondria; however, these observations remain to be confirmed by simultaneous Ca2+ and pH measurements during physiological stimulation (18). LETM1 and UCP2/3 independently contribute two molecularly distinct pathways for mitochondrial Ca2+ uptake in endothelial cells. Knockdown of LETM1 resulted in highly depleted sequestration of entering Ca2+, but had no effect on Ca2+ uptake at ER-mitochondrial junctions, suggesting that LETM1 and UCP2/3 contribute Ca2+ uptake from different sources and at different Ca2+ concentrations (22). However, it has been proposed that LETM1 works as a Ca2+/H+ antiporter in mitochondria, but also that LETM1 functions as a mitochondrial K+/H+ exchanger (12). Measurements in isolated mitochondria labeled with K+ and H+ fluorescent dyes confirmed the presence of a K+/H+ exchanger in the inner mitochondrial membrane of yeast (12). Mutants in the yeast LETM1 homolog Mdm38, which lacks the two Ca2+ binding sites present in LETM1, cause loss of mitochondrial K+/H+ exchange activity, resulting in highly abundant K+ within the mitochondrial matrix and low membrane potential (ΔΨm), followed by water influx and organelle swelling (23,24). LETM1 expression restores Mdm38 and potassium acetate indicating a defect in K+/H+ exchange activity-induced swelling (23). Furthermore, Mdm38 depletion resulted in an early loss of K+/H+ exchange-mediated swelling capacity of mitochondria, loss of ΔΨm and mitophagy during the extensive interaction of mitochondria with vacuoles (24,25). These effects strongly support the notion that K+/H+ exchange activity is the primary cause of morphological changes in mitochondria, which in turn trigger the process of mitophagy (24). This evidence of mitophagy induction via Mdm38 depletion feasibly suggests that LETM1 regulates mitophagy and cell viability.
Figure 2.
Regulation of ion-channels in mitochondria by LETM1. MCU drives massive calcium entry with high cytosolic Ca2+ concentrations (>10 µM) in microdomains near Ca+2 release channels on ER but LETM1 functions as Ca2+/H+ antiporter, at low cytosolic Ca2+ concentrations (>100 nM). The pH gradient generated by the ETC limits its action. It can also extrude Ca2+ along with the NCLX during Ca2+ overload. Furthermore, it also functions as the K+/H+ exchanger as well as a Mg2+ transporter. ETC, electron transport chain; MCU, Mitochondrial Ca2+ uniporter; NCLX, Na+-Ca+ exchanger; VDAC, voltage dependent anion channel; SR, sarcoplasmic Reticulum; ER, endoplasmic reticulum; LETM1, leucine zipper/EF-hand-containing transmembrane protein 1.
Mitochondrial Mg2+ transport processes may play an important role in cellular Mg2+ homeostasis and the regulation of cell and mitochondrial functions (26). MRS2 encodes a mitochondrial membrane protein, that is an essential component of the major electrophoretic Mg2+ influx system in mitochondria (26). Mutant alleles of MRS2 and its overexpression increase intra-mitochondrial Mg2+ concentrations. Two yeast homolog genes of LETM1, MRS7 and YOL027, are multicopy suppressors of MRS2D (disruptiing the open reading frames of MRS2 and defective in mitochondrial Mg2+ influx, respectively), whereas Yo1027p overexpression may improve Mg2+ influx in MRS2D cells (23,26). Furthermore, Mdm38 mutation suppresses the growth deficiency caused by mutation in the MRS2 gene (27,28). Together, these findings suggest LETM1 acts as an Mg2+ transporter in the mitochondrion.
