Abstract
In plant cells, calcium (Ca2+) serves as a versatile intracellular messenger, participating in several fundamental and important biological processes. Recent studies have shown that the actin cytoskeleton is not only an upstream regulator of Ca2+ signaling, but also a downstream regulator. Ca2+ has been shown to regulates actin dynamics and rearrangements via different mechanisms in plants, and on this basis, the upstream signaling encoded within the Ca2+ transient can be decoded. Moreover, actin dynamics have also been proposed to act as an upstream of Ca2+, adjust Ca2+ oscillations, and establish cytosolic Ca2+ ([Ca2+]cyt) gradients in plant cells. In the current review, we focus on the advances in uncovering the relationship between the actin cytoskeleton and calcium in plant cells and summarize our current understanding of this relationship.
Keywords: Calcium (Ca2+), actin, ABPs, channels, pollen tube, ROP, CDPK
1. Introduction
In plants, temporary and spatial changes in cellular Ca2+ concentrations play vital roles in growth, development, and signal transduction, such as tip growth of pollen tubes and root hairs, stomatal movement, salt or osmotic stress response, temperature and hormone response, as well as beneficial and pathogenic associations with microorganisms [1,2,3,4,5]. Changes in Ca2+ concentration often serve as triggers for calcium sensors or adapters, such as calcium-dependent protein kinases (CDPKs), calcineurin B-like protein (CBL) family, and Ca2+-dependent ABPs [1,2,6,7,8]. However, the underlying mechanisms of decoding Ca2+ signals and changes of cellular Ca2+ are largely unknown. Recent advances suggest that the actin cytoskeleton plays an important role in both the upstream and downstream of Ca2+ signaling.
The actin cytoskeleton—consisting of two forms of actin, globular actin (G-actin) and filamentous actin (F-actin)—is highly conserved in eukaryotic cells [9,10,11]. The two forms of actin are dynamically converted and regulated by a plethora of actin-binding proteins (ABPs) [9,10,11]. This dynamic conversion and regulation are essential for a variety of plant physiological processes [11,12,13,14,15]. Ca2+ can remodel the actin cytoskeleton by directly binding to ABPs to activate or inactivate their activity, or by indirectly regulating their activity via calcium-stimulated protein kinases, such as CDPKs [11,16,17,18,19]. In addition, the actin cytoskeleton can alter cellular Ca2+ homeostasis by regulating the influx and efflux of Ca2+ [20,21,22,23,24,25]. For example, the dynamics of actin have been proposed to act upstream of Ca2+, adjust Ca2+ oscillations, and establish free cytosolic Ca2+ ([Ca2+]cyt) gradients in plant cells [23,24]. This review mainly focuses on the relationship between the actin cytoskeleton and calcium in plant cells.
2. The Actin Cytoskeleton Adjusts Calcium Homeostasis
In plant cells, the resting [Ca2+]cyt is maintained in the submicromolar range (about 100 nM) under normal conditions, whereas [Ca2+] is 1–10 mM in the cell wall and vacuole. Moreover, even the endoplasmic reticulum (ER) is expected to contain large amounts of Ca2+ [1,2,26]. These Ca2+ stockpiles can be used for elevating the [Ca2+]cyt level under stress conditions or growth signaling [1,2,26]. Once a stress trigger or growth signal is received, the cytosolic concentration of calcium in plant cells can increase suddenly to the micromolar level, but Ca2+ is toxic to plants if high levels of the ion remain in the cytosol for a long period [1,2,26]. Therefore, plants use their apoplast or intracellular organelles, such as the vacuole and ER, to take up and store excess Ca2+ [1,2,26]. In normal conditions, [Ca2+]cyt homeostasis plays a vital role in a myriad of physiological functions, and changes in [Ca2+]cyt result from the influx and efflux of external and internal Ca2+ stores [1,2,26,27,28]. The influx of extracellular Ca2+ occurs mainly based on the plasma membrane (PM) calcium-permeable channels, and the efflux of Ca2+ is due to its release from intracellular Ca2+ stores, such as those in the vacuole, ER, mitochondria, and chloroplasts [1,2,29].
Intriguingly, many studies have revealed that the dynamics of actin cytoskeleton act as a signal transducer, contributing to the regulation of calcium-permeable channel activity [30,31,32]. For example, in plant pollen tubes, Wang et al. reported that the actin depolymerization reagents cytochalasin D (CD) and cytochalasin B (CB) significantly increased [Ca2+]cyt levels, and that this increase in [Ca2+]cyt was abolished by the calcium channel blocker La3+ or Gd3+ [20]. Moreover, the effects of actin depolymerization reagents on the channel were prevented by pretreatment with phalloidin, a stabilizer of actin filaments [20]. In Vicia faba guard cells, Zhang et al. found that disruption of actin dynamics activated stretch-activated (SA) Ca2+ channels, while stabilization of actin filaments blocked the activation of these SA channels under stretching or hypotonic treatment [33].
The reorganization and dynamics of the actin cytoskeleton serve as a link that also transfers external signals to induce calcium influx. For instance, in response to cold stimuli, disruption of actin filaments leads to Ca2+ influx, whereas stabilization of actin filaments blocks the cold-induced Ca2+ influx in plant cells [34,35,36]. Changes in [Ca2+]cyt concentration in response to gravity are also widely accepted in plant biology [37,38]. Toyota et al. reported that the increase in [Ca2+]cyt through mechanosensitive (MS) channels induced by changes in the gravity vector was attenuated by actin-specific reagents, implying that actin dynamics adjust calcium homeostasis by regulating the activity of MS Ca2+-permeable channels [39,40]. Regarding the underlying molecular mechanisms, plant annexins are considered to be as unconventional Ca2+-permeable channels and are involved in many developmental and stress-related processes [41,42,43,44]. Based on a few studies, annexins, such as AtANN5 and AnxGb6, can bind to F-actin and may function as scaffolding proteins for calcium and the actin cytoskeleton [45,46]. Therefore, actin dynamics may regulate the activity of Ca2+-permeable channels by influencing the annexin-actin interaction [43]. [Ca2+]cyt changes may also derive from the release of the ion from the mitochondria and vacuole [23,24,47,48]. A study on Arabidopsis root hairs indicated that disruption of actin dynamics by latrunculin B (Lat B) and jasplakinolide (Jas) decreased the Ca2+ concentration in the mitochondria and induced an instantaneous elevation of [Ca2+]cyt, followed by a continuous decrease [23]. Moreover, Ca2+ concentration gradients exist in mitochondria from the tip region to the basal region of the root hair, and this Ca2+ gradient can be disrupted by actin-specific reagents, such as Lat B and Jas [23]. These findings suggest that a highly organized and dynamic actin cytoskeleton is vital for maintaining calcium homeostasis in root hairs.
Actin dynamics and Ca2+ play important roles in response to salt stress in plants, and the Arp2/3 complex, a nucleation factor of actin filaments that consists of seven subunits, was shown to integrate these two components [24]. Zhao et al. found that the disruption of actin dynamics by Lat B treatment increased [Ca2+]cyt in response to salt stress, and plants lacking the subunit proteins in the Arp2/3 complex showed enhanced increases in [Ca2+]cyt in response to salt stress, decreased mitochondria movement, and hypersensitivity to salt. Furthermore, ARP2/3 complex promotes actin assembly around mitochondria and drives mitochondrial movement [24]. Similar to the mechanism in plants, Boldogh et al. demonstrated that the mitochondrial motility in yeast is driven by actin polymerization and that this process requires the Arp2/3 complex [47]. These reports suggest that actin dynamics regulate mitochondria-dependent Ca2+ homeostasis in response to salt stress. In stomatal cells, although the actin-specific agents Jas and Lat B have no effect on resting tonoplast efflux, these two agents have opposite effects on the tonoplast efflux responding to exogenous abscisic acid (ABA). When treated with exogenous ABA, Jas reduced the ABA-induced transient stimulation of tonoplast efflux, while Lat B enhanced it [48], suggesting that actin dynamics adjust cellular calcium homeostasis by regulating calcium efflux from the vacuole.
Taken together, the reports above suggest that actin dynamics act as upstreams of calcium and adjust cellular calcium homeostasis by regulating the influx and efflux of Ca2+.
3. The Actin Cytoskeleton Acts a Potential Downstream of Calcium Signaling
Calcium signaling also regulates the dynamics of the actin cytoskeleton [12,18,19,49,50]. Pollen tube elongation depends on actin cytoskeleton remodeling, and actin dynamics are associated with the oscillation of the Ca2+ concentration gradients at the tip region of the pollen tube [49,50,51,52,53,54,55,56], suggesting that the Ca2+ gradient might precisely regulate actin dynamics to promote tube growth. In the tip-growing cell (e.g., protonema in the moss Physcomitrella patens) high [Ca2+]cyt promotes actin filament disassembly, but low [Ca2+]cyt promotes the assembly of a tip-localized actin spot. Moreover, abolishing the Ca2+ gradient leads to dramatic actin accumulation at the protonema tip. Together, these data indicate that the tip calcium gradient regulates actin accumulation to promote tip growth [57]. Calcium signaling is known to regulate actin dynamics through three main pathways: 1, calcium directly binds to ABPs to precisely remodel the actin cytoskeleton; 2, calcium-stimulated protein kinases, such as CDPKs, phosphorylate ABPs to adjust their activity and thus regulate actin dynamics; and 3, the Rho family of small GTPases (ROP GTPases) signaling pathway mediates actin cytoskeleton regulation via Ca2+.