LETM1 and its partners cooperate to influence mitochondria morphology, respiration and biogenesis
Yeast Mdm38, a homolog of LETM1 was identified in a comprehensive genome-wide screen for mutants with mitochondrial morphology defects (29). Within LETM1-knockdown cells, mitochondrial swelling and the disorganization of cristae structures were observed using electron microscopy (11). LETM1 knockdown caused mitochondria to become dot-like structures and lose their tubular networks to a significantly greater extent than fragmented mitochondria observed in OPA1-knockdown cells (11,30). Images of mitochondria lacking LETM1 were reminiscent of observations following overexpression of pro-fission proteins such as Fis1 (31) or knockdown of pro-fusion proteins sfiguch as OPA1 (32). In both cases, inhibition of the dynamin-related protein Drp1-dependent fission machinery, by silencing or using a dominant negative, caused the Drp1K38A mutant to recover its mitochondrial morphology (33,34). Nevertheless, Drp1 inhibition in LETM1 knockdown cells, did not recover the fragmented phenotype, indicating that blockage of the fission machinery is not induced by reduction of LETM1 and that lack of LETM1 causes Drp1-independent mitochondrial fragmentation (30). Moreover, following the silencing of both Drp1 and LETM1, mitochondria remained partly swollen, suggesting that mitochondrial membrane fusion is unaffected by downregulation of LETM1 or co-silencing of Drp1 (11). These results suggest that LETM1 is not directly implicated in mitochondrial membrane fission and fusion. Thus, neither lack nor excess of LETM1 is beneficial to cells, and fragmented mitochondria and swollen cristae were observed in LETM1-overexpressed HeLa cells (14).
LETM1 downregulation caused reduced numbers, and morphological changes in cristae, leading to a substantially lower membrane potential (ΔΨm), which is consistent with reports that isolated mitochondria from yeast Mdm38 mutants exhibited low membrane potential (23,35); however, no significant changes in ΔΨm were observed between controls and LETM1-transduced cells (14). LETM1 knockdown caused the disassembly of three different proton pumps complex I (NADH dehydrogenase), III (cytochrome b complex), and IV (cytochrome c oxidase) (36), suggesting that LETM1 regulates the biogenesis of respiratory chains. Low membrane potential appears to be a consequence of the failure to form super-complexes: complexes I, III and IV of the respiratory chains (11).
Therefore, LETM1 is critical for respiratory chain biogenesis, being physically associated with mitochondrial ribosomes to mediate membrane insertion of several proteins of nuclear and mitochondrial origin, and facilitating their transport across the inner membrane (24,35). A ribosome-associated site has been identified on the LETM1 ribosome binding domain (RBD); it displays a matrix-exposed 14-3-3-like fold, which is critical for respiratory chain assembly through the regulation of Cox1 and Cytb translation (37). Mitochondrial ribosomal protein L36 (MRPL36) has been reported to be associated with the inner-membrane (38). The addition of puromycin was used to access possible interactions between LETM1 and MRPL36; LETM1 associated with MRPL36 both in vivo and in vitro. MRPL36 siRNA significantly recovered LETM1-mediated ATP reduction, suggesting that LETM1 may regulate the mitochondrial translation system and reduce mitochondrial biogenesis through association with MRPL36 (14). The inhibition of mitochondrial biogenesis by LETM1 offers a possible explanation of WHS phenotypes, especially neuromuscular defects and seizures, which likely reflect oxidative phosphorylation defects and thus resembles classical mitochondrial encephalomyopathies such as mitochondrial encephalomyopathy, lactic acidosis, and stroke like episodes (35).
Cells lacking mitochondrial DNA lose active respiratory chains, but maintain mitochondrial tubular networks, indicating that mitochondrial swelling caused by LETM1 knockdown is not caused by the disassembly of respiratory chains (11). Human AAA-ATPase BCS1L, which is a mitochondria inner membrane protein, is responsible for human disorders and the assembly of respiratory chains (39–41). BCS1L interacts specifically with LETM1, stimulating the formation of the LETM1 major complex; thus, BCS1L and LETM1 function in different process to maintain mitochondria and form tubular network structures (11).
Role of LETM1 in mitochondrial quality control
The term mitophagy was first introduced by Dr J.J. Lemasters for the selective autophagic degradation of damaged and dysfunctional mitochondria (42). An accumulation of studies suggest that mitochondrial dysfunction and morphological changes, which are interrelated factors, are responsible for the induction of mitophagy (24,43,44). Mitochondrial fission produces two subsets of daughter mitochondria with either increased or decreased membrane potential. The depolarized daughter mitochondrion, which is incapable of fusing into the polarized network, is removed through the process of mitophagy (44). Loss of K+/H+ activity and subsequent decrease in membrane potential due to Mdm38 depletion leads to mitochondrial fragmentation into discrete spheres and their association with vacuoles, suggestive of mitophagy induction. Furthermore, the depletion of DNM1, a mitochondrial fission protein, and Mdm38 leads to the continued fusion of swollen mitochondria, indicating the blockage of selective removal by mitophagy (24). LETM1 overexpression has been reported to result in a mitochondrial ATP decrease, mitochondrial dysmorphology, swollen mitochondria cristae, and fragmentation in HeLa cells in an OPA1-dependent manner (45). The mitochondria within autophagosomes with reduced OPA1 levels (44) and fragmented discrete mitochondria targeted by autophagic vacuoles in an Mdm38-depleted yeast strain point toward a possible role for LETM1 in mitophagy induction. Mitochondrial depolarization also leads to the stabilization and accumulation of PINK1 in the outer membrane of the mitochondrion (46), which can recruit parkin; the depolarized, damaged mitochondria are subsequently removed by mitophagy (47). Interestingly, PINK1-phosphorylated LETM1 has been shown to modulate mitochondrial Ca2+ transport and protect neurons against mitochondrial stress (48). These studies paved the way for further studies to reveal the role of LETM1 in the quality control machinery of mitochondria.
3. New insights into tumorigenesis
Tumors have been reported in several WHS cases (49–52). In particular, the discovery of neuroblastoma in a child with WHS implicated the association of these two phenotypes (51). Because WHS is rare, the occurrence of these tumors raises concern that cancer may be a component feature of WHS. LETM1 is highly expressed in many human malignancies and is correlated with poor prognosis (14,53,54). LETM1 knockdown by siRNA repressed proliferation, migration, and invasion in bladder cancer cells (53). Several oncogenic proteins in the Wnt/β-catenin signaling pathway (β-catenin, cyclin D1 and c-Myc) were significantly decreased by LETM1 siRNA. These finding clearly indicate the roles of LETM1 in tumor progression. However, the potential molecular mechanism of LETM1-mediated tumorigenesis remains to be elucidated.
Because mitochondrial dysfunction has been implicated in a wide variety of human diseases, including cancers and age-related disorders, mitochondria have emerged as effective targets for anticancer therapy (55–57). Mitochondria function as central components of cell survival through ATP production and govern cell fate by mitochondrial membrane-dependent cell death signaling (58). Loss of Mdm38 results in a variety of phenotypic effects, including defects of respiratory chain, altered mitochondrial morphology, osmotic swelling, and mitophagy (24). Downregulation of LETM1 causes Drp1-independent fragmentation of the mitochondrial network, but is not associated with respiratory chain, whereas overexpression of LETM1 leads to mitochondrial fragmentation through OPA1 modulation (30). Although the functions and mechanisms of LETM1 with respect to cell viability and tumorigenesis remain controversial, accumulating data suggest that LETM1 will be a crucial candidate to clarify how mitochondria regulate the normal life of the cell.
Maintenance of mitochondrial morphology by LETM1 is required for cell viability
The regulation of mitochondrial volume and morphology has been associated with a wide range of important biological functions and pathologies. Any disruption in the osmotic balance between mitochondria and the cytosol, mainly as a result of intracellular ion fluxes (particularly K+, due to its higher concentration), induces water movement between these two compartments, leading to loss of mitochondrial volume and homeostasis, which then cause morphological changes (59). Fission-fusion events can also result in mitochondrial morphological changes, which are modulated by a complex network of cytosolic and mitochondrial proteins and are coordinated to respond to specific cellular demands (60,61). Osmotic swelling, which indicates pathological states in mitochondria, has been found to activate downstream cascades, notably determining the viability of the cell as a whole (61).
Mitochondrial swelling also plays an important role in the release of cytochrome c, which is associated with apoptotic cell death (59). Adenovirus-meditated LETM1 overexpression leads to mitochondrial dysmorphology, swollen mitochondria cristae, and an increase in mitochondrial fragmentation, as shown by electron microscopy, and sensitizes the cell to apoptosis in an OPA-1-dependent manner (45).
However, long-term LETM1 overexpression leads to necrotic cell death via decreases in intracellular ATP levels, in a time-dependent manner, because it is independent of AIF nuclear translocation and caspase activation. A previous study found that the addition of extra glucose led to a reduction in total mitochondrial mass and the amount of ATP per cell, and partial recovery, supporting the notion that LETM1-mediated cell death is influenced by energy deprivation (14). However, downregulation of LETM1 has also been shown to result in caspase-independent and Bcl-2-insensitive necrotic cell death. To date, it remains unknown how both the gain and loss of LETM1 causes similar phenotypes in cells.