3.1. Calcium Directly Binds to ABPs to Regulate Their Activity and Effect on Actin Dynamics
Actin dynamics are regulated spatially and temporally by different classes of ABPs, such as G-actin sequestration proteins, nucleating proteins, severing, and depolymerizing proteins, bundling and crosslinking proteins and end-capping proteins [9]. The activities of ABPs are regulated by several signaling factors, such as calcium (Ca2+), pH, phosphatidylinositol (4,5) bisphosphate (PIP2), and phosphatidic acid (PA) [2,11]. Among these signaling factors, Ca2+ is the most important second messenger for the regulation of plant development and stress signaling [2,11].
From pollen hydration and germination to pollen tube tip growth, the process is accompanied by actin rearrangement regulated via numerous ABPs and calcium oscillations [14,15,58,59]. In dehydrated pollen grains, short actin fragments were the main form. In hydrated pollen grains, long, thin actin filaments appear. Upon germination, parallel actin cables encircle the shank of the newly growing tube and highly dynamic actin structures form in the tip [12,58]. During pollen tube tip growth, highly dynamic actin filaments are present in the apical dome; short actin filaments are arranged into a mesh ring or fringe-like F-actin structure in the subapical region; long, thick actin bundles are parallelly aligned in the shank region; and overall, a tip-focused calcium gradient is established in the pollen tube, in which maximum calcium concentrations can reach 1–3 μM in the tip [14,15]. Therefore, pollen tubes have been used as a model for the study of actin dynamics and calcium oscillations [14,15,59,60]. Over the past few years, based on this system, many studies have found that several Ca2+-dependent ABPs regulate actin dynamics in the tip growth of pollen tubes, including profilin, LIM domain-containing proteins (LILIM1 and PLIM2c), MICROTUBULE-ASSOCIATED PROTEIN18 (MAP18), MICROTUBULE-DESTABILIZING PROTEIN25 (MDP25), Rho-like GTPase of plants (ROP)-interactive and CRIB motif-containing protein1 (RIC1), and Villins (VLNs) [49,61,62,63,64,65,66,67]. In addition, in other physiological processes, a few ABPs, such as OsVLN2, VLN4 and fragmin-like protein, have also been characterized as regulating actin dynamics in a Ca2+-dependent manner [68,69,70]. These Ca2+-dependent/regulated ABPs are discussed below and summarized in Table 1 and Figure 1.
Table 1.
Summary of calcium-dependent actin-binding proteins.
| ABPs | Species | Tissues | Activities on Actin Organization | References |
|---|---|---|---|---|
| 115ABP | Lilium longiflorum | Pollen tube | MF nucleation, capping, and bundling | [71,72] |
| 135ABP | Lilium longiflorum | Pollen tube | MF nucleation, capping, and bundling | [73,74] |
| ABP29 | Lilium longiflorum | Pollen tube | MF nucleation, capping, and severing | [12] |
| ABP41 | Lilium davidii | Pollen tube | MF severing and capping | [62,75] |
| AnxGb6 | Gossypium barbadense | Fiber | Actin binding | [45] |
| Fragmin-like 42-kD protein | Mimosa pudica | Petiole | MF severing | [68] |
| LILIM1 | Lilium longiflorum | Pollen tube | MF bundling | [61] |
| MAP18 | Arabidopsis thaliana | Pollen tube | MF severing | [65] |
| MdMVG | Malus domestica | Pollen tube | MF severing | [75] |
| MDP25 | Arabidopsis thaliana | Pollen tube | MF severing | [49,76] |
| Mimosa annexin | Mimosa pudica | Pulvinus | MF binding | [77] |
| Myosin | Lilium longiflorum | None | MF binding | [78] |
| OsVLN2 | Oryza sativa | Roots and Shoots | MF bundling, severing, and capping | [70] |
| P34/35 | Lycopersicon esculentum | None | MF binding | [79] |
| PLIM2c | Arabidopsis thaliana | Pollen and Pollen tube | MF bundling | [64] |
| PrABP80 | Papaver rhoeas | None | MF nucleation, capping and severing | [80] |
| Profilin | Zea mays | None | Sequester G-Actin | [81,82,83] |
| RIC1 | Arabidopsis thaliana | Pollen tube | MF severing and capping | [67] |
| VLN2/5 | Arabidopsis thaliana | Pollen tube | MF bundling, severing and capping | [63,66] |
| VLN2/3 | Arabidopsis thaliana | Sclerenchyma | MF bundling, severing and capping | [84,85] |
| VLN4 | Arabidopsis thaliana | Root hair | MF bundling, severing and capping | [69] |
MF indicates actin filaments.
Figure 1.
Simplified scheme showing calcium regulation of actin dynamics via direct and indirect pathways. CDPKs, calcium-dependent protein kinases; CBL/CIPK, calcineurin B-like protein and CBL-interacting protein kinases; ADFs, actin-depolymerizing factors; ABPs, actin-binding proteins.
Among these Ca2+-dependent ABPs, the major group is the villin/gelsolin/fragmin superfamily, which is a class of multifunctional ABPs that remodel actin dynamics by nucleating, severing, depolymerizing, and bundling actin [86,87]. These ABPs typically possess two to six tandem gelsolin-like (G) homologous domains, which contain at least a conserved Ca2+-binding site, based on protein crystallography [86,88,89,90]. In plants, the first two villins to be characterized were 135ABP and 115ABP from lily (Lilium) pollen, which can bind and bundle actin filaments in a Ca2+-dependent manner [71,73,74]. There are five villin genes (VLN1-VLN5) in the Arabidopsis genome, and all of them bundle actin filaments in a Ca2+-insensitive manner [63,69,84,85,91]. In addition, with the exception of VILLIN1 (VLN1), these VLNs (VLN2-VLN5) exhibit nucleating, severing, and capping activities in a Ca2+-sensitive manner [63,69,84,85,91]. Moreover, there are two types of Ca2+ binding sites in the villin/gelsolin/fragmin superfamily, and as regards to gelsilin, Ca2+ binds type-1 sites at the interface of gelsolin is important for its interaction with actin, while Ca2+ binds type-2 sites buried within gelsolin activate this protein [92]. Khurana et al. found that all five VLNs in Arabidopsis have just one type-1 site in the G1 domain. In contrast to VLN1, which has only two type-2 sites in the G2/4 domains, VLN2-5 has one type-2 site in the G1 domain and another two to three type-2 sites in the G2/4/6 domains [84]. These results suggest that VLNs with a type-2 site in the G1 domain and a greater number of type-2 sites will be Ca2+-sensitive [84]. Furthermore, several studies demonstrated that a strong tip-focused calcium gradient is vital for VLN-mediated actin-filament severing to promote actin turnover, which may be correlated with actin dynamics and calcium gradients in pollen tubes [63,69,84,85,91]. In Arabdopsis root hairs, VLN4 caps and severs actin filaments in the presence of 5 μM Ca2+ but fails to do so in the presence of 0.5 μM Ca2+; these Ca2+ concentrations resemble those at the tip region and the shank region, respectively. Therefore, it may be that VLN4 needs to stabilize the actin cytoskeleton via its bundling activity in the shank region, while it promotes actin turnover by severing and capping actin filaments at the tip region [69]. To gain further insight, point mutations of calcium-binding site of VLN4 may be needed in the future. Moreover, Wu et al. also found that OsVLN2 exhibits conserved Ca2+-dependent actin filament severing, actin filament bundling, and actin filament capping activities. OsVLN2 promotes recycling of PIN2 and polar auxin transport by regulating actin dynamics to modulate morphogenesis of plant architecture [70].
In Papaver rhoeas (poppy) pollen, Huang et al. isolated and characterized an 80 kDa gelsolin-like protein, PrABP80, which possesses six tandem G domains and exhibits Ca2+-dependent actin-filament-severing and barbed-end-capping activities. This calcium-mediated actin filament depolymerization is vital for the self-incompatibility response in P. rhoeas pollen [80]. A fragmin-like protein with an apparent molecular mass of 42 kDa has also been identified in Mimosa pudica, which possesses three G domains, severs actin filaments, and enhances actin polymerization in a Ca2+-dependent manner [68]. From Lilium davidii pollen, the Ren group also identified a fragmin-like protein, LdABP41, which possesses three G domains, and the smallest member of this superfamily, ABP29, which possesses only two G domains; they found that these two proteins nucleate and sever actin filaments in a Ca2+-sensitive manner [12,75]. Recently, an ABP containing domains of myosin, villin, and GRAM (MdMVG) that directly binds and severs actin filaments in a Ca2+-dependent manner was identified in apples (Malus domestica). Moreover, MdMVG can physically interact with S-RNase, and this interaction inhibits the actin filament-severing activity of MdMVG in vitro. Therefore, S-RNase interacts with MdMVG to inhibit its actin filament-severing activity, while Ca2+ binds to MdMVG to enhance its severing activity and then regulates self-pollen tube growth during the early stage of self-pollination induction [93].