Mitochondrial morphology is also strongly associated with energy metabolism, as mitochondria with increased respiration levels appear in morphologically interconnected networks with enlarged cristae compartments, whereas those with low respiration and therefore high glycolysis levels are fragmented, with smaller inter-cristae space (62). LETM1 has been demonstrated to cause such fragmented morphology (24,45) along with altered energy metabolism leading to tumorigenesis (14). Taken together, these findings demonstrate that the role of LETM1 in mitochondrial volume and morphological homeostasis is critical to determining cell viability and correlation with tumorigenesis.
LETM1 contributes to cancerous metabolic alteration
In 1931, Otto Warburg was awarded the Nobel Prize for oncology for his breakthrough hypothesis and research on cancerous metabolism (63). He observed that cancer cells are predominantly dependent on energy produced by glycolysis, rather than by pyruvate oxidation in mitochondria (57). Although the exact mechanisms responsible for this metabolic alteration remain to be elucidated, mitochondrial respiratory defects, due in part to mitochondrial DNA mutations/deletions and hypoxia, are thought to be important contributing factors. Mitochondrial respiration deficiency and an increase in ATP production via glycolysis, leading to activation of the PKB/Akt survival pathway through NADH-mediated inactivation of PTEN, is a novel mechanism contributing to altered cancerous metabolism (64). Consistent with these results, Piao and colleagues demonstrated that LETM1 overexpression (45); led to increased ATP production via glycolysis and increased lactate production, also causing PTEN inactivation and PKB activation, suggesting that LETM1-induced mitochondrial dysfunction resulted in PKB activation which increased the ratio of NADH/NADPH to inactivate PTEN enzyme activity. -Similar levels of LETM1 expression in cancerous tissue and in overexpressed HeLa cells, along with the detection of LETM1 in two bands from six patients who had undergone surgery for malignant cancer, strongly suggest that LETM1 overexpression is a feature of altered cancerous metabolism accompanied by metastasis (14). In contrast, AMPK activation and consequent inhibition of G1/S cell cycle progression, as well as decreased PKB and mTOR phosphorylation by LETM1 overexpression can alter lung cancer cell growth (65). Using liquid chromatography tandem mass spectrometry and a power law global error model for reliable bio-signature mapping of hepatocellular carcinoma,, LETM1 was found to have increased expression level, and its potential translocation to the tumor nuclear fraction was inferred, especially to the peripheral nuclear region and outer nucleus as confirmed by western blot, immunohistochemical, and immunofluorescent analyses (66). Collectively, these finding underscore the putative role of LETM1 in altered cancerous metabolism and indicate the need for further study to explore the roles of LETM1 in different tumors, and establish it as an effective therapeutic target in cancer-treatment.
Association of LETM1 with mitochondrial ribosomes and mitochondrial ATP regulation
Mutations in mitochondrial DNA have been shown to play a key role in tumorigenesis within various organs, as these mutations lead to the malformation or/and malfunctioning of the mitochondrial respiratory chain, compelling cells to depend on glycolysis to fulfill their ATP demand (67,68). The biogenesis of respiratory chain complexes requires the synthesis of proteins encoded by the mitochondrial genome and their subsequent insertion into the inner membrane. Mdm38 has been found to be associated with newly synthesized mitochondrial proteins via the ribosome, and is specifically required for efficient membrane insertion of cytochrome b and Atp6, and polytopic membrane proteins of complexes III and V, respectively (35). The LETM1 ortholog Mdm38 plays a role in respiratory chain function at the cellular level, as demonstrated by growth defects and reduced ∆ψ observed in Mdm38∆ mitochondria. LETM1 partially rescues growth defects in Mdm38∆ cells, suggesting that both proteins fulfill similar cellular functions (23).
An LETM1 RBD has been shown to be important to respiratory chain assembly through regulation of Cox1 and Cytb translation; this matrix-exposed domain displays a 14-3-3-like fold (37). LETM1 overexpression has been reported to decrease mitochondrial ATP production, oxygen consumption, and MRPL36 depletion through the use of siRNA-MRPL36 to significantly revert the LETM1-mediated ATP decrease, suggesting a functional association between LETM1 and mitochondrial ribosomes (14). Thus, the association of the LETM1 family with mitochondrial ribosomes and its consequent role in the mitochondrial translation system and biogenesis highlights the putative role of LETM1 in tumorigenesis.