LIM is a family of proteins containing two LIM domains that essentially consist of two zinc fingers linked together by a short, two-amino-acid spacer and function as a module for protein–protein interaction [94]. Plant LIMs have been found to directly bind to actin filaments and bundle them into thick bundles [61,64,95,96,97]. To date, many LIMs have been identified in different species, including Arabidopsis, cotton (Gossypium hirsutum), lily (Lilium longiflorum), sunflower (Helianthus annuus), and tobacco (Nicotiana tabacum) [61,64,96,97,98,99,100], but only LILIM1 from lily and PLIM2c from Arabidopsis can bind to actin filaments and bundle them in response to Ca2+, and their activity is downregulated by high concentrations of Ca2+ [61,64]. One possible reason for this pattern is that LlLIM1 and PLIM2c are expressed in pollen tubes and regulate actin dynamics in response to calcium gradients in the pollen tube.
Two Arabidopsis PM-associated cation-binding proteins (PCaPs), namely, PCaP1 and PCaP2, which can bind to the PM and to calcium, have been proven to be microtubule (MT)-associated proteins, named MICROTUBULE-DESTABILIZING PROTEIN25 (MDP25) and MICROTUBULE-ASSOCIATED PROTEIN18 (MAP18), respectively [76,101,102,103,104]. Recently, these two MT-associated proteins were found to also regulate actin dynamics in pollen tubes [49,65]. Zhu et al. found that MAP18 exhibits Ca2+-dependent actin-filament-severing activity, and this activity is essential for determining the direction of pollen tube growth [65]. Moreover, MAP18 can modulate actin dynamics in tip-growing cells to regulate the direction of pollen tube growth and proper positioning of the nucleus in root hairs [65,105]. The second MT-associated protein, MDP25, also exhibits actin filament severing activity in the negative regulation of pollen tube growth. Furthermore, the calcium-binding site, formed by VEEKK residues, is responsible for Ca2+-dependent actin filament-severing activity [49,102]. Additionally, another MT-associated protein, RIC1, which belongs to the Rho-like GTPase of plants (ROP)-interactive, CRIB motif-containing protein family and which reorders MTs via promoting the MT-severing activity of katanin [106,107,108], was identified to regulate actin dynamics at the apical PM as well as the cytosol in pollen tubes [67]. Zhou et al. found that RIC1 could bind and cap the barbed ends of actin filaments and sever them in a Ca2+-dependent manner, and the distribution of RIC1 at the apical PM exhibits oscillation in concert with pollen tube growth. Moreover, high concentrations of Ca2+ enhance actin-filament-severing activity of RIC1 to regulate the abundance and oscillatory amplitude of fine actin filaments in pollen tubes [67]. Considering that the negative regulator of pollen tube elongation, MDP25, located at the apical PM, also exhibits actin filament severing activity [49], these studies suggest that Ca2+ might coordinate diverse actin filament severing proteins to regulate the proper organization, abundance, and dynamics of actin filaments for pollen tube tip growth [67].
Profilin as a key player in the early step of actin assembly is a G-actin binding protein with a molecular mass of 12–15 kDa capable of maintaining a pool of monomeric actin in cells [109,110,111,112,113]. The role of profilin is a condition-dependent dual function in actin polymerization and depolymerization [109,110,111,112,113]. Several studies have demonstrated that the binding of birch pollen profilin to muscle actin and the G-actin sequestering activity of maize profilin1/5 are regulated by calcium [81,82,83]. Myosin is a motor protein that uses energy to travel along actin filaments, and this actomyosin-based transport system is a key feature of cellular structure and dynamics, such as cell division and cytoplasmic streaming [114,115]. Yokota et al. first reported that Ca2+ could inhibit the travel of 170 kDa myosin along the actin filaments responsible for cytoplasmic streaming. Moreover, the light chain of myosin-CaM might be involved in Ca2+ regulation [78]. Annexin is another potential candidate that may link the actin cytoskeleton with calcium signaling in plants, since several vertebrate annexins have been proven to bind actin filaments in a Ca2+-dependent manner both in vitro and in vivo [116,117,118,119,120]. Similar to the annexins in animals, a few plant annexins have also been shown to bind actin filaments in a Ca2+-dependent manner, such as p34 and p35 from tomato, annexin from Mimosa, and AnxGb6 from cotton [45,77,79].
Collectively, these studies demonstrate that calcium can regulate actin dynamics by directly binding to ABPs to regulate their activity.
3.2. Calcium Indirectly Regulates the Actin Dynamics Via Calcium-Stimulated Protein Kinases, CDPKs
Posttranslational modifications are very important for the activity of some proteins [121,122]. Among various modifications, phosphorylation is widely involved in the activation and inactivation of ABPs in eukaryotes [123,124]. In plants, Ca2+ has been demonstrated to activate the CDPK family; the activated CDPKs subsequently transfer Ca2+ signals to downstream phosphorylation substrates to decode information related to Ca2+ oscillations and spikes [6,125,126]. The CDPK family was previously proven to colocalize with actin filaments, but CDPKs do not directly interact with actin [127,128], suggesting that CDPKs might interact with ABPs to localize to actin and that actin dynamics might be indirectly regulated by Ca2+ via activation/deactivation of CDPKs.
ADF/cofilin is a family of ABPs that can bind both G-actin and actin filaments and then depolymerize and sever actin filaments to promote rapid actin turnover [129,130,131,132]. Moreover, several two-dimensional electrophoresis studies revealed that both phosphorylated and unphosphorylated forms of ADFs exist in plant extracts from Arabidopsis, maize, tobacco, and moss [133,134,135,136]. The Hussey group found that maize actin-depolymerizing factor 3 (ZmADF3) could be phosphorylated at Ser-6 by CDPKs, and this phosphorylation inhibits an actin filament severing or depolymerizing activity of ZmADF3 [18,19]. However, which of the CDPKs phosphorylate these plant ADFs is not yet known [18,19]. Uno et al. used AtCPK4 and AtCPK11 as baits to isolate putative CDPK-interacting proteins by yeast two-hybrid (Y2H) screening, and their results showed an ADF among the CDPK-interacting proteins [137]. Recently, Dong et al. found that AtCDPK6 can phosphorylate AtADF1, predominantly at its Ser-6, and overexpression of AtCDPK6 can depress the activity of wild-type AtADF1 in severing/depolymerizing actin filaments, but not that of a mutant of AtADF1(S6A) in seedling root cells [138]. While ADF/cofilin is phosphorylated at Ser-3 in animal cells, the equivalent Ser-6 is conserved in plant ADFs [18,133], suggesting that ADFs could be phosphorylated at Ser-6 by calcium-stimulated protein kinases in plants. These results imply that calcium could activate CDPKs to phosphorylate ADFs and thus indirectly adjust actin dynamics (Figure 1).
Additionally, the CBL family, which serves as another Ca2+ sensor in plants, has been suggested to be involved in regulating the dynamics of the actin cytoskeleton [139]. CBLs perceive calcium signals to activate specific protein kinases, namely, CBL-interacting protein kinases (CIPKs); they then form CBL/CIPK complexes to relay the signals to downstream responses [8,140,141,142,143]. AtCBL4, also known as SOS3, which senses salt-elicited Ca2+ signals, interacts with and activates CIPK24/SOS2 to participate in salt-stress sensing and tolerance [144]. Ye et al. found that a loss of function in SOS3 disrupts the arrangement of actin filaments; actin assembly and arrangement in sos3 are abnormal in response to salt stress, and external calcium or a low concentration of latrunculin A (Lat A) can partially rescue this phenomenon [139]. Collectively, these results suggest that the actin cytoskeleton is closely related to CBL/CIPK pathway that is possibly integrated by calcium signaling (Figure 1).
3.3. ROP GTPase Signaling Mediates Actin Cytoskeleton Regulation by Calcium
In plants, ROP GTPase, also called RAC-GTPase, plays a fundamental role in several important cellular processes, such as tip growth of pollen tubes and root hairs, regulation of the actin cytoskeleton, and hormone and stress response [107,145,146,147]. Similar to the Rho family proteins in fungi and mammalian cells, ROP GTPases regulate plant actin and MT cytoskeletal organization and dynamics [51,59,107,148,149,150]. ROP GTPases, serving as important molecular switches, mediate several signaling pathways in which calcium and the actin and MT cytoskeleton act downstream of the ROP GTPase signaling pathway [51,59,107,149].
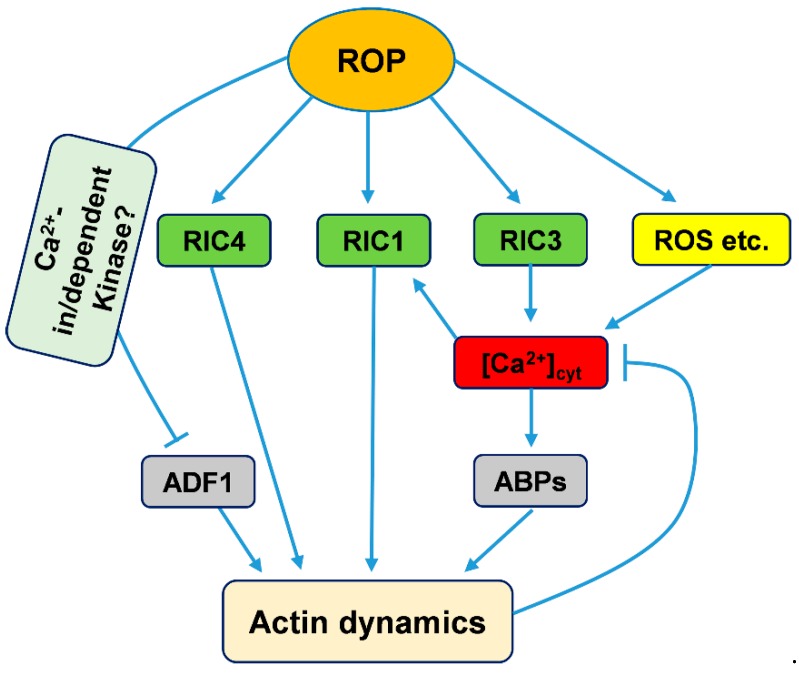
During pollen tube tip growth, ROP GTPase has been found to be involved in maintaining the Ca2+ gradient by interacting with reactive oxygen species (ROS), phosphoinositides, and pH gradient signaling in the tip regions of pollen tubes [59,150,151,152,153]. Furthermore, ROP GTPases can control Ca2+ gradients through their downstream plant-specific family of ROP effectors, RICs [52,146]. Two RICs, RIC3 and RIC4, adjust the tip Ca2+ gradients: RIC3 directly stimulates Ca2+ influx in the tube apex, while RIC4-mediated actin assembly might inhibit the accumulation of Ca2+ at the tip [56,146,154]. Gu et al. found that ROP GTPase could activate RIC3 and RIC4, which promote the formation of a tip Ca2+ gradient and the assembly of tip actin filaments, respectively. Then, elevated Ca2+ might induce actin disassembly through some ABPs, such as profilin or members of the villin/gelsolin/fragmin family [59,155]. Based on these results, there is a check-and-balance model encompassing ROP GTPase, RIC3 and RIC4 control of actin dynamics, the Ca2+ gradient and tip growth [56,155]. Together with the above study of RIC1, ROP GTPase regulates the organization and dynamics of actin mediated by Ca2+ gradients mainly via its downstream effectors, the RIC family (Figure 2).
Figure 2.
Working model to explain ROP signaling pathway mediation of actin cytoskeleton regulation by calcium in pollen tubes. ROP, ROP GTPases; RIC, ROP-interactive and CRIB motif-containing protein; ROS, Reactive oxygen species.
Chen et al. found that NtADF1(S6A) with a nonphosphorylatable Ala substitution at the Ser-6 position shows high activity to counteract the inhibiting effect of NtRac1 overexpression on pollen tube tip growth, while the phosphomimic form of NtADF1(S6D) shows a reduced ability to counteract this inhibiting effect [135]. Moreover, overexpression of NtRac1 diminishes the actin-binding activity of NtADF1 but has no effect on the association of NtADF1(S6A) with actin filaments in pollen tubes [135]. Given that the phosphorylation of ADF/Cofilin inhibits its actin-filament-severing and depolymerizing activity, these reports suggest that ROP GTPase could regulate the organization and dynamics of actin through phosphorylation modification [135]. However, the mechanism underlying this phosphorylation remains unknown. In pollen tubes, the Hussey group showed that CDPKs could phosphorylate ZmADF3 to decorate its actin filament severing/depolymerizing activity, and that CDPKs can be activated by Ca2+ [18,19]. In addition, ROP GTPase promotes calcium accumulation in the tips of pollen tubes [59]. Overall, we speculate that ROP GTPase increases calcium levels to activate CDPKs, and then phosphorylates ADF to regulate its actin filament severing and depolymerizing activities.
4. Outlook
Overall, recent studies have shown that calcium can adjust the actin cytoskeleton by directly binding to ABPs and regulating their activity or by indirectly regulating their activity via calcium-stimulated protein kinases, such as CDPKs [17,18]. On the other hand, actin dynamics could maintain calcium homeostasis by regulating the activity of Ca2+-permeable channels [20]. Although tremendous progress in understanding the connection between the actin cytoskeleton and calcium has been made, many questions remain unanswered. The phosphorylation of ABPs by calcium-stimulated protein kinases is not well understood, and the calcium-stimulated protein kinases involved in phosphorylating ABPs and their phosphorylation substrates have yet to be identified. Moreover, it is unknown how the actin cytoskeleton regulates the activity of calcium-permeable channels, and one of the possible ways is that actin cytoskeleton adjusts their location on the membrane or the process of intracellular transport of calcium channels or receptors. In summary, future studies should focus on the signaling pathways through which calcium transduces environmental or developmental changes to the actin cytoskeleton and the mechanism(s) through which calcium homeostasis is regulated by the actin cytoskeleton.
Acknowledgments
We thank members of the Xiang laboratory for helpful discussion. We are grateful to Hui Su (Northwest University) and Jingen Zhu (The Catholic University of America) for their valuable comments on the manuscript. We also thank the anonymous reviewers for their efforts and constructive advice to improve the review.
Abbreviations
| ABA | abscisic acid |
| ABPs | actin-binding proteins |
| ADF | actin-depolymerizing factor |
| CB | cytochalasin B |
| CBL | calcineurin B-like protein |
| CD | cytochalasin D |
| CDPKs | calcium-dependent protein kinases |
| CIPKs | CBL-interacting protein kinases |
| ER | endoplasmic reticulum |
| F-actin | filamentous actin |
| G-actin | globular actin |
| Jas | jasplakinolide |
| Lat A | latrunculin A |
| Lat B | latrunculin B |
| MAP18 | MICROTUBULE-ASSOCIATED PROTEIN18 |
| MDP25 | MICROTUBULE-DESTABILIZING PROTEIN25 |
| MS | mechanosensitive |
| MT | microtubule |
| PA | phosphatidic acid |
| PM | plasma membrane |
| PIP2 | phosphatidylinositol (4,5) bisphosphate |
| ROP GTPases | The rho family of small GTPases |
| ROS | reactive oxygen species |
| SA | stretch-activated |
| VLN | VILLIN |
| Y2H | yeast two-hybrid |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
Author Contributions
Conceptualization and writing, Y.X. and D.Q.