4. Conclusion
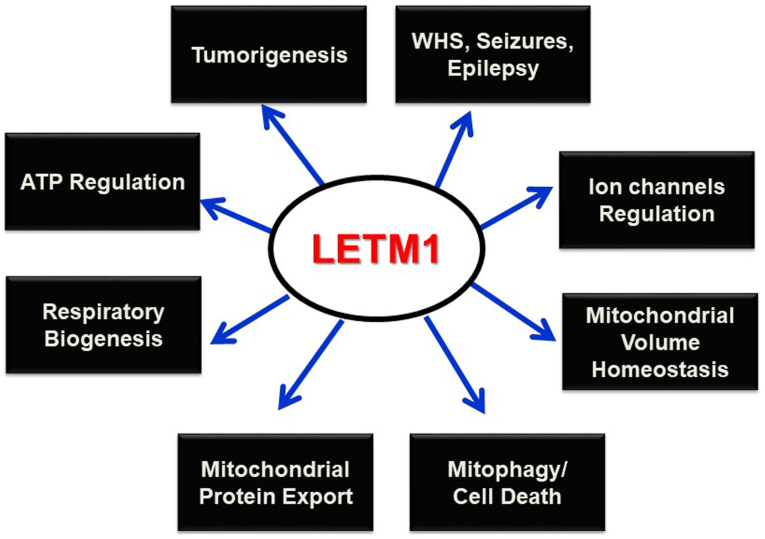
LETM1 has been cloned in an attempt to identify the genes deleted in WHS (6), and was found to be responsible for seizure development (4,10). Since this discovery, LETM1 has been shown to play prominent roles in mitochondrial K+ and Ca2+ homeostasis, volume, and morphology maintenance, and respiratory chain biogenesis, and to be indispensable in maintaining organelles and cellular physiological integrity. Its potential interaction with mitochondrial ribosomes and biogenesis, and regulation of mitochondrial ATP and morphological homeostasis underscore its putative candidacy in altered metabolism and subsequent tumorigenesis. Mitophagy has been reported as the selective degradation of depolarized mitochondria, as a result of lost K+/H+ exchange activity due to Mdm38 depletion (24); however, this process must be further explored to determine the contributing roles of the PINK/PARKIN pathway and LETM1. Studies of the possible interactions between LETM1 and mitochondrial ribosomes and its functional relevance as a translocase in mitochondrial protein synthesis and export machinery may help to establish this protein as a potential therapeutic target for various diseases such as WHS, epilepsy, cancer, and other pathophysiological conditions (Fig. 3).
Figure 3.
Schematic representation of proposed LETM1 function. Schematic diagram depicting the proposed physiological as well as pathophysiological roles of LETM1. It shows the functions of LETM1 in the mitochondria and the consequences of loss in these functions. LETM1, leucine zipper/EF-hand-containing transmembrane protein 1.
Acknowledgements
Not applicable.
Funding
This work was financially supported by research fund of Chungnam National University (Jongsun Park) and by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (grant nos. NRF-2012M3A9B6055302, NRF-2015R1A2A2A01003597 and NRF-2016K1A3A1A08953546).
Availability of data and materials
All data used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YL conceived the present study. QT analyzed the LETM1 literature and made the substantial contribution in the finalization of this manuscript. LP assessed the experimental data. RS and SP contributed to data interpretation and writing the discussion. JiP contributed to data interpretation and discussion, particularly regarding the mitochondria function of LETM1. JoP contributed to designing the study and was involved in data interpretation and writing the discussion. JoP also approved the final manuscript for publication. All authors reviewed the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schlickum S, Moghekar A, Simpson JC, Steglich C, O'Brien RJ, Winterpacht A, Endele SU. LETM1, a gene deleted in Wolf-Hirschhorn syndrome, encodes an evolutionarily conserved mitochondrial protein. Genomics. 2004;83:254–261. doi: 10.1016/j.ygeno.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 2.McQuibban AG, Joza N, Megighian A, Scorzeto M, Zanini D, Reipert S, Richter C, Schweyen RJ, Nowikovsky K. A Drosophila mutant of LETM1, a candidate gene for seizures in Wolf-Hirschhorn syndrome. Hum Mol Genet. 2010;19:987–1000. doi: 10.1093/hmg/ddp563. [DOI] [PubMed] [Google Scholar]
- 3.South ST, Bleyl SB, Carey JC. Two unique patients with novel microdeletions in 4p16.3 that exclude the WHS critical regions: Implications for critical region designation. Am J Med Genet A. 2007;143A:2137–2142. doi: 10.1002/ajmg.a.31900. [DOI] [PubMed] [Google Scholar]
- 4.Rauch A, Schellmoser S, Kraus C, Dörr HG, Trautmann U, Altherr MR, Pfeiffer RA, Reis A. First known microdeletion within the Wolf-Hirschhorn syndrome critical region refines genotype-phenotype correlation. Am J Med Genet. 2001;99:338–342. doi: 10.1002/ajmg.1203. [DOI] [PubMed] [Google Scholar]