Funding
This research was funded by grants from the National Natural Science Foundation of China (grant numbers 31470283, 31700161, and 31722005) and the Fundamental Research Funds for the Central Universities (grant number lzujbky-2017-k14).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hepler P.K. Calcium: A central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodd A.N., Kudla J., Sanders D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 3.Clark G., Roux S.J. Role of Ca2+ in mediating plant responses to extracellular ATP and ADP. Int. J. Mol. Sci. 2018;19:3590. doi: 10.3390/ijms19113590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipfel C., Oldroyd G.E. Plant signalling in symbiosis and immunity. Nature. 2017;543:328–336. doi: 10.1038/nature22009. [DOI] [PubMed] [Google Scholar]
- 5.Aldon D., Mbengue M., Mazars C., Galaud J.P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018;19:3. doi: 10.3390/ijms19030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto K., Kudla J. Calcium decoding mechanisms in plants. Biochimie. 2011;93:2054–2059. doi: 10.1016/j.biochi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Ranty B., Aldon D., Cotelle V., Galaud J.P., Thuleau P., Mazars C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 2016;7:327. doi: 10.3389/fpls.2016.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudla J., Becker D., Grill E., Hedrich R., Hippler M., Kummer U., Parniske M., Romeis T., Schumacher K. Advances and current challenges in calcium signaling. New Phytol. 2018;218:414–431. doi: 10.1111/nph.14966. [DOI] [PubMed] [Google Scholar]
- 9.Staiger C.J., Blanchoin L. Actin dynamics: Old friends with new stories. Curr. Opin. Plant Biol. 2006;9:554–562. doi: 10.1016/j.pbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Higaki T., Sano T., Hasezawa S. Actin microfilament dynamics and actin side-binding proteins in plants. Curr. Opin. Plant Biol. 2007;10:549–556. doi: 10.1016/j.pbi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Blanchoin L., Staiger C.J. Signaling to actin stochastic dynamics. Annu. Rev. Plant Biol. 2015;66:415–440. doi: 10.1146/annurev-arplant-050213-040327. [DOI] [PubMed] [Google Scholar]
- 12.Xiang Y., Huang X., Wang T., Zhang Y., Liu Q., Hussey P.J., Ren H. ACTIN BINDING PROTEIN 29 from Lilium pollen plays an important role in dynamic actin remodeling. Plant Cell. 2007;19:1930–1946. doi: 10.1105/tpc.106.048413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perico C., Sparkes I. Plant organelle dynamics: Cytoskeletal control and membrane contact sites. New Phytol. 2018;220:381–394. doi: 10.1111/nph.15365. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y. The cytoskeleton in the pollen tube. Curr. Opin. Plant Biol. 2015;28:111–119. doi: 10.1016/j.pbi.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Qu X., Jiang Y., Chang M., Liu X., Zhang R., Huang S. Organization and regulation of the actin cytoskeleton in the pollen tube. Front. Plant Sci. 2015;5:786. doi: 10.3389/fpls.2014.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada M., Kong S.G. Actin-mediated movement of chloroplasts. J. Cell Sci. 2018;131:210310. doi: 10.1242/jcs.210310. [DOI] [PubMed] [Google Scholar]
- 17.Smertenko A.P., Jiang C.J., Simmons N.J., Weeds A.G., Davies D.R., Hussey P.J. Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J. 1998;14:187–193. doi: 10.1046/j.1365-313X.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 18.Allwood E.G., Smertenko A.P., Hussey P.J. Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 2001;499:97–100. doi: 10.1016/S0014-5793(01)02528-5. [DOI] [PubMed] [Google Scholar]
- 19.Smertenko A.P., Deeks M.J., Hussey P.J. Strategies of actin reorganisation in plant cells. J. Cell Sci. 2010;123:3019–3028. doi: 10.1242/jcs.071126. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y.F., Fan L.M., Zhang W.Z., Zhang W., Wu W.H. Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004;136:3892–3904. doi: 10.1104/pp.104.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cárdenas L., Vidali L., Domínguez J., Pérez H., Sánchez F., Hepler P.K., Quinto C. Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 1998;116:871–877. doi: 10.1104/pp.116.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cárdenas L., Lovy-Wheeler A., Kunkel J.G., Hepler P.K. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 2008;146:1611–1621. doi: 10.1104/pp.107.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Zhu Y., Ling Y., Zhang H., Liu P., Baluska F., Samaj J., Lin J., Wang Q. Disruption of actin filaments induces mitochondrial Ca2+ release to the cytoplasm and [Ca2+]c changes in Arabidopsis root hairs. BMC Plant Biol. 2010;10:53. doi: 10.1186/1471-2229-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Pan Z., Zhang Y., Qu X., Zhang Y., Yang Y., Jiang X., Huang S., Yuan M., Schumaker K.S., et al. The actin-related Protein2/3 complex regulates mitochondrial-associated calcium signaling during salt stress in Arabidopsis. Plant Cell. 2013;25:4544–4559. doi: 10.1105/tpc.113.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D.H., Acharya B., Liu W., Zhang W. Interaction between calcium and actin in guard cell and pollen signaling networks. Plants. 2013;2:615–634. doi: 10.3390/plants2040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins T.V., Evans M.J., Woolfenden H.C., Morris R.J. Towards the physics of calcium signalling in plants. Plants. 2013;2:541–588. doi: 10.3390/plants2040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demidchik V., Shabala S., Isayenkov S., Cuin T., Pottosin I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018;220:49–69. doi: 10.1111/nph.15266. [DOI] [PubMed] [Google Scholar]
- 28.Kurusu T., Kuchitsu K., Nakano M., Nakayama Y., Iida H. Plant mechanosensing and Ca2+ transport. Trends Plant Sci. 2013;18:227–233. doi: 10.1016/j.tplants.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Costa A., Navazio L., Szabo I. The contribution of organelles to plant intracellular calcium signalling. J. Exp. Bot. 2018;69:4175–4193. doi: 10.1093/jxb/ery185. [DOI] [PubMed] [Google Scholar]
- 30.Schwiebert E.M., Mills J.W., Stanton B.A. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J. Biol. Chem. 1994;269:7081–7089. [PubMed] [Google Scholar]
- 31.Cantiello H.F. Role of actin filament organization in cell volume and ion channel regulation. J. Exp. Zool. 1997;279:425–435. doi: 10.1002/(SICI)1097-010X(19971201)279:5<425::AID-JEZ4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Janmey P. The cytoskeleton and cell signaling: Component localization and mechanical coupling. Physiol. Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W., Fan L.M. Actin dynamics regulates voltage-dependent calcium-permeable channels of the vicia faba guard cell plasma membrane. J. Integr. Plant Biol. 2009;51:912–921. doi: 10.1111/j.1744-7909.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 34.Mazars C., Thion L., Thuleau P., Graziana A., Knight M.R., Moreau M., Ranjeva R. Organization of cytoskeleton controls the changes in cytosolic calcium of cold-shocked Nicotiana plumbaginifolia protoplasts. Cell Calcium. 1997;22:413–420. doi: 10.1016/S0143-4160(97)90025-7. [DOI] [PubMed] [Google Scholar]
- 35.Orvar B.L., Sangwan V., Omann F., Dhindsa R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23:785–794. doi: 10.1046/j.1365-313x.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 36.Konopka-Postupolska D., Clark G. Annexins as overlooked regulators of membrane trafficking in plant cells. Int. J. Mol. Sci. 2017;18:863. doi: 10.3390/ijms18040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasano J.M., Massa G.D., Gilroy S. Ionic signaling in plant responses to gravity and touch. J. Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 38.Bushart T.J., Cannon A., Clark G., Roux S.J. Structure and function of CrACA1, the major PM-type Ca2+-ATPase, expressed at the peak of the gravity-directed trans-cell calcium current in spores of the fern Ceratopteris richardii. Plant Biol. 2014;16:151–157. doi: 10.1111/plb.12107. [DOI] [PubMed] [Google Scholar]
- 39.Toyota M., Furuichi T., Tatsumi H., Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2008;146:505–514. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsumi H., Furuichi T., Nakano M., Toyota M., Hayakawa K., Sokabe M., Iida H. Mechanosensitive channels are activated by stress in the actin stress fibres, and could be involved in gravity sensing in plants. Plant Biol. 2014;16:18–22. doi: 10.1111/plb.12095. [DOI] [PubMed] [Google Scholar]
- 41.Gerke V., Moss S.E. Annexins: From structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 42.Gerke V., Creutz C.E., Moss S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Boil. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 43.Davies J.M. Annexin-mediated calcium signalling in plants. Plants (Basel) 2014;3:128–140. doi: 10.3390/plants3010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Song J., Clark G., Roux S.J. ANN1 and ANN2 function in post-phloem sugar transport in root tips to affect primary root growth. Plant Physiol. 2018;178:390–401. doi: 10.1104/pp.18.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y., Wang J., Zhang L., Zuo K. A cotton annexin protein AnxGb6 regulates fiber elongation through its interaction with actin 1. PLoS ONE. 2013;8:e66160. doi: 10.1371/journal.pone.0066160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J., Wu X., Yuan S., Qian D., Nan Q., An L., Xiang Y. Annexin5 plays a vital role in Arabidopsis pollen development via Ca2+-dependent membrane trafficking. PLoS ONE. 2014;9:e102407. doi: 10.1371/journal.pone.0102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boldogh I.R., Yang H.C., Nowakowski W.D., Karmon S.L., Hays L.G., Yates J.R., Pon L.A. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc. Natl. Acad. Sci. USA. 2001;98:3162–3167. doi: 10.1073/pnas.051494698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacRobbie E.A., Kurup S. Signalling mechanisms in the regulation of vacuolar ion release in guard cells. New Phytol. 2007;175:630–640. doi: 10.1111/j.1469-8137.2007.02131.x. [DOI] [PubMed] [Google Scholar]
- 49.Qin T., Liu X.M., Li J.J., Sun J.B., Song L.N., Mao T.L. Arabidopsis microtubule-destabilizing protein25 functions in pollen tube growth by severing actin filaments. Plant Cell. 2014;26:325–339. doi: 10.1105/tpc.113.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian D., Nan Q., Yang Y., Li H., Zhou Y., Zhu J., Bai Q., Zhang P., An L., Xiang Y. Gelsolin-like domain 3 plays vital roles in regulating the activities of the lily villin/gelsolin/fragmin superfamily. PLoS ONE. 2015;10:e0143174. doi: 10.1371/journal.pone.0143174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu Y., Wu G., Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang J.U., Gu Y., Lee Y.J., Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell. 2005;16:5385–5399. doi: 10.1091/mbc.e05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai G., Parrotta L., Cresti M. Organelle trafficking, the cytoskeleton, and pollen tube growth. J. Integr. Plant Biol. 2015;57:63–78. doi: 10.1111/jipb.12289. [DOI] [PubMed] [Google Scholar]
- 54.Szymanski D., Staiger C.J. The actin cytoskeleton: Functional arrays for cytoplasmic organization and cell shape control. Plant Physiol. 2018;176:106–118. doi: 10.1104/pp.17.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monteiro D., Liu Q., Lisboa S., Scherer G.E., Quader H., Malho R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J. Exp. Bot. 2005;56:1665–1674. doi: 10.1093/jxb/eri163. [DOI] [PubMed] [Google Scholar]
- 56.Yan A., Xu G., Yang Z.B. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc. Natl. Acad. Sci. USA. 2009;106:22002–22007. doi: 10.1073/pnas.0910811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bascom B.C., Jr., Winship L.J., Bezanilla M. Simultaneous imaging and functional studies reveal a tight correlation between calcium and actin networks. Proc. Natl. Acad. Sci. USA. 2018;115:E2869–E2878. doi: 10.1073/pnas.1711037115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren H., Xiang Y. The function of actin-binding proteins in pollen tube growth. Protoplasma. 2007;230:171–182. doi: 10.1007/s00709-006-0231-x. [DOI] [PubMed] [Google Scholar]
- 59.Fu Y. The actin cytoskeleton and signaling network during pollen tube tip growth. J. Integr. Plant Biol. 2010;52:131–137. doi: 10.1111/j.1744-7909.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 60.Guan Y., Guo J., Li H., Yang Z. Signaling in pollen tube growth: Crosstalk, feedback, and missing links. Mol. Plant. 2013;6:1053–1064. doi: 10.1093/mp/sst070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H.J., Wan A.R., Jauh G.Y. An actin-binding protein, LlLIM1, mediates calcium and hydrogen regulation of actin dynamics in pollen tubes. Plant Physiol. 2008;147:1619–1636. doi: 10.1104/pp.108.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T., Xiang Y., Hou J., Ren H.Y. ABP41 is involved in the pollen tube development via fragmenting actin filaments. Mol. Plant. 2008;1:1048–1055. doi: 10.1093/mp/ssn073. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H., Qu X.L., Bao C.C., Khurana P., Wang Q.N., Xie Y.R., Zheng Y., Chen N., Blanchoin L., Staiger C.J., et al. Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell. 2010;22:2749–2767. doi: 10.1105/tpc.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papuga J., Hoffmann C., Dieterle M., Moes D., Moreau F., Tholl S., Steinmetz A., Thomas C. Arabidopsis LIM proteins: A family of actin bundlers with distinct expression patterns and modes of regulation. Plant Cell. 2010;22:3034–3052. doi: 10.1105/tpc.110.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L., Zhang Y., Kang E., Xu Q., Wang M., Rui Y., Liu B., Yuan M., Fu Y. MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell. 2013;25:851–867. doi: 10.1105/tpc.113.110528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qu X.L., Zhang H., Xie Y.R., Wang J., Chen N.Z., Huang S.J. Arabidopsis villins promote actin turnover at pollen tube tips and facilitate the construction of actin collars. Plant Cell. 2013;25:1803–1817. doi: 10.1105/tpc.113.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z., Shi H., Chen B., Zhang R., Huang S., Fu Y. Arabidopsis RIC1 severs actin filaments at the apex to regulate pollen tube growth. Plant Cell. 2015;27:1140–1161. doi: 10.1105/tpc.114.135400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamashiro S., Kameyama K., Kanzawa N., Tamiya T., Mabuchi I., Tsuchiya T. The gelsolin/fragmin family protein identified in the higher plant Mimosa pudica. J. Biochem. 2001;130:243–249. doi: 10.1093/oxfordjournals.jbchem.a002978. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Xiao Y., Du F., Cao L., Dong H., Ren H. Arabidopsis VILLIN4 is involved in root hair growth through regulating actin organization in a Ca2+-dependent manner. New Phytol. 2011;190:667–682. doi: 10.1111/j.1469-8137.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 70.Wu S., Xie Y., Zhang J., Ren Y., Zhang X., Wang J., Guo X., Wu F., Sheng P., Wang J., et al. VLN2 regulates plant architecture by affecting microfilament dynamics and polar auxin transport in rice. Plant Cell. 2015;27:2829–2845. doi: 10.1105/tpc.15.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakayasu T., Yokota E., Shimmen T. Purification of an actin-binding protein composed of 115-kDa polypeptide from pollen tubes of lily. Biochem. Biophys. Res. Commun. 1998;249:61–65. doi: 10.1006/bbrc.1998.9088. [DOI] [PubMed] [Google Scholar]
- 72.Yokota E., Vidali L., Tominaga M., Tahara H., Orii H., Morizane Y., Hepler P.K., Shimmen T. Plant 115-kDa actin filament bundling protein, P-115-ABP, is a homologue of plant villin and is widely distributed in cells. Plant Cell Physiol. 2003;44:1088–1099. doi: 10.1093/pcp/pcg132. [DOI] [PubMed] [Google Scholar]
- 73.Vidali L., Yokota E., Cheung A.Y., Shimmen T., Hepler P.K. The 135 kDa actin-bundling protein from Lilium longiflorum pollen is the plant homologue of villin. Protoplasma. 1999;209:283–291. doi: 10.1007/BF01453456. [DOI] [Google Scholar]
- 74.Yokota E., Shimmen T. The 135-kDa actin-bundling protein from lily pollen tubes arranges F-actin into bundles with uniform polarity. Planta. 1999;209:264–266. doi: 10.1007/s004250050631. [DOI] [PubMed] [Google Scholar]
- 75.Fan X., Hou J., Chen X., Chaudhry F., Staiger C.J., Ren H. Identification and characterization of a Ca2+-dependent actin filament-severing protein from lily pollen. Plant Physiol. 2004;136:3979–3989. doi: 10.1104/pp.104.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J., Wang X., Qin T., Zhang Y., Liu X., Sun J., Zhou Y., Zhu L., Zhang Z., Yuan M., et al. MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell. 2011;23:4411–4427. doi: 10.1105/tpc.111.092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoshino D., Hayashi A., Temmei Y., Kanzawa N., Tsuchiya T. Biochemical and immunohistochemical characterization of Mimosa annexin. Planta. 2004;219:867–875. doi: 10.1007/s00425-004-1285-7. [DOI] [PubMed] [Google Scholar]
- 78.Yokota E., Muto S., Shimmen T. Inhibitory regulation of higher-plant myosin by Ca2+ ions. Plant Physiol. 1999;119:231–240. doi: 10.1104/pp.119.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calvert C.M., Gant S.J., Bowles D.J. Tomato annexins p34 and p35 bind to F-actin and display nucleotide phosphodiesterase activity inhibited by phospholipid binding. Plant Cell. 1996;8:333–342. doi: 10.1105/tpc.8.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang S., Blanchoin L., Chaudhry F., Franklin-Tong V.E., Staiger C.J. A gelsolin-like protein from Papaver rhoeas pollen (PrABP80) stimulates calcium-regulated severing and depolymerization of actin filaments. J. Biol. Chem. 2004;279:23364–23375. doi: 10.1074/jbc.M312973200. [DOI] [PubMed] [Google Scholar]
- 81.Giehl K., Valenta R., Rothkegel M., Ronsiek M., Mannherz H.G., Jockusch B.M. Interaction of plant profilin with mammalian actin. Eur. J. Biochem. 1994;226:681–689. doi: 10.1111/j.1432-1033.1994.tb20096.x. [DOI] [PubMed] [Google Scholar]