- 5.Zollino M, Lecce R, Fischetto R, Murdolo M, Faravelli F, Selicorni A, Buttè C, Memo L, Capovilla G, Neri G. Mapping the Wolf-Hirschhorn syndrome phenotype outside the currently accepted WHS critical region and defining a new critical region, WHSCR-2. Am J Hum Genet. 2003;72:590–597. doi: 10.1086/367925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endele S, Fuhry M, Pak SJ, Zabel BU, Winterpacht A. LETM1, a novel gene encoding a putative EF-hand Ca(2+)-binding protein, flanks the Wolf-Hirschhorn syndrome (WHS) critical region and is deleted in most WHS patients. Genomics. 1999;60:218–225. doi: 10.1006/geno.1999.5881. [DOI] [PubMed] [Google Scholar]
- 7.Simon R, Bergemann AD. Mouse models of Wolf-Hirschhorn syndrome. Am J Med Genet C Semin Med Genet. 2008;148C:275–280. doi: 10.1002/ajmg.c.30184. [DOI] [PubMed] [Google Scholar]
- 8.Piekorz RP, Hoffmeyer A, Duntsch CD, McKay C, Nakajima H, Sexl V, Snyder L, Rehg J, Ihle JN. The centrosomal protein TACC3 is essential for hematopoietic stem cell function and genetically interfaces with p53-regulated apoptosis. EMBO J. 2002;21:653–664. doi: 10.1093/emboj/21.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa A, van der Bliek AM. Inverse correlation between expression of the Wolfs Hirschhorn candidate gene Letm1 and mitochondrial volume in C. elegans and in mammalian cells. Hum Mol Genet. 2007;16:2061–2071. doi: 10.1093/hmg/ddm154. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Chen G, Lu Y, Liu J, Fang M, Luo J, Cao Q, Wang X. Association of mitochondrial letm1 with epileptic seizures. Cereb Cortex. 2013;24:2533–2540. doi: 10.1093/cercor/bht118. [DOI] [PubMed] [Google Scholar]
- 11.Tamai S, Iida H, Yokota S, Sayano T, Kiguchiya S, Ishihara N, Hayashi J, Mihara K, Oka T. Characterization of the mitochondrial protein LETM1, which maintains the mitochondrial tubular shapes and interacts with the AAA-ATPase BCS1L. J Cell Sci. 2008;121:2588–2600. doi: 10.1242/jcs.026625. [DOI] [PubMed] [Google Scholar]
- 12.Froschauer E, Nowikovsky K, Schweyen RJ. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim Biophys Acta. 2005;1711:41–48. doi: 10.1016/j.bbamem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Perkins SJ, Bonner A. Structure determinations of human and chimaeric antibodies by solution scattering and constrained molecular modelling. Biochem Soc Trans. 2008;36:37–42. doi: 10.1042/BST0360037. [DOI] [PubMed] [Google Scholar]
- 14.Piao L, Li Y, Kim SJ, Byun HS, Huang SM, Hwang SK, Yang KJ, Park KA, Won M, Hong J, et al. Association of LETM1 and MRPL36 contributes to the regulation of mitochondrial ATP production and necrotic cell death. Cancer Res. 2009;69:3397–3404. doi: 10.1158/0008-5472.CAN-08-3235. [DOI] [PubMed] [Google Scholar]
- 15.Shao J, Fu Z, Ji Y, Guan X, Guo S, Ding Z, Yang X, Cong Y, Shen Y. Leucine zipper-EF-hand containing transmembrane protein 1 (LETM1) forms a Ca2+/H+ antiporter. Sci Rep. 2016;6:34174. doi: 10.1038/srep34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Kang MG, Shin S, Kwak C, Kwon T, Seo JK, Kim JS, Rhee HW. Architecture mapping of the inner mitochondrial membrane proteome by chemical tools in live cells. J Am Chem Soc. 2017;139:3651–3662. doi: 10.1021/jacs.6b10418. [DOI] [PubMed] [Google Scholar]
- 17.Stec I, van Ommen GJ, den Dunnen JT. WHSC1L1, on human chromosome 8p11.2, closely resembles WHSC1 and maps to a duplicated region shared with 4p16.3. Genomics. 2001;76:5–8. doi: 10.1006/geno.2001.6581. [DOI] [PubMed] [Google Scholar]
- 18.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardi P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 20.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 21.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Waldeck-Weiermair M, Jean-Quartier C, Rost R, Khan MJ, Vishnu N, Bondarenko AI, Imamura H, Malli R, Graier WF. Leucine zipper EF hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J Biol Chem. 2011;286:28444–28455. doi: 10.1074/jbc.M111.244517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, Wiesenberger G, Schweyen RJ. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J Biol Chem. 2004;279:30307–30315. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- 24.Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ. 2007;14:1647–1656. doi: 10.1038/sj.cdd.4402167. [DOI] [PubMed] [Google Scholar]