- 82.Perelroizen I., Didry D., Christensen H., Chua N.H., Carlier M.F. Role of nucleotide exchange and hydrolysis in the function of profilin in actin assembly. J. Biol. Chem. 1996;271:12302–12309. doi: 10.1074/jbc.271.21.12302. [DOI] [PubMed] [Google Scholar]
- 83.Kovar D.R., Drøbak B.K., Staiger C.J. Maize profilin isoforms are functionally distinct. Plant Cell. 2000;12:583–598. doi: 10.1105/tpc.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khurana P., Henty J.L., Huang S., Staiger A.M., Blanchoin L., Staiger C.J. Arabidopsis VILLIN1 and VILLIN3 have overlapping and distinct activities in actin bundle formation and turnover. Plant Cell. 2010;22:2727–2748. doi: 10.1105/tpc.110.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bao C., Wang J., Zhang R., Zhang B., Zhang H., Zhou Y., Huang S. Arabidopsis VILLIN2 and VILLIN3 act redundantly in sclerenchyma development via bundling of actin filaments. Plant J. 2012;71:962–975. doi: 10.1111/j.1365-313X.2012.05044.x. [DOI] [PubMed] [Google Scholar]
- 86.Sun H.Q., Yamamoto M., Mejillano M., Yin H.L. Gelsolin, a multifunctional actin regulatory protein. J. Biol. Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 87.Huang S., Qu X., Zhang R. Plant villins: Versatile actin regulatory proteins. J. Integr. Plant Biol. 2015;57:40–49. doi: 10.1111/jipb.12293. [DOI] [PubMed] [Google Scholar]
- 88.Nag S., Larsson M., Robinson R.C., Burtnick L.D. Gelsolin: The tail of a molecular gymnast. Cytoskeleton. 2013;70:360–384. doi: 10.1002/cm.21117. [DOI] [PubMed] [Google Scholar]
- 89.Burtnick L.D., Koepf E.K., Grimes J., Jones E.Y., Stuart D.I., McLaughlin P.J., Robinson R.C. The crystal structure of plasma gelsolin: Implications for actin severing, capping, and nucleation. Cell. 1997;90:661–670. doi: 10.1016/S0092-8674(00)80527-9. [DOI] [PubMed] [Google Scholar]
- 90.Kazmirski S.L., Isaacson R.L., An C., Buckle A., Johnson C.M., Daggett V., Fersht A.R. Loss of a metal-binding site in gelsolin leads to familial amyloidosis-Finnish type. Nat. Struct. Biol. 2002;9:112–116. doi: 10.1038/nsb745. [DOI] [PubMed] [Google Scholar]
- 91.Huang S., Robinson R.C., Gao L.Y., Matsumoto T., Brunet A., Blanchoin L., Staiger C.J. Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization. Plant Cell. 2005;17:486–501. doi: 10.1105/tpc.104.028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choe H., Burtnick L.D., Mejillano M., Yin H.L., Robinson R.C., Choe S. The calcium activation of gelsolin: Insights from the 3A˚ structure of the G4-G6/actin complex. J. Mol. Biol. 2002;324:691–702. doi: 10.1016/S0022-2836(02)01131-2. [DOI] [PubMed] [Google Scholar]
- 93.Yang Q., Meng D., Gu Z., Li W., Chen Q., Li Y., Yuan H., Yu J., Liu C., Li T. Apple S-RNase interacts with an actin-binding protein, MdMVG, to reduce pollen tube growth by inhibiting its actin-severing activity at the early stage of self-pollination induction. Plant J. 2018;95:41–56. doi: 10.1111/tpj.13929. [DOI] [PubMed] [Google Scholar]
- 94.Smith M.A., Hoffman L.M., Beckerle M.C. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014;24:575–583. doi: 10.1016/j.tcb.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moes D., Gatti S., Hoffmann C., Dieterle M., Moreau F., Neumann K., Schumacher M., Diederich M., Grill E., Shen W.H., et al. A LIM domain protein from tobacco involved in actin-bundling and histone gene transcription. Mol. Plant. 2013;6:483–502. doi: 10.1093/mp/sss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas C., Hoffmann C., Dieterle M., Van Troys M., Ampe C., Steinmetz A. Tobacco WLIM1 is a novel F-actin binding protein involved in actin cytoskeleton remodeling. Plant Cell. 2006;18:2194–2206. doi: 10.1105/tpc.106.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomas C., Moreau F., Dieterle M., Hoffmann C., Gatti S., Hofmann C., Van Troys M., Ampe C., Steinmetz A. The LIM domains of WLIM1 define a new class of actin bundling modules. J. Biol. Chem. 2007;282:33599–33608. doi: 10.1074/jbc.M703691200. [DOI] [PubMed] [Google Scholar]
- 98.Luo M., Xiao Y.H., Hou L., Luo X.Y., Li D.M., Pei Y. Cloning and expression analysis of a LIM-domain protein gene from cotton (Gossypium hirsuturm L.) Yi Chuan Xue Bao. 2003;30:175–182. [PubMed] [Google Scholar]
- 99.Li Y., Jiang J., Li L., Wang X.L., Wang N.N., Li D.D., Li X.B. A cotton LIM domain-containing protein (GhWLIM5) is involved in bundling actin filaments. Plant Physiol. Biochem. 2013;66:34–40. doi: 10.1016/j.plaphy.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 100.Han L.B., Li Y.B., Wang H.Y., Wu X.M., Li C.L., Luo M., Wu S.J., Kong Z.S., Pei Y., Jiao G.L., et al. The dual functions of WLIM1a in cell elongation and secondary wall formation in developing cotton fibers. Plant Cell. 2013;25:4421–4438. doi: 10.1105/tpc.113.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ide Y., Nagasaki N., Tomioka R., Suito M., Kamiya T., Maeshima M. Molecular properties of a novel, hydrophilic cation-binding protein associated with the plasma membrane. J. Exp. Bot. 2007;58:1173–1183. doi: 10.1093/jxb/erl284. [DOI] [PubMed] [Google Scholar]
- 102.Nagasaki N., Tomioka R., Maeshima M. A hydrophilic cation-binding protein of Arabidopsis thaliana, AtPCaP1, is localized to plasma membrane via N-myristoylation and interacts with calmodulin and the phosphatidylinositol phosphates PtdIns (3,4,5) P (3) and PtdIns (3,5) P (2) FEBS J. 2008;275:2267–2282. doi: 10.1111/j.1742-4658.2008.06379.x. [DOI] [PubMed] [Google Scholar]
- 103.Wang X., Zhu L., Liu B.Q., Wang C., Jin L.F., Zhao Q., Yuan M. Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell. 2007;19:877–889. doi: 10.1105/tpc.106.048579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kato M., Nagasaki-Takeuchi N., Ide Y., Maeshima M. An Arabidopsis hydrophilic Ca2+-binding protein with a PEVK-rich domain, PCaP2, is associated with the plasma membrane and interacts with calmodulin and phosphatidylinositol phosphates. Plant Cell Physiol. 2010;51:366–379. doi: 10.1093/pcp/pcq003. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Kang E., Yuan M., Fu Y., Zhu L. PCaP2 regulates nuclear positioning in growing Arabidopsis thaliana root hairs by modulating filamentous actin organization. Plant Cell Rep. 2015;34:1317–1330. doi: 10.1007/s00299-015-1789-6. [DOI] [PubMed] [Google Scholar]
- 106.Fu Y., Gu Y., Zheng Z., Wasteneys G., Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 107.Fu Y., Xu T., Zhu L., Wen M., Yang Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr. Biol. 2009;19:1827–1832. doi: 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin D., Cao L., Zhou Z., Zhu L., Ehrhardt D., Yang Z., Fu Y. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr. Biol. 2013;23:290–297. doi: 10.1016/j.cub.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 109.Sun T., Li S., Ren H. Profilin as a regulator of the membrane-actin cytoskeleton interface in plant cells. Front. Plant Sci. 2013;4:512. doi: 10.3389/fpls.2013.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao L., Henty-Ridilla J.L., Blanchoin L., Staiger C.J. Profilin-dependent nucleation and assembly of actin filaments controls cell elongation in Arabidopsis. Plant Physiol. 2016;170:220–323. doi: 10.1104/pp.15.01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu X., Qu X., Jiang Y., Chang M., Zhang R., Wu Y., Fu Y., Huang S. Profilin regulates apical actin polymerization to control polarized pollen tube growth. Mol. Plant. 2015;8:1694–1709. doi: 10.1016/j.molp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 112.Fan T., Zhai H., Shi W., Wang J., Jia H., Xiang Y., An L. Overexpression of profilin 3 affects cell elongation and F-actin organization in Arabidopsis thaliana. Plant Cell Rep. 2013;32:149–160. doi: 10.1007/s00299-012-1349-2. [DOI] [PubMed] [Google Scholar]
- 113.Sun H., Qiao Z., Chua K.P., Tursic A., Liu X., Gao Y.G., Mu Y., Hou X., Miao Y. Profilin Negatively regulates formin-mediated actin assembly to modulate PAMP-triggered plant immunity. Curr. Biol. 2018;28:1882–1895. doi: 10.1016/j.cub.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 114.Guhathakurta P., Prochniewicz E., Thomas D.D. Actin-Myosin interaction: Structure, function and drug discovery. Int. J. Mol. Sci. 2018;19:2628. doi: 10.3390/ijms19092628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pollard T.D., Cooper J.A. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schlaepfer D.D., Haigler H.T. Characterization of Ca2+-dependent phospholipid binding and phosphorylation of lipocortin I. J. Biol. Chem. 1987;262:6931–6937. [PubMed] [Google Scholar]
- 117.Khanna N.C., Helwig E.D., Ikebuchi N.W., Fitzpatrick S., Bajwa R., Waisman D.M. Purification and characterization of annexin proteins from bovine lung. Biochemistry. 1990;29:4852–4862. doi: 10.1021/bi00472a015. [DOI] [PubMed] [Google Scholar]
- 118.Traverso V., Morris J.F., Flower R.J., Buckingham J. Lipocortin 1 (annexin 1) in patches associated with the membrane of a lung adenocarcinoma cell line and in the cell cytoplasm. J. Cell Sci. 1998;111:1405–1418. doi: 10.1242/jcs.111.10.1405. [DOI] [PubMed] [Google Scholar]