- 25.Mijaljica D, Prescott M, Devenish RJ. Different fates of mitochondria: Alternative ways for degradation? Autophagy. 2007;3:4–9. doi: 10.4161/auto.3011. [DOI] [PubMed] [Google Scholar]
- 26.Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldherr M, Ragnini A, Schweyer RJ, Boguski MS. MRS6-yeast homologue of the choroideraemia gene. Nat Genet. 1993;3:193–194. doi: 10.1038/ng0393-193. [DOI] [PubMed] [Google Scholar]
- 28.Gregan J, Kolisek M, Schweyen RJ. Mitochondrial Mg(2+) homeostasis is critical for group II intron splicing in vivo. Genes Dev. 2001;15:2229–2237. doi: 10.1101/gad.201301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W, Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet. 2008;17:201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- 31.Alirol E, James D, Huber D, Marchetto A, Vergani L, Martinou JC, Scorrano L. The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol Biol Cell. 2006;17:4593–4605. doi: 10.1091/mbc.e06-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 34.Sesaki H, Southard SM, Yaffe MP, Jensen RE. Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol Biol Cell. 2003;14:2342–2356. doi: 10.1091/mbc.e02-12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frazier AE, Taylor RD, Mick DU, Warscheid B, Stoepel N, Meyer HE, Ryan MT, Guiard B, Rehling P. Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery. J Cell Biol. 2006;172:553–564. doi: 10.1083/jcb.200505060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lupo D, Vollmer C, Deckers M, Mick DU, Tews I, Sinning I, Rehling P. Mdm38 is a 14-3-3-like receptor and associates with the protein synthesis machinery at the inner mitochondrial membrane. Traffic. 2011;12:1457–1466. doi: 10.1111/j.1600-0854.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 38.Ott M, Prestele M, Bauerschmitt H, Funes S, Bonnefoy N, Herrmann JM. Mba1, a membrane-associated ribosome receptor in mitochondria. EMBO J. 2006;25:1603–1610. doi: 10.1038/sj.emboj.7601070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Lonlay P, Valnot I, Barrientos A, Gorbatyuk M, Tzagoloff A, Taanman JW, Benayoun E, Chrétien D, Kadhom N, Lombès A, et al. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat Genet. 2001;29:57–60. doi: 10.1038/ng706. [DOI] [PubMed] [Google Scholar]
- 40.Visapää I, Fellman V, Vesa J, Dasvarma A, Hutton JL, Kumar V, Payne GS, Makarow M, Van Coster R, Taylor RW, et al. GRACILE syndrome, a lethal metabolic disorder with iron overload, is caused by a point mutation in BCS1L. Am J Hum Genet. 2002;71:863–876. doi: 10.1086/342773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinson JT, Fantin VR, Schönberger J, Breivik N, Siem G, McDonough B, Sharma P, Keogh I, Godinho R, Santos F, et al. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N Engl J Med. 2007;356:809–819. doi: 10.1056/NEJMoa055262. [DOI] [PubMed] [Google Scholar]
- 42.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 43.Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 44.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piao L, Li Y, Kim SJ, Sohn KC, Yang KJ, Park KA, Byun HS, Won M, Hong J, Hur GM, et al. Regulation of OPA1-mediated mitochondrial fusion by leucine zipper/EF-hand-containing transmembrane protein-1 plays a role in apoptosis. Cell Signal. 2009;21:767–777. doi: 10.1016/j.cellsig.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang E, Qu D, Huang T, Rizzi N, Boonying W, Krolak D, Ciana P, Woulfe J, Klein C, Slack RS, et al. PINK1-mediated phosphorylation of LETM1 regulates mitochondrial calcium transport and protects neurons against mitochondrial stress. Nat Commun. 2017;8:1399. doi: 10.1038/s41467-017-01435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battaglia A, Calhoun ARUL, Lortz A, Carey JC. Risk of hepatic neoplasms in Wolf-Hirschhorn syndrome (4p-): Four new cases and review of the literature. Am J Med Genet A. 2018;176:2389–2394. doi: 10.1002/ajmg.a.40469. [DOI] [PubMed] [Google Scholar]
- 50.Bayhan T, Aydin B, Yalcin B, Orhan D, Akyuz C. Hepatoblastoma and Wolf-Hirschhorn syndrome: Coincidence or a new feature of a rare disease? Pediatr Int. 2017;59:1028–1029. doi: 10.1111/ped.13345. [DOI] [PubMed] [Google Scholar]
- 51.Ozcan A, Acer H, Ciraci S, Gumus H, Karakukcu M, Patiroglu T, Ozdemir MA, Unal E. Neuroblastoma in a child with Wolf-Hirschhorn syndrome. J Pediatr Hematol Oncol. 2017;39:e224–e226. doi: 10.1097/MPH.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 52.Prunotto G, Cianci P, Cereda A, Scatigno A, Fossati C, Maitz S, Biondi A, Selicorni A. Two cases of hepatic adenomas in patients with Wolf-Hirschhorn syndrome: A new rare complication? Am J Med Genet A. 2013;161A:1759–1762. doi: 10.1002/ajmg.a.35966. [DOI] [PubMed] [Google Scholar]
- 53.Huang B, Zhang J, Zhang X, Huang C, Hu G, Li S, Xie T, Liu M, Xu Y. Suppression of LETM1 by siRNA inhibits cell proliferation and invasion of bladder cancer cells. Oncol Rep. 2017;38:2935–2940. doi: 10.3892/or.2017.5959. [DOI] [PubMed] [Google Scholar]
- 54.Li N, Zheng Y, Xuan C, Lin Z, Piao L, Liu S. LETM1 overexpression is correlated with the clinical features and survival outcome of breast cancer. Int J Clin Exp Pathol. 2015;8:12893–12900. [PMC free article] [PubMed] [Google Scholar]
- 55.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/S0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 56.Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb Symp Quant Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 57.Don AS, Hogg PJ. Mitochondria as cancer drug targets. Trends Mol Med. 2004;10:372–378. doi: 10.1016/j.molmed.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 59.Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007;292:C157–C163. doi: 10.1152/ajpcell.00272.2006. [DOI] [PubMed] [Google Scholar]
- 60.Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene. 2006;25:4717–4724. doi: 10.1038/sj.onc.1209605. [DOI] [PubMed] [Google Scholar]
- 61.Nowikovsky K, Schweyen RJ, Bernardi P. Pathophysiology of mitochondrial volume homeostasis: Potassium transport and permeability transition. Biochim Biophys Acta. 2009;1787:345–350. doi: 10.1016/j.bbabio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Alirol E, Martinou JC. Mitochondria and cancer: Is there a morphological connection? Oncogene. 2006;25:4706–4716. doi: 10.1038/sj.onc.1209600. [DOI] [PubMed] [Google Scholar]
- 63.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang SK, Piao L, Lim HT, Minai-Tehrani A, Yu KN, Ha YC, Chae CH, Lee KH, Beck GR, Park J, Cho MH. Suppression of lung tumorigenesis by leucine zipper/EF hand-containing transmembrane-1. PLoS One. 2010;5:e12535. doi: 10.1371/journal.pone.0012535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YY, McKinney KQ, Ghosh S, Iannitti DA, Martinie JB, Caballes FR, Russo MW, Ahrens WA, Lundgren DH, Han DK, et al. Subcellular tissue proteomics of hepatocellular carcinoma for molecular signature discovery. J Proteome Res. 2011;10:5070–5083. doi: 10.1021/pr2005204. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 68.Tran Q, Park J, Lee H, Hong Y, Hong S, Park S, Park J, Kim SH. TMEM39A and human diseases: A brief review. Toxicol Res. 2017;33:205–209. doi: 10.5487/TR.2017.33.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used and/or analyzed during the present study are available from the corresponding author on reasonable request.