- 119.Babiychuk E.B., Palstra R.J., Schaller J., Kämpfer U., Draeger A. Annexin VI participates in the formation of a reversible, membrane-cytoskeleton complex in smooth muscle cells. J. Biol. Chem. 1999;274:35191–35195. doi: 10.1074/jbc.274.49.35191. [DOI] [PubMed] [Google Scholar]
- 120.Tzima E., Trotter P.J., Orchard M.A., Walker J.H. Annexin V relocates to the platelet cytoskeleton upon activation and binds to a specific isoform of actin. Eur. J. Biochem. 2000;267:4720–4730. doi: 10.1046/j.1432-1327.2000.01525.x. [DOI] [PubMed] [Google Scholar]
- 121.Pedrazzini E., Vitale A. Protein biosynthesis and maturation in the ER. Methods Mol. Biol. 2018;1675:179–189. doi: 10.1007/978-1-4939-7389-7_14. [DOI] [PubMed] [Google Scholar]
- 122.Arsova B., Watt M., Usadel B. Monitoring of plant protein post-translational modifications using targeted proteomics. Front. Plant Sci. 2018;9:1168. doi: 10.3389/fpls.2018.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013;25:457–469. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 124.Miao Y., Tipakornsaowapak T., Zheng L., Mu Y., Lewellyn E. Phospho-regulation of intrinsically disordered proteins for actin assembly and endocytosis. FEBS J. 2018;285:2762–2784. doi: 10.1111/febs.14493. [DOI] [PubMed] [Google Scholar]
- 125.Myers C., Romanowsky S.M., Barron Y.D., Garg S., Azuse C.L., Curran A., Davis R.M., Hatton J., Harmon A.C., Harper J.F. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009;59:528–539. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 126.Shi S., Li S., Asim M., Mao J., Xu D., Ullah Z., Liu G., Wang Q., Liu H. The Arabidopsis Calcium-dependent protein kinases (CDPKs) and their roles in plant growth regulation and abiotic stress responses. Int. J. Mol. Sci. 2018;19:1900. doi: 10.3390/ijms19071900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Putnam-Evans C., Harmon A.C., Palevitz B.A., Fechheimer M., Cormier M.J. Calcium-dependent protein kinase is localized with F-actin in plant cells. Cell Motil. Cytoskeleton. 1989;12:12–22. doi: 10.1002/cm.970120103. [DOI] [Google Scholar]
- 128.Chehab E.W., Patharkar O.R., Hegeman A.D., Taybi T., Cushman J.C. Autophosphorylation and subcellular localization dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiol. 2004;135:1430–1446. doi: 10.1104/pp.103.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carlier M.F., Santolini J., Lanrent V., Melki R., Didry D., Hong Y., Xia G.X., Chua N.H., Pantolni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: Implication in actin-based motility. J. Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galkin V.E., Orlova A., Kudryashov D.S., Solodukhin A., Reisler E., Schröder G.F., Egelman E.H. Remodeling of actin filaments by ADF/cofilin proteins. Proc. Natl. Acad. Sci. USA. 2011;108:20568–20572. doi: 10.1073/pnas.1110109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suarez C., Roland J., Boujemaa-Paterski R., Kang H., McCullough B.R., Reymann A.C., Guérin C., Martiel J.L., De la Cruz E.M., Blanchoin L. Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr. Biol. 2011;21:862–868. doi: 10.1016/j.cub.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nan Q., Qian D., Niu Y., He Y.X., Tong S.F., Niu Z.M., Ma J., Yang Y., An L., Wan D., et al. Plant actin depolymerizing factors possess opposing biochemical properties arising from key amino acid changes throughout evolution. Plant Cell. 2017;29:395–408. doi: 10.1105/tpc.16.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang C.J., Weeds A.G., Hussey P.J. The maize actin depolymerizing factor, ZmADF3, redistributes to the growing tip of elongating root hairs and can be induced to translocate into the nucleus with actin. Plant J. 1997;12:1035–1043. doi: 10.1046/j.1365-313X.1997.12051035.x. [DOI] [PubMed] [Google Scholar]
- 134.Dong C.H., Xia G.X., Hong Y., Ramachandran S., Kost B., Chua N.H. ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell. 2001;13:1333–1346. doi: 10.1105/tpc.13.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen C.Y., Cheung A.Y., Wu H.M. Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell. 2003;15:237–249. doi: 10.1105/tpc.007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Augustine R.C., Vidali L., Kleinman K.P., Bezanilla M. Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J. 2008;54:863–875. doi: 10.1111/j.1365-313X.2008.03451.x. [DOI] [PubMed] [Google Scholar]
- 137.Uno Y., Rodriguez M.M.A., Maher E., Cushman J.C. Identification of proteins that interact with catalytically active calcium-dependent protein kinases from Arabidopsis. Mol. Genet. Genom. 2009;281:375–390. doi: 10.1007/s00438-008-0419-1. [DOI] [PubMed] [Google Scholar]
- 138.Dong C.H., Hong Y. Arabidopsis CDPK6 phosphorylates ADF1 at N-terminal serine 6 predominantly. Plant Cell Rep. 2013;32:1715–1728. doi: 10.1007/s00299-013-1482-6. [DOI] [PubMed] [Google Scholar]
- 139.Ye J., Zhang W., Guo Y. Arabidopsis SOS3 plays an important role in salt tolerance by mediating calcium-dependent microfilament reorganization. Plant Cell Rep. 2013;32:139–148. doi: 10.1007/s00299-012-1348-3. [DOI] [PubMed] [Google Scholar]
- 140.Sanyal S.K., Kanwar P., Samtani H., Kaur K., Jha S.K., Pandey G.K. Alternative splicing of CIPK3 results in distinct target selection to propagate aba signaling in Arabidopsis. Front. Plant Sci. 2017;8:1924. doi: 10.3389/fpls.2017.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y., Li T., John S.J., Chen M., Chang J., Yang G., He G. A CBL-interacting protein kinase TaCIPK27 confers drought tolerance and exogenous ABA sensitivity in transgenic Arabidopsis. Plant Physiol. Biochem. 2017;123:103–113. doi: 10.1016/j.plaphy.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 142.Bender K.W., Zielinski R.E., Huber S.C. Revisiting paradigms of ca2+ signaling protein kinase regulation in plants. Biochem. J. 2018;475:207–223. doi: 10.1042/BCJ20170022. [DOI] [PubMed] [Google Scholar]
- 143.Wang X., Hao L., Zhu B., Jiang Z. Plant calcium signaling in response to potassium deficiency. Int. J. Mol. Sci. 2018;19:3456. doi: 10.3390/ijms19113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu W.Z., Deng M., Li L., Yang B., Li H., Deng H., Jiang Y.Q. Rapeseed calcineurin B-like protein CBL4, interacting with CBL-interacting protein kinase CIPK24, modulates salt tolerance in plants. Biochem. Biophys. Res. Commun. 2015;467:467–471. doi: 10.1016/j.bbrc.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 145.Rivero C., Traubenik S., Zanetti M.E., Blanco F.A. Small GTPases in plant biotic interactions. Small GTPases. 2017;23:1–11. doi: 10.1080/21541248.2017.1333557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feiquelman G., Fu Y., Yalovsky S. Rop GTPases structure-function and signaling pathyways. Plant Physiol. 2018;176:57–59. doi: 10.1104/pp.17.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bascom C.J.R., Burkart G.M., Mallett D.R., O’Sullivan J.E., Tomaszewski A.J., Walsh K., Bezanilla M. Systematic survey of the function of ROP regulators and effectors during tip growth in the moss P. patens. J. Exp. Bot. 2018 doi: 10.1093/jxb/ery376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakamura M., Claes A.R., Grebe T., Hermkes R., Viotti C., Ikeda Y., Grebe M. Auxin and ROP GTPase signaling of polar nuclear migration in root epidermal hair cells. Plant Physiol. 2018;176:378–391. doi: 10.1104/pp.17.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li C., Lu H., Li W., Yuan M., Fu Y. A ROP2-RIC1 pathway fine-tunes microtubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ. 2017;40:1127–1142. doi: 10.1111/pce.12905. [DOI] [PubMed] [Google Scholar]
- 150.Burkart G.M., Baskin T.I., Bezanilla M. A family of ROP proteins that suppresses actin dynamics, and is essential for polarized growth and cell adhesion. J. Cell Sci. 2015;128:2553–2564. doi: 10.1242/jcs.172445. [DOI] [PubMed] [Google Scholar]
- 151.Bloch D., Pleskot R., Pejchar P., Potocký M., Trpkošová P., Cwiklik L., Vukašinovi N., Sternberg H., Yalovsky S., Zárský V. Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol. 2016;172:980–1002. doi: 10.1104/pp.16.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bloch D., Yalovsky S. Cell polarity signaling. Curr. Opin. Plant Biol. 2013;16:734–742. doi: 10.1016/j.pbi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 153.Michard E., Simon A.A., Tavares B., Wudick M.M., Feijó J.A. Signaling with ions: The keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol. 2017;173:91–111. doi: 10.1104/pp.16.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu G., Gu Y., Li S., Yang Z. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell. 2001;13:2841–2856. doi: 10.1105/tpc.13.12.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gu Y., Wang Z., Yang Z. ROP/RAC GTPase: An old new master regulator for plant signaling. Curr. Opin. Plant Biol. 2004;7:527–536. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